Abstract
Background
Repeat cross sectional surveys document the trend of prevalence rates for non-communicable diseases and their risk factors. In this study, we compare the prevalence rates for risk factors for cardiovascular disease in urban and rural Vellore between 1991–1994 and 2010–2012.
Methods
Cross sectional survey was carried out in 1991–1994 in a rural block in Vellore district and in Vellore town, to study the prevalence of cardiovascular risk factors among adults aged 30–60 years. A repeat survey was done in 2010–2012 using the WHO STEPS method. In both surveys, socio-demographic and behavioral history, physical measurements, biochemical measurements, and medical history were obtained. Age adjusted rates were used to compare the rates in the two surveys.
Results
In the rural areas, there was a three times increase in diabetes and body mass index (BMI) ≥25 kg/m2 (overweight/obese) with a doubling of the prevalence of hypertension. In urban areas there was a tripling of diabetes, doubling of proportion with BMI ≥ 25 kg/m2 and 50% increase in prevalence of hypertension. While the proportion of male current smokers reduced by 50% in both rural and urban Vellore, lifetime abstainers to alcohol decreased in the rural area from 46.8% to 37.5% (p < 0.001).
Conclusions
There has been an alarming rise in diabetes, hypertension, and overweight/obese with an even greater increase in rural areas. Alcohol use is increasing while smoking is on the decline. Primary prevention programs are required urgently to stem the rising incidence of non-communicable diseases in India.
Keywords: Non-communicable diseases, WHO STEPS, Diabetes, Tobacco, Alcohol
1. Introduction
The prevalence of coronary heart disease in India has been found to be progressively increasing from approximately 6.5% in urban areas in the 1960s to 10.5% in 2000, and from 2% in the 1970s in the rural areas to about 4.5% in 2000.1 Traditional risk factors such as diet, physical activity, abnormal lipids, diabetes, and hypertension have been shown to account for most of the risk for myocardial infarction worldwide.2 Various cross sectional surveys throughout the country have shown the increasing prevalence of cardiovascular risk factors such as diabetes and hypertension.3, 4, 5, 6 However, evidence from repeated cross-sectional surveys in the same location is limited with only a few such periodic surveys e.g. Jaipur, Chennai.7, 8, 9, 10 Cardiovascular risk factor surveillance is an important component of control programs for non-communicable diseases (NCDs) and repeated cross sectional surveys drawn from the same population are useful for monitoring population trends.11
This paper compares the findings from two cross sectional studies conducted in urban and rural areas of Vellore, Tamil Nadu, in 1991–1994 and 2010–2012, assessing the changes in prevalence of risk factors for coronary heart disease. The detailed results of the repeat survey conducted in 2010–2012 have been published separately.12
2. Methods
2.1. Study design
A cross sectional study done in 1991–1994 in Vellore town and a rural block of Vellore district was repeated in 2010–2012 in the same location. These surveys were conducted as part of a multi-centric study with the other centers being rural and urban Delhi.
2.2. Setting and sample selection
In 1991–1994 a study was conducted in 20 urban clusters of Vellore town and 23 clusters of Kaniyambadi, a rural block in Vellore district, using probability proportional to population size, to document the prevalence of coronary heart disease and its risk factors.
A repeat survey was done in 48 urban clusters (12 consecutive clusters each from four urban zones) and nine randomly selected clusters out of the 23 rural clusters surveyed earlier, between June 2010 to December 2012, in the same town and rural block.
In the first survey, all individuals aged 30–60 years currently residing in the selected urban and rural clusters were invited to participate. In the repeat survey, the first 40 consecutive households from a randomly selected street in each of the 48 urban clusters were selected for the survey in the urban area, while all eligible individuals aged 30–64 years in the selected rural clusters were invited. As the first survey was done among adults aged 30–60 years and the second among 30–64 years, the comparison of results in this paper is restricted to the population aged 30–60 years in both surveys. There were no other exclusion criteria except age as mentioned.
