Summary
Mesenchymal stromal cells (MSCs) are promising therapeutic candidates given their potent immunomodulatory and anti-inflammatory secretome. However, controlling the MSC secretome post-transplantation is considered a major challenge that hinders their clinical efficacy. To address this, we used a microparticle-based engineering approach to non-genetically modulate pro-inflammatory pathways in human MSCs (hMSCs) under simulated inflammatory conditions. Here we show that microparticles loaded with TPCA-1, a small-molecule NF-κB inhibitor, when delivered to hMSCs can attenuate secretion of pro-inflammatory factors for at least 6 days in vitro. Conditioned medium (CM) derived from TPCA-1-loaded hMSCs also showed reduced ability to attract human monocytes and prevented differentiation of human cardiac fibroblasts to myofibroblasts, compared with CM from untreated or TPCA-1-preconditioned hMSCs. Thus, we provide a broadly applicable bioengineering solution to facilitate intracellular sustained release of agents that modulate signaling. We propose that this approach could be harnessed to improve control over MSC secretome post-transplantation, especially to prevent adverse remodeling post-myocardial infarction.
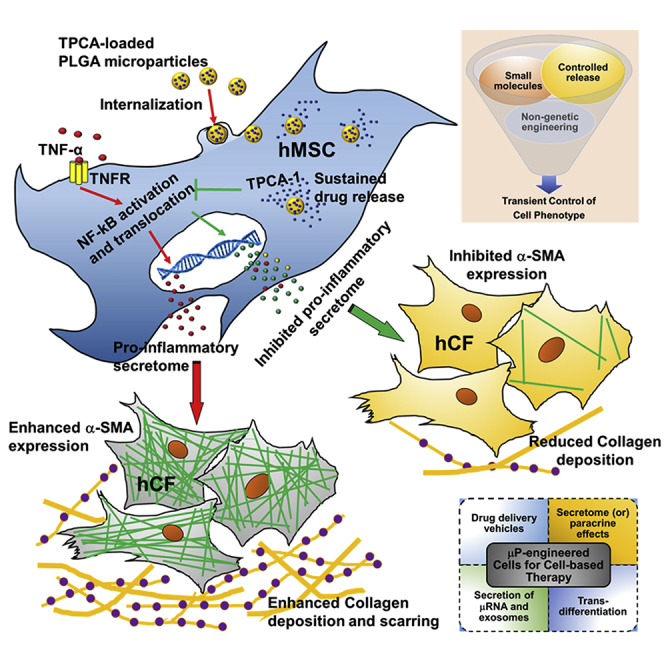
Graphical Abstract
Highlights
-
•
Soluble TPCA-1 attenuates pro-inflammatory secretome in TNF-α-stimulated hMSCs
-
•
TPCA preconditioning fails to inhibit pro-inflammatory secretome in TNF-hMSCs
-
•
TPCA-μP-hMSCs demonstrate sustained inhibition of pro-inflammatory secretome
-
•
Engineered hMSCs inhibit α-SMA expression and collagen deposition in cardiac fibroblasts
Inamdar, Karp, and colleagues have integrated small-molecule-mediated and microparticle engineering approaches to modulate the MSC secretome. Through this, the pro-inflammatory components were inhibited, yet generally the beneficial anti-inflammatory and pro-angiogenic components were maintained. In vitro functional relevance was demonstrated by inhibiting key processes of cardiac fibrosis. It is anticipated that this non-genetic, transient cell engineering approach has broad therapeutic relevance.
Introduction
Mesenchymal stromal cells (MSCs; also known as bone marrow stromal cells and earlier known as mesenchymal stem cells) are being explored as therapeutics in over 550 clinical trials registered with the US Food and Drug Administration (www.FDA.gov) for the treatment of a wide range of diseases (Ankrum et al., 2014c). Their immune-evasive properties (Ankrum et al., 2014c) and safe transplant record, allowing allogeneic administration without an immunosuppressive regimen, positions MSCs as an appealing candidate for a potential off-the-shelf product. One of the primary mechanisms exploited in MSC therapeutics is a secretome-based paracrine effect as evidenced in many pre-clinical studies (Ranganath et al., 2012). However, controlling the MSC secretome post-transplantation is considered a major challenge that hinders their clinical efficacy. For instance, upon transplantation, MSCs are subjected to a complex inflammatory milieu (soluble mediators and immune cells) in most injury settings. MSCs not only secrete anti-inflammatory factors, but also produce pro-inflammatory factors that may compromise their therapeutic efficacy. Table S1 lists a few in vitro and in vivo conditions that demonstrate the complex microenvironment under which MSCs switch between anti-inflammatory and pro-inflammatory phenotypes.
Levels of pro- or anti-inflammatory cytokines are not always predictive of the response, possibly due to the dynamic cytokine combinations (and concentrations) present in the cell microenvironment (Table S1). For example, relatively low inflammatory stimulus (<20 ng/ml tumor necrosis factor alpha [TNF-α] alone or along with interferon-γ [IFN-γ]) can polarize MSCs toward pro-inflammatory effects (Bernardo and Fibbe, 2013) resulting in increased inflammation characterized by T cell proliferation and transplant rejection. Conversely, exposure to high levels of the inflammatory cytokine TNF-α has been shown in certain studies to result in MSC-mediated anti-inflammatory effects via secretion of potent mediators such as TSG6, PGE2, STC-1, IL-1Ra, and sTNFR1 as demonstrated in multiple inflammation-associated disease models (Prockop and Oh, 2012, Ylostalo et al., 2012). These effects are mediated via molecular pathways such as NF-κB, PI3K, Akt, and JAK-STAT (Ranganath et al., 2012). However, it is not clear that low and high levels of TNF-α always exert the same effect on anti- versus pro-inflammatory MSC secretome. NF-κB is a central regulator of the anti-inflammatory secretome response in monolayer (Yagi et al., 2010), spheroid MSCs (Bartosh et al., 2013, Ylostalo et al., 2012) and TNF-α-mediated (20 ng/ml for 120 min) apoptosis (Peng et al., 2011). Given that NF-κB can promote secretion of pro-inflammatory components in the MSC secretome (Lee et al., 2010), we hypothesized that NF-κB inhibition via small molecules in MSC subjected to a representative inflammatory stimulus (10 ng/ml TNF-α) would inhibit their pro-inflammatory responses.
Adverse remodeling or cardiac fibrosis due to differentiation of cardiac fibroblasts (CF) into cardiac myofibroblasts (CMF) with pro-inflammatory phenotype and collagen deposition is the leading cause for heart failure. The secretome from exogenous MSCs has anti-fibrotic and angiogenic effects that can reduce scar formation (Preda et al., 2014) and improve ejection fraction when administered early or prior to adverse remodeling (Preda et al., 2014, Tang et al., 2010, Williams et al., 2013). Unfortunately in many cases, due to poor prognosis, MSCs may not be administered in time to prevent adverse remodeling to inhibit CF differentiation to CMF (Virag and Murry, 2003) or to prevent myocardial expression of TNF-α (Bozkurt et al., 1998, Mann, 2001). Also, when administered following an adverse remodeling event including CMF differentiation, MSCs may assume a pro-inflammatory phenotype and secretome (Naftali-Shani et al., 2013) under TNF-α (typically ∼5 pg/mg of total protein in myocardial infarction (MI) rat myocardium which is not significantly higher than 1–3 pg/mg protein in control rat myocardium) (Moro et al., 2007) resulting in impaired heart function. Hence, if the pro-inflammatory response of hMSCs can be suppressed it may maximize efficacy.
While the MSC phenotype can be controlled under regulated conditions in vitro, the in vivo response of MSCs post-transplantation is poorly controlled as it is dictated by highly dynamic and complex host microenvironments (Discher et al., 2009, Rodrigues et al., 2010). Factors including MSC tissue source (Melief et al., 2013, Naftali-Shani et al., 2013), donor and batch-to-batch variability with respect to cytokine secretion and response to inflammatory stimuli (Zhukareva et al., 2010), gender (Crisostomo et al., 2007), and age (Liang et al., 2013) also affect the response of MSCs. Thus, it is important to develop approaches to control the MSC secretome post-transplantation regardless of their source or expansion conditions. We hypothesized that engineering MSCs to induce a specific secretome profile under a simulated host microenvironment may maximize their therapeutic utility. Previously, the MSC secretome has been regulated via genetic engineering (Gnecchi et al., 2006, Wang et al., 2009) or cytokine/small-molecule preconditioning approaches (Crisostomo et al., 2008, Mias et al., 2008). Genetically engineered human MSCs (hMSCs) pose challenging long-term regulatory hurdles given that the potential tumorigenicity has not been well characterized, and while preconditioning hMSCs with cytokines/small molecules may be safer, the phenotype-altering effects are transient.
In this study, we aimed to modulate the hMSC secretome with a small molecule, specifically inhibiting the pro-inflammatory components while maintaining few anti-inflammatory and angiogenic components. Through this strategy, we sought to minimize CMF differentiation and inhibit total collagen deposition, which are primary mediators of cardiac fibrosis. Using TNF-α stimulated NF-κB activation as a model of inflammation, we show that microparticle-mediated continuous delivery of an NF-κB inhibitor can provide sustained inhibition of the hMSC pro-inflammatory secretome and thereby inhibit monocyte migration, CMF differentiation, and collagen deposition.
Results
Profiling the hMSC Secretome upon TNF-α Stimulation
To identify relevant targets for inhibition of the pro-inflammatory secretome, we profiled hMSCs treated with the pro-inflammatory stimulus TNF-α by quantifying over 250 cytokines and chemokines in the hMSC conditioned medium (CM) using antibody arrays (Figure 1A). See Table 1 for details of the experimental groups. Upon TNF-α stimulation, there was notable increase in the secretion of multiple cytokines and chemokines in the CM compared with control-hMSCs (Figure 1B). While constitutively secreted pro-inflammatory cytokines IL-6, IL-8, MCP-1, and MCP-3 showed modest increase (∼19-, ∼5-, ∼2.6-, and ∼4-fold, respectively), TNF-α stimulation resulted in secretion of pro-inflammatory cytokines RANTES (CCL5), ENA78, GRO, and GCP-2 at significantly elevated concentrations (∼4,000-, ∼133-, ∼17-, and ∼63-fold, respectively) compared with control-hMSCs (Figure 1B).
Figure 1.
Characterization of hMSC Pro-inflammatory Secretome upon TNF-α Stimulation and TPCA-1 Treatment
(A) Fluorescence images of cytokine antibody arrays obtained via laser scanning microplate reader from multiple hMSC secretome samples.
(B) Concentration profile of hMSC secretome obtained via quantitative analysis of RayBiotech Quantibody arrays using GenePix Pro software. Of 250 cytokines, chemokines, and growth factors profiled, mediators that were statistically significant among the three hMSC samples are reported. @ refers to 1/10th of the actual concentration of ENA78. $ and NS indicate p < 0.01 and not significant between secretome from control-hMSC and TNF-hMSC, respectively; ∗ and NS indicate p < 0.01 and not significant between secretome from TNF-hMSC and TNF + TPCA-hMSC, respectively; # and NS indicate p < 0.01 and not significant between secretome from control-hMSC and TNF + TPCA-hMSC, respectively.
(C) Fold change in concentration of hMSC secretome components normalized to concentration of secretome components in TNF-hMSC. p values indicate significance (∗p < 0.01) between secretome from TNF-hMSC and TNF + TPCA-hMSC. Data represent the average of duplicates from three independent experiments from two donor MSCs. Error bars represent ± SD.
Table 1.
Abbreviations and Expanded Forms of the Experimental Groups
| Abbreviations | Description of the Experimental Groups |
|---|---|
| Control-hMSC | treated with 1% DMSO for 48 hr |
| TNF-hMSC | treated with 10 ng/ml TNF-α and 1% DMSO for 48 hr |
| TNF + TPCA-hMSC | treated with 10 ng/ml TNF-α and 10 μM TPCA-1 for 48 hr |
| TPCA-hMSC | treated with 10 μM TPCA-1 for 48 hr |
| TPCApre + TNF-hMSC | pre-treated with 10 μM TPCA-1 for 24 hr and after removal of TPCA-1, treated with 10 ng/ml TNF-α every 48 hr |
| TPCApre-hMSC | pre-treated with 10 μM TPCA-1 for 24 hr |
| μP-hMSC | loaded with 200 μg/ml blank microparticles and incubated for 24 hr |
| TNF + μP-hMSC | loaded with 200 μg/ml blank microparticles, incubated for 24 hr and treated with 10 ng/ml TNF-α every 48 hr |
| TPCAμP-hMSC | loaded with 200 μg/ml TPCA-microparticles and incubated for 24 hr |
| TNF + TPCAμP-hMSC | loaded with 200 μg/ml TPCA-microparticles, incubated for 24 hr and treated with 10 ng/ml TNF-α every 48 hr |
TPCA-1 Inhibits Secretion of Pro-inflammatory Cytokines from TNF-α-Stimulated hMSCs
To investigate whether the increased secretion of pro-inflammatory factors upon TNF-α stimulation in hMSCs is mediated by NF-κB, we used small-molecule inhibitors of NF-κB, namely PSI (proteasome inhibitor) and TPCA-1 (IKKβ inhibitor). PSI was highly toxic to the cells (IC50 ∼3 μM; where IC50 is the median inhibitory concentration) when treated for 48 hr (Figure S1A), but at lower concentration (10 nM) PSI did not inhibit secretion of IL-6, a pro-inflammatory cytokine secreted at high levels upon TNF-α stimulation of hMSCs (Figure S1B). In contrast, TPCA-1 had low toxicity on hMSCs (IC50 ∼90 μM) when treated for 48 hr (Figure S1C) and 10 μM concentration was chosen for future experiments. In addition, TPCA-1 treatment at 10 μM did not inhibit osteogenic and adipogenic differentiation of hMSCs (Figures S2A and S2B).
To test the utility of TPCA-1 as a reversible modulator of the NF-κB mediated pro-inflammatory secretome, we tested its effect on cytokine production by TNF-α activated hMSCs. Control (control-hMSC), TNF-α-treated (TNF-hMSC), and TNF-α/TPCA-1-treated hMSC (TNF + TPCA-hMSC) were incubated for 48 hr in serum-free medium. Sustained TPCA-1 treatment in TNF + TPCA-hMSCs significantly reduced the secreted levels of pro-inflammatory mediators MCP-1, RANTES, GCP-2, LIF, and MCP-3 to lower than or equivalent to the basal levels of control-hMSCs (Figure 1B). Secreted levels of IL-6, IL-8, and GRO were significantly lower than levels secreted by TNF-hMSCs. In addition, reduction in cytokine secretion levels for TNF + TPCA-hMSC CM versus TNF-hMSC CM were as follows: IL-6 (∼6-fold), IL-8 (∼1.2-fold), LIF (∼9-fold), MCP-1 (∼7-fold), MCP-3 (∼3-fold), RANTES (∼5,400-fold), ENA78 (∼45-fold), GRO (∼6-fold), and GCP-2 (∼58-fold) (Figure 1C).
Next, we sought to examine if TPCA-1 would inhibit the beneficial paracrine effect of hMSCs mediated by several pathways including JAK-STAT, PI3/Akt, and MAPK (Ranganath et al., 2012). Concentrations of growth factors, cytokines, and anti-inflammatory mediators were quantified from Quantibody arrays. Secretion of most growth factors (Figures S3A and S3B) and cytokines (Figures S3C and S3D) was unaffected even upon TNF-α stimulation and TPCA-1 treatment, except for HGF-1 the secretion of which was significantly inhibited upon TPCA-1 treatment (Figure S3A). However, secretion of platelet-derived growth factor-AA and anti-inflammatory cytokine TSG-6 was significantly augmented upon TNF-α stimulation and maintained at elevated levels even upon TPCA-1 treatment (Figures S3A and S3D).
Functionality of the inhibited pro-inflammatory hMSC secretome was characterized by investigating transwell migration of human monocytes in vitro (Figure S4A). Figure S4B indicates that TPCA-1 treatment significantly inhibited transwell migration of human monocytes in response to TNF-hMSC secretome. This, along with the inhibition of pro-inflammatory secretome by TPCA-1 prompted us to develop a non-genetic, autonomous controlled release approach for modulating pro-inflammatory secretome in hMSCs.
Surface Modified PLGA Microparticles Delivering TPCA-1 are Internalized into hMSCs
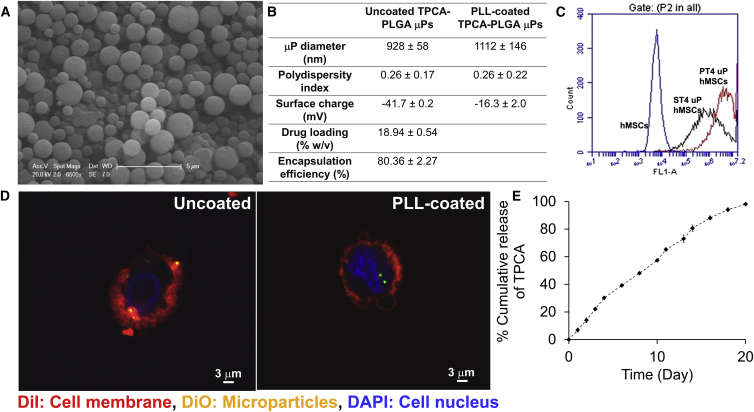
Previously, we demonstrated a particle-in-cell approach where 1–2 μm drug-loaded poly(D,L-lactide-co-glycolide) (PLGA) microparticles were internalized into hMSCs and retained at least for 7 days and potentially weeks (Sarkar et al., 2011). Drugs released from these particle-engineered hMSCs demonstrated sustained phenotype-altering effects (Ankrum et al., 2014a, Sarkar et al., 2011). In this study, we developed 1–2 μm PLGA microparticles encapsulating TPCA-1 using the single-emulsion solvent evaporation technique. PLGA with a glycolic acid to lactic acid ratio of 50:50 and 10 kDa molecular weight was chosen to formulate the microparticles to ensure faster degradation. The microparticles were round in shape with smooth surface morphology (Figure 2A) with a relatively homogeneous size distribution (PDI 0.26) (Figure 2B). Given the anionic surface property of PLGA used in this study, the surface of the microparticles was negatively charged (Figure 2B). Flow cytometry analysis of fluorescent dye-loaded microparticles (DμPs) revealed their association with hMSCs (Figure 2C), and confocal imaging confirmed that they were only associated with the cell membrane and not completely internalized (Figure 2D). To ensure internalization, the microparticles were treated with cationic polymer poly-L-lysine (PLL) resulting in reduced negative surface charge (Figure 2B). Flow cytometry analysis confirmed that PLL-coated microparticles had greater interaction with hMSCs (increase in average fluorescent intensity, Figure 2C), and confocal imaging revealed internalization (Figure 2D). The microparticles were formulated with 19% (w/w) TPCA-1 loading and 80% encapsulation efficiency (Figure 2B), and PLL coating did not affect these parameters. The microparticles released TPCA-1 completely within 20 days of incubation in PBS at 37°C with a near zero-order release kinetics (Figure 2E). The microparticles degraded almost completely after 20 days suggesting that the drug was encapsulated. Incubation of hMSCs with TPCAμP concentrations of up to 200 μg/ml for 7 days did not affect cell viability (Figure S1D).
Figure 2.
Characterization of TPCA-1-Delivering PLGA Microparticles and Internalization in hMSCs
(A) Representative scanning electron microscope image of TPCAμPs showing size, shape, and morphology.
(B) Physicochemical properties of TPCAμPs.
(C) Representative plot of flow cytometry analysis of association of uncoated and PLL-coated DμPs in hMSCs.
(D) Representative confocal images depicting membrane association of uncoated DμPs and cytosolic association of PLL-coated DμPs (red, DiI-stained cell membrane; green, DμPs; blue, DAPI-stained nucleus).
(E) Profile of TPCA release kinetics from 6 mg of TPCAμPs into PBS at 37°C. Error bars represent ±SD.
TPCAμPs Sustainably Inhibit Pro-inflammatory Secretome in TNF-α-Stimulated hMSCs
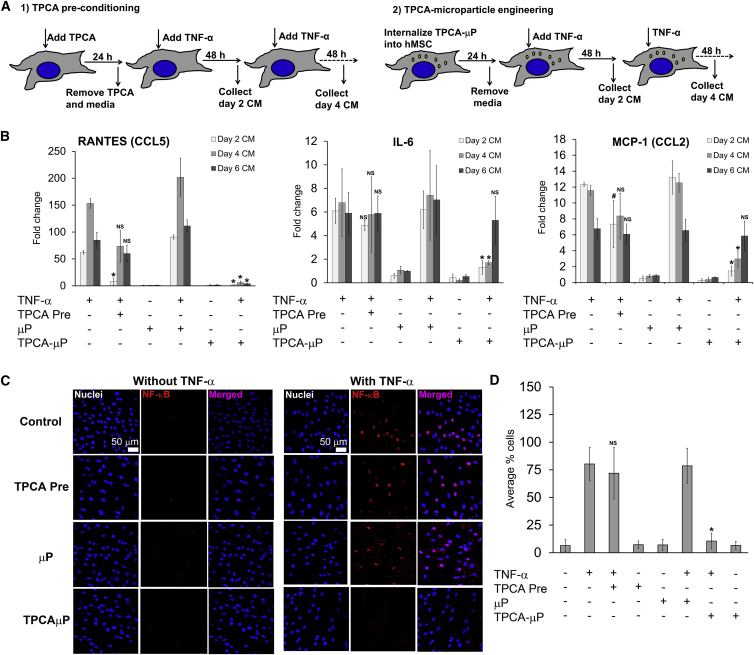
Next, we compared our microparticle engineering regimen with the conventional preconditioning regimen to investigate if our approach would provide sustained modulation of the hMSC secretome under TNF-α stimulation. From the pro-inflammatory secretome profiled earlier, IL-6, MCP-1 (CCL2), and RANTES (CCL5) were selected for this study as they are known to significantly mediate inflammatory responses in many cell types. Soluble TPCA-1 preconditioning (Figure 3A) could inhibit secretion of RANTES from TNF-hMSC significantly for only 2 days (Figure 3B). Soluble TPCA-1 preconditioning was unable to inhibit IL-6 and MCP-1 secretion in TPCApre + TNF-hMSCs (Figure 3B) throughout the assay. μP-hMSCs displayed a secretory profile similar to control-hMSCs and, upon TNF-α stimulation, secreted upregulated levels of RANTES, IL-6, and MCP-1 similar to TNF-hMSCs. Notably, TPCAμP-hMSCs, upon repeated TNF-α stimulation, displayed significant inhibition in secreted levels of RANTES (low to basal level) until 6 days and IL-6 and MCP-1 until 4 days (Figure 3B).
Figure 3.
Quantitative Analysis of Secretion of Pro-inflammatory Mediators in hMSC Conditioned Medium and NF-κB Nuclear Translocation in TPCA Preconditioned and Microparticle-Engineered hMSCs on Days 2, 4, and 6
(A) Experimental design for comparing effects of TPCA preconditioning and microparticle engineering regimens in TNF-hMSC.
(B) Fold change in secreted level of RANTES (CCL5), IL-6, and MCP-1 (CCL2) as indicated in hMSCs normalized to the secreted level of control-hMSC. Data represent the average of triplicates from two different hMSC donors. NS represents non-significance versus TNF-hMSC and ∗ indicates p < 0.01 versus μP + TNF-hMSC CM.
(C) Representative fluorescence images at 40× magnification of hMSCs without microparticles and hMSCs internalized with microparticles after 30 min of TNF-α (10 ng/ml) stimulation. Blue, DAPI (nucleus); red, NF-κB.
(D) Quantitative analysis of percentage of hMSCs positive for nuclear NF-κB. Each data point is the average of five random fields of view, and data represent the average of triplicates from three independent experiments from two donor hMSCs. NS represents non-significance versus TNF-hMSC, ∗ indicates p < 0.01 versus μP + TNF-hMSC CM, and # represents p < 0.01 versus TPCApre + TNF-hMSC CM. Error bars represent ±SD.
Intracellular TPCA Released from Microparticles Inhibits NF-κB Nuclear Translocation in TNF-α-Stimulated hMSCs
We then sought to examine the effect of TNF-α and TPCA (exogenous and intracellularly released from microparticles) on NF-κB signaling in hMSCs. The experimental design is similar to the one depicted in Figure 3A except that the cells were stained 30 min after TNF-α stimulation due to the rapid nuclear translocation of NF-κB (p65 subunit). The p65 subunit of NF-κB complex was absent in the nuclei of about 90% of untreated and μP-hMSCs, whereas stimulation with TNF-α resulted in the translocation of NF-κB into the nuclei of TNF-hMSCs and TNF + μP-hMSCs (Figures 3C and 3D). Preconditioning of hMSCs with TPCA also did not inhibit nuclear translocation of NF-κB suggesting the effect of TPCA is significantly attenuated due to its removal from the media. In contrast, significantly fewer TNF + TPCAμP-hMSCs (about 20% cells) contained NF-κB in their nuclei (Figure 3D), suggesting that binding of TPCA to IKK-2 inhibits TNF-α from activating NF-κB. This suggests that hMSCs could be transiently controlled for response to TNF-α by loading them with TPCAμPs.
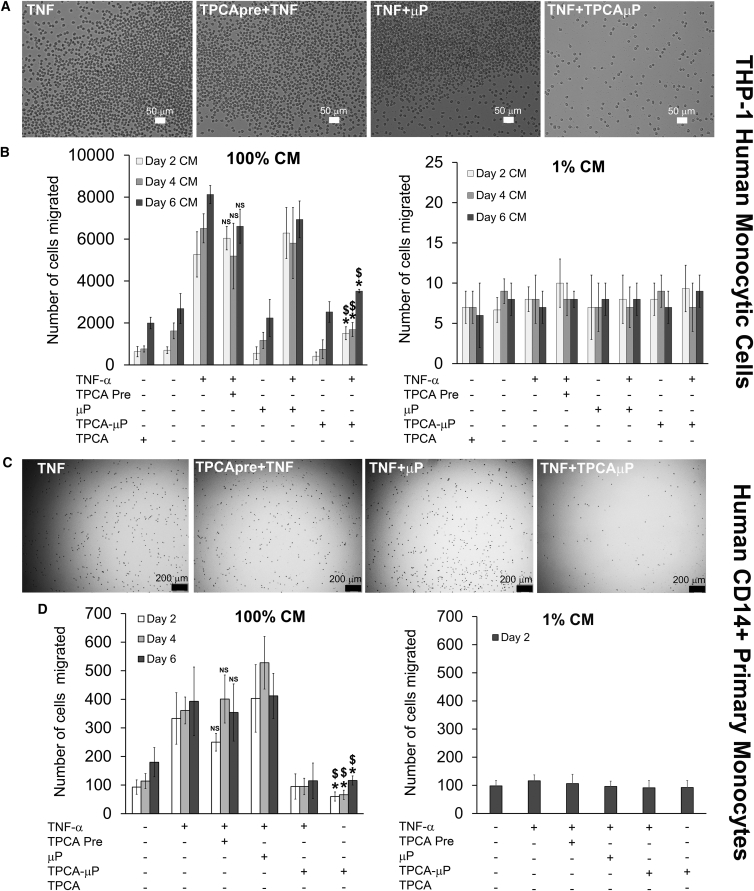
Secretome from TNF-α-Treated-TPCAμP-hMSCs Sustainably Inhibits Transwell Migration of Human Monocytic Cell Line and Human Primary CD14+ Monocytes
Timely inhibition of monocyte migration is necessary to attenuate irreversible scar formation after MI. To assess functionality of the inhibited secretome from TNF + TPCAμP-hMSCs, we investigated transwell migration of a human monocytic cell line (THP-1) and human primary CD14+ monocytes (Figure S4A) in response to hMSC secretome (Figures 4A and 4D). Figures 4B and 4D show that the migration of THP-1 and primary monocytes was dose dependent. This is evident from the large number of cells that migrated toward 100% CM, while 1% CM attracted few cells. CM from days 2, 4, and 6 of TNF-hMSCs augmented migration of THP-1 cells and primary monocytes across the transwell approximately 4- to 8-fold (Figure 4B) and approximately 4-fold (Figure 4D) that of CM from control-hMSCs, respectively. CM from days 2, 4, and 6 of the soluble TPCA preconditioning regimen was ineffective in inhibiting migration of cells across the transwell, even though less significant reduction was observed for day 4 and day 6 CM versus CM from TNF-α-stimulated hMSCs. Conversely, CM from day 2 of TNF + TPCAμP-hMSCs significantly inhibited migration (3.7-fold and p < 0.01) versus TNF_BμP-hMSCs, and the effect was sustained through day 4 and day 6 although less significantly (2.5-fold, p < 0.05 and 1.6-fold, p < 0.01, respectively). A 4- to 5-fold reduction was observed in the number of primary monocytes migrated with CM from TNF + TPCAμP-hMSCs versus CM from TNF_BμP-hMSCs. Direct comparison of CM from days 2, 4, and 6 reveals that particle-engineered hMSCs are significantly more efficient in reducing migration of monocytes than soluble factor preconditioned hMSCs.
Figure 4.
Dose-Dependent Transwell Migration of Human Monocytic THP-1 Cells and Human Primary Monocytes for 2 h in Response to Conditioned Medium from TPCA Preconditioned and Microparticle-Engineered hMSCs
(A) Representative bright-field images of migrated THP-1 cells in the bottom of the wells.
(B) Quantitative analysis of the number of THP-1 cells migrated into the bottom well in response to 100% CM or 1% CM from TPCA-1 preconditioned and microparticle-engineered hMSCs. Data represent the average of triplicates from three independent experiments.
(C) Representative bright-field images of migrated primary macrophages in the bottom of the wells.
(D) Quantitative analysis of the number of primary macrophages migrated into the bottom well in response to 100% CM or 1% CM from TPCA-1 preconditioned and microparticle-engineered hMSCs.
Data represent the average of triplicates from three independent experiments. NS represents non-significance versus TNF-hMSC CM, whereas ∗ represents p < 0.01 versus T-hMSC CM and $ represents p < 0.01 versus μP + TNF-hMSC CM. Error bars represent ±SD.
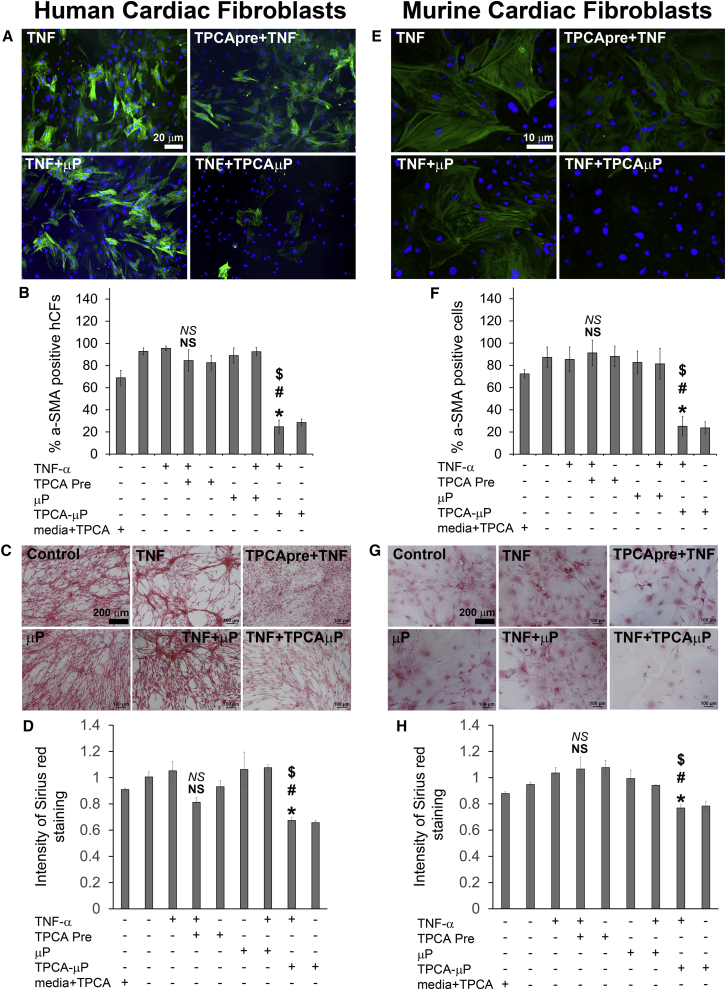
Secretome from TNF-α-Treated-TPCAμP-hMSCs Inhibits α-SMA Expression and Collagen Production in Human and Murine Cardiac Fibroblasts
CF differentiate into myofibroblasts owing mainly to stimulation by TGF-β, but other pro-inflammatory mediators such as IL-6 and MCP-1 also mediate such pathological differentiation leading to irreversible scar formation upon acute myocardial infarction. Hence we investigated whether the secretome from TNF + TPCAμP-hMSCs would inhibit α-smooth muscle actin (α-SMA; myofibroblast specific marker) expression and collagen production in CMF. Human (hCF) and mouse (mCF) CF were treated with CM from different hMSC groups for 96 h at 37°C and later immunostained for α-SMA and imaged. hCF receiving day 2 CM from control-hMSCs or TNF-hMSCs were significantly positive for α-SMA confirming their CMF phenotype (Figure 5A). CM from TPCApre + TNF-hMSCs could only inhibit α-SMA expression in about 18% of hCF in comparison with CM from control-hMSCs (Figures 5A and 5B). CM from TNF + μP-hMSCs had a similar effect on α-SMA expression in hCF in comparison with CM from TNF-hMSCs. However, day 2 CM from TNF + TPCAμP-hMSC inhibited α-SMA expression in about 80% of hCF versus CM from control-hMSCs. In addition, CM from particle-engineered hMSCs reduced the α-SMA expression in hCF 4-fold more than CM from preconditioned hMSCs. hMSC media alone or hMSC media with TPCA-1 did not inhibit α-SMA expression (Figure 5B) in hCF. Next, we sought to examine collagen deposition by hMSC CM-treated hCF and mCF using Sirius red staining assay. None of the hMSC CM or hMSC media or hCF media showed detectable collagen levels when assayed alone for Sirius red staining (Figures S5A and S5B). On the other hand, when hCF or mCF were treated in various hMSC media, we could detect significant levels of collagen (Figures 5C, 5D, 5G, and 5H). Within the groups, hCF treated in control-hMSC CM, TNF-hMSC CM, μP-hMSC CM, and TNF + μP-hMSC CM groups had significantly higher levels of collagen as quantified by relative staining intensity in Figures 5D and 5H. hCF treated with hMSC media or hMSC media + TPCA-1, secreted collagen at similar levels to control-hMSC CM. Soluble TPCA-1 preconditioned hMSC CM did not significantly inhibit collagen secretion from hCF, but, hCF in TNF + TPCAμP-hMSC CM secreted 35% lower amount of collagen compared with control-hMSC CM-treated hCF.
Figure 5.
Analysis of α-SMA Expression and Collagen Deposition of Human and Murine CF in Response to Conditioned Media of TPCA Preconditioned and Microparticle-Engineered hMSCs
(A) Representative fluorescence images (4×) of hCF treated with day 2 CM from each hMSC group (green, α-SMA; blue, DAPI).
(B) Quantitative analysis of the number of α-SMA + hCF treated with day 2 CM from each hMSC group.
(C) Representative bright-field images of hCF treated with day 2 CM from each hMSC group.
(D) Quantitative analysis of relative Sirius red staining intensity from hCF in response to CM from each hMSC group.
(E) Representative fluorescence images (10×) of mCF treated with day 2 CM from each hMSC group (green, α-SMA; blue, DAPI).
(F) Quantitative analysis of number of α-SMApos mCF treated with CM from each hMSC group.
(G) Representative bright-field images of mCF treated with day 2 CM from each hMSC group.
(H) Quantitative analysis of relative Sirius red staining intensity from mCF in response to day 2 CM from each hMSC group.
NS represents not significant versus hMSC media + TPCA; NS represents not significant versus TNF-hMSC CM; ∗ indicates p < 0.01 versus hMSC media + TPCA; $ indicates p < 0.01 versus TPCApre + TNF-hMSC CM; # indicates p < 0.01 versus TNF + μP-hMSC CM. All data represent the average of triplicates from three independent experiments from two different donor hMSCs. Error bars represent ±SD.
As a first step toward validation in an animal model in vivo, we chose to analyze whether TPCAμP regulated hMSC secretome would also affect mouse CF differentiation. The effect of hMSC CM on mCF was similar to hCF (Figures 5E and 5F) with respect to α-SMA expression. mCF treated with hMSC media alone or hMSC media with soluble TPCA-1 had elevated levels of α-SMA expression (data not shown) in mCF. However, hMSC CM did not seem to inhibit collagen production of mCF to the extent of hCF (Figures 5G and 5H). More importantly, soluble TPCA-1 preconditioned hMSC CM did not significantly inhibit collagen secretion from mCF. However, compared with CM from TNF + μP-hMSC, TNF + TPCAμP-hMSC CM reduced collagen secretion from mCF by about 24%. This suggests that the inhibited pro-inflammatory secretome from particle-engineered hMSCs attenuate the adoption of fibrotic phenotype changes in both hCF and mCF.
Discussion
The MSC secretome is being explored in pre-clinical and clinical trials as one of the primary mediators of cardiovascular repair via immunomodulatory effects (Ranganath et al., 2012). However, to date, approaches have not met with success as initially hoped. The immunomodulatory property of MSCs presents an unpredictable therapeutic outcome as it is affected by inter-cellular and inter-donor heterogeneity, the MSC source (Melief et al., 2013, Naftali-Shani et al., 2013, Ortiz et al., 2007), and the host microenvironment (Prockop, 2013). In view of these heterogeneities, there exists an urgent need to develop a platform for controlling the MSC secretome post-transplantation. To address this, we subjected hMSCs to a simulated inflammatory microenvironment by TNF-α stimulation (10 ng/ml) and characterized the secretome. It is important to consider that the levels of TNF-α vary in the host microenvironment, and thus MSC response cannot be easily predicted. The pro-inflammatory effect of TNF-α is generally mediated by NF-κB. In view of this, we investigated whether NF-κB inhibition in TNF-α-stimulated MSCs could inhibit the pro-inflammatory secretome sufficiently to suppress outcomes of inflammation. We demonstrate that the pro-inflammatory phenotype of hMSCs under an inflammatory stimulus can be transiently inhibited using intracellular release of a small-molecule inhibitor of NF-κB (TPCA-1) via microparticles. This in turn is sufficient to suppress differentiation of CF to CMF.
In hMSCs, TNF-α mediates the upregulation of pro-inflammatory gene expression and protein secretion via NF-κB (Carrero et al., 2012, Lee et al., 2010). Of 250 secreted factors profiled, we found that TNF-hMSCs secreted nine pro-inflammatory mediators at significantly higher concentrations compared with control-hMSCs. These mediators, barring RANTES (CCL5), are constitutively secreted, and TNF-α further augments their secretion levels. Secretion of RANTES, however, seems exclusively to be the result of TNF-α stimulation since it is not constitutively secreted.
The pro-inflammatory secretome from MSCs is reported to have deleterious effects in several disease models (Table S1). Pro-inflammatory mediators such as IL-6 and MCP-1 mediate differentiation of CF to CMF and collagen deposition, leading to adverse remodeling and heart failure. MCP-1 attracts circulating monocytes and induces differentiation to M1 macrophages, leading to collagen deposition and adverse fibrosis in aging hearts (Cieslik et al., 2014). Macrophages aggravate the inflammatory response by releasing elevated levels of pro-inflammatory cytokines including IL-6, MCP-1, IL-8 which in turn mediate differentiation of CF to CMF. Hence, therapeutic interventions are primarily aimed at reducing CMF differentiation thereby preventing irreversible scar formation and maximizing viable myocardium. The negative effects of pro-inflammatory mediators under disease settings such as cardiac fibrosis provided the insight for inhibiting their secreted levels from hMSCs to maximize the benefits of hMSC-based therapy.
Initiation and progression of cardiac fibrosis may occur due to secretion of TNF-α and IL-6 by infused MSCs (Naftali-Shani et al., 2013). Naive BM-MSCs can be pro-inflammatory and hence not sufficiently beneficial in improving ischemic myocardium and might require pretreatment to maximize efficacy (Eun et al., 2011). For example, infused TNFR2 and TNFR1/R2 knockout mMSCs (into isolated rat hearts) after ischemia-reperfusion injury offered either no benefit or reduced cardioprotection whereas infused TNFR1 knockout mMSCs provided greater cardioprotection, measured via myocardial TNF-α levels and left ventricular diastolic performance compared with WT mMSCs. This suggests that by controlling TNFR signaling, MSC phenotype could be modulated between pro-inflammatory to anti-inflammatory (Kelly et al., 2010). TNF-α stimulation (10 ng/ml for 48 hr) also results in a significantly elevated concentration of pro-inflammatory mediators in the hMSC secretome (Lee et al., 2010) stimulating migration of human monocytes in vitro that might aggravate inflammation post-MI. Hence, we sought to non-genetically and transiently inhibit secretion levels of pro-inflammatory mediators from hMSCs under TNF-α stimulation.
TPCA-1 reversibly binds to IKKβ and prevents it from phosphorylating IκBα for degradation and thereby blocks nuclear translocation of NF-κB (Kondo et al., 2008). Our results demonstrate that TPCA-1 may inhibit the stimulatory action of TNF-α on NF-κB. TPCA-1 partially inhibits secretion of constitutively secreted pro-inflammatory factors such as IL-6 and MCP-1, which suggests that constitutive secretion is not entirely dependent on NF-κB activation. The reduction of RANTES, GRO, and GCP-2 to basal levels may indicate that NF-κB activation mediates the secretion of these cytokines upon TNF-α treatment as TPCA-1 abrogates NF-κB activation. Our cytokine array results confirm that TPCA-1 abrogates the effect of TNF-α on production of pro-inflammatory cytokines by hMSCs. In addition, we demonstrated that TPCA-1 treatment did not inhibit secretion of beneficial paracrine factors such as growth factors, cytokines, and anti-inflammatory mediator TSG-6, which when secreted from lung embolized hMSCs attenuate MI in mice (Lee et al., 2009). Hence, angiogenic and TSG-6-mediated anti-inflammatory properties of hMSCs may not be compromised upon TPCA-1 treatment. In addition, continuous intracellular TPCA exposure to hMSCs might yield sustained binding to IKKβ, resulting in the inhibition of NF-κB and overcome reversible inhibition of IKKβ. TPCA-1 has non-specific effects of IKKβ inhibition on many cell types with potential undesirable side effects. In addition, MSCs demonstrate a “hit and run effect” of immune modulation (Levy et al., 2013) and are cleared from the body within a week post-transplantation. Hence it is desirable to intracellularly deliver TPCA-1 into hMSCs at a controlled rate for transient modulation of the pro-inflammatory secretome.
In this study, we developed an engineering platform to intracellularly deliver TPCA-1 to hMSCs. μPs of 1–2 μm were internalized following anionic surface modification. By choosing PLGA polymer with a lactic acid to glycolic acid ratio of 50:50 and low molecular weight of 10 kDa, we tailored the release kinetics of TPCA-1 to achieve a concentration to inhibit IKK2. Upon preconditioning of hMSCs with soluble TPCA prior to stimulation with TNF-α, the hMSC secretome was similar to TNF-α stimulation alone (TNF-hMSC) suggesting that IKKβ inhibition may require continued presence of TPCA to prevent NF-κB activation. The sustained intracellular release of TPCA-1 enabled prolonged inhibition of IKKβ and competitively inhibited TNF-α from activating NF-κB mediated pro-inflammatory cytokine secretion. NF-κB signaling in cells is oscillatory in nature, and gene expression seems to depend on its dynamics (Nelson et al., 2004). Hence, under continuous TNF-α stimulus, NF-κB activation might be sustained. However, the effect of intracellular TPCA inhibition in microparticle-engineered hMSCs was maintained after multiple rounds of TNF-α stimulation suggesting that microparticle-engineered hMSCs are able to control the pro-inflammatory phenotype under sustained inflammatory stimulus.
To demonstrate the functional relevance of microparticle-mediated hMSC secretome regulation, we focused on cardiac fibrosis. IL-6 (Ma et al., 2012), MCP-1, and other pro-inflammatory mediators augment monocyte migration (Lee et al., 2010) and induce differentiation of CF to CMF, which are the primary scar-forming cells post-MI. Expectedly, we found that while the elevated levels of pro-inflammatory mediators in CM from preconditioned hMSCs are ineffective in inhibiting monocyte migration, CM from TNF + TPCAμP-hMSCs effectively inhibited transwell migration of THP-1 cells by at least 2- to 3-fold, corresponding to reduced levels of pro-inflammatory mediators as seen by our secretome analysis.
Control-hMSC CM also induced significant differentiation of CF to CMF demonstrating the presence of pro-inflammatory mediators secreted by hMSCs as well as those likely to be present in the culture serum. CM from control-hMSCs and μP-hMSCs have lower levels of pro-inflammatory factors (compared with TNF-α-stimulated hMSCs) yet the number of α-SMA+ CMF remained unchanged when CF were treated with these CM, suggesting that the secretome may lack anti-inflammatory modulators and growth factors. In addition, treatment with TPCA-1 alone did not cause a reduction in the number of α-SMA+ CMF, suggesting that an hMSC-mediated paracrine function is essential. While TPCA-1 pretreatment of hMSCs before TNF-α stimulation showed a significant reduction in CMF numbers, this was greatly enhanced when TPCA-1 was available intracellularly via the microparticles. CM from TNF + TPCAμP-hMSC likely prevented differentiation of CF to CMF due to the continued intracellular inhibition of IKK-mediated NF-κB activation, thus preventing the release of an hMSC pro-inflammatory secretome. Surprisingly, the CMF number was reduced by over 2-fold, suggesting that TPCA-1 may activate hMSC pathways that revert the CMF phenotype. We cannot rule out the possibility that other contributors in the hMSC secretome that were not profiled might also be contributing, and their action is facilitated by intracellular TPCA-1. For instance, in an in vitro 3D model of cardiac fibrosis under hypoxic conditions, reversal of CMF to the CF phenotype was shown to be due to reduced MSC TGF-β levels (Galie and Stegemann, 2014). Our assay revealed a similar trend in terms of collagen production from CF. CF treated with CM from control-hMSCs, or TPCApre + TNF-hMSCs or μP-hMSCs secreted elevated levels of collagen into the media suggesting that only inhibited levels of pro-inflammatory mediators could not be implicated in the reduction in collagen secretion. The reduced number of α-SMA+ CMF in CM from TNF + TPCAμP-hMSCs possibly contributed to the lower secretion level of collagen in the media (Figure 5B). Upon de-differentiation or lowered α-SMA expression, it is possible that CMFs lose collagen secretion ability. In regions of MI, CF switch to the myofibroblast phenotype due to stress from the infarct scar (Tomasek et al., 2002). High expression of α-SMA typical in such CMF (Teunissen et al., 2007) has been implicated for remodeling due to their high contractility (Santiago et al., 2010). In addition, the collagen secretion capacity of CMF is very high (Petrov et al., 2002). Overall, attenuation in the number of collagen-secreting α-SMA+ CMF could be beneficial in preventing pathological remodeling or irreversible scar formation and allowing cardiac regeneration.
Here we have demonstrated that the pro-inflammatory hMSC secretome could be inhibited using a microparticle engineering approach delivering an intracellular NF-κB inhibitor, TPCA-1. It is however important to note that the MSC secretome composition may change depending on the level of TNF-α encountered in vivo following transplantation. Nevertheless, a similar approach could be beneficial in inflammatory disease settings such as chronic inflammation and macrophage-mediated atherosclerosis. The approach of microparticle engineering of an exogenous cell population by modulating a central regulatory pathway, may find application in other cell types and pathways and could provide an attractive strategy for harnessing any cell secretome for therapy. This approach could also be potentially employed to modulate the composition of extracellular vesicles (exosomes) for therapy.
Experimental Procedures
Mesenchymal Stromal Cell Culture and Characterization
Human MSCs and human and murine CF culture and characterization are described in Supplemental Experimental Procedures.
Stimulation of hMSCs with TNF-α and TPCA-1 Treatment
Primary hMSCs derived from bone marrow of healthy consenting donors were cultured in standard media and used at passage number 3–6 (see Supplemental Experimental Procedures). 30,000 cells per well in a 24-well plate were cultured in serum-free MesenCult XF media (STEMCELL Technologies) for 24 hr and then treated for 48 hr with either (1) 0.1% (v/v) DMSO (Sigma) (control-hMSC), or (2) 10 ng/ml TNF-α (Sigma-Aldrich) (TNF-hMSC), or (3) 10 μM TPCA-1 2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide) (Calbiochem), or (4) 10 μM TPCA-1 and stimulating with 10 ng/ml TNF-α (TNF + TPCA-hMSC), followed by conditioned media (CM) collection. Metabolic activity of cells was measured using the MTS assay (see Supplemental Information).
Secretome Profiling of Stimulated hMSCs
Quantitative measurement of 250 human cytokines in the stimulated hMSC CM was performed using Quantibody Human Cytokine Array 5000 (Ray Biotech) according to the manufacturer's instructions. Fluorescence signals at 532 nm from the arrays were obtained by scanning the slides using an Axon GenePix 4300A microarray laser scanner (Molecular Devices). Spot intensity was quantified into concentration values using standard curves based on linear regression and analyzed (fold change in concentration) using GenePix Pro 7 software. See Supplemental Information for details.
PLGA Microparticle Fabrication, Characterization, and Engineering of hMSCs
TPCA-1, or Rhodamine 6G dye (Sigma) or DiI dye (Cell Signaling) were encapsulated in PLGA microparticles using the single-emulsion technique. To enhance microparticle uptake and loading in hMSCs, the surface of PLGA microparticles was modified as described before (Ankrum et al., 2014b). See Supplemental Information for details.
Analysis of Microparticle-hMSC Interaction
DiO-loaded-microparticle internalized hMSCs (DμP-hMSCs) were trypsinized and analyzed by flow cytometry to characterize the association of microparticles with hMSCs. The mean fluorescence of the gated cell population was quantified. hMSCs with no particles and blank particles served as controls. DμP internalization was characterized by confocal microscopy imaging (Zeiss LSM510) of fixed cells followed by image analysis. Microparticles within the plane of the nucleus and inside the boundaries of the cell membrane were interpreted to be internalized. Cytotoxicity of TPCAμPs on hMSCs was measured using the MTS assay as described earlier.
Release Kinetics of TPCA-1 from Microparticles
In vitro release kinetics of TPCA-1 from TPCAμPs was profiled by suspending 6 mg of TPCA μPs in 1 ml of PBS and loading into a 1.5 × 6 cm polytetrafluoroethylene dialysis membrane (Thermo Fisher Scientific) with 8–10 kDa molecular weight cut off. The membrane was then introduced into 20 ml of PBS and incubated at 37°C with shaking. PBS was sampled at specific time points and drug was extracted using dichloromethane (DCM). Following evaporation of DCM and dissolution of the drug in DMSO, the concentration of TPCA-1 was measured by determining absorbance at 320 nm using a UV-visible spectrophotometer.
Analysis of the Composition and Kinetics of Preconditioned and Microparticle-Engineered hMSC Secretome
The experimental procedure is described in Figure 3A. The effect of TPCA preconditioning or internalized TPCAμPs on TNF-α-treated hMSC secretome was investigated by performing an ELISA of CM to quantify MCP-1, IL-6, and RANTES concentrations. See Supplemental Information for details.
Statistical Analysis
Single-factor ANOVA was used for multiple pairwise comparisons with two-factor t test with Bonferroni correction post hoc analysis. p < 0.01 was considered significant.
See Supplemental Information for additional methods.
Author Contributions
S.H.R. and O.L. designed experiments and contributed to discussions; S.H.R., Z.T., and K.M., performed experiments. J.M.K. and M.S.I. conceptualized and supervised the experiments. S.H.R., J.M.K., and M.S.I., wrote the paper.
Acknowledgments
We thank Abhishek Sinha, Chandan Kadur, James Ankrum, and Mamta Jain for helpful and enlightening discussions. Funding supported by the Indo-US Science and Technology Forum (IUSSTF) to J.M.K. and M.S.I.; JNCASR grant to M.S.I.; and NIH grant HL097172, and HL095722 to J.M.K. J.M.K. consults in the field of cell therapy for Stempeutics and Mesoblast.
Published: June 2, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.05.003.
Contributor Information
Jeffrey M. Karp, Email: jeffkarp.bwh@gmail.com.
Maneesha S. Inamdar, Email: inamdar@jncasr.ac.in.
Supplemental Information
References
- Ankrum J.A., Dastidar R.G., Ong J.F., Levy O., Karp J.M. Performance-enhanced mesenchymal stem cells via intracellular delivery of steroids. Sci. Rep. 2014;4:4645. doi: 10.1038/srep04645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrum J.A., Miranda O.R., Ng K.S., Sarkar D., Xu C., Karp J.M. Engineering cells with intracellular agent-loaded microparticles to control cell phenotype. Nat. Protoc. 2014;9:233–245. doi: 10.1038/nprot.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosh T.J., Ylostalo J.H., Bazhanov N., Kuhlman J., Prockop D.J. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1) Stem Cells. 2013;31:2443–2456. doi: 10.1002/stem.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo M.E., Fibbe W.E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Bozkurt B., Kribbs S.B., Clubb F.J., Jr., Michael L.H., Didenko V.V., Hornsby P.J., Seta Y., Oral H., Spinale F.G., Mann D.L. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- Carrero R., Cerrada I., Lledo E., Dopazo J., Garcia-Garcia F., Rubio M.P., Trigueros C., Dorronsoro A., Ruiz-Sauri A., Montero J.A. IL1beta induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-kappaB. Stem Cell Rev. 2012;8:905–916. doi: 10.1007/s12015-012-9364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik K.A., Trial J., Crawford J.R., Taffet G.E., Entman M.L. Adverse fibrosis in the aging heart depends on signaling between myeloid and mesenchymal cells; role of inflammatory fibroblasts. J. Mol. Cell Cardiol. 2014;70:56–63. doi: 10.1016/j.yjmcc.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisostomo P.R., Wang M., Herring C.M., Markel T.A., Meldrum K.K., Lillemoe K.D., Meldrum D.R. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1) J. Mol. Cell Cardiol. 2007;42:142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisostomo P.R., Wang Y., Markel T.A., Wang M., Lahm T., Meldrum D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- Discher D.E., Mooney D.J., Zandstra P.W. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun L.Y., Song H., Choi E., Lee T.G., Moon D.W., Hwang D., Byun K.H., Sul J.H., Hwang K.C. Implanted bone marrow-derived mesenchymal stem cells fail to metabolically stabilize or recover electromechanical function in infarcted hearts. Tissue Cell. 2011;43:238–245. doi: 10.1016/j.tice.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Galie P.A., Stegemann J.P. Injection of mesenchymal stromal cells into a mechanically stimulated in vitro model of cardiac fibrosis has paracrine effects on resident fibroblasts. Cytotherapy. 2014;16:906–914. doi: 10.1016/j.jcyt.2014.01.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M., He H.M., Noiseux N., Liang O.D., Zhang L.M., Morello F., Mu H., Melo L.G., Pratt R.E., Ingwall J.S. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- Kelly M.L., Wang M., Crisostomo P.R., Abarbanell A.M., Herrmann J.L., Weil B.R., Meldrum D.R. TNF receptor 2, not TNF receptor 1, enhances mesenchymal stem cell-mediated cardiac protection following acute ischemia. Shock. 2010;33:602–607. doi: 10.1097/SHK.0b013e3181cc0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y., Fukuda K., Adachi T., Nishida T. Inhibition by a selective IkappaB kinase-2 inhibitor of interleukin-1-induced collagen degradation by corneal fibroblasts in three-dimensional culture. Invest Ophthalmol. Vis. Sci. 2008;49:4850–4857. doi: 10.1167/iovs.08-1897. [DOI] [PubMed] [Google Scholar]
- Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., Semprun-Prieto L., Delafontaine P., Prockop D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.J., Kim J., Kim M.Y., Bae Y.S., Ryu S.H., Lee T.G., Kim J.H. Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J. Proteome Res. 2010;9:1754–1762. doi: 10.1021/pr900898n. [DOI] [PubMed] [Google Scholar]
- Levy O., Zhao W., Mortensen L.J., Leblanc S., Tsang K., Fu M., Phillips J.A., Sagar V., Anandakumaran P., Ngai J. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood. 2013;122:e23–32. doi: 10.1182/blood-2013-04-495119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Hou H., Yi W., Yang G., Gu C., Lau W.B., Gao E., Ma X., Lu Z., Wei X. Increased expression of pigment epithelium-derived factor in aged mesenchymal stem cells impairs their therapeutic efficacy for attenuating myocardial infarction injury. Eur. Heart J. 2013;34:1681–1690. doi: 10.1093/eurheartj/ehr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F., Li Y., Jia L., Han Y., Cheng J., Li H., Qi Y., Du J. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS One. 2012;7:e35144. doi: 10.1371/journal.pone.0035144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D.L. Recent insights into the role of tumor necrosis factor in the failing heart. Heart Fail Rev. 2001;6:71–80. doi: 10.1023/a:1011449708842. [DOI] [PubMed] [Google Scholar]
- Melief S.M., Zwaginga J.J., Fibbe W.E., Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013;2:455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mias C., Trouche E., Seguelas M.H., Calcagno F., Dignat-George F., Sabatier F., Piercecchi-Marti M.D., Daniel L., Bianchi P., Calise D. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749–1757. doi: 10.1634/stemcells.2007-1000. [DOI] [PubMed] [Google Scholar]
- Moro C., Jouan M.G., Rakotovao A., Toufektsian M.C., Ormezzano O., Nagy N., Tosaki A., de Leiris J., Boucher F. Delayed expression of cytokines after reperfused myocardial infarction: possible trigger for cardiac dysfunction and ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3014–H3019. doi: 10.1152/ajpheart.00797.2007. [DOI] [PubMed] [Google Scholar]
- Naftali-Shani N., Itzhaki-Alfia A., Landa-Rouben N., Kain D., Holbova R., Adutler-Lieber S., Molotski N., Asher E., Grupper A., Millet E. The origin of human mesenchymal stromal cells dictates their reparative properties. J. Am. Heart Assoc. 2013;2:e000253. doi: 10.1161/JAHA.113.000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.E., Ihekwaba A.E., Elliott M., Johnson J.R., Gibney C.A., Foreman B.E., Nelson G., See V., Horton C.A., Spiller D.G. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- Ortiz L.A., DuTreil M., Fattman C., Pandey A.C., Torres G., Go K., Phinney D.G. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C.F., Han Y.L., Jie D., Yan C.H., Jian K., Bo L., Jie L. Overexpression of cellular repressor of E1A-stimulated genes inhibits TNF-alpha-induced apoptosis via NF-kappaB in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2011;406:601–607. doi: 10.1016/j.bbrc.2011.02.100. [DOI] [PubMed] [Google Scholar]
- Petrov V.V., Fagard R.H., Lijnen P.J. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–263. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- Preda M.B., Ronningen T., Burlacu A., Simionescu M., Moskaug J.O., Valen G. Remote transplantation of mesenchymal stem cells protects the heart against ischemia-reperfusion injury. Stem Cells. 2014;32:2123–2134. doi: 10.1002/stem.1687. [DOI] [PubMed] [Google Scholar]
- Prockop D.J. Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31:2042–2046. doi: 10.1002/stem.1400. [DOI] [PubMed] [Google Scholar]
- Prockop D.J., Oh J.Y. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol. Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath S.H., Levy O., Inamdar M.S., Karp J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M., Griffith L.G., Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res. Ther. 2010;1:32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J.J., Dangerfield A.L., Rattan S.G., Bathe K.L., Cunnington R.H., Raizman J.E., Bedosky K.M., Freed D.H., Kardami E., Dixon I.M. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev. Dyn. 2010;239:1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- Sarkar D., Ankrum J.A., Teo G.S.L., Carman C.V., Karp J.M. Cellular and extracellular programming of cell fate through engineered intracrine-, paracrine-, and endocrine-like mechanisms. Biomaterials. 2011;32:3053–3061. doi: 10.1016/j.biomaterials.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.M., Wang J.N., Guo L.Y., Kong X., Yang J.Y., Zheng F., Zhang L., Huang Y.Z. Mesenchymal stem cells modified with stromal cell-derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol. Cells. 2010;29:9–19. doi: 10.1007/s10059-010-0001-7. [DOI] [PubMed] [Google Scholar]
- Teunissen B.E., Smeets P.J., Willemsen P.H., De Windt L.J., Van der Vusse G.J., Van Bilsen M. Activation of PPARdelta inhibits cardiac fibroblast proliferation and the transdifferentiation into myofibroblasts. Cardiovasc. Res. 2007;75:519–529. doi: 10.1016/j.cardiores.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Virag J.I., Murry C.E. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am. J. Pathol. 2003;163:2433–2440. doi: 10.1016/S0002-9440(10)63598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Tan J., Wang Y., Meldrum K.K., Dinarello C.A., Meldrum D.R. IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Proc. Natl. Acad. Sci. USA. 2009;106:17499–17504. doi: 10.1073/pnas.0908924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A.R., Suncion V.Y., McCall F., Guerra D., Mather J., Zambrano J.P., Heldman A.W., Hare J.M. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. J. Am. Heart Assoc. 2013;2:e000140. doi: 10.1161/JAHA.113.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H., Soto-Gutierrez A., Navarro-Alvarez N., Nahmias Y., Goldwasser Y., Kitagawa Y., Tilles A.W., Tompkins R.G., Parekkadan B., Yarmush M.L. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol. Ther. 2010;18:1857–1864. doi: 10.1038/mt.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylostalo J.H., Bartosh T.J., Coble K., Prockop D.J. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukareva V., Obrocka M., Houle J.D., Fischer I., Neuhuber B. Secretion profile of human bone marrow stromal cells: donor variability and response to inflammatory stimuli. Cytokine. 2010;50:317–321. doi: 10.1016/j.cyto.2010.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.