Abstract
Background
Early childhood is characterized by dramatic gains in emotion regulation skills that support social adjustment and mental health. Understanding the physiological substrates of healthy emotion regulation may offer new directions for altering trajectories towards initiation and escalation of substance abuse. Here, we describe the intersections between parasympathetic and sympathetic tone, emotion regulation and prosocial behavior in a high-risk sample of preschoolers.
Method
Fifty-two 3 – 6 year old children completed an assessment of attention regulation in response to affective stimuli. Cardiac respiratory sinus arrhythmia, an index of parasympathetic tone, and pre-ejection period, a marker of sympathetic activation, were recorded at rest and while children engaged in social interactions with their mothers and an unfamiliar research assistant. Mothers reported on children’s emotional reactivity and prosocial behavior.
Results
Controlling for age and psychosocial risk, higher parasympathetic tone predicted better attention regulation in response to angry emotion and higher levels of prosocial behavior, whereas a reciprocal pattern of higher parasympathetic tone and lower sympathetic arousal predicted better attention in response to positive emotion and lower emotional reactivity. Children exposed to fewer risk factors and higher levels of maternal warmth were more able to sustain a high level of parasympathetic tone during interaction episodes.
Conclusions
Findings suggest that autonomic measures represent biomarkers for socio-emotional competence in young children. They also point to the importance of early experiences in the establishment of physiological regulation and the promise of family-based intervention to promote healthy emotion regulation and prevent substance dependence in high-risk populations.
Keywords: Preschool, autonomic system, vagal tone, socio-emotional development, emotion regulation
1. INTRODUCTION
In theories of developmental psychopathology, the strategies children use to overcome early developmental milestones become part of their personalities, guiding habitual responses to challenges across the lifespan. Children exposed to early adversity, including household stress, poverty, violence and maltreatment, are at increased risk for psychiatric morbidity, early drug use and substance dependence (Blanco et al., 2013; Chassin et al., 2013; Enoch, 2011; Sitnick et al., 2014). Moreover, higher levels of childhood adversity predict greater levels of dependence in adult substance users (Banducci et al., 2014). To understand heterogeneity in these outcomes and enhance preventative strategies, it is necessary to take a lifespan approach, focusing on the etiological mechanisms that progressively canalize some children’s development along these poor trajectories while other children remain resilient. Here, we focus on a critical developmental skill that typically begins to develop in early childhood, the ability to manage and effectively respond to emotion.
Emotion regulation is a multi-dimensional construct incorporating both the ability to modulate one’s own emotional arousal and intensity, and the recognition of and socially appropriate response to others’ emotions (Thompson, 1994). Theories of emotion regulation stress the importance of both bottom-up processing of emotional cues and top-down regulation of attention towards these cues (Cole et al., 1994). That is, both the experience of emotion and use of strategies to regulate emotions reflect adaptive responses of the organism to his/her environment (Kim and Cicchetti, 2010). Emotion regulation is also an important dimension of interpersonal competence, allowing an individual to gauge and adjust his/her level of emotional expressiveness according to social demands and expectations (Denham et al., 2003; Jones et al., 2013). Indeed, deficits in emotion regulation are a hallmark of psychopathology and, as emphasized throughout this volume, invariably accompany substance dependence (Aldao et al., 2010; Berking et al., 2011; Dorard et al., 2008).
The preschool years are a time of dramatic improvement in children’s ability to regulate their emotions (Kopp, 1989). During these years, youngsters shift from being primarily co-regulated in the context of close caregiver-child interactions to showing increased capacity for autonomous regulation (Cole et al., 1994). They become more proficient at recognizing expressions such as anger, fear and surprise (Smith and Walden, 1998). They also make gains in language skills that enable them to label emotions and better articulate and understand feedback regarding their own and others’ emotional states. Between 3 and 4 years of age, children are able to successfully navigate tasks that involve taking the perspectives of others (Carlson et al., 2013; Wimmer and Perner, 1983). Notably, the preschool period is also characterized by marked growth in higher-order cognitive control competencies (e.g., working memory, inhibitory control) that allow children to manage and coordinate attentional and behavioral responses to competing neural and sensory inputs (Clark et al., 2012; Garon et al., 2008). The convergence of these new skills enables children to implement complex strategies for managing emotion that foster their ability to engage in socially appropriate, empathic peer interactions by the time they reach kindergarten (Denham et al., 2003; Stansbury and Sigman, 2000).
Theory and research suggest that the environment plays a critical role in shaping these emotional competencies (Blandon et al., 2008; Feng et al., 2008). In infancy, primary caregivers act as emotional regulators through their responses to infant cues (Stern, 1977). Early caregiver-child interactions provide a training ground for emotion recognition and modulation: parents model, shape and reinforce children’s emotional responses and act as supports in times of emotional arousal (Eisenberg et al., 2009; Thompson, 1994; Tronick, 1989). Accordingly, sensitive, warm and responsive parenting is associated with more effective emotion regulation in young children, whereas harsh, negative and physically abusive parenting is associated with poor emotion regulation and dysregulated attention in the face of negative emotion (Calkins et al., 2001; Hastings et al., 2008; Maughan and Cicchetti, 2002; Pollak et al., 2005). More broadly, chronic exposure to environmental stress, unpredictability, and a negative emotional climate may lead children to develop ineffective or atypical regulatory strategies (Morris et al., 2007). Not only are children exposed to such stress more likely to encounter caregivers who provide models of ineffective emotion regulation, but chronic activation of physiological stress response systems may alter biological set-points for arousal, compromise the integrity of these systems, and condition atypical responses to emotional challenge or threat (Evans and English, 2002; Evans, 2003; Nederhof et al., 2015). In a recent neuroimaging study with adults, for example, childhood poverty was associated with reduced neural responses in brain regions that are central to emotion regulation – the dorso- and ventrolateral prefrontal regions (Kim et al., 2013). Importantly, the relation of childhood poverty to neural activation in these core emotional processing networks was mediated by chronic early stress exposure. Taken together, studies provide compelling evidence that a child’s environmental context shapes his/her bio-behavioral responses to emotion, with cascading implications for social competence and mental health.
Although there is widespread recognition of the importance of emotion regulation for healthy development, the construct has proven difficult to measure at a behavioral level, particularly during the preschool period when children’s regulatory skills are just emerging and emotional expression may fluctuate rapidly from one moment the next. Consequently, some researchers have employed physiological measures as indices of emotional arousal and regulation. In Porges’ (1995) polyvagal theory, the distinct branches of the mammalian autonomic nervous system evolved to serve different adaptive needs. The sympathetic nervous system, Porges contends, is a phylogenetically ancient system that theoretically mobilizes the body’s resources for fight or flight. Pre-ejection period (PEP), a measure of the time window between the depolarization of the cardiac ventricles to the opening of the aortic valve, is thought to capture this sympathetic influence, with shorter PEP indicative of greater sympathetic activation and accelerated heart rate (Alkon et al., 2003; Stern et al., 2001). The parasympathetic system is a more recently evolved system that theoretically supports effective social engagement, warmth, and proximity through the regulation of attention and the control of muscles involved in facial expressions that facilitate social engagement and communication (Porges, 1998; 2001). Respiratory sinus arrhythmia (RSA), a measure of beat-to-beat variability in heart rate across the respiration cycle, provides an approximation of the parasympathetic influence of the vagus nerve on the heart. Via its connections to the heart’s sinostriatal node, the myelinated vagus presumably is able to inhibit sympathetic activation, conserve metabolic resources, and promote calm and self-soothing in non-threatening contexts (Porges, 2007). Through dynamic engagement or withdrawal of vagal influence on heart rate, the parasympathetic system can respond quickly to changing demands for vigilance or socio-emotional engagement. Importantly, the vagus has numerous afferent and efferent inputs from areas in the limbic system and ventromedial frontal cortex that are fundamentally involved in emotion regulation (Berntson et al., 2007; Thayer and Lane, 2000).
