Abstract
Purpose
Concurrent chemoradiotherapy (CCRT) for squamous cell carcinoma of the head and neck (SCCHN) increases both local tumor control and toxicity. This study evaluates clinical factors that are associated with and might predict severe late toxicity after CCRT.
Methods
Patients were analyzed from a subset of three previously reported Radiation Therapy Oncology Group (RTOG) trials of CCRT for locally advanced SCCHN (RTOG 91-11, 97-03, and 99-14). Severe late toxicity was defined in this secondary analysis as chronic grade 3 to 4 pharyngeal/laryngeal toxicity (RTOG/European Organisation for the Research and Treatment of Cancer late toxicity scoring system) and/or requirement for a feeding tube ≥ 2 years after registration and/or potential treatment-related death (eg, pneumonia) within 3 years. Case-control analysis was performed, with a multivariable logistic regression model that included pretreatment and treatment potential factors.
Results
A total of 230 patients were assessable for this analysis: 99 patients with severe late toxicities and 131 controls; thus, 43% of assessable patients had a severe late toxicity. On multivariable analysis, significant variables correlated with the development of severe late toxicity were older age (odds ratio 1.05 per year; P = .001); advanced T stage (odds ratio, 3.07; P = .0036); larynx/hypopharynx primary site (odds ratio, 4.17; P = .0041); and neck dissection after CRT (odds ratio, 2.39; P = .018).
Conclusion
Severe late toxicity after CCRT is common. Older age, advanced T-stage, and larynx/hypopharynx primary site were strong independent risk factors. Neck dissection after CCRT was associated with an increased risk of these complications.
INTRODUCTION
Concurrent chemoradiotherapy (CCRT) is a standard treatment for patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN) treated nonsurgically. Meta-analyses show an improved 5-year survival by approximately 8% when CCRT is compared with radiation therapy alone.1,2 The advantage of this approach with respect to disease-free survival and locoregional control is greater than 8%.3-10
While there are undisputed advantages to CCRT for locoregional control, it increases toxicity when compared with radiation therapy alone.11 Many studies have focused on acute toxicity, particularly mucositis, as summarized in a meta-analysis by Trotti et al.12 Comprehensive data on late toxicity from randomized trials of radiation therapy with or without chemotherapy, however, are sparse. Late toxicity may include long-term severe dysphagia and its related effects, including dependence on a feeding tube, and have a profound effect on quality of life. The increased incidence of these serious, potentially permanent effects after CCRT is of concern, leading some to question as to whether chemoradiotherapy is truly a major improvement in the therapeutic ratio over radiation therapy alone.13
Starting approximately 15 years ago, the Radiation Therapy Oncology Group (RTOG) conducted a series of prospective clinical trials using CCRT for locally advanced SCCHN. General data on efficacy and early and subacute toxicity have been reported.14-16 It is likely, however, that each individual study is underpowered for a thorough analysis of late effects, given sample size and patient attrition due to mortality and other causes. Consequently, we performed a secondary analysis of severe late toxicities from these several trials, specifically focusing on late toxicities and mortality related to pharyngolaryngeal dysfunction. An analysis of potential factors associated with severe late toxicities was undertaken.
METHODS
As noted earlier, the three prospective trials analyzed for this article have been previously reported. All three studies required an acceptable performance status (60% to 100% by Karnofsky scale); nonmetastatic stage III/IV SCCHN; and good hematologic, renal, hepatic, and cardiovascular function. A brief description of these three studies follows.
RTOG 91-11
RTOG 91-11 was a phase III trial of larynx-preserving radiation therapy or chemoradiotherapy for selected stage III/IV larynx cancer.14 For this analysis, only the CCRT arm was studied; this treatment in this arm consisted of 70 Gy in conventionally fractionated radiation therapy—2 Gy once daily—plus three cycles of high-dose cisplatin (100 mg/m2, weeks 1, 4, and 7). There were 172 patients in this arm from RTOG 91-11; 88 patients were assessable for this analysis of late toxicity.
RTOG 97-03
RTOG 97-03 was a phase IIR trial of several novel regimens of CCRT for stage III/IV head and neck cancer (excluding patients who were eligible for RTOG 91-11). 17 This study included three arms. Arms 1 and 3 utilized conventionally fractionated radiation therapy as per 91-11. Arm 1 chemotherapy was infusional fluorouracil (FU) and cisplatin, both given daily during the last 2 weeks of fractionated radiation therapy. Arm 3 chemotherapy was once weekly cisplatin (20 mg/m2/wk) and paclitaxel (30 mg/m2/wk). Arm 2 chemoradiotherapy was modeled on the prospective phase II trials performed by the University of Chicago. In arm 2, although the total fractionated radiation therapy dose remained 70 Gy in 2 Gy fractions, it was delivered over 13 weeks (week-on, week-off technique); chemotherapy in arm 2 consisted of concurrent infusional FU and hydroxyurea. There were 231 patients in RTOG 97-03; 102 patients were assessable for this analysis of late toxicity.
