Abstract
Introduction
Nerve sparing during robotic radical prostatectomy (RRP) considerably improves post-operative potency and urinary continence as long as it does not compromise oncological outcome. Excision of the neurovascular bundle (NVB) is often performed in patients with intermediate and high risk prostate cancer to reduce the risk of positive surgical margin raising the risk of urinary incontinence and impotence. We present the first UK series outcomes of such patients who underwent an intra-operative frozen section (IOFS) analysis of the prostate during RRP allowing nerve sparing.
Patients and Methods
We prospectively analysed the data of 40 patients who underwent an IOFS during RRP at our centre from November 2012 until November 2014. Our IOFS technique involved whole lateral circumferential analysis of the prostate during RRP with the corresponding neurovascular tissue. An intrafascial nerve spare was performed and the specimen was removed intra-operatively via an extension of the 12 mm Autosuture™ camera port without undocking robotic arms. It was then painted by the surgeon and sprayed with “Ink Aid” prior to frozen section analysis. The corresponding NVB was excised if the histopathologist found a positive surgical margin on frozen section.
Results
Median time to extract the specimen, wound closure and re-establishment of pneumoperitoneum increased the operative time by 8 min. Median blood loss for IOFS was 130 ± 97 ml vs. 90 ± 72 ml (p = NS). IOFS was not associated with major complications or with blood transfusion. PSM decreased significantly from non-IOFS RRP series of 28.7 to 7.8% (p < 0.05). Intra-operative PSM on the prostate specimen was seen in 8/40 margin analysis (20%) leading to an excision of the contra-lateral nerve bundle. On analysis of the nerve bundle on a paraffin embedded block, 6 nerve bundle matched tumor on the specimen whereas 2 NVB were retrospectively removed unnecessarily in our series. All 40 patients have undetectable PSA at a mean follow up of 21.2 months (SD 7.79). Functional data at 18 months confirms a reduction in the urinary incontinence from 37% in the IOFS group vs 57% in the non-IOFS group (p = NS). IOFS technique has resulted in a significant increase in intravesical nerve sparing in both T2/T3 patients with intermediate and high risk prostate cancer when appropriately counselled and selected (T2 from 100% in the IOFS group versus 67% and T3 from 100% in the IOFS group to 42%) (p < 0.05).
Conclusion
Introduction of the IOFS analysis during intrafascial nerve spare RRP has reduced PSM and the rate of urinary incontinence.
Key Words: Prostate adenocarcinoma, RRP
Introduction
When a patient is referred for a robotic radical prostatectomy (RRP) the potential side effects of this surgical procedure include the risk of impotence and urinary incontinence [1,2]. There is a benefit in functional outcomes in terms of post RRP potency and urinary continence when a nerve sparing (NS) RRP is performed [3,4]. Hence, it is important to ensure that whilst offering patients a NS-RRP there should be no compromise to oncological outcome [5,6,7,8].
In the United Kingdom (UK) there is no official screening programme for prostate cancer so prostate cancer presents later [10]. Robotic prostatectomy surgeons in the UK are seeing more patients with intermediate and high-risk prostate cancer who are sexually active and potent at the time of diagnosis of prostate cancer. In this cohort of patients it is important to offer a NS-RRP in order to give these patients the best chance of preserving potency and continence whilst ensuring a negative surgical margin and optimum oncological control during a NS-RRP. Intra-operative frozen section (IOFS) during RRP is a safe oncological technique that allows optimum oncological control whilst giving the best and safest change of nerve sparing in which the prostate is removed intra-operatively and analysed for a positive margin. If the margin is confirmed to be positive then the adjacent neurovascular bundle (NVB) is excised.
In November 2012, we commenced our IOFS technique in patients with intermediate and high D'Amico stratification prostate cancer [9] in whom we tend to normally perform a wide local excision but proceed to performing an IOFS and NS-RRP after careful counselling. We present the results of our first 40 cases with IOFS making this the first reported UK case series with this technique.
Patients and Methods
We prospectively analysed and present the details of our first 40 consecutive patients who underwent an intra-operative frozen section analysis (IOFS) of the prostate during robotic radical prostatectomy (RRP) from November 2012 until November 2014. All 40 patients were diagnosed with intermediate and high-risk prostate cancer and would have normally undergone a wide local excision as against a NS-RRP. However, in view of the patient's age, functional status and the importance of persevering post-operative potency and reducing the risk of post RRP urinary incontinence, all patients were counselled on IOFS of the prostate during a NSRRP and elected to undergo this procedure.
The procedure was performed by Mr J Adshead, Consultant urological and robotic surgeon (November 2012 onwards) and Mr N Vasdev, Consultant urological and robotic surgeon (August 2014 onwards). All IOFS analyses were performed by Dr S Agarwal, Consultant histo-pathologist. The pre-operative data collected included patient age, co-morbidities, presentation PSA, transrectal ultrasound of prostate volume, detailed prostate pathology (Gleason score, number of cores, percentage of tumor involved), MRI location of tumor and stage, pre-operative bone scan result, patient performance and anesthetic status. Intra-operative parameters collected include operative time, robotic console time, prolongation of operative time with the introduction of frozen section technique, type of nerve spare performed, blood loss, intra-operative complications (i.e., injury to rectum during excision of neurovascular bundle) and type of bladder neck reconstruction required (bladder neck preservation, anterior bladder neck reconstruction or posterior bladder neck reconstruction).