Recent meta-analytic findings provide support for polyvagal theory, showing that higher resting RSA is associated with increased levels of empathy, positive emotional expression and sustained attention, whereas lower resting RSA is associated with higher levels of disruptive and aggressive behavior (Graziano and Derefinko, 2013). Moreover, in a few recent studies, these associations have proven quite specific, with RSA relating to children’s emotional or reward-related regulation as opposed to measures of cognitive control in non-emotional contexts (Conradt et al., 2014). Although there has been considerably less attention to sympathetic influences on emotion regulation early in life, low sympathetic activation appears to confer risk for antisocial behavior, attention deficits and reward insensitivity (Beauchaine et al., 2013; Crowell et al., 2006; Muñoz and Anastassiou-Hadjicharalambous, 2011), whereas high sympathetic activation is associated with anxious and inhibited behavior (Scarpa et al., 1997).
Studies have seldom considered the additive or interactive influence of parasympathetic and sympathetic branches of the autonomic nervous system in young children, an important oversight given that the two systems can act independently and that both systems influence heart rate (Alkon et al., 2003; Cacioppo et al., 1994; Quigley and Stifter, 2006). Berntson and colleagues (1991) proposed that the autonomic system can act in one of five ways: 1) reciprocal parasympathetic activation is characterized by parasympathetic activation coupled with sympathetic withdrawal; 2) reciprocal sympathetic activation is the inverse of this; 3) co-activation involves activation in both parasympathetic and sympathetic systems; 4) co-inhibition involves decreased activity in both autonomic branches, whereas 5) uncoupled activation involves activation in one system that is uncorrelated with activation in the other. It is likely that these distinct patterns have implications for emotional dysregulation and social competence. In a study by El Sheikh et al. (2009), for instance, co-inhibition and co-activation in response to emotion induction both were associated with increased problem behavior in high-risk adolescents, whereas reciprocal parasympathetic activation was associated with the lowest levels of problem behavior.
In sum, the establishment of core regulatory processes during the preschool years forms the foundation for social competence, deficits in which are centrally implicated in substance use trajectories (e.g., Lynne-Landsman et al., 2010). Delineation of the physiological correlates of emotion regulation in preschoolers facing high levels of adversity may help to identify risk biomarkers and inform approaches to substance abuse prevention. In this study, we drew on polyvagal theory as a framework for understanding the intersections between physiological regulation, emotional regulation and socio-emotional competence in young children at risk. Specifically, our first aim was to examine the relation of children’s autonomic response patterns to their emotion regulation and prosocial behavior. In keeping with the proposal that the ventral vagal system is integral to emotional regulation and expression, we postulated that higher parasympathetic tone (i.e. higher RSA) would be associated with a greater capacity to regulate attention in response to emotion, reduced negative reactivity in response to emotion and with the display of positive, prosocial behavior. In line with El Sheikh et al. (2009), we also predicted that co-inhibition, characterized by low activation of both autonomic branches (i.e., low RSA and long PEP), would predict less optimal emotional regulation and reduced prosocial behavior. Our second aim was to examine the relations of children’s socio-familial experiences (i.e., exposure to cumulative psychosocial risk and experience of warm/affiliative parenting) to their autonomic and behavioral regulation of emotion. We hypothesized that children with exposure to higher levels of cumulative psychosocial risk and lower parental warmth/affiliation would show poorer regulation and lower parasympathetic tone.
2. METHOD AND MATERIALS
2.1 Participants
Fifty-two mothers and their preschool-aged children from high adversity backgrounds were recruited through Child Welfare and Early Head Start, as well as through an ongoing study of mothers who had been involved with Child Welfare Services as children. Interested mothers contacted a university-based laboratory and were carefully screened to ensure that they met study inclusion criteria: having primary custody of a child in the preschool age range at the time of enrollment, no mother or child history of head trauma or congenital disorder, maternal age > 18 years, and fluency in English. At a mean age of 30.81 (SD = 6.81) years, the majority of mothers (64.8%) were unmarried and 11.5% had not completed high school. Maternal ethnicity breakdown was 69% Caucasian, 12% Hispanic, 2% African American, 2% Native American, 15% Mixed/Multiple Race. Children had a mean age of 4.27 (SD = .88) years and 46% were male. Child ethnicity breakdown was 56% Caucasian, 27% Hispanic, and 17% Mixed Race. This was a particularly disadvantaged sample, with 92% of mothers meeting criteria for state financial support (i.e., social security, Food stamps, TANF, unemployment or free lunch) and 87% receiving state support for medical care (i.e., Medicaid, CHIP or Social Security). Average household income was $2,207 (SD =1,139) per month before taxes.
2.2 Procedure
A university institutional review board approved all study procedures. Study activities were completed during a single laboratory visit lasting approximately 3 hours. After mothers had provided written informed consent, both mother and child were fitted with three disposable Bionomadix electrocardiogram (ECG) electrodes in a modified Lead II placement that included the right clavicle, lower left rib cage and lower right abdomen. Eight additional electrodes were placed in a tetrapolar configuration of electrode pairs on either side of the neck and the sternum to record cardiac impedance. These electrodes transmitted continuous ECG data via a Biopac MP-150 wireless system (BioNomadix, Biopac) at a sampling rate of 1000 Hz. A research assistant monitored physiological signals from a separate room throughout the session to ensure data integrity.
After a cardiac signal had been established, dyads were seated separately in a dimly-lit room and encouraged to relax while they watched a 5-minute relaxing video (“Baby Einstein”). This provided resting baseline measures for both RSA and PEP. Following the baseline recording, children participated in two joint interaction episodes of identical format, administered in counterbalanced order across families (based on Fries et al., 2005). One joint interaction was completed with the mother. The other was conducted with an unfamiliar female research assistant. For each joint interaction, the child was seated on the adult’s lap in front of a computer monitor. The monitor provided instructions for a series of interactive activities for the dyad to complete, including counting each other’s fingers, pointing to parts of each other’s faces (i.e., nose, hair, ears) and whispering a story to each other. All instructions were presented for fixed time intervals and the story told by the research assistant was always the same.
After completing the joint interactions, mothers and children each completed a series of tasks in separate rooms. The child tasks were administered in a fixed sequence interspersed with 1-minute rest periods. Regular breaks and snacks were provided. Mothers completed questionnaires and interviews during the break intervals and/or at the end of the laboratory session. Mothers were paid upon completion of the visit and children received stickers and toys as compensation for their participation in the study.
