Abstract
AIM: To investigate the prognostic significance of insulin-like growth factor-binding protein 3 (IGFBP-3) in patients with cirrhosis.
METHODS: Prospective study that included two cohorts: outpatients with stable cirrhosis (n = 138) and patients hospitalized for acute decompensation (n = 189). Development of complications, mortality or liver transplantation was assessed by periodical phone calls and during outpatient visits. The cohort of stable cirrhosis also underwent clinical and laboratory evaluation yearly (2013 and 2014) in predefined study visits. In patients with stable cirrhosis, IGFBP-3 levels were measured at baseline (2012) and at second re-evaluation (2014). In hospitalized subjects, IGFBP-3 levels were measured in serum samples collected in the first and in the third day after admission and stored at -80 °C. IGFBP-3 levels were measured by immunochemiluminescence.
RESULTS: IGFBP-3 levels were lower in hospitalized patients as compared to outpatients (0.94 mcg/mL vs 1.69 mcg/mL, P < 0.001) and increased after liver transplantation (3.81 mcg/mL vs 1.33 mcg/mL, P = 0.008). During the follow-up of the stable cohort, 17 patients died and 11 received liver transplantation. Bivariate analysis showed that death or transplant was associated with lower IGFBP-3 levels (1.44 mcg/mL vs 1.74 mcg/mL, P = 0.027). The Kaplan-Meier transplant-free survival probability was 88.6% in patients with IGFBP-3 ≥ 1.67 mcg/mL and 72.1% for those with IGFBP3 < 1.67 mcg/mL (P = 0.015). In the hospitalized cohort, 30-d mortality was 24.3% and was independently associated with creatinine, INR, SpO2/FiO2 ratio and IGFBP-3 levels in the logistic regression. The 90-d transplant-free survival probability was 80.4% in patients with IGFBP-3 ≥ 0.86 mcg/mL and 56.1% for those with IGFBP3 < 0.86 mcg/mL (P < 0.001).
CONCLUSION: Lower IGFBP-3 levels were associated with worse outcomes in patients with cirrhosis, and might represent a promising prognostic tool that can be incorporated in clinical practice.
Keywords: Liver cirrhosis, Acute decompensation, Insulin-like growth factor binding protein 3, Acute-on-chronic liver failure, Prognosis
Core tip: Insulin-like growth factor-binding protein 3 (IGFBP-3) levels are decreased in cirrhosis and seem to correlate with the intensity of hepatic dysfunction, but its prognostic significance is uncertain. In this prospective cohort study, IGFBP-3 levels correlated with variables associated with the intensity of liver dysfunction in both outpatients with stable cirrhosis and in subjects hospitalized for acute decompensation. IGFBP-3 levels increased significantly after discharge and after liver transplantation. Lower IGFBP-3 levels were associated with poor outcomes in both outpatients with stable cirrhosis and in those hospitalized for acute decompensation, suggesting that it can be used in clinical practice as a prognostic biomarker in cirrhosis.
INTRODUCTION
Cirrhosis is a late stage progressive hepatic fibrosis characterized by distortion of hepatic architecture and the formation of regenerative nodules. Although considered potentially reversible in some clinical scenarios, in its advanced stages, liver cirrhosis is associated with high mortality and the only treatment option may be liver transplantation[1]. Patients with cirrhosis are susceptible to a variety of complications such as variceal bleeding, ascites, spontaneous bacterial peritonitis (SBP), hepatic encephalopathy, hepatocellular carcinoma, hepatorenal syndrome, hepatopulmonary syndrome and portal vein thrombosis[1]. The prognosis of cirrhosis is highly variable because it is influenced by a number of factors including etiology, severity, presence of complications, and comorbidities. Mortality rate increases significantly once decompensation occurs[2].
Changes in the growth hormone-insulin-like growth factor (GH-IGF) axis occur as a result of liver disease and has been reported in cirrhosis[3,4]. GH and IGF-I have important anabolic effects on the metabolism of proteins, carbohydrates and lipids[5]. GH is a peptide hormone released from anterior pituitary that stimulates growth, cell reproduction and regenerations while exerting metabolic effects on bone, cartilage, fat, muscles, heart and the immune system[6-8].
In the liver, GH activation of GH receptors induces IGF-I gene transcription, and the subsequent synthesis and release of IGF-I to plasma[9]. However, the actions of IGF-I are tightly controlled by the binding of IGF to binding proteins (IGFBP-1 to -6). The IGFBPs carry IGFs in the serum and regulate their activity and bioavailability[10]. IGFBP-3 is the major binding protein of IGF and carries 80%-90% of circulating IGF-I. The IGFBP-3 is predominantly produced in the liver and synthesized in Kupffer cells. It forms a 150 kDa ternary complex with IGFs and the acid-labile subunit[7,9].
Low serum concentrations in both IGF-I and IGFBP-3 have been reported in patients with cirrhosis vs healthy controls. This likely reflects decreased hepatic synthesis function[5,11-13]. In fact, some studies reported that IGFBP-3 serum levels are abnormally low in patients with liver cirrhosis and correlated with several variables associated with the intensity of liver dysfunction[5,7]. Even though these reports suggest the clinical utility of monitoring circulating IGFBP-3 in cirrhosis, there is little data on the prognostic significance of this biomarker. The aim of this study was to investigate the relationship between serum IGFBP-3 levels and prognosis in both outpatients with stable cirrhosis and in patients hospitalized for acute decompensation (AD).
MATERIALS AND METHODS
Patients
This prospective study included two cohorts of adult patients (≥ 18 years of age) with liver cirrhosis in the University Hospital of the Federal University of Santa Catarina. The diagnosis of cirrhosis was established either histologically (when available) or by combination of clinical, imaging and laboratory findings in patients with evidence of portal hypertension. The first cohort was comprised of patients with stable cirrhosis in the outpatient clinic. In this case, patients in the following situations were excluded: Diagnosis of hepatocellular carcinoma; interferon-based therapy over the last 30 d; or refusal or inability of the patient to understand the terms of the informed consent.