RTOG 99-14
RTOG 99-14 was a phase II trial of accelerated radiation therapy with concurrent chemotherapy for stage III/IV head and neck cancer.16 This single arm phase II study consisted of accelerated concomitant boost radiotherapy to 72 Gy over 6 weeks (as per the concomitant boost arm of RTOG 90-03), with two cycles of high-dose cisplatin (100 mg/m2 weeks 1 and 4). There were 76 patients in RTOG 99-14; 40 patients were assessable for this analysis of late toxicity.
All of these studies used conventional radiation therapy techniques, mostly two-dimensional planning and delivery. No patient received intensity modulated radiation therapy.
For this report, a severe late toxicity was defined as any or all of the following events: grade 3 or higher toxicity (RTOG/European Organisation for the Research and Treatment of Cancer late toxicity criteria) present more than 180 days after the start of fractionated radiation therapy and clearly related to dysfunction of the larynx and/or pharynx (eg, dysphagia); requirement for a feeding tube/gastrostomy 2 years or longer after the start of fractionated radiation therapy; death without cancer progression and from an uncertain cause in which laryngeal dysfunction was suspected to be a contributing factor (eg, pneumonia) ≤ 3 years from the date of random assignment. Patients who died of unknown causes were included in this category. Review of these deaths was performed by one of the study authors (M.M.) in a manner blinded to any of the patient's clinical pretreatment and/or treatment related characteristics. Patients who suffered one or more qualifying severe late toxicity events were only considered to be one case. Patients with severe laryngopharynx dysfunction due to cancer, before the start of treatment, were excluded because of the potential confounding nature of tumor destruction of critical normal tissues (See Table 1). In RTOG 91-11, the determination of severe pretreatment laryngopharynx dysfunction was based on patients’ on-study data collection form, which scored airway obstruction and dysphagia on a 4-point scale (none, mild, moderate, severe/life-threatening); patients with severe/life-threatening airway obstruction and/or dysphagia based on this form were excluded. In RTOG 91-11, data on pretreatment use of feeding tubes were not collected. In RTOG 97-03 and RTOG 99-14, pretreatment feeding tube data were collected, and this was used as the primary means of defining patients with pretreatment severe laryngopharynx dysfunction. Patients with missing/unassessable data or early death from acute toxicity were also excluded.
Table 1.
Summary of Patients Excluded From This Analysis
| Characteristic | Radiation Therapy Oncology Group Study |
||||||
|---|---|---|---|---|---|---|---|
| 91-11 | 97-03 | 99-14 | Total | ||||
| Original No. of patients | 172 | 231 | 76 | 479 | |||
| Reason for exclusion | |||||||
| Severe pretreatment airway obstruction | 15 | — | — | 15 | |||
| Severe pretreatment dysphagia | 5 | — | — | 5 | |||
| Pretreatment feeding tube dependence | — | 62 | 18 | 80 | |||
| Total excluded due to severe pretreatment laryngopharynx dysfunction | 20 | 62 | 18 | 100 | |||
| Death from acute toxicity | 2 | 3 | 1 | 6 | |||
| Tumor recurrence/death < 3 years follow-up | 52 | 62 | 16 | 130 | |||
| Missing data | 10 | 2 | 1 | 13 | |||
| Grand total excluded | 84 | 129 | 36 | 249 | |||
| Total analyzable for this study | 88 | 102 | 40 | 230 | |||
Statistical Analysis
Frequency tables with counts and percentages were used to describe pretreatment and treatment characteristics for each group. Univariate and multivariable logistic regression models were used to identify associations of pretreatment and treatment-related factors with severe late toxicity. All models were stratified by the five treatment arms described earlier. The following factors were studied: age (continuous variable); sex; race (nonblack v black); Karnofsky performance status (60 to 80 v 90 to 100); hemoglobin (continuous variable); weight loss pretreatment (continuous variable); T-stage (T1/2 v T3/4); N stage (Nx/0/1 v N2 v N3); tumor site (oral cavity/oropharynx v larynx/hypopharynx); radiation therapy dose received as assessed by late effects biologically equivalent dose (BED) model (total radiation therapy dose multiplied by (1+ [dose per fraction]/3): continuous variable); chemotherapy dose received (< 85% of planned dose v > 85% of planned dose); and postradiation therapy neck dissection (yes v no). Variables’ levels were grouped in order to avoid small cell counts. A stepwise selection procedure was used to build the multivariable logistic regression model using the above pretreatment/treatment variables. Entry criterion was set at P < .05. The odds ratios for each variable in the final model along with their 95% CIs and P values are reported. The odds ratios estimate how much more (less) likely it is to be in the case group versus the control group among patients with the specific variable level's characteristic compared with those patients in the reference level, after stratifying for treatment arm. The cumulative incidence method was used to estimate time to severe late toxicity and levels for pretreatment/treatment-related variables were compared using the Gray's test.18,19
RESULTS
The original, potential patient population from these three studies was 479. However, there were 130 patients excluded because of locoregional failure or death due to cancer, 100 patients were excluded because of severe pretreatment laryngopharynx dysfunction due to tumor; 13 patients were excluded because of missing data; and six patients were excluded because of early death due to acute toxicity (Table 1). Thus, the overall assessable sample size for this report was 230 patients. The median follow-up for the patient population was 2.96 years.
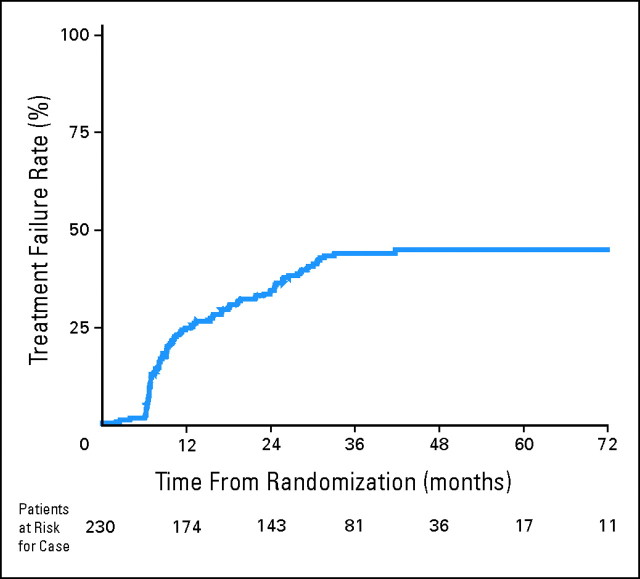
Of these 230 patients, 99 patients had severe late toxicity and 131 controls did not have severe late toxicity. This results in a crude rate of late toxicity of 43%. It should be noted that if the entire population of patients (N = 479) from all three studies are analyzed (as is often performed for studies of late effects), the crude rate would appear to be 21%—considerably lower than the data reported here. An actuarial plot of time to severe late toxicity for all 230 assessable patients is shown in Figure 1.
Fig 1.
Time to severe late toxicity (shown in the graph as Treatment Failure Rates): all assessable patients.
The pretreatment characteristics of these 230 patients are presented in Table 2, including both pretreatment and treatment-related characteristics. Table 3 presents an accounting of the types of late toxicity events observed in this analysis; most were related to swallowing function (particularly in RTOG 97-03 and RTOG 99-14) or laryngeal dysfunction (RTOG 91-11).
Table 2.
Characteristics of Patients With Severe Late Toxicities and Controls
| Characteristic | Patients (n = 99) |
Controls (n = 131) |
||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Age, years | ||||||
| Median | 60 | 56 | ||||
| Range | 33-78 | 26-78 | ||||
| ≤ 70 | 85 | 86 | 118 | 90 | ||
| > 70 | 14 | 14 | 13 | 10 | ||
| Sex | ||||||
| Male | 78 | 79 | 99 | 76 | ||
| Female | 21 | 21 | 32 | 24 | ||
| Race | ||||||
| Nonblack | 90 | 91 | 120 | 92 | ||
| Black | 9 | 9 | 11 | 8 | ||
| KPS | ||||||
| 60-80 | 24 | 24 | 20 | 15 | ||
| 90-100 | 75 | 76 | 111 | 85 | ||
| Hemoglobin, g/dL | ||||||
| Median | 14.3 | 14.2 | ||||
| Range | 7.1-18.2 | 9.9-18.2 | ||||
| ≤ 13.5 | 28 | 28 | 43 | 33 | ||
| > 13.5 | 71 | 72 | 88 | 67 | ||
| Weight loss in previous 6 months, kg | ||||||
| Mean | 3.9 | 2.8 | ||||
| ≤ 5 | 78 | 79 | 112 | 86 | ||
| > 5 | 21 | 21 | 19 | 14 | ||
| T stage | ||||||
| 1/2 | 18 | 18 | 39 | 30 | ||
| 3/4 | 81 | 82 | 92 | 70 | ||
| N stage | ||||||
| X/0/1 | 47 | 47 | 63 | 48 | ||
| 2 | 42 | 42 | 58 | 44 | ||
| 3 | 10 | 10 | 10 | 8 | ||
| Tumor site | ||||||
| Oral cavity/oropharynx | 42 | 42 | 71 | 54 | ||
| Oral cavity | 7 | 7 | 5 | 4 | ||
| Oropharynx | 35 | 35 | 66 | 50 | ||
| Larynx/hypopharynx | 57 | 58 | 60 | 46 | ||
| Larynx | 41 | 41 | 51 | 39 | ||
| Hypopharynx | 16 | 16 | 9 | 7 | ||
| Radiotherapy dose-intensity delivered, BED Gy | ||||||
| Mean | 115 | 116 | ||||
| Median | 117 | 117 | ||||
| Range | 67-117 | 111-126 | ||||
| Neck dissection after RT | ||||||
| Yes | 26* | 26 | 21 | 16 | ||
| No | 73 | 74 | 110 | 84 | ||
| Chemotherapy dose-intensity delivered, % | ||||||
| < 85 | 22 | 22 | 29 | 22 | ||
| ≥ 85 | 77 | 78 | 102 | 78 | ||
Abbreviations: KPS, Karnofsky performance status; BED, biologically equivalent dose; RT, radiation therapy.
Two of these patients had their neck dissection after experiencing a severe late toxicity.
Table 3.
Types of Late Toxicity Events Seen by Trial
| Variable | 91-11 | 97-03 | 99-14 | Total | |
|---|---|---|---|---|---|
| Feeding tube dependence > 2 years post-radiation therapy | —* | 29* | 29 | ||
| RTOG late toxicity criteria, grade 3+ | |||||
| Pharyngeal dysfunction | 16 | 28 | 19 | 63 | |
| Laryngeal dysfunction | 22 | 6 | 0 | 28 | |
| Death | 11 | 9 | 2 | 22 | |
| Other (eg, infection, fistula) | 3 | 0 | 1 | 4 | |
| Any | 38† | 40† | 21† | 99† | |
| No severe late toxicity event (controls) | 50 | 62 | 19 | 13 | |
Abbreviation: RTOG, Radiation Therapy Oncology Group.
Feeding tube data were not collected at all in RTOG study 91-11.
Numbers do not always add up along columns, due to some patients having more than one toxicity event.
Patients with severe toxicities were more likely to be older and/or to have larger T-stage and/or larynx/hypopharynx primary cancer. On univariate analysis, there were no statistically significant differences in the rates of late effects based on each individual study/arm.
Univariate logistic regression analysis of pretreatment and treatment-related variables is presented in Table 4. Actuarial estimates of time to severe late toxicity as a function of T stage, primary tumor site, and neck dissection are shown in Figures 2A to 2C, respectively. The most significant pretreatment factor associated with severe late toxicity was age, analyzed as a continuous variable (P = .0038)—older patients were significantly more likely to have severe late toxicity. T stage (T3-T4 more likely to have severe late toxicity) and tumor site (larynx/hypopharynx more likely to have severe late toxicity) were also statistically significant factors. On univariate analysis, none of the treatment-related variables were statistically significant except BED (P < .0001), with a paradoxical negative association between BED and severe late toxicity. The P value for potential association between severe late toxicity and neck dissection after radiation therapy was .145.
Table 4.
Univariate and Multivariable Logistic Regression Models to Identify Covariates that are Associated With Severe Late Toxicity
| Covariate | Univariate Analysis |
Multivariate Analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | P | Odds Ratio | 95% CI | P | ||||
| Age | ||||||||
| Continuous variable | 1.043* | .0038 | 1.05* | 1.02 to 1.09 | .001 | |||
| Sex | ||||||||
| Female | RL | |||||||
| Male | 1.140 | .6846 | ||||||
| Race | ||||||||
| Nonblack | RL | |||||||
| Black | 1.165 | .7458 | ||||||
| KPS | ||||||||
| 60-80 | 1.892 | .0612 | ||||||
| 90-100 | RL | |||||||
| Hemoglobin, g/dL | ||||||||
| Continuous variable | 1.005 | .9528 | ||||||
| Weight loss, kg | ||||||||
| Continuous variable | 1.018 | .3733 | ||||||
| T stage | ||||||||
| T1/T2 | RL | RL | ||||||
| T3/T4 | 2.041 | .0349 | 3.07 | 1.444 to 6.54 | .0036 | |||
| N stage | ||||||||
| NX/N0/N1 | RL | |||||||
| N2 | 0.942 | .8464 | ||||||
| N3 | 1.297 | .6108 | ||||||
| Tumor site | ||||||||
| Oral cavity/oropharynx | RL | RL | ||||||
| Larynx/hypopharynx | 2.955 | .0131 | 4.17 | 1.57 to 11.03 | .0041 | |||
| BED (toxicities) based on actual dose/Fx, Gy | ||||||||
| Continuous variable | 0.842 | < .0001 | ||||||
| Neck dissection after RT† | ||||||||
| Yes | 1.632 | .145 | 2.39 | 1.16 to 4.92 | .018 | |||
| No | RL | RL | ||||||
| Chemotherapy received relative to the protocol amount, % | ||||||||
| < 85 | 1.033 | .9216 | ||||||
| ≥ 85 | RL | |||||||
Abbreviations: KPS, Karnofsky performance status; RL, reference level; BED, biologically equivalent dose; Fx, fraction; RT, radiation therapy.
The odds ratio of 1.043 for age indicates that for each one year increase in age, patients have 1.043 times higher odds of being in the case group (having a severe late toxicity) than being in the control group (not having a severe late toxicity).
This excludes two patients who had neck dissection after having already experiencing a severe late toxicity.
Fig 2.
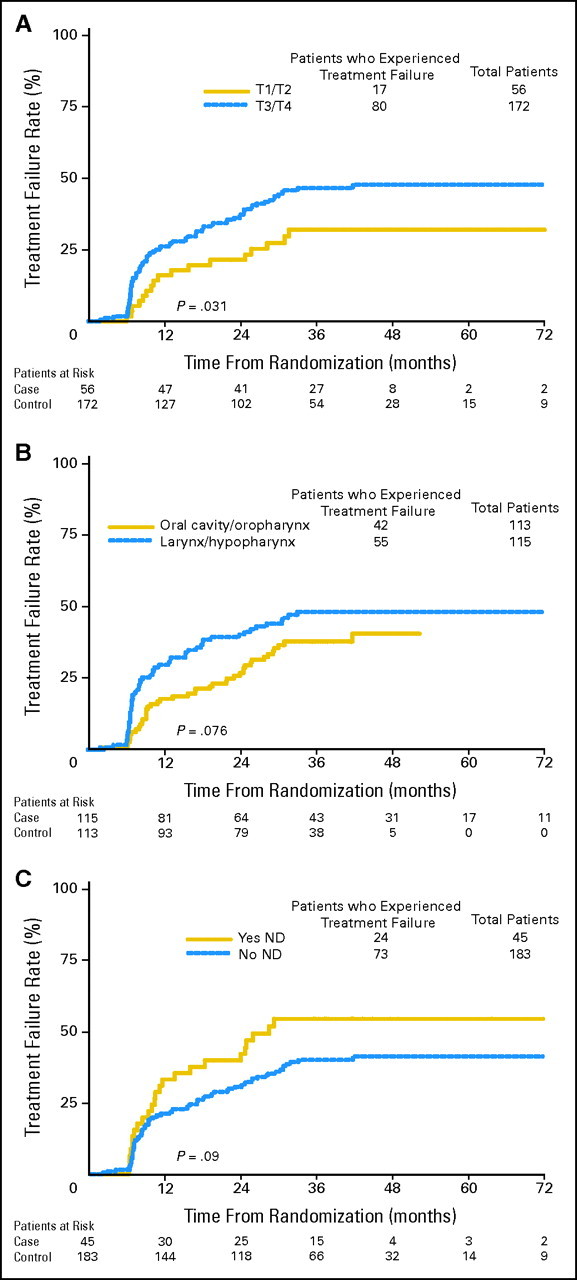
Time to severe late toxicity (shown in the graphs as Treatment Failure Rate): subgroup analyses based on patient/treatment characteristics. All graphs exclude two patients who had neck dissection after already experiencing a severe late toxicity. (A) Time to severe late toxicity by T stage. Advanced T stage is associated with a higher likelihood of severe late toxicity (P value from Gray's test = .031). (B) Time to severe late toxicity by primary tumor site. Larynx/hypopharynx cancer is associated with a statistically nonsignificant higher likelihood of severe late toxicity (P value from Gray's test = .076). (C) Time to severe late toxicity by neck dissection (ND). ND is associated with a statistically nonsignificant higher likelihood of severe late toxicity (P value from Gray's test = .09).
The results of a multivariable logistic regression model analysis are presented in Table 4. Age, T stage, and tumor site remained statistically significant. In addition, a positive association between post-treatment neck dissection and severe late toxicity was noted (P = .02). Specifically, of the 230 patients in this study, 47 (20%) underwent post-treatment neck dissection; this included 22% of the oral cavity/oropharynx patients and 19% of the larynx/hypopharynx patients. These 47 patients had a crude rate of severe late toxicity of 55% (compared with 40% for the 183 patients who did not undergo neck dissection).
Of note, besides neck dissection, other treatment-related factors were not associated with severe late effects. Although the most aggressive radiation therapy fractionation trial (RTOG 99-14, which used concomitant boost fractionated radiation therapy plus cisplatin) numerically had the highest crude rate of severe late toxicity (21 of 40; 53%), there were no statistically significant differences among the trial arms.
As noted in Table 4, radiation therapy dose delivered (as analyzed as BED) was significant on univariate analysis (with a paradoxical relationship in which lower radiation therapy dose was associated with higher risk) but fell out of the multivariable model. The amount of chemotherapy delivered was not statistically significant in either model.