The details of the IOFS analysis were collected evaluating the number and location of the on table positive margin with correlation of the final pathology. The post-operative parameters collected will include length of post-operative stay, post-operative complication as per the Clavien Dindo system, length of catheter and final post-operative pathology. The interim oncological data on biochemical recurrence and the number of patients who have received adjuvant radiotherapy was recorded.
Robotic Radical Prostatectomy Technique
All patients were admitted on the evening before surgery and received a phosphate enema. At induction all patient received a single dose of broad spectrum antibiotics. Patients received a standard general anaesthetic consisting of fentanyl 100 µg, midazolam 2 mg and propofol induction and intubation facilitated by atracurium. In addition paracetamol 1 g, ketorolac 30 mg, ondansetron 4 mg and dexamethasone 6.6 mg were given. Anesthesia was maintained with oxygen, air and Desflurane through a circle system and positive pressure ventilation. Muscle relaxation was maintained with atracurium infusion. The caudal block administered contained 40 ml 0.25% bupivicaine, 150 µg clonidine and 100 µg fentanyl. Bupivacaine 0.5% 20 ml were infiltrated to the skin wounds at the end of the operation. Regular paracetomol was prescribed postoperatively and ketorolac 30 mg im and oramorph 20 mg cyclizine were available on an as required basis. A clear view of the face and vigilance in positioning are important preventative measures. The patient is transferred onto a non-slip gel mat on the operating table.
As the prostate specimen needs to be removed during the robotic prostatectomy we have modified our approach on the initial port placement. During a standard RRP the initial incision made is supraumblical and a 12mm Excel™ port is inserted. However, as the specimen needs to be removed during the robotic radical prostatectomy we use a premeasured cut down method to open the rectus sheath to the size required for extraction. Interrupted 2/0 Ethibond™ sutures are applied and a 12 mm Autosuture™ balloon port is placed (fig. 1). Once the balloon port is inflated, 3 × 8 mm robotic, a 13 mm × Surgiquest™ and a 5 mm × assistant port is placed in situ (fig. 2).
Fig. 1.

Autosuture™ port.
Fig. 2.
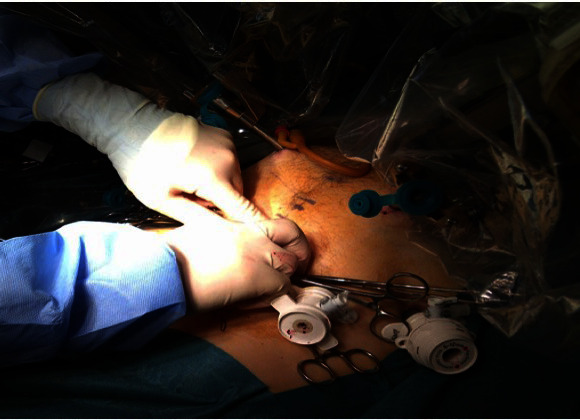
Placement of Autosuture™ port during Robotic Radical Prostatectomy with placement of interrupted 2/0 Ethibond™ sutures.
Nerve Sparing Technique and Apical Dissection during Robotic Radical Prostatectomy During a radical robotic prostatectomy an intrafascial or interfascial nerve spare is performed and the adjacent NVB is carefully separated from the prostate. An intrafascial NS is performed for low volume/low risk disease and interfascial NS is performed for higher volume disease. The pneumoperitoneum pressure is increased to 20 mmHg transiently and the dorsal vein complex is divided. The assistant does not use any suction and the pressure of 20 mmHg allows minimal bleeding from the open dorsal vein complex. The dorsal vein complex is then oversewn with a 3/0 V-Loc™ barbed unidirectional polyglyconate suture. The step allows the complete dissection of the urethral sphincter complex and the prostate is separated from the sphincter urethrae. The anterior urethra is then exposed to be divided until the urinary catheter has been seen. The catheter is then withdrawn under vision. The left arm was used to retract the rectourethralis. Using the fourth arm the prostate is pulled caudally in order to expose the dorsal urethra completely.
Specimen Removal The prostate specimen is placed in a 12 mm Endo-Catch™ bag that is inserted in via the 13 mm Surgiquest™ port. In order to prevent damage to organs due to a loss or pneumoperitoneum during specimen removal, we withdraw and remove all instruments from the robotic cannulae (Monopolar scissors from Arm 1, Maryland bipolar scissors from Arm 2 and Prograsp forceps from Arm 4). Only the camera robotic port arm is docked briefly to remove the specimen. All robotic ports are pushed in under vision by 4-5 cm to prevent the loss of the port position when pneumoperitoneum collapses (fig. 3). Under vision the specimen bag is withdrawn and extracted via extending the umbilical incision. The specimen is then handed to the console surgeon who commences the preparation and painting process of the prostate specimen prior to frozen section analysis. This step is described in detail below. The AutoSuture™ balloon port is inserted and pneumoperitoneum is created by closing the preplaced Ethibond sutures around the balloon port.
Fig. 3.
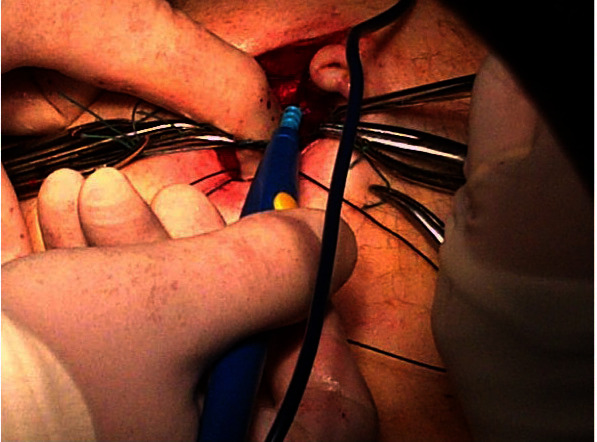
Advancement of ports when pneumoperitoneum collapses prior to removal of specimen intra-operatively.