The second cohort included patients admitted to the emergency room due to AD of liver cirrhosis. In this group, the following exclusion criteria were adopted: Hospitalization for elective procedures; admissions not related to complications of liver cirrhosis; and hepatocellular carcinoma outside Milan criteria. Exclusion criteria for both groups also included: Pregnancy; chronic renal failure requiring hemodialysis; severe heart disease; severe chronic pulmonary disease; and active extrahepatic cancer. The study protocol complies with ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee on Human Research of the Federal University of Santa Catarina.
Methods
This initial cohort was initially evaluated from June to October 2012. The development of complications, mortality, or liver transplantation was assessed by periodic phone calls and during outpatient visits. Patients also underwent clinical and laboratory evaluation yearly (2013 and 2014) in predefined study visits. The second cohort included subjects hospitalized for AD between January 2011 and November 2013. AD was defined by acute development of hepatic encephalopathy, large ascites, gastrointestinal bleeding, bacterial infection or any combination of these. Patients were evaluated within 24 h of admission by one of the researchers involved in the study. They were followed during their hospital stay and 30- and 90-d mortality was evaluated by phone call in case of hospital discharge. In case of more than one hospital admission during the study period-only the most recent hospitalization was considered.
The following clinical variables were collected for all patients: Age, gender, etiology of cirrhosis, history of previous decompensation, current complications of cirrhosis, active alcoholism and regular propranolol and omeprazole use. All subjects underwent laboratory evaluation including total leukocytes, serum sodium, creatinine, international normalized ratio (INR), albumin, C-reactive protein (CRP), total bilirubin and IGFBP-3. In patients hospitalized for AD of cirrhosis, blood samples were obtained on the first and third day after admission.
Active alcoholism was defined as an average overall consumption of 21 or more drinks per week for men and 14 or more drinks per week for women during the 4 wk before enrollment (one standard drink is equal to 12 g absolute alcohol)[14].
Hospitalized individuals with a suspected infection at admission received a clinical examination to confirm this diagnosis and to establish the primary source of infection. Diagnosis of infection was made according to the criteria of Center for Disease Control[15]. A diagnostic paracentesis was performed in all patients with ascites at admission. SBP was diagnosed when the neutrophil count in the ascitic fluid was ≥ 250 neutrophils/mm3 in the absence of an intra-abdominal source of infection regardless of negative culture[16]. All patients with SBP received ceftriaxone plus weight-based intravenous albumin in the first and third day after diagnosis. Hepatic encephalopathy was graded according to West-Haven criteria[17]. If this was present, a precipitant event was actively investigated and lactulose was initiated, and the dose was adjusted as needed. All subjects with acute variceal bleeding received intravenous octreotide, an antibiotic (either oral quinolone or intravenous ceftriaxone), and underwent urgent therapeutic endoscopy after stabilization.
The severity of liver disease was estimated using the Child-Pugh classification system[18] and model for end-stage liver disease (MELD)[19] calculated based on laboratory tests performed at admission in the case of hospitalized patients. Acute-on-chronic liver failure (ACLF) was defined as proposed by the EASL-CLIF Consortium[20].
IGFBP-3 serum levels
In patients with stable cirrhosis, IGFBP-3 levels were measured at baseline (2012) and at second re-evaluation (2014). In hospitalized subjects, IGFBP-3 levels were measured in serum samples collected in the first and in the third day after admission and stored at -80 °C until use. The IGFBP-3 levels were measured by immunochemiluminescence (Immulite® 2000, Diagnostic Products Corp., Los Angeles, CA, united States). The reported analytical sensitivity of this assay is 0.50 mcg/mL.
Statistical analysis
The normality of variable distribution was determined using the Kolmogorov-Smirnov test. The correlation between numerical variables was evaluated using Spearman’s correlation coefficient. Continuous variables were compared using the Student’s t-test in case of a normal distribution or Mann-Whitney test in the remaining cases. Categorical variables were evaluated with a χ2 test or Fisher's exact test, as appropriate. Multiple logistic regression analysis (forward stepwise regression) was used to investigate factors independently associated with death or liver transplantation during follow-up period. The best cutoffs of IGFBP-3 for predicting mortality, in both cohorts, were chosen based on the receiver operating characteristics (ROC) curves. Survival curves were calculated using the Kaplan-Meier method and survival differences between groups were compared using a log-rank test. Wilcoxon signed rank-test was used for comparing IGFBP-3 at two times. All tests were performed using SPSS software, version 22.0 (SPSS, Chicago, IL, united States). A P value of less than 0.05 was considered statistically significant.
RESULTS
Characteristics of included patients
The study included 138 patients with stable cirrhosis and 189 subjects hospitalized for AD of cirrhosis. Table 1 lists the characteristics of the included patients. In the cohort of stable cirrhosis, the mean age was 53.6 ± 12.5 years, 92.8% were Caucasians with a predominance of men (70.3%). A previous history of cirrhosis decompensation was observed in 75.4% of the sample and only 3.6% of subjects reported active alcoholism during the past month. Regular propranolol and PPI use was 63.0% and 50.0% of the patients, respectively. The mean MELD score was 9.84 ± 2.28 and 66.7% of subjects were Child-Pugh A.
Table 1.