DISCUSSION
This retrospective analysis of several prospective trials shows that the rate of severe late toxicity after CCRT for SCCHN is high, particularly with the analysis methodology used here. Specifically, in this study, patients with severe pretreatment laryngopharynx dysfunction and patients with early tumor recurrence were excluded a priori from this analysis. Thus, the number of patients at risk for a severe late toxicity event is much smaller than the original treated population. This technique closely approximates the use of actuarial analysis of late complications, a technique which yields a higher rate of complications than simply reporting crude rate of complications, as reported by Bentzen et al.20 A true actuarial analysis of late complications in head and neck cancer is difficult because it is not easy to ascertain a date of onset of a late complication in any one individual patient (Figs 2A to 2C). Our sample size of 230 patients makes this one of the largest studies of late toxicity in the CCRT era.
In this study, several factors that correlated with severe late toxicity were identified. Because this is a retrospective stud, the data must be considered hypothesis generating rather than definitive. Some caveats result from the fact that these studies were conducted over a 10-year time period (approximately 1991 to 2001), with variations in eligibility, treatment, and data collection techniques. A second problem inherent to retrospective studies like ours is that a number of potentially important factors may not have been collected at all. For example, our database does not include information on tumor volume, cardiopulmonary comorbidity, and amount of tobacco consumed in follow-up.
However, it is logical to believe that age, tumor site, and tumor stage would predict for greater likelihood of severe late toxicity. The finding that post-treatment neck dissection was significantly associated with severe late toxicity was somewhat more surprising, although this has been reported previously. The number of patients undergoing post-treatment neck dissection was relatively small (20%, despite more than 50% of the patients having N2-N3 disease), and thus these data can not be considered conclusive. It is possible that selection bias could lead to this association; for example, patients with larger volume neck disease may be more likely to undergo neck dissection and may be more likely to have neck tumor–related damage to adjacent normal tissues unrelated to the neck dissection. It is possible, though, that disturbance of the soft tissues of the neck via post-treatment neck dissection could cause added swallowing dysfunction, for example by increasing fibrosis in the neck and thus limiting the mobility of the laryngopharynx. It should be noted that a similar report of an association between severe late toxicity and post-treatment neck dissection was recently reported by researchers at Fox Chase Cancer Center.21 If these findings are validated in additional, larger data sets, there may be important implications with respect to the controversy regarding neck dissection after chemoradiotherapy for patients with advanced neck disease.
The lack of significant association between cumulative radiation therapy dose delivery (or chemotherapy dose delivery) and severe late toxicity may be due to the narrow dose range prescribed and the generally excellent compliance. We are currently analyzing the detailed radiation therapy records (simulation films, dosimetry, and treatment records) available at RTOG headquarters in order to estimate the doses received by individual normal tissue substructures within the head and neck. Several recent single-institution studies have rigorously analyzed the relationships between radiation therapy dose-volume histograms for normal structures and the risk and severity of toxicities.22,23
Considering the widespread acceptance of CCRT for SCCHN over the past 10 years, there are relatively few detailed studies of late toxicities. Groupe d’ Oncologie et de Radiotherapie de la Tete et du Cou reported long-term follow-up from their randomized trial of radiation therapy alone versus FU, carboplatin, and radiation therapy for oropharynx cancer; they did not find a significant difference in severe late toxicity.24 However, there were fewer than 50 long-term survivors in that study. Staar8 reported that 51% of long-term survivors (> 2 years) after a very intense combination of accelerated fractionation radiation therapy and chemotherapy were dependent on feeding tubes. With longer follow-up, that alarmingly high rate did decrease, and was not significantly worse than accelerated radiation therapy alone but the number of assessable patients was relatively small.25 Shiley reported that four (31%) of 13 cancer-free survivors (> 1 year) after chemoradiotherapy required tube feedings for some or all of their nutrition.26
These data suggest that the CCRT has reached the limits of acceptable long-term toxicity. Dose intensity can not be easily increased without some new and effective technique(s) of protection against late effects. In the future, these may include modern techniques in radiation therapy technology27,28 or biopharmacologic radioprotectors.29-31 Presently, however, these techniques have only succeeded in reducing xerostomia, not severe late dysphagia. Emphasis should therefore be on careful patient selection for aggressive treatment and swallowing exercises before during and after radiation therapy.32,33 Some patients may benefit from more invasive procedures, such as dilation of hypopharyngeal/esophageal stricture under anesthesia.
For some patient subpopulations the risks of CCRT may outweigh the benefits. For example, subgroup analysis of a meta-analysis suggested that there was no significant survival benefit to CCRT in patients older than age 70.1 Our data may add to the controversy regarding treatment of the elderly patient with head and neck cancer—if there is no significant survival benefit and a significant increase in late toxicity with concurrent chemoradiotherapy, should it be the standard of care in this patient population?