Intra-operative Frozen Section Protocol
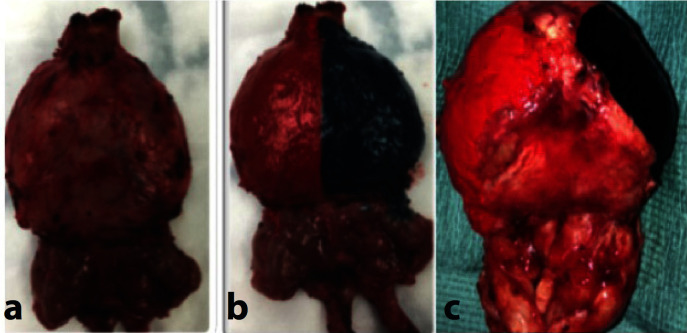
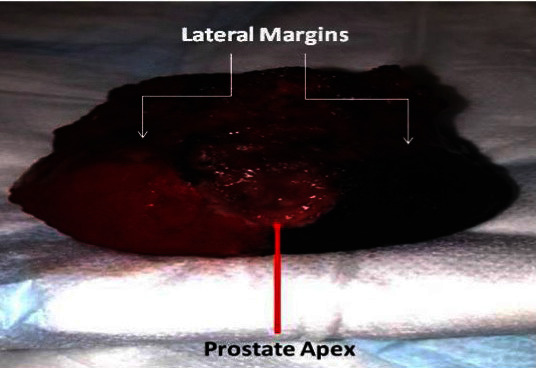
The specimen is then taken by the console surgeon and taken for frozen section analysis. Our IOFS analysis protocol involves inking the specimen in theatre prior to transferring the specimen to pathology for frozen section analysis. The protocol for inking different margins is as follows: red color for the right margin and violet color for the left margin (fig. 3). The specimen is then sprayed with “Ink Aid” which is a tissue marking dye setting solution. This is done to fix the ink on the fresh specimen. The specimen is then taken to histopathology department frozen section room (fig. 4). If intrafascial NS complete preservation of the “Veil of Aphrodite” then the ink needs to come to the midline as whole posterior of the prostate is clean (fig. 4a, 4b). If interfascial NS is performed then ink is only required in the base plate between the cut edge of Denovillers (fig. 4c).
Fig. 4.
Appearance of specimen on excision intraoperatively (a), Intrafascial NS for lower risk disease (b) Interfascial NS for intermediate / high risk disease (c).
The specimen is then sprayed with “Ink Aid” which is a tissue marking dye setting solution. This is done to fix the ink on the fresh specimen. The specimen is then taken to histopathology department frozen section room.
The process of inking the specimen in our series has a mean time of 6 min (range 4-12 min). This step adds on no significant additional time to the total operative timeas during this time of inking the specimen, the pneumoperitoneum is established, robotic ports are re-positioned and the camera arm is re-docked. The console surgeon then returns to complete the urethrovesical anastomosis and a pelvic lymph node dissection if indicated.
Pathological Processing of Specimen
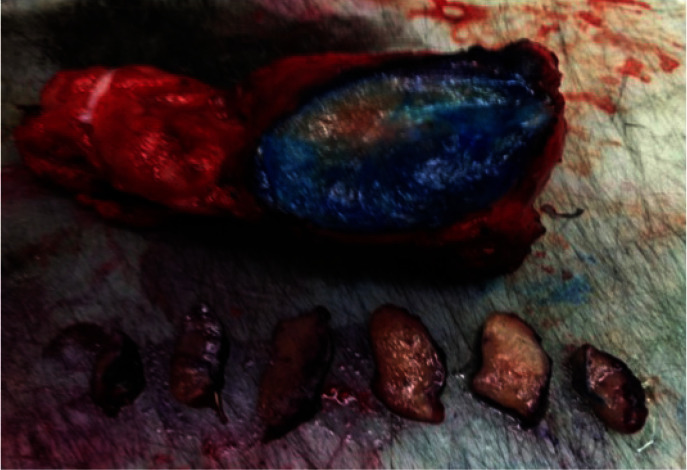
The entire inked margin is sectioned in one smooth incision. This is done by a straight surgical blade. A dot of blue ink is added at the apical margin for the anatomical orientation of the tissue (fig. 5)
Fig. 5.
Sectioning of prostate.
The sectioned slice of tissue is further serially sectioned into 3-4 mm slices. All the slices are then arranged in a serial manner to maintain the anatomic orientation. Each slice is then put on a cryostamp (and covered with plastic embedding medium OCT). Following this, the cryostamp with the tissue is sprayed with liquid CO2. This is done to freeze the tissue to enable section cutting The cryostamp is then fixed in the cryostat which is maintained at a temp of −24 °C. Sections are cut at 3-4 µm thickness and stained with hematoxylin and eosin stain (fig. 6).
Fig. 6.

Cryostamp processing of the prostate.