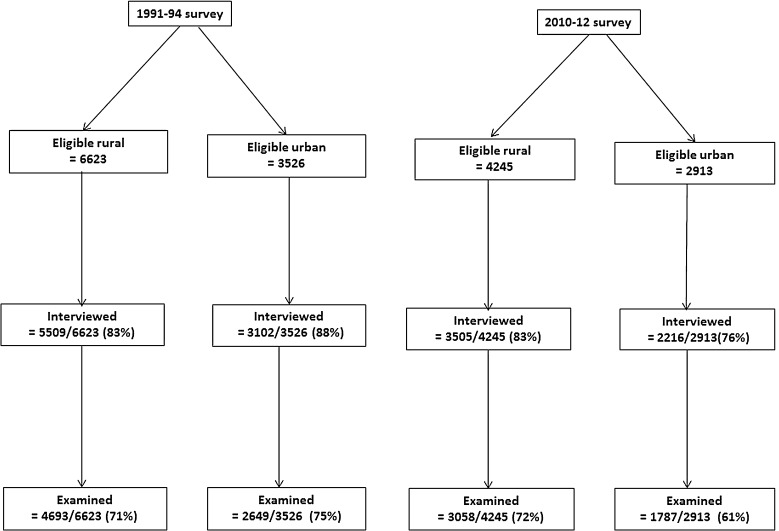
The eligible populations (all those aged 30–60 years) in the study areas and response rates obtained in the two surveys are shown in Fig. 1. Overall the numbers of participants examined in the first survey were 7342 (4693 rural, 2649 urban) and 4845 (3058 rural, 1787 urban) in the second survey (Fig. 1).
Fig. 1.
Eligible population (all those aged 30–60 years) and response rates.
2.3. Measurements and data collection
In the first survey, the questionnaires used were prepared jointly by both the study sites in conjunction with experts at the Indian Council of Medical Research, pre-tested and checked for reliability using repeated pilot surveys before administration by field workers through house-to-house interviews. In the repeat survey, the WHO STEPS method and questionnaire13 were used with information being collected by trained field workers through home visits and details of the methodology have been described in an earlier paper.12 In both surveys, special clinics were arranged at the villages/wards for the interviewed participants to collect clinical data, physical measurements, and fasting blood samples.
While in the 1991 survey, a random zero sphygmomanometer was used to record blood pressure, taking an average of three readings, the second survey used an automated apparatus (Omron HEM 7080), taking an average of two readings. Height was measured using an SECA 213 stadiometer and weight using a digital weighing machine (Essae, accuracy 0.01 kg). Venous blood samples were collected early morning after an overnight fasting of at least 8 hours. In both surveys plasma glucose was tested using the enzymatic calorimetric glucose-oxidase peroxidase method and lipids by calorimetric CHOD-PAP method, using autoanalysers. In the first survey accuracy of the biochemical measurements was checked by analyzing quality control sera from M/S Boehringer Mannheim Co., West Germany along with every batch of samples. The values obtained from the quality control sera and the study samples were comparable throughout the study period of the first survey. The quality control methods for the second survey have been described in the earlier paper.12
Written informed consent was obtained from the participants, and the study was approved by the Institutional Research Committee and Ethics Board of the tertiary healthcare institution conducting the study.
2.4. Statistical methods
Sample sizes calculated for the second survey based on expected prevalence of CHD of 1.7% in the rural area and 6% in the urban area were 5000 and 3000 respectively, as described in the earlier publication.12 Age adjustment was done by direct standardization of the results of the second survey to the survey population in 1991–1994, to enable comparison of the rates obtained in both surveys. Comparison of proportions was done using the chi-square test and of means using the independent t-test. Due to lack of complete availability of raw data from the initial survey and differences in data collection tools and definitions, only those parameters could be compared for which comparable information was available from both surveys. Therefore, the comparison is restricted to comparison of prevalence rates of current smoking, lifetime abstainers (alcohol), diabetes (fasting blood sugar ≥126 mg% or on medication), hypertension (blood pressure ≥140/90 mm Hg or on medication), body mass index (BMI) ≥25 kg/m2 to define overweight/obese, according to the definitions proposed in the WHO STEPS methodology,13 as well as total cholesterol >200 mg% or on medication, triglycerides >190 mg% and HDL cholesterol ≤40 mg%.14 Comparison of prevalence of diabetes in the urban area was restricted to comparison of persons with fasting blood sugar >140 mg% or on medication in both surveys, as this was the only comparable information available from the earlier survey.