2.3 Measures
2.3.1 Cumulative Psychosocial Risk Index
Mothers completed a comprehensive demographic interview including questions regarding socio-demographic circumstances, financial and residential stressors and stressful life events. Based on their responses, we calculated a multi-measure index of cumulative risk across socio-demographic, physical environment and psychosocial domains (e.g., Evans and Kim, 2007). Risk was scored discretely as present (1) or not present (0) for each of the following factors: 1) mother did not complete high school, 2) mother is a single parent, and 3) household income to needs ratio below 1.0. Additional scores of 1 were allocated if families fell into the top quartile for questions regarding of the following stressors: 4) household crowding and 5) housing problems 6) parent-child separation events, 7) family turmoil events, and 8) exposure to violence. Scores were summed to create a cumulative risk index ranging from 0 to 8.
2.3.2 Autonomic Physiology
Complete cleaning and processing of ECG and cardiac impedance data was performed offline using MindWare 3.10 software (MindWare Technologies, Ltd., Gahanna, OH). Trained research assistants visually inspected all data to ensure that R spikes were conclusively identified. The cardiac impedence wave provided an estimate of respiration and was used to ensure that the frequency of respiration was within the range necessary for RSA calculation (Berntson et al., 2007). The RSA data were Fast-Fourier transformed to decompose component frequencies, with the frequency band set to .24–1.04 Hz for children to capture high frequency variability. Spectral densities were computed from 30 second epochs (Berntson, 1997). PEP was calculated by superimposing the ECG on the 30 second ensemble average of the impedance wave (derivative of the Z0 wave) and calculating the time between the Q point on the ECG and the B point on the impedance wave (Lozano et al., 2007). Trained research assistants entered the electrode distance and visually inspected the all data to ensure that the Z and R wave points identified by MindWare software did not deviate substantially over the course of successive intervals and that calculated PEP was within a feasible range (80–130 s). The RSA and PEP values for each 30 second epoch were averaged for the resting baseline, maternal-child joint interaction and examiner-child joint interaction tasks, respectively. Scatter plots were used to ensure that RSA and PEP data were normally distributed. Complete RSA data were available for 90% of children and PEP was available for 94% of the mother-child episodes and 92% of the researcher-child episodes, with remaining data unable to be scored due to artifact, inability to calculate RSA or PEP, or equipment failure.
2.3.3 Behavioral Measures
2.3.3.1 Emotion Regulation
An Emotional Oddball Task (adapted from Pollak et al., 2001), programmed in E-prime, provided a measure of attention regulation in response to emotional stimuli. In blocks 1 and 4, children were instructed to press a button in response to happy faces and inhibit responses to neutral or angry emotions, whereas in blocks 2 and 3, children were instructed to respond only to angry faces. Faces were chosen from the NIMSTIM (Tottenham et al., 2009) facial stimulus set and were equated for the number of open and closed-mouth expressions. Based on pilot data suggesting that young children were unable to respond at quicker presentation rates, stimulus presentation was set at 1 second with an inter-stimulus interval of 250ms. Blocks 1 and 2 each consisted of 18 trials with happy and angry expressions occurring with equal frequency. Blocks 3 and 4 consisted of 20 trials each (8 neutral faces, 8 go trials and 4 non-target emotions). The task showed good reliability in this sample (Cronbach’s α = .88; Spearman Brown split half = .76). The dependent variable was d′ (standardized hits – false alarms) averaged across the happy and angry blocks respectively. Although task accuracy and d′ were highly correlated (r = .82, p < .001), d′ was selected on the basis that it takes into account that children can achieve relatively high accuracy even when they incorrectly respond to all non-target stimuli. Data for 5 children were excluded because their d′ values were below 0, suggesting that had misunderstood the task.
2.3.3.2
The Child Behavior Checklist (CBCL; Achenbach and Rescorla, 2000) Emotional Reactivity Scale was used to assess children’s emotional reactivity. The CBCL is a diagnostic checklist designed to identify symptoms of psychopathology in young children. The Emotional Reactivity scale includes items such as, “rapid shifts between sadness and excitement” or “sudden changes in mood or feelings”. Items are rated on a scale of 0 (not true) to 2 (very/often true). The CBCL is one of the most widely used measures of child behavior problems and its 7-factor structure has been validated in international samples (Ivanova et al., 2010). Test re-test reliability over 8 days for the normative sample was .87 and inter-rater agreement for mothers and fathers was .64 (Achenbach and Rescorla, 2000). Chronbach’s α in our sample was .77.
2.3.3.3 Prosocial Behavior
Mothers rated their children’s levels of prosocial warmth/affiliation using the Structural Analysis of Social Behavior (SASB) – Intrex scale (Benjamin, 2000). The SASB is a comprehensive circumplex model of interpersonal behavior, with the left-right warmth/affiliation dimension ranging from hate/attack to active love and affiliation (Benjamin, 1974). The SASB circumplex model has been validated in clinical and non-clinical populations (Lorr and Strack, 1999; Monsen and Lippe, 2007; Pincus et al., 1998) and the Intrex scales used in this study show good internal consistency (Benjamin, 1974; Lorr and Strack, 1999). Ratings are completed on a scale of 0 (never/not at all) to 100 (always/perfectly). Here, we used the intransitive surface, which reflects typically child-like responses towards others.
2.3.3.4 Maternal Warmth/Affiliation
Mothers also rated their own levels of warmth and affiliation towards their child on the SASB-Intrex using the transitive surface, which reflects behavior directed towards the child.
2.4 Statistical Methods
Data analysis was performed in SAS 9.3 and SPSS 19 and involved four major phases. In phase 1, we examined descriptive statistics and physiological responses to the joint interaction episodes. In phase 2, we used robust regression analysis with M estimation (Huber, 1973) to examine the predictive relations of cumulative psychosocial risk, baseline RSA and baseline PEP to measures of emotional regulation and prosocial behavior. Robust regression provides a useful alternative to ordinary least squares regression, particularly in small sample sizes, because it down-weights the influence of outliers on parameter estimates (Rousseeuw and Leroy, 1987). Interactions were tested by multiplying the centered RSA and PEP variables and entering these into the regression models. Models were constructed using a backwards trimming approach, with α set at .05. In phase 3, we examined the relation of autonomic reactivity, measured during the joint interaction tasks, to measures of child emotion regulation and prosocial behavior. RSA and PEP measures collected during the joint interaction episodes were entered into robust regression models simultaneous with baseline RSA and PEP scores. Here, pertinent model estimates reflect the predictive value of joint episode RSA or PEP scores over and above scores collected during the resting baseline period. In phase 4, we examined the relations of a) cumulative psychosocial risk and b) maternal warmth/affiliation to children’s baseline RSA and PEP, as well as to RSA and PEP responses to the joint episodes. For the latter models, the familial factors were entered after accounting for baseline RSA and PEP, with the joint task RSA and PEP scores used as dependent variables. Child age was covaried in all analyses, as it is related both to vagal measures and to attention task performance.
3. RESULTS
3.1 Descriptive Analysis of Cardiac Physiology and Behavioral Measures
Table 1 provides the descriptive statistics for all measures included in analyses. Of note, paired samples t-tests indicated that children generally showed an expected drop in average RSA relative to baseline when interacting with their mothers [t(47) = 6.39, p <.001] and with the unfamiliar research assistant, t(47) = 5.08, p <.001. PEP, on the other hand, showed only a trend toward an increase during the joint task with mothers [t(48) = 1.71, p = .093] and showed no significant change during the joint episode with the researcher relative to baseline, t(47) =−1.15, p = .256.