Approximately 10 tissue sections are generated per margin assessment. These are then examined by our department's uropathologist. The aim is to assess the adequacy of the margins. The presence of even a single neoplastic gland at the inked margin is reported as a positive margin. The result is then conveyed to the surgeon and is followed by a re- resection of the ipsilateral neurovascular bundle. The re-resected margin is further assessed by a paraffin wax block as a part of the routine process (fig. 7).
Fig. 7.
Paraffin wax block of prostate specimen.
Pathology Reporting Protocol
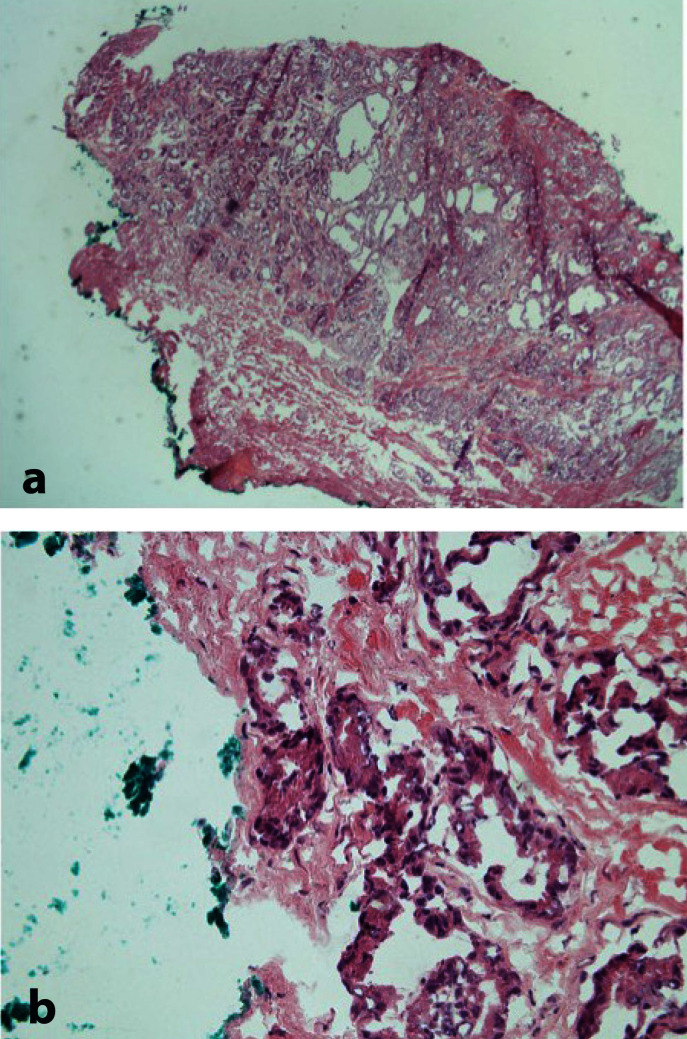
A positive surgical margin on frozen section is reported as the presence of one invasive malignant gland that contact with the inked margin (fig. 8).
Fig. 8.
Positive painted surgical margin (a. Low power) and (b. High power).
Secondary Excision of the Neurovascular Bundle
When the pathologist confirms the presence of a positive margin, a secondary resection of the ipsilateral NVB is performed. This is performed by placing the fourth arm on the bladder to retract the bladder medially and the urethrovesical anastomosis does not hinder visualization of the NVB. The caudal aspect of the NVB is identified and clipped with a Hem-O-Loc™ clip. The NVB is released and excised. The bundle is placed in an EndoCatch bag and sent for final pathological analysis.
Statistical Analysis
Statistical analyses between the groups (IOFS vs. Non-IOFS during RRP) using a commercially available computer software package (Graphpad prism 5). Patients' age and preoperative PSA values were evaluated as continuous variables, using Student t-test. Pearson X2 test was used to evaluate the association among categorized variables. In addition, the logistic regression model was used to determine statistical significance of predictors in a multivariate setting. P < 0.05 was considered to be statistically significant.
Baseline characteristics of patients undergoing an IOFS and a NS-RRP versus those who underwent a wide local excision during a RRP were compared. The 2 groups were compared using the X2 test. Propensity score matching was performed to correct imbalances based on preoperative PSA, pT stage, and postoperative Gleason score. The key parameters being primarily compared were operative time, blood loss, NS frequency, and positive surgical margin.
Results
Pre-operative Parameters
From November 2012 until November 2014, 40 patients with intermediate and high-risk D'Amico stratification prostate cancer underwent an IOFS during a RRP. The mean age of this cohort was 58.62 ± 6.7 years and BMI was 26 (range 22-34). The median serum PSA at presentation was 9.96 ng/ml (range 3-22 ng/ml). Clinical staging on the basis of pre-operative PSA, digital rectal examination and pre-operative MRI scan was found to be T2a-T2c disease in 33 patients (82.5%) and T3 disease in 7 patients (17.5%). Pre-operative number of cores positive on transrectal ultrasound scan biopsies was 61.1% (217 biopsy cores positive from 355 total cores biopsied). The mean pre-operative Gleason score was Gleason 3 + 4 = 7 (n = 23, 57.5%), Gleason 4 + 3 = 7 (n = 14, 35%) and Gleason 4 + 4 = 8 (n = 3, 7.5%) (Table 1).
Table 1.
Pre-operative baseline characteristics of 40 patients who underwent intra-operative frozen section analysis and robotic radical prostatectomy
| Parameter | N | % |
|---|---|---|
| Number of patients | 40 | N/A |
| Preoperative PSA (ng/ml) | ||
| < 4 | 1 | 2.5 |
| 4–10 | 16 | 40 |
| 10–20 | 19 | 47.5 |
| < 20 | 4 | 20 |
| Preoperative clinical T stage | ||
| T2B | 21 | 52.5 |
| T2C | 12 | 30 |
| T3A | 6 | 15 |
| T3B | 1 | 2.5 |
| Preoperative Gleason score | ||
| Gleason 3 + 4 | 23 | 57.5 |
| Gleason 4 + 3 | 14 | 35 |
| Gleason 4 + 4 | 3 | 7.5 |
All patients were discussed at the multidisciplinary team meeting on diagnosis of prostate cancer. Patients were subsequently seen in the outpatient clinic and the multidisciplinary team outcomes were discussed with the patients. All patients were also seen by our colleague clinical oncologist to discuss options of radical radiotherapy and brachytherapy.
Intra-operative Parameters
With the introduction of the use of IOFS analysis during RRP the median time added to the entire procedure was 8 min (range 5-37 min). The median time from specimen removal to reporting was 31 min (range 18-47 min). The median intra-operative blood loss with IOFS analysis is 50 ± 97 ml. The intra-operative transfusion rate for this cohort was 0%. There were no intra-operative complications associated including bowel injuries, rectal injury, port site hernia or acute bleed. Pelvic lymph node dissection was performed in 30% of cases (n = 12) with a median lymph node number of 14/case (range 4-26). None of the patients had any lymph node positive on final pathology. Median duration for post RRP removal of catheter was 7 days (range 6-10 days).
All patients with both T2 and T3 were counselled in detail pre-operatively with the role of excising the ipsilateral nerve bundle in the event of a positive surgical margin being diagnosed on IOFS. Using our new technique on IOFS a propensity score shows an increase in NS across all patients with intermediate and high risk prostate cancer with all pT stages from 57.8 to 100% using the IOFS (p < 0.05). In patients with pT2 stage disease with intermediate and high-risk prostate cancer, intrafascial NS increased from 57 to 100% (p < 0.05) and in patients with T3a disease from 32 to 100% (p < 0.0001). In patients with T3b disease we did not perform an interfascial NS and hence the increase was from 0 to 100%.
In patient cohorts based on propensity scores, the positive surgical margin significantly reduced statistically from an overall positive surgical margin rate of our entire robotic series to date across all stage pT prostate cancer (n = 620) of 24.7 to 7.8% (p < 0.05). In patients with T2 intermediate and high risk prostate cancer (n = 28) who did not undergo IOFS analysis and a standard wide local excision, the reduction in the T2 positive surgical margin rates with the introduction of IOFS during robotic radical prostatectomy was 17.8 to 0% (p < 0.0001). None of our patients with T2 disease underwent an IOFS during a positive surgical margin. In patients with T3 intermediate and high risk prostate cancer (n = 12) who did not undergo IOFS analysis and a standard wide local excision, the reduction in the T3 positive surgical margin rates with the introduction of IOFS during robotic radical prostatectomy was 48 to 33% (p = NS).
The location of the positive surgical margin was broken down into the following locations - apical and lateral (These are the margins that are aimed to be reduced during intra-operative frozen section analysis) and margin positivity at the urethra, bladder neck, basal, anterior, rectal, seminal vesicle) (fig. 9).
Fig. 9.
Location of positive surgical margins during robotic radical prostatectomy.
Dr S Agarwal, consultant histopathlogist, performed a total of 80 frozen section analysis. The incidence of positive margin on the lateral/apex margin on frozen section analysis was seen in 10 cases ranging from 1-4 mm and the ipsilateral NVB was excised. On performing further pathological analysis of the excised NVB using the paraffin wax block with immunostaining, 6 NVBs had a foci of tumor in correlation to the specimen at the matched point. Four NVBs did not have any tumor present and on further analysis of the main prostate specimen, there was no tumor identified at the lateral and apex indicating a “precautionary over-diagnosis” by the histopathologist leading to the excision of the NVB. In the later 4 cases in which an over diagnosis of the presence of a false positive diagnosis had taken place were case 3, 5, 7 and 11. The application of IOFS has a sensitivity of 75% and specificity of 75% when compared with permanent sections after re-embedding in paraffin sections. The false negative of IOFS analysis were all characterized by < 0.5 mm positive surgical margin.
At a current mean follow up of 21.2 months (SD 7.79, range 12-36 months) none of the patients have had a biochemical recurrence. The long term oncological data with IOFS will be published as the effect on long term biochemical recurrence free survival.
The main benefit of performing a nerve spare as against performing a wide local excision is to evaluate the functional (urinary continence and erectile function) in these patients. In our cohort of 40 patients, 85% of patients are now pad-free, 12% patients wear a precautionary continence pad during exercise and 3% of patients are on > 1 pad.
The incidence of patients having spontaneous erections rigid enough for intercourse following (with or without a PDE5 inhibitor) bilateral nerve spare using the IOFS technique is 83% in comparison to 73% previously (p = NS). In those patients in whom the ipsilateral NVB has bundle has been excised the incidence of spontaneous erections is 67% in comparison to 43% previously (p = NS). Longer follow up is required to evaluate the functional data and we will publish our results in due course.
The implementation of the IOFS technique has resulted in a significant increase in NS in both T2/T3 patients with intermediate and high risk prostate cancer when appropriately counselled and selected (Table 2).
Table 2.
Impact of Oncological and Functional outcome with implementation of Intra-operative frozen section of prostate
| Non IOFS | IOFS | p | |
|---|---|---|---|
| T2 positive margin rate | 15 % | 0 % | P < 0.05 |
| T3 negative margin rate | 48% | 76% | P < 0.05 |
| Bilateral NS potency | 72% | 83% | P = NS |
| Unilateral NS potency | 43% | 67% | P = NS |
IOFS = Intra-operative frozen section
There was one Clavien grade 2 complication which was a wound infection requiring antibiotics making the 30 day complication of 2.5%. None of the patients in the series had a urine leak necessitating prolonged catheterisation.
Discussion
There is currently no screening programme for prostate cancer in the UK, because it has not been proven that the benefits would outweigh the risks. However, men above the age of 50 years can request for a PSA level to be checked in the UK in primary care. With an increase in PSA levels being performed in the community and patients now presenting with localized or locally advanced prostate cancer [9] on further investigations there is a trend to see younger patients with intermediate and high risk prostate cancer who are potent and have no lower urinary tract symptoms at presentation [10]. In this selected group of patients it is important to offer a NS prostatectomy to ensure a superior functional outcome from the perspective of erectile function and continence without any compromise to the oncological outcome whilst ensuring negative surgical margins.
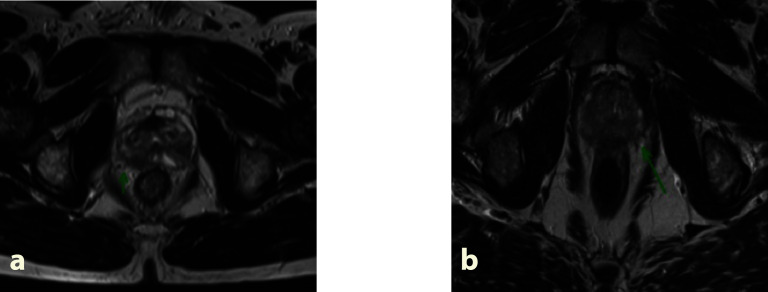
The main aim at the pre-IOFS RRP stage is to ensure that patients are staged very accurately. An MRI of the prostate is a useful staging tool but is still being evaluated to assess NVB involvement at diagnosis [11]. With the advent of 3T MRI of the prostate, there is better evaluation of extra capsular extension prior to NS-RRP which in turn help to guide decision-making about NVB preservation, with comparatively high specificity and negative predictive value [11]. For optimal NS outcome, the pre-operative search for extracapsular extension in the prostate and the accurate staging of prostate cancer appear to be key points in the pre-therapeutic workup for indicating whether the NS approach is feasible. In our series we have retrospectively evaluated the pre-operative MRI scans of the prostate prior to IOFS in NS-RRP in those patients in whom the NVB was excised during RRP at the time of NS-RRP to evaluate if the pre-operative MRI could have predicted NVB involvement as confirmed on final pathology. The sensitivity and specificity of pre-operative MRI in the 6 patients with NVB involvement with tumor was 57 and 44% (fig. 10). With the increase use of 3T MRI compared to 1.5T, there is improved signal resolution and the radiologist is more confident in identifying the NVB which emerges from the prostate posteriolaterally at the base level. Features suggestive of NVB involvement are: irregular capsule bulge, periprostatic fat infiltration, obliteration of the rectoprostatic angle, abutment of the tumor on the NVB, or asymmetry of the NVB adjacent to the tumor.
Fig. 10.
Preoperative MRI pre robotic prostatectomy. a Tumor has early breach of capsule and very close to right neurovascular bundle. b Asymmetric thickening of Left Neurovascular bundle, possible sign of involvement.
All patients in our series who underwent an IOFS analysis underwent an intrafascial dissection or interfascial dissection of the NVB. During an intrafascial dissection the plane of dissection during a RRP is medial or internal to the prostatic fascia at the anterolateral and posterolateral aspect of the prostate and also this dissection plane remains anterior to the posterior prostatic fascia/seminal vesicle fascia. During an intrafascial dissection a part of the posterior prostatic fascia/seminal vesicle fascia remains on the posterior surface of the prostate specimen where it is fused with the capsule in the midline. During a RRP we commence this dissection at 6 o'clock positions where the specimen is fused with the capsule in the midline as the posterior prostatic fascia/seminal vesicle fascia is recognized as a single layer structure. In all our patients we performed a high release of the NVB, this exact location of release of this plane during a RRP is slightly more difficult to identify due to the multilayered disposition of the fascia at the posterolateral border of the prostate. The advantage of the intrafascial approach allows a whole-thickness preservation of the NVB laterally and therefore a complete preservation of the neurovascular bundle is possible, as the neurovascular bundle remains preserved on the lateral prostatic fascia. The intrafascial approach has the highest risk of capsular penetration during RRP and risks a positive margin [10].
During a RRP we also perform an interfascial dissection of the NVB, which is considered as a dissection outside or lateral to the prostatic fascia at the anterolateral and posterolateral aspects of the prostate. The interfascial nerve spare dissection combined with a dissection medial to the NVB at both 5 and 7 o'clock or at 2 and 10 o'clock position located lateral to the prostate. The interfascial plane of dissection is developed so that the posterolateral prostate plane still remains covered with a layer of fascia. During the interfascial technique the NVB may be prone to partial resection at the apex, but the interfascial technique does not allow a complete preservation of all nerve fibres dispersed on the anterolateral surface of the prostate. The interfascial approach does result in a safer oncological dissection in comparison to the interfascial technique.
The relationship between the NVB and prostatic fascia continues to be evaluated. Research has shown that the NVB is located between the prostate capsule and either the levator ani fascia or the posterior prostatic fascia/seminal vesicle fascia. Further research by Kourambas et al. [13] found that on an axial section the neurovascular bundle was actually in an “H” shape fascial structure flanking the prostate. The left and right posterior prostatic fascia represented the upper region of the “H” and the horizontal bar of the “H” was represented by the pararectal fascia [12]. The most important landmark in order to prevent damage to the NVB during robotic prostatectomy is that the posterior prostatic fascia/seminal vesicle fascia is multiple layered and within this layer is a large concentration of nerve fibers found. The fibers are located not only ventral to the posterior prostatic fascia/seminal vesicle fascia but also dorsal to the posterior prostatic fascia/seminal vesicle fascia [13].
The data from our series in relationship to oncological outcome is very encouraging. We have seen the reduction in the T2 positive surgical margin rates with the introduction of IOFS during RRP was 17.8 to 0% (p < 0.0001). In patients with T3 intermediate and high-risk prostate cancer (n = 12) who did not undergo IOFS analysis and a standard wide local excision, the reduction in the T3 positive surgical margin rates with the introduction of IOFS during RRP was 48 to 33% (p = NS). At a mean follow up of 20 months none of our patients have had a biochemical recurrence. The importance of achieving negative surgical margin during RRP is important as a positive surgical margin during a RRP is related to the incidence of biochemical recurrence in the long term for patients and the possible need for adjuvant therapy [14,15]. There is a strong association between the risk of biochemical recurrence and the presence of positive margin at final histology following a radical prostatectomy. However, the data on the incidence of metastatic progression in these patients and death related to prostate cancer is not readily available [15,16]. Due to the current follow up of our patients being a mean of 21.2 months longer follow up is required to validate these results further. One patient in our series with adverse histology T3b has had adjuvant radiotherapy.
Our results are in corroboration with the experience of the Martini clinic who has published extensively on neurovascular structure-adjacent frozen section examination (NeuroSAFE) procedure for da Vinci robot-assisted prostatectomy. Beyer et al. [17] (Martini clinic) analysed the outcomes of 1,178 patients undergoing robot-assisted prostatectomy with NeuroSAFE. Results from this paper confirmed that it is feasible and secure adaption of the neurovascular structure-adjacent frozen section examination (NeuroSAFE) procedure for da-Vinci robot-assisted prostatectomy. The authors showed that there was no increased blood loss and operating room time. The results concluded that the authors maximized the nerve-sparing frequency and could reduce positive surgical margins even in non-organ-confined tumors. Furthermore Schlomm et al. [18] from the same group reported on 5,392 patients who had NeuroSAFE radical prostatectomy with either an open or a robotic approach. In NeuroSAFE radical prostatectomy, frequency of NS was significantly higher and positive surgical margin rates were significantly lower. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases NS frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: experience after 11,069 consecutive patients.
In our limited repertoire of cases, we have experienced numerous technical difficulties. Each case has been a new experience with better learning of the process and sequential modification of the process with each case.
Maintenance of the temperature in the cryostat—Up to 20 blocks are cut sequentially. This is a time consuming process and often the temperature rises in the cryostat. We often have to wait for the temperature to stabilise before resuming the process.
Irregularity in slicing of the tissue—The pathologist does serial slicing of the tissue. Any irregularity in sectioning leads to incomplete inked margins. These then have to be reassessed by multiple deeper frozen section levels. This again delays the process significantly.
Initially, in an attempt to reduce the number of blocks and the time of the process, thick slices of tissue were sectioned by the pathologist. This turned out to be a false economy of time as this resulted in poor freezing of tissue, leading to incomplete margins and increased levels on all blocks which further increased the total time of the process.
Proper orientation of the chuck in the cryostat—The aim of the process is to assess margins. Therefore the chuck is oriented in a way that the inked margin of the tissue is sectioned first. This reduces the incidence of incomplete margins, thus alleviating the need for further levels.
Histological interpretation of sections—The quality of a frozen section is not as good as a routine paraffin wax section. Microscopically, neurovascular bundles can be over interpreted as a focus of adenocarcinoma.
The study has a few limitations. The numbers are small. However our early experience is encouraging. Perhaps future randomised control trials comparing IOFS vs. non-IOFS RRP will ensure avoiding associated bias involved with non-randomised studies such as the current study. The authors however would like to raise ethical concerns with randomised control trial comparing IOFS vs. non-IOFS cohorts. The evidence by the Martini clinic in favour of the NeuroSafe technique is compelling. Similarly our early results are encouraging. With this technique being able to maintain functional outcomes without compromising oncological outcomes it would seem unfair not to offer it to non-IOFS cohort if local histopathology expertise is indeed available in a randomised control setting. Another limitation is the specimens were evaluated by one histo-pathologist. Validation of these results by a second pathologist would have confirmed inter-observer reliability. We also did not use validated questionaries' to evaluate continence and functional outcomes. Future research will have to concentrate on cost analysis of the frozen section technique. Prospective comparative studies between the frozen section technique and pre-operative MRI in predicting nerve bundle will be of interest as well. Furthermore long-term oncological outcomes will be vital as well.
Conclusion
In our series there is a reduction in the positive surgical margin from 17.8 to 0% in patients with T2 disease and from 34 to 22% in patients with T3 disease with the introduction of IOFS analysis during intrafascial NS-RRP. Functional data at a mean follow up of 18 months confirms a reduction in the rate of urinary incontinence (i.e. pad free) status to be better in patients undergoing IOFS and intrafascial nerve spare during RRP when compared to patients undergoing a standard wide local excision (37 vs. 57%). There were no major intra-operative complications (i.e. Clavien Dindo ≥ 3) with the introduction of the IOFS during intrafascial NS-RRP. Larger patient numbers and longer follow up is required to validate these results further, but our initial data are very encouraging allowing us to offer IOFS safely to patients with intermediate and high risk prostate cancer who would have normally undergone a wide local excision that would effect post operative urinary continence and erectile function as this technique involves the excision of the bilateral NVB.
References
- 1.Budaus L, Isbarn H, Schlomm T, Heinzer H, Haese A, Steuber T, Salomon G, Huland H, Graefen M. Current technique of open intrafascial nerve-sparing retropubic prostatectomy. Eur Urol. 2009;56:317–324. doi: 10.1016/j.eururo.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 2.Eastham JA, Kattan MW, Rogers E, Goad JR, Ohori M, Boone TB, Scardino PT. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156:1707–1713. [PubMed] [Google Scholar]
- 3.Palisaar RJ, Noldus J, Graefen M, Erbersdobler A, Haese A, Huland H. Influence of nerve-sparing (NS) procedure during radical prostatectomy (RP) on margin status and biochemical failure. Eur Urol. 2005;47:176–184. doi: 10.1016/j.eururo.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Bianco FJ Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66(5 suppl):83–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 5.Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, Menon M, Montorsi F, Myers RP, Rocco B, Villers A. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57:179–192. doi: 10.1016/j.eururo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Sofer M, Hamilton-Nelson KL, Schlesselman JJ, Soloway MS. Risk of positive margins and biochemical recurrence in relation to nerve-sparing radical prostatectomy. J Clin Oncol. 2002;20:1853–1858. doi: 10.1200/JCO.2002.07.069. [DOI] [PubMed] [Google Scholar]
- 7.Simon MA, Kim S, Soloway MS. Prostate specific antigen recurrence rates are low after radical retropubic prostatectomy and positive margins. J Urol. 2006;175:140–145. doi: 10.1016/S0022-5347(05)00050-9. [DOI] [PubMed] [Google Scholar]
- 8.Vasdev N, Bishop C, Kass-Iliyya A, Hamid S, McNicholas TA, Prasad V, Mohan SG, Lane T, Boustead G, Adshead JM. Developing a robotic prostatectomy service and a robotic fellowship programme-defining the learning curve. Curr Urol. 2013;7:136–144. doi: 10.1159/000356266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 10.Hoyland K, Vasdev N, Abrof A, Boustead G. Post-radical prostatectomy incontinence: etiology and prevention. Rev Urol. 2014;16:181–188. [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka K, Shigemura K, Muramaki M, Takahashi S, Miyake H, Fujisawa M. Efficacy of using three-tesla magnetic resonance imaging diagnosis of capsule invasion for decision-making about neurovascular bundle preservation in robotic-assisted radical prostatectomy. Korean J Urol. 2013;54:437–441. doi: 10.4111/kju.2013.54.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savera AT, Kaul S, Badani K, Stark AT, Shah NL, Menon M. Robotic radical prostatectomy with the “Veil of Aphrodite” technique: histologic evidence of enhanced nerve sparing. Eur Urol. 2006;49:1065–1074. doi: 10.1016/j.eururo.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Kourambas, Angus DG, Hosking P, Chou ST. A histological study of Denonvilliers' fascia and its relationship to the neurovascular bundle. Br J Urol. 1998;82:408–410. doi: 10.1046/j.1464-410x.1998.00749.x. [DOI] [PubMed] [Google Scholar]
- 14.Moon A, Vasdev N, Veeraterpillay R, O'Riordon A, Durkan G, Johnson M, Soomro N. Oncological outcomes in low, intermediate and high D'Amico risk patients undergoing laparoscopic radical prostatectomy. J Clin Urol. 2013;6:295–301. [Google Scholar]
- 15.Kass-Iliyya A, Vasdev N, Soomro N, Durkan GC. Urinary function and health-related quality of life of patients after laparoscopic radical prostatectomy: two methods of assessment for 112 patients with up to six years of follow-up. J Clin Urol. 2013;6:32–44. [Google Scholar]
- 16.Wright JL, Dalkin BL, True LD, Ellis WJ, Stanford JL, Lange PH, Lin DW. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. J Urol. 2010;183:2213–2218. doi: 10.1016/j.juro.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyer B, Schlomm T, Tennstedt P, Boehm K, Adam M, Schiffmann J, Sauter G, Wittmer C, Steuber T, Graefen M, Huland H, Haese A. A feasible and time-efficient adaptation of NeuroSAFE for da Vinci robot-assisted radical prostatectomy. Eur Urol. 2014;66:138–144. doi: 10.1016/j.eururo.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Schlomm T, Tennstedt P, Huxhold C, Steuber T, Salomon G, Michl U, Heinzer H, Hansen J, Budäus L, Steurer S, Wittmer C, Minner S, Haese A, Sauter G, Graefen M, Huland H. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: experience after 11,069 consecutive patients. Eur Urol. 2012;62:333–340. doi: 10.1016/j.eururo.2012.04.057. [DOI] [PubMed] [Google Scholar]