3. Results
Socio-demographic characteristics of the two surveyed populations are shown in Table 1. The educational level of the population in this area improved considerably between the two surveys. As there was a difference in age distribution of the two survey populations, with a higher proportion of participants aged below 40 years in the first survey (Table 1), age adjusted rates were calculated to compare prevalence rates of various risk factors.
Table 1.
Distribution of socio-demographic factors in the two surveys.
| Characteristics | Rural (% of total) |
Urban (% of total) |
||||||
|---|---|---|---|---|---|---|---|---|
| Males |
Females |
Males |
Females |
|||||
| 1991 | 2010 | 1991 | 2010 | 1991 | 2010 | 1991 | 2010 | |
| Age (years) | ||||||||
| 30–39 years | 791 (38.4) | 482 (31.8) | 1148 (43.6) | 706 (35.5) | 494 (42.9) | 341 (35.1) | 694 (46.3) | 495 (39.8) |
| 40–49 years | 646 (31.4) | 544 (35.8) | 837 (31.8) | 672 (33.8) | 320 (27.9) | 364 (37.4) | 404 (26.9) | 417 (33.5) |
| 50–60 years | 622 (30.2) | 502 (33.1) | 649 (24.6) | 609 (30.6) | 335 (29.2) | 267 (27.5) | 402 (26.8) | 332 (26.7) |
| Total | 2059 | 1518 | 2634 | 1987 | 1149 | 972 | 1500 | 1244 |
| Education | ||||||||
| ≤8 years | 1535 (74.6) | 752 (49.7) | 2468 (93.7) | 1563 (79.2) | 705 (61.4) | 399 (41.3) | 1194 (79.6) | 704 (56.9) |
| >8 years | 524 (25.4) | 760 (50.3) | 166 (6.3) | 410 (20.8) | 444 (38.6) | 568 (58.7) | 306 (20.4) | 534 (43.1) |
| Occupation | ||||||||
| Unskilled labor | 589 (28.6) | 531 (35.7) | 749 (28.4) | 840 (42.3) | 136 (11.8) | 188 (19.7) | 76 (5.1) | 156 (12.5) |
| Unemployed/housewives | 13 (6.7) | 50 (3.4) | 1451 (55.1) | 880 (44.3) | 63 (5.5) | 64 (6.7) | 1254 (83.6) | 871 (70.0) |
| Others | 1332 (64.7) | 907 (60.9) | 434 (16.5) | 267 (13.4) | 950 (82.7) | 704 (73.6) | 170 (11.3) | 217 (17.4) |
In the rural population, the proportion of current smokers in the surveyed population decreased by 50% between the two surveys, while the proportion of those who reported lifetime abstinence to alcohol decreased by 20% (Table 2). While no female in the rural area reported ever consuming alcohol in 1991–1994, there was a small proportion (1.7%) that did so in 2010–2012. The frequency of alcohol use among male current drinkers decreased in the rural area from 65.2% consuming alcohol at least once a week in 1991–1994, to only 47.7% doing so in 2010–2012 (chi-square p < 0.001). However, there was no significant reduction in frequency of alcohol intake in the urban area, with 56.5% of male current drinkers in 1991–1994 consuming alcohol at least once a week and 53.5% consuming at least once a week in 2010–2012 (chi-square p = 0.681).
Table 2.