Table 1.
Descriptive Statistics for Child Autonomic Measures, Child Task Performance and Maternal Ratings of Child Behavior
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Child Autonomic Measures | ||||
| Baseline RSA | 6.24 | 1.23 | 3.89 | 9.74 |
| Baseline PEP | 88.69 | 8.28 | 70.80 | 103.10 |
| RSA Joint Task with Mom | 5.68 | .98 | 2.97 | 8.50 |
| RSA Joint Task with RA | 5.80 | 1.09 | 2.61 | 8.72 |
| PEP Joint Task with Mom | 89.55 | 8.67 | 70.10 | 106.8 |
| PEP Joint Task with RA | 89.14 | 8.57 | 69.00 | 103.38 |
| Child Emotional Regulation | ||||
| Oddball task Happy blocks d′ | 1.69 | 1.04 | 0.13 | 3.56 |
| Oddball task Angry blocks d′ | 1.61 | 0.69 | 0.12 | 2.60 |
| CBCL Emotional reactivity | 4.04 | 3.17 | 0.00 | 13.00 |
| SASB Child prosocial behavior | 139.11 | 46.46 | −8.62 | 207.86 |
| Socio-Familial Context | ||||
| Cumulative Risk Score | 3.02 | 1.79 | .00 | 7.00 |
| SASB Maternal warmth/affiliation | 152.11 | 39.06 | −.54 | 143.25 |
3.2 Intersections between Resting Parasympathetic and Sympathetic Tone and Children’s Emotion Regulation
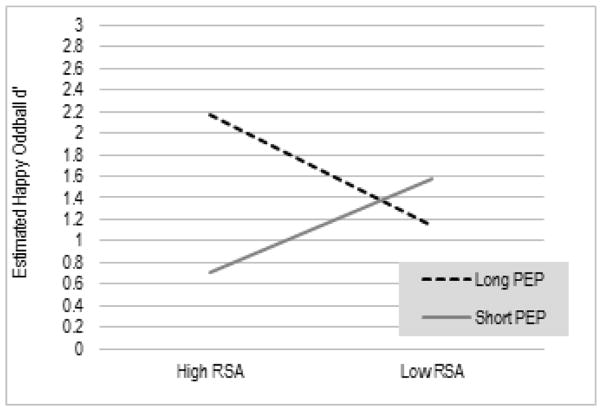
Table 2 shows the correlations of RSA and PEP values collected during the baseline rest period and joint task episodes with all child outcome variables. The relations of baseline RSA and PEP to each of the behavioral outcomes were assessed using a series of robust regression models. Cumulative psychosocial risk scores did not predict performance on any measure. After accounting for child age and cumulative risk, resting RSA and PEP scores interacted to predict children’s performance on the happy blocks of the Emotional Oddball task, EstRSA (SE) = .02 (.11), p = .84, EstPEP = .03 (.02), p = .12, EstRSA x PEP = .05 (.02), p = .009; R2 = .37. This interaction is illustrated in Figure 1, which shows that children with a reciprocal parasympathetic activation pattern, characterized by higher baseline parasympathetic tone and lower baseline sympathetic activation, were better able to attend and respond to positive emotional stimuli. Conversely, children who showed a co-activation pattern of high parasympathetic tone coupled with high sympathetic activation performed less well on the happy trials of this emotion regulation task.
Table 2.
Correlations between autonomic measures, behavioral measures of child emotion regulation and prosocial behavior, and socio-familial characteristics
| Episode
|
||||||
|---|---|---|---|---|---|---|
| Baseline RSA | Baseline PEP | RSA with mom | PEP with mom | RSA with RA | PEP with RA | |
| Oddball task happy blocks d′ | .05 | .30 | .07 | .18 | .14 | .25 |
| Oddball task angry blocks d′ | .44** | −.15 | .35* | −.20 | .42** | −.20 |
| CBCL Emotional reactivity | −.22 | .27+ | −.28+ | .40** | −.25 | .37* |
| SASB Child prosocial behavior | .30+ | .06 | .39* | .10 | .38* | .02 |
| Cumulative risk | .14 | .01 | −.04 | .01 | −.02 | .02 |
| SASB Maternal warmth/affiliation | .02 | .25 | .18 | .29+ | .05 | .27+ |
RSA: Respiratory sinus arrhythmia; PEP: Pre-ejection period; RA: Research assistant
Figure 1.
Interaction between RSA and PEP in relation to children’s performance on happy Emotional Oddball Task trials
Note: Estimates are based on RSA and PEP scores > 1 SD above and < 1 SD below the mean.
For the angry blocks of the emotion regulation task, there were no interactions between RSA and PEP. However, there was a main effect of RSA. Specifically, children with higher baseline RSA were better able to discriminate angry emotions, Est (SE) = .20 (.07), p = .004, R2 = .48. In sum, findings for this emotion regulation task indicate that children who show greater parasympathetic tone are better able to focus attention in response to negative, angry emotion, whereas children who display a reciprocal pattern of high parasympathetic tone coupled with low sympathetic activation are better able to focus attention in response to positive emotion.
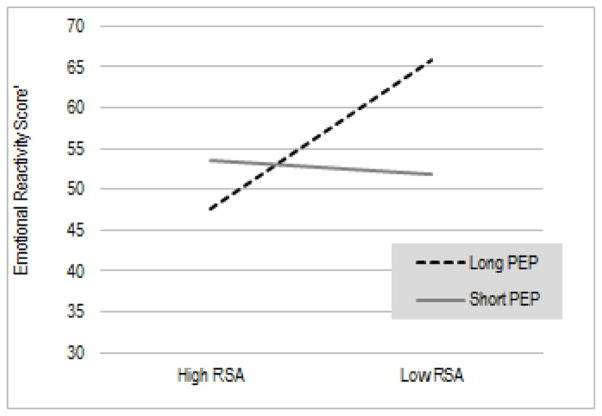
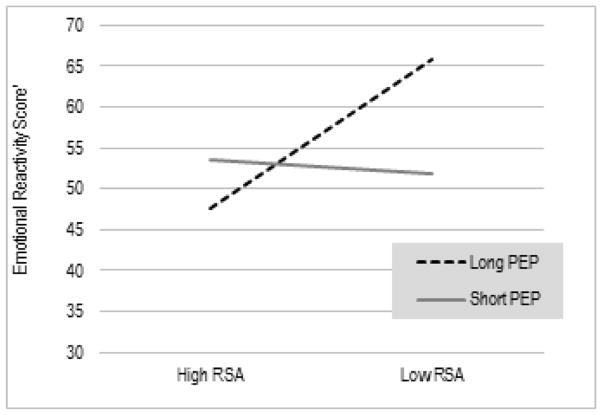
Another significant interaction emerged for maternal reports of children’s emotional reactivity on the CBCL. As illustrated in Figure 2, here again, high baseline parasympathetic tone was most optimal when accompanied by low baseline sympathetic activation, as children with this pattern of reciprocal parasympathetic activation showed the lowest levels of emotional reactivity, EstRSA (SE) = −.74 (.29), p = .007, EstPEP = .09 (.04), p = .03, EstRSA x PEP = −.11 (.05), p = .01; R2 = .40. Conversely, children with a co-inhibition pattern, characterized by low parasympathetic and low sympathetic activation, were rated by their mothers as most emotionally reactive.