Demographic, clinical and biochemical features of included patients n (%)
| Stable cirrhosis (n = 138) | Acute decompensation (n = 189) | |
| Age; yr, mean ± SD | 53.62 ± 12.52 | 53.58 ± 11.56 |
| Caucasians | 128 (92.8) | 129 (68.6) |
| Male gender | 97 (70.3) | 138 (73.0) |
| Etiology of cirrhosis | ||
| Alcohol | 42 (30.4) | 68 (36.0) |
| Hepatitis C | 50 (36.2) | 78 (41.3) |
| Hepatitis B | 6 (4.3) | 8 (4.2) |
| Cryptogenic | 14 (10.1) | 15 (7.9) |
| Other | 26 (18.8) | 20 (10.6) |
| Previous decompensation | 104 (75.4) | 120 (63.5) |
| Active alcoholism | 5 (3.6) | 68 (36.0) |
| Propranolol | 87(63.0) | 74 (40.2) |
| PPI | 69 (50.0) | 43 (23.4) |
| Complication at evaluation | ||
| Ascites | 28 (20.3) | 92 (48.7) |
| Hepatic encephalopathy | 14 (10.1) | 112 (59.3) |
| Gastrointestinal bleeding | 0 | 99 (52.4) |
| Bacterial infection | 0 | 50 (26.6) |
| ACLF | 0 | 45 (23.8) |
| Laboratory data | ||
| Leucocyte count (× 109), median | 4.90 | 7.20 |
| Sodium (meq/L), median | 138 | 135 |
| Creatinine (mg/dL), median | 0.90 | 1.1 |
| INR, median | 1.20 | 1.41 |
| Albumin (g/dL), mean ± SD | 3.44 ± 0.46 | 2.35 ± 0.69 |
| CRP (mg/L), median | 3.5 | 10.05 |
| Total bilirubin (mg/dL), median | 1.00 | 2.10 |
| IGFBP-3 (mcg/mL), median | 1.69 | 0.94 |
| Child-Pugh classification | ||
| A | 92 (66.7) | 23 (12.2) |
| B | 43 (31.2) | 91 (48.1) |
| C | 3 (2.2) | 75 (39.7) |
| MELD score, mean ± SD | 9.84 ± 2.28 | 16.32 ± 6.53 |
PPI: Proton-pump inhibitors; ACFL: Acute-on-chronic liver failure; INR: International normalized ratio; CRP: C-reactive protein; IGFBP-3: Insulin-like growth factor-binding protein 3; MELD: Model for end-stage liver disease.
In the group hospitalized for AD, the mean age was 53.6 ± 11.6 years, 68.6% were Caucasians and 73.0% were males. Previous decompensation was reported by 63.5% of the sample, and active alcoholism was present in 36.0% of the sample. Upon admission, upper gastrointestinal bleeding was observed in 52.4% of cases, ascites in 48.7%, hepatic encephalopathy in 59.3%, bacterial infections in 26.6% and ACLF in 23.8%. In hospitalized patients, propranolol and PPI use prior to admission was reported in 40.2% and 23.4% of the patients, respectively. The mean MELD score was 16.32 ± 6.53 and 39.7% of subjects were Child-Pugh C. Patients hospitalized for AD of cirrhosis exhibited significantly lower median IGFBP-3 vs outpatients (0.94 mcg/mL vs 1.69 mcg/mL, P < 0.001).
IGFBP-3 in outpatients with stable cirrhosis
In patients with stable cirrhosis, IGFBP-3 levels were positively correlated with total leukocytes (r = 0.215, P = 0.011) and albumin levels (r = 0.579, P < 0.001). A negative correlation was observed between IGFBP-3 levels and INR (r = -0.412, P < 0.001), total bilirubin (r = -0.329, P < 0.001), CRP (r = -0.265, P = 0.002) and MELD (r = -0.327, P < 0.001). No significant correlations were observed between IGFBP-3 and other studied variables.
Significantly lower IGFBP-3 median levels were observed in Child-Pugh B/C patients (1.38 mcg/mL vs 1.88 mcg/mL, P < 0.001), in those with previous decompensation of cirrhosis (1.58 mcg/mL vs 2.19 mcg/mL, P = 0.012), hospitalization secondary to complications of cirrhosis (1.57 mcg/mL vs 1.95 mcg/mL, P = 0.052), and those with a history of ascites (1.53 mcg/mL vs 1.85 mcg/mL, P = 0.024). Previous variceal bleeding or hepatic encephalopathy as well as an etiology of cirrhosis had no impact on IGFBP-3 levels (P > 0.05).
The median follow-up of patients with stable cirrhosis was 20 mo. During the study period, 17 patients died and 11 received liver transplantation. Bivariate analysis showed that progression to death or liver transplantation was associated with previous ascites (70.4% vs 42.3%, P = 0.009), history of hospitalization for complications of cirrhosis (88.9% vs 65.8%, P = 0.018), and Child-Pugh B/C (70.4% vs 24.3%, P < 0.001) (Table 2). In addition, those with unfavorable outcome showed a higher median total bilirubin (1.80 mg/dL vs 0.90 mg/dL, P < 0.001), INR (1.27 vs 1.16, P < 0.001), CRP (4.51 mg/L vs 3.50 mg/L, P = 0.014), MELD score (11.98 ± 2.24 vs 9.32 ± 1.97, P < 0.001) and lower sodium (136.00 mEq/L vs 138.00 mEq/L, P = 0.022), albumin (3.11 ± 0.39 g/dL vs 3.52 ± 0.44 g/dL, P < 0.001) and IGFBP-3 values (1.44 mcg/mL vs 1.74 mcg/mL, P = 0.027). A stepwise forward logistic regression analysis was performed including the following variables with P < 0.05 in bivariate analysis: Previous ascites, history of hospitalization for complications of cirrhosis, albumin, INR, total bilirubin, CRP, sodium, and IGFBP-3. Multivariate analysis showed that only albumin (OR = 0.183, 95%CI: 0.053-0.631, P = 0.007) and total bilirubin (OR = 2.482, 95%CI: 1.358-4.538, P = 0.003) were independently associated with death or liver transplantation during follow-up. However, at the end of follow-up, Kaplan-Meier survival probability was 88.6% for patients with IGFBP-3 ≥ 1.67 mcg/mL and 72.1% for those with IGFBP-3 < 1.67 mcg/mL. The survival was significantly shorter for those with lower IGFBP-3 values (20.24 mo, 95%CI: 18.32-22.17) as compared to the remaining subjects (23.06 mo, 95%CI: 21.72-24.40) (P = 0.015) (Figure 1).
