Summary
Treatment with several Wnt/β-catenin signaling pathway regulators can change the cellular reprogramming efficiency; however, the dynamics and role of endogenous Wnt/β-catenin signaling in reprogramming remain largely unanswered. Here we identify the upregulation of WNT2 and subsequent β-catenin nuclear accumulation as key events in reprogramming. Transient nuclear accumulation of β-catenin occurs early in MEF reprogramming. Wnt2 is strongly expressed in the early stage of reprogramming. Wnt2 knockdown suppresses the nuclear accumulation of β-catenin and reduces the reprogramming efficiency. WNT2 overexpression promotes β-catenin nuclear accumulation and enhances the reprogramming efficiency. WNT2 contributes to the promotion of cell proliferation. Experiments with several drugs that control the Wnt pathway also indicate the importance of β-catenin nuclear accumulation in reprogramming. Our findings reveal the role of WNT2/β-catenin signaling in reprogramming.
Graphical Abstract

Highlights
-
•
Nuclear accumulation of β-catenin occurs in the early stage of MEF reprogramming
-
•
Wnt2 expression is transiently increased during MEF reprogramming
-
•
WNT2 promotes both the β-catenin nuclear accumulation and the reprogramming process
-
•
Nuclear accumulation of β-catenin is important for MEF reprogramming
In this article, Nishida and colleagues revealed the behavior, dynamics, and role of endogenous Wnt/β-catenin signaling in reprogramming. Transient expression of Wnt2 and β-catenin nuclear accumulation occurs in the early stage of reprogramming. WNT2 promotes both the β-catenin nuclear accumulation and the reprogramming process. These findings uncover the importance of WNT2/β-catenin signaling in reprogramming.
Introduction
Somatic cells can be reprogrammed to induced pluripotent stem cells (iPSCs) by ectopic expression of defined transcription factors (OCT3/4, KLF4, SOX2, and c-MYC, hereafter referred to as OKSM) (Takahashi and Yamanaka, 2006). While the changes in gene expressions and epigenetic modifications during reprogramming have been well studied (Hussein et al., 2014, Koche et al., 2011, Koga et al., 2014, Mikkelsen et al., 2008, O'Malley et al., 2013, Polo et al., 2012), the changes in activities of signaling pathways have not been extensively studied.
The Wnt signaling pathway controls the pluripotency of embryonic stem cells (ESCs) (Sato et al., 2004). Wnt ligands inhibit GSK3 activity, resulting in β-catenin stabilization. Stabilized β-catenin then translocates into the nucleus and regulates gene expression. Mouse ESCs secrete Wnt ligands, and the autocrine Wnt activity is required for the maintenance of pluripotency (ten Berge et al., 2011). Mouse ESCs can even be maintained in the so-called 2i culture condition, the GSK3 inhibitor plus the MEK inhibitor (Ying et al., 2008). While Wnt/β-catenin signaling activates self-renewal of ESCs, it also plays a critical role in the initiation of differentiation (Murry and Keller, 2008), suggesting its divergent role in ESCs.
The role of Wnt/β-catenin signaling in reprogramming has also been investigated. Exogenously introduced WNT3A enhances fibroblast reprogramming in the absence of c-Myc (Marson et al., 2008). Knockdown or knockout of T cell factors or treatments with several drugs that control the Wnt pathway can change the reprogramming efficiency (Aulicino et al., 2014, Ho et al., 2013, Lluis et al., 2011, Ross et al., 2014, Zhang et al., 2014). However, it remains controversial whether endogenous Wnt/β-catenin signaling has a stimulatory or inhibitory effect on reprogramming. Furthermore, the dynamics and role of endogenous Wnt ligands or β-catenin in reprogramming remain largely unanswered.
In this study, we find that transient upregulation of WNT2 induces β-catenin nuclear accumulation and promotes cellular reprogramming.