Figure 2.
Interaction between RSA and PEP in relation to children’s parent-rated emotional reactivity
Note: Estimates are based on RSA and PEP scores > 1SD above and < 1 SD below the mean. Higher scores indicate greater difficulties regulating emotional reactivity.
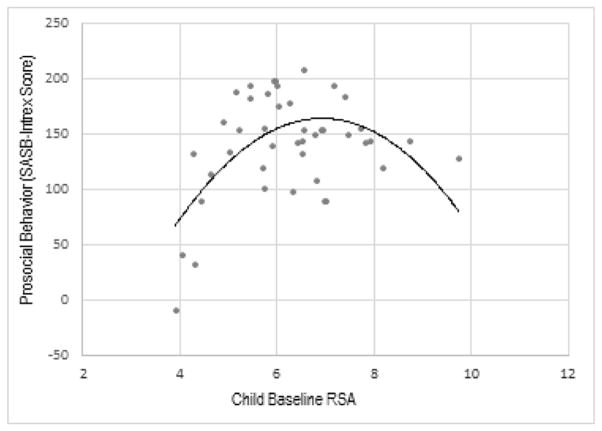
Finally, in terms of children’s prosocial behavior, there was a quadratic effect of RSA, EstRSA (SE) = 15.05 (5.07), p = .003, EstRSA x RSA (SE) = −9.39 (2.86), p = .001 (see Figure 3). That is, children with the lowest baseline RSA scores showed the lowest levels of prosocial behavior, whereas children with moderately high baseline RSA showed the highest levels of prosocial behavior. Overall then, higher RSA and a pattern of reciprocal parasympathetic activation were associated with better emotion regulation and increased levels of prosocial behavior in this preschool sample.
Figure 3.
Quadratic relation of resting RSA to child prosocial behavior
3.3. Autonomic Responses to Social Interaction, Emotion Regulation and Prosocial Behavior
The correlations in Table 2 indicate that there were some associations between children’s RSA and PEP scores collected during the social interaction episodes and their levels of emotion regulation, emotional reactivity, and prosocial behavior. In the next set of analyses, we examined whether autonomic reactivity during social interactions with mother and with a female research assistant was related to each of these socio-emotional outcomes. Specifically, we examined the relation of residual RSA and PEP scores during social interaction to children’s emotion regulation and prosocial behavior after accounting for child age, cumulative risk and baseline RSA and PEP scores. Controlling for baseline RSA and PEP, children with lower parasympathetic tone [Est (SE) = −4.81 (2.21), p = .030] and those with lower sympathetic activation [Est (SE) = .82 (.26), p = .001] during the maternal-child joint episode were rated by their mothers as being more emotionally reactive. There were no other associations between children’s autonomic responses to joint interaction and their emotion regulation or prosocial behavior.
3.4 Relations of Children’s Contextual Experiences with their Autonomic Regulation
In the final set of analyses, we examined whether children’s socio-familial experiences, including their exposure to cumulative risk and their experience of warm, sensitive interactions with their mothers, were associated with autonomic measures. A series of robust linear regression models showed that cumulative risk was not associated with child baseline parasympathetic (RSA Est (SE) = .13 (.11), p = .223) or sympathetic tone (PEP Est (SE) = −.13 (.80), p = .869) after controlling for child age. However, children with higher levels of cumulative risk showed lower parasympathetic tone during both of the joint social interaction tasks after controlling for baseline RSA, Estmother-child (SE) = −.08 (.04), p = .025; Estresearch assistant-child (SE) = −.09 (.04), p = .037. This effect is illustrated in Figure 4, which shows that children with greater levels of cumulative risk showed greater RSA withdrawal during the joint interactions with both their mothers and with strangers. Importantly, follow-up scatter plots revealed that this was a linear effect, where children who experienced the least cumulative risk showed RSA augmentation during the joint episodes.
Figure 4.
Changes in RSA from baseline to interaction with a) mother and b) with a research assistant (RA) in children with low and high cumulative risk scores.
For illustrative purposes, children are grouped by high (> 3 risk factors) and low (3 or fewer risk factors) cumulative risk scores. Error bars represent standard errors of the mean. While cumulative risk was not associated with baseline RSA, children with higher levels of cumulative risk showed a greater drop in RSA during the social interaction episodes.
A similar pattern emerged in models examining the relation of maternal warm/affiliative behavior in relation to child autonomic measures. Maternal warmth was not associated with child baseline parasympathetic (Est (SE) = .01 (.01), p = .859) or sympathetic tone, Est (SE) = −.01 (.07), p = .996. However, after accounting for baseline RSA, higher levels of maternal warmth were associated with higher child parasympathetic tone during the mother-child joint interaction, Est (SE) = .01 (.01), p = .033. There was no relation of maternal warmth to children’s parasympathetic tone during the joint task with the research assistant, Est (SE) = .00 (.00), p = .426.
4. DISCUSSION
Early-emerging deficits in emotion regulation are considered a primary risk factor for future substance dependence (Kober, 2014). Understanding the biological substrates of emotion regulation may therefore provide greater leverage for interventions to disrupt substance use trajectories. In this study, we examined the physiological correlates of emotion regulation and interpersonal competence in a high-risk sample of preschoolers. The key findings to emerge from the study are that children’s patterns of autonomic regulation predict their competence in fundamental aspects of emotion regulation: attention to emotional stimuli, modulating their own emotional reactivity, and responding with warm, prosocial affiliation toward others. Moreover, children’s early experiences, in the form of cumulative psychosocial risk and levels of maternal warmth, are reflected in their patterns of physiological reactivity during social interactions. These findings support current theories regarding the role of autonomic function in emotion regulation (Porges et al., 1994; Thayer and Lane, 2000), with important implications for prevention science.
Higher parasympathetic tone generally emerged as a correlate of stronger emotion regulation in this preschool sample, although in some cases children’s levels of sympathetic activation moderated this association. Children with higher resting parasympathetic tone showed better regulation of attention in the face of negative emotion, as measured by the angry trials of the Emotional Oddball task, which is in keeping with several studies indicating that high resting RSA correlates with multiple positive outcomes in children (Beauchaine, 2001; Graziano and Derefinko, 2013). With respect to children’s ability to regulate attention to positive, happy emotion, the picture was more complex. Here, children who performed most accurately during the happy trials of the Emotional Oddball task showed a reciprocal pattern of autonomic physiology, characterized by higher resting parasympathetic tone and lower sympathetic tone. Similarly, children whose mothers reported them as having lower levels of emotional reactivity also showed this reciprocal autonomic pattern. This pattern of high parasympathetic tone coupled with low sympathetic arousal may be particularly conducive to emotional regulation because it signifies a calm physiological state with low heart rate that fosters an ability to focus and attend in the context of minimal threat (El-Sheikh and Erath, 2011). Conversely, a pattern of co-inhibition, characterized by low activation of both the parasympathetic and sympathetic systems when at rest, predicted higher levels of emotional reactivity. In previous studies, this co-inhibition pattern has been shown to be a vulnerability factor for children exposed to high levels of parental conflict, increasing the propensity to externalizing behavior (El-Sheikh et al., 2009). This co-inhibition pattern may signify a failure of both autonomic branches to regulate physiological homeostasis, leading to deficient emotion regulation.