Table 2.
Factors associated with mortality or liver transplantations among patients with stable cirrhosis n (%)
| Survivors (n = 111) | Death/liver transplantation (n = 27) | P value | |
| Age (yr), mean ± SD | 54.33 ± 12.11 | 50.67 ± 13.91 | 0.173 |
| Male gender | 79 (71.2) | 18 (66.7) | 0.646 |
| Etiology of cirrhosis | |||
| Alcohol | 34 (30.6) | 8 (29.6) | 0.919 |
| Hepatitis C | 42 (37.8) | 8 (29.6) | 0.426 |
| Hepatitis B | 4 (3.6) | 2 (7.4) | 0.334 |
| Cryptogenic | 10 (9.0) | 4 (14.8) | 0.475 |
| Other | 21 (18.9) | 5 (18.5) | 0.962 |
| Previous hospitalization | 73 (65.8) | 24 (88.9) | 0.018 |
| Active alcoholism | 3 (2.7) | 2 (7.4) | 0.252 |
| Propranolol | 67 (60.4) | 20 (74.1) | 0.185 |
| PPI | 54 (48.6) | 15 (55.6) | 0.520 |
| Complication at evaluation | |||
| Ascites | 19 (17.1) | 9 (33.3) | 0.060 |
| Hepatic encephalopathy | 8 (7.2) | 6 (22.2) | 0.032 |
| Previous ascites | 47 (42.3) | 19 (70.4) | 0.009 |
| Laboratory data | |||
| Leucocyte count (× 109), median | 4.93 | 4.83 | 0.548 |
| Sodium (meq/L), median | 138.00 | 136.00 | 0.022 |
| Creatinine (mg/dL), median | 0.9 | 0.8 | 0.480 |
| INR, median | 1.16 | 1.27 | < 0.001 |
| Albumin (g/dL), mean ± SD | 3.52 ± 0.44 | 3.11 ± 0.39 | < 0.001 |
| CRP (mg/L), median | 3.5 | 4.51 | 0.014 |
| Total bilirubin (mg/dL), median | 0.9 | 1.8 | < 0.001 |
| IGFBP-3 (mcg/mL), median | 1.74 | 1.44 | 0.027 |
| Child-Pugh B/C | 27 (24.3) | 19 (70.4) | < 0.001 |
| MELD score, mean ± SD | 9.32 ± 1.97 | 11.98 ± 2.24 | < 0.001 |
PPI: Proton-pump inhibitors; INR: International normalized ratio; CRP: C-reactive protein; IGFBP-3: Insulin-like growth factor-binding protein 3; MELD: Model for end-stage liver disease.
Figure 1.
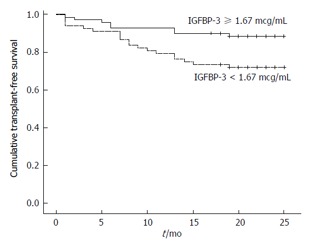
Kaplan-Meier transplant-free survival of 138 outpatients with cirrhosis stratified according to insulin-like growth factor-binding protein 3 cut-off level of 1.67 mcg/mL. Survival probability after a median follow-up of 20 mo was 88.6% for patients with IGFBP-3 ≥ 1.67 mcg/mL and 72.1% for those with IGFBP-3 < 1.67 mcg/mL (P = 0.015). IGFBP-3: Insulin-like growth factor-binding protein 3.
Twenty-seven patients developed variceal bleeding during follow-up. Those patients exhibited similar baseline IGFBP-3 levels when compared to those who did not develop this complication (1.74 mcg/mL vs 1.69 mcg/mL, P = 0.478).
One-hundred and nine patients underwent laboratory evaluation in 2014, and 12 subjects refused blood collection. Of those who did not receive liver transplantation, IGFBP-3 significantly decreased at the second assessment (1.67 mcg/mL vs 1.74 mcg/mL, P = 0.013). However, in patients who underwent liver transplantation, a significant increase in IGFBP-3 was observed (3.81 mcg/mL vs 1.33 mcg/mL, P = 0.008). Actually, IGFBP-3 increased in all nine patients, and its levels were restored to normal after transplantation in five subjects.
Prognostic significance of IGFBP-3 in patients hospitalized for AD of cirrhosis
In hospitalized patients, IGFBP-3 levels were positively correlated with sodium (r = 0.173, P = 0.018) and albumin levels (r = 0.422, P < 0.001). A negative correlation was observed between IGFBP-3 levels and INR (r = -0.437, P < 0.001), total bilirubin (r = -0.278, P < 0.001), CRP (r = -0.365, P < 0.001), MELD (r = -0.373, P < 0.001), and CLIF-SOFA (r = -0.410, P < 0.001). Significantly lower levels of IGFBP-3 were observed in Child-Pugh C patients (0.73 mcg/mL vs 1.13 mcg/mL, P < 0.001), in those with ascites (0.76 mcg/mL vs 1.22 mcg/mL, P < 0.001), hepatic encephalopathy (0.85 mcg/mL vs 1.12 mcg/mL, P = 0.001), ACLF (0.78 mcg/mL vs 1.02 mcg/mL, P = 0.007) and bacterial infection at admission (0.66 mcg/mL vs 1.11 mcg/mL, P < 0.001). The etiology of cirrhosis had no impact on IGFBP-3 levels (P > 0.05).