Our study has several limitations that should be discussed. First, it is a meta-analysis of three separate clinical trials, each of which had somewhat different eligibility criteria, chemoradiotherapy regimen, and year of activation. However, all of the patients did receive treatment that would be considered appropriate standard of care in today's oncology clinic. Second, our exclusion of patients with pre-existing severe laryngopharynx dysfunction from this analysis can be considered controversial. Although patients were excluded a priori, determining pre-existing severe laryngopharynx dysfunction is subjective. However, it should be noted that the determination of post-treatment severe laryngopharynx dysfunction (toxicity) is also subjective. It is extremely difficult to determine if severe dysfunction after treatment is the result of treatment or the result of the pre-existing cancer. By excluding patients with pretreatment severe laryngopharynx dysfunction, we attempted to isolate the influence of treatment on outcomes. Third, our study is an exploratory analysis; while it is one of the larger series on late toxicity after chemoradiotherapy, the number of patients and number of events are relatively small. We plan to address this in the future with an analysis of the recently completed trial, RTOG 0129. This was a randomized trial of standard fractionation versus accelerated fractionation radiation therapy (with cisplatin in both arms). Preliminary acute and subacute toxicity data showed no significant differences between the two arms.34 It is premature at this time to perform a detailed analysis of efficacy or late toxicity from that study. It is possible that with improved knowledge and experience with CCRT and supportive care available in the 21st century, outcomes may be improved in RTOG 0129 compared with historical controls.
Ultimately, it should be remembered that for most patients with head and neck cancer, the highest priority is cure and length of survival.35 Excessive concern about treatment toxicity should not prevent the use of proven aggressive multimodality treatment, provided the patient is well informed about the potential late sequelae of these aggressive treatment regimens.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Mitchell Machtay
Administrative support: Mitchell Machtay, Jennifer Moughan, K. Kian Ang
Provision of study materials or patients: Mitchell Machtay, Andrew Trotti, Adam S. Garden, Randal S. Weber, Jay S. Cooper, Arlene Forastiere, K. Kian Ang
Collection and assembly of data: Jennifer Moughan
Data analysis and interpretation: Mitchell Machtay, Jennifer Moughan, Andrew Trotti
Manuscript writing: Mitchell Machtay, Jennifer Moughan, Andrew Trotti, Adam S. Garden, Randal S. Weber, Jay S. Cooper, Arlene Forastiere, K. Kian Ang
Final approval of manuscript: Mitchell Machtay, Jennifer Moughan, Andrew Trotti, Adam S. Garden, Randal S. Weber, Jay S. Cooper, Arlene Forastiere, K. Kian Ang
Footnotes
published online ahead of print at www.jco.org on June 16, 2008.
Supported by Grants No. CA21661 and CA32115 from the National Cancer Institute and by a grant from the Commonwealth of Pennsylvania C.U.R.E. Program (Tobacco Settlement Act 77-2001).
Presented in part at the 42nd Annual Meeting American Society of Clinical Oncology, Atlanta, GA, June 2-6, 2006.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Bourhis J, LeMaitre A, Baujat B, et al: Individual patients' data meta-analyses in head and neck cancer. Curr Opin Oncol 19:188-194, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, Bourhis J, Domenge C, et al: Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: Three meta-analyses of updated individual data. Lancet 355:949-955, 2000 [PubMed] [Google Scholar]
- 3.Brizel DM, Albers MA, Fisher SR, et al: Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med 328:1798-1804, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Wendt TG, Grabenbauer CG, Rodel CM, et al: Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: A randomized multicenter study. J Clin Oncol 16:1318-1324, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Calais G, Alfonsi M, Bardet E, et al: Randomized study comparing radiation alone RT versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 91:2081-2086, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Adelstein DA, Lavertu P, Saxton JP, et al: Mature results of a phase III trial comparing concurrent chemoradiotherapy with radiation therapy alone in patients with stage III and IV squamous cell carcinoma of the head and neck. Cancer 88:876-883, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Olmi P, Crispino S, Fallai C, et al: Locoregionally advanced carcinoma of the oropharynx: Conventional radiotherapy vs. accelerated hyperfractionated radiotherapy vs. concomitant radiotherapy and chemotherapy – a multicenter randomized trial. Int J Radiat Oncol Biol 55:78-92, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Staar S, Rudat V, Stuetzer H, et al: Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy: Results of a multicentric randomized German trial in advanced head and neck cancer. Int J Radiat Oncol Biol Phys 50:1161-1171, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Budach V, Stuschke M, Budach W, et al: Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: Final results of the radiotherapy cooperative clinical trials group of the German Cancer Society 95-06 prospective randomized trial. J Clin Oncol 23:1125-1135, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bensadoun RJ, Benezery K, Dassonville O, et al: French multicenter phase III randomized study testing concurrent