There was also a positive relation between parasympathetic tone and prosocial behavior, although this relation was U-shaped, where children with moderately high resting RSA were rated as most prosocial. This positive association between RSA and prosocial behavior is in keeping with theoretical postulations that vagal tone is particularly important for interpersonal behavior and that it allows for sustained attention in the context of safe social interactions (Porges and Furman, 2011; Porges, 2001). In a set of studies with adults, Kogan et al. (2014) also found evidence for a U-shaped relation of vagal reactivity to prosocial behavior (see also Beauchaine et al., 2007; Marcovitch et al., 2011 for similar quadratic relations to RSA withdrawal). Kogan et al. (2014) contend that some degree of sympathetic arousal (i.e., moderate parasympathetic tone) is necessary to effectively demonstrate empathy for others while maintaining a calm, attentive state. This may be a plausible explanation for our results as well.
Contrary to expectations, cumulative psychosocial risk did not relate to performance on any of the behavioral measures of emotion regulation. However, children exposed to fewer risk factors and to higher levels of maternal warmth maintained higher levels of parasympathetic tone in response to the joint interaction episodes, whereas greater risk exposure predicted greater parasympathetic withdrawal during social interaction. In sum, different social experiences were associated with different physiological regulation strategies during social interaction. There are opposing findings regarding parasympathetic reactivity, with some studies suggesting that parasympathetic withdrawal during laboratory tasks reflects a positive, adaptive response to challenge and others suggesting that excessive parasympathetic withdrawal is indicative of dysregulation (Beauchaine, 2015; Calkins and Keane, 2004). In safe interpersonal environments, however, polyvagal theory would suggest that high parasympathetic tone is conducive to increased social engagement, whereas parasympathetic withdrawal may reflect greater physiological arousal and an anxious or defensive stance. Consistent with these assumptions, our data indicate that a predictable, warm and safe home environment likely supports higher levels of parasympathetic tone during social tasks, whereas less predictable, high-risk environments may condition a more reactive, vigilant approach to social interaction.
Findings have important implications for prevention science and, in particular, for drug abuse prevention. Children who lack effective regulatory skills are more likely to experience early academic and behavioral problems, entering school with deficits that tend to grow larger over time as they fall further behind their peers (Espy et al., 2011; La Paro and Pianta, 2000) and putting them on a trajectory of heightened risk for early onset drug and alcohol abuse problems (Hawkins et al., 1992). Indeed, in one study, deficits in preschool self-regulation, including high emotional lability and low frustration tolerance, predicted higher substance use in adulthood (Moffitt et al., 2011). There is some suggestion too that adults with substance dependence exhibit reduced resting parasympathetic tone (Ingjaldsson et al., 2003) and that reduced parasympathetic tone in these adults is associated with less ability to modulate alcohol craving (Eddie et al., 2014; Quintana et al., 2013). In addition, blunted sympathetic response to reward has been related to increased rates of substance use in adolescents (Brenner and Beauchaine, 2011; Evans et al., 2013). Of course, longitudinal studies will be necessary to determine whether patterns of autonomic function in preschoolers represent risk biomarkers for future substance dependence. However, the current study represents an important first step in establishing these associations, as it suggests that emotion regulation has a physiological signature as early as the preschool years and that children from households with a higher levels of risk already show deviations in their physiological response patterns.
The relation of environmental factors to children’s physiological responsiveness in our study also suggests that there may be some leverage in family-based interventions to promote physiological regulation and prevent early initiation of substance abuse. In one set of studies, lower baseline RSA predicted greater responsiveness to parent-child interaction training (Bagner et al., 2012; Graziano et al., 2012). Conceivably, there is scope for incorporating autonomic metrics into the process of parent-child interaction coaching through real-time visualization of child physiological activity in response to parent-child interactions. Such real-time feedback might be useful in tailoring parent behavior to child levels of arousal. Others have incorporated autonomic measures into kindergarten-based interventions to reduce externalizing behavior, finding that vagal tone predicts children’s responsiveness to intervention (Beauchaine et al., 2013; Gatzke-Kopp et al., 2013). While these studies hint at the utility of autonomic measures as moderators or end-points for intervention, our findings suggest that additional consideration of sympathetic markers will be relevant in the future, as parasympathetic and sympathetic tone jointly predicted different patterns of socio-emotional competence and emotion regulation in our high-risk sample.
Findings should be interpreted with the caveat that the sample is small and the majority of the sample was facing significant economic and familial challenges. To fully determine the relation of environmental risk to children’s emotion regulation, we would require greater variability in exposure to adverse experiences. Even within this relatively constrained sample, though, autonomic measures related meaningfully to child risk exposure and outcome measures. Additionally, we did not measure respiration directly in this study and there have been suggestions that respiration may a confounding factor in the measurement of parasympathetic tone (Denver et al., 2007). Finally, we focused here on physiological data from baseline and the joint interaction episodes. Although physiological recordings during children’s attention task performance are pending, it is likely that there will be a greater loss of data for tasks administered later in the assessment session.
This study is unique in its consideration of both sympathetic and parasympathetic contributions to emotion regulation in the preschool period. Critically, the study demonstrates the intersecting implications of these systems for children’s emerging emotional competence. Emotion regulation forms the cornerstone of social competence and has been repeatedly implicated in pathways to early substance use. Interventions that target autonomic reactivity and enable children to meet the developmental challenge of establishing healthy emotion regulation strategies offer great promise for fostering resilience and preventing substance dependence in children at risk.
Highlights.
Higher parasympathetic tone predicts prosocial competence in at risk preschoolers
Parasympathetic and sympathetic measures reciprocally predict emotion regulation
Children at lower social risk had higher parasympathetic tone during social interactions
Early familial experiences may shape the physiological regulation of emotion
The autonomic system is a promising target for substance use prevention efforts
Acknowledgments
This project was supported by start-up funds and by a National Institutes of Health Research grant R01 MH079328 awarded to Elizabeth A. Skowron, as well as P50DA035763, R01HD075716, and P50MH078105 to Philip A. Fisher and 5P50DA035763-03 to Leslie Leve and Philip A. Fisher. We are grateful to members of the University of Oregon Prevention Science Institute, including Rose Jeffries and Mora A. Reinka, for their assistance with data collection, scoring, and coding. Special thanks to the many families who were willing to share their time and experiences to contribute to this research.
Footnotes
Author Contributions
All authors have contributed substantially to study completion and the preparation of this article.
Caron Clark assisted with study design and data collection and completed the analysis and preparation of the manuscript.
Elizabeth Skowron conceptualized the study and helped to prepare the manuscript.
Ryan Guiliano assisted with data collection and study design and reviewed the manuscript, providing editorial input.
Phil Fisher provided consultation on the design of the study, assisted with participant recruitment, and provided feedback and editorial guidance regarding this manuscript.