Overall 30-d mortality was 24.3%, and it was associated with active alcoholism in bivariate analysis (Table 3) (50.0% vs 31.5%, P = 0.023) as well as ascites (76.1% vs 39.9%, P < 0.001), hepatic encephalopathy (84.8% vs 51.0%, P < 0.001), bacterial infection (48.9% vs 19.6%, P < 0.001), Child-Pugh C (73.9% vs 28.7%, P < 0.001), ACLF at admission (63.0% vs 11.2%, P < 0.001), lower median SpO2/FiO2 ratio (442.86 vs 461.90, P < 0.001), and higher MELD score (22.44 ± 6.95 vs 14.35 ± 5.00, P < 0.001). The 30-d mortality was also related to higher median leucocyte count (8.46 × 109/L vs 6.59 × 109/L, P = 0.005), creatinine (1.80 mg/dL vs 1.00 mg/dL, P < 0.001), INR (1.60 vs 1.38, P < 0.001), CRP (24.80 mg/L vs 8.40 mg/L, P = 0.002), total bilirubin (3.10 mg/dL vs 1.51 mg/dL, P < 0.001) as well as lower mean albumin (1.98 ± 0.51 g/dL vs 2.47 ± 0.70 g/dL, P < 0.001), lower median sodium (134.00 mEq/L vs 136.00 mEq/L, P = 0.011), and IGFBP-3 levels (0.63 mcg/mL vs 1.05 mcg/mL, P < 0.001).
Table 3.
Factors associated with 30-d mortality among patients hospitalized for acute decompensation of cirrhosis n (%)
| Survivors (n = 143) | Deaths (n = 46) | P value | |
| Age (yr), mean ± SD | 53.61 ± 11.54 | 53.49 ± 11.75 | 0.952 |
| Male gender | 101 (70.6) | 37 (80.4) | 0.192 |
| Etiology of cirrhosis | |||
| Alcohol | 47 (32.9) | 21 (49.7) | 0.116 |
| Hepatitis C | 61 (42.7) | 17 (37.0) | 0.495 |
| Hepatitis B | 6 (4.2) | 2 (4.3) | 1.000 |
| Cryptogenic | 14 (9.8) | 1 (2.2) | 0.123 |
| Other | 15 (10.5) | 5 (10.9) | 1.000 |
| Previous decompensation | 88 (61.5) | 32 (69.6) | 0.325 |
| Active alcoholism | 45 (31.5) | 23 (50.0) | 0.023 |
| Complication at evaluation | |||
| Ascites | 57 (39.9) | 35 (76.1) | < 0.001 |
| Hepatic encephalopathy | 73 (51.0) | 39 (84.8) | < 0.001 |
| Gastrointestinal bleeding | 81 (56.6) | 18 (39.1) | 0.039 |
| Bacterial infection | 28 (19.6) | 22 (48.9) | < 0.001 |
| ACLF | 16 (11.2) | 29 (63.0) | < 0.001 |
| Laboratory data | |||
| Leucocyte count (× 109), median | 6.59 | 8.46 | 0.005 |
| Sodium (meq/L), median | 136.00 | 134.00 | 0.011 |
| Creatinine (mg/dL), median | 1.00 | 1.80 | < 0.001 |
| INR, median | 1.38 | 1.60 | < 0.001 |
| Albumin (g/dL), mean ± SD | 2.47 ± 0.70 | 1.98 ± 0.51 | < 0.001 |
| CRP (mg/L), median | 8.40 | 24.8 | 0.002 |
| Total bilirubin (mg/dL), median | 1.51 | 3.10 | < 0.001 |
| IGFBP-3 (mcg/mL), median | 1.05 | 0.63 | < 0.001 |
| Child-Pugh C | 41 (28.7) | 34 (73.9) | < 0.001 |
| MELD score, mean ± SD | 14.35 ± 5.00 | 22.44 ± 6.95 | < 0.001 |
| Vital signs | |||
| MAP (mmHg), mean ± SD | 85.11 ± (13.95) | 80.33 ± (15.27) | 0.053 |
| Heart rate (BPM), mean ± SD | 81.27 ± 19.19 | 88.66 ± 16.40 | 0.022 |
| SpO2/FiO2 ratio, median | 461.9 | 442.86 | < 0.001 |
INR: International normalized ratio; CRP: C-reactive protein; IGFBP-3: Insulin-like growth factor-binding protein 3; MELD: Model for end-stage liver disease; MAP: Mean arterial pressure; SpO2/FiO2: Oxygen saturation to fraction of inspired oxygen ratio; ACLF: Acute-on-chronic liver failure.
A stepwise forward logistic regression analysis was performed including the following variables with P < 0.010 in the bivariate analysis: Ascites, hepatic encephalopathy, infection at admission, SpO2/FiO2 ratio, leukocyte count, creatinine, INR, albumin, CRP, total bilirubin, and IGFBP-3. In this regression analysis, the 30-day mortality was independently associated with creatinine (OR = 5.331, 95%CI: 2.563-11.090, P < 0.001), INR (OR = 5.830, 95%CI: 1.492-22.785, P = 0.011), SpO2/FiO2 ratio (OR = 0.985, 95%CI: 0.975-0.995, P = 0.004), and IGFBP-3 (OR = 0.332, 95%CI: 0.120-0.915, P = 0.033).