Results
Nuclear Accumulation of β-Catenin Occurs during MEF Reprogramming
Wnt/β-catenin signaling and c-MYC could play a partially redundant functional role in reprogramming (Marson et al., 2008). Moreover, OKSM-induced reprogramming produces numerous partially reprogrammed cells (Nakagawa et al., 2008, Wernig et al., 2008). Therefore, an OKS method would be more suitable for tracing successful reprogramming. First, we examined the subcellular localization of β-catenin. Mouse embryonic fibroblasts (MEFs) were infected with retroviruses encoding either three transcription factors (OKS) or mCherry (control) at day 0. In control samples, β-catenin was faintly detected in the cytoplasm, but not at all in the nucleus (Figure 1A). In contrast, in OKS-introduced cells there appeared a fraction of cells, which exhibited nuclear accumulation of β-catenin at day 2 (Figure 1B). Both the number of nuclear β-catenin-positive cells and the staining intensity of nuclear β-catenin were increased at days 4 and 6. At days 8 and 10, when iPSC-like colonies began to emerge, β-catenin became localized at or near the plasma membrane in colonies, not in the nucleus, suggesting that β-catenin may function as a scaffolding protein for E-cadherin in the later stages of reprogramming (Li et al., 2010, Perez-Moreno and Fuchs, 2006, Samavarchi-Tehrani et al., 2010). Nuclear accumulation of β-catenin was decreased in the later stages (Figure 1C), suggesting that Wnt/β-catenin signaling is transiently activated during MEF reprogramming.
Figure 1.
Transient Nuclear Accumulation of β-Catenin Occurs during MEF Reprogramming
(A) Control MEFs, which were infected with a retrovirus encoding mCherry instead of OKS, were stained for β-catenin (green). Hoechst, blue. Higher-magnification images are shown to the right. Representative of three independent experiments. Scale bars, 200 μm.
(B) OKS-introduced MEFs were stained for β-catenin (green). Hoechst, blue. Higher-magnification images are shown to the right. Representative of three independent experiments. Scale bars, 200 μm.
(C) 1 × 104 cells were plated on the cell-culture chamber at day 3. The cells with β-catenin nuclear accumulation per cell-culture chamber were counted. Data are shown as means ± SEM (n = 3 independent experiments).
(D and E) MEFs were infected with a lentivirus encoding 7xTcf-eGFP (7TGP) together with retroviruses encoding OKS at day 0. (D) Schematic description of 7TGP and fluorescence-activated cell sorting (FACS) plots of 7TGP expression at day 6 are shown on the left. 7TGP-positive cells are outlined in green. The percentages of 7TGP-positive cells are shown on the right. Data are shown as means ± SEM (n = 3 independent experiments). The p value was calculated by Student's t test. ∗∗∗p < 0.001 compared with control cells. (E) At day 6, OKS and 7TGP infected-MEFs were sorted on the basis of the 7TGP expression intensity as in Figure S1A. Sorted cells were plated on new feeder layer-coated plates and incubated for 2 weeks. Representative images of AP staining and the numbers of AP-positive colonies are shown. Scale bars, 5 mm. The numbers of NANOG-positive colonies were evaluated by anti-NANOG staining (right). Data are shown as means ± SEM (n = 4 independent experiments). ∗∗∗p < 0.001 compared with 7TGP-negative cells (Student's t test).
Next, we used 7xTcf-eGFP (7TGP) as a reporter of Wnt/β-catenin activity (Figure 1D; Fuerer and Nusse, 2010). MEFs were infected with a lentivirus carrying 7TGP. In the absence of OKS, cells remained 7TGP negative. In contrast, about 5% of the OKS-introduced MEFs became 7TGP-positive cells at day 6. Wnt/β-catenin signaling target genes, Axin2 and CyclinD1, were more highly expressed in 7TGP-positive cells than in 7TGP-negative cells (Figures S1A and S1B). Previous studies have shown that E-cadherin (epidermal marker) is upregulated while Thy1 (fibroblast-associated marker) and Slug (mesenchymal marker) are downregulated during MEF reprogramming (Li et al., 2010, Samavarchi-Tehrani et al., 2010, Stadtfeld et al., 2008). Our results showed that E-cadherin was more highly expressed in 7TGP-positive cells than in 7TGP-negative cells, and Thy1 and Slug were more highly expressed in 7TGP-negative cells (Figure S1C), suggesting that 7TGP-positive cells are in a more advanced state. 7TGP-positive cells expressed higher levels of OKS factors (Figure S1D). To investigate whether 7TGP-positive cells become iPSCs, we sorted OSK-introduced MEFs into 7TGP-positive and -negative cells and evaluated the reprogramming efficiencies. More alkaline phosphatase (AP)-positive and NANOG-positive iPSC colonies were generated from 7TGP-positive cells (Figure 1E), suggesting that transient activation of Wnt/β-catenin signaling represents successful reprogramming.