The authors have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T, Rescorla L. Manual For The ASEBA Preschool Forms And Profiles. University of Vermont; Burlington: 2000. [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin Psychol Rev. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Dev Psychobiol. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Bagner DM, Graziano Pa, Jaccard J, Sheinkopf SJ, Vohr BR, Lester BM. An initial investigation of baseline respiratory sinus arrhythmia as a moderator of treatment outcome for young children born premature with externalizing behavior problems. Behav Ther. 2012;43:652–65. doi: 10.1016/j.beth.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banducci AN, Hoffman E, Lejuez CW, Koenen KC. The relationship between child abuse and negative outcomes among substance users: psychopathology, health, and comorbidities. Addict Behav. 2014;39:1522–1527. doi: 10.1016/j.addbeh.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T. Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Curr Opin Psychol. 2015;3:43–47. doi: 10.1016/j.copsyc.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol Psychol. 2007;74:174–84. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T, Gatzke-Kopp L, Neuhaus E, Chipman J, Reid MJ, Webster-Stratton C. Sympathetic- and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. J Consult Clin Psychol. 2013;81:481–93. doi: 10.1037/a0032302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LS. Intrex User Manual. University of Utah; Salt Lake City, UT: 2000. [Google Scholar]
- Benjamin LS. Structural analysis of social behavior. Psychol Rev. 1974;81:392–425. doi: 10.1037/h0037024. [DOI] [Google Scholar]
- Berking M, Margraf M, Ebert D, Wupperman P, Hofmann SG, Junghanns K. Deficits in emotion-regulation skills predict alcohol use during and after cognitive-behavioral therapy for alcohol dependence. J Consult Clin Psychol. 2011;79:307–318. doi: 10.1037/a0023421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG. Heart rate variability: orgins, methods and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev. 1991;98:459–487. doi: 10.1037/0033-295X.98.4.459. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Lozano D. Cardiovascular psychophysiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- Blanco C, Rafful C, Wall MM, Ridenour TA, Wang S, Kendler KS. Towards a comprehensive developmental model of cannabis use disorders. Addiction. 2013;109:284–294. doi: 10.1111/add.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O’Brien M. Individual differences in trajectories of emotion regulation processes: the effects of maternal depressive symptomatology and children’s physiological regulation. Dev Psychol. 2008;44:1110–1123. doi: 10.1037/0012-1649.44.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP. Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: a pilot study. Psychophysiology. 2011;48:1588–1596. doi: 10.1111/j.1469-8986.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JH, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: stability, continuity, and implications for childhood adjustment. Dev Psychobiol. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Smith CL, Gill KL, Johnson MC. Maternal interactive style across contexts: relations to emotional, behavioral and physiological regulation during toddlerhood. Soc Dev. 2001;7:350–369. doi: 10.1111/1467-9507.00072. [DOI] [Google Scholar]
- Carlson SM, Moses LJ, Moses L. Individual differences in inhibitory control and childrens theory of mind. Child Dev. 2013;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Chassin L, Sher KJ, Hussong A, Curran P. The developmental psychopathology of alcohol use and alcohol disorders: research achievements and future directions. Dev Pscychopathol. 2013;25:1567–1584. doi: 10.1017/S0954579413000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CAC, Sheffield TD, Chevalier N, Nelson JM, Wiebe SA, Espy KA. Charting early trajectories of executive control with the shape school. Dev Psychol. 2012;49:1481–1493. doi: 10.1037/a0030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PM, Michel MK, Teti LO. The development of emotion regulation and dysregulation: a clinical perspective. Monogr Soc Res Child Dev. 1994;59:73–100. doi: 10.1111/j.1540-5834.1994.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Conradt E, Degarmo D, Fisher P, Abar B, Lester BM, Lagasse LL, Shankaran S, Bada H, Bauer CR, Whitaker TM, Hammond Ja. The contributions of early adverse experiences and trajectories of respiratory sinus arrhythmia on the development of neurobehavioral disinhibition among children with prenatal substance exposure. Dev Psychopathol. 2014;26:901–916. doi: 10.1017/S095457941400056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine T, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. J Abnorm Psychol. 2006;115:174–8. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Denham Sa, Blair Ka, DeMulder E, Levitas J, Sawyer K, Auerbach-Major S, Queenan P. Preschool emotional competence: pathway to social competence? Child Dev. 2003;74:238–256. doi: 10.1111/1467-8624.00533. [DOI] [PubMed] [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol Psychol. 2007;74:286–94. doi: 10.1016/j.biopsycho.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorard G, Berthoz S, Phan O, Corcos M, Bungener C. Affect dysregulation in cannabis abusers: a study in adolescents and young adults. Eur Child Adolesc Psychiatry. 2008;17:274–282. doi: 10.1007/s00787-007-0663-7. [DOI] [PubMed] [Google Scholar]
- Eddie D, Kim C, Lehrer P, Deneke E, Bates ME. A pilot study of brief heart rate variability biofeedback to reduce craving in young adult men receiving inpatient treatment for substance use disorders. Appl Psychophysiol Biofeedback. 2014;39:181–92. doi: 10.1007/s10484-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Cumberlan A, Spinrad TL. Parental socialization of emotion. Psychol Inq. 2009;9:241–273. doi: 10.1207/s15327965pli0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Erath Sa. Family conflict, autonomic nervous system functioning, and child adaptation: state of the science and future directions. Dev Psychopathol. 2011;23:703–721. doi: 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Ii Interactions among marital conflict, sympathetic, and parasympathetic nervous systems activity in the prediction of children’s externalizing problems. Monogr Soc Res Child Dev. 2009;74:19–34. doi: 10.1111/j.1540-5834.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Sheffield TD, Wiebe SA, Clark CAC, Moehr MJ. Executive control and dimensions of problem behaviors in preschool children. J Child Psychol Psychiatry Allied Discip. 2011;52:33–46. doi: 10.1111/j.1469-7610.2010.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BE, Greaves-Lord K, Euser AS, Tulen JHM, Franken IHa, Huizink AC. Determinants of physiological and perceived physiological stress reactivity in children and adolescents. PLoS One. 2013;8:e61724. doi: 10.1371/journal.pone.0061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. doi:10.1111%2F1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18:953–958. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Feng X, Shaw DS, Kovacs M, Lane T, O’Rourke FE, Alarcon JH. Emotion regulation in preschoolers: the roles of behavioral inhibition, maternal affective behavior, and maternal depression. J Child Psychol Psychiatry Allied Discip. 2008;49:132–141. doi: 10.1111/j.1469-7610.2007.01828.x. [DOI] [PubMed] [Google Scholar]
- Fries ABW, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Smith IM. Executive function in preschoolers: a review using an integrative framework. Psychol Bull. 2008;134:31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Greenberg M, Bierman K. Children’s parasympathetic reactivity to specific emotions moderates response to intervention for early-onset aggression. J Clin Child Adolesc Psychol. 2013:37–41. doi: 10.1080/15374416.2013.862801. [DOI] [PubMed] [Google Scholar]