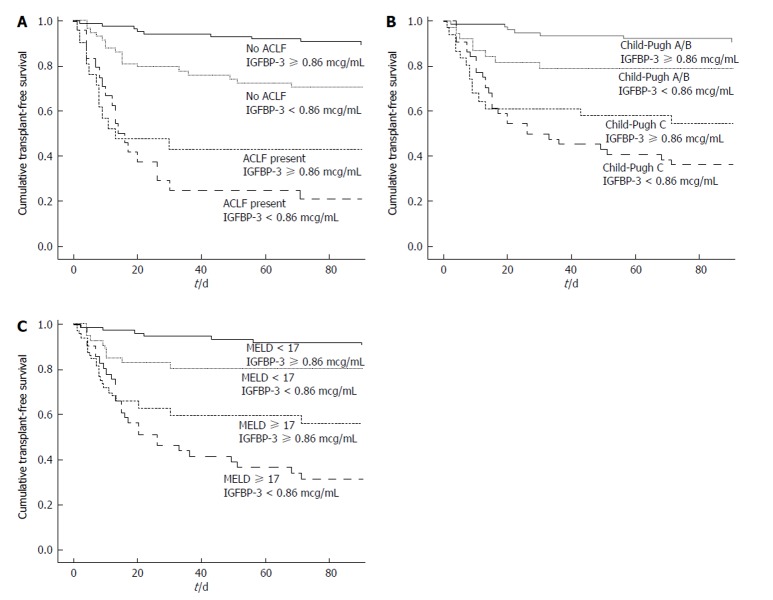
Fifty-four patients died and three subjects underwent liver transplantation during 90 d of follow-up. The Kaplan-Meier survival probability at 90-d was 80.4% in patients with IGFBP-3 ≥ 0.86 mcg/mL and 56.1% for subjects with IGFBP-3 < 0.86 mcg/mL (P < 0.001) (Figure 2A). For the prediction of 90-d mortality, the IGFBP-3 at a cutoff of 0.86 mcg/mL showed a sensitivity of 63% and a specificity of 65%. The negative predictive value was 80% with a positive predictive value of only 44%. Figure 2A exhibited Kaplan-Meier curves for mortality during the follow-up period according to the presence of ACLF and IGFBP-3 categories. The Kaplan-Meier survival probability at 90-d was 89.5% in patients without ACLF and IGFBP-3 ≥ 0.86 mcg/mL. It was 70.7% for those with IGFBP-3 < 0.86 mcg/mL only, 42.9% for those with ACLF only, and 20.8% for patients with both ACLF and IGFBP-3 < 0.86 mcg/mL (P < 0.001, long-rank test). Similarly, the 90-d survival was 90.8% in patients with Child-Pugh A/B and with IGFBP-3 ≥ 0.86 mcg/mL, 78.9% for those with IGFBP-3 < 0.86 mcg/mL only, 54.8% for those with Child-Pugh C only, and 36.4% for Child-Pugh C patients with IGFBP-3 < 0.86 mcg/mL (P < 0.001) (Figure 2B).
Figure 2.
Cumulative 90-d transplant-free survival of hospitalized patients with cirrhosis according to insulin-like growth factor-binding protein 3, acute-on-chronic liver failure, Child-Pugh and model for end-stage liver disease. The 90-d survival was 89.5% in patients without ACLF and with IGFBP-3 ≥ 0.86 mcg/mL, 70.7% for those with IGFBP-3 < 0.86 mcg/mL only, 42.9% for those with ACLF only and 20.8% for patients with both ACLF and IGFBP-3 < 0.86 mcg/mL (A: P < 0.001). Similarly, survival probability was 90.8% in patients Child-Pugh A/B and with IGFBP-3 ≥ 0.86 mcg/mL, 78.9% for those with IGFBP-3 < 0.86 mcg/mL only, 54.8% for those Child-Pugh C only and 36.4% for Child-Pugh C patients with IGFBP-3 < 0.86 mcg/mL (B: P < 0.001). The 90-d Kaplan-Meier survival probability was 90.7% in patients with MELD < 17 and IGFBP-3 ≥ 0.86 mcg/mL, 80.5% for those with MELD < 17 and IGFBP-3 < 0.86 mcg/mL, 56.3% for those with MELD ≥ 17 and IGFBP-3 ≥ 0.86 mcg/mL and 31.7% for patients with both MELD ≥ 17 and IGFBP-3 < 0.86 mcg/mL (C: P < 0.001). ACLF: Acute-on-chronic liver failure; IGFBP-3: Insulin-like growth factor-binding protein 3; MELD: Model for end-stage liver disease.
The MELD score was dichotomized as ≥ 17 and < 17 based on ROC curve. The 90-d Kaplan-Meier survival probability was 90.7% in patients with MELD < 17 and IGFBP-3 ≥ 0.86 mcg/mL, 80.5% for those with MELD < 17 and IGFBP-3 < 0.86 mcg/mL, 56.3% for those with MELD ≥ 17 and IGFBP-3 ≥ 0.86 mcg/mL, and 31.7% for patients with both MELD ≥ 17 and IGFBP-3 < 0.86 mcg/mL (P < 0.001) (Figure 2C).
The IGFBP-3 levels were available for 163 patients at the third day of hospitalization and showed a significant decline vs admission values (0.76 mcg/mL vs 0.94 mcg/mL, P < 0.001). However, neither the magnitude nor the occurrence of IGFBP-3 decline was related to the severity of cirrhosis or bad prognosis (data not shown). In addition, IGFBP-3 measured at the third day showed similar performance to admission levels when used at its best cutoff for prediction of 90-d mortality (0.68 mcg/mL). These metrics offered a sensitivity of 64%, specificity of 65%, negative predictive value of 83% and positive predictive value of 41%.
IGFBP-3 levels after hospital discharge
The 30 patients evaluated during hospitalization underwent laboratory analysis within a median 105 days after discharge. These were compared at two time points to investigate the impact of AD on IGFBP-3 levels. Vs inpatient assessment, significantly higher median IGFBP-3 levels were observed with outpatient evaluation (1.51 mcg/mL vs 1.07 mcg/mL, P < 0.001). Likewise, an increase in IGFBP-3 levels at outpatient evaluation were observed in 24 out of 30 patients included in this analysis (80%).
DISCUSSION
Even though the course of cirrhosis varies according to several factors, the need for prognostic markers and scoring systems is critical to manage individuals facing different therapeutic options[21]. It was previously shown that IGFBP-3 levels in cirrhosis are related to the severity of liver dysfunction. This marker undergoes only slight influence from other factors not related to liver synthesis capacity. Therefore, IGFBP-3 is a potential and underexplored prognostic biomarker in liver cirrhosis.