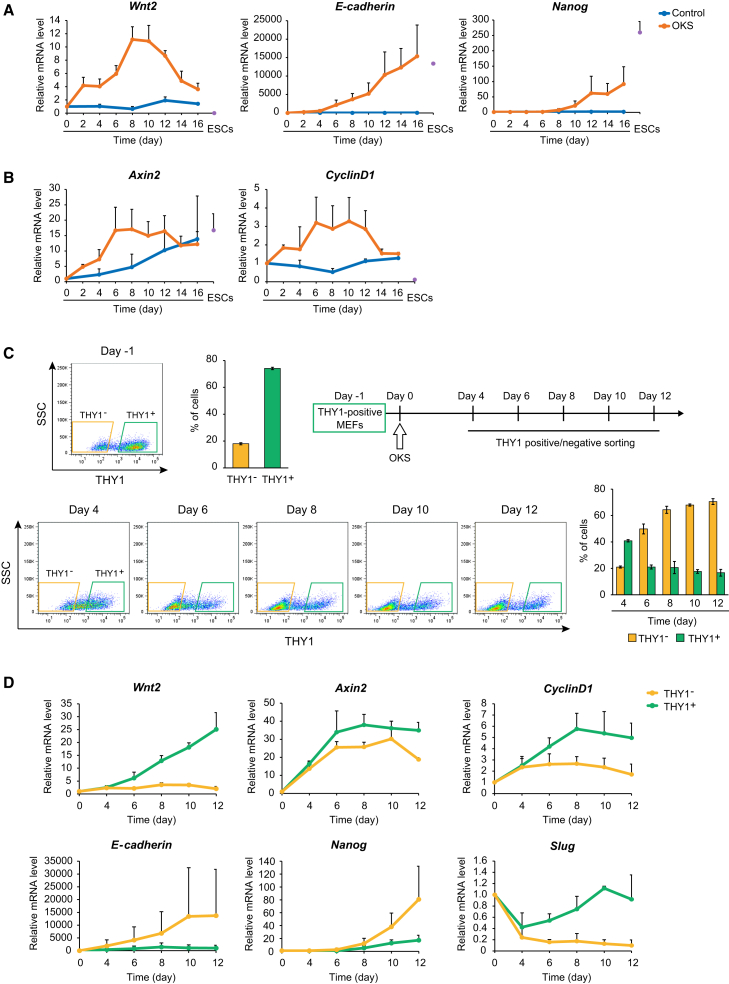
Wnt2 Expression Is Transiently Increased during MEF Reprogramming
By examining our microarray data (Koga et al., 2014), we found that Wnt2 was specifically upregulated prominently during reprogramming (Figure S2A). qRT-PCR analysis showed the transient Wnt2 expression (Figure 2A). The expression level of Wnt2 was quite low in ESCs. The increase in Wnt2 expression preceded those of E-cadherin and Nanog. The timing of Wnt2 upregulation is similar to that of β-catenin nuclear accumulation. It was reported that WNT2 activates Wnt/β-catenin signaling in several processes (Fu et al., 2011, Goss et al., 2009, You et al., 2004). The expression patterns of Axin2 and CyclinD1 were similar to that of Wnt2, suggesting that WNT2 induces Wnt/β-catenin signaling target genes during reprogramming (Figure 2B).
Figure 2.
Wnt2 Expression Is Transiently Increased during MEF Reprogramming
(A) qRT-PCR analysis. Each mRNA level was normalized to the GAPDH level, and the value at day 0 was set to 1. Data are shown as means ± SEM (n = 3 independent experiments).
(B) qRT-PCR analysis. Data are shown as means ± SEM (n = 3 independent experiments).