twice-a-day radiotherapy and cisplatin/5-fluorouracil chemotherapy (BiRCF) in unresectable pharyngeal carcinoma: Results at 2 years (FNCLCC-GORTEC). Int J Radiat Oncol Biol Phys 64:983-994, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Henk JM: Controlled trials of synchronous chemotherapy with radiotherapy in head and neck cancer: Overview of radiation morbidity. Clin Oncol (Royal Coll Radiol) 9:308-312, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Trotti A, Bellm LA, Epstein JB, et al: Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol 66:253-262, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Stuben G, Thews O, Pottgen C, et al: Recombinant human erythropoietin increases the radiosensitivity of xenografted human tumours in anaemic nude mice. J Cancer Res Clin Oncol 127:346-350, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forastiere AA, Goepfert H, Maor M, et al: Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091-2098, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Garden AS, Harris J, Vokes EE, et al: Preliminary results of Radiation Therapy Oncology Group 97-03: A randomized phase II trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. J Clin Oncol 22:2856-2864, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Ang KK, Harris J, Garden A, et al: Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: Radiation Therapy Oncology Group phase II trial 99-14. J Clin Oncol 23:3008-3015, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Garden AS, Pajak T, Vokes E, et al: Preliminary results of RTOG 9703: A phase II randomized trial of concurrent radiation (RT) and chemotherapy for advanced squamous cell carcinomas (SCC) of the head and neck. Proc Am Soc Clin Oncol 20:223a, 2001. (abstr 891) [DOI] [PubMed] [Google Scholar]
- 18.Kalbfleisch JD, Prentice RL: The Statistical Analysis of Failure Time Data. New York, NY, John Wiley & Sons, 1980, pp 167-169
- 19.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist 16:1141-1154, 1988 [Google Scholar]
- 20.Bentzen SM, Vaeth M, Pedersen DE, et al: Why actuarial estimates should be used in reporting late normal tissue effects of cancer treatment… now! Int J Radiat Oncol Biol Phys 32:1531-1534, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Lango M, Ende K, Ahmad S, et al: Neck dissection following organ preservation protocols prolongs feeding tube dependence in patients with advanced head and neck cancer. J Clin Oncol 24:286s, 2006. (suppl; abstr 5525) [Google Scholar]
- 22.Dornfeld K, Simmons JR, Karnell L, et al: Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys 68:750-757, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Eisbruch A, Schwartz M, Rasch C, et al: Dysphagia and aspiration after chemoradiotherapy for head and neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 60:1425-1439, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Denis F, Garaud P, Bardet E, et al: Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 22:69-76, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Semaru R, Mueller RP, Stuetzer H, Staar S, et al: Efficacy of intensified hyperfractionated and accelerated radiotherapy and concurrent chemotherapy with carboplatin and 5-fluorouracil: Updated results of a randomized multicentric trial in advanced head and neck cancer. Int J Radiat Oncol Biol Phys 64:1308-1316, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Shiley SG, Hargunani C, Skoner JM, et al: Swallowing function after chemoradiation for advanced stage oropharyngeal cancer. Otolaryngol Head Neck Surg 134:455-459, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Bucci MK, Bevan A, Roach MR: Advances in radiation therapy: Conventional to 3D, to IMRT, to 4D, and beyond. CA Cancer J Clin 55:117-134, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Feng FY, Kim HM, Lyden TH, et al: Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: Early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys 68:1289-1298, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kouvaris JR, Kouloulias VE, Vlahos LJ: Amifostine: The first selective-target and broad spectrum radioprotector. Oncologist 12:738-747, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ning S, Shuii C, Khan WB, et al: Effects of keratinocyte growth factor on the proliferation and radiation survival of human squamous cell carcinoma cell lines in vitro and in vivo. Int J Radiat Oncol Biol Phys 40:177-187, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Greenberger JS, Epperly MW: Review: Antioxidant gene therapeutic approaches to normal tissue radioprotection and tumor radiosensitization. In Vivo 21:141-146, 2007 [PubMed] [Google Scholar]
- 32.Mittal BB, Pauloski BR, Haraf DJ, et al: Swallowing dysfunction – preventative and rehabilitation strategies in patients with head and neck cancers treated with surgery, radiotherapy and chemotherapy: A critical review. Int J Radiat Oncol Biol Phys 57:1219-1230, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal DI, Lewin JS, Eisbruch A: Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol 24:2636-2643, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Ang KK, Pajak T, Rosenthal D, et al: A phase III trial to compare standard versus accelerated fractionation in combination with concurrent cisplatin for head and neck carcinomas (RTOG 0129): Report of compliance and toxicity. Int J Radiat Oncol Biol Phys 69, 2008. (suppl; abstr 21) [Google Scholar]
- 35.List MA, Stracks J, Colangelo L, et al: How do head and neck cancer patients prioritize treatment outcomes before initiating treatment? J Clin Oncol 18:877-884, 2000 [DOI] [PubMed] [Google Scholar]