Here, the IGFBP-3 levels correlated with several variables directly or indirectly associated with the intensity of liver dysfunction in both stable and decompensated patients. These findings agree with previous studies, which demonstrated an association between lower IGFBP-3 levels and the severity of liver disease[5,7,10,21-24]. In a recent study including patients with AD of cirrhosis, we showed that patients with more severe liver dysfunction exhibited lower IGFBP-3 levels - these levels were not influenced by other parameters such as gender, etiology of cirrhosis and comorbidities[25]. In addition, this study showed that IGFBP-3 increased significantly after liver transplantation as well as after hospital discharge. These findings reinforce the impact of hepatic synthetic function on IGFBP-3 levels and support its utility as a potential biomarker for assessment of liver function.
In patients with stable cirrhosis, death or liver transplantation during follow-up was associated with lower IGFBP-3 levels in bivariate analysis. In addition, transplant-free survival was significantly shorter in subjects with IGFBP3 < 1.67 mcg/mL. Data about the prognostic significance of IGFBP-3 in cirrhosis are scarce. IGFBP-3 was evaluated in 354 patients with alcohol-induced liver disease from a large multicenter trial of the effect of malotilate on survival[26]. The mean follow-up period was 569 d and low IGFBP-3 levels were associated with poor prognosis, especially at a cutoff of 1.35 mcg/mL[26]. The difference between this cutoff and that in the present study probably reflects methodological issues or disparities in the severity of the disease across the cohorts. It is important to note that the European study included both patients with and without cirrhosis-no detailed analysis of only those with cirrhosis was provided[26]. In our data, IGFBP-3 was not associated with death or liver transplantation in the logistic regression analysis in stable cirrhosis. This can be explained by the relatively low number of events in this cohort. Nevertheless, these results indicate the potential of IGFBP-3 as a prognostic marker in stable cirrhosis.
In patients hospitalized for AD of cirrhosis, lower IGFBP-3 levels were associated with short-term mortality in both bivariate and multivariate analysis. There is no data about IGFBP-3 prognostic value in this setting. It is possible that suppressed IGFBP-3 reflects the acute deterioration of hepatic function as suggested by its lower levels in hospitalized cirrhosis vs outpatients. The Kaplan-Meier survival probability at 90 d was significantly worse in patients with IGFBP3 < 0.86 mcg/mL vs control subjects (56.1% vs 80.4%, P < 0.001). This cutoff is markedly lower than the limits suggested by Møller et al[26] as well as in our study for stable cirrhosis. This likely reflects the severe deterioration of hepatic function in AD of cirrhosis.
The significance of IGFBP-3 in AD of cirrhosis was evaluated according to two of the most important prognostic parameters in this setting: The presence of ACLF and Child-Pugh Classification. The ACLF definition used here was based on a modified version of the SOFA score (CLIF-SOFA) and was first proposed by the EAS-CLIF Consortium in a large multicenter trial and subsequently validated[20,27]. Patients who presented with higher IGFBP-3 and without ACLF showed good prognosis (90-d survival approximately 90%). However, even in the absence of ACLF, low IGFBP-3 was associated with worse prognosis and a 90-d survival of 70.7%. Similarly, in the presence of ACLF, higher IGFBP-3 was associated with 90-d survival of 42.9% vs 20.8% for those with both ACLF and low IGFBP-3. These results indicate that combining IGFBP-3 and ACLF definition provided a well-defined four-level stratification for short-term prognosis in patients with cirrhosis hospitalized for AD. Similar results were observed for Child-Pugh Classification and MELD score although with a less clear stratification than that observed by using ACLF.
This study does have some limitations. The relatively small number of events in the stable cohort may have influenced the results - especially concerning regression analysis. In fact, regarding stable patients with cirrhosis there is still a need for validation of our results in larger cohorts with a longer follow-up before this biomarker is incorporated into clinical practice. Another limitation that we should highlight is the fact that we included a very heterogeneous population in distinct clinical scenarios and no specific treatment guidelines were created for the purpose of this study. Therefore, variations in the approach to specific cases are expected. In addition, the underlying liver disease status (if active or not) was not investigated. However, this issue is common in almost all studies investigating biomarkers in clinical settings. In addition, patients were evaluated according to standardized charts developed specifically for the purpose of the study and were followed both in outpatient clinic and in the ward by the same medical team. This minimized the impact of non-standardized approach.
In conclusion, in patients with cirrhosis IGFBP-3 levels correlated with several variables associated with severity of liver disease and improved significantly after discharge and after liver transplantation, indicating the impact of impaired hepatic function on its levels. Lower IGFBP-3 levels were associated with worse long-term prognosis in outpatients with stable cirrhosis and worse short-term prognosis in those hospitalized for AD. These findings suggest that measurement of circulating IGFBP-3 is of prognostic relevance and can be incorporated into clinical practice to improve the care of patients with liver cirrhosis.
COMMENTS
Background
insulin-like growth factor-binding protein 3 (IGFBP-3) is the major binding protein of insulin-like growth factor (IGF) system, carrying 80%-90% of circulating IGF-I. IGFBP-3 has predominantly hepatic production and decreased IGFBP-3 serum levels have been reported in patients with cirrhosis and seem to correlate with hepatic dysfunction intensity. Although preliminary reports indicate a clinical applicability for the assessment of circulating IGFBP-3 in cirrhosis, there are very few data on prognostic significance of this biomarker in this context.
Research frontiers
Defining the prognosis of patients with cirrhosis is of great relevance in order to select appropriate candidates for distinct therapeutic approaches, such as liver transplantation.
Innovations and breakthroughs
In the present study, IGFBP-3 levels correlated with several variables associated with hepatic dysfunction. Lower IGFBP-3 levels were associated with worse long-term prognosis in outpatients with stable cirrhosis and worse short-term prognosis in those hospitalized for acute decompensation.
Applications
These data suggested that IGFBP-3 levels can be used solely or in combination with other models (including Child-Pugh, model for end-stage liver disease and acute-on-chronic liver failure definition) to evaluate the prognosis of patients with liver cirrhosis.
