Abstract Abstract
Background
Carolina bays are elliptic, directionally aligned basins of disputed origin that occur on the Atlantic Coastal Plain from the Delmarva Peninsula to southern Georgia. In southeastern North Carolina, several large, natural, lacustrine systems (i.e., Carolina bay lakes) exist within the geomorphological features known as Carolina bays. Within the current distribution of Carolina bays, Bladen and Columbus counties (North Carolina) contain the only known examples of Carolina bay lakes. The Carolina bay lakes can be split into two major divisions, the “Bladen Lakes Group” which is characterized as being relatively unproductive (dystrophic – oligotrophic), and Lake Waccamaw, which stands alone in Columbus County and is known for its high productivity and species richness. Although there have been several studies conducted on these unique lentic systems, none have documented the flora comprehensively.
New information
Over the 2013−2014 growing seasons, the littoral zone flora of Carolina bay lakes was surveyed and vouchered. Literature reviews and herbarium crawls complemented this fieldwork to produce an inventory of the vascular plant species. This survey detected 205 taxa (species/subspecies and varieties) in 136 genera and 80 vascular plant families. Thirty-one species (15.2%) are of conservation concern. Lake Waccamaw exhibited the highest species richness with 145 catalogued taxa and 26 species of conservation concern. Across all sites, the Cyperaceae (25 spp.), Poaceae (21 spp.), Asteraceae (13 spp.), Ericaceae (8 spp.), Juncaceae (8 spp.), and Lentibulariaceae (6 spp.) were the six most species-rich vascular plant families encountered. A guide to the littoral zone flora of Carolina bay lakes is presented herein, including dichotomous keys, species accounts (including abundance, habitat, phenology, and exsiccatae), as well as images of living species and vouchered specimens.
Keywords: North American southeastern Coastal Plain lakes, floristics, aquatic, emersed vegetation
Introduction
Carolina bays are shallow elliptical depressions of disputed origin aligned in a northwest-southeast direction on the Atlantic Coastal Plain of the eastern United States from the Delmarva Peninsula to southern Georgia (Tuomey 1848, Glenn 1895, Melton and Schriever 1933, Prouty 1952, LeBlond 1995, Sharitz 2003). In southeastern North Carolina, several large, natural, lacustrine systems exist within the geomorphological features known as Carolina bays. Within the current distribution of Carolina bays, Bladen and Columbus counties in North Carolina contain the only known examples of Carolina bay lakes. Carolina bay lakes can be split into two major divisions, the “Bladen Lake Group”, which are dystrophic to oligotrophic and relatively unproductive, and Lake Waccamaw, which stands alone in Columbus County and is known for its high productivity, species richness, and rates of endemism (Weiss and Kuenzler 1976, Casterlin et al. 1984, LeBlond 1995, North Carolina Division of Water Quality, Environmental Sciences Section, Intensive Survey Unit 2009, North Carolina Division of Water Quality, Environmental Sciences Section, Intensive Survey Unit 2012, Schafale 2012).
Although there have been several studies conducted on these unique lentic (freshwater) systems (Prouty 1935, Eyles 1941, Hubbs and Raney 1946, Frey 1949, Frey 1951a, Frey 1951b, Frey 1954, Louder 1962, Casterlin et al. 1984, Newman and Schalles 1990; see also Suppl. material 1), none have focused comprehensively on their vegetation. Several manuals, guides, and broader floristic works are available on wetlands of North Carolina and the eastern United States (Suppl. material 2), but few floras have followed the guidelines and recommendations of Palmer et al. (1995) and Denslow et al. (2010) and documented the site-specific aquatic flora of wetlands, streams, rivers, ponds, or lakes in North Carolina (Sieren and Warr 1992, Warren et al. 2004). Nifong (1998) estimated the occurrence of 620 functionally intact, unaltered, Carolina bays remaining in the Coastal Plain of the Carolinas, and an annual rate of loss of about 36 functionally intact North Carolina bays to development, agriculture, silviculture, and other means. It is imperative that the few remaining unaltered bays be studied, especially Carolina bay lakes, considering that with increasing demotechnic growth (Wetzel 2001) and insecure protection status of isolated wetlands (Sharitz 2003), many freshwater systems, including Carolina bays and bay lakes, face an uncertain future.
A narrow time frame exists to study the few remaining natural freshwater systems not affected by severe degradation. Denslow et al. (2010) found only one aquatic flora (Sieren and Warr 1992) within the state of North Carolina between the years 1834−2009, showing a neglect of aquatic systems in floristic studies within the state. To help fill this gap in knowledge, the objectives of this study were to (1) inventory the littoral zone vascular flora of Carolina bay lakes through the collection of voucher specimens, (2) provide a comprehensive checklist of the littoral zone vascular flora based on integration of new and historic collections and reports, and (3) create an illustrated guide based on the checklist.
Background
Lake Ecosystems and Abiotic Factors
Catchment Area
Lakes (also referred to as lentic systems along with ponds) exhibit physical and chemical characteristics unique to the soils, vegetation, and land use activities present on immediately surrounding lands; thus, no two lakes are exactly the same (Moss et al. 1996, Brönmark and Hansson 2005). All lakes occur within catchment areas. A catchment area can also be referred to as a watershed or drainage basin, which is simply the zone of land surrounding a lake that drains precipitation into the lake basin (Brönmark and Hansson 2005). The area, geology, edaphic (soil) properties, land use, and vegetation of catchment areas affect the acidity, water color, nutrient input, and chemical composition of lakes (Wetzel 2001, Brönmark and Hansson 2005). Large catchment areas have a more pronounced impact on the chemical properties of lakes because they drain more precipitation, and thus the potential for more nutrients, into the lake basin. Consequently, land use activities that release excessive nutrient inputs into large catchment areas (e.g., intensive agriculture) are likely to cause eutrophication (Casterlin et al. 1984, Brönmark and Hansson 2005).
Water Color
The observed color of natural lake waters is caused by the selective absorption of wavelengths as light penetrates through the water column (Wetzel 2001). Organic matter (i.e., dead and decomposing plant and animal parts) is the principal determinant of water color in lakes (Juday and Birge 1933, Rasmussen et al. 1989, Brönmark and Hansson 2005). Due to differences in wavelength absorption, waters with little dissolved organic matter, such as hardwater lakes or glacial streams, appear blue/green, and, in contrast, lakes containing much dissolved organic matter in the form of humic substances (e.g., Carolina bay lakes and bogs) appear yellow/red or “tea-stained” in color. Humic substances are large molecules formed as a result of decomposing organic matter; they are difficult for the microbial community to degrade and are long-lived within the lake system (Brönmark and Hansson 2005).
Trophic status
Trophic status refers to the rate at which organic matter is supplied by or transported into a lake. Humic substances are the most common component in allochthonous organic matter; consequently, wetlands that receive the bulk of their organic matter from allochthonous sources (e.g., Carolina bay lakes, bogs, pocosins) are heavily “tea-stained” and are commonly referred to in the southeastern United States as “black water” lakes, streams, rivers, ponds. Lakes receiving the majority of their organic matter from allochthonous sources have been given the term dystrophic. Dystrophic lakes have low productivity and are often acidic due to large quantities of allochthonous humic input.
Phosphorous is limiting in freshwater systems and is therefore a useful determinant for production. Phosphorous concentrations are easier to quantify than carbon content and production, and, as a result, trophic status is often classified based on phosphorous content (Brönmark and Hansson 2005). Oligotrophic lakes experience low productivity associated with autochthonous carbon production and low levels of phosphorous and nitrogen. Eutrophic lakes experience high productivity associated with autochthonous production and high levels of phosphorous and nitrogen.
pH
The unit commonly used to measure acidity is pH. It is technically defined as the reciprocal of the activity of free hydrogen ions (H+; Covington et al. 1985). Because pH is measured on a logarithmic scale, a change of one unit in pH corresponds to a ten-fold increase in hydrogen ions (Brönmark and Hansson 2005). pH is measured on a scale of 1–14; most lakes possess a pH between 6 and 9, but extreme cases of acidity (1–5) and alkalinity (10–14) also exist depending upon various abiotic and biotic conditions within a lake’s catchment area (see above; Brönmark and Hansson 2005). Geological and hydrological conditions within catchment areas primarily control the pH of lakes; however, acid rain can also affect the pH of lakes. In North America, coal-fired power plants and other industries emit sulfur dioxide (SO2) into the atmosphere. As weather systems make their way across North America from west to east, they pick up this sulfur dioxide (SO2) and deposit it across the landscape in the form of precipitation (i.e., acid rain). The cumulative effects of acid rain deposition on both terrestrial and aquatic systems is known to be most severe in the eastern United States; this is due to the region's geographic location in relation to broad-scale weather paterns and industries emitting sulfur dioxides (Schindler 1988).
Photosynthesis and respiration are also known to affect the pH of waters by influencing the amount of carbon dioxide (CO2) in the water column. When CO2 is taken up and stored by aquatic macrophytes, phytoplankton, and algae during photosynthesis, free hydrogen ions (H+) are neutralized or taken up by carbonates, bicarbonates, and hydroxides, causing a reduction in H+ and thus a higher pH. Respiration adds CO2 into the system, thus releasing free H+ into the water column and lowering the pH (Brönmark and Hansson 2005). Because photosynthesis and respiration can cause fluctuating differences in pH within a 24-hour cycle, alkalinity is typically considered to be a better measurement of a lake's acidification status (Brönmark and Hansson 2005).
Alkalinity
Alkalinity refers to a lake's ability to neutralize strong inorganic acids (i.e., it is a measure of how sensitive a lake is to acidification). It is now used synonymously with acid neutralizing capacity (ANC; Wetzel 2001). Today, alkalinity is generally expressed in milliequivalents per liter (meq/L), but has commonly been recorded in the past in milligrams per liter (mg/L; Brönmark and Hansson 2005). Lakes with an alkalinity above 0.5 meq/L have good buffering capacities, whereas lakes with alkalinities below 0.01 meq/L have little or no buffering capacities (Wetzel 2001,Brönmark and Hansson 2005). Lakes with low alkalinities are susceptible to drops in pH with only small additions of acid (H+), whereas lakes with high alkalinities can withstand the addition of acid (H+) into their systems without proportional drops in pH (Brönmark and Hansson 2005).
Wetzel (2001) noted that the property of alkalinity in most fresh waters is imparted by the presence of carbonates (i.e., carbonate, bicarbonate, calcium carbonate). Carbonates and hydroxides remove hydrogen ions (H+) from lakes, thus neutralizing their acidity (i.e., raising the pH to a more basic status). Lake Waccamaw, the largest Carolina bay lake, has a high alkalinity (7.0−12 mg/L or 0.14−0.24 meq/L; Weiss and Kuenzler 1976) due to the presence of both subsurface and surficial limestone deposits within and around the lake. As a result, it possesses a neutral to basic pH (6.8−8.5 s.u.) and has the ability to handle larger additions of acid.
Carolina Bays, Bay Lakes, and Pocosins
Carolina Bays
The core concentration of Carolina bays occurs in southeastern North Carolina and northeastern South Carolina (Ross 2003; Fig. 1). Although these depressions share the same elliptical shape, they vary dramatically in length along their long axis from 50 m to 8 km (with some as large as 3,600 ha; Prouty 1935, Thom 1970, Savage 1982, Sharitz and Gibbons 1982). Nifong (1982) suggested that there are fewer than 13,000 bays (unaltered and altered) left in the Coastal Plain of the Carolinas, as opposed to the 400,000 proposed by Prouty (1935). It was not until the early 20th century that researchers fully recognized the magnitude and extent of Carolina bay distribution by the use of airplanes and soon-to-be aerial imagery.
Figure 1.

Core distribution of Carolina bays. Carolina bays are known to occur from the Delmarva Peninsula south to southern Georgia. Although many historical texts frequently cite the distribution range of Carolina bays as occurring from New Jersey south to Florida, the more narrow range from the Delmarva Peninsula to southern Georgia is more accurate. Conversations with state agencies and personnel from all states included in the broader range of Carolina bays confirm their “apparent absence” in southern New Jersey and northern Florida. The core distribution of Carolina bays is located in northeast South Carolina and southeast North Carolina (darker gray). The bays in this region would be considered “classic” Carolina bays (i.e., matching all of the well-known and consistent geomorphological criteria in the literature), whose geomorphology is described well by Prouty (1952) and Ross (2003). Toward the peripheries of the known Carolina bay distribution range, the term Carolina bay tends to be used loosely and is not used in its strictest sense (i.e., depression wetlands; Chick Gaddy, pers. comm.). Figure taken from Ross (2003).
Savage (1982) declared that: “When seen from the air, Carolina bays are an astounding, unforgettable revelation. But though hundreds of thousands lie clearly visible, scattered across the Atlantic Coastal Plain from Maryland to northern Florida, they are often all but unrecognizable to the uninitiated eyes of groundlings”. The first aerial images produced of the Atlantic Coastal Plain exposed Carolina bays to both citizens and scientists on a broad scale; moreover, they initiated a flurry of scientific research on Carolina bay distribution, numbers, origin, vegetation, and soils.
The term bay is used to describe these landscape features not because they commonly contain hydric soils or are inundated with water, but because of the presence of three species of bay tree typically found within and around their elliptical boundaries (i.e., Magnolia virginiana L. [sweetbay; Magnoliaceae], Persea palustris (Raf.) Sarg. [swamp bay; Lauraceae], and Gordonia lasianthus (L.) J. Ellis [loblollybay; Theaceae]. Traditionally, the term “bay” tree has been used when speaking of the laurel trees within the Lauraceae family. While Persea palustris may be properly referred to as a “bay” tree, Gordonia lasianthus and Magnolia virginiana may not (sensu stricto), hence their common names being one word (i.e., loblollybay and sweetbay). Gordonia lasianthus and Magnolia virginiana bear a noticeable morphological resemblence to the laurels of the Lauraceae; thus, they are generally referred to as “bay” trees (sensu lato). North of Virginia, these mysterious landscape features are referred to as Delmarva potholes, bays, or basins (Tiner and Burke 1995, Lide 1997, Sharitz 2003, Tiner 2003). The inability to agree upon a clear-cut definition and universal name for these unique geological features has caused some discrepancy among estimates of bay numbers (Lide 1997).
Collectively, Carolina bays and pocosins represent the largest total acreage of palustrine wetlands in the Carolinas (Wilson 1962, Richardson 1983, Richardson and Gibbons 1993, Nifong 1998). Pocosins occur on the Atlantic Coastal Plain from southern Virginia to northern Florida (essentially the same range as Carolina bays). Unlike Carolina bays, pocosins have been poorly mapped throughout the whole of their range. Wilson (1962) and Richardson (1981) comprehensively mapped the pocosins of North Carolina. It is estimated that ca. 70% of the nation's pocosin habitat occurs in North Carolina and that over 50% of the state's palustrine wetlands are comprised of pocosins (Richardson and Gibbons 2003). Richardson (1981) suggested that ca. 8,300 km2 (3,200 mi2) of unaltered pocosins were drained for other land uses between 1962 and 1979; and ca. 3,700 km2 (1,450 mi2) of unaltered pocosins remained in North Carolina in 1980. Based on the presence of wetland soils (i.e., “soils formed under conditions of saturation, flooding, or ponding long enough during the growing season to develop anaerobic conditions in the upper part” [Vepraskas and Richardson 2001]), North Carolina is estimated to have contained nearly 7.5 million acres (3.03 million hectares) of wetlands prior to European settlement of the state; 95% of these wetlands were located in the Coastal Plain (North Carolina Division of Environmental Management 1994).
Geographic location, soil depth, soil type, surrounding land use, varying hydrology, and fire regimes interact to create vastly different vegetative and wetland assemblages within Carolina bays. Nifong (1998) summarized this diversity, noting that bays included “in some form, virtually every non-marine wetland system found on the southeastern Coastal Plain, including brackish marsh, freshwater pond, freshwater marsh, freshwater prairie, pocosin, bay forest, bog, swamp forest, depression meadow, cypress savanna, and longleaf pine savanna communities, among others”. Other communities found within Carolina bays include Pinus taeda L. (loblolly pine) plantations, cropland, and open lakes (Carolina bay lakes).
Carolina bays can be divided into two classes based on soil substrate: clay-based bays and peat-based bays. The vast majority of Carolina bay literature has referenced peat-based bays, frequently using terms such as “pocosin” or “evergreen shrub bog” to describe the vegetation growing over deep organic soils. However, there are about 27 bays (as of 1982) located in the Carolinas that contain clay subsoil not overlain with sand or peat (Kelley and Batson 1955, Nifong 1982). These clay-based bays are restricted to Cumberland, Scotland, Hoke, and Robeson Counties in North Carolina. The vegetative physiognomy of clay-based bays differs from peat-based bays in that the structure is more open in the former (i.e., they have a sparse overstory of Taxodium and an herbaceous understory composed mostly of herbaceous taxa). However, clay-based bays do share some of the classical Carolina bay morphology features (e.g., elliptical boundaries, varying size, sand rims) with peat-based Carolina bays.
Clay-based bays are species-rich communities, often supporting rare taxa within their boundaries (Nifong 1982). Clay-based bays in high quality condition typically have an open canopy with a species-rich herbaceous understory. Fire and water level fluctuations are two disturbance regimes that account for the diversity found in these bays (Sutter and Kral 1994, Nifong 1998). Peat based bays are more prevalent throughout the Coastal Plain of the Carolinas. Peat-based bays are not as restricted to the inner Coastal Plain and are not as floristically rich as high quality clay-based bays.
Bladen County, North Carolina, is well-known for its many Carolina bays. Nifong (1998) found 617 Carolina bays within Bladen County; of these, 325 were classified as fully vegetated and 292 were classified as cleared (i.e., > 50% of their natural vegetation removed). Bladen County hosts the densest cluster of unaltered bays in the state (the county is fourth densest for bays in any condition). The majority of the bays in Bladen County are found in the Cape Fear River Valley, between the Cape Fear River and the South and Black Rivers. All of these bays are considered peat-based bays. Among extent Carolina bay lakes, all but one occur in Bladen County.
Carolina bays should not be confused with pocosins; they are two distinct physiographic features that just so happen to coexist with one another on the Atlantic Coastal Plain. These two landscape features differ from one another and using the terms synonymously is a common mistake among both laymen and professionals (Ross 2003). The term pocosin originated as an eastern Algonquian term meaning “swamp-on-a-hill” (Richardson 1983). It is defined by Ross (2003) as “a Coastal Plain wetland area of variable shape and size in an area of poor surface drainage whose vegetation is mostly broad-leafed evergreen shrubs and Pinus serotina Michx. growing on organic peaty soils” and by Brinson (1991) as “ecosystems dominated by woody, predominantly evergreen species and that normally occur on histosols (organic peat or muck soils ≥ 40 cm deep) or on soils with a histic epipedon (uppermost soil horizon used to classify a soil)”. Pocosins typically are located on broad, flat, interstream areas or near estuaries where rising sea levels affect their hydrology and hinder their drainage. Although there may be “pocosin-like vegetation” within a Carolina bay, the features are structurally of different origins. Unlike Carolina bays, the origin of pocosins is generally more understood (Whitehead 1972, Whitehead 1981, Brinson 1991, Richardson and Gibbons 1993).
Brinson (1991) attributed pocosin formation and subsequent persistence to two factors: climate and topography. Climate, he attested, “determines the exchange of matter and thermal energy between pocosins and the atmosphere”. The bulk of this exhange is in the form of precipitation, much of which is lost to evapotranspiration following its input. Brinson (1991) added “while the muted topographic relief of the Atlantic Coastal Plain is probably the main contributor to pocosin formation, the feedback between climate and topography is likely essential”. In summary, pocosins have formed in landscape positions with low topgraphic relief where the regional climate and lack of surficial hydrologic connections with adjacent wetland systems interact to form ombrotrophic conditions. Here, organic matter in the form of dead terrestrial vegetation is deposited onto wetland soils and accumlates at a slow, consistent rate through geologic time, resulting in the formation of pocosins.
Historically, the Atlantic and Gulf Coastal Plains supported a heterogeneous landscape of longleaf pine savannas, xeric sandhills, upland mixed-pine hardwoods, pocosins, Carolina bays, bottomland hardwood forests, natural lakes, and black and brown-water river systems (Garren 1943, Christensen 1999). However, it is now a highly fragmented and fire-suppressed region dominated by agriculture, residential developments, and large cities with few large intact parcels of natural ecosystems remaining. Demotechnic growth (Wetzel 2001, Dudgeon et al. 2006), global warming (Smith and Tirpak 1989), increasing agricultural production (Tilman et al. 2002), fire supression (Nowacki and Abrams 2008, Palmquist et al. 2014), urbanization (Terando et al. 2014), shoreline development (Radomski and Goeman 2001, Ford and Flaspohler 2010, Frost and Hicks 2012), and introduction of invasive species (Pimentel et al. 2005) continue to threaten and encroach upon the few “natural”, intact, terrestrial and freshwater ecosystems remaining in the Southeast, including Carolina bays and bay lakes.
Carolina bays are valuable components of our national and state natural heritage (Nifong 1998). Their variable hydrology and size, presence of rare and endemic taxa, and isolated landscape position, make them valuable habitats for southeastern flora and fauna and provide important ecosystem services (Suppl. material 4). Unfortunately, Carolina bays and other palustrine wetland systems have suffered from extensive habitat loss and degradation during the past three centuries (Bennett and Nelson 1991, Mitsch and Gosselink 1993, North Carolina Division of Environmental Management 1996, Kirkman et al. 1996, Nifong 1998). Using 1988 aerial imagery, Nifong (1998) found 8,057 Carolina bays in the state of North Carolina. Of these 8,057 total bays, 6,331 (79%) had more than half of their natural vegetation removed.
Sharitz and Gibbons (1982) and Nifong (1998) suggested several ways to better preserve and manage Carolina bays in the future. For an excellent review on the copious amount of Carolina bay literature available, see Ross (2000), Ross (2003); and for detail specifically about bays in the Carolinas, see Nifong (1998).
Carolina Bay Lakes
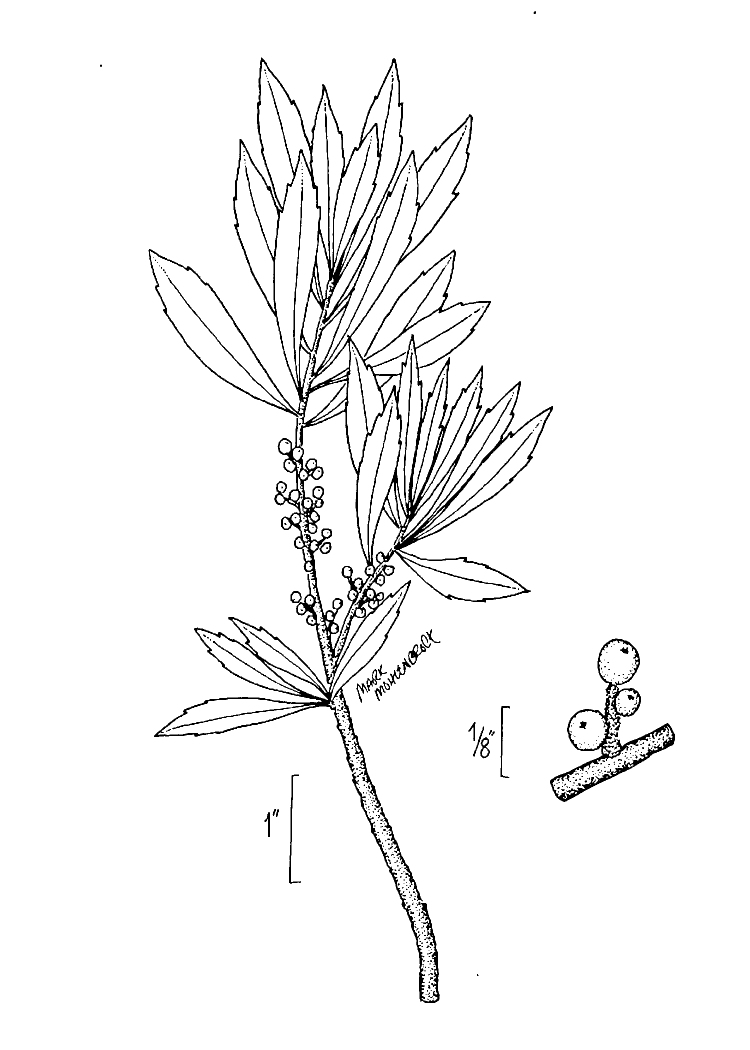
Several Carolina bays in southeastern North Carolina contain large (i.e., > 50 hectares) natural lakes within their elliptic boundaries (Frey 1949), thereby giving them the name Carolina bay lakes. Each lake is located in the southernmost portion of the elliptical feature known as a Carolina bay (Fig. 2). The northern portions of the bays (i.e., the portions not inundated by lake waters) contain organic, peaty soils and a unique vegetative assemblage comprised of bay trees (Gordonia lasianthus, Magnolia virginiana, Persea palustris), ericaceous shrubs (e.g., Chamaedaphne calyculata (L.) Moench, Eubotrys racemosa (L.) Nutt., Kalmia L., Lyonia Nutt., Rhododendron L., Vaccinium L., Zenobia pulverulenta (W. Bartram ex Willd.) Pollard), and several other species well-associated with nutrient-poor soils (e.g., Chamaecyparis thyoides (L.) Britton, Sterns & Poggenb., Nyssa biflora Walter, Pinus serotina, and Smilax laurifolia L.).
Figure 2.

Position of Carolina bay lakes within Carolina bays. Carolina bay lakes are located in the southeasternmost portions of Carolina bays. The northern portions of the bays (i.e., the portion not inundated by lake waters) support shrub-bog plants over organic soils. Here, Salters (top left) and Jones (middle right) Lakes exemplify the typical bay lake position within Carolina bays. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
Nine Carolina bay lakes (i.e., Bakers Lake, Bay Tree Lake, Horseshoe Lake, Jones Lake, Lake Waccamaw, Little Singletary Lake, Salters Lake, Singletary Lake and White Lake) are known to exist within the known distribution of Carolina bays. All nine lakes occur in Bladen and Columbus counties, North Carolina (Frey 1949, LeBlond 1995, LeBlond and Grant 2005; Fig. 3). Carolina bay lakes, with the exception of Lake Waccamaw and White Lake, are nutrient poor because they receive the bulk of their hydrologic inputs in the form of precipitation. These lakes are also characteristically dystrophic due to the dominance of organic soils within their catchment area. Organic soils do not allow for the rapid decomposition of plant and animal matter, resulting in the high amount of humic substances found in the water column.
Figure 3.