(C) THY1-positive MEFs were collected 1 day before the OKS introduction as the starting material (day −1). (Upper left) The FACS plot of THY1 expression and the percentages of THY1-neagtive and -positive cells at day −1. (Upper right) OKS-introduced MEFs were sorted at the indicated day based on the THY1 expression intensity. (Lower) FACS plots of THY1 expression and the percentages of THY1-neagtive and -positive cells. Data are shown as means ± SEM (n = 3 independent experiments).
(D) qRT-PCR analysis. Data are shown as means ± SEM (n = 3 independent experiments).
We sorted cells based on the expression level of THY1 (Figure 2C). As previously shown, expression patterns of E-cadherin, Nanog, and Slug in THY1-negative and -positive cells suggest that THY1-negative cells are in a more advanced state (Figure 2D) (Polo et al., 2012). Wnt2 was expressed in both THY1-positive and -negative cells at day 4 and are then expressed much more highly in THY1-positive cells, suggesting that Wnt2 is expressed in the early stage of reprogramming. Axin2 and CyclinD1 were upregulated in both THY1-postive and -negative cells in the early stage. At day 6, Axin2 and CyclinD1 show slightly lower expression in THY1-negative cells. The THY1-negative population at day 6 may contain both types of cells: the cells with nuclear accumulated β-catenin and the cells that have gone through the transient β-catenin nuclear accumulation. The expression of Wnt/β-catenin target genes may be downregulated immediately after the transient β-catenin nuclear accumulation is completed.
We investigated the role of OKS in Wnt2 expression. Ectopic expression of any single factor did not induce Wnt2 expression (Figure S2B). However, OK, OS, and OKS resulted in a marked increase in Wnt2 expression, suggesting that OCT3/4 may play a major role in Wnt2 induction in cooperation with KLF4 and/or SOX2. We investigated whether OCT3/4 binds to the Wnt2 promoter region. There are three potential OCT3/4-binding sites in the upstream region (−5,000 bp) of Wnt2 (Figure S2C; Nishimoto et al., 2003). Chromatin immunoprecipitation assays showed that OCT3/4 was likely to bind to at least one site (site A) (Figure S2D), suggesting that OCT3/4 may directly regulate the expression of Wnt2. We noted that KLF4-binding motifs and SOX2-binding motifs exist near site A.
WNT2 Regulates Nuclear Accumulation of β-Catenin and Promotes MEF Reprogramming
We performed Wnt2 knockdown by using short-hairpin RNAs (shRNAs) against Wnt2. The Wnt2 expression levels were markedly reduced by each Wnt2 shRNA (Figure 3A). The Wnt2 knockdown markedly reduced the number of nuclear β-catenin-positive cells, the numbers of AP-positive colonies, the numbers of NANOG-positive iPSC colonies, total cell numbers, and the percentages of NANOG-positive cells (Figures 3B–3E), suggesting that WNT2 is required for both the β-catenin nuclear accumulation and the reprogramming progression.
Figure 3.
WNT2 Promotes β-Catenin Nuclear Accumulation and Reprogramming
(A–E) MEFs were infected with a lentivirus encoding Wnt2 shRNAs (sh-Wnt2 #1 or sh-Wnt2 #2) or the control shRNA (sh-LacZ) together with retroviruses encoding OKS at day 0. (A) Knockdown efficiency of Wnt2 was evaluated by qRT-PCR analysis. Data are shown as means ± SEM (n = 4 independent experiments). Wnt2 expression levels at day 4 were determined. ∗∗∗p < 0.001 compared with control shRNA-expressing cells (Student's t test). (B) Cells at day 5 were stained for β-catenin. (Left) Representative images of β-catenin staining. Scale bars, 100 μm. (Right) Numbers of cells with β-catenin nuclear accumulation per cell-culture chamber. Data are shown as means ± SEM (n = 3 independent experiments). ∗p < 0.05, ∗∗∗p < 0.001 compared with control shRNA-expressing cells (Student's t test). (C) Cells at days 11–14 were stained for AP activity. (Left) Representative images of AP staining. Scale bars, 5 mm. (Right) Numbers of AP-positive colonies. Data are shown as means ± SEM (n = 3 independent experiments). ∗∗∗p < 0.001 compared with control shRNA-expressing cells (Student's t test). (D) Numbers of NANOG-positive colonies at days 11–14. Data are shown as means ± SEM (n = 3 independent experiments). ∗∗∗p < 0.001 compared with control shRNA-expressing cells (Student's t test). (E) (Left) The total cell numbers were counted at day 12. (Center and right) FACS plots of NANOG expression and the percentages of NANOG-positive cells, respectively. Data are shown as means ± SEM (n = 3 independent experiments). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 compared with control shRNA-expressing cells (Student's t test).