Peer-review
This prospective study investigated the prognostic significance of IGFBP-3 in patients with cirrhosis. This is an interesting study, and this manuscript could provide useful information to readers.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Federal University of Santa Catarina Institutional Review Board.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: Nothing to report.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at leo-jf@uol.com.br. Participants gave informed consent for data sharing.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 26, 2016
First decision: March 23, 2016
Article in press: May 27, 2016
P- Reviewer: Bruha R, Invernizzi P, Niu ZS, Yoshida H S- Editor: Gong ZM L- Editor: A E- Editor: Liu SQ
References
- 1.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 2.Di Martino V, Weil D, Cervoni JP, Thevenot T. New prognostic markers in liver cirrhosis. World J Hepatol. 2015;7:1244–1250. doi: 10.4254/wjh.v7.i9.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonefeld K, Møller S. Insulin-like growth factor-I and the liver. Liver Int. 2011;31:911–919. doi: 10.1111/j.1478-3231.2010.02428.x. [DOI] [PubMed] [Google Scholar]
- 4.Donaghy AJ, Delhanty PJ, Ho KK, Williams R, Baxter RC. Regulation of the growth hormone receptor/binding protein, insulin-like growth factor ternary complex system in human cirrhosis. J Hepatol. 2002;36:751–758. doi: 10.1016/s0168-8278(02)00049-1. [DOI] [PubMed] [Google Scholar]
- 5.Wu YL, Ye J, Zhang S, Zhong J, Xi RP. Clinical significance of serum IGF-I, IGF-II and IGFBP-3 in liver cirrhosis. World J Gastroenterol. 2004;10:2740–2743. doi: 10.3748/wjg.v10.i18.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–210. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 7.Colakoğlu O, Taşkiran B, Colakoğlu G, Kizildağ S, Ari Ozcan F, Unsal B. Serum insulin like growth factor-1 (IGF-1) and insulin like growth factor binding protein-3 (IGFBP-3) levels in liver cirrhosis. Turk J Gastroenterol. 2007;18:245–249. [PubMed] [Google Scholar]
- 8.Audí L, Fernández-Cancio M, Camats N, Carrascosa A. Growth hormone deficiency: an update. Minerva Endocrinol. 2013;38:1–16. [PubMed] [Google Scholar]
- 9.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 10.Donaghy A, Ross R, Gimson A, Hughes SC, Holly J, Williams R. Growth hormone, insulinlike growth factor-1, and insulinlike growth factor binding proteins 1 and 3 in chronic liver disease. Hepatology. 1995;21:680–688. [PubMed] [Google Scholar]
- 11.Ferry RJ, Cerri RW, Cohen P. Insulin-like growth factor binding proteins: new proteins, new functions. Horm Res. 1999;51:53–67. doi: 10.1159/000023315. [DOI] [PubMed] [Google Scholar]
- 12.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 13.Møller S, Juul A, Becker U, Flyvbjerg A, Skakkebaek NE, Henriksen JH. Concentrations, release, and disposal of insulin-like growth factor (IGF)-binding proteins (IGFBP), IGF-I, and growth hormone in different vascular beds in patients with cirrhosis. J Clin Endocrinol Metab. 1995;80:1148–1157. doi: 10.1210/jcem.80.4.7536200. [DOI] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 16.Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537–547. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- 18.Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlich J, Mendel H, Pichler L, Wichlas M, Kreil A, et al. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879–885. doi: 10.1136/gut.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 20.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1-9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Shaarawy M, Fikry MA, Massoud BA, Lotfy S. Insulin-like growth factor binding protein-3: a novel biomarker for the assessment of the synthetic capacity of hepatocytes in liver cirrhosis. J Clin Endocrinol Metab. 1998;83:3316–3319. doi: 10.1210/jcem.83.9.5082. [DOI] [PubMed] [Google Scholar]
- 22.Scharf JG, Schmitz F, Frystyk J, Skjaerbaek C, Moesus H, Blum WF, Ramadori G, Hartmann H. Insulin-like growth factor-I serum concentrations and patterns of insulin-like growth factor binding proteins in patients with chronic liver disease. J Hepatol. 1996;25:689–699. doi: 10.1016/s0168-8278(96)80240-6. [DOI] [PubMed] [Google Scholar]
- 23.Assy N, Hochberg Z, Amit T, Shen-Orr Z, Enat R, Baruch Y. Growth hormone-stimulated insulin-like growth factor (IGF) I and IGF-binding protein-3 in liver cirrhosis. J Hepatol. 1997;27:796–802. doi: 10.1016/s0168-8278(97)80315-7. [DOI] [PubMed] [Google Scholar]
- 24.Rehem RN, El-Shikh WM. Serum IGF-1, IGF-2 and IGFBP-3 as parameters in the assessment of liver dysfunction in patients with hepatic cirrhosis and in the diagnosis of hepatocellular carcinoma. Hepatogastroenterology. 2011;58:949–954. [PubMed] [Google Scholar]
- 25.Ronsoni MF, Lazzarotto C, Fayad L, Silva MC, Nogueira CL, Bazzo ML, Narciso-Schiavon JL, Dantas-Corrêa EB, Schiavon Lde L. IGF-I and IGFBP-3 serum levels in patients hospitalized for complications of liver cirrhosis. Ann Hepatol. 2013;12:456–463. [PubMed] [Google Scholar]
- 26.Møller S, Becker U, Juul A, Skakkebaek NE, Christensen E. Prognostic value of insulinlike growth factor I and its binding protein in patients with alcohol-induced liver disease. EMALD group. Hepatology. 1996;23:1073–1078. doi: 10.1002/hep.510230521. [DOI] [PubMed] [Google Scholar]
- 27.Silva PE, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516–1523. doi: 10.1111/liv.12597. [DOI] [PubMed] [Google Scholar]