Trend of risk factors among rural participants between 1991–1994 and 2010–2012.
| Risk factors | Prevalence rates in males %, 95% CI |
Prevalence rates in females %, 95% CI |
||||
|---|---|---|---|---|---|---|
| 1991 | 2010 | 2010 age adjusted ratesa | 1991 | 2010 | 2010 age adjusted ratesa | |
| Current smokers | 45.9, 43.7–48.1 | 24.4,* 20.1–28.7 | 23.4, 20.9–25.8 | 0 | 0 | 0 |
| Lifetime abstainers (alcohol) | 46.8, 44.6–49.0 | 37.5,* 35.0–40.0 | 37.9, 34.8–41.2 | 100 | 98.3,* 97.7–98.9 | 98.5, 94.0–100 |
| BMI ≥ 25 kg/m2 (overweight/obese) | 8.5, 7.3–9.7 | 27.0,* 21.9–31.9 | 27.0, 24.1–29.9 | 11.0, 9.8–12.2 | 35.2,* 27.9–42.6 | 34.9, 32.1–37.7 |
| Total cholesterol >200 mg% or on medication | 13.8, 11.9–15.7 | 27.5,* 21.6–33.3 | 26.7, 23.8–29.6 | 13.7, 12.1–15.3 | 24.6,* 17.9–31.2 | 22.9, 20.6–25.2 |
| HDL ≤ 40 mg% | 69.1, 66.6–71.6 | 64.7, 61.9–67.5 | 64.8, 60.2–69.5 | 61.7, 59.4–64.0 | 53.6,* 51.1–56.1 | 53.7, 50.1–57.3 |
| Triglycerides >190 mg% | 12.9, 11.1–14.7 | 19.3,* 15.9–22.6 | 19.2, 16.7–21.8 | 8.7, 7.4–10.0 | 10.2, 8.6–11.9 | 9.5, 8.0–10.9 |
| BP ≥ 140/90 mm Hg or on medication | 7.9, 6.7–9.1 | 18.0,* 12.6–23.4 | 17.0, 16.8–17.3 | 7.4, 6.4–8.4 | 13.3,* 10.9–15.6 | 12.1, 11.9–12.2 |
| Fasting sugar ≥126 mg% or on medication | 3.6, 2.6–4.6 | 11.4,* 8.7–14.1 | 10.1, 8.4–11.8 | 2.5, 1.8–3.2 | 9.6,* 6.9–12.3 | 8.4, 7.1–9.7 |
Adjusted to 1991 survey population.
Chi-square p < 0.001, comparison of rates in 1991 and 2010.
The prevalence rates of diabetes (fasting sugar ≥126 mg% or on medication) and BMI ≥ 25 kg/m2 (overweight/obese) among 30–60 year olds tripled during the 20-year period (Table 2). The prevalence rates of hypertension (blood pressure ≥140/90 mm Hg or on medication) and high total cholesterol doubled in the rural area, even after adjusting for age (Table 2). There was also a significant increase in prevalence rates of triglycerides >190 mg% among rural males.
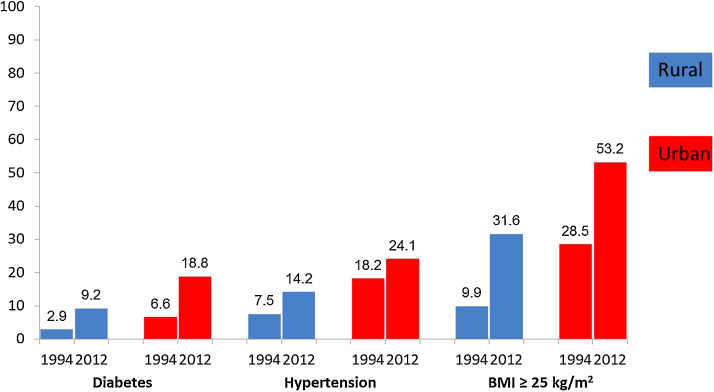
A similar pattern of increase in risk factors was observed in the urban areas with a few differences, although the increases were less than the rural area, except for the proportion with low HDL (≤40 mg%) which increased only in the urban sample (Table 3). The proportion of lifetime abstainers to alcohol did not change significantly between the two surveys while current smoking reduced by 50%. The rates of diabetes and BMI ≥ 25 kg/m2 (overweight/obese) doubled while there was no change in rates of hypertriglyceridemia (>190 mg%) (Table 3). The increase in prevalence of diabetes, hypertension, and overweight/obese in the rural and urban samples between 1991–1994 and 2010–2012 is depicted in Fig. 2.
Table 3.