Geographic location of all nine Carolina bay lakes (green text boxes). Bladen County (light yellow) supports eight of the nine Carolina bay lakes known to exist; all eight lakes occur within the Cape Fear River Valley between the Cape Fear River and South River. Bay Tree Lake is the largest Carolina bay lake in Bladen County; the smallest is Bakers Lake. Lake Waccamaw is the largest Carolina bay and bay lake in North Carolina and is the only bay lake known to exist in Columbus County (tan). Baseline vector data obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map Produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
Although some Carolina bays may contain shallow marshes or ponds (Bennett and Nelson 1991, Nifong 1998), these are not considered lakes. There is no universally accepted technical definition that distinguishes a lake from a pond (Heinonen et al. 2008); however, it seems reasonable to accept as distinguishing that lakes have a clearly defined littoral and profundal zone, a larger overall size (>8 hectares), a shoreline exposed to wave dynamics, greater water depth, a mixing of the water column by wind induced turbulence, and the ability to retain the bulk of their water volume even in years of drought (Cowardin et al. 1979, Moss et al. 1996, Williams et al. 2004, Biggs et al. 2005, Brönmark and Hansson 2005).
Carolina bays are considered to be geographically isolated wetlands with their primary water source coming directly from precipitation (Sharitz 2003, Tiner 2003). Although the vast majority of Carolina bays lack surface water connections to outside aquatic systems, Carolina bay lakes are an exception. Carolina bay lakes all contain drainage outlets--usually along their southern shorelines, but in the northwest for White Lake (Frey 1949)--that release excess water into the Cape Fear and Waccamaw River drainages during periods of high precipitation. However, during years of scarce rainfall, these lakes are more or less isolated from surrounding lotic systems and are confined to their basins (N. Howell, pers. obs.).
Lacustrine Zonation (derived from Wetzel 2001)
Lakes, including Carolina bay lakes, can be divided into distinct transitional zones, moving from the shoreline to the center of the lake (Fig. 4).
Figure 4.
Lacustrine zonation. EPI = epilittoral zone, EU = eulittoral zone. Aerial imagery obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map Produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014). Illustration (top right) by Nathan Howell.
(1) Epilittoral zone: The zone that lies entirely above the lake surface and is not influenced by the spray of surf. This zone can be thought of as the terrestrial or upland zone; the highest water levels never reach it and it is not affected by lakeshore dynamics or hydrology.
(2) Supralittoral zone: The zone that lies entirely above the lake surface and is influenced by the spray of the surf.
(3) Eulittoral zone: The zone encompassing the entire region of the shoreline from the highest and lowest seasonal water levels. This zone experiences natural disturbances such as water level fluctuations and wave dynamics.
(4) Infralittoral zone: This zone is subdivided into three zones in relation to the occurrence and distribution of the major classes of aquatic macrophytes: upper infralittoral zone where emergent rooted macrophytes persist; middle infralittoral zone where floating-leaved rooted macrophytes occur; and lower infralittoral zone where submersed-rooted, adnate, or free-floating macrophytes occur. The eulittoral and infralittoral zones collectively constitute the littoral zone.
(5) Littoriprofundal zone: The zone occupied by photosynthetic algae and bacteria, often associated with the metalimnion (i.e., the stratum between the epilimnion and hypolimnion representing a marked thermal change; also synonymous with thermocline) of stratified lakes.
(6) Profundal zone: The zone that consists of the remainder of the vegetation free sediments.
The Littoral Zone
The littoral zone of lakes (i.e., the eulittoral and infralittoral zones) is an important transition zone between adjacent uplands and the deeper pelagic area of the lake. This zone contains vascular macrophytes (i.e., aquatic vascular plants large enough to see with the naked eye) that have evolved from their terrestrial ancestors to cope with the physical and physiological demands of persisting in an aquatic environment (Sculthorpe 1967, Wetzel 2001, Brönmark and Hansson 2005, Keddy 2010). The vascular macrophytes and coarse woody debris that exist in this zone provide critical habitat for zooplankton, photosynthetic and heterotrophic microflora, macroinvertebrates, herpetofauna, avifauna, fish, and mammals (Brusnyk and Gilbert 1983, Pieczynska 1990, North Carolina Division of Environmental Management 1996, Wetzel 2001, Keddy 2010, Ewert et al. 2011). The littoral zone is characterized by having high productivity, including some of the highest rates of organic matter synthesis in the biosphere (Wetzel 2001).
Aquatic Macrophytes (derived from Wetzel 2001)
Aquatic macrophytes may be divided into four classes. Moving from the shoreline out to deeper water, these classes are as follows [taxa vouchered or reported from Carolina bay lakes are indicated by c]:
(1) Emergent macrophytes: Species rooted in saturated and inundated soils with a water depth up to 1.5 meters; root systems remain in anoxic soil conditions while leaves and reproductive organs stay above the water surface. These plants are often rhizomatous, stoloniferous, or cormous with the potential to reproduce asexually. Heterophyllous (i.e., when a plant exhibits vegetative polymorphism, having morphologically different submersed and aerial organs) species may also be emergent. Examples of genera that may be grouped in this category include Carex L.c, Cephalanthus L.c, Cladium P. Brownec, Juncus L.c, Panicum L.c, Pontederia L.c, Rhynchospora Vahlc, Scirpus L.c, and Typha L.
(2) Floating-leaved macrophytes: Species rooted in the substratum with floating leaves attached to long flexible petioles or on short petioles attached to an ascending stem.
Submersed leaves precede the floating leaves in heterophyllous species. Reproductive organs remain atop or above the water surface. Examples of genera grouped into this category include Brasenia Schreb.c, Nelumbo Adans.c, Nuphar Sm.c, Nymphaea L.c, Nymphoides Ség.c, and Potamogeton Lc.
(3) Submersed macrophytes: Species that remain completely submersed in the water column, but are rooted to the substratum. Leaf morphology is highly variable in this group, from finely dissected to very broad, and reproductive organs may be emersed, floating, or submersed. Examples of genera included in this group are Ceratophyllum L., Isoetes L., and Myriophyllum Lc.
(4) Freely floating macrophytes: Species that remain unattached to the substratum and are completely dependent upon the nutrients in the water column for survival. Reproductive organs may be floating or aerial. Examples of genera include Azolla Lam., Eichhornia Kunth, Hydrocharis L., Limnobium Rich., Trapa L., and Utricularia Lc.
Factors affecting Aquatic Macrophyte Richness in Lakes
Lacoul and Freedman (2006) provided a thorough review on how various environmental influences affect aquatic plants in freshwater systems. A few of these environmental factors are reviewed below.
Latitude
It is well known that generally the number of species occuring at the equator greatly exceeds that of the temperate and northern latitudes (Edmonds 1997). Although this general rule applies across most groups of taxa, it does not seem to apply to aquatic plants. Crow (1993) found that aquatic plants are more diverse in temperate rather than tropical latitudes. When comparing temperate wetland floras to those of tropical climes, this pattern is reinforced (Stuckey 1975, Henry and Scott 1984, Peet and Allard 1993, Ruch et al. 2009). Because Carolina bay lakes differ little in latitude, this factor does not significantly affect species richness in these systems.
pH and Alkalinity
Peat-based Carolina bays are known to have acidic (< 7 pH), nutrient poor, organic soils (Daniels et al. 1984, Leab 1990, Newman and Schalles 1990). In many respects, these isolated wetlands of the Southeast are quite similar to the peatlands of the northern United States and Canada. Floristic diversity in peatlands has been shown to increase with increased levels of calcium and alkalinity in the groundwater (Glaser et al. 1990, Vitt and Chee 1990). Similarly, aquatic macrophyte richness of lakes tends to be lower in unproductive lakes with low pH (e.g., Carolina bay lakes) and higher in more productive lakes with higher alkalinities (Roelofs 1983, Roberts et al. 1985, Rørslett 1991, Dodson et al. 2000, Vestergaard and Sand-Jensen 2000, Søndergaard et al. 2005).
Water Color
Waters with increased levels of humic substances are typically, dystrophic, acidic, and tea-stained. Tea-stained waters are not as transparent as lakes with low humic substances, thus humic lakes have a shallow euphotic zone and a narrow littoral zone, reducing the abundance and depth at which aquatic macrophytes may grow (Spence 1982). Vestergaard and Sand-Jensen (2000) also saw decreased richness in aquatic macrophytes when water transparency was low. An excellent example of how increased humic substances affect water transparency and macrophyte richness and composition can be seen when comparing White Lake to the other Carolina bay lakes. White Lake is an oligotrophic lake with transparent water due to the presence of natural springs on the lake floor. Secchi depths commonly reach to the bottom of the lake (3m/10 ft) and submerged aquatic macrophytes are able to colonize the deepest portions of the lake with ease (i.e., the euphotic zone is deep compared to the other bay lakes).
Hydrography
Frey (1949) documented the morphometry and hydrography of the Carolina bay lakes and determined that the southern portions of the lakes possessed a gentle, tapering hydrography while the northern portions possessed a steep hydrography. Floristic inventories by the first author confirm that aquatic macrophyte richness is higher along southern shorelines; so much so, that the surveying of northern shorelines was abandoned early in the life of the project. A broad sandy terrace occurring along the southern shore of Lake Waccamaw (Fig. 5) creates a wide littoral zone compared to other Carolina bay lakes. This stretch of shoreline, with its gentle hydrography, is known to support over 140 species of wetland plants, while the Bladen lakes, with their comparatively steeper hydrography, are known to support < 55 wetland plant taxa (see floristic summary).
Figure 5.
Broad, shallow, sandy terrace along Lake Waccamaw’s southern shoreline. The gentle relief of this terrace creates a wide littoral zone. Wide littoral zones are more floristically diverse and contain more available area for the establishment of aquatic macrophytes. Alternatively, narrow littoral zones do not have much area for the establishment of aquatic macrophytes and are species-poor. Aerial imagery obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
Lake Size
As a general rule, species richness usually increases with increasing area (Arrhenius 1921, Williams 1964, Connor and McCoy 1979, Rosenzweig and M.L. 1995, Søndergaard et al. 2005). Findlay and Houlahan (1997) found that species richness increased with area sampled for birds, mammals, hepertofauna, and plants in southeastern Ontario wetlands. Results from this work also support these findings with Bakers Lake (i.e., the smallest bay lake) supporting the least diverse littoral zone flora and Lake Waccamaw (i.e., the largest bay lake) supporting the most species-rich littoral zone flora. Other large natural lakes of North Carolina Coastal Plain (e.g., Lake Phelps, Lake Mattamuskeet, Lake Waccamaw) are known to support diverse shoreline floras, more so than the smaller lakes of the region (Lynch and Peacock 1982, Schafale 2012; N. Howell, pers. obs.).
Water Level Variation, Disturbance, and Soil Fertility
Keddy and Fraser (2000) summarized factors that govern littoral zone diversity irrespective of geographic location or size. Three environmental factors (i.e., water levels, soil fertility, and disturbance) govern the composition and floral diversity of littoral zones. Shorelines exposed to intermediate levels of natural disturbances will support a richer flora than those experiencing little to no disturbances and those experiencing extremely harsh disturbances. Natural disturbances may include wave action, ice scour, water level fluctuations, fire, or grazing. If water level fluctuations were absent from a lake or similar waterbody (e.g., in a permanently impounded pond), a two-staged littoral zone would result, with aquatic macrophytes in the aquatic zone and shrubs and trees in the terrestrial zone. Under long-term water level fluctuations, a multi-staged littoral zone would result, leading to increased heterogeneity and a richer flora. Keddy and Fraser (2000) attested that “simply changing water levels from one year to the next doubles the number of vegetation types”. Rørslett (1991) observed that northern European lakes experiencing water level fluctuations of 1–2 meters per year showed greater macrophyte richness than sites experiencing little or intense disturbances. Carolina bay lakes historically would have experienced long-term water level fluctuations, but the installation of water control structures (i.e., dams) in some of the lakes outlet channels has resulted in more stabilized systems (N. Howell, pers. obs.).
Shorelines exposed to frequent disturbances typically have silt and clay stripped from them; and consequently, contain few nutrients. Sheltered shorelines receive clay and silt deposits and therefore contain a higher nutrient content. Foreshores will have a distinct vegetative community characterized as having low biomass and rare species, while backshores (bays or backwater areas sheltered from disturbance) will support a higher biomass community composed of a few clonal dominants (Keddy 2010). Macrophyte richness is always higher in areas of intermediate disturbance. Eutrophification of littoral zones causes increased soil fertility, which increases biomass and negatively impacts macrophyte richness and rare plant taxa.
Study Sites
Bakers Lake
Bakers Lake (30.35 hectares; 75 acres) is a small, privately owned, Carolina bay lake, located in northwestern Bladen County between Little Singletary Lake and the Cape Fear River north of Thoroughfare Bay, ca. 1.5−2 miles east of the intersection of SR 1318 (Old River Road) and SR 1320 (Middle Road; LeBlond and Grant 2005; Fig. 6). This site is located along the northwest boundary of the Bladen Lakes Macrosite, a large tract of undeveloped and relatively unfragmented land between the Cape Fear, South, and Black River systems (LeBlond and Grant 2005; Figs 7, 8). The macrosite extends from southern Cumberland County, through Bladen County, and into southwestern Pender County. This large area is given the name “macrosite” because it contains numerous “standard sites” (i.e., smaller tracts of land with high ecological integrity) that are strongly geographically associated with one another. The majority of the macrosite is located in Bladen County and contains the largest concentration of unaltered, intact, Carolina bays.
Figure 6.

Bakers Lake and surrounding lands. Bakers Lake is located in northern Bladen County and is surrounded by a mix of agriculture and forestland. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
Figure 7.

Bladen Lakes Macrosite (vector). The Bladen Lakes Macrosite (hatched pattern) is a large area encompassing parts of southern Cumberland County, eastern Bladen County, and northwest Pender County. Historically, macrosites were established by the North Carolina Natural Heritage Program (NCNHP) in efforts to identify large, intact, natural areas that withheld numerous other smaller natural areas within their boundaries. The NCNHP no longer uses macrosites as viable natural area boundaries, but it is useful to show the extent of the Bladen Lakes Macrosite boundary. When moving from north to south, the lands are as follows: Bushy Lake State Natural Area (teal green), Suggs Mill Pond Gameland (light mint green), Bladen Lakes State Forest (forest green), Jones Lake State Park (pink), Bay Tree Lake State Park (orange), and Singletary Lake State Park (yellow). Lake Waccamaw State Park (neon green) can be seen farther south along with Friar and Brown Marsh Swamps in Columbus County. Baseline vector data obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
Figure 8.

Bladen Lakes Macrosite (ortho). The North Carolina Natural Heritage Program no longer uses macrosites as viable natural area boundaries, but here it is useful to show the extent of the Bladen Lakes Macrosite boundary. Note the large areas of fragmented land surrounding the macrosite and the relatively unfragmented land within the boundaries of the macrosite. This large tract of land contains one of the largest remaining portions of intact unaltered Carolina bay complexes known to exist. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
Dr. Clemuel Johnson and wife Nancy Johnson, of Elizabethtown, have owned Bakers Lake and surrounding lands (451.40 hectares; 1,155.45 acres) since 1980. Prior to the Johnson’s ownership, Agnes Holden Williams owned the lake and surrounding lands. Ms. Williams’ father acquired the land from an unknown seller during the early 20th century. This seller was able to successfully purchase the lake before 1929, when North Carolina legislation mandated that all lakes greater than 50 acres in size be made property of the state.
Bakers Lake forms the headwaters of Phillips Creek, which drains southward into the Cape Fear River. Bakers Lake Natural Area (i.e., Bakers Lake bay and immediate surrounding lands) is known to support five natural community types (i.e., Pond Pine Woodland – Typic Subtype (S3,G3), Peatland Atlantic White Cedar Forest (S1,G2), Low Pocosin – Gallberry/Fetterbush Subtype (S2,G2), Sand Barren – Typic Subtype (S2,G2), and Natural Lake Shoreline – Cypress Subtype (S2,G3; LeBlond and Grant 2005). Bakers Lake has been known to support heron rookeries and small populations of the state rare Anhinga (Anhinga anhinga [W2; S3B, G5]; LeGrand et al. 2014) during the spring and summer months (S. Clark, pers. comm.; N. Howell, pers. obs.). In addition, the site provides important stopover habitat for large flocks of migrating waterfowl (e.g., Aix sponsa [Wood Duck], Anas americana [American Widgeon], Anas clypeata [Northern Shoveler], Anas crecca [Green-winged Teal], Anas discors [Blue-winged Teal], Anas platyrhynchos [Mallard], Anas strepera [Gadwall], Aythya collaris [Ring-necked Duck], Aythya valisineria [Canvasback], Branta canadensis [Canada Goose], Bucephela albeola [Bufflehead], Lyphodytes cucullatus [Hooded Merganser], Oxyura jamaicensis [Ruddy Duck; G. German and S. Clark, pers. comm; N. Howell pers. obs.).
Anthropogenic disturbances (i.e., silvicultural practices, dam installation in the outflow channel, agricultural fields, confined animal feeding operations (CAFOs), fire supression, and rural residential development) have either been documented on site or on adjacent properties (LeBlond and Grant 2005; S. Clark, pers. comm.). These disturbances have lowered the integrity of several of the aforementioned natural community types within and adjacent to Bakers Lake Natural Area (N. Howell, pers. obs.), but restoration potential is still relatively high. The installation of a flashboard riser system in the outflow channel has altered the natural hydrology of the lake and caused natural water level fluctuations to essentially cease. Following the installation of the dam, the lake consistently stays at a high level, thus narrowing the littoral zone and forcing aquatic macrophytes to occur at or just below the maximum annual high water mark (N. Howell, pers. obs.).
The water quality of Baker’s Lake has not been formally tested by state agencies, but appears high in humic substances (N. Howell, pers. obs.) and the chemistry is likely similar to that of the other Bladen lakes. The lake is here considered dystrophic and relatively unproductive.
Bay Tree Lake
Bay Tree Lake (formerly Black Lake; 588.81 hectares; 1,455 acres) is a large, state-owned Carolina bay lake, located in east-central Bladen County along NC Hwy 41 east of White Lake and west of NC Hwy 210. Bay Tree Lake is part of Bay Tree Lake State Park, a 1,006.85 hectare (2,488 acre) park that includes Bay Tree Lake bay and large parcels of land lying to the north and west of Bay Tree Lake (Fig. 9).
Figure 9.

Bay Tree Lake State Park (highlighted in green) and surrounding lands. Lands surrounding Bay Tree Lake State Park to the south are privately owned and have been partially converted to agriculture. Black Creek Bay and several others in the vicinity have been cleared of their original vegetation and converted to agriculture (primarily blueberry farms in this area). Historically, Horsepen Bay was a peat-filled Carolina bay. During the development of the residential community seen along the northeast shoreline (Bay Tree Resorts), it was turned into a body of open water. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
The North Carolina General Assembly passed legislation in 1911 confirming the status of Bay Tree Lake as a state-owned public trust resource (North Carolina Division of Parks and Recreation, Planning and Development Section 2006b). Historically, Bay Tree Lake was not included within the original natural area site boundary determined by the North Carolina Natural Heritage Program (NCNHP) due to high levels of shoreline disturbance. Today, the lake is considered part of the natural area due to the presence of three rare dragonflies (Gomphus australis [Clearlake Clubtail], Gomphus cavillaris brimleyi [Brimley’s “Sandhill” Clubtail], and Progomphus bellei [Belle’s Sanddragon]) that utilize the lake throughout their life cycle.
In January 1965, a private land development group had the option to purchase 5,665.59 hectares (14,000 acres) of land surrounding Bay Tree Lake with the intent of creating an inland resort community (North Carolina Division of Parks and Recreation, Planning and Development Section 1996a). Later that year, a proposal was constructed and sent to the North Carolina Department of Conservation and Development concerning the drainage of Bay Tree Lake. The purpose for draining the lake was to improve the quality of the water and lake bottom for recreational purposes (e.g., swimming and boating). Permission to lower lake levels 4 feet was granted in 1965 and in January of 1966, the development group made a request to completely drain the lake where peat deposits and debris could be taken from the lake bottom (North Carolina Division of Parks and Recreation, Planning and Development Section 1996a).
The purpose of the drainage project was to release tannic, tea-colored, waters from the lake and divert all incoming tannic waters from a northerly adjacent swamp to below the outflow channel. Drainage of the lake was completed in the winter of 1966. The lake remained dry for 5 years while developers removed debris and peat deposits and imported large quantities of white sand, which would later be distributed around the entirety of the lakeshore. In 1970, the lakes outflow channel was plugged and the lake began to refill (North Carolina Division of Parks and Recreation, Planning and Development Section 1996a). After two years, the lake had nearly reached its original water levels. Shortly after residential lots went for sale, a breach of the lake rim occurred and tea-stained waters were allowed to re-enter the lake. The breach was plugged within 24 hours, but the lake had already returned to its original dystrophic condition (North Carolina Division of Parks and Recreation, Planning and Development Section 1996a). The lake has not been significantly altered since and remains in a dystrophic condition to this day.
Bay Tree Lake State Park contains five natural community types (Mesic Pine Savanna – Coastal Plain Subtype [S2,G2G3]; Sand Barren – Typic Subtype [S2,G2]; Small Depression Drawdown Meadow – Typic Subtype [S2S3,G2?]; Small Depression Pocosin – Blueberry Subtype [S2,G3?]; and Xeric Sandhill Scrub – Typic Subtype [S3S4,G3?]. A Natural Lake Shoreline community was not assigned to Bay Tree Lake by the NCNHP due to the shoreline’s disturbance history. The present authors agree with this determination and have chosen not to assign a natural lake shoreline community to this site. However, it is worth noting that the shoreline flora of Bay Tree Lake differs only slightly from the other Bladen Lakes.
Bay Tree Lake forms the headwaters of Lake Creek, a small blackwater creek that drains southeast to the South River (the boundary between Bladen and Sampson counties). Much of the land surounding Bay Tree Lake State Park has been cleared for agriculture (particularly blueberry farms) and has limited the landscape connectivity between it and other intact natural areas. Several bay complexes occur in the immediate vicinity of Bay Tree Lake including Beagle Bay, Black Creek Bay, Causeway Bay, Cooley Bay, Horsepen Bay (now an artificially created lake/pond), Floodgate Bay, Kelso Bay, and Spring Bay. A residential resort community is located along the north and east shorelines of the lake. The boundaries of this community have continued to extend around the east and southeast shorelines. Residential development, agricultural expansion, severe offroad vehicle use, and fire supression are the primary threats to biological diversity within and around Bay Tree Lake State Park (N. Howell pers. obs.). Available water quality parameters for Bay Tree Lake are provided in Table 1.
Table 1.
Water Quality Data for Bay Tree Lake (Bladen County, North Carolina). Frey (1949) sampled Bay Tree Lake 6 times during the Summer and Fall of 1947. Weiss and Kuenzler (1976) sampled Bay Tree Lake twice in 1974 (March 22 and June 6) and 4 times in 1975 (April 7, June 10, August 5, October 6). North Carolina Division of Water Quality, Environmental Sciences Section, Intensive Survey Unit (2009) (DWQ) sampled Bay Tree Lake 4 times in 2008 (June 24, July 29, August 18, October 2). Value ranges have been provided where applicable to show variability. Units are as follows: km2 = squared kilometers, ha = hectares, km = kilometers, m = meters, °C = degrees celsius, mg/L – milligrams per liter, meq/L = milliequivalents per liter, s.u. = standard units, μg/L = micrograms per liter.
| Frey (1949) | Weiss & Kuenzler (1976) | DWQ 2009 | |
| Trophic Status | − | − | Dystrophic |
| Watershed Area (km2) | − | − | 10.36 |
| Surface Area (ha) | − | 573.84 | − |
| Max Width (km) | − | 1.77 | − |
| Max Length (km) | − | 3.05 | − |
| Max Depth (m) | − | 1.83 | − |
| Mean Depth (m) | − | − | 0.9 |
| Secchi Depth (m) | 0.55 | 0.3−0.4 | 1.4−1.8 |
| Min Temp. (°C) | − | 13.3 | 23.2 |
| Max Temp. (°C) | − | 30.5 | 30 |
| Dissolved Oxygen (mg/L) | 6.4 | 7.1−10.9 | 6.8−8 |
| Alkalinity (meq/L) | − | 0.159−0.231 | − |
| pH (s.u.) | 4.4 | 6.3−7.1 | 4.1−4.5 |
| Total N (mg/L) | − | 0.48−1.568 | − |
| Total P (mg/L) | − | 0.13−0.238 | − |
| Chlorophyll-A (μg/L) | − | − | 2−6 |
Horseshoe Lake
Horseshoe Lake (also known as Suggs Mill Pond; 109 hectares; ca. 270 acres) is an irregularly shaped Carolina bay lake located in northern Bladen County south of Bushy Lake State Natural Area, east of Little Singletary Lake, north of SR 1325 (Gum Springs Rd), and west of SR 1002 (Old Fayetteville Rd). Horseshoe Lake is one of two Carolina bay lakes within Suggs Mill Pond Game Land (4469.34 hectares; 11,044 acres; Fig. 10), the other being Little Singletary Lake. Suggs Mill Pond Game Land is owned by the State of North Carolina and the North Carolina Wildlife Resources Commission (NCWRC) and is located in northern Bladen County and southern Cumberland County. This game land is located in the northwestern portion of the Bladen Lakes Macrosite and contains one of the largest remaining examples of unaltered Carolina bay complexes.
Figure 10.

Suggs Mill Pond Game Land (outlined in green) and surrounding lands. Lands north of the red dividing line occur in Cumberland County while lands south of the red line occur in Bladen County. Suggs Mill Pond Game Land contains two large bay lakes within its boundary. Little Singletary Lake is located along the western boundary of the property and Horseshoe Lake (aka Suggs Mill Pond) is located in the center of the property. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
The state first gained rights to the property in 1994 when a 62-acre (25 ha) parcel was donated to the NCWRC from Canal Woods Industries. Thereafter, much of the remaining property was purchased from Canal Woods. The fact that Horseshoe Lake and Little Singletary lake were not owned by the state of North Carolina until the mid-1990s suggests that these lakes were involved in a similar ownership situation as Bakers Lake (i.e., these lakes must have been privately owned prior to 1929 when legislation mandated that all lakes greater than 50 acres (20.2 ha) in size be released to the state of North Carolina). Suggs Mill Pond Game Land is one of four North Carolina game lands enrolled in the Cooperative Upland habitat Restoration and Enhancement program (CURE), where management for early successional habitat is the top priority (Allen et al. 2015). Traditionally, hunters and fishermen were primary users of Suggs Mill Pond Game Land, but an increasing number of non-traditional users (i.e., birders, canoers, hikers, photographers, and researchers) visit the site regularly.
The largest bay on site contains a horsehoe-shaped artificial impoundment (Horseshoe Lake). Horseshoe Lake forms the headwaters of Ellis Creek, which drains southwest to the Cape Fear River. Although an old milldam currently maintains Horseshoe Lake, it is thought that a smaller body of open water may have been present prior to the dam’s installation in the late 19th or early 20th centuries. Horseshoe Lake was formed subsequent to the dam installation, as water levels began to rise into the peat-filled Carolina bay. Today, it is best described as a semi-permanent impoundment; however, the presence of floating bogs within the lake makes it unique from other semi-permanent impoundments in North Carolina. Parts of the lake support patches of the rare floating bog community (the largest extent known from the state), which is dominated by sedges, orchids, carnivorous plants, and ericaceous shrubs. Other portions comprise the Coastal Plain Semipermanent Impoundment community, which is characterized by open water, dominated by floating-leaved macrophytes, and a sparse overstory of Taxodium ascendens Brongn.
The floating bog community type is quite unique. Manifestations of this community type occur just above the water surface and range in size from ca. 10 × 10 m to a few hectares in size (N. Howell, pers. obs.). Some bogs may contain well-developed herbaceous vegetation in addition to small (e.g., < 3 m tall) trees of Chamaecyparis thyoides, Nyssa biflora, and Taxodium ascendens, while others contain a strictly herbaceous component. Exposed portions of peat can be seen around the peripheries of some bogs; here, Drosera intermedia Hayne, Eleocharis baldwinii (Torr.) Chapm. /E. vivipara Link, Pogonia ophioglossoides (L.) Ker Gawl, Utricularia striata Leconte ex Torr., Utricularia purpurea Walter, and other small-statured herbaceous plants can be seen colonizing the apparently young peat formations. Isolated floating bogs (i.e., bogs surrounded by open water and separated from adjacent bogs and upland habitats) of varying size show a consistent zonation pattern. Small statured herbaceous taxa colonize the outer periphery and are slowly replaced by larger herbaceous taxa (Andropogon glaucopsis, Dulichium arundinaceum (L.) Britton, Hypericum virginicum L., Rhexia nashii Small, Rhynchospora alba (L.) Vahl, Rhynchospora inundata (Oakes) Fernald, Xyris fimbriata Elliott, and Xyris smalliana Nash) and woody species (Acer rubrum L., Chamaecyparis thyiodes, Decodon verticillatus (L.) Elliott, Nyssa biflora, and Taxodium ascendens) when moving toward the center. Thus, a dome-shaped appearance is typically seen.
Few examples of floating bogs or mats of vegetation are known to science. The floating peat mats of New Hampshire are most similar to those of Horseshoe Lake. These peat mats possess the same general structure and abiotic conditions as those of Horseshoe Lake and are known to contain several overlapping taxa, inculding Drosera intermedia, Dulichium arundinaceum, Eleocharis R. Br. spp., Hypericum virginicum, Nymphaea odorata W.T. Aiton, Rhynchospora alba, and Utricularia spp. (New Hampshire Division of Forests and Lands 2015).
A separate but similar case of floating vegetation mats, forming as a result of dam installation, has been observed at Goose Creek Reservoir in South Carolina (Hunt 1943). In 1933, a dam was installed on Goose Creek, ca. 12 miles north of Charleston, subsequently flooding historic rice plantations that had reverted to brackish marsh vegetation. Hunt (1943) described the zonation (looking across to the center of the mat from the outer periphery) of a typical floating mat as follows: (1) pioneer zone (i.e., the outer margins of the mats): Alternanthera philoxeroides (Mart.) Griseb., Bidens laevis (L.) Britton, Sterns & Poggenb., Boehmeria cylindrica (L.) Sw., Habenaria repens, Hydrocotyle ranunculoides L.f., Persicaria glabra (Willd.) M. Gómez, and Sacciolepis striata (L.) Nash, (2) the cat-tail/shrub zone: Kosteletzkya pentacarpos (L.) Ledeb., Typha latifolia L., and Salix nigra Marshall, and (3) the main body: Acer rubrum, Apios americana Medik., Decodon verticillatus, Mikania scandens (L.) Willd., Panicum virgatum L. var. virgatum, Persea palustris, Rubus L. spp., and Taxodium distichum (L.) Rich.
The floating “sudd” vegetation of the upper Nile River is also somewhat similar, forming large floating mats of marsh vegetation both along the margins and within the river. Denny (1984) gave a general description of the sudd vegetation as seen only from a boat. Several taxa commonly observed along the margins of the Sudd included: Ceratophyllum demersum L., Cyperus papyrus L., Eichhornia crassipes (Mart.) Solms, Phragmites karka (Retz.) Trin. ex Steud., Typha domingensis Pers., Vossia cuspidata (Roxb.) Griff. A complete checklist of the vascular plants collected from this vegetative study can be found in the attached appendix of Denny (1984).
Eleven natural community types exist within Suggs Mill Pond Game Land, but the low and high pocosin communities are dominant, comprising 48% (2,119.74 hectares; 5,238 acres) of the site (Allen et al. 2015). Lakes and impoundments make up 8.6% (381.21 hectares; 942 acres) of the total acreage of the game land. Fair to high quality landscape connections exist between Suggs Mill Pond Game Land and adjacent natural areas within the Bladen Lakes Macrosite (i.e., Bushy Lake State Natural Area, Charlie Long Mill Pond/Big Colly Bay Natural Area, Jessups Pond, Mill Pond Bay Natural Area, and White Pond Bay Natural Area; LeBlond and Grant 2005). These connections to other large natural areas provide relatively uninterrupted habitat for the movement of plants and animals. Records of Horseshoe Lake’s water quality are lacking, but the lakes water appears high in humic substances and the chemistry is more than likely similar to the other Bladen Lakes. The lake is dystrophic and probably exhibits a pH of < 5.
Jones Lake
Jones Lake (91.05 hectares; 225 acres) is one of two dystrophic Carolina bay lakes located within Jones Lake State Park (893.54 hectares; 2,208 acres; Fig. 11), the other being Salters Lake. This lake is located in central Bladen County four miles north of Elizabethtown west of NC Hwy 242 and east of NC Hwy 53. Jones Lake State Parkforms the headwaters of an unnamed tributary of Turnbull Creek, which drains into the Cape Fear River. The state park sits on a sandy terrace (of Upper Pleistocene age) of the Cape Fear River (Soller 1988). Jones Lake was originally referred to as Woodward’s Lake, after Samuel Woodward, justice of the peace for the area in 1734 (North Carolina Division of Parks and Recreation, Planning and Development Section 2006b). It is believed that the lake later received its current name from Isaac Jones, an adjacent landowner to Samuel Wooodward, on whose land Elizabethtown was later established in 1773. Jones Lake State Park was established in 1939 and became the first state park specifically devoted to African Americans (North Carolina Department of Conservation and Development 1940).
Figure 11.

Jones Lake State Park (outlined in green) and surrounding lands. Jones Lake State Park is located between state highways 53 and 242, north of the Cape Fear River. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
LeBlond and Grant (2005) described both Jones and Salters Lakes as “among the very best examples of Carolina bay lakes in nearly pristine condition”. Jones Lake State Park is connected by fair to high quality landscape connections to Bethel Flatwoods, Cotton Bay Sand Ridge, Tatum Mill Pond/Cypress Bay, and Turnbull Creek Swamp natural areas.
Eleven natural community types have been described from Jones Lake State Park (i.e., Bay Forest, Coastal Plain Small Stream Swamp, High Pocosin, Low Pocosin, Natural Lake Shoreline, Peatland Atlantic White Cedar Forest, Pine/Scrub Oak Sandhill Mixed Oak Variant, Pond Pine Woodland, Wet Pine Flatwoods Wet Spodosol Variant, Xeric Sandhill Scrub Coastal Plain Variant, Xeric Sandhill Scrub Sandbarren Variant; LeBlond and Grant 2005, Schafale 2012), several of which are of extremely high quality and globally rare, such as the Low Pocosin, Peatland Atlantic White Cedar Forest, and Xeric Sandhill Scrub (LeBlond and Grant 2005, Schafale 2012). Available water quality parameters for Jones Lake are provided in Table 2.
Table 2.
Water Quality Data for Jones Lake (Bladen County, North Carolina). Frey (1949) sampled Jones Lake 9 times during the Summer and Fall of 1947. Weiss and Kuenzler (1976) sampled Jones Lake twice in 1974 (March 22 and June 6) and 4 times in 1975 (April 7, June 10, August 5, October 6). North Carolina Division of Water Quality, Environmental Sciences Section, Intensive Survey Unit 2009 (DWQ) sampled Jones Lake 5 times in 2008 (May 29, June 25, July 15, September 10, September 24). Value ranges have been provided where applicable to show variability. Units are as follows: km2 = squared kilometers, ha = hectares, km = kilometers, m = meters, °C = degrees celsius, mg/L – milligrams per liter, meq/L = milliequivalents per liter, s.u. = standard units, μg/L = micrograms per liter.
| Frey (1949) | Weiss & Kuenzler (1976) | DWQ 2009 | |
| Trophic Status | − | − | Dystrophic |
| Watershed Area (km2) | − | − | 5.18 |
| Surface Area (ha) | − | 90.65 | − |
| Max Width (km) | − | 0.48 | − |
| Max Length (km) | − | 0.80 | − |
| Max Depth (m) | − | 2.13 | − |
| Mean Depth (m) | − | − | 0.9 |
| Secchi Depth (m) | 0.73 | 0.3−1.22 | 1.3−2.4 |
| Min Temp. (°C) | − | 14 | 21.9 |
| Max Temp. (°C) | − | 30.5 | 29.6 |
| Dissolved Oxygen (mg/L) | 5.7 | 6.7−10.6 | 6.2−7.5 |
| Alkalinity (meq/L) | − | 0−0.002 | − |
| pH (s.u.) | 4.34 | 3.1−4.8 | 3.6−4.2 |
| Total N (mg/L) | − | 0.32−0.73 | − |
| Total P (mg/L) | − | 0.013−0.025 | − |
| Chlorophyll-A (μg/L) | − | − | 1−11 |
Lake Waccamaw
Lake Waccamaw is located south of the township of Lake Waccamaw, between Friar Swamp to the northeast, and the Waccamaw River to the south. It is the only Carolina bay lake located in Columbus County and is the largest Carolina bay and bay lake (3,617.48 hectares; 8,939 acres) in North Carolina (LeBlond 1995). Lake Waccamaw is the third largest lake in North Carolina behind Lake Mattamuskeet and Lake Phelps. The lake is part of Lake Waccamaw State Park (4,327.70 hectares; 10,694 acres; Fig. 12), which also includes lands directly abutting the lake’s southern shoreline. Stager and Cahoon (1987) estimated Lake Waccamaw to be ca. 15,000 years old or less.
Figure 12.

Lake Waccamaw State Park (outlined in green) and surrounding lands. Lake Waccamaw State Park is a large state park encompassing Lake Waccamaw and adjacent swampland and uplands. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
Prior to European civilization in the Southeast, the Waccamaw-Sioux Native American peoples, one of five Native American tribes known to inhabit the Cape Fear Region, inhabited the lands surrounding the lake (North Carolina Division of Parks and Recreation, Planning and Development Section 2006a). Native American artifacts, including dugout canoes, dating back to 1015−315 B.P. have been found within and around Lake Waccamaw. In the early 18th century, an unknown young man traveled through Columbus County on his way from north Georgia and, upon seeing Lake Waccamaw, described it as “the most pleasantest place that ever I saw in my life. It is at least eighteen miles round, surrounded with exceeding good land, as oak of all sorts, hickory and fine cypress swamps” (Gentleman 1737).
This bay lake differs from the Bladen lakes in its larger size, neutral pH, mesotrophic status, and presence of alluvial hydrologic inputs (Big Creek). Tea-stained waters from Friar Swamp are delivered into northeast Lake Waccamaw via Big Creek, the largest of several creeks draining into the lake from Friar Swamp. Lake Waccamaw forms the headwaters of the Waccamaw River, a species-rich river system known to support several rare plant (e.g., Fimbristylis perpusilla R.M. Harper ex Small & Britton, Ilex amelanchier M.A. Curtis ex Chapm., Lipocarpha micrantha (Vahl) G.C. Tucker, Oldenlandia boscii (DC.) Chapm., Rhynchospora decurrens Chapm., and Sabatia kennedyana Fernald) and animal taxa (Alligator mississipiensis [American Alligator], Elliptio folliculata [Pod Lance], Etheostoma perlongum [Waccamaw Darter], Lampsilis ochracea [Tidewater Mucket], Menidia extensa [Waccamaw Silverside], Noturus spp. 2 [Broadtail Madtom], and Procambarus leptodactylus [Pee Dee Lotic Crayfish; LeBlond 1995]).
Much of the land surrounding Lake Waccamaw has been converted to agriculture (north of the lake) and loblolly pine plantations (south of the lake). A small portion of Lake Waccamaw’s bay is still present on the northern end.
The Coastal Plain Marl Outcrop occurs along a roughly 394 m (1,000 ft.) stretch of northern shoreline and is characterized by having vertical and overhanging low cliffs in the supralittoral zone of the lake. Portions of these cliffs are submerged in the upper eulittoral zone, but local residents privately own terrestrial portions. This marl community is known for supporting the only naturally occuring population of Venus hair fern (Adiantum capillus-veneris L.) in the state.
Shoreline residential development extends along the northern shores of the lake from the lake outlet (southwest corner of lake) to just south of Big Creek. These shorelines support the globally rare Natural Lake Shoreline Marsh (Lake Waccamaw Pondlily Subtype) community. Undeveloped shorelines (i.e., Natural Lake Shoreline Swamp – Lake Waccamaw Subtype) occur from just south of Big Creek to the lake’s outlet. Historically, Lake Waccamaw experienced wide-ranging water level fluctuations determined by precipitation. In 1925, a poorly constructed dam was built at the lakes outlet in an effort to stabilize lake levels for increased recreational use. Before construction began, lake levels were so low that vehicles could be driven to the construction site on the dried lake bed (North Carolina Division of Parks and Recreation, Planning and Development Section 2006a).
The physical and hydrographic nature of Lake Waccamaw’s shoreline also differs from the other bay lakes. Lake Waccamaw’s shoreline is sandy around its entire periphery (Frey 1949), whereas the Bladen lakes may be either sandy or peaty along their shorelines.
A broad, sandy, terrace (lacking in Bladen lakes) is also present along the southeast shoreline of Lake Waccamaw (Fig. 5). This shallow underwater terrace extends perpendicularly out into the lake as far as 305 m (1,000 ft.; Frey 1949). The gentle relief of the terrace gradually extends shoreward resulting in a shallow, broad, littoral zone. This littoral zone is the most floristically rich of all Carolina bay lakes and is rivaled only by Lake Phelps in Washington County, North Carolina (N. Howell, pers. obs.). Varying water depths in the littoral zone of Lake Waccamaw result in the temporary and sometimes permanent presence of offshore sandbars and islands. This hydrographical heterogeneity in the littoral zone increases the floristic richness. A more detailed review of the lakes shoreline flora is provided in the floristic summary section and in Suppl. material 6.
The buffering effect of subsurface and surficial limestone on the naturally acidic waters of Lake Waccamaw result in an unusually diverse fauna. Lake Waccamaw contains the largest number of endemic animal species (i.e., endemic to this lake and nowhere else in the world; 10 taxa) of any site in North Carolina (Hubbs and Raney 1946, LeBlond 1995). An additional species, Fundulus waccamawensis (Waccamaw Killfish), is found only in waters within and adjacent to Lake Waccamaw and Lake Phelps (Washington County, North Carolina). Six other faunal taxa known to be rare but not endemic also occur within or adjacent to the lake. Available water quality parameters for Lake Waccamaw are provided in Table 3.
Table 3.
Water Quality Data for Lake Waccamaw (Columbus County, North Carolina). Frey (1949) sampled Lake Waccamaw 8 times during the Summer and Fall of 1947. Weiss and Kuenzler (1976) sampled Lake Waccamaw twice in 1974 (March 22 and June 6) and 4 times in 1975 (April 7, June 10, August 5, October 6). North Carolina Division of Water Quality, Environmental Sciences Section, Intensive Survey Unit (2012) (DWQ) sampled Lake Waccamaw 5 times in 2011 (May 4, June 8, September 1, July 20, and August 17). Value ranges have been provided where applicable to show variability. Units are as follows: km2 = squared kilometers, ha = hectares, km = kilometers, m = meters, °C = degrees celsius, mg/L – milligrams per liter, meq/L = milliequivalents per liter, s.u. = standard units, μg/L = micrograms per liter.
| Frey (1949) | Weiss & Kuenzler (1976) | DWQ 2012 | |
| Trophic Status | − | − | Mesotrophic |
| Watershed Area (km2) | − | − | 181.29 |
| Surface Area (ha) | − | 3617.08 | − |
| Max Width (km) | − | 5.47 | − |
| Max Length (km) | − | 8.36 | − |
| Max Depth (m) | − | 3.35 | − |
| Mean Depth (m) | − | − | 1.5 |
| Secchi Depth (m) | 1.34 | 0.61−2.38 | 1.1−1.9 |
| Min Temp. (°C) | − | 14 | 23.5 |
| Max Temp. (°C) | − | 31.5 | 29.9 |
| Dissolved Oxygen (mg/L) | 5.2 | 7.8−11 | 6.9−8.1 |
| Alkalinity (meq/L) | − | 0.14−0.24 | − |
| pH (s.u.) | 6.95 | 6.8−7.5 | 7.0−8.5 |
| Total N (mg/L) | − | 0.297−1.56 | − |
| Total P (mg/L) | − | 0.017 − .055 | − |
| Chlorophyll-A (μg/L) | − | − | 2.8−8 |
Little Singletary Lake
Little Singletary Lake (626 acres; 253.33 hectares) is located in the western half of Suggs Mill Pond Game Land (Fig. 10). Unlike Horseshoe Lake, Little Singletary Lake is natural in origin and exhibits a more “typical” bay lake physiognomy. Little Singletary Lake forms the headwaters of Lake Run, a tributary of Ellis Creek, which drains into the Cape Fear River. Relatively intact landscape connections exist to the northeast (Horseshoe Lake), southeast (Marshy Bay Natural Area), and southwest (Cedar Swamp Seep Natural Area) from Little Singletary Lake.
Lands abutting the southern shoreline are privately owned and were once subject to residential development. Remnants of bulkheads and recreational piers can still be seen today along the southeast shoreline. The North Carolina Wildlife Resources Commission gained property rights to all remaining lands surrounding Little Singletary Lake before residential development could ensue. On June 20, 2011, a lightning caused wildfire (Simmons Road Fire) started just west of Little Singletary Lake and by August 18th, had burned over 2,023 hectares (5,000 acres) of Carolina bay and pocosin habitat, much of which surrounded Little Singletary Lake. During growing seasons of extreme drought, water levels have been known to recede low enough to reveal a clean sandy lake bottom 90−275 m (100−300 yds) out into the lake (G. Lewis, pers. comm.). Native American projectile points have been found on this lake bottom during drought years (G. Lewis, pers. comm.).
The water quality of Little Singletary Lake has not been documented by state agencies. The water appears high in humic substances and is likely similar to the other Bladen lakes (i.e., dystrophic, acidic, shallow, nutrient poor).
Salters Lake
Salters Lake (127.47 hectares; 315 acres) is the larger of the two Carolina bay lakes located in Jones Lake State Park (Fig. 11). Salters Lake was named after Sallie Salter, a revolutionary war hero who spied on the Tories while encamped at Elizabethtown. Her spying played a role in the defeat over the Tories on August 28, 1771, at the battle of Elizabethtown, where 70 Whigs defeated 400 Tories (JNorth Carolina Division of Parks and Recreation, Planning and Development Section 2006b).
Salters Lake is similar to Jones Lake in many respects, but quite possibly could be the most “pristine” of all Carolina bay lakes. Salters Lake has no shoreline development, appreciable recreational activities (e.g., outboard motor use), immediate surrounding agricultural (crop or animal production) land use, water level control structures, or historical manipulation of any kind. Natural communities and landscape features for Salters Lake are the same as those for Jones Lake (above). Available water quality parameters for Salters Lake are provided in Table 4.
Table 4.
Water Quality Data for Salters Lake (Bladen County, North Carolina). Frey (1949) sampled Salters Lake 7 times during the Summer and Fall of 1947. Weiss and Kuenzler (1976) sampled Salters Lake twice in 1974 (March 22 and June 6). North Carolina Division of Water Quality, Environmental Sciences Section, Intensive Survey Unit 2009 (DWQ) sampled Salters Lake 4 times in 2008 (June 25, July 15, August 20, September 24). Value ranges have been provided where applicable to show variability. Units are as follows: km2 = squared kilometers, ha = hectares, km = kilometers, m = meters, °C = degrees celsius, mg/L – milligrams per liter, meq/L = milliequivalents per liter, s.u. = standard units, μg/L = micrograms per liter.
| Frey (1949) | Weiss & Kuenzler (1976) | DWQ 2009 | |
| Trophic Status | − | − | Dystrophic |
| Watershed Area (km2) | − | − | 7.77 |
| Surface Area (ha) | − | 127.47 | − |
| Max Width (km) | − | 0.80 | − |
| Max Length (km) | − | 1.12 | − |
| Max Depth (m) | − | 1.82 | − |
| Mean Depth (m) | − | − | 2.13 |
| Secchi Depth (m) | 0.55 | 0.6−0.91 | − |
| Min Temp. (°C) | − | 15 | 21.7 |
| Max Temp. (°C) | − | 25.4 | 31.2 |
| Dissolved Oxygen (mg/L) | 6 | 7.9−10.1 | 6.5 – 8.1 |
| Alkalinity (meq/L) | − | 0.0019 | − |
| pH (s.u.) | 4.49 | 4.1−4.8 | 3.6 – 4.1 |
| Total N (mg/L) | − | 0.293−0.374 | − |
| Total P (mg/L) | − | 0.015−0.016 | − |
| Chlorophyll-A (μg/L) | − | − | 4.7 – 26 |
Singletary Lake
Singletary Lake (233.09 hectares; 576 acres) is located within Singletary Lake State Park (494.12 hectares; 1,221 acres; Fig. 13). This lake was named after Richard Singletary, who received the grant of land in 1729 (North Carolina Division of Parks and Recreation, Planning and Development Section 1996b). Singletary Lake State Park is located just southeast of White Lake in central-southeast Bladen County between the Cape Fear River and Colly Swamp. Singletary Lake forms the headwaters of Lake Drain Creek, which drains into Big Colly Creek, which drains to the Black River, which drains into the Cape Fear River.
Figure 13.

Singletary Lake State Park (outlined in green) and surrounding lands. Singletary Lake State Park is primarily comprised of lands immediately surrounding Singletary Lake. In addition to the lands surrounding Singletary Lake, White Lake is also managed by Singletary Lake State Park. Singletary Lake State Park is located north of the Cape Fear River and State Hwy 53 and southeast of White Lake. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
Singletary Lake is similar to the other Bladen lakes in that it is dystrophic, acidic, and nutrient poor. It contains high quality examples of the Natural Lake Shoreline Swamp (Cypress Subtype) and Natural Lake Shoreline Marsh (Typic Subtype) communities. LeBlond and Grant (2005) described this lake’s shoreline community as “one of the most aesthetically pleasing natural communities in the North Carolina Coastal Plain”. A direct landscape connection exists between Singletary Lake and Colly Swamp and the Black River to the northeast. Fair quality landscape connections exist between the state park and the Cape Fear River to the southwest. Available water quality parameters for Singletary Lake are provided in Table 5.
Table 5.
Water Quality Data for Singletary Lake (Bladen County, North Carolina). Frey (1949) sampled Singletary Lake 10 times during the Summer and Fall of 1947. Weiss and Kuenzler (1976) sampled Singletary Lake twice in 1974 (March 22 and June 6) and four times in 1975 (April 7, June 10, August 5, October 6). North Carolina Division of Water Quality, Environmental Sciences Section, Intensive Survey Unit (2009) (DWQ) sampled Singletary Lake 5 times in 2008 (June 25, July 15, August 20, September 24). Value ranges have been provided where applicable to show variability. Units are as follows: km2 = squared kilometers, ha = hectares, km = kilometers, m = meters, °C = degrees celsius, mg/L – milligrams per liter, meq/L = milliequivalents per liter, s.u. = standard units, μg/L = micrograms per liter.
| Frey (1949) | Weiss & Kuenzler (1976) | DWQ 2009 | |
| Trophic Status | − | − | Dystrophic |
| Watershed Area (km2) | − | − | 5.18 |
| Surface Area (ha) | − | 231.48 | − |
| Max Width (km) | − | 0.64 | − |
| Max Length (km) | − | 2.09 | − |
| Max Depth (m) | − | 2.74 | − |
| Mean Depth (m) | − | − | 2.13 |
| Secchi Depth (m) | 0.76 | 0.48−1.21 | 0.6−1 |
| Min Temp. (°C) | − | 13.8 | 24.8 |
| Max Temp. (°C) | − | 31 | 30.6 |
| Dissolved Oxygen (mg/L) | 6.6 | 7.3−11.2 | 6−7.8 |
| Alkalinity (meq/L) | − | 0.0019 | − |
| pH (s.u.) | 4.5 | 3.2−4.6 | 3.9−4.2 |
| Total N (mg/L) | − | 0.255−0.515 | − |
| Total P (mg/L) | − | 0.018−0.075 | − |
| Chlorophyll-A (μg/L) | − | − | 4.8−44 |
White Lake
Although not included in the sampling aspect of this study, White Lake is unique and deserves a brief summary. White Lake (432.20 hectares; 1,068 acres) is a large Carolina bay lake located in east-central Bladen County about 6 miles east of Elizabethtown, just east of the intersection of NC Hwy 53 and U.S. Hwy 701 (Fig. 14). White Lake is owned by the state of North Carolina, and is managed by Singletary Lake State park. Unlike all of the remaining bay lakes, White lake’s water is clear and not tea-stained. This feature has made it an incredibly attractive location for development and vacationers. This lake is primarily used for recreation (e.g., water sports, swimming, fishing) and essentially all of its shoreline is residentially and commercially developed.
Figure 14.

White Lake and surrounding lands. Like the majority of Carolina bay lakes, White Lake is a state-owned lake. All but a very small portion of White Lake’s shoreline has been altered. Aerial imagery, transportation, and hydrography layers obtained from NRCS Geospatial Data Gateway: https://gdg.sc.egov.usda.gov. Map produced by Nathan Howell using ArcGis Desktop: Version 10.2.2. (Environmental Systems Research Institute (ESRI) 2014).
White Lake’s remarkable water clarity is attributed to the presence of artesian springs on the lake bottom (Wells and Boyce 1953). The clarity of the lake’s water yields a deep euphotic zone (i.e., sunlight can penetrate through the entirety of the water column) with submerged aquatic macrophytes (e.g., Myriophyllum humile (Raf.) Morong; N. Howell pers. obs.) present at the lakes deepest depths. White Lake receives its hydrologic inputs principally in two forms, precipitation and groundwater (through springs). Although this lake is primarily fed by springs, its overall water levels are determined by the regional water table (i.e., during drought years, White Lake’s water levels will drop just like all other bay lakes). Another unique feature of White Lake is the location of its outlet channel. White Lake’s outlet channel is located in the northwestern section of the lake as opposed to the southeastern section where it occurs in all other bay lakes. Frey (1954) reported that William Bartram, a renowned naturalist who documented the flora, fauna, and Native American culture of the southeastern United States in the 18th century, operated a sawmill on White Lake during the 20 years following 1770. A map in Bartram and Harper (1942) shows that White Lake was formerly called Lake Bartram. Available water quality parameters for White Lake are provided in Table 6.
Table 6.
Water Quality Data for White Lake (Bladen County, North Carolina). Frey (1949) sampled White Lake 8 times during the Summer and Fall of 1947. Weiss and Kuenzler (1976) sampled White Lake twice in 1974 (March 22 and June 6). North Carolina Division of Water Quality, Environmental Sciences Section, Intensive Survey Unit (2009) (DWQ) sampled White Lake 5 times in 2008 (May 27, June 24, July 29, August 11, and October 2). Value ranges have been provided where applicable to show variability. Units are as follows: km2 = squared kilometers, ha = hectares, km = kilometers, m = meters, °C = degrees celsius, mg/L – milligrams per liter, meq/L = milliequivalents per liter, s.u. = standard units, μg/L = micrograms per liter.
| Frey (1949) | Weiss & Kuenzler (1976) | DWQ 2009 | |
| Trophic Status | − | − | Oligotrophic |
| Watershed Area (mi2) | − | − | − |
| Surface Area (ha) | − | 432.2 | − |
| Max Width (km) | − | 1.61 | − |
| Max Length (km) | − | 2.57 | − |
| Max Depth (m) | − | 3.35 | − |
| Mean Depth (m) | − | − | 3.04 |
| Secchi Depth (m) | 3.35 | 3.35 | 3.35 |
| Min Temp. (°C) | − | 15.1 | 22.3 |
| Max Temp. (°C) | − | 26.1 | 30.1 |
| Dissolved Oxygen (mg/L) | 6.7 | 8.6−10.1 | 6.8−8.2 |
| Alkalinity (meq/L) | − | 0.0019−.0099 | − |
| pH (s.u.) | 4.92 | 4.6−4.8 | 4.6−5.2 |
| Total N (mg/L) | − | 0.123−0.211 | − |
| Total P (mg/L) | − | 0.010−0.017 | − |
| Chlorophyll-A (μg/L) | − | − | 4.8−44 |
Climate
Bladen Lake Group (Bladen County, NC)
Climate data from the nearest weather station to the Bladen County bay lakes, ca. 1.6 kilometers away in Elizabethtown, North Carolina (Bladen County: 34.68° N, -78.58°W; 30.5 m elev.), show that during the thirty-year period between 1971-2000, the average annual temperature was 16.44 °C (61.6 °F) and mean annual precipitation 1,254.76 mm (49.4 in). Average daily maximum and minimum temperatures were 22.83 °C (73.1 °F) and 10.11 °C (50.2 °F; State Climate Office of North Carolina 2014; Fig. 15).
Walter climate diagrams for weather stations closest to the Bladen Lakes (Bladen County, NC; a) and Lake Waccamaw (Columbus County, NC; b), based on data from the State Climate Office of North Carolina (2014). At the top left of each figure, the town closest to the weather station is listed as well as the elevation of the weather station in meters and the number of years climate data were recorded (30). At the top right of each figure, the mean annual temperature and precipitation over thirty years for each site is listed. Climate data for these figures were recorded from 1971 to 2000. Solid black areas in the diagrams represent “excess rainfall”. When the precipitation curve rises above 100 mm, there is an excess amount of precipitation present that plants do not need in order to survive. Areas marked with vertical lines between the temperature curve and the 100 mm precipitation mark on the secondary y-axis represent a “wet period”. These diagrams show that plants in these two locations are not water-stressed (i.e., the precipitation curve does not drop below the temperature curve for the 30-year climatic period).
Figure 15a.

Figure 15b.

The lowest temperature recorded for Bladen County was -14.4 °C (6 °F) on January 17, 1977 (Leab 1990). The highest recorded temperature for Bladen County was 37.7 °C (100 °F) on July 20, 1977 (Leab 1990). Monthly average temperatures were highest in July and August and lowest in December and January. Monthly precipitation amounts were also highest in July and August, while the lowest monthly precipitation amounts were in April and November (State Climate Office of North Carolina 2014; Fig. 15). The annual growing season, defined as the number of days in five out of ten years during which the daily minimum air temperature exceeds -2.2 °C (28 °F), is 243 days in Bladen County (weather data recorded from 1957-1979; Leab 1990).
Lake Waccamaw (Columbus County, NC)
Climate data from the nearest weather station to Lake Waccamaw, ca. 16 km away in Whiteville, North Carolina (Columbus County: 34.27287° N, -78.71499° W; 29.8 meters above sea level), show that for the 30-year period between 1971 and 2000, the average annual temperature was 17.16 °C (62.9 °F) and mean annual precipitation 1,275.08 mm (50.2 in). The average daily maximum and minimum temperatures over the same thirty-year period were 24.3 °C (75.8 °F) and 10 °C (50 °F; State Climate Office of North Carolina 2014; Fig. 15).
The lowest temperature recorded for Columbus County was -15 °C (5 °F) on February 12, 1973 (Spruill 1990). The highest recorded temperature for Columbus County was 40.5 °C (105 °F) on June 27, 1954 (Spruill 1990). Monthly average temperatures were highest in July and August and lowest in January and February. Monthly precipitation amounts were also highest in July and August, while the lowest monthly precipitation amounts were in April and November (State Climate Office of North Carolina 2014; Fig. 15). The annual growing season, defined as the number of days in five out of ten years during which the daily minimum air temperature exceeds -2.2 °C (28 °F), is 240 days in Columbus County (weather data recorded from 1951-1981; Spruill 1990).
Plant Communities
Four plant community types and two subtypes can be distinguished within the littoral zone of Carolina bay lakes (Schafale 2012; Table 7). Of these four community types and subtypes, three are globally critically imperiled (Natural Lake Shoreline Swamp – Lake Waccamaw Subtype; Natural Lake Shoreline Marsh – Typic Subtype; Natural Lake Shoreline Marsh − Lake Waccamaw Pondlily Subtype), while the others do not have a conservation ranking (Table 7).
Table 7.
Plant community types occurring within the littoral zone of Carolina bay lakes. Community types follow Schafale (2012); rank designations follow Robinson and Finnegan (2014). Community types are presented in order of increasing species richness. The Natural Lake Shoreline Marsh (Lake Waccamaw Pondlily Subtype) typically supports a couple of dominant taxa (i.e., Nuphar sagittifolia and Eriocaulon aquaticum) with several other co-dominants. The Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) is known to contain 140+ taxa.
|
Species
Richness |
Plant Community Types |
State
Rank |
Global
Rank |
| Lowest Highest |
Natural Lake Shoreline Marsh (Lake Waccamaw Pondlily Subtype) | S1 | G1 |
| Coastal Plain Semipermanent Impoundment | S4 | G4G5 | |
| floating Bog | S1 | G1? | |
| Natural Lake Shoreline Swamp (Cypress Subtype) | S2 | G3 | |
| Natural Lake Shoreline Marsh (Typic Subtype) | S1 | G1 | |
| Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) | S1 | G1 | |
| S1 = Critically Imperiled, 1–5 occurrences in state; S2 = Imperiled, 6–20 occurrences in state; S4 = Apparently Secure, 101–1000 occurrences in state; G1 = Critically Imperiled, 1–5 occurrences in the world; G3 = Vulnerable, 21–100 occurrences in the world; G4 = Apparently Secure, 101–1000 occurrences in the world; G5 = Secure, 1001+ occurrences in the world. | |||
Natural Lake Shoreline Swamp (Cypress Subtype; S2G3) [Taxodium distichum – T. ascendens / Panicum hemitomon Schult. Woodland (CES203.044)].
This natural community type covers Carolina bay lake shorelines with narrow littoral zones characterized by an absent to sparse herbaceous component and a nearly closed canopy of Chamaecyparis Spach, Nyssa L., or Taxodium Rich. in the upper eulittoral zone. If a cross-section of this littoral zone were to be drawn, the epilittoral vegetation would abruptly coincide with the littoral zone (i.e., a zone of emergent herbaceous vegetation is lacking where it typically would occur between the epilittoral and infralittoral zones). This “two-staged” zonation pattern typical of this community type is directly attributable to the steeper hydrography and narrow littoral zone. The Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) and the Natural Lake Shoreline Marsh community types can be distinguished from the depauperate Natural Lake Shoreline Swamp (Cypress Subtype) community type by having a broader littoral zone, a well-developed zone of herbaceous emergent macrophytes, a sparse to open canopy of Nyssa, Taxodium, or other obligate wetland hardwoods, and the absence of Nuphar sagittifolia (Walter) Pursh. Examples of this community type are found at Bakers Lake, and the western, northern, and eastern shorelines of Jones, Salters, Little Singletary, and Singletary Lakes.
Natural Lake Shoreline Swamp (Lake Waccamaw Subtype; S1G1) [Taxodium distichum – T. ascendens / Panicum hemitomon – Sclerolepis uniflora (Walter) Britton, Sterns & Poggenb. Woodland (CEGL004465)].
This natural community type covers the southern shoreline of Lake Waccamaw located between Big Creek and the lake’s outlet on the southwest shore. This stretch of natural shoreline is characterized by gentle hydrography, which results in a broad littoral zone, and a species-rich flora dominated by emergent herbaceous macrophytes, many of which are rare. Emergent macrophytes typical of this community type include Cladium mariscoides (Muhl.) Torr., Eriocaulon aqutaicum (Hill) Druce, Panicum hemitomon, Sclerolepis uniflora, and Xyris smalliana, among others. This community type can be distinguished from the species-poor Natural Lake Shoreline Swamp (Cypress Subtype) community type by its broader littoral zone and species-rich herbaceous component (95 taxa). It can be distinguished from the Natural Lake Shoreline Marsh community types by the absence or only irregular presence of Nuphar sagittifolia and the unique assemblage of diverse herbaceous taxa (e.g., Bacopa caroliniana (Walter) B.L. Rob., Boltonia asteroides (L.) L’Hér. var. glastifolia, Cladium mariscoides, Ludwigia brevipes (B.H. Long ex Britton, A. Braun & Small) Eames, L. sphaerocarpa Elliott, and Sclerolepis uniflora).
Natural Lake Shoreline Marsh (Typic Subtype; S1G1) [Panicum hemitomon – Juncus spp. Coastal Plain Lakeshore Herbaceous Vegetation (CEGL004307)].
This natural community type covers the southern shorelines of the Bladen Lakes. The southern shorelines have a broader littoral zone than the remaining portions of the lakes. Consequently, they support a more diverse emergent herbaceous component. Herbs found in this community type include Eleocharis baldwinii, E. equisetoides (Elliott) Torr., E. vivipara, Juncus pelocarpus E. Mey., Panicum hemitomon, Panicum verrucosum Muhl., Rhexia nashii, Rhynchospora distans, Saccharum giganteum (Walter) Pers., Sacciolepis striata, Scirpus cyperinus (l.) Kunth, and Xyris smalliana. This community type is also characterized as having a sparse to open canopy of Nyssa and Taxodium. This community type can be distinguished from the Natural Lake Shoreline Marsh (Lake Waccamaw Pondlily Subtype) by the absence of Nuphar sagittifolia and from the Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) by the occurence of < 30 herbaceous taxa, none of which include the unique and rare herbs found at Lake Waccamaw. Examples of this community type include the southern shorelines of Jones, Little Singletary, Salters, and Singletary Lakes.
Natural Lake Shoreline Marsh (Lake Waccamaw Pond-lily Subtype; S1G1) [Nuphar sagittifola – Eriocaulon aquaticum Lakeshore Herbaceous Vegetation (CEGL004297)].
This natural community type covers the western, northern, and eastern shorelines of Lake Waccamaw (i.e., where residential and commercial development is present). It is the only Natural Lake Shoreline community type dominated by Nuphar sagittifolia (a distinguishing feature) and Eriocaulon aquaticum. Nuphar sagittifolia is essentially absent from the Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) community type save for small stands around the mouth of Big Creek and around the dam at the lakes outlet.
floating Bog [Rhynchospora alba Saturated Herbaceous Vegetation (CEGL004463)]
This natural community type covers the rare examples of vegetation occuring on floating peat mats in deep water of natural or artificial ponds and lakes. Horseshoe Lake is the only Carolina bay lake known to support floating bogs. The floating bogs of Horseshoe Lake are the largest in the state. These floating bogs are saturated and nutrient-poor, supporting taxa that characteristically inhabit such stressful conditions (e.g., Calopogon tuberosus (L.) Britton, Sterns & Poggenb., Drosera intermedia, Dulichium arundinaceum, Hypericum virginicum, Pogonia ophioglossoides, Rhynchospora alba, R. inundata, and Xyris fimbriata). This community type’s “floating” nature and the presence of the aforementioned plant taxa sets it apart from all others.
Coastal Plain Semipermanent Impoundment (Cypress-Gum Subtype; G4G5) [Taxodium distichum / Lemna minor L. Forest (CEGL002420)]
All portions of Horseshoe Lake not considered floating Bog fall into the Coastal Plain Semipermanent Impoundment community type. This community type is characterized by a sparse to absent canopy of Taxodium ascendens with sporadically occurring beds of floating-leaved and submersed aquatics (e.g., Brasenia schreberi J.F. Gmel, Cabomba caroliniana A. Gray, Nymphaea odorata ssp. odorata, and Utricularia spp.). This community type can be distinguished from all others by the sparse presence of Taxodium throughout the lake with floating-leaved and submersed aquatics occurring underneath.
Floristic Summary
Across All Sites
The littoral zone vascular flora of Carolina bay lakes, based on vouchered collections, reports, and personal observations, consists of 205 taxa (170 species, 4 subspecies, 30 varieties, 1 hybrid) in 136 genera and 80 vascular plant families (Table 8; Suppl. material 6). Of these 205 taxa, 186 (90.7%) are vouchered and 19 (9.3%) are known only from reports (Peet et al. 2013a, Peet et al. 2013b, North Carolina Natural Heritage Program 2014; NCSU Crop Science Department [Rob Richardson and Justin Nawrocki, pers. comm., April 9, 2015]). Of the 186 vouchered taxa, 157 (84.4%) were collected by the first author; the remaining 29 (15.6%) vouchered taxa were collected from Carolina bay lake shorelines by others and were found by completing systematic searches of major herbaria (DUKE, NCSC, and NCU). Nineteen taxa (9.3%) are listed as significantly rare and twelve taxa (5.8%) are on the NCNHP Watch List (Table 9). Four taxa (1.9%) are Federal Species of Concern (Ludwigia brevipes; Nuphar sagittifolia; Rhexia aristosa Britton; Sagittaria weatherbiana). Pair-wise comparisons of species similarity for all bays are provided in Table 10.
Table 8.
Summary of vascular plant taxa collected or reported from Carolina bay lake littoral zones
| Species and Subspecies/Varieties | |||||
| Group | Families | Genera | Native | Exotic | Total |
| Basal Angiosperms & Magnoliids | 4 | 6 | 6 | 0 | 6 |
| Pteridophytes | 6 | 7 | 7 | 0 | 7 |
| Gymnosperms | 2 | 3 | 5 | 0 | 5 |
| Monocotyledons | 17 | 41 | 84 | 2 | 86 |
| Eudicotyledons | 51 | 79 | 98 | 3 | 101 |
| Total | 80 | 136 | 200 | 5 | 205 |
Table 9.
List of North Carolina Significantly Rare and Watch List taxa collected or reported from Carolina bay lake littoral zones. Status and rank designations follow Robinson and Finnegan (2014). Taxa for which voucher specimens have been collected (by the first author or others) are indicated with a check mark (✓) in the second column. The taxonomy followed in this work and that of Robinson and Finnegan (2014) differ in one instance in the following table: Luziola fluitans (Michx.) Terrell & H. Rob. var. fluitans (as Luziola fluitans (Michx.) Terrell & H. Rob. sensu Robinson and Finnegan 2014). See Martínez-y-Pérez et al. (2008) in addition to the FNA treatment for reasons of further division to an infraspecific rank.
| Taxon | Vouchered ? | State Status | Fed. Status | State Rank | Global Rank | |
| Significantly Rare: | ||||||
| 1 | Bacopa caroliniana (Walter) B.L. Rob. | ✓ | T | − | S1 | G4G5 |
| 2 | Boltonia asteroides (L.) L’Hér var. glastifolia (Hill) Fernald | ✓ | SR−O | − | S2 | G5TNR |
| 3 | Cladium mariscoides (Muhl) Torr. | ✓ | SR−O | − | S3 | G5 |
| 4 | Eleocharis vivipara Link | ✓ | E | − | S1 | G5 |
| 5 | Epidendrum magnoliae Muhl. | ✓ | T | − | S1S2 | G4 |
| 6 | Eriocaulon aquaticum (Hill) Druce | ✓ | SC−V | − | S2 | G5 |
| 7 | Ludwigia brevipes (Long) Eames | ✓ | SR−T | FSC | S1S2 | G2G3 |
| 8 | Ludwigia sphaerocarpa Elliott | ✓ | E | − | S1 | G5 |
| 9 | Luziola fluitans (Michx.) Terrell & H. Rob. var. fluitans | ✓ | SR−P | − | S2 | G4,G5 |
| 10 | Lycopus angustifolius Elliott | ✓ | SR−P | − | S1 | G4?Q |
| 11 | Rhexia aristosa Britton | SC−V | FSC | S3 | G3,G4 | |
| 12 | Rhynchospora alba (L.) Vahl | ✓ | SR−P | − | S2 | G5 |
| 13 | Sagittaria filiformis J.G. Sm. | ✓ | SR−P | − | SH | G4,G5 |
| 14 | Sagittaria isoetiformis J.G. Sm. | ✓ | T | − | S2 | G4? |
| 15 | Sagittaria weatherbiana Fernald | ✓ | E | FSC | S2 | G3G4 |
| 16 | Sclerolepis uniflora (Walter) Britton, Sterns & Poggenb. | ✓ | SR−T | − | S2 | G4 |
| 17 | Spiranthes laciniata (Small) Ames | ✓ | SC−V | − | S2 | G4,G5 |
| 18 | Utricularia cornuta Michx. | ✓ | T | − | S1S2 | G5 |
| 19 | Utricularia resupinata B.D. Greene ex Bigelow | ✓ | E | − | S1 | G4 |
| Watch List: | ||||||
| 1 | Dichanthelium dichotomum (L.) Gould var. roanokense (Ashe) LeBlond | ✓ | W1 | − | S2 | G5T4? |
| 2 | Dichanthelium erectifolium (Nash) Gould & C.A. Clark | ✓ | W1 | − | S2 | G4 |
| 3 | Dryopteris ludoviciana (Kunze) Small | ✓ | W1 | − | S2 | G4 |
| 4 | Eleocharis equisetoides (Elliott) Torr. | ✓ | W1 | − | S3 | G4 |
| 5 | Habaneria repens Nutt. | W1 | − | S2 | G5 | |
| 6 | Nelumbo lutea Willd. | ✓ | W7 | − | S2 | G4 |
| 7 | Nuphar sagittifolia (Walter) Pursh | ✓ | W1 | FSC | S2 | G5T2 |
| 8 | Rhexia cubensis Griseb. | ✓ | W1 | − | S3 | G4G5 |
| 9 | Rhynchospora inundata (Oakes) Fernald | ✓ | W1 | − | S3 | G4? |
| 10 | Rhynchospora nitens (Vahl) A. Gray | ✓ | W1 | − | S3 | G4? |
| 11 | Xyris iridifolia Chapm. | W7 | − | S2 | G4G5T4T | |
| 12 | Xyris smalliana Nash | ✓ | W1 | − | S3 | G5 |
| STATE STATUS: E = Endangered; T = Threatened; SC-V = Special Concern-Vulnerable; SR = Significantly Rare: −T = Throughout; −P = Periphery of Range; −O = Other; W = Watchlist: W1 = rare but relatively secure; W7 = rare and poorly known. FEDERAL STATUS: FSC = Federal Species of Concern. STATE RANK: SH = historical (known only from historical populations in the state); S1 = Critically Imperiled, 1–5 populations in the state; S2 = Imperiled, 6–20 populations in the state; S3 = Vulnerable, 21–100 populations in the state. FEDERAL RANK: G2 = Imperiled, 6–20 populations in the world; G3 = Vulnerable, 21–100 populations in the world; G4 = Apparently Secure, 101–1000 populations in the world; G5 = Secure, 1001+ populations in the world; T# = Global rank of a subspecies or variety; NR = Not Ranked; Q = Questionable taxonomy; ? = Uncertain. | ||||||
Table 10.
Sørenson’s Similarity Index for Carolina bay lakes. Values in this table are represented as percentiles (i.e., when looking in the second column from the left under Bakers Lake, Bakers Lake is considered to be 16.4% similar to Bay Tree Lake, 23.5% similar to Horseshoe Lake, and 40.8% similar to Jones Lake). Based solely on littoral zone plant taxa, Jones Lake and Singletary Lake are 83.3% alike.
|
Bakers
Lake |
Bay Tree
Lake |
Horseshoe
Lake |
Jones
Lake |
Lake
Waccamaw |
Little
SingletaryLake |
Salters
Lake |
Singletary
Lake |
|
| Bakers Lake | 100 | 16.4 | 23.5 | 40.8 | 12.4 | 39.3 | 41.0 | 41.5 |
| Bay Tree Lake | 16.4 | 100 | 37.4 | 38.6 | 33.0 | 46.3 | 32.4 | 41.3 |
| Horseshoe Lake | 23.5 | 37.4 | 100 | 38.6 | 26.7 | 42.2 | 24.7 | 48.3 |
| Jones Lake | 40.8 | 38.6 | 38.5 | 100 | 20.5 | 42.5 | 55.6 | 83.3 |
| Lake Waccamaw | 12.4 | 33.0 | 26.7 | 20.5 | 100 | 22.5 | 21.3 | 28.9 |
| Little Singletary Lake | 39.3 | 46.3 | 42.2 | 42.5 | 22.5 | 100 | 29.5 | 56.0 |
| Salters Lake | 41.0 | 32.4 | 24.7 | 55.5 | 21.3 | 29.5 | 100 | 51.7 |
| Singletary Lake | 41.5 | 41.3 | 48.3 | 83.3 | 28.9 | 56.0 | 51.7 | 100 |
Among all taxa treated in this guide, the major vascular plant groups consisted of the following total taxa: Eudicotyledons (101 taxa; 86 species, 1 subspecies, 13 varieties, 1 hybrid), monocotyledons (86 taxa; 71 species, 1 subspecies, 14 varieties), pteridophytes (7 taxa; 6 species and 1 subspecies), gymnosperms (5 species), basal angiosperms (4 taxa; 3 species and 1 subspecies), and magnoliids (2 taxa; 1 species and 1 variety; Table 8; Fig. 16). The richest families in the eudicotyledons are Asteraceae (13 taxa; 11 species, 1 variety, 1 hybrid), Ericaceae (8 taxa; 6 species, 2 varieties), Lentibulariaceae (6 taxa), Melastomataceae (5 taxa; 4 species, 1 variety), Hypericaceae (4 taxa; 3 species, 1 variety), and Rosaceae (4 taxa; Fig. 17). The richest genera in the eudicotyledons are Utricularia (6 taxa), Rhexia L. (5 taxa), and Hypericum L. (4 taxa). The richest families in the monocotyledons are Cyperaceae (25 taxa; 20 species, 5 varieties), Poaceae (21 taxa; 17 species, 4 varieties), Juncaceae (8 taxa), Orchidaceae (5 taxa; 4 species, 1 variety), Alismataceae (4 taxa), Smilacaceae (4 taxa), and Xyridaceae (4 taxa: Fig. 17). The richest genera in the monocotyledons are Rhynchospora (9 taxa; 8 species, 1 variety), Juncus (8 taxa), Dichanthelium (Hitchc. & Chase) Gould (6 taxa; 5 species, 1 variety), Carex (4 taxa; 3 species, 1 variety), Eleocharis (4 taxa; 3 species, 1 variety), Sagittaria L. (4 taxa), Smilax L. (4 taxa), and Xyris L. (4 taxa).
Figure 16.

Distribution of plant habit across all Carolina bay lakes. Lakes dominated by herbs have broader littoral zones, which encourage the establishment of herbaceous emergent macrophytes. Lakes dominated by trees and shrubs have narrow littoral zones, which discourage the establishment of herbaceous emergent macrophytes.
Figure 17.

The thirteen most species-rich vascular plant families across all Carolina bay lakes. Cyperaceae (orange), Ericaceae (yellow), Juncaceae (dull green), Poaceae (purple), Smilacaceae (neon green), and Xyridaceae (black) consistently occur across all sites.
Among all taxa treated in this guide, the most species-rich habit is herbs (140 taxa; 119 species, 2 subspecies, 18 varieties, 1 hybrid), followed by trees and shrubs (51 taxa; 42 species, 1 subspecies, 8 varieties), and vines (14 taxa, 12 species, 2 varieties; Fig. 16). Among the herbs, Cyperaceae (25 taxa), Poaceae (20 taxa), Asteraceae (11 taxa), Juncaceae (8 taxa), Lentibulariaceae (6 taxa), Melastomataceae (5 taxa), and Orchidaceae (5 taxa) are the most species-rich families. Among trees and shrubs, the Ericaceae (8 taxa) and Rosaceae (4 taxa) were the most species-rich families. Among vines, the Smilacaceae (4 taxa) and Vitaceae (2 taxa) were the most species rich families.
Among the natural community types included in this work, the Natural Lake Shoreline Swamp (Lake Waccamaw Subtype) is the most species-rich (145 taxa) and the Natural Lake Shoreline Marsh (Lake Waccamaw Pondlily Subtype) is the least species-rich (< 10 taxa; Table 7). Five exotic taxa are known to occur in the bay lakes, four (Alternanthera philoxeroides [Amaranthaceae], Colocasia esculenta (L.) Schott [Araceae], Hydrilla verticillata (L.F.) Royle [Hydrocharitaceae], Triadica sebifera (L.) Small [Euphorbiaceae]) from Lake Waccamaw and one (Hypochaeris radicata L. [Asteraceae]) from Bay Tree Lake.
Individual Lakes
Among the lakes, the largest number of littoral zone taxa (i.e., species, subspecies, and varieties) occurred in Lake Waccamaw (145 taxa), followed by Bay Tree Lake (56 taxa) and Horseshoe Lake (52 taxa; Table 11). The least number of littoral zone taxa occurred in Bakers Lake (18 taxa).
Table 11.
Number of taxa (species, subspecies, and varieties) by major taxonomic group across study sites. Sites are arranged from taxonomically richest to most depauperate. BALA = Bakers Lake; BATR = Bay Tree Lake; HOLA = Horseshoe Lake; JOLA = Jones Lake; LAWA = Lake Waccamaw; LISI = Little Singletary Lake; SALA = Salters Lake; SILA = Singletary Lake.
| LAWA | BATR | HOLA | LISI | SILA | JOLA | SALA | BALA | |
| Pteridophytes | 7 | 3 | 1 | 2 | 1 | 1 | 1 | 1 |
| Gymnosperms | 2 | 3 | 3 | 3 | 5 | 4 | 2 | 1 |
| Basal angiosperms | 3 | -- | 3 | -- | 1 | -- | -- | -- |
| Magnoliids | 2 | -- | -- | 1 | 2 | 2 | 2 | 2 |
| Monocots | 60 | 23 | 21 | 17 | 9 | 10 | 5 | 3 |
| Eudicots | 71 | 27 | 24 | 16 | 18 | 16 | 12 | 11 |
| Total | 145 | 56 | 52 | 39 | 36 | 33 | 22 | 18 |
Bakers Lake
The littoral zone vascular flora of Bakers Lake is depauperate with respect to the other bay lakes (Table 11). A total of 18 taxa (14 species, 4 varieties) in 17 genera and 14 vascular plant families were found in this lake’s littoral zone (Suppl. material 6). All but one taxon (Tillandsia usneoides) from Bakers Lake were collected by the first author (i.e., there were no reports or historical vouchers). The richest eudicotyledonous family was Ericaceae (5 taxa; Fig. 17).
The most species-rich habit class was trees and shrubs (14 taxa; 10 species, 4 varieties), followed by herbs (3 taxa), and vines (1 taxa; Fig. 16). Among the trees and shrubs, the Ericaceae (5 taxa) is the most species-rich family. No exotic taxa or taxa of conservation concern occured at this site. One species (Rhus copallinum L.) was unique to this Carolina bay lake (i.e., it was not found/reported from any other bay lake in this study; Suppl. material 5).
Bay Tree Lake
The littoral zone vascular flora of Bay Tree Lake is comprised of 56 taxa (48 species, 2 subspecies, and 6 varieties), in 47 genera and 34 vascular plant families (Table 11; Suppl. material 6). All but 2 taxa from Bay Tree Lake were vouchered; Decodon verticillatus and Pontederia cordata L. var. cordata were personal observations. No species of conservation concern were collected or reported from Bay Tree Lake’s littoral zone. One exotic taxon (Hypochaeris radicata) was collected from this site (Suppl. material 6). Twelve taxa are unique to this bay lake (i.e., they were not found/reported from any other bay lake in this study; Suppl. material 6: [Amelanchier canadensis (L.) Medik., Carex longii Mack., Cyperus odoratus L. var. odoratus, Diodia virginiana L., Fuirena pumila (Torr.) Spreng., Hypochaeris radicata, Juncus acuminatus Michx., Krigia virginica (L.) Willd., Nuttallanthus canadensis (L.) D.A. Sutton, Panicum virgatum, Rumex hastatulus Baldwin, Smilax glauca Walter, and Stipulicida setacea Michx. var. setacea]).
The richest eudicotyledon families are Asteraceae (3 taxa), followed by Ericaceae (2 taxa) and Aquifoliaceae (2 taxa;) . The richest monocotyledonous families are Poaceae (7 taxa; 6 species, 1 subspecies), Cyperaceae (5 taxa; 4 species, 1 variety), and Juncaceae (5 taxa). The richest monocotyledon genera are Juncus (5 taxa; 3 species, 1 subspecies, 1 variety) and Panicum (3 taxa).
The most species-rich habit class was herbs (35 taxa; 29 species, 2 subspecies, 4 varieties), followed by trees and shrubs (16 taxa; 15 species, 1 variety), and vines (4 species, 1 variety; Fig. 16). Among the herbs, Poaceae (7 taxa; 6 species, 1 subspecies), Cyperaceae (5 taxa; 4 species, 1 variety), Juncaceae (5 taxa), and Asteraceae (3 taxa) are the most species-rich families. Among the trees and shrubs, Cupressaceae (3 taxa), Aquifoliaceae (2 taxa), and Ericaceae (2 taxa) are the most species-rich families.
Horseshoe Lake
The littoral zone vascular flora of Horseshoe Lake is comprised of 52 taxa (45 species, 2 subspecies, and 5 varieties), in 41 genera and 29 vascular plant families (Table 11; Suppl. material 6). All but three taxa from Horseshoe Lake were vouchered; Eleocharis baldwinii/vivipara, Rhexia aristosa, and Tillandsia usneoides were the only taxa not vouchered from the site. No exotic taxa were collected from this site. Sixteen taxa are unique to this bay lake (i.e., they were not found/reported from any other bay lake in this study; Suppl. material 6). Five taxa of conservation concern were collected or reported from this site (Rhexia aristosa, Rhynchospora alba, Rhynchospora inundata, Sagittaria isoetiformis J.G. Sm., and Xyris smalliana; Table 9).
The richest eudicotyledon families are Ericaceae (4 taxa), Lentibulariaceae (3 taxa) and Melastomataceae (3 taxa). The richest eudicotyledonous genera are Rhexia (3 taxa), Utricularia (3 taxa), followed by Hypericum (2 taxa). The richest monocotyledonous families are Cyperaceae (5 taxa), Juncaceae (4 taxa), Poaceae (3 taxa), followed by Orchidaceae (2 taxa), Smilacaceae (2 taxa) and Xyridaceae (2 taxa). The richest monocotyledonous genera are Juncus (4 taxa), followed by Rhynchospora (2 taxa), Smilax (2 taxa), and Xyris (2 taxa).
The most species-rich habit class was herbs (38 taxa; 31 species, 2 subspecies, 4 varieties), followed by trees and shrubs (11 taxa; 10 species, 1 variety), and vines (3 taxa; Fig. 16). Among the herbs, Cyperaceae (6 taxa), Juncaceae (4 taxa), followed by Lentibulariaceae (3 taxa), Melastomataceae (3 taxa), Poaceae (3 taxa), Orchidaceae (2 taxa), and Xyridaceae (2 taxa) are the most species-rich families. Among the trees and shrubs, the most species-rich family is Ericaceae (4 taxa).
Jones Lake
The littoral zone vascular flora of Jones Lake is comprised of 33 taxa (29 species, 1 subspecies, and 3 varieties), in 31 genera and 23 vascular plant families (Table 11; Suppl. material 6). All taxa, save for Cyrilla racemiflora, were vouchered by the first author or others. No exotic taxa were collected from this site. Two taxa are unique to this bay lake (i.e., they were not found/reported from any other bay lake in this study; Suppl. material 6: [Cyperus polystachyos Rottb., Rhynchospora inexpansa (Michx.) Vahl]). Xyris smalliana was the only species of conservation concern collected from this site (Table 9).
The richest eudicotyledonous family is Ericaceae (5 taxa). The richest eudicotyledonous genus is Lyonia (2 taxa; 1 species, 1 variety). The richest monocotyledonous families are Cyperaceae (3 taxa) and Poaceae (3 taxa). Monocotyledons are comprised of ten different genera.
The most species-rich habit class was trees and shrubs (20 taxa; 16 species, 1 subspecies, 3 varieties), followed by herbs (11 taxa), and vines (2 taxa; Fig. 16). Among the herbs, Cyperaceae (3 taxa) and Poaceae (3 taxa) are the most species-rich families. Among the trees and shrubs, Ericaceae (5 taxa) and Cupressaceae (3 taxa) are the most species-rich families.
Lake Waccamaw
The littoral zone vascular flora of Lake Waccamaw is comprised of 145 taxa (122 species, 3 subspecies, 19 varieties, 1 hybrid), in 111 genera and 72 vascular plant families (Table 11; Suppl. material 6). Of the 145 total catalogued taxa, 127 are vouchered and 18 are known only from reports (Suppl. material 6). Twenty-six species of conservation concern were collected or reported from Lake Waccamaw’s littoral zone. Four exotic taxa (Alternanthera philoxeroides [Amaranthaceae], Colocasia esculenta [Araceae], Hydrilla verticillata [Hydrocharitaceae], Triadica sebifera [Euphorbiaceae]) are known from this site. Ninety-five taxa are unique to Lake Waccamaw (i.e., they were not found/reported from any other bay lake in this study; Suppl. material 6).
The richest eudicotyledonous families are Asteraceae (10 taxa; 8 species, 1 variety, 1 hybrid), followed by Lentibulariaceae (4 taxa), Ericaceae (3 taxa), Rosaceae (3 taxa), and Salicaceae (3 taxa). The richest eudicotyledonous genera are Utricularia (4 taxa), Eupatorium L. (2 taxa), Hypericum (2 taxa), Ludwigia L. (2 taxa), Nyssa (2 taxa), and Salix L. (2 taxa). The richest monocotyledonous families are Poaceae (17 taxa; 13 species, 1 subspecies, 3 varieties), Cyperaceae (14 taxa; 11 species, 3 varieties), Alismataceae (4 taxa), Juncaceae (3 taxa), Orchidaceae (3 taxa), and Smilacaceae (3 taxa). The richest monocotyledonous genera are Dichanthelium (Hitchc. & Chase) Gould (6 taxa; 5 species and 1 variety), Rhynchospora (6 taxa; 5 species and 1 variety), Sagittaria L. (4 taxa), Juncus (3 taxa; 3 species, 1 subspecies, 1 variety) and Smilax L. (3 taxa).
The most species-rich habit class was herbs (96 taxa; 80 species, 3 subspecies, 13 varieties, 1 hybrid), followed by trees and shrubs (36 taxa; 32 species, 4 varieties), and vines (13 taxa; 11 species, 2 varieties; Fig. 16). Among the herbs, the Poaceae (16 taxa; 13 species, 1 subspecies, 2 varieties), Cyperaceae (14 taxa; 11 species, 3 varieties), Asteraceae (8 taxa; 6 species, 1 variety, 1 hybrid), Alismataceae (4 taxa), Lentibulariaceae (4 taxa), Juncaceae (3 taxa), and Orchidaceae (3 taxa) are the most species-rich families. Among the trees and shrubs, the Ericaceae (3 taxa), Rosaceae (3 taxa), Salicaceae (3 taxa), Betulaceae (2 taxa), Cupressaceae (2 taxa), Nyssaceae (2 taxa), and Sapindaceae (2 taxa) are the most species-rich families.
Little Singletary Lake
The littoral zone flora of Littoral Singletary Lake is comprised of 39 taxa (35 species, 1 subspecies, 3 varieties), in 32 genera and 21 vascular plant families (Table 11; Suppl. material 6). All of the 39 total catalogued taxa were vouchered (i.e., no taxa were known strictly from reports or observations; Suppl. material 6). Two species of conservation concern (i.e., Eleocharis equisetoides and Eleocharis vivipara) were collected from Little Singletary Lake’s littoral zone (Table 9). No exotic taxa are known from this site. Three taxa are unique to Little Singletary Lake (i.e., they were not found/reported from any other bay lake in this study; Suppl. material 6: [Agrostis hyemalis (Walter) Britton, Sterns & Poggenb., Rhexia virginica L., and Xyris jupicai Rich.])
The richest eudicotyledonous genus is Rhexia (2 taxa). The richest monocotyledonous families are Cyperaceae (6 taxa; 5 species and 1 variety), Juncaceae (4 taxa; 2 species, 1 subspecies, 1 variety), and Poaceae (3 taxa). The richest monocotyledonous genera are Juncus (4 taxa), Eleocharis (3 taxa), and Panicum (2 taxa).
The most species-rich habit class was herbs (23 taxa; 20 species, 1 subspecies, 2 varieties), followed by trees and shrubs (15 taxa; 14 species and 1 variety), and vines (1 taxon; Fig. 16). Among the herbs, the Cyperaceae (6 taxa), Juncaceae (4 taxa), and Poaceae (3 taxa) are the most species-rich families. Among the trees and shrubs, the Ericaceae (5 taxa) is the most species-rich family.
Salters Lake
The littoral zone flora of Salters Lake is comprised of 22 taxa (16 species, 2 subspecies, 4 varieties), in 18 genera and 16 vascular plant families (Table 11; Suppl. material 6). Twenty of the twenty-three total catalogued taxa were vouchered; Decodon verticillatus, Nyssa biflora, and Xyris iridifolia, were reports or personal observations (Suppl. material 6). Two species of conservation concern (i.e., Xyris iridifolia and Xyris smalliana) were collected/reported from Salters Lake’s littoral zone (Suppl. material 6; Table 9). No exotic taxa are known from this site. One taxon is unique to Salters Lake (i.e., not found/reported from any other bay lake in this study; Suppl. material 5: [Xyris iridifolia])
The richest eudicotyledon family is Ericaceae (5 taxa). The richest eudicotyledonous genera are Lyonia (2 taxa) and Vaccinium (2 taxa). The richest monocotyledonous family is Xyridaceae (2 taxa). The richest monocotyledon genus is Xyris (2 taxa).
The most species-rich habit class was trees and shrubs (15 taxa; 11 species, 1 subspecies, 3 varieties), herbs (5 taxa; 4 species and 1 subspecies), and vines (2 taxa; Fig. 16). Among the trees and shrubs, the Ericaceae (5 taxa) and Cupressaceae (2 taxa) are the most species-rich families. Among the herbs, the Xyridaceae (2 taxa) is the most species-rich family.
Singletary Lake
The littoral zone vascular flora of Singletary Lake is comprised of 36 taxa (32 species, 1 subspecies, 3 varieties), in 30 genera and 22 vascular plant families (Table 11; Suppl. material 6). All thirty-six total catalogued taxa were vouchered (i.e., none were reports or personal observations; Suppl. material 6). One taxon from Singletary Lake’s littoral zone is of conservation concern (i.e., Xyris smalliana; Suppl. material 6; Table 9). No exotic taxa are known from this site. One taxon is unique to Salters Lake (i.e., not found/reported from any other bay lake in this study; Suppl. material 6: [Rhododendron viscosum (L.) Torr. var. serrulatum (Small) H.E. Ahles]).
The richest eudicotyledonous families are Ericaceae (7 taxa) and Rosaceae (2 taxa). The richest eudicotyledonous genus is Vaccinium (2 taxa). The richest monocotyledonous families are Juncaceae (3 taxa), Poaceae (2 taxa), and Xyridaceae (2 taxa). The richest monocotyledonous genera are Juncus (3 taxa) and Xyris (2 taxa).
The most species-rich habit class was trees and shrubs (22 taxa; 19 species and 3 varieties), herbs (11 taxa; 10 species and 1 subspecies), and vines (3 taxa; Fig. 16). Among the trees and shrubs, the Ericaceae (7 taxa), Cupressaceae (3 taxa), Pinaceae (2 taxa), and Rosaceae (2 taxa) are the most species-rich families. Among the herbs, the Juncaceae (3 taxa), Poaceae (2 taxa), and Xyridaceae (2 taxa) are the most species-rich families.
White Lake
White Lake was not included in this study due to the severity of the lake’s shoreline development. A provisional checklist of plants known to occur within the littoral zone of White Lake (from historical vouchers, personal observation, and literature review) is provided in Suppl. material 7. The intent of the provisional checklist is to provide a baseline for future research in this lake.
Materials and methods
This work is restricted to the littoral zone vascular flora of unaltered Carolina bay lake shorelines. The littoral zone was defined as the zone of vegetation occurring between the maximum annual high water mark and the point at which submerged aquatic plants cease to persist (Fig. 4). Unaltered shorelines were defined as those lacking residential or commercial development (therefore, the entirety of White Lake and the developed shorelines of Lake Waccamaw and Bay Tree Lake were not included in this inventory).
During the 2013 and 2014 growing seasons, 36 total visits were made to the eight study sites meeting the criteria articulated above (i.e., Bakers Lake, Bay Tree Lake, Horseshoe Lake, Jones Lake, Lake Waccamaw, Little Singletary Lake, Salters Lake, Singletary Lake), resulting in 121 field hours and the identification of 204 taxa (species, subspecies, and varieties). A 10-foot aluminum boat with a transom-mounted trolling motor was used to transport equipment along Carolina bay lake shorelines. Where water was too shallow for the use of the trolling motor, we walked and pulled the boat by rope. GPS locations (NAD 83) were taken at numerous intervals and associated with all specimens collected within 30 m of each point. Digital photographs of plant habit and overall morphology were taken prior to collection using a Panasonic Lumix FZ−150. Plant specimens were pressed while in the field. Tissue samples were taken in the field and dessicated with blue indicating silica gel (purchased from Delta Enterprises Inc.) in ziploc bags. Voucher specimens and tissue samples were deposited respectively at the North Carolina State University Vascular Plant Herbarium (NCSC) and its DNA bank. The entirety of Carolina bay lake shorelines was surveyed, but it was quickly observed that all shorelines, save for the southernmost, were relatively depauperate. All taxa occurring along western, northern, and eastern shorelines could be found within the littoral zone of the southern shoreline, but the inverse did not hold true. The significantly gentler hydrography (see Frey 1949 for lake longitudinal profiles), and consequently wider littoral zone of southern shorelines, produces a more species-rich macrophyte community. Consequently, survey time was much longer on the southern, more diverse shorelines of Carolina bay lakes.
The flora is organized by the following major vascular plant groups: (1) pteridophytes, (2) gymnosperms, (3) monocots, and (4) basal angiosperms, magnoliids, and eudicotyledons. Dichotomomous keys are provided to each major group, as well as to families, genera, and species within each group. Notes are provided above some keys to aid in the identification process. Within each group, taxa are arranged alphabetically, by family, then genus, then species.
The following information is provided for each taxon account: taxon concept mapping, basionym, conservation status, habit, habitat, flowering and fruiting phenology, abundance, and presence/absence data for each site (Suppl. material 3). Unless stated otherwise, accepted taxon concepts follow Weakley (2012) and are tied to those in the following major works: RAB = Radford et al. (1968); GW = Godfrey and Wooten (1979), Godfrey and Wooten (1981); FNA = Flora of North America (pteridophytes: Blechnaceae [Cranfill 1993], Dryopteridaceae [Smith 1993b, Wagner and Montgomery 1993, Smith 1993b], Lycopodiaceae [Wagner and Beitel 1993], Osmundaceae [Whetstone and Atkinson 1993, Polypodiaceae [Andrews and Windham 1993]; gymnosperms: Cupressaceae [Michener 1993, Watson 1993, Watson and Eckenwalder 1993], Pinaceae [Kral 1993]; monocots: Alismataceae [Durand 2000], Araceae [Thompson 2000], Bromeliaceae [Luther and Brown 2000], Burmanniaceae [Lewis 2002], Cyperaceae [Ball and Reznicek 2002, Ball et al. 2002b, Kral 2002a, Kral 2002b, Kral and Persoon 2002, Mastrogiuseppe 2002, Mastrogiuseppe et al. 2002, Reznicek 2002, Reznicek and Catling 2002, Smith et al. 2002, Tucker 2002, Tucker et al. 2002], Eriocaulaceae [Kral 2000a], Haemodoraceae [Robertson 2002], Hydrocharitaceae [Haynes 2000a, Haynes 2000b], Hypoxidaceae [Herndon 2002], Juncaceae [Brooks and Clemants 2000], Mayacaceae [Faden 2000], Orchidaceae [Goldman et al. 2002, Hágsater et al. 2002, Romero-Gonzáles et al. 2002, Sheviak 2002, Sheviak and Brown 2002, Sheviak and Catling 2002], Poaceae [Barkworth 2003a, Barkworth 2003b, Campbell 2003, Clark and Triplett 2007, Daniel 2007, Freckmann and Lelong 2003a, Freckmann and Lelong 2003b, Harvey 2007, Peterson 2003, Terrell 2007, Wipff 2003], Pontederiaceae [Adanson et al. 2002], Smilacaceae [Holmes 2002], Xyridaceae [Kral 2000a]; basal angiosperms, magnoliids, and eudicots: Altingiaceae [Meyer 1997a], Amaranthaceae [Clemants 2003], Asteraceae [Bogler 2006, Chambers and O'Kennon 2006, Haines 2006, Holmes 2006, Karaman-Castro and Urbatsch 2006, Lamont 2006, Nesom 2006a, Nesom 2006b, Semple and Cook 2006, Siripun and Schilling 2006, Strother and Weedon 2006, Sundberg and Bogler 2006], Betulaceae [Furlow 1997], Cabombaceae [Wiersema 1997b], Caryophyllaceae [Swanson and Rabeler 2005], Clethraceae [Tucker and Jones 2009], Cyrillaceae [Lemke 2009], Ebenaceae [Eckenwalder 2009], Ericaceae [Dorr 2009, Fabijan 2009, Judd 2009, Judd and Kron 2009, Tucker 2009b, Tucker 2009a, Vander Kloet, S.P. 2009], Fagaeae [Jensen 1997], Iteaceae [Morin 2009], Juglandaceae [Stone 1997], Lauraceae [Wofford 1993], Magnoliaceae [Meyer 1997b], Myricaceae [Bornstein 1997], Nelumbonaceae [Wiersema 1997a], Nymphaeaceae [Wiersema and Hellquist 1997], Platanaceae [Kaul 1997], Polygonaceae [Mosyaking 2005], Ranunculaceae [Pringle 1997], Salicaceae [Argus et al. 2010], Sarraceniaceae [Mellichamp and Chase 2009], Theaceae [Prince 2009], Ulmaceae [Sherman-Broyles 1997]). Three symbols are used to relate whether our taxon concepts used here are equivalent (=), narrower (<), or broader (>) than those of other works. For example, the statement “= RAB, FNA” means that the taxon concept, as well as the species name used here, is the same as that used in RAB and FNA (see Dryopteris ludoviciana (Kunze) Small). The use of a “less than” symbol (e.g., “< Onoclea sensibilis L. – RAB, FNA”), indicates that the taxon concept used here is narrower than that used by RAB and FNA (alternatively, a “greater than” symbol would mean that the concept of a particular taxon is broader than in the cited works). An equals symbol followed by a different species name than the one bolded, indicates that the taxon concept used here is the same as in the work cited, except that the taxon was treated under a different name in the work cited (see Sagittaria filiformis J.G. Sm. vs. Sagittaria stagnorum Small).
Abundance estimates following the recommendations of Palmer et al. (1995) are provided for each lake in which a taxon was collected or observed by the current author (Table 12; Suppl. material 3). Taxa designated as “exotic” are not native to North America and are indicated by an asterisk preceding the scientific name. The conservation status and rank of species of conservation concern precede the habitat description in each taxon entry (e.g., E, FSC; S1, G2. “Habitat description”). Conservation status and rank of species are designated according to NatureServe (2012), the North Carolina Plant Conservation Program (2010), and the North Carolina Natural Heritage Program List of Rare Plants (Robinson and Finnegan 2014). Unvouchered taxa (i.e., those known only from reports or personal observations) are given one of four symbols in taxon entries (• = the first author observed the species while in the field, but was not able to collect a viable voucher specimen, ♦ = the taxon was reported by the Carolina Vegetation Survey (Peet et al. 2013a, Peet et al. 2013b), = ► the taxon was reported by the North Carolina Natural Heritage Program (North Carolina Natural Heritage Program 2014), ¤ = the taxon was reported by the North Carolina State University Crop Science Program; Rob Richardson and Justin Nawrocki, pers. comm, April 9, 2015).
Table 12.
Descriptions for estimating the abundance of taxa (adapted from Palmer et al. 1995)
| Density | Description |
| Abundant | Dominant or co-dominant in one or more communities. |
| Frequent | Easily seen or found in one or more common communities but not dominant in any common community |
| Occasional | Widely scattered but not difficult to find |
| Infrequent | Difficult to find with few individuals or colonies but found in several locations |
| Rare | Very difficult to find and limited to one or very few locations or uncommon communities |
When available, digital photographs and line drawings were obtained from: Britton and Brown (1913), Center for Aquatic and Invasive Plants, University of Florida, IFAS (2015), Hitchcock and Chase (1951), Mickel (1979), and United States Department of Agriculture, Natural Resources Conservation Service (USDA- NRCS) (2015).
In addition, relevant historical vouchers are cited based on systematic searches of the three major herbaria−DUKE, NCSC, and NCU. Unfortunately, it is not uncommon to find historical specimens containing vague habitat or locality descriptions. For a taxon to be included in the present study, a clear label statement referencing Carolina bay lake shoreline habitat was required (e.g., “collected from peat-drained lake bed of Suggs Mill Pond”). Herbarium vouchers meeting this criterion were annotated (following taxon concepts accepted here) and their label information was subsequently entered into spreadsheets for organization. Label information for new collections resulting from this study was captured in a DarwinCore compliant spreadsheet for upload to the online portal of the Southeastern Regional Network of Expertise and Collections (www.sernecportal.org), which feeds into iDigBio and the Global Biodiversity Data Facility (GBIF).
Checklists
PTERIDOPHYTES
Families represented: 6
Blechnaceae
Anchistea virginica
(L.) C. Presl
Anchistea virginica Basionym: Blechnum virginicum L.
Anchistea virginica Taxon concept: [= Woodwardia virginica (L.) Sm. − RAB, FNA, Weakley]
Distribution
Bakers Lake (Infrequent): Howell BALA−14 (NCSC!)
Bay Tree Lake (Occasional): Howell BATR−4, 24 (NCSC!)
Jones Lake (Rare): Howell JOLA−44 (NCSC!)
Lake Waccamaw (Infrequent): Howell LAWA−59 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−42 (NCSC!)
Notes
Perennial herbs. Upper eulittoral zone; typically found in saturated soils or rooted on logs, stumps, and other debris (NLSS–C, NLSS–LW, NLSM–T). Jun–Sep. Fig. 18
Anchistea virginica (digital photographs taken by Nathan Howell)
Figure 18a.
Specimen: Howell BATR−24 (NCSC)
Figure 18b.
Stipe; note the dark purple coloration.
Figure 18c.
Mature frond
Figure 18d.
Pinna; note the chain-like venation pattern along the sides of leaflet midveins.
Lorinseria areolata
(L.) C. Presl
Lorinseria areolata Basionym: Acrostichum areolatum L.
Lorinseria areolata Taxon concept: [= Woodwardia areolata (L.) T. Moore − RAB, FNA, Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−5, 26 (NCSC!)
Lake Waccamaw: Wilbur 84200 (DUKE!)
Little Singletary Lake (Occasional): Howell LISI–6 (NCSC!)
Singletary Lake: Hueske s.n. (NCU!)
Notes
Perennial herbs. Upper eulittoral zone; typically found in saturated soils or rooted on logs, stumps, and other debris (NLSS–C, NLSS–LW, NLSM–T) . May–Sep. Fig. 19
Lorinseria areolata (digital photographs taken by Nathan Howell)
Figure 19a.
Specimen: Howell LISI–6 (NCSC)
Figure 19b.
Mature frond
Figure 19c.
Frond underside
Figure 19d.
Pinnae
Dryopteridaceae
Dryopteris ludoviciana
(Kunze) Small
Dryopteris ludoviciana Basionym: Aspidium ludovicianum Kunze
Dryopteris ludoviciana Taxon concept: [= RAB, FNA, Weakley]
Distribution
Lake Waccamaw: Bennedict 1247 & 2298 (NCU!); Blomquist & Correll 7625 (NCU!)
Notes
Perennial herbs. Juncture of eulittoral and supralittoral zones (NLSS–LW). Jun–Sep. This species was not encountered by the first author, but voucher specimens (see above) place it within close proximity of Lake Waccamaw’s shoreline (i.e., it has the potential to occur at the uppermost portions of the littoral zone where the swamp forest adjoins the shoreline community on the southwest side of the lake). Fig. 20
Figure 20.

Dryopteris ludoviciana (from Mickel 1979)
Lycopodiaceae
Lycopodiella appressa
(Chapm.) Cranfill
Lycopodiella appressa Basionym: Lycopodium inundatum L. var. appressum Chapm.
Lycopodiella appressa Taxon concept: [= Lycopodium appressum (Chapm.) F.E. Lloyd & Underw. − RAB; = FNA, Weakley]
Distribution
Bay Tree Lake: Wilbur 48656 (DUKE!)
Horseshoe Lake (Infrequent): Howell HOLA−52 (NCSC!)
Lake Waccamaw (Infrequent): Howell LAWA−110 (NCSC!)
Notes
Perennial herbs. Upper eulittoral zone; usually in association with saturated peaty to sandy soils (NLSS–LW, CPSI–CG). Jul–Sep. Fig. 21
Lycopodiella appressa (digital photographs taken by Nathan Howell)
Figure 21a.
Specimen: Howell LAWA–110 (NCSC)
Figure 21b.
Specimen: Howell HOLA–52 (NCSC)
Figure 21c.
Rooting stems where making contact with soil
Figure 21d.
Terminal strobilus
Onocleaceae
Onoclea sensibilis
L.
Onoclea sensibilis Taxon concept: [< O. sensibilis L. – RAB, FNA; = Weakley]
Distribution
Lake Waccamaw: Wilbur 84220 (DUKE!)
Notes
Perennial herbs. Upper eulittoral zone (NLSS−LW). May−Jun. This species was not encountered by the first author in the field, but a single voucher (see above) places it within close proximity to Lake Waccamaw’s southwest shoreline. Fig. 22
Figure 22.
Onoclea sensibilis (digital photograph taken by Nathan Howell)
Osmundaceae
Osmunda spectabilis
Willd.
Osmunda spectabilis Taxon concept: [< O. regalis L. var. spectabilis (Willd.) A. Gray − RAB, FNA; = Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−58, 87, 90 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; sometimes establishing itself on old stumps and logs (NLSS–LW). Mar–Jun. Fig. 23
Osmunda spectabilis (digital photographs taken by Nathan Howell)
Figure 23a.
Specimen: LAWA-90 (NCSC)
Figure 23b.
Habit
Figure 23c.
Sterile frond
Figure 23d.
Sterile and fertile fronds
Polypodiaceae
Pleopeltis polypodioides michauxianavar.michauxiana
(Weath.) E.G. Andrews & Windham
Pleopeltis polypodioides michauxianavar.michauxiana Basionym: Polypodium polypodioides (L.) Watt var. michauxianum Weath.
Pleopeltis polypodioides michauxianavar.michauxiana Taxon concept: [< Polypodium polypodioides (L.) Watt – RAB; = FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−47 (NCSC!)
Salters Lake (Infrequent): Howell SALA−1 (NCSC!)
Notes
Perennial, frequently epiphytic, herbs. Eulittoral zone; commonly on large limbs and trunks of Taxodium and Nyssa (NLSS−C, NLSS−LW). Jun−Oct. Fig. 24
Pleopeltis polypodioides var. michauxiana (digital photographs taken by Nathan Howell)
Figure 24a.
Specimen: Howell LAWA-47 (NCSC)
Figure 24b.
Specimen: Howell SALA-1 (NCSC)
Figure 24c.
Habit
Figure 24d.
Habit
GYMNOSPERMS
Families represented: 2
Cupressaceae
Chamaecyparis thyoides
(L.) Britton, Sterns, & Poggenb.
Chamaecyparis thyoides Basionym: Cupressus thyoides L.
Chamaecyparis thyoides Taxon concept: [= RAB, FNA, Weakley]
Distribution
Bay Tree Lake (Infrequent): Howell BATR−2 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−2, 13 (NCSC!)
Jones Lake (Occasional): Brown s.n. (NCSC!); Howell JOLA−1, 23 (NCSC!); Lance s.n. (NCU!); Russell 1304 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−8, 26 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−14 (NCSC!)
Notes
Trees. At or just below the juncture of the supralittoral and eulittoral zones; often in saturated peaty or sandy soil (NLSS–C, NLSS–LW, NLSM–T). Mar–Apr; Oct– Nov. Fig. 25
Chamaecyparis thyoides (digital photographs taken by Nathan Howell)
Figure 25a.
Specimen: Howell HOLA-2 (NCSC)
Figure 25b.
Bark
Figure 25c.
Leaves and developing seed cones
Figure 25d.
Leaves and mature seed cones
Taxodium ascendens
Brongn.
Taxodium ascendens Taxon concept: [= RAB; < T. distichum L. var. imbricarium (Nutt.) Croom − FNA; = Weakley]
Distribution
Bakers Lake (Abundant): Howell BALA−15 (NCSC!)
Bay Tree Lake (Abundant): Howell BATR−7 (NCSC!)
Horseshoe Lake (Abundant): Howell HOLA−10 (NCSC!)
Jones Lake (Abundant): Howell JOLA−3, 22 (NCSC!); Krings 508 (NCSC!); Wilbur 57584 (DUKE!)
Lake Waccamaw (Abundant): Howell LAWA−13 (NCSC!)
Little Singletary Lake (Abundant): Howell LISI−4, 20 (NCSC!)
Salters Lake (Abundnat): Howell SALA−8 (NCSC!)
Singletary Lake (Abundant): Howell SILA−13 (NCSC!); Wilbur 27966 (DUKE!)
Notes
Trees. Eulittoral zone (NLSS–C, NLSS–LW, NLSM–T, NLSM−LWP, CPSI–CG). Mar– Apr; Oct. Fig. 26
Taxodium ascendens (digital photographs taken by Nathan Howell)
Figure 26a.
Specimen: Howell BALA-15 (NCSC)
Figure 26b.
Habit
Figure 26c.
Leaves
Figure 26d.
Pollen cones
Taxodium distichum
(L.) Rich.
Taxodium distichum Basionym: Cupressus disticha L.
Taxodium distichum Taxon concept: [= RAB; < T. distichum (L.) Rich. var. distichum – FNA; = Weakley]
Distribution
Bay Tree Lake: Wilbur 61464 (DUKE!)
Jones Lake: Stone 3704 (DUKE!)
Lake Waccamaw: ♦
Salters Lake: Beckman & Linnenburger 38 (DUKE!)
Singletary Lake: Crosby 4032 (DUKE!)
Notes
Trees. Eulittoral zone (NLSS–C, NLSS–LW, NLSM−T). Infrequent. Mar–Apr; Oct. Fig. 27
Figure 27.

Taxodium distichum (from Britton and Brown 1913)
Pinaceae
Pinus serotina
Michx.
Pinus serotina Taxon concept: [= RAB, FNA, Weakley]
Distribution
Jones Lake (Rare): Howell JOLA−14 (NCSC!)
Singletary Lake (Rare): Howell SILA−37 (NCSC!)
Notes
Trees. Juncture of supralittoral and eulittoral zones (NLSS–C). Apr–Aug (or any time of the year in response to fire). Fig. 28
Pinus serotina (digital photographs taken by Nathan Howell)
Figure 28a.
Specimen: Howell SILA-37 (NCSC)
Figure 28b.
Specimen: Howell JOLA-14 (NCSC)
Figure 28c.
Habit
Figure 28d.
Mature seed cones
Pinus taeda
L.
Pinus taeda Taxon concept: [= RAB, FNA, Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−71 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−27 (NCSC!)
Singletary Lake (Rare): Howell SILA−12 (NCSC!)
Notes
Trees. Juncture of supralittoral and eulittoral zones (NLSS–C, NLSS–LW). Mar–Apr; Oct– Nov. Fig. 29
Pinus taeda (digital photographs taken by Nathan Howell)
Figure 29a.
Specimen: Howell LAWA-71 (NCSC)
Figure 29b.
Specimen: Howell SILA-12 (NCSC)
Figure 29c.
Young trees
Figure 29d.
Mature seed cones
MONOCOTYLEDONS
Families represented: 17
Alismataceae
Sagittaria filiformis
J.G. Sm.
Sagittaria filiformis Taxon concept: [= S. stagnorum Small – GW; S. subulata L. Buchenau var. gracillima (S. Watson) J.G. Sm.; = FNA, Weakley]
Ecological interactions
Conservation status
SR−P; SH, G4G5.
Distribution
Lake Waccamaw: Blomquist & Schuster 16191 (DUKE!)
Notes
Perennial herbs. Eulittoral and infralittoral zones (NLSS−LW, NLSM−LWP). May−Sep. The first author has not encountered this taxon in the field, but a single voucher specimen (see above) confirms its historic presence within the lake. Fig. 30
Figure 30.

Sagittaria filiformis (from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Sagittaria graminea
Michx.
Sagittaria graminea Taxon concept: [= S. graminea Michx. var. graminea – RAB, GW; = S. graminea Michx. ssp. graminea – FNA; = Weakley]
Distribution
Lake Waccamaw (Frequent): Howell LAWA−19, 57 (NCSC!); Radford s.n. (NCU!); ♦
Notes
Perennial herbs. Eulittoral zone (NLSS−LW, NLSM−LWP). May−Nov. Fig. 31
Figure 31.

Sagittaria graminea (from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Sagittaria isoetiformis
J.G. Sm.
Sagittaria isoetiformis Taxon concept: [< S. teres S. Watson (misapplied) – RAB; = GW, FNA, Weakley]
Ecological interactions
Conservation status
State T; S2, G4?.
Distribution
Horseshoe Lake (Infrequent): Grant s.n. (NCU!); Howell HOLA−34 (NCSC!)
Lake Waccamaw: LeBlond 5792D (NCU!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW, NLSM−LWP, CPSI−CG, FB). Jun−Sep. Fig. 32
Sagittaria isoetiformis (digital photographs taken by Nathan Howell)
Figure 32a.
Specimen: Howell HOLA-34 (NCSC)
Figure 32b.
Leaf
Figure 32c.
Flower and floral buds
Figure 32d.
Flower and floral buds
Figure 32e.

Inflorescence (note bract)
Figure 32f.

Inflorescence bract detail
Sagittaria weatherbiana
Fernald
Sagittaria weatherbiana Taxon concept: [= S. graminea Michx. var. weatherbiana – RAB, GW; = S. graminea Michx. ssp. weatherbiana – FNA; = Weakley]
Ecological interactions
Conservation status
State E, FSC; S2, G3G4.
Distribution
Lake Waccamaw: Adams s.n. (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW, NLSM−LWP). Apr−Jun.
Araceae
Colocasia esculenta
(L.) Schott
Colocasia esculenta Basionym: Arum esculentum L.
Colocasia esculenta Taxon concept: [= GW, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−93 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW, NLSM−LWP). “Generally infertile in our area” (Weakley 2012). This species is exotic and has become naturalized in roadside ditches, canals, and portions of the lakes shoreline. It spreads by way of rhizome dispersal, which is almost cartainly caused by residential homeowners digging up rhizomes from their flower beds and either tossing them into the lake or into the canals that surround the lake. Fig. 33
Colocasia esculenta (digital photographs taken by Nathan Howell)
Figure 33a.
Specimen: Howell LAWA-93 (NCSC)
Figure 33b.
Habit
Wolffia
Horkel ex Schleid.
Notes
The first author has not encountered taxa within this genus in the field; however, the Carolina Vegetation Survey reported “Wolffia spp.” from the southwest side Lake Waccamaw. Although a species-level identification has not been made, a key to the two species most likely to inhabit this location is provided in the Identification Keys section below.
Bromeliaceae
Tillandsia usneoides
(L.) L.
Tillandsia usneoides Basionym: Renealmia usneoides L.
Tillandsia usneoides Taxon concept: [= RAB, FNA, Weakley]
Distribution
Bakers Lake (Occasional): •
Bay Tree Lake (Frequent): Howell BATR−49 (NCSC!)
Horseshoe Lake (Occasional): •
Jones Lake (Occasional): Howell JOLA–33 (NCSC!)
Lake Waccamaw (Abundant): Howell LAWA−46, 84 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−18 (NCSC!)
Salters Lake (Occasional): Howell SALA−9 (NCSC!)
Singletary Lake (Frequent): Howell SILA−6, 20 (NCSC!)
Notes
Perennial, epiphytic herbs. Eulittoral zone; common in low-hanging limbs of Taxodium or Nyssa (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Apr−Jun. Fig. 34
Tillandsia usneoides (digital photographs taken by Nathan Howell)
Figure 34a.
Specimen: Howell LAWA-46 (NCSC)
Figure 34b.
Habit (draped on branches of Taxodium ascendens)
Figure 34c.
Flower
Figure 34d.
Capsule
Burmanniaceae
Burmannia capitata
(Walter ex J.F. Gmel.) Mart.
Burmannia capitata Basionym: Vogelia capitata Walter ex J.F. Gmel.
Burmannia capitata Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw: LeBlond & Franklin 6578 (NCU!)
Notes
Annual herbs. Eulittoral zone (NLSS−LW). Jul−Nov. Fig. 35
Figure 35.

Burmannia capitata (digital photograph taken by Alexander Krings)
Cyperaceae
Carex alata
Torr.
Carex alata Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−98 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; usually at or just below the juncture of the supralittoral and eulittoral zones (NLSS−LW). May−Jun. Fig. 36
Carex alata (digital photograph taken by Nathan Howell)
Figure 36a.
Specimen: Howell LAWA-98 (NCSC)
Figure 36b.
Inflorescence
Carex longii
Mack.
Carex longii Taxon concept: [< C. albolutescens Schwein. – RAB, GW; = FNA, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−34 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; typically at or just below the juncture of the supralittoral and eulittoral zones. May−Jun. Fig. 37
Carex longii (digital photographs taken by Nathan Howell)
Figure 37a.
Specimen: Howell BATR-34 (NCSC)
Figure 37b.
Inflorescence
Carex lupulina
Muhl. ex Willd.
Carex lupulina Taxon concept: [= RAB; < C. lupulina Muhl. ex Willd. – GW (see C. lupuliformis); = FNA, Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−136 (NCSC!)
Notes
Perennial herbs. Juncture of the eulittoral and supralittoral zones (NLSS−LW). Jun−Sep. A taxon of bottomland forests throughout the state, this large-fruited sedge occurs where bottomland swamp forests abut the shoreline of Lake Waccamaw. Fig. 38
Carex lupulina (digital photograph taken by Nathan Howell)
Figure 38a.
Specimen: Howell LAWA-136 (NCSC)
Figure 38b.
Inflorescence
Carex striatavar.brevis
L.H. Bailey
Carex striatavar.brevis Taxon concept: [< C. walteriana L.H. Bailey – RAB, GW; = FNA, Weakley]
Distribution
Horseshoe Lake: Buell 2279 (DUKE!, NCSC!)
Notes
Perennial herbs. Eulittoral zone; typically in acidic, saturated, peaty soils (CPSI−CG, FB). May−Jun. Fig. 39
Figure 39.

Carex striata var. brevis (from Britton and Brown 1913)
Cladium mariscoides
(Muhl.) Torr.
Cladium mariscoides Basionym: Schoenus mariscoides Muhl.
Cladium mariscoides Taxon concept: [= RAB, FNA, Weakley]
Ecological interactions
Conservation status
SR–O; S3, G5.
Distribution
Lake Waccamaw (Abundant): Howell LAWA−16, 146 (NCSC!); LeBlond 3862 (NCU!); Wilbur 49778, 49789 (DUKE!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Sep. This taxon is the principal sedge component of the natural shoreline community of Lake Waccamaw. Fig. 40
Cladium mariscoides (digital photographs taken by Nathan Howell)
Figure 40a.
Specimen: Howell LAWA-16 (NCSC)
Figure 40b.
Specimen: Howell LAWA-146 (NCSC)
Figure 40c.
Inflorescence
Figure 40d.
Inflorescence
Cyperus erythrorhizos
Muhl.
Cyperus erythrorhizos Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Horseshoe Lake: Buell 2263 (DUKE!); Rothfels, Burge, Duke Natural History Society 2403 (DUKE!)
Notes
Annual herbs. floating bogs; saturated, acidic, peaty soil (FB). Jul−Sep. Fig. 41
Figure 41.
Cyperus erythrorhizos (from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Cyperus odoratusvar.odoratus
Cyperus odoratusvar.odoratus Taxon concept: [= C. odoratus L. – RAB, GW; < C. odoratus L. – FNA; = Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−63 (NCSC!)
Notes
Annual or short-lived perennial herbs. Eulittoral zone; typically on moist sandy beaches at or just below the maximum annual high water mark. Jul−Sep. Fig. 42
Cyperus odoratus var. odoratus (digital photograph taken by Nathan Howell)
Figure 42a.
Specimen: Howell BATR-63 (NCSC)
Figure 42b.
Inflorescence
Cyperus polystachyos
Rottb.
Cyperus polystachyos Taxon concept: [> C. polystachyos Rottb. var. texensis (Torr.) Fernald – RAB; < C. polystachyos – GW; = FNA, Weakley]
Distribution
Jones Lake (Rare): Howell JOLA−43 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; usually in sandy moist soil just below the maximum annual high water mark (NLSM−T). Jul−Oct. Fig. 43
Cyperus polystachyos (illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 43a.
Specimen: Howell JOLA-43 (NCSC)
Figure 43b.
Illustration
Dulichium arundinaceumvar.arundinaceum
Dulichium arundinaceumvar.arundinaceum Basionym: Cyperus arundinaceus L.
Dulichium arundinaceumvar.arundinaceum Taxon concept: [< D. arundinaceum (L.) Britton – RAB, GW; = FNA, Weakley]
Distribution
Horseshoe Lake (Occasional): Beal 4345 (NCSC!); Buell s.n. (DUKE!); Howell HOLA−32 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−26, 77 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−41 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; calm, quiet waters along shorelines or on floating bogs (NLSS−C, NLSS−LW, CPSI−CG, FB). Jul–Oct. Fig. 44
Dulichium arundinaceum (digital photographs taken by Nathan Howell)
Figure 44a.
Specimen: Howell LAWA-26 (NCSC)
Figure 44b.
Specimen: Howell LAWA-77 (NCSC)
Figure 44c.
Habit
Figure 44d.
Leaves (3-ranked)
Eleocharis baldwinii
Chapm.
Eleocharis baldwinii Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Infrequent): Howell BATR−36, 40 (NCSC!)
Horseshoe Lake: • (The first author has observed Eleocharis baldwinii/vivipara around the peripheries of floating bogs and along saturated peaty shores, but voucher specimens were not collected. These two species are unidentifiable from a distance and the use of a hand lens is needed to distinguish one from the other.)
Little Singletary Lake (Rare): Howell LISI−43 (NCSC!)
Notes
Annual (?) herbs. Eulittoral zone and infralittoral zones; typically submersed in shallow water or on sarurated organic to sandy soils above current lake levels (NLSS−C, NLSM−T). Jul−Sep. Fig. 45
Eleocharis baldwinii (digital photographs taken by Nathan Howell)
Figure 45a.
Specimen: Howell BATR-40 (NCSC)
Figure 45b.
Specimen: Howell LISI-43 (NCSC)
Figure 45c.
Habit
Figure 45d.
Habit
Eleocharis equisetoides
(Elliott) Torr.
Eleocharis equisetoides Basionym: Scirpus equisetoides Elliott
Eleocharis equisetoides Taxon concept: [= RAB, GW, FNA, Weakley]
Ecological interactions
Conservation status
W1; S3, G4.
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−67, 155 (NCSC!)
Little Singletary Lake (Rare): Howell LISI−38 (NCSC!)
Notes
Perennial herbs. Eulittoral and infralittoral zones; calm, quiet waters along shorelines (NLSS−C, NLSS−LW). Jun−Sep. Fig. 46
Eleocharis equisetoides (digital photographs taken by Nathan Howell)
Figure 46a.
Specimen: Howell LAWA-67 (NCSC)
Figure 46b.
Specimen: Howell LAWA-155 (NCSC)
Figure 46c.
Habit
Figure 46d.
Inflorescence detail
Eleocharis olivaceavar.olivacea
Eleocharis olivaceavar.olivacea Taxon concept: [< E. flavescens (Poir.) Urb. – RAB; < E. olivacea Torr. – GW; < E. flavescens (Poir.) Urb. var. olivacea (Torr.) Gleason – FNA; = Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−78 (NCSC!); LeBlond 3987 (NCU!)
Notes
Perennial herbs. Eulittoral and infralittoral zones; calm, quiet waters along shorelines (NLSS−LW). Jun−Sep. Fig. 47
Eleocharis olivacea var. olivacea (digital photograph taken by Nathan Howell)
Figure 47a.
Specimen: Howell LAWA-78 (NCSC)
Figure 47b.
Habit
Eleocharis vivipara
Link
Eleocharis vivipara Taxon concept: [= RAB, GW, FNA, Weakley]
Ecological interactions
Conservation status
State E; S1, G5.
Distribution
Horseshoe Lake: • (The first author has observed Eleocharis baldwinii/vivipara around the peripheries of floating bogs and along saturated peaty shores, but voucher specimens were not collected. These two species are unidentifiable from a distance and the use of a hand lens is needed to distinguish one from the other.)
Little Singletary Lake (Rare): Howell LISI−53 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (calm, quiet waters) or boggy, saturated, organic soils at or just below the maximum annual high water mark (NLSS−C, NLSM−T). Jul−Sep. Fig. 48
Eleocharis vivipara (digital photograph taken by Nathan Howell)
Figure 48a.
Specimen: Howell LISI-53 (NCSC)
Figure 48b.
Habit
Fimbristylis autumnalis
(L.) Roem. & Schult.
Fimbristylis autumnalis Basionym: Scirpus autumnalis L.
Fimbristylis autumnalis Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw: Radford 677 (NCU!)
Notes
Annual herbs. Eulittoral zone; wet, sandy, disturbed areas (NLSS−LW). Jun−Oct. Fig. 49
Figure 49.
Fimbristylis autumnalis (from Britton and Brown 1913)
Fuirena pumila
(Torr.) Spreng.
Fuirena pumila Basionym: Fuirena squarrosa Michx. var. pumila Torr.
Fuirena pumila Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−62 (NCSC!); Wilbur 57396 (DUKE!)
Notes
Annual herbs. Eulittoral zone; typically in moist sandy soil at high water mark. Jul−Oct. Fig. 50
Fuirena pumila (digital photograph taken by Nathan Howell)
Figure 50a.
Specimen: Howell BATR-62 (NCSC)
Figure 50b.
Inflorescence detail
Rhynchospora alba
(L.) Vahl
Rhynchospora alba Basionym: Schoenus albus L.
Rhynchospora alba Taxon concept: [= RAB, GW, FNA, Weakley]
Ecological interactions
Conservation status
SR−P; S2, G5.
Distribution
Horseshoe Lake (Occasional): Howell HOLA−45 (NCSC!)
Notes
Perennial herbs. floating bogs of Horseshoe Lake (FB). Jul−Oct. Fig. 51
Rhynchospora alba (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 51a.
Specimen: Howell HOLA-45 (NCSC)
Figure 51b.
Illustration
Figure 51c.
Inflorescences
Figure 51d.
Inflorescences
Rhynchospora corniculata
(Lam.) A. Gray
Rhynchospora corniculata Basionym: Schoenus corniculatus Lam.
Rhynchospora corniculata Taxon concept: [= RAB, GW, FNA; < R. corniculata (L.) A. Gray var. corniculata − Weakley]
Distribution
Lake Waccamaw (Occasional): Howell LAWA−135, 163 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Sep. Fig. 52
Rhynchospora corniculata (digital photographs taken by Nathan Howell)
Figure 52a.
Specimen: Howell LAWA-135 (NCSC)
Figure 52b.
Specimen: Howell LAWA-163 (NCSC)
Figure 52c.
Inflorescence
Figure 52d.
Inflorescence detail
Rhynchospora distans
(Michx.) Vahl
Rhynchospora distans Basionym: Schoenus distans Michx.
Rhynchospora distans Taxon concept: [< R. fascicularis (Michx.) Vahl – RAB, GW, FNA; = Weakley]
Distribution
Bakers Lake (Rare): Howell BALA−2 (NCSC!)
Lake Waccamaw: Wilbur 49814 (DUKE!)
Little Singletary Lake (Rare): Howell LISI−33 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; typically at the high water mark in moist sandy soil (NLSS−C). Jun−Sep. Fig. 53
Figure 53.
Rhynchospora distans (Howell BALA-2, NCSC)
Rhynchospora elliottii
A. Dietr.
Rhynchospora elliottii Taxon concept: [= R. schoenoides (Elliott) Wood – RAB; = GW, FNA, Weakley]
Distribution
Lake Waccamaw: ♦
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Sep.
Rhynchospora inexpansa
(Michx.) Vahl
Rhynchospora inexpansa Basionym: Schoenus inexpansus Michx.
Rhynchospora inexpansa Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Jones Lake: Beal 799 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS–C). Jul–Sep. Fig. 54
Figure 54.
Rhynchospora inexpansa (illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Rhynchospora inundata
(Oakes) Fernald
Rhynchospora inundata Basionym: Ceratoschoenus macrostachyus (Torr. ex A. Gray) A. Gray var. inundatus Oakes
Rhynchospora inundata Taxon concept: [= RAB, GW, FNA, Weakley]
Ecological interactions
Conservation status
W1; S3, G4?
Distribution
Horseshoe Lake (Infrequent): Howell HOLA−53 (NCSC!); Grant s.n. (NCU!); Rothfels, Burge, Duke Nat. Hist. Soc. 2401 (DUKE!)
Notes
Perennial herbs. Eulittoral zone of shorelines and on floating bogs (CPSI−CG, FB). Jul−Sep. Fig. 55
Rhynchospora inundata (digital photograph taken by Nathan Howell)
Figure 55a.
Specimen: Howell HOLA-53 (NCSC)
Figure 55b.
Inflorescence
Rhynchospora latifolia
(Baldwin) W.W. Thomas
Rhynchospora latifolia Basionym: Dichromena latifolia Baldwin
Rhynchospora latifolia Taxon concept: [= Dichromena latifolia Baldwin ex Elliott – RAB, GW; = FNA, Weakley]
Distribution
Lake Waccamaw: Radford 723 (NCU!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). May−Sep. Fig. 56
Figure 56.
Rhynchospora latifolia (illustration from Britton and Brown 1913)
Rhynchospora macrostachya
Torr. ex A. Gray
Rhynchospora macrostachya Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−130 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Sep. Fig. 57
Rhynchospora macrostachya (digital photograph taken by Nathan Howell)
Figure 57a.
Specimen: Howell LAWA-130 (NCSC)
Figure 57b.
Achene detail
Rhynchospora nitens
(Vahl) A. Gray
Rhynchospora nitens Basionym: Scirpus nitens Vahl
Rhynchospora nitens Taxon concept: [= Psilocarya nitens (Vahl) Alph. Wood – RAB, GW; = FNA, Weakley]
Ecological interactions
Conservation status
W1; S3, G4?
Distribution
Lake Waccamaw: Wilbur 49781 (DUKE!)
Notes
Annual herbs. Eulittoral zone (NLSS−LW). Jul−Aug. Fig. 58
Figure 58.
Rhynchospora nitens (illustration from Britton and Brown 1913)
Scirpus cyperinus
(L.) Kunth
Scirpus cyperinus Basionym: Eriophorum cyperinum L.
Scirpus cyperinus Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−58 (NCSC!)
Jones Lake (Occasional): Howell JOLA−4, 45 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−166 (NCSC!)
Little Singletary Lake (Occasional): •
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSS−LW, NLSM−T). Jul−Sep. Fig. 59
Scirpus cyperinus (digital photographs taken by Nathan Howell)
Figure 59a.
Specimen: Howell BATR-58 (NCSC)
Figure 59b.
Specimen: Howell LAWA-166 (NCSC)
Figure 59c.
Inflorescence
Figure 59d.
Inflorescence detail
Eriocaulaceae
Eriocaulon aquaticum
(Hill) Druce
Eriocaulon aquaticum Basionym: Cespa aquatica Hill
Eriocaulon aquaticum Taxon concept: [> E. pellucidum Michx. – RAB; = E. septangulare – GW; = FNA, Weakley]
Ecological interactions
Conservation status
SC−V; S2, G5.
Distribution
Lake Waccamaw (Abundant): Howell LAWA−5, 52 (NCSC!); Lynch 185 (NCSC!); Wilbur 49802 (DUKE!)
Notes
Perennial herbs. Eulittoral and infralittoral zones (NLSS−LW, NLSM−LWP). Jul−Oct. A dominant species in the littoral zone of Lake Waccamaw. Fig. 60
Eriocaulon aquaticum (digital photographs taken by Nathan Howell)
Figure 60a.
Specimen: Howell LAWA-5 (NCSC)
Figure 60b.
Specimen: Howell LAWA-52 (NCSC)
Figure 60c.
Habit
Figure 60d.
Leaves
Figure 60e.
Inflorescence
Figure 60f.
Inflorescence
Haemodoraceae
Lachnanthes caroliniana
(Lam.) Dandy
Lachnanthes caroliniana Basionym: Dilatris caroliniana Lam.
Lachnanthes caroliniana Taxon concept: [= RAB, GW, FNA, Weakley]]
Distribution
Bay Tree Lake (Infrequent): Howell BATR−50, 51 (NCSC!)
Horseshoe Lake (Infrequent): Howell HOLA−51 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−107 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−25, 51 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; typically in saturated soils at or below the maximum annual high water mark (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Jun–early Sep; Sep–Nov. Fig. 61
Lachnanthes caroliniana (digital photographs taken by Nathan Howell):
Figure 61a.
Specimen: Howell BATR-50 (NCSC)
Figure 61b.
Specimen: Howell HOLA-51 (NCSC)
Figure 61c.
Roots
Figure 61d.
Inflorescence
Hydrocharitaceae
Hydrilla verticillata
(L. f.) Royle
Hydrilla verticillata Basionym: Serpicula verticillata L.f.
Hydrilla verticillata Taxon concept: [= GW, FNA, Weakley]
Distribution
Lake Waccamaw: ¤
Notes
Perennial herbs. Infralittoral zone (NLSS−LW, NLSM−LWP). Jun−Aug. This exotic, invasive taxon is native to warm climates of the Old World. Hydrilla verticillata was introduced to Florida in 1950 as an ornamental and has since become a terrible aquatic invasive throughout the Southeast. Where introduced, H. verticillata chokes out native submersed aquatic vegetation (e.g., Ceratophyllum, Myriophyllum, Najas, Potomogeton, Vallisneria), negatively impacts recreational activities and alters natural hydrology and water chemistry (Ramey and Peichel 2001). Fig. 62
Figure 62.

Hydrilla verticillata (from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Najas guadalupensisvar.guadalupensis
Najas guadalupensisvar.guadalupensis Basionym: Caulinia guadalupensis Spreng.
Najas guadalupensisvar.guadalupensis Taxon concept: [< N. guadalupensis (Spreng.) Magnus – RAB, GW; = N. guadalupensis ssp. guadalupensis – FNA; = Weakley]
Distribution
Lake Waccamaw: Blomquist & Schuster 16190 (DUKE!)
Notes
Annual herbs. Infralittoral zone (NLSS−LW, NLSM−LWP). Jul−Sep. Fig. 63
Figure 63.

Najas guadalupensis (from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Hypoxidaceae
Hypoxis curtissii
Rose
Hypoxis curtissii Taxon concept: [= H. hirsuta (L.) Coville var. leptocarpa (Engelm. & A. Gray) Fernald – RAB; = H. leptocarpa (Engelm. & A. Gray) Small – GW; = FNA, Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−60 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; at the high water mark in moist to saturated soil (NLSS−LW). Mar−Jun; May−Jul. Fig. 64
Hypoxis curtissii (digital photographs taken by Nathan Howell)
Figure 64a.
Specimen: Howell LAWA-60 (NCSC)
Figure 64b.
Habit
Figure 64c.
Flower
Figure 64d.
Flower
Juncaceae
Juncus acuminatus
Michx.
Juncus acuminatus Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−15 (NCSC!)
Notes
Perennial herbs. Eulittoral zone. May−Aug. Fig. 65
Juncus acuminatus (illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 65a.
Specimen: Howell BATR-15 (NCSC)
Figure 65b.
Illustration
Juncus biflorus
Elliott
Juncus biflorus Taxon concept: [= RAB; < J. marginatus Rostk. – GW, FNA; = Weakley]
Distribution
Little Singletary Lake (Rare): Howell LISI−58 (NCSC!)
Singletary Lake: Beal 796 (NCSC!)
Notes
Perennial herbs. Juncture of the eulittoral and supralittoral zones; usually in wet soils at or just below the high water mark (NLSM−T, NLSS−C). Jun−Oct. Fig. 66
Juncus biflorus (digital photograph taken by Nathan Howell)
Figure 66a.
Specimen: Howell LISI-58 (NCSC)
Figure 66b.
Inflorescence
Juncus canadensis
J. Gay ex Laharpe
Juncus canadensis Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−32, 170 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Oct. Fig. 67
Juncus canadensis (illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 67a.
Specimen: Howell LAWA-167 (NCSC)
Figure 67b.
Illustration
Juncus coriaceus
Mack.
Juncus coriaceus Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Horseshoe Lake: Beal 828 (NCSC!)
Notes
Perennial herbs. Juncture of eulittoral and supralittoral zones (CPSI−CG). Jun−Sep. Fig. 68
Figure 68.
Juncus coriaceus (from Britton and Brown 1913)
Juncus effusus solutusvar.effusus
(Fernald & Wiegand) Hämet-Ahti
Juncus effusus solutusvar.effusus Taxon concept: [< J. effusus – RAB, GW, FNA; = Weakley]
Distribution
Bay Tree Lake (Occassional): Howell BATR−6 (NCSC!)
Little Singletary Lake (Occassional): Howell LISI−3 (NCSC!)
Horseshoe Lake (Occassiona): Howell HOLA−8 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSM−T, CPSI−CG). Jun−Sep. Fig. 69
Juncus effusus (digital photographs taken by Nathan Howell; illustration from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Figure 69a.
Specimen: Howell HOLA-8 (NCSC)
Figure 69b.

Illustration
Figure 69c.
Habit
Figure 69d.
Inflorescence
Juncus pelocarpus
E. Mey.
Juncus pelocarpus Taxon concept: [> J. abortivus Chapm. – RAB, GW; = FNA, Weakley]
Distribution
Bay Tree Lake (Frequent): Howell BATR−61 (NCSC!); Wilbur 57415 (DUKE!)
Horseshoe Lake: Wilbur 2264, 81465 (DUKE!)
Jones Lake (Occasional): Howell JOLA−17, 35 (NCSC!); Wilbur 57582 (DUKE!)
Lake Waccamaw (Frequent): Howell LAWA−3 (NCSC!); Wilbur s. n., 84188 (DUKE!)
Singletary Lake (Occasional): Howell SILA−31 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jul−Oct. Fig. 70
Juncus pelocarpus (digital photographs taken by Nathan Howell)
Figure 70a.
Specimen: Howell BATR-61 (NCSC)
Figure 70b.
Specimen: Howell LAWA-3 (NCSC)
Figure 70c.

Flower
Figure 70d.
Inflorescence
Juncus repens
Michx.
Juncus repens Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−8 (NCSC!); Wilbur 57395 (DUKE!)
Horseshoe Lake (Occasional): Beal 4348 (NCSC!); Howell HOLA−14 (NCSC!); Wilbur & Menchi Ho 83792 (DUKE!)
Lake Waccamaw (Occasional): Howell LAWA−30, 31 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−19, 44 (NCSC!)
Singletary Lake (Occasional): Howell SILA−1, 32 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jun−Oct. Fig. 71
Juncus repens (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 71a.
Specimen: Howell SILA-32 (NCSC)
Figure 71b.
Illustration
Figure 71c.
Habit
Figure 71d.
Habit
Juncus scirpoidesvar.scirpoides
Juncus scirpoidesvar.scirpoides Taxon concept: [< J. scirpoides Lam. – RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Infrequent): Howell BATR−27, 66 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−55 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSM−T). Jun−Sep. Fig. 72
Juncus scirpoides (digital photographs taken by Nathan Howell)
Figure 72a.
Specimen: Howell BATR-27 (NCSC)
Figure 72b.
Specimen: Howell BATR-66 (NCSC)
Figure 72c.
Inflorescence
Figure 72d.
Inflorescence
Mayacaceae
Mayaca fluviatilis
Aubl.
Mayaca fluviatilis Taxon concept: [> M. aubletii Michx. – RAB; > M. fluviatilis Aubl. – RAB; = GW, FNA, Weakley]
Distribution
Lake Waccamaw: ¤
Notes
Perennial herbs. Eulittoral and infralittoral zones (NLSS−LW). May−Jul. Fig. 73
Figure 73.

Mayaca fluviatilis (from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Orchidaceae
Calopogon tuberosusvar.tuberosus
Calopogon tuberosusvar.tuberosus Basionym: Limodorum tuberosum L.
Calopogon tuberosusvar.tuberosus Taxon concept: [< C. pulchellus R. Brown − RAB; < C. tuberosus (L.) Britton, Sterns, & Poggenb. – GW; = FNA, Weakley]
Distribution
Horseshoe Lake (Occasional): Howell HOLA−24, 39 (NCSC!)
Notes
Perennial herbs. floating bogs (CPSI–CG, FB). Apr–Jul. Fig. 74
Calopogon tuberosus (digital photograph taken by Nathan Howell)
Figure 74a.
Specimen: Howell HOLA-24 (NCSC)
Figure 74b.
Flower
Epidendrum magnoliae
Muhl.
Epidendrum magnoliae Taxon concept: [< E. conopseum R. Br. – RAB; = FNA, Weakley]
Ecological interactions
Conservation status
T; S1S2, G4.
Distribution
Lake Waccamaw: Correll & Blomquist 4900 (DUKE!)
Notes
Perennial, epiphytic herbs. Eulittoral zone; typically on limbs and trunks of Taxodium ascendens, Taxodium distichum, Nyssa aquatica, Nyssa biflora, Liquidambar styraciflua, and possibly other bottomland tree species in the shoreline of Lake Waccamaw (NLSS– LW, NLSS–C). Jul–Oct. This species usually co-occurs with Pleopeltis polypodiodes. The first author observed a vegetative specimen on the edge of Big Creek ca. 50–70 meters from the shoreline of Lake Waccamaw. The specimen was on a large Nyssa aquatica limb, ca. 25–30 meters above the water, and was co- occuring with Pleopeltis polypodioides.
Habenaria repens
Nutt.
Habenaria repens Taxon concept: [= RAB, GW, FNA, Weakley]
Ecological interactions
Conservation status
W1; S2, G5.
Distribution
Lake Waccamaw: ►
Notes
Perennial herbs. Eulittoral zone (NLSS–LW). Apr–Nov.
Pogonia ophioglossoides
(L.) Ker Gawl.
Pogonia ophioglossoides Basionym: Arethusa ophioglossoides L.
Pogonia ophioglossoides Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Horseshoe Lake (Occasional): Howell HOLA−30 (NCSC!)
Notes
Perennial herbs. floating bogs (CPSI–CG, FB). Mar–Jun. Fig. 75
Pogonia ophioglossoides (digital photograph taken by Nathan Howell)
Figure 75a.
Specimen: Howell HOLA-30 (NCSC)
Figure 75b.
Flower
Spiranthes laciniata
(Small) Ames
Spiranthes laciniata Basionym: Gyrostachys laciniata Small
Spiranthes laciniata Taxon concept: [= RAB; < S. × laciniata – GW; = FNA, Weakley]
Ecological interactions
Conservation status
SC−V; S2, G4,G5.
Distribution
Lake Waccamaw (Occasional): Howell LAWA−105, 106, 116 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS–LW). May–Aug. Fig. 76
Spiranthes laciniata (digital photographs taken by Nathan Howell)
Figure 76a.
Specimen: Howell LAWA-105 (NCSC)
Figure 76b.
Leaf
Figure 76c.
Inflorescence
Figure 76d.
Inflorescence (detail)
Poaceae
Agrostis hyemalis
(Walter) Britton, Sterns & Poggenb.
Agrostis hyemalis Basionym: Cornucopiae hyemalis Walter
Agrostis hyemalis Taxon concept: [< A. hyemalis (Walter) Britton, Sterns & Poggenb. – RAB; = A. hiemalis (Walter) Britton, Sterns & Poggenb. – GW; = FNA, Weakley]
Distribution
Little Singletary Lake (Rare): Howell LISI−37 (NCSC!)
Notes
Perennial herbs. Juncture of supralittoral and eulittoral zones; typically in moist sandy soils (NLSM−T). Mar−Jul. Fig. 77
Agrostis hyemalis (digital photographs taken by Nathan Howell; illustration from Hitchcock and Chase 1951)
Figure 77a.
Specimen: Howell LISI-37 (NCSC)
Figure 77b.
Illustration
Figure 77c.
Base of inflorescence
Figure 77d.
Inflorescence, including spikelets
Andropogon glaucopsis
Steud.
Andropogon glaucopsis Taxon concept: [< A. virginicus L. – RAB; = GW; = A. glomeratus var. glaucopsis (Elliott) C. Mohr − FNA; = Weakley]
Distribution
Horseshoe Lake: Buell s.n. (DUKE!, NCSC!)
Jones Lake (Infrequent): Howell JOLA–16 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (CPSI−CG, FB). Sep−Oct. Fig. 78
Figure 78.
Andropogon glaucopsis (Howell JOLA-16, NCSC)
Andropogon virginicusvar.virginicus
Andropogon virginicusvar.virginicus Taxon concept: [< A. virginicus L. – RAB; = FNA, Weakley]
Distribution
Lake Waccamaw: ♦
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Sep−Oct. Fig. 79
Figure 79.
Andropogon virginicus (illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Arundinaria tecta
(Walter) Muhl.
Arundinaria tecta Basionym: Arundo tecta Walter
Arundinaria tecta Taxon concept: [< A. gigantea (Walter) Muhl. – RAB, GW; = FNA, Weakley]
Distribution
Lake Waccamaw: Bennedict 4350 (DUKE!)
Notes
Arborescent herbs. Eulittoral zone; at or just below the mean annual high water mark (NLSS−LW). Apr−Jul. The first author has not encountered this taxon in the field, but a single voucher specimen (see above) places it within the immediate vicinity. Fig. 80
Figure 80.
Arundinaria tecta (from Hitchcock and Chase 1951)
Coleataenia longifoliavar.longifolia
Coleataenia longifoliavar.longifolia Basionym: Panicum longifolium Torr.
Coleataenia longifoliavar.longifolia Taxon concept: [= Panicum longifolium Torr. var. longifolium – RAB; < Panicum longifolium Torr. – GW; = Panicum rigidulum Bosc ex Nees ssp. pubescens (Vasey) Freckmann & Lelong − FNA; = Weakley]
Distribution
Bay Tree Lake (Infrequent): Howell BATR – 68 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA −145, 147, 164, 168 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Oct. Fig. 81
Figure 81.
Coleataenia longifolia (illustration from Britton and Brown 1913)
Coleataenia tenera
(Beyr. ex Trin.) Soreng
Coleataenia tenera Basionym: Panicum tenerum Bey. ex Trin.
Coleataenia tenera Taxon concept: [= Panicum tenerum Bey. ex Trin. – RAB, GW, FNA; = Weakley]
Distribution
Lake Waccamaw: ►
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jun−Sep.
Dichanthelium boreale
(Nash) Freckmann
Dichanthelium boreale Basionym: Panicum boreale Nash
Dichanthelium boreale Taxon concept: [> Panicum bicknellii Nash– RAB; > D. boreale (Nash) Freckmann – FNA; = Weakley]
Distribution
Lake Waccamaw: Blomquist 957 (DUKE!)
Notes
Perennial herbs. Eulittoral zone (NLS−LW). Apr−Sep. Fig. 82
Figure 82.
Dichanthelium boreale (illustration from Britton and Brown 1913)
Dichanthelium dichotomumvar.roanokense
(Ashe) LeBlond
Dichanthelium dichotomumvar.roanokense Basionym: Panicum roanokense Nash
Dichanthelium dichotomumvar.roanokense Taxon concept: [< D. dichotomum (L.) Gould – RAB, GW; < D. dichotomum (L.) Gould) ssp. roanokense (Ashe) Freckmann & Lelong – FNA; = Weakley]
Distribution
Lake Waccamaw: Ashe s.n. (NCU!)
Notes
Perennial herbs. Eulittoral zone; moist to peaty lakeshores (NLSS−LW). May−Sep.
Dichanthelium erectifolium
(Nash) Gould & C.A. Clark
Dichanthelium erectifolium Basionym: Panicum erectifolium Nash
Dichanthelium erectifolium Taxon concept: [= Panicum erectifolium Nash – RAB, GW; = FNA, Weakley]
Ecological interactions
Conservation status
W2; S2, G4.
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−111 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; moist sandy to peaty shores (NLSS−LW). May−Aug. Fig. 83
Dichanthelium erectifolium (digital photographs taken by Nathan Howell)
Figure 83a.
Specimen: Howell LAWA-111 (NCSC)
Figure 83b.
Specimen: Howell LAWA-127 (NCSC)
Figure 83c.
Culm and leaf
Figure 83d.
Inflorescence
Dichanthelium lancearium
Dichanthelium lancearium Taxon concept: [= Panicum lancearium Trinius – RAB; < D. portoricense (Desv. ex Ham.) B.F. Hansen & Wunderlin ssp. patulum (Scribner & Merrill) Freckmann & Lelong – FNA; = Weakley]
Distribution
Lake Waccamaw: Blomquist & Correll 9383 (NCU!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). May−Sep.
Dichanthelium mattamuskeetense
(Ashe) Mohlenbr.
Dichanthelium mattamuskeetense Basionym: Panicum mattamuskeetense Ashe
Dichanthelium mattamuskeetense Taxon concept: [< Panicum dichotomum L. – RAB, GW; < D. dichotomum (L.) Gould ssp. mattamuskeetense (Ashe) Freckmann & Lelong – FNA; = Weakley]
Distribution
Lake Waccamaw: Blomquist & Correll 9385 (DUKE!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). May−Oct. Fig. 84
Figure 84.
Dichanthelium mattamuskeetense (illustration from Britton and Brown 1913)
Dichanthelium portoricense
(Desv. ex Ham.) B.F. Hansen & Wunderlin
Dichanthelium portoricense Basionym: Panicum portoricense Desv. ex Ham.
Dichanthelium portoricense Taxon concept: [= Panicum portoricense Desv. ex Ham. – RAB; = D. portoricense (Desv. ex Ham.) B.F. Hansen & Wunderlin ssp. portoricense – FNA; = Weakley]
Distribution
Bay Tree Lake (Infrequent): Howell BATR – 52 (NCSC!)
Lake Waccamaw: Blomquist & Correll 9383 (NCU!)
Notes
Perennial herbs. Eulittoral zone; moist sandy to peaty shores (NLSS−LW). May−Sep. Fig. 85
Figure 85.
Dichanthelium portoricense (Howell BATR-52, NCSC)
Eragrostis elliottii
S. Watson
Eragrostis elliottii Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−67 (NCSC!)
Lake Waccamaw: ►
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Sep−Oct. Fig. 86
Figure 86.
Eragrostis elliottii (Howell BATR-67, NCSC)
Eragrostis refracta
(Muhl. ex Elliott) Scribn.
Eragrostis refracta Basionym: Poa refracta Muhl. ex Elliott
Eragrostis refracta Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw: ►
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Oct.
Luziola fluitansvar.fluitans
Luziola fluitansvar.fluitans Basionym: Zizania fluitans Michx.
Luziola fluitansvar.fluitans Taxon concept: [= Hydrochloa carolinensis P. Beauv. – RAB, GW; = FNA, Weakley]
Distribution
Lake Waccamaw (Occasional): Bolser MEH107 (NCU!); Howell LAWA−51 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Aug−Oct. Fig. 87
Luziola fluitans (digital photographs taken by Nathan Howell; illustration from Hitchcock and Chase 1951)
Figure 87a.
Specimen: Howell LAWA-51 (NCSC)
Figure 87b.
Illustration
Figure 87c.
Habit
Figure 87d.
Leaves
Panicum hemitomon
Schult.
Panicum hemitomon Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Abundant): Howell BATR−18 (NCSC!)
Horseshoe Lake (Infrequent): Howell HOLA−23 (NCSC!)
Lake Waccamaw (Abundant): Blomquist 1399 (DUKE!); Blomquist & Correll 9379 (DUKE!, NCU!); Howell LAWA−79 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−35 (NCSC!)
Salters Lake (Occasional): Howell SALA−14 (NCSC!)
Singletary Lake (Occasional): Blomquist 1400 (DUKE!); Howell SILA−17 (NCSC!); Wilbur 60947 (DUKE!)
Notes
Perennial herbs. Eulittoral and infralitoral zones (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jun−Jul. Fig. 88
Panicum hemitomon (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 88a.
Specimen: Howell BATR-18 (NCSC)
Figure 88b.
Illustration
Figure 88c.
Habit
Figure 88d.
Inflorescences
Panicum verrucosum
Muhl.
Panicum verrucosum Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Infrequent): Howell BATR−53 (NCSC!)
Jones Lake (Rare): Howell JOLA−40, 41 (NCSC!)
Little Singletary Lake (Rare): Howell LISI−50 (NCSC!)
Notes
Annual herbs. Eulittoral zone (NLSM−T, NLSS−C). Aug−Oct. Fig. 89
Panicum verrucosum (illustration from Britton and Brown 1913)
Figure 89a.
Specimen: Howell BATR-53 (NCSC)
Figure 89b.
Illustration
Panicum virgatumvar.virgatum
Panicum virgatumvar.virgatum Taxon concept: [< P. virgatum – RAB, GW, FNA; = Weakley]
Distribution
Bay Tree Lake: Wilbur 57420 (DUKE!)
Notes
Perennial herbs. Eulittoral zone. Jun−Oct. Fig. 90
Figure 90.
Panicum virgatum (illustration from Hitchcock and Chase 1951)
Saccharum giganteum
(Walter) Pers.
Saccharum giganteum Basionym: Anthoxanthum giganteum Walter
Saccharum giganteum Taxon concept: [= Erianthus giganteus (Walter) P. Beauv. – RAB, GW; = FNA. Weakley]
Distribution
Jones Lake (Rare): Howell JOLA−37 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−7, 160 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSS−LW). Sep−Oct. Fig. 91
Saccharum giganteum (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 91a.
Specimen: Howell LAWA-7 (NCSC)
Figure 91b.
lllustration
Figure 91c.
Culm and leaf blade
Figure 91d.
Inflorescence
Sacciolepis striata
(L.) Nash
Sacciolepis striata Basionym: Holcus striatus L.
Sacciolepis striata Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−43, 54, 55 (NCSC!); Wilbur 48657, 57394 (DUKE!)
Horseshoe Lake: Rothfels, Burge, Duke Natural History Society 2398 (DUKE!)
Lake Waccamaw (Occasional): Howell LAWA−131 (NCSC!)
Singletary Lake (Occasional): Beal 3225 (NCSC!); Frey s.n. (NCU!); Howell SILA−38 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jul−Oct. Fig. 92
Sacciolepis striata (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 92a.
Specimen: Howell BATR-55 (NCSC)
Figure 92b.
Illustration
Figure 92c.
Leaf sheath
Figure 92d.
Inflorescence
Sphenopholis obtusata
(Michx.) Scribn.
Sphenopholis obtusata Basionym: Aria obtusata Michx.
Sphenopholis obtusata Taxon concept: [= RAB, FNA, Weakley]
Distribution
Lake Waccamaw: Blomquist 1492 (DUKE!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Apr−May. Fig. 93
Figure 93.
Sphenopholis obtusata (illustration from Hitchcock and Chase 1951)
Pontederiaceae
Pontederia cordatavar.cordata
Pontederia cordatavar.cordata Taxon concept: [< P. cordata − RAB; = GW; < P. cordata – FNA; = Weakley]
Distribution
Bay Tree Lake (Rare): •Lake Waccamaw (Occasional): Howell LAWA−15, 50, 159 (NCSC!); Matthews s.n. (NCU!); Wilbur 59382 (DUKE!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). May−Oct. Fig. 94
Pontederia cordata var. cordata (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 94a.
Specimen: Howell LAWA-159 (NCSC)
Figure 94b.
Illustration
Figure 94c.
Leaves
Figure 94d.
Inflorescence
Pontederia cordatavar.lancifolia
(Muhl.) Torr.
Pontederia cordatavar.lancifolia Taxon concept: [< P. cordata – RAB; = GW; < P. cordata – FNA; = Weakley]
Distribution
Lake Waccamaw: ♦
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). May−Oct.
Potamogetonaceae
Potamogeton pulcher
Tuck.
Potamogeton pulcher Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw: ►
Notes
Perennial herbs. Eulittoral and infralittoral zones (NLSS−LW, NLSM−LWP). Jun−Sep. Fig. 95
Figure 95.
Potamogeton pulcher (illustration from Britton and Brown 1913)
Potamogeton pusillus
L.
Potamogeton pusillus Taxon concept: [< P. berchtoldii Fieber – RAB; = GW; > P. pusillus L. ssp. pusillus – FNA; > P. pusillus L. var. pusillus − Weakley]
Distribution
Lake Waccamaw: ¤
Notes
Annual herbs. Eulittoral and infralittoral zones (NLSS−LW, NLSM−LWP). May−Sep.
Smilacaceae
Smilax glauca
Walter
Smilax glauca Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−29 (NCSC!)
Notes
Perennial vines. Juncture of eulittoral and supralittoral zones. Late Apr−Early Jun; Sep−Nov and persisting. Fig. 96
Smilax glauca (digital photographs taken by Alexander Krings)
Figure 96a.
Specimen: Howell BATR-29 (NCSC)
Figure 96b.

Leaf (abaxial surface)
Figure 96c.

Leaf showing contrast between abaxial surface (left) and adaxial surface (right)
Figure 96d.

Fruits
Smilax laurifolia
L.
Smilax laurifolia Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bakers Lake (Frequent): Howell BALA−13 (NCSC!)
Bay Tree Lake (Occasional): Howell BATR−44 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−3 (NCSC!)
Jones Lake (Frequent): Howell JOLA−7 (NCSC!)
Lake Waccamaw (Infrequent): Howell LAWA−34 (NCSC!)
Salters Lake (Frequent): Howell SALA−4, 15 (NCSC!)
Singletary Lake (Frequent): Howell SILA−9 (NCSC!)
Notes
Perennial vines. Eulittoral zone; typically at the maximum annual high water mark in saturated organic to sandy soils (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jul−Aug; Sep−Oct and persisting. Fig. 97
Smilax laurifolia (digital photographs taken by Nathan Howell; illustrations from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 97a.
Specimen: Howell SALA-15 (NCSC)
Figure 97b.
Illustration
Figure 97c.
Habit
Figure 97d.
Infructescence
Smilax rotundifolia
L.
Smilax rotundifolia Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw: ♦
Notes
Perennial vines. Juncture of eulittoral and supralittoral (NLSS−LW). Apr−May; Sep−Oct and persisting. Fig. 98
Smilax rotundifolia (digital photographs taken by Alexander Krings; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 98a.
Illustration
Figure 98b.

Leaf (adaxial surface)
Figure 98c.

Stem (with prickles), petiole, and withered stipular tendrils (at base of petiole)
Figure 98d.

Denticulations at base of leaf blade (arrowed)
Smilax walteri
Pursh
Smilax walteri Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Horseshoe Lake (Occasional): Howell HOLA−13, 21, 25 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−29, 55, 162 (NCSC!)
Notes
Perennial vines. Eulittoral zone (NLSS−LW, CPSI−CG). Late Apr−May; Sep−Nov and persisting. Fig. 99
Smilax walteri (digital photographs taken by Nathan Howell [leaf and flower] and Alexander Krings [fruits])
Figure 99a.
Specimen: Howell LAWA-55 (NCSC)
Figure 99b.
Leaf (adaxial surface)
Figure 99c.
Flower
Figure 99d.

Fruits
Xyridaceae
Xyris fimbriata
Elliott
Xyris fimbriata Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Horseshoe Lake: Rothfels, Burge, Duke Natural History Society 2400, 2404 (DUKE!)
Lake Waccamaw: ►
Singletary Lake: Frey s.n. (NCU!)
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSM−T, NLSS−LW, CPSI–CG). Sep−Oct. Fig. 100
Figure 100.
Xyris fimbriata (illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Xyris iridifolia
Chapm.
Xyris iridifolia Taxon concept: [= RAB, GW; < X. laxifolia Mart. var. iridifolia (Chapm.) Kral – FNA; = Weakley]
Ecological interactions
Conservation status
W7; S2, G4G5T4T.
Distribution
Salters Lake: ♦
Notes
Perennial herbs. Eulittoral zone (NLSS–C). Jul–Sep.
Xyris jupicai
Rch.
Xyris jupicai Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Little Singletary Lake (Infrequent): Howell LISI−46 (NCSC!)
Notes
Annual, rarely biennial, herbs. Eulittoral zone (NLSS−C, NLSM−T). Jul−Sep. Fig. 101
Xyris jupicai (illustration from Britton and Brown 1913)
Figure 101a.
Specimen: Howell LISI-46 (NCSC)
Figure 101b.
Illustration
Xyris smalliana
Nash
Xyris smalliana Taxon concept: [= RAB, GW, FNA, Weakley]
Ecological interactions
Conservation status
W1; S3, G5.
Distribution
Horseshoe Lake: R.L Wilbur 81092 (DUKE!)
Jones Lake (Occasional): Howell JOLA−42 (NCSC!)
Lake Waccamaw (Abundant): Howell LAWA−114, 125, 142, 144 (NCSC!); LeBlond 3996 (NCU)
Salters Lake: Beckman & Linnenburger 24 (DUKE!); Grant s.n. (NCU)
Singletary Lake (Occasional): Howell SILA−29 (NCSC!); Rothfels & O’ Reilly, Shaw Lab s.n. (DUKE!)
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSS−LW, NLSM–T, CPSI–CG). Jul−Aug. Fig. 102
Xyris smalliana (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 102a.
Specimen: Howell LAWA-114 (NCSC)
Figure 102b.
Illustration
Figure 102c.
Inflorescence
Figure 102d.
Flowers
BASAL ANGIOSPERMS, MAGNOLIIDS, and EUDICOTYLEDONS
Families represented: 55 (BA: 2; M: 2; E: 51)
Altingiaceae
Liquidambar styraciflua
L.
Liquidambar styraciflua Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Infrequent): Howell BATR−47 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−45, 133 (NCSC!)
Notes
Trees. Juncture of eulittoral and supralittoral zones (NLSS–LW). Apr–May; Aug–Sep. Fig. 103
Liquidambar styraciflua (digital photographs taken by Nathan Howell [leaves] and Alexander Krings [twig, staminate flowers, fruit]; illustration from Britton and Brown 1913)
Figure 103a.
Specimen: Howell LAWA-133 (NCSC)
Figure 103b.
Illustration
Figure 103c.
Twig, bud, and leaf scars
Figure 103d.
Leaves
Figure 103e.
Staminate flowers
Figure 103f.
Fruit
Amaranthaceae
Alternanthera philoxeroides
(Mart.) Griseb.
Alternanthera philoxeroides Basionym: Bucholzia philoxeroides Mart.
Alternanthera philoxeroides Taxon concept: [= RAB, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Beal 543, 1776 (DUKE!); Howell LAWA−65 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; calm, quiet waters (NLSS–LW). Apr–Oct. Fig. 104
Alternanthera philoxeroides (digital photographs taken by Nathan Howell [habit, node] and Alexander Krings [leaf, inflorescence]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 104a.
Specimen: Howell LAWA-65 (NCSC)
Figure 104b.
Illustration
Figure 104c.
Habit
Figure 104d.
Node
Figure 104e.

Leaf
Figure 104f.

Inflorescence
Anacardiaceae
Rhus copallinumvar.copallinum
Rhus copallinumvar.copallinum Taxon concept: [< R. copallina L. – RAB; = Weakley]
Distribution
Bakers Lake (Rare): Howell BALA−10 (NCSC!)
Notes
Shrubs or small trees. Juncture of eulittoral and supralittoral zones (NLSS−C). Jul−Sep; Aug−Oct. Fig. 105
Rhus copallinum (digital photographs taken by Nathan Howell [infructescence] and Alexander Krings [stem, leaf, infructescence detail]; illustration from Britton and Brown 1913)
Figure 105a.
Specimen: Howell BALA-10 (NCSC)
Figure 105b.
Illustration
Figure 105c.

Stem
Figure 105d.

Imparipinnate leaf (with winged rachis)
Figure 105e.
Infructescence
Figure 105f.
Infructescence (detail)
Toxicodendron radicansvar.radicans
Toxicodendron radicansvar.radicans Taxon concept: [< Rhus radicans L. – RAB; < T. radicans (L.) Kuntze – GW; = Weakley]
Distribution
Bay Tree Lake (Infrequent): •
Lake Waccamaw (Occasional): Howell LAWA−82, 152 (NCSC!)
Notes
Shrubs or lianas. Eulittoral zone; typically growing on woody shrubs and trees at or just below the high water mark (NLSS−LW, NLSM−LWP). Late Apr−May; Aug−Oct. Fig. 106
Toxicodendron radicans (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 106a.
Specimen: Howell LAWA-152 (NCSC)
Figure 106b.
lllustration
Figure 106c.
Habit
Figure 106d.
Climbing stem with adventitious roots
Figure 106e.
Inflorescence
Figure 106f.
Fruits
Apiaceae
Centella asiatica
(L.) Urb.
Centella asiatica Basionym: Hydrocotyle asiatica L.
Centella asiatica Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Frequent): Howell LAWA−25, 115 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS–LW). Jun–Aug; Jul–Sep. Fig. 107
Centella asiatica (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 107a.
Specimen: Howell LAWA-115 (NCSC)
Figure 107b.
Illustration
Figure 107c.
Leaves
Figure 107d.
Flower
Cicuta maculatavar.maculata
Cicuta maculatavar.maculata Taxon concept: [= C. maculata L. – RAB, GW; = Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−121 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; moist soils at or just below the mean annual high water mark, also on sandbars and peninsular islands stranded in the littoral zone (NLSS–LW). May−Aug; Jul−Sep. Fig. 108
Cicuta maculata (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 108a.
Specimen: Howell LAWA-121 (NCSC)
Figure 108b.
Illustration
Figure 108c.
Leaf
Figure 108d.
Inflorescence
Aquifoliaceae
Ilex coriacea
(Pursh) Chapm.
Ilex coriacea Basionym: Prinos coriaceus Pursh
Ilex coriacea Taxon concept: [= RAB, GW, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−16 (NCSC!)
Jones Lake (Occasional): Howell JOLA−10, 32 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS−C). Apr−May; Sep−Oct. Fig. 109
Ilex coriacea (digital photographs taken by Nathan Howell)
Figure 109a.
Specimen: Howell BATR-16 (NCSC)
Figure 109b.
Leaves
Figure 109c.
Flower
Figure 109d.
Fruits
Ilex glabra
(L.) A. Gray
Ilex glabra Basionym: Prinos glaber L.
Ilex glabra Taxon concept: [= RAB, GW, Weakley]
Distribution
Bakers Lake (Rare): Howell BALA−9 (NCSC!)
Bay Tree Lake (Rare): Howell BATR−3, 59 (NCSC!)
Lake Waccamaw (Infrequent): Howell LAWA−9, 153 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSS−LW). May−Jun; Sep−Nov. Fig. 110
Ilex glabra (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 110a.
Specimen: Howell LAWA-9 (NCSC)
Figure 110b.
Illustration
Figure 110c.
Leaves
Figure 110d.
Fruits
Araliaceae
Hydrocotyle umbellata
L.
Hydrocotyle umbellata Taxon concept: [= RAB, GW, RAB, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−45 (NCSC; this specimen is sterile and tentatively referred here)
Lake Waccamaw (Frequent): Howell LAWA−24, 53 (NCSC; these specimens are sterile and tentatively referred here)
Notes
Perennial herbs. Eulittoral zone (NLSS–LW). Apr–Sep. Fig. 111
Hydrocotyle umbellata (illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 111a.
Specimen: Howell LAWA-24 (NCSC)
Figure 111b.
Illustration
Asteraceae
Baccharis halimifolia
L.
Baccharis halimifolia Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−88 (NCSC!)
Notes
Shrubs. Eulittoral zone; can be found on saturated soil at or just below the mean annual high water mark or growing from the bases of Taxodium in the littoral zone (NLSS−LW). Aug−Oct. Fig. 112f
Baccharis halimifolia (digital photographs taken by Alexander Krings; illustration from Britton and Brown 1913)
Figure 112a.
Specimen: Howell LAWA-88 (NCSC)
Figure 112b.
Illustration
Figure 112c.

Stem
Figure 112d.

Leaf
Figure 112e.

Staminate capitulescence
Figure 112f.

Pistillate capitulescence
Bidens laevis
(L.) Britton, Sterns & Poggenb.
Bidens laevis Basionym: Helianthus laevis L.
Bidens laevis Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw: Radford 683 (NCU!)
Notes
Annual herbs. Eulittoral zone; wet sandy beaches and sand bars (NLSS−LW). (Aug−Nov). The first author did not encounter this taxon in the field, but a single voucher confirms its historical presence. Fig. 113
Figure 113.

Bidens laevis (illustration from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Boltonia asteroidesvar.glastifolia
(Hill) Fernald
Boltonia asteroidesvar.glastifolia Basionym: Matricaria glastifolia Hill
Boltonia asteroidesvar.glastifolia Taxon concept: [< B. asteroides (L.) L’Hér – RAB; < Boltonia spp. – GW (formal treatment of the genus lacking); < B. asteroides (L.) L’Hér var. asteroides – FNA; = Weakley]
Ecological interactions
Conservation status
SR−O; S2,G5TNR.
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−1, 158 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Aug−Sep. Fig. 114
Boltonia asteroides var. glastifolia (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 114a.
Specimen: Howell LAWA-158 (NCSC)
Figure 114b.
Illustration
Figure 114c.
Stem and leaf
Figure 114d.
Radiate head
Erigeron vernus
(L.) Torr. & A. Gray
Erigeron vernus Basionym: Aster vernus L.
Erigeron vernus Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw: ♦
Notes
Biennial or short-lived perennial herbs. Eulittoral zone (NLSS−LW). Late Mar−Jun. Fig. 115
Figure 115.
Erigeron vernus (illustration from Britton and Brown 1913)
Eupatorium capillifolium
(Lam.) Small ex Porter & Britton
Eupatorium capillifolium Basionym: Artemisia capillifolia Lam.
Eupatorium capillifolium Taxon concept: [= E. capillifolium (Lam.) Small ex Porter & Britton var. capillifolium – RAB; = GW, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA– 141 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; usually in a stunted form where detritus has washed ashore just under the maximum annual high water mark (NLSS−LW). Sep−Nov. Fig. 116
Eupatorium capillifolium (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 116a.
Specimen: Howell LAWA-141 (NCSC)
Figure 116b.
Illustration
Figure 116c.
Leaf
Figure 116d.
Leaf
Eupatorium mohrii paludicola
E.E. Schilling & LeBlond
Distribution
Lake Waccamaw (Rare): Howell LAWA−6 (NCSC!)
Notes
Perennial herbs. Eutlittoral zone (NLSS−LW). Aug−Oct. Fig. 117
Eupatorium mohrii × paludicola (digital photographs taken by Nathan Howell)
Figure 117a.
Specimen: Howell LAWA-6 (NCSC)
Figure 117b.
Stem and leaves
Figure 117c.
Capitulescence
Figure 117d.
Capitulescence (detail)
Euthamia caroliniana
(L.) Greene ex Porter & Britton
Euthamia caroliniana Basionym: Erigeron carolinianus L.
Euthamia caroliniana Taxon concept: [> Solidago microcephala (Nutt.) Bush – RAB; > Solidago tenuifolia Pursh – RAB; < E. tenuifolia – GW (also see E. hirtipes); > E. minor (Michx.) Greene – GW; = FNA, Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−12 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Sep−Dec. Fig. 118
Euthamia caroliniana (digital photographs taken by Nathan Howell [capitulescence] and Alexander Krings [leaves]; illustration from Britton and Brown 1913)
Figure 118a.
Specimen: Howell LAWA-12 (NCSC)
Figure 118b.
Illustration
Figure 118c.
Leaves
Figure 118d.
Capitulescence
Hypochaeris radicata
L.
Hypochaeris radicata Taxon concept: [= Hypochoeris radicata L. – RAB; = FNA, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−32 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; moist sandy shores. Apr−Oct. Fig. 119
Hypochaeris radicata (digital photographs taken by Nathan Howell)
Figure 119a.
Specimen: Howell BATR-32 (NCSC)
Figure 119b.
Habit
Figure 119c.
Basal rosette
Figure 119d.
Ligulate head
Krigia virginica
(L.) Willd.
Krigia virginica Basionym: Hyoseris virginica L.
Krigia virginica Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−20 (NCSC!)
Notes
Annual herbs. Eulittoral zone; moist sandy shores. Late Mar−Jul. Fig. 120
Krigia virginica (digital photographs taken by Nathan Howell)
Figure 120a.
Specimen: Howell BATR-20 (NCSC)
Figure 120b.
Basal rosette
Figure 120c.
Capitulescence
Figure 120d.
Capitulescence (lateral)
Mikania scandens
(L.) Willd.
Mikania scandens Basionym: Eupatorium scandens L.
Mikania scandens Taxon concept: [= RAB, GW, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−161 (NCSC!)
Notes
Perennial, sometimes lianescent, vines. Eulittoral zone; usually sprawling and climbing on small shrubs and trees (NLSS−LW). Jun−Oct. Fig. 121
Mikania scandens (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 121a.
Specimen: Howell LAWA-161 (NCSC)
Figure 121b.
Illustration
Figure 121c.
Leaves
Figure 121d.
Capitulescence
Pluchea baccharis
(Mill.) Pruski
Pluchea baccharis Basionym: Conyza baccharis Mill.
Pluchea baccharis Taxon concept: [= P. rosea R.K. Godfrey – RAB; = P. rosea R.K. Godfrey var. rosea – GW; = FNA, Weakley]
Distribution
Lake Waccamaw (Frequent): Howell LAWA−2, 101, 148 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jun−Jul. Fig. 122
Pluchea baccharis (digital photographs taken by Nathan Howell)
Figure 122a.
Specimen: Howell LAWA-101 (NCSC)
Figure 122b.
Stem and leaves
Figure 122c.
Capitulescence
Figure 122d.
Capitulescence
Sclerolepis uniflora
(Walter) Britton, Sterns & Poggenb.
Sclerolepis uniflora Basionym: Ethulia uniflora Walter
Sclerolepis uniflora Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Frequent): Howell LAWA−18, 23, 103, 108 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). May−Aug; Jul−Oct. Fig. 123
Sclerolepis uniflora (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 123a.
Specimen: Howell LAWA-18 (NCSC)
Figure 123b.
Illustration
Figure 123c.
Habit
Figure 123d.
Capitulescence
Solidago fistulosa
Mill.
Solidago fistulosa Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−64, 65 (NCSC!)
Horseshoe Lake: Buell 2266 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; saturated peaty to sandy soils at or just below the mean annual high water mark (CPSI−CG). Aug−Nov. Fig. 124
Solidago fistulosa (digital photographs taken by Nathan Howell)
Figure 124a.
Specimen: Howell BATR-64 (NCSC)
Figure 124b.
Habit
Figure 124c.
Stem and leaves
Figure 124d.
Capitulescence
Betulaceae
Alnus serrulata
(Aiton) Willd.
Alnus serrulata Basionym: Betula serrulata Aiton
Alnus serrulata Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Occasional): Howell LAWA−39, 170 (NCSC!); Matthews 683 (NCU!)
Notes
Shrubs. Eulittoral zone (NLSS–LW). Feb–Mar; Aug–Oct. Fig. 125
Alnus serrulata (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 125a.
Specimen: Howell LAWA-11 (NCSC)
Figure 125b.
Illustration
Figure 125c.
Twig and axillary bud
Figure 125d.
Leaf
Figure 125e.
Staminate catkin
Figure 125f.
Pistillate catkin
Betula nigra
L.
Betula nigra Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−13, 19 (NCSC!)
Lake Waccamaw (Occasional): Blomquist 15004 (DUKE!); Howell LAWA−37, 63 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−29 (NCSC!)
Notes
Trees. Eulittoral zone (NLSS–LW). Mar–Apr; May–Jun. Fig. 126
Betula nigra (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 126a.
Specimen: Howell LAWA-63 (NCSC)
Figure 126b.
Illustration
Figure 126c.
Bark (young tree on right)
Figure 126d.
Pistillate catkin
Bignoniaceae
Campsis radicans
(L.) Bureau
Campsis radicans Basionym: Bignonia radicans L.
Campsis radicans Taxon concept: [= RAB, GW, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−81 (NCSC!)
Notes
Lianas. Eulittoral zone; climbing on young trees and shrubs at or just below the mean annual high water mark (NLSS−LW). Jun−Jul; Sep−Oct. Fig. 127
Campsis radicans (digital photographs taken by Alexander Krings; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 127a.
Specimen: Howell-LAWA 81 (NCSC)
Figure 127b.
lllustration
Figure 127c.

Flower
Figure 127d.

Fruit
Cabombaceae
Brasenia schreberi
J.F. Gmel.
Brasenia schreberi Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Horseshoe Lake (Infrequent): Howell HOLA−43 (NCSC!)
Notes
Perennial herbs. Infralittoral zone (CPSI–CG). Jun–Oct. Fig. 128
Brasenia schreberi (digital photographs taken by Nathan Howell; illustration from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Figure 128a.
Specimen: Howell HOLA-43 (NCSC)
Figure 128b.

Illustration
Figure 128c.
Habit
Figure 128d.
Flower (with hymenopteran visitor)
Cabomba caroliniana
A. Gray
Cabomba caroliniana Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Horseshoe Lake (Infrequent): Howell HOLA−26 (NCSC!)
Notes
Perennial herbs. Infralittoral Zone (CPSI–CG). May–Sep. Fig. 129
Cabomba caroliniana (illustration from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Figure 129a.
Specimen: Howell HOLA-26 (NCSC)
Figure 129b.

Illustration
Campanulaceae
Lobelia glandulosa
Walter
Lobelia glandulosa Taxon concept: [= RAB, GW, Weakley]
Distribution
Lake Waccamaw: ¤
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Sep−Oct.
Lobelia nuttallii
Roem. & Schult.
Lobelia nuttallii Taxon concept: [= RAB, GW, Weakley]
Distribution
Horseshoe Lake (Rare): Howell HOLA−47 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; moist sandy soil at or just below the high water mark (CPSI−CG). May−Nov. Fig. 130
Lobelia nuttallii (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 130a.
Specimen: Howell HOLA-47 (NCSC)
Figure 130b.
Illustration
Figure 130c.
Cauline leaf
Figure 130d.
Inflorescences
Caryophyllaceae
Stipulicida setaceavar.setacea
Stipulicida setaceavar.setacea Taxon concept: [< S. setacea Michx. – RAB; = FNA, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−21 (NCSC!)
Notes
Annual, or short-lived perennial, herbs. Juncture of eulittoral and supralittoral zones. May−Aug. Fig. 131
Stipulicida setacea (digital photographs taken by Nathan Howell)
Figure 131a.
Specimen: Howell BATR-21 (NCSC)
Figure 131b.
Habit
Figure 131c.
Habit
Figure 131d.
Flowers
Clethraceae
Clethra alnifolia
L.
Clethra alnifolia Taxon concept: [< C. alnifolia L. var. alnifolia – RAB; = GW, FNA, Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−12 (NCSC!)
Jones Lake (Occasional): Howell JOLA−5 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−42, 132, 150 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−7, 57 (NCSC!)
Singletary Lake (Occasional): Howell SILA−15, 28 (NCSC!)
Notes
Shrubs. Juncture of supralittoral and eulittoral zones; can also establish itself on stumps, logs, and tree bases in the eulittoral zone (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jun−Jul; Sep−Oct. Fig. 132
Clethra alnifolia (digital photographs taken by Nathan Howell [leaves and flowers] and Alexander Krings [twig and fruits]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 132a.
Specimen: Howell LAWA-150 (NCSC)
Figure 132b.
Illustration
Figure 132c.
Twig, showing leaf scar
Figure 132d.
Leaves
Figure 132e.
Flowers
Figure 132f.
Fruits
Cyrillaceae
Cyrilla racemiflora
L.
Cyrilla racemiflora Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Frequent): Howell BATR−38, 41, 60 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−37 (NCSC!)
Jones Lake (Occasional): •
Lake Waccamaw (Frequent): Howell LAWA−100 (NCSC!)
Little Singletary Lake (Frequent): Howell LISI−32 (NCSC!)
Singletary Lake (Frequent): Howell SILA−11, 23 (NCSC!)
Notes
Shrubs or small trees. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSM−T, NLSS−LW, CPSI−CG). May−Jul; Sep−Oct. Fig. 133
Cyrilla racemiflora (digital photographs taken by Nathan Howell [inflorescence and infructescence] and Alexander Krings [twig and leaves]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 133a.
Specimen: Howell LAWA-100 (NCSC)
Figure 133b.
Illustration
Figure 133c.
Twig, leaf scar, and bud
Figure 133d.
Leaves
Figure 133e.
Inflorescence
Figure 133f.
Infructescence
Droseraceae
Drosera intermedia
Hayne
Drosera intermedia Taxon concept: [= RAB, GW, Weakