Abstract
AIM: To study the effect of a new anti-CD163-dexamethasone conjugate targeting activated macrophages on the hepatic acute phase response in rats.
METHODS: Wistar rats were injected intravenous with either the CD163 targeted dexamethasone-conjugate (0.02 mg/kg) or free dexamethasone (0.02 or 1 mg/kg) 24 h prior to lipopolysaccharide (LPS) (2.5 mg/kg intraperitoneal). We measured plasma concentrations of tumour necrosis factor-α (TNF-α) and interleukin 6 (IL-6) 2 h post-LPS and liver mRNAs and serum concentrations of the rat acute phase protein α-2-macroglobulin (α-2-M) 24 h after LPS. Also, plasma concentrations of alanine aminotransferase and bilirubin were measured at termination of the study. Spleen weight served as an indicator of systemic steroid effects.
RESULTS: The conjugate halved the α-2-M liver mRNA (3.3 ± 0.6 vs 6.8 ± 1.1, P < 0.01) and serum protein (201 ± 48 μg/mL vs 389 ± 67 μg/mL, P = 0.04) after LPS compared to low dose dexamethasone treated animals, while none of the free dexamethasone doses had an effect on liver mRNA or serum levels of α-2-M. Also, the conjugate reduced TNF-α (7208 ± 1977 pg/mL vs 21583 ± 7117 pg/mL, P = 0.03) and IL-6 (15685 ± 3779 pg/mL vs 25715 ± 4036 pg/mL, P = 0.03) compared to the low dose dexamethasone. The high dose dexamethasone dose decreased the spleen weight (421 ± 11 mg vs 465 ± 12 mg, P < 0.05) compared to controls, an effect not seen in any other group.
CONCLUSION: Low-dose anti-CD163-dexamethasone conjugate effectively decreased the hepatic acute phase response to LPS. This indicates an anti-inflammatory potential of the conjugate in vivo.
Keywords: Acute phase response, Dexamethasone, Endotoxin, Hemoglobin scavenger receptor CD163, Cytokines, Inflammation, Rats
Core tip: We aimed to study the effect of a new anti-CD163-dexamethasone conjugate targeting activated macrophages on the hepatic acute phase response in rats. The central finding of the study was a reduction in liver mRNA and plasma levels of the acute phase protein α-2-macroglobulin, and plasma tumour necrosis factor-α and interleukin 6 by administration of the conjugate prior to a lipopolysaccharide-induced inflammatory response. This anti-acute phase effect exceeded that of the therapeutic dexamethasone dose and did not cause systemic adverse effects. Thus, the antibody conjugate may be a potential candidate in future anti-inflammatory macrophage-directed therapy, e.g., in liver diseases with Kupffer cells activation.
INTRODUCTION
In conditions with macrophage proliferation and activation, CD163, a haemoglobin-haptoglobin scavenger receptor expressed exclusively on monocytes and macrophages[1,2], is up-regulated[3,4]. Following toll-like receptor activation by inflammatory stimuli like lipopolysaccharide (LPS), receptor shedding to circulation as soluble CD163 (sCD163) is increased, and within hours upregulated on the cell surface[5]. As an example, hepatic macrophages (Kupffer cells) are activated and sCD163 is increased in patients with liver cirrhosis who chronically experience some degree of endotoxemia and acute phase response[6,7] and this may be involved in the development of the serious cirrhosis complications[6,8].
We have recently constructed a conjugate of CD163 antibody and the potent corticosteroid dexamethasone (anti-CD163mAb-dexa) specifically targeting dexamethasone to activated macrophages[9]. The conjugate reduces the LPS-stimulated cytokine release from activated macrophages in vitro and in vivo in rats and pigs[9,10]. The effect is obtained with very low concentration of dexamethasone, thereby minimizing steroid-induced systemic effects. A fifty-fold higher concentration of non-conjugated dexamethasone is needed to obtain the same anti-inflammatory response[9].
Exposure to LPS is a standard method to induce an acute phase response with a large increase in pro-inflammatory cytokines and hepatic synthesis and release of acute phase proteins[11,12]. While the conjugate reduces the LPS-mediated cytokine response in rats it remains unknown whether it also inhibits the hepatic acute phase protein synthesis response.
To approach this issue we measured the gene expression in liver tissue and serum concentrations of the prevailing acute phase protein α-2-macroglobulin (α-2-M) 24 h post-LPS exposure in rats. α-2-M is a hepatocyte-derived inhibitor of a wide range of proteinases that can be activated during inflammation[13]. Further, we compared plasma concentrations of tumour necrosis factor-α (TNF-α) and interleukin 6 (IL-6) 2 h post-LPS exposure. Spleen weight served as an indicator of systemic steroid effects.
MATERIALS AND METHODS
Animals
The animal protocol was designed to minimize pain or discomfort to the animals. Female Wistar rats (body weight 190-210 g; Taconic M and B, Ejby, Denmark) were housed at 21 °C ± 2 °C with a 12-h artificial light cycle. Two or three animals were housed in each cage, with free access to tap water and standard food (Altromin, Lage, Germany) and acclimatized for one week. Food intake and body weight were registered at the beginning and at the end of the experimental procedures. The study was performed in accordance with local and national guidelines for animal welfare and approved by the national Animal Ethics Committee, protocol No. 2010/561-1918.
Design
Forty animals were allocated in 5 groups of 8: One control group receiving only vehicle (PBS pH 7.4) intravenously and four groups injected intravenously with either vehicle, anti-CD163mAb-dexa (0.02 mg/kg dexamethasone), high dose free dexamethasone (1 mg/kg) (Sigma-Aldrich, Brøndby, Denmark), or low dose free dexamethasone (0.02 mg/kg). The high (“therapeutic”) dose gives maximal steroid efficacy in other rat studies[14,15] and the low dose was the same as in the anti-CD163mAb-dexa. After 24 h, 0.5 mL of saline (controls) or LPS dissolved in 0.5 mL saline (2.5 mg/kg) (from Ecsherichia coli 0111:B4 obtained from Sigma-Aldrich, Brøndby, Denmark; product No. L2630) was injected intraperitoneally. Two hours later and following anaesthesia with inhalation of isofluran 2%-3% (Forene®, Abbott Laboratories, Gentofte, Denmark), a blood sample for determination of plasma TNF-α and IL-6 was drawn from a retrobulbary venous plexus using heparinised micropipettes. After an overnight 12-h fast the animals were anaesthetised with a subcutaneous injection of fentanyl/fluanisone (Hypnorm®, Jansen Pharma, Birkerød, Denmark) 0.5 mL/kg and midazolam (Dormicum®, La Roche, Basel, Schwitzerland) 2.5 mg/kg. All blood was collected for blood analyses and approximately 200 mg of liver tissue was snap-frozen in liquid N2, and stored at -80 °C. Finally, the spleen was weighed. In all animals we measured liver mRNA levels and serum concentrations of α-2-M and plasma concentrations of alanine aminotransferase and bilirubin at termination of the study.
Liver tissue
mRNA levels of α-2-M were determined by slot blot hybridization as previously described[16].
Blood analyses
The concentrations of α-2-M in serum were evaluated by rat ELISA (Immunology Consultants Laboratory, Newberg, OR, United States). The plasma concentrations of TNF-α and IL-6 were determined by immunoassay (R and D Systems, Minneapolis, MN, United States, both). Samples were analysed in duplicate and all assays had intra- and inter-assay coefficients of variance below 5% and 10%, respectively. Plasma concentrations of alanine aminotransferase and bilirubin were determined by standard clinical biochemical analytical methods.
Statistical analysis
Data were analysed using the Kruskal-Wallis One Way Analysis of Variance on Ranks; when significant, post-hoc tests were performed among groups by the Mann-Whitney rank sum test. Data are presented as the mean ± SEM. Differences were considered significant with P-values < 0.05. A statistical review of the study was performed by a biomedical statistician.
RESULTS
Body and spleen weight
LPS induced a body weight loss in all the intervention groups (P < 0.05) (Table 1) and there was no difference among these groups. The high dose dexamethasone dose decreased the spleen weight (P < 0.05), an effect not seen in any other group (Table 1).
Table 1.
Weights, liver function tests, and cytokines.
| Controls | LPS | Anti-CD163-dexa plus LPS | High dexa plus LPS | Low dexa plus LPS | |
| Body weight | 199 ± 1 | 196 ± 2 | 207 ± 2g | 204 ± 3 | 206 ± 3g |
| Weight loss | 11 ± 1 | 14 ± 3 | 22 ± 2a | 23 ± 2a | 21 ± 1a |
| Spleen weight | 465 ± 12 | 512 ± 31 | 492 ± 23 | 421 ± 11a | 483 ± 23 |
| ALT | 42 ± 3 | 61 ± 16 | 57 ± 20 | 48 ± 9 | 77 ± 31 |
| Bilirubin | 3.0 ± 0.0 | 3.3 ± 0.3 | 3.1 ± 0.1 | 3.6 ± 0.4 | 4.0 ± 0.4 |
| TNF-α | 0 ± 0 | 26817 ± 9780a | 7208 ± 1977a,c | 16891 ± 4210a | 21583 ± 7117a |
| IL-6 | 0 ± 0 | 23075 ± 6758a | 15685 ± 3779a,c,e | 32964 ± 8294a | 25715 ± 4036a |
Body weight (g), body weight loss (g), spleen weight (mg), plasma alanine aminotransferase (U/L), and bilirubin (μmol/L) in controls (n = 8) and in animals injected with LPS 24 h after vehicle (n = 8), anti-CD163mAb-dexa (n = 8), high dose (n = 8) and low dose (n = 8) dexamethasone at termination of study. Plasma TNF-α (pg/mL) and IL-6 (pg/mL) are measured 2 h after saline (controls) or LPS injection.
P < 0.05 vs controls;
P < 0.05 vs low dose free dexamethasone group;
P < 0.05 vs high dose free dexamethasone group;
P < 0.05 vs vehicle. ALT: Alanine aminotransferase; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6; LPS: Lipopolysaccharide.
Acute phase protein liver mRNA and serum levels
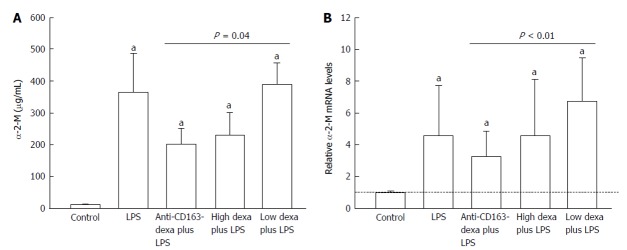
LPS increased the liver mRNA and serum levels of α-2-M several fold in all groups (P < 0.01) (Figure 1). Anti-CD163mAb-dexa approximately halved the α-2-M liver mRNA (P < 0.01) and serum response (P = 0.04) compared to low dose dexamethasone treated animals, while no free dexamethasone dose had any effect on liver mRNA or serum levels of α-2-M compared to vehicle (Figure 1).
Figure 1.
Relative levels of serum levels (A) and liver mRNA (B) of α-2-macroglobulin. Changes in serum levels (μg/mL) (A) and liver mRNA (% of controls) (B) of α-2-macroglobulin (α-2-M) in controls (n = 8) and in animals injected with LPS 24 h after vehicle (n = 8), anti-CD163mAb-dexa (n = 8), high dose (n = 8) and low dose (n = 8) dexamethasone. mRNA results from LPS-injected animals are presented as relative levels compared to control animals. Bars represent the mean and SEM. aP < 0.05 vs controls. LPS: Lipopolysaccharide; SEM: Standard error of mean.
TNF-α and IL-6
LPS markedly increased plasma TNF-α and IL-6 in all groups (P < 0.001). There was a trend for reduced TNF-α (P = 0.08) after anti-CD163mAb-dexa compared to vehicle and significantly so vs the low dose dexamethasone (P = 0.03). Also, the anti-CD163mAb-dexa decreased IL-6 compared to both dexamethasone doses (P < 0.05). None of the free dexamethasone doses had an effect on TNF-α or IL-6 (Table 1).
Plasma-alanine transferase and bilirubin
LPS had no effect on these measures at termination of the study (Table 1).
DISCUSSION
The central finding of this study was the reduction in liver mRNA and plasma α-2-M, and plasma TNF-α and IL-6 by the administration of the anti-CD163-dexa conjugate prior to the LPS-induced inflammatory response. This anti-acute phase effect much exceeded that of the therapeutic dexamethasone dose and did not cause systemic adverse effects, as evidenced by reduced spleen weight in the group treated with high dose free dexamethasone. This study completes the chain of evidence that the conjugate not only suppresses the LPS elicited IL signaling but also the ultimate effect on synthesis and release of hepatic acute phase proteins that effectuate the acute phase response.
The increase in plasma α-2-M after LPS reflects de novo synthesis as almost no such protein is present under non-induced conditions[17] in contrast to conditions with ongoing low grade inflammation such as cirrhosis[18]. LPS as assumed caused a marked systemic acute phase response reflected in increased liver mRNA and plasma α-2-M, TNF-α, and IL-6. In contrast to the equal amount of free dexamethasone, the anti-CD163mAb-dexa efficiently suppressed this response. Still, however, the acute phase response to some extent serves to restore homeostasis and one needs to be aware that suppression of the response might not be entirely beneficial entailing a potential risk using the conjugate long term.
The anti-inflammatory effects of glucocorticoids are related to a decrease in lymphocyte expansion and cell survival and also a reduction in the expression of pro-inflammatory cytokines originating from macrophages[19]. However, as glucocorticoids bind to the ubiquitous intracellular glucocorticoid steroid receptor present in most cell types they also exert serious systemic metabolic side effects. Thus dexamethasone causes the spleen to undergo a corticosteroid-induced weight reduction due to lymphocyte depletion[20]. Accordingly, the high dose dexamethasone in our study decreased the spleen weight as compared with the other groups reflecting systemic non-macrophages effects. In contrast, the conjugate did not affect spleen weight and was still found to exert a potent anti-inflammatory effect.
In our animal model, the conjugate was given as a pre-emptive dose prior to the induction of the acute phase response as we aimed at establishing a proof-of-concept position of the conjugate’s effects. We believe our findings support further studies on interference with on-going inflammation in relevant experimental models. Such studies are also essential for monitoring of long term effects of the conjugate.
In conclusion, the anti-CD163-dexa conjugate demonstrated potent effects in reducing the acute phase proteins without evident systemic side effects during an endotoxin-induced acute phase response in rats. The effect much exceeded that of a therapeutic dose of dexamethasone. Thus, the antibody conjugate may be a potential candidate in future anti-inflammatory macrophage-directed therapy, e.g., in liver diseases with Kupffer cells activation[7].
ACKNOWLEDGMENTS
We are indebted to Rikke Andersen, Birgitte Nielsen, and Kirsten Priisholm for their skilled technical assistance.
COMMENTS
Background
In conditions with macrophage proliferation and activation, CD163, a scavenger receptor expressed exclusively on monocytes and macrophages, is up-regulated. As an example, hepatic macrophages (Kupffer cells) are activated and CD163 is increased in patients with liver cirrhosis who chronically experience some degree of endotoxemia and acute phase response.
Research frontiers
The authors have recently constructed a conjugate of CD163 antibody and the potent corticosteroid dexamethasone (anti-CD163mAb-dexa) specifically targeting dexamethasone to activated macrophages.
Innovations and breakthroughs
The anti-CD163-dexa conjugate exerts an anti-inflammatory effect, which is obtained with very low concentration of dexamethasone, thereby minimizing steroid-induced systemic effects.
Applications
The antibody conjugate may be a potential candidate in future anti-inflammatory macrophage-directed therapy, e.g., in liver diseases with Kupffer cells activation.
Peer-review
This is an experimental report written by Thomsen et al, which indicates an efficacy of dexamethasone-conjugated anti-CD163 against lipopolysaccharide-induced acute inflammatory reaction. The well-designed study was carried out using firm methods.
Footnotes
Supported by The NOVO Nordisk foundation; the Aarhus University Research Foundation; and Clinical Institute, Aarhus University, Denmark.
Institutional animal care and use committee statement: The study was performed in accordance with local and national guidelines for animal welfare and reviewed and approved by the national Animal Ethics Committee, protocol No. 2010/561-1918.
Conflict-of-interest statement: Møller HJ, Graversen JH and Moestrup SK are inventors for the CD163-dexamethasone conjugate and minority shareholders in Affinicon Aps. All other authors have nothing to disclose.
Data sharing statement: Dataset is available from the corresponding author at karethom@rm.dk.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 22, 2016
First decision: March 24, 2016
Article in press: June 2, 2016
P- Reviewer: Ikura Y, Liu ZH, Pan JJ, Tsoulfas G, Zhu X S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 2.Moestrup SK, Møller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36:347–354. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 3.Møller HJ, de Fost M, Aerts H, Hollak C, Moestrup SK. Plasma level of the macrophage-derived soluble CD163 is increased and positively correlates with severity in Gaucher’s disease. Eur J Haematol. 2004;72:135–139. doi: 10.1046/j.0902-4441.2003.00193.x. [DOI] [PubMed] [Google Scholar]
- 4.Schaer DJ, Schleiffenbaum B, Kurrer M, Imhof A, Bächli E, Fehr J, Moller HJ, Moestrup SK, Schaffner A. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol. 2005;74:6–10. doi: 10.1111/j.1600-0609.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 5.Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, Givan AL, Wallace PK, Yeager MP, Guyre PM. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002;72:711–717. [PubMed] [Google Scholar]
- 6.Grønbaek H, Sandahl TD, Mortensen C, Vilstrup H, Møller HJ, Møller S. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment Pharmacol Ther. 2012;36:173–180. doi: 10.1111/j.1365-2036.2012.05134.x. [DOI] [PubMed] [Google Scholar]
- 7.Sandahl TD, Grønbaek H, Møller HJ, Støy S, Thomsen KL, Dige AK, Agnholt J, Hamilton-Dutoit S, Thiel S, Vilstrup H. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: a prospective cohort study. Am J Gastroenterol. 2014;109:1749–1756. doi: 10.1038/ajg.2014.262. [DOI] [PubMed] [Google Scholar]
- 8.Mookerjee RP, Sen S, Davies NA, Hodges SJ, Williams R, Jalan R. Tumour necrosis factor alpha is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut. 2003;52:1182–1187. doi: 10.1136/gut.52.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graversen JH, Svendsen P, Dagnæs-Hansen F, Dal J, Anton G, Etzerodt A, Petersen MD, Christensen PA, Møller HJ, Moestrup SK. Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Mol Ther. 2012;20:1550–1558. doi: 10.1038/mt.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granfeldt A, Hvas CL, Graversen JH, Christensen PA, Petersen MD, Anton G, Svendsen P, Sølling C, Etzerodt A, Tønnesen E, et al. Targeting dexamethasone to macrophages in a porcine endotoxemic model. Crit Care Med. 2013;41:e309–e318. doi: 10.1097/CCM.0b013e31828a45ef. [DOI] [PubMed] [Google Scholar]
- 11.Milland J, Tsykin A, Thomas T, Aldred AR, Cole T, Schreiber G. Gene expression in regenerating and acute-phase rat liver. Am J Physiol. 1990;259:G340–G347. doi: 10.1152/ajpgi.1990.259.3.G340. [DOI] [PubMed] [Google Scholar]
- 12.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 13.Rehman AA, Ahsan H, Khan FH. α-2-Macroglobulin: a physiological guardian. J Cell Physiol. 2013;228:1665–1675. doi: 10.1002/jcp.24266. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Whiteman M, Moore PK. Dexamethasone inhibits lipopolysaccharide-induced hydrogen sulphide biosynthesis in intact cells and in an animal model of endotoxic shock. J Cell Mol Med. 2009;13:2684–2692. doi: 10.1111/j.1582-4934.2008.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori Y, Murakami Y, Atsuta H, Minamino N, Kangawa K, Kasai K. Glucocorticoid regulation of adrenomedullin in a rat model of endotoxic shock. Life Sci. 1998;62:PL181–PL189. doi: 10.1016/s0024-3205(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen SS, Grøfte T, Tygstrup N, Vilstrup H. Synthesis of acute phase proteins in rats with cirrhosis exposed to lipopolysaccharide. Comp Hepatol. 2006;5:3. doi: 10.1186/1476-5926-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiger T, Andus T, Klapproth J, Hirano T, Kishimoto T, Heinrich PC. Induction of rat acute-phase proteins by interleukin 6 in vivo. Eur J Immunol. 1988;18:717–721. doi: 10.1002/eji.1830180510. [DOI] [PubMed] [Google Scholar]
- 18.Naveau S, Poynard T, Benattar C, Bedossa P, Chaput JC. Alpha-2-macroglobulin and hepatic fibrosis. Diagnostic interest. Dig Dis Sci. 1994;39:2426–2432. doi: 10.1007/BF02087661. [DOI] [PubMed] [Google Scholar]
- 19.McColl A, Michlewska S, Dransfield I, Rossi AG. Effects of glucocorticoids on apoptosis and clearance of apoptotic cells. ScientificWorldJournal. 2007;7:1165–1181. doi: 10.1100/tsw.2007.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rungruang T, Chaweeborisuit P, Klosek SK. Effect of malaria infection and dexamethasone on spleen morphology and histology. Southeast Asian J Trop Med Public Health. 2010;41:1290–1296. [PubMed] [Google Scholar]