(F–I) MEFs were infected with a retrovirus encoding Wnt2 together with the retroviruses encoding OKS. (F) (Left) Cells at day 4 were stained for β-catenin. Representative images of β-catenin staining. Scale bars, 100 μm. (Right) Numbers of cells with β-catenin nuclear accumulation per cell-culture chamber. Data are shown as means ± SEM (n = 3 independent experiments). ∗∗∗p < 0.001 (Student's t test). (G) Cells at days 11–14 were stained for AP activity. (Left) Representative images of AP staining. Scale bars, 5 mm. (Right) Numbers of AP-positive colonies. Data are shown as means ± SEM (n = 3 independent experiments). ∗∗p < 0.01 (Student's t test). (H) Numbers of NANOG-positive colonies at day 11–14. Data are shown as means ± SEM (n = 3 independent experiments). ∗p < 0.05 (Student's t test). (I) (Left) Total cell numbers at day 12. (Center and right) FACS plots of NANOG expression and the percentages of NANOG-positive cells, respectively. Data are shown as means ± SEM (n = 3 independent experiments). ∗p < 0.05, ∗∗p < 0.01 compared with control cells (Student's t test).
Next we performed WNT2 overexpression, which increased the number of nuclear β-catenin-positive cells as well as the numbers of AP-positive colonies, the numbers of NANOG-positive iPSC colonies, total cell numbers, and the percentages of NANOG-positive cells (Figures 3F–3I). These results indicate that WNT2 promotes both the β-catenin nuclear accumulation and the reprogramming process.
At day 5, the rate of cell proliferation was increased in OKS-introduced MEFs and was further increased by the ectopic expression of WNT2 (Figure S3A). In contrast, it was reduced by Wnt2 knockdown (Figure S3B). The proliferative potential was higher in THY1-negative cells than in THY1-positive cells, suggesting that cell proliferation is promoted in the early stage (Figure S3C). Thus, WNT2 regulates cell proliferation in the early stage of reprogramming.
Nuclear Accumulation of β-Catenin Is Important for MEF Reprogramming
OKS-introduced MEFs were treated with several small molecules that inhibit (IWR-1, XAV939, and IWP-2) or activate (CHIR99021) the Wnt pathway. IWR-1 and XAV939 promote AXIN stabilization and thereby decrease β-catenin levels. IWP-2 is an inhibitor of PORCUPINE, which is necessary for the secretion of Wnt ligands. CHIR99021 inhibits GSK3 and thereby stabilizes β-catenin. The numbers of nuclear β-catenin-positive cells, AP-positive colonies, and NANOG-positive colonies were reduced by IWR-1, XAV939, or IWP-2, and increased by CHIR99021 (Figure 4A). These effects were more prominent in the early and middle stages than in the late stage (Figure S4A). Thus, β-catenin nuclear accumulation in the early stage of reprogramming may be important for efficient reprogramming.
Figure 4.
β-Catenin Nuclear Accumulation Is Important for MEF Reprogramming
(A) Treatment with an inhibitor (IWR-1, XAV939, or IWP-2), or an activator (CHIR99021) of the Wnt signaling pathway, or DMSO (control). (Upper) Representative images of β-catenin staining at day 4. Scale bars, 100 μm. (Lower left) Numbers of cells with β-catenin nuclear accumulation. (Lower center and right) Numbers of AP-positive and NANOG-positive colonies, respectively. Data are shown as means ± SEM (n = 3 independent experiments). ∗p < 0.05, ∗∗∗p < 0.001 compared with control cells treated with DMSO (Student's t test).
(B) shRNA-expressing, OKS-introduced MEFs were treated with CHIR99021 or DMSO. (Left) Representative images of AP staining at day 14. Scale bars, 5 mm. (Center and right) Numbers of AP-positive and NANOG-positive colonies, respectively. Data are shown as means ± SEM (n = 3 independent experiments). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student's t test).
(C) OKS and WNT2-introduced MEFs were treated with IWR-1, XAV939, or DMSO. (Left) Representative images of AP staining at day 14. Scale bars, 5 mm. (Center and right) Numbers of AP-positive and NANOG-positive colonies, respectively. Data are shown as means ± SEM (n = 3 independent experiments). ∗∗∗p < 0.001 (Student's t test).
We then performed rescue experiments. Treatment of Wnt2-knockdown, OKS-introduced MEFs with CHIR99021, a β-catenin stabilizer, restored the number of nuclear β-catenin-positive cells, the reprogramming efficiency, and the rate of cell proliferation (Figures 4B, S4B, and S4C). Furthermore, treatment of WNT2-overexpressed cells with a β-catenin destabilizer, IWR-1 or XAV939, reduced the increased reprogramming efficiency (Figure 4C). These results support our idea that WNT2 promotes reprogramming by inducing β-catenin nuclear accumulation.
Discussion
We demonstrated that transient upregulation of WNT2 induces β-catenin nuclear accumulation and promotes reprogramming.
β-Catenin nuclear accumulation decreased after day 6 while Wnt2 expression still remained high until day 10. There may be two possibilities to explain this time difference between Wnt2 downregulation and nuclear β-catenin diminution. (1) β-Catenin can function as a scaffolding protein for E-cadherin and the binding of E-cadherin to β-catenin prevents β-catenin nuclear localization (Orsulic et al., 1999). It can be speculated that while WNT2 promotes the stabilization of β-catenin in the early to middle stage of reprogramming, E-cadherin binds to β-catenin and prevents β-catenin nuclear localization in the middle stage of reprogramming (days 8–10). (2) Wnt/β-catenin signaling induces the transcription of Axin2, which promotes phosphorylation and degradation of β-catenin (Behrens et al., 1998, Ikeda et al., 1998, Jho et al., 2002). During reprogramming this negative feedback could occur, and thus β-catenin may be degraded in the middle phase of reprogramming, although Wnt2 is still expressed.
Our results showed that Wnt2 was upregulated in the early stage of reprogramming and expressed mainly in THY1-positive cells. A transition from a THY1-positive state to a THY1-negative state also occurred in the early stage. WNT2 may act on THY1-positive cells to change them to THY1-negative cells. Alternatively, it is possible that WNT2 secreted from THY1-positive cells supports the reprogramming of THY1-negative cells. Whether WNT2 acts on THY1-positive or -negative cells in an autocrine or paracrine manner should be examined in future studies.
WNT2 promotes metastasis in several cancers (Fu et al., 2011, Jiang et al., 2014, Pu et al., 2009). Epithelial-to-mesenchymal transition takes place in the initiation of metastasis. On the other hand, mesenchymal-to-epithelial transition (MET) occurs in the early stage of fibroblast reprogramming (Li et al., 2010, Samavarchi-Tehrani et al., 2010). Thus, prolonged activation of WNT2/β-catenin signaling might result in the inhibition of MET during reprogramming. In fact, our results showed that the expression of Axin2 and CyclinD1 remained high in THY1-positive cells during the middle to late phase. It would be important that WNT2/β-catenin signaling is “on” in the early stage and switches to “off” in later stages.
Several studies have investigated the involvement of Wnt signaling in different stages of reprogramming. However, their results have been controversial (Aulicino et al., 2014, Ho et al., 2013, Ross et al., 2014, Zhang et al., 2014). The reprogramming-inducing systems (retroviral infection or doxycycline-inducible system; OKSM or OKS) or donor cell types (fibroblasts or neural stem cells) differ among these studies. In ESCs, the Wnt/β-catenin signaling pathway contributes to the maintenance of pluripotency, and on the contrary plays a critical role in early development (Miki et al., 2011), implying the context-dependent role of Wnt/β-catenin signaling. In cell-fusion-mediated reprogramming, the level of Wnt/β-catenin signaling activity is critical for successful reprogramming; very high or very low activities have an inhibitory effect on reprogramming (Lluis et al., 2008). Therefore, one hypothesis for the opposite effects of endogenous Wnt/β-catenin signaling on reprogramming is that different reprogramming systems may cause different Wnt/β-catenin activity levels. The changes in Wnt/β-catenin activity levels in different reprogramming systems should be investigated in future studies.
Experimental Procedures
Cell-Culture Protocols
MEFs were isolated from E14.5 embryos of ICR mice. MEFs and HEK293T were maintained in DMEM containing 10% fetal bovine serum (FBS). Plat-E cells were maintained in DMEM containing 10% FBS, 1 μg/ml puromycin, and 10 μg/ml blasticidin S. The cells undergoing reprogramming were cultured in standard mouse ESC medium containing 15% ES-FBS (Thermo Science) and 1,000 U/ml ESGRO LIF (Millipore). ESCs were cultured and passed on gelatin coated-dishes in mouse ESC medium with 1,500 U/ml LIF. All mouse experiments were conducted in accordance with the Regulation on Animal Experimentation at Kyoto University and approved by the Animal Experimentation Committee of Kyoto University.
Author Contributions
M.N.-K. conceived the study. M.K. and M.N.-K. designed and performed most of the experiments and analyzed the data. J.L. performed qRT-PCR and chromatin immunoprecipitation experiments and ESC culture, together with M.K. and M.N.-K. E.N. supervised the project. M.K., M.N.-K., and E.N. wrote the manuscript.
Acknowledgments
This work was supported by JST, CREST and MEXT (to E.N.), and MEXT Scholarship Program (to J.L.).
Published: May 19, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.04.012.
Contributor Information
May Nakajima-Koyama, Email: mnakajima.m07@lif.kyoto-u.ac.jp.
Eisuke Nishida, Email: nishida@lif.kyoto-u.ac.jp.
Supplemental Information
References
- Aulicino F., Theka I., Ombrato L., Lluis F., Cosma M.P. Temporal perturbation of the Wnt signaling pathway in the control of cell reprogramming is modulated by TCF1. Stem Cell Rep. 2014;2:707–720. doi: 10.1016/j.stemcr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Jerchow B.A., Wurtele M., Grimm J., Asbrand C., Wirtz R., Kuhl M., Wedlich D., Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Fu L., Zhang C., Zhang L.Y., Dong S.S., Lu L.H., Chen J., Dai Y., Li Y., Kong K.L., Kwong D.L. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/beta-catenin signalling pathway. Gut. 2011;60:1635–1643. doi: 10.1136/gut.2011.241638. [DOI] [PubMed] [Google Scholar]
- Fuerer C., Nusse R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PLoS One. 2010;5:e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss A.M., Tian Y., Tsukiyama T., Cohen E.D., Zhou D., Lu M.M., Yamaguchi T.P., Morrisey E.E. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R., Papp B., Hoffman J.A., Merrill B.J., Plath K. Stage-specific regulation of reprogramming to induced pluripotent stem cells by Wnt signaling and T cell factor proteins. Cell Rep. 2013;3:2113–2126. doi: 10.1016/j.celrep.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein S.M., Puri M.C., Tonge P.D., Benevento M., Corso A.J., Clancy J.L., Mosbergen R., Li M., Lee D.S., Cloonan N. Genome-wide characterization of the routes to pluripotency. Nature. 2014;516:198–206. doi: 10.1038/nature14046. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Kishida S., Yamamoto H., Murai H., Koyama S., Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho E.H., Zhang T., Domon C., Joo C.K., Freund J.N., Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Li Q., He C., Li F., Sheng H., Shen X., Zhang X., Zhu S., Chen H., Chen X. Activation of the Wnt pathway through Wnt2 promotes metastasis in pancreatic cancer. Am. J. Cancer Res. 2014;4:537–544. [PMC free article] [PubMed] [Google Scholar]