Trend of risk factors among urban participants between 1991–1994 and 2010–2012.
| Risk factors | Prevalence rates in males %, 95% CI |
Prevalence rates in females %, 95% CI |
||||
|---|---|---|---|---|---|---|
| 1991 | 2010 | 2010: age adjusted ratesa | 1991 | 2010 | 2010: age adjusted ratesa | |
| Current smokers | 47.4, 44.5–50.3 | 26.2,* 23.4–29.0 | 25.9, 22.7–29.1 | 0 | 0 | 0 |
| Lifetime abstainers (alcohol) | 56.6, 53.7–59.5 | 57.5, 54.3–60.7 | 57.7, 52.8–62.5 | 100 | 99.8, 99.5–100 | 99.9, 94.2–100 |
| BMI ≥ 25 kg/m2 (overweight/obese) | 23.0, 20.3–25.7 | 45.3,* 40.9–49.7 | 43.5, 38.7–48.2 | 32.8, 30.2–35.5 | 61.1,* 57.1–65.2 | 59.8, 55.0–64.5 |
| Total cholesterol >200 mg% or on medication | 24.8, 22.1–27.6 | 34.7,* 30.0–39.4 | 33.4, 29.1–37.7 | 24.8, 22.4–27.2 | 31.0, 27.6–34.4 | 29.4, 26.1–32.8 |
| HDL ≤ 40 mg% | 70.3, 67.4–73.2 | 84.5,* 81.7–87.3 | 84.4, 77.4–91.4 | 58.4, 55.6–61.2 | 76.7,* 74.0–79.4 | 77.1, 71.5–82.6 |
| Triglycerides >190 mg% | 21.0, 18.4–23.6 | 23.9, 20.1–27.8 | 23.3, 19.7–26.9 | 15.9, 13.8–17.9 | 14.2, 10.8–17.5 | 13.2, 10.9–15.4 |
| BP ≥ 140/90 mm Hg or on medication | 19.6, 17.3–21.9 | 28.6,* 25.1–32.0 | 26.6, 22.9–30.2 | 17.2, 15.3–19.2 | 23.7,* 20.9–26.5 | 22.4, 19.5–25.2 |
| Fasting sugar >140 mg% or on medicationb | 7.5, 5.8–9.2 | 21.4,* 18.2–24.6 | 20.2, 16.9–23.5 | 5.9, 4.6–7.2 | 19.3,* 16.7–21.9 | 17.7, 15.2–20.3 |
Adjusted to 1991 survey population.
Definition used in 1991 and raw data unavailable for re-classification.
Chi-square p = 0.000, for comparison of rates in 1991 and 2010.
Fig. 2.
Trends of diabetes, hypertension and overweight among adults aged 30–60 years.
The mean values of most risk factors also increased in both the urban and rural population (Table 4) except HDL, systolic blood pressure, and triglycerides in rural females. The greatest increase was seen in mean waist circumference of urban females which increased by 23% with an increase of mean BMI of 14%. Mean triglyceride levels among rural males increased by 19.2%, while there was a significant decline in HDL values among urban participants with the maximum being a 16% decline in HDL among urban males (Table 4).
Table 4.
Comparison of mean values of risk factors in 1991–1994 and 2010–2012.