- Graziano Pa, Bagner DM, Sheinkopf SJ, Vohr BR, Lester BM. Evidence-based intervention for young children born premature: preliminary evidence for associated changes in physiological regulation. Infant Behav Dev. 2012;35:417–28. doi: 10.1016/j.infbeh.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: a meta-analysis. Biol Psychol. 2013;94:22–37. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, Sullivan C. Applying the polyvagal theory to children’s emotion regulation: social context, socialization, and adjustment. Biol Psychol. 2008;79:299–306. doi: 10.1016/j.biopsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: Implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Huber PJ. Robust regression: asymptotics, conjectures and Monte Carlo. Ann Stat. 1973;1:799–821. doi: 10.1214/aos/1176342503. [DOI] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol Psychiatry. 2003;54:1427–1436. doi: 10.1016/S0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Ivanova MY, Achenbach TM, Rescorla LA, Harder VS, Ang RP, Bilenberg N, Bjarnadottir G, Capron C, De Pauw SSW, Dias P, Dobrean A, Doepfner M, Duyme M, Eapen V, Erol N, Esmaeili EM, Ezpeleta L, Frigerio A, Gonalves, et al. Preschool psychopathology reported by parents in 23 societies: testing the seven-syndrome model of the child behavior checklist for ages 1.55. J Am Acad Child Adolesc Psychiatry. 2010;49:1215–1224. doi: 10.1016/j.jaac.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KM, Champion PR, Woodward LJ. Social competence of preschool children born very preterm. Early Hum Dev. 2013;89:795–802. doi: 10.1016/j.earlhumdev.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. J Child Psychol Psychiatry Allied Discip. 2010;51:706–716. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci USA. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110/-/DCSupplemental. www.pnas.org/cgi/doi/10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H. Handbook of Emotion Regulation. Guildford Press; London: 2014. Emotion regulation in substance use disorders; p. 669. [Google Scholar]
- Kogan A, Oveis C, Carr E, Gruber J. Vagal activity is quadratically related to prosocial traits, prosocial emotions, and observer perceptions of prosociality. J Pers Soc Psychol. 2014;107:1051–1063. doi: 10.1037/a0037509. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Regulation of distress and negative emotions: Aadevelopmental view. Dev Psychol. 1989;25:343–352. [Google Scholar]
- La Paro KM, Pianta RC. Predicting children’s competence in the early school years: a meta-analytic review. Rev Educ Res. 2000;70:443–484. doi: 10.3102/00346543070004443. [DOI] [Google Scholar]
- Lorr M, Strack S. A study ol Benjamin’s eiglit-facet structufal analysis ol social beliavior (SASB) model. J Clin Psychol. 1999;55:207–215. doi: 10.1002/(sici)1097-4679(199902)55:2<207::aid-jclp8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, Berntson GG. Where to B in dZ/dt. Psychophysiology. 2007;44:113–119. doi: 10.1111/j.1469-8986.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Lynne-Landsman SD, Bradshaw CP, Ialongo NS. Testing a developmental cascade model of adolescent substance use trajectories and young adult adjustment. Dev Psychopathol. 2010;22:933–948. doi: 10.1017/S0954579410000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, Leerkes EM, Brien MO, Blankson AN. Moderate vagal withdrawal in 3.5-year-old children is associated with optimal performance on executive function tasks. Dev Pscychobiol. 2011;52:603–608. doi: 10.1002/dev.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan A, Cicchetti D. Impact of child maltreatment and interadult violence on children’s emotion regulation abilities and socioemotional adjustment. Child Dev. 2002;73:1525–1542. doi: 10.1111/1467-8624.00488. http://dx.doi.org/10.1111/1467-8624.00488. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Houts R, Poulton R, Roberts BW, Ross S, Sears MR, Thomson WM, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsen JT, Von Der Lippe AL. Validation of the SASB Introject Surface in a Norwegian clinical and nonclinical sample. J Pers Assess. 2007;88:235–245. doi: 10.1080/00223890701268108. [DOI] [PubMed] [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR. The role of the family context in the development of emotion regulation. Soc Dev. 2007;16:361–388. doi: 10.1111/j.1467-9507.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz LC, Anastassiou-Hadjicharalambous X. Disinhibited behaviors in young children: relations with impulsivity and autonomic psychophysiology. Biol Psychol. 2011;86:349–359. doi: 10.1016/j.biopsycho.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Marceau K, Shirtcliff Ea, Hastings PD, Oldehinkel AJ. Autonomic and adrenocortical interactions predict mental health in late adolescence: the TRAILS Study. J Abnorm Child Psychol. 2015;43:847–861. doi: 10.1007/s10802-014-9958-6. [DOI] [PubMed] [Google Scholar]
- Pincus AL, Gurtman MB, Ruiz Ma. Structural analysis of social behavior (SASB): circumplex analyses and structural relations with the interpersonal circle and the five-factor model of personality. J Pers Soc Psychol. 1998;74:1629–1645. doi: 10.1037/0022-3514.74.6.1629. [DOI] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology. 2001;38:267–274. doi: 10.1017/S0048577201990808. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Vardi S, Putzer Bechner AM, Curtin JJ. Physically abused children’s regulation of attention in response to hostility. Child Dev. 2005;76:968–77. doi: 10.1111/j.1467-8624.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/S0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. Love: an emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology. 1998;23:837–861. doi: 10.1016/S0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-A. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-roosevelt JA, Maiti AK. Vagal one and the physiological regulation. Monogr Soc Res Child Dev. 1994;59:167–186. [PubMed] [Google Scholar]
- Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behaviour : a polyvagal perspective. Infant Child Dev. 2011;118:106–118. doi: 10.1002/icd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley KS, Stifter Ca. A comparative validation of sympathetic reactivity in children and adults. Psychophysiology. 2006;43:357–365. doi: 10.1111/j.1469-8986.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, McGregor IS, Hickie IB, Kemp AH. Heart rate variability predicts alcohol craving in alcohol dependent outpatients: further evidence for HRV as a psychophysiological marker of self-regulation. Drug Alcohol Depend. 2013;132:395–398. doi: 10.1016/j.drugalcdep.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Rousseeuw PJ, Leroy AM. Robust Regression and Outlier Detection, Wiley Series in Probability and Statistics. John Wiley & Sons, Inc; Hoboken, NJ, USA: 1987. [Google Scholar]
- Scarpa a, Raine a, Venables PH, Mednick Sa. Heart rate and skin conductance in behaviorally inhibited Mauritian children. J Abnorm Psychol. 1997;106:182–190. doi: 10.1037/0021-843X.106.2.182. [DOI] [PubMed] [Google Scholar]
- Sitnick SL, Shaw DS, Hyde LW. Precursors of adolescent substance use from early childhood and early adolescence: Testing a developmental cascade model. Dev Psychopathol. 2014;26:125–140. doi: 10.1017/S0954579413000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Walden T. Developmental trends in emotion understanding among a diverse sample of African-American preschool children. J Appl Dev Psychol. 1998;19:177–197. [Google Scholar]
- Stansbury K, Sigman M. Responses of preschoolers in two frustrating episodes: emergence of complex strategies for emotion regulation. J Genet Psychol. 2000;161:182–202. doi: 10.1080/00221320009596705. [DOI] [PubMed] [Google Scholar]
- Stern D. The First Relationship: Mother And Infant. Harvard University Press; Cambridge, MA: 1977. [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Physiological Recording. Oxford University Press; New York: 2001. [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: a theme in search of definition. Monogr Soc Res Child Dev. 1994;59:25–52. doi: 10.2307/1166137. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare Ta, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick EZ. Emotions and emotional communication in infants. Am Psychol. 1989;44:112–119. doi: 10.1037/0003-066X.44.2.112. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: Representation and constraircing function of wrong bekfs in young children’s understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]