- Koche R.P., Smith Z.D., Adli M., Gu H., Ku M., Gnirke A., Bernstein B.E., Meissner A. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell. 2011;8:96–105. doi: 10.1016/j.stem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M., Matsuda M., Kawamura T., Sogo T., Shigeno A., Nishida E., Ebisuya M. Foxd1 is a mediator and indicator of the cell reprogramming process. Nat. Commun. 2014;5:3197. doi: 10.1038/ncomms4197. [DOI] [PubMed] [Google Scholar]
- Li R., Liang J., Ni S., Zhou T., Qing X., Li H., He W., Chen J., Li F., Zhuang Q. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Lluis F., Pedone E., Pepe S., Cosma M.P. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell. 2008;3:493–507. doi: 10.1016/j.stem.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Lluis F., Ombrato L., Pedone E., Pepe S., Merrill B.J., Cosma M.P. T-cell factor 3 (Tcf3) deletion increases somatic cell reprogramming by inducing epigenome modifications. Proc. Natl. Acad. Sci. USA. 2011;108:11912–11917. doi: 10.1073/pnas.1017402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A., Foreman R., Chevalier B., Bilodeau S., Kahn M., Young R.A., Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Yasuda S.Y., Kahn M. Wnt/beta-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem Cell Rev. 2011;7:836–846. doi: 10.1007/s12015-011-9275-1. [DOI] [PubMed] [Google Scholar]
- Mikkelsen T.S., Hanna J., Zhang X., Ku M., Wernig M., Schorderet P., Bernstein B.E., Jaenisch R., Lander E.S., Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C.E., Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T., Okita K., Mochiduki Y., Takizawa N., Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nishimoto M., Miyagi S., Katayanagi T., Tomioka M., Muramatsu M., Okuda A. The embryonic Octamer factor 3/4 displays distinct DNA binding specificity from those of other Octamer factors. Biochem. Biophys. Res. Commun. 2003;302:581–586. doi: 10.1016/s0006-291x(03)00218-3. [DOI] [PubMed] [Google Scholar]
- O'Malley J., Skylaki S., Iwabuchi K.A., Chantzoura E., Ruetz T., Johnsson A., Tomlinson S.R., Linnarsson S., Kaji K. High-resolution analysis with novel cell-surface markers identifies routes to iPS cells. Nature. 2013;499:88–91. doi: 10.1038/nature12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsulic S., Huber O., Aberle H., Arnold S., Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J. Cell Sci. 1999;112:1237–1245. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M., Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev. Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo J.M., Anderssen E., Walsh R.M., Schwarz B.A., Nefzger C.M., Lim S.M., Borkent M., Apostolou E., Alaei S., Cloutier J. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu P., Zhang Z., Kang C., Jiang R., Jia Z., Wang G., Jiang H. Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2009;16:351–361. doi: 10.1038/cgt.2008.78. [DOI] [PubMed] [Google Scholar]
- Ross J., Busch J., Mintz E., Ng D., Stanley A., Brafman D., Sutton V.R., Van den Veyver I., Willert K. A rare human syndrome provides genetic evidence that WNT signaling is required for reprogramming of fibroblasts to induced pluripotent stem cells. Cell Rep. 2014;9:1770–1780. doi: 10.1016/j.celrep.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P., Golipour A., David L., Sung H.K., Beyer T.A., Datti A., Woltjen K., Nagy A., Wrana J.L. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M., Maherali N., Breault D.T., Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- ten Berge D., Kurek D., Blauwkamp T., Koole W., Maas A., Eroglu E., Siu R.K., Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M., Meissner A., Cassady J.P., Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L., He B., Xu Z., Uematsu K., Mazieres J., Fujii N., Mikami I., Reguart N., McIntosh J.K., Kashani-Sabet M. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64:5385–5389. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- Zhang P., Chang W.H., Fong B., Gao F., Liu C., Al Alam D., Bellusci S., Lu W. Regulation of induced pluripotent stem (iPS) cell induction by Wnt/beta-catenin signaling. J. Biol. Chem. 2014;289:9221–9232. doi: 10.1074/jbc.M113.542845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.