| Risk factors | Rural: mean, SD and mean difference |
Urban: mean, SD and mean difference |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males |
Females |
Males |
Females |
|||||||||
| 1991 (a) | 2010 (b) | b − a | 1991 (a) | 2010 (b) | b − a | 1991 (a) | 2010 (b) | b − a | 1991(a) | 2010 (b) | b − a | |
| Physical measurements | ||||||||||||
| BMI (kg/m2) | 20.3, 3.2 |
22.6, 3.9 |
2.3 | 20.6, 3.7 |
23.5, 4.7 |
2.9 | 22.4, 4.2 | 24.5, 4.4 |
2.1 | 23.4, 5.1 |
26.5, 5.3 |
3.1 |
| Waist circumference (cm) | 74.7, 8.7 |
82.9, 11.0 |
8.2 | 68.6, 8.9 |
81.0, 12.0 |
12.4 | 80.6, 11.0 |
89.9, 11.3 |
9.3 | 74.7, 11.6 |
91.9, 11.0 |
17.2 |
| Systolic blood pressure (mm Hg) | 110.0, 16.2 |
118.5, 17.9 |
8.5 | 110.3, 16.0 |
111.9, 12.0 |
1.6 | 117.4, 17.5 |
121.2, 18.7 |
3.8 | 118.2, 20.2 |
115.2, 19.0 |
3.0 |
| Diastolic blood pressure (mm Hg) | 72.0, 11.1 |
77.7, 17.0 |
5.7 | 70.8, 9.6 |
74.7, 10.9 |
3.9 | 77.0, 11.1 |
79.1, 12.4 |
2.1 | 75.4, 11.5 |
76.5 11.3 |
1.1* |
| Biochemical risk factors | ||||||||||||
| Fasting blood glucose (mg%) | 91.2, 28.1 |
99.0, 39.5 |
7.8 | 90.0, 21.6 |
97.8, 34.8 |
7.8 | 99.3, 35.7 |
109.6, 49.9 |
10.3 | 98.7, 36.8 |
109.7, 49.0 |
11.0 |
| Total cholesterol (mg%) | 158.8, 39.4 |
175.6, 46.3 |
16.8 | 161.3, 38.0 |
173.7, 42.3 |
12.4 | 176.9, 41.1 |
184.5, 41.0 |
7.6 | 177.1, 40.8 |
180.6, 41.2 |
3.5† |
| Triglycerides (mg%) | 118.1, 75.2 |
140.8, 116.0 |
22.7 | 109.5, 61.6 |
111.6 72.4 |
2.1‡ | 139.2, 77.0 |
154.6, 108.6 |
15.4 | 133.5, 74.4 |
122.4, 76.7 |
−11.1 |
| HDL (mg%) | 37.2, 11.0 |
37.3, 11.9 |
0.3‡ | 38.6, 9.9 |
40.0, 11.6 |
1.4 | 36.6, 11.8 |
30.6, 12.9 |
−6.0 | 39.1, 11.9 |
33.6, 11.1 |
−5.5 |
Independent t-test, p = 0.000 for all comparisons between 1991 and 2010, except those marked *p = 0.01, †p = 0.044, ‡p > 0.05.
4. Discussion
Although repeated cross sectional surveys are useful to measure trends of non-communicable diseases and their risk factors, there have been only a few such studies in India, such as in urban Jaipur and Chennai.7, 8, 9 Utilization of the same standardized methodology in repeated surveys would enable direct comparison of results. With the availability of the WHO STEPS methodology, this should now be possible in India, where cross sectional surveys are now increasing using this method.5, 6, 15, 16, 17 This study attempts to measure trends in risk factors in a district in south India, comparing data available from a survey done in the pre-STEPS era to a second survey done using the STEPS method in the same location, and using the comparison of standard indicators prescribed by the STEPS methodology.
There has been a marked decrease in smoking in both rural and urban Vellore since 1991, similar to international and national trends among men.18 This is probably a reflection of both improvement in education as well as the numerous nationwide tobacco control efforts which have decreased this behavior. We did not find a trend of increase in smoking among women as reported elsewhere.19 Alcohol consumption has increased in the rural areas as seen by the decrease in percent of lifetime abstainers with no change in consumption rates in the urban areas. This pattern of increase in proportion of population consuming alcohol is consistent with other studies in the region and the country and points to the need for more measures to be taken to reduce its use.20, 21, 22, 23 However, the frequency of use by current drinkers appears to have decreased since 1991–1994 with fewer drinkers reporting at least once-a-week consumption in the rural areas.
The prevalence of diabetes has almost tripled and hypertension doubled confirming the trends seen throughout the country.3, 4, 24, 25 The prevalence of overweight/obesity was high in both the rural and urban areas with a doubling of rates since 1991, consistent with global trends of rising overweight and mean BMI values.26, 27, 28 Similar trends of rising diabetes and obesity were also reported from a study done in 1999 in three villages near Chennai in comparison to a previous study 14 years earlier in a similar location.9 The rise in total cholesterol and lowering of HDL values (in urban participants) in our study was also consistent with that seen in other studies from India and south Asia.8, 29
The rise in prevalence of overweight/obese, hypertension, diabetes, and dyslipidemia is probably related to lifestyle changes, which are expected to have occurred with the changing socio economic status of the population in the 20-year period between the two surveys. Physical activity levels in the two surveys could not be compared directly as the initial survey did not use the STEPS questionnaire. However, the proportion who reported moderate or heavy physical activity in 1991–1994 was 90% in urban and 95% in rural areas, while only 37% and 57% respectively were classified as having moderate or vigorous activity in 2010 using the STEPS questionnaire, indicating a trend towards lower physical activity. Further details such as LDL levels, quantity of alcohol consumption, and dietary practices although assessed in both surveys could not be compared due to differences in methods and definitions used.
As these two surveys were conducted in a single Municipal Corporation and rural block of south India, it is generalizable only to similar areas in south India. The previous survey did not use the WHO STEPS questionnaire, which was unavailable at the time, and hence it was possible to obtain comparative data only for major diseases and conditions. However, the definitions were standardized using the WHO STEPS methodology in order to obtain comparative indicators for both surveys. The other strengths of this study were the large sample sizes, similar methods used for most of the major risk factors, quality control assessments for biochemical data in both surveys and availability of socio-economic indicators for both study periods.
The implications of the rising trends of cardiovascular risk factors in both rural and urban India include the rising burden on society, families and healthcare systems as well as the need for prevention. Among females, the larger increase in some risk factors such as waist circumference and BMI indicates the need for greater interventions targeting women. It would be wise to increase the quantum of interventions for primary prevention of these risk factors in the population. In the long term, this will yield greater benefit than secondary prevention screening programs, which currently are the main focus of intervention for diseases such as diabetes, hypertension, and cancer both in the government program30 as well as the private health sector. Feasible, locally acceptable, and affordable primary prevention interventions focused on improving physical activity and diet and decreasing tobacco and alcohol use need to be studied and advocated by both governmental and non-governmental organizations using a multifactorial approach. It is vital that sentinel centers are identified to be continually involved in surveillance and monitoring for non-communicable diseases which need to be implemented in various parts of the country in order to spur further action as well as evaluate ongoing programs.31, 32
Conflicts of interest
The authors have none to declare.
Funding
This study was funded by the Indian Council of Medical Research, New Delhi. No. 50/3/TF/DV/06-NCD-II.
Acknowledgments
The surveys were funded by the Indian Council of Medical Research. Grant No. 50/3/TF/DV/06-NCD-II.
References
- 1.Gupta R., Joshi P., Mohan V., Reddy K.S., Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S., Hawken S., Ounpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 3.Mohan V., Sandeep S., Deepa R. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–230. [PubMed] [Google Scholar]
- 4.Gupta R. Trends in hypertension epidemiology in India. J Hum Hypertens. 2004;18:73–78. doi: 10.1038/sj.jhh.1001633. [DOI] [PubMed] [Google Scholar]
- 5.National Institute of Medical Statistics, Indian Council of Medical Research (ICMR), 2009, IDSP. Non-Communicable Disease Risk Factors Survey, Phase-I, States of India, 2007–08. Available at: http://www.icmr.nic.in/final/IDSP-NCD%20Reports/Phase-1%20States%20of%20India.pdf.
- 6.Thankappan K.R., Shah B., Mathur P. Risk factor profile for chronic non-communicable diseases: results of a community-based study in Kerala, India. Indian J Med Res. 2010;131:53–63. [PubMed] [Google Scholar]
- 7.Mohan V., Deepa M., Deepa R. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India – the Chennai Urban Rural Epidemiology Study (CURES-17) Diabetologia. 2006;49:1175–1178. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 8.Gupta R., Gupta V.P., Sarna M. Prevalence of coronary heart disease and risk factors in an urban Indian population: Jaipur Heart Watch-2. Indian Heart J. 2002;54:59–66. [PubMed] [Google Scholar]
- 9.Ramachandran A., Snehalatha C., Baskar A.D. Temporal changes in prevalence of diabetes and impaired glucose tolerance associated with lifestyle transition occurring in the rural population in India. Diabetologia. 2004;47:860–865. doi: 10.1007/s00125-004-1387-6. [DOI] [PubMed] [Google Scholar]
- 10.Raban M.Z., Rakhi Dandona R., Dandona L. Availability of data for monitoring non communicable disease risk factors in India. Bull World Health Organ. 2012;90:20–29. doi: 10.2471/BLT.11.091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonita R. Strengthening NCD prevention through risk factor surveillance. Glob Health Action. 2009;28:2. doi: 10.3402/gha.v2i0.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oommen A.M., Abraham V.J., George K. Prevalence of risk factors for non-communicable diseases in rural and urban Tamil Nadu. Indian J Med Res. 2015 doi: 10.4103/0971-5916.198668. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. STEP wise approach to surveillance. Available at: http://www.who.int/chp/steps/en/.
- 14.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15.Anand K., Shah B., Gupta V. Risk factors for non-communicable disease in urban Haryana: a study using the STEPS approach. Indian Heart J. 2008;60:9–18. [PubMed] [Google Scholar]
- 16.Krishnan A., Shah B., Lal V. Prevalence of risk factors for non-communicable disease in a rural area of Faridabad district of Haryana. Indian J Public Health. 2008;52:117–124. [PubMed] [Google Scholar]
- 17.Bhagyalaxmi A., Atul T., Shikha J. Prevalence of risk factors of non-communicable diseases in a district of Gujarat, India. J Health Popul Nutr. 2013:78–85. doi: 10.3329/jhpn.v31i1.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng M., Freeman M.K., Fleming T.D. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 19.Goel S., Tripathy J.P., Singh R.J. Smoking trends among women in India: analysis of nationally representative surveys (1993–2009) South Asian J Cancer. 2014;3:200–202. doi: 10.4103/2278-330X.142958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John A., Barman A., Bal D. Hazardous alcohol use in rural southern India: nature, prevalence and risk factors. Natl Med J India. 2009;22:123–125. [PubMed] [Google Scholar]
- 21.Kim S., Rifkin S., John S.M. Nature, prevalence and risk factors of alcohol use in an urban slum of Southern India. Natl Med J India. 2013;26:203–209. [PMC free article] [PubMed] [Google Scholar]
- 22.Gururaj G., Murthy P., Girish N. NIMHANS; Bangalore, India: 2011. Alcohol Related Harm: Implications for Public Health and Policy in India. [Publication No. 73] [Google Scholar]
- 23.Prasad R. Alcohol use on the rise in India. Lancet. 2009;373:17–18. doi: 10.1016/s0140-6736(08)61939-x. [DOI] [PubMed] [Google Scholar]
- 24.Danaei G., Finucane M.M., Lu Y. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 25.Danaei G., Finucane M.M., Lin J.K. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 26.Finucane M.M., Stevens G.A., Cowan M.J. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens G.A., Singh G.M., Lu Y. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balarajan Y., Villamor E. Nationally representative surveys show recent increases in the prevalence of overweight and obesity among women of reproductive age in Bangladesh, Nepal, and India. J Nutr. 2009;139:2139–2144. doi: 10.3945/jn.109.112029. [DOI] [PubMed] [Google Scholar]
- 29.Farzadfar F., Finucane M.M., Danaei G. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet. 2011;377:578–586. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 30.National Program for prevention and control of cancer, diabetes, cardiovascular disease and stroke. Operational guidelines. Available at: http://health.bih.nic.in/Docs/Guidelines/Guidelines-NPCDCS.pdf.
- 31.Shah B., Mathur P. Surveillance of cardiovascular disease risk factors in India: the need & scope. Indian J Med Res. 2010;132:634–642. doi: 10.4103/0971-5916.73420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebrahim S. Surveillance and monitoring: a vital investment for the changing burdens of disease. Int J Epidemiol. 2011;40:1139–1143. doi: 10.1093/ije/dyr144. [DOI] [PubMed] [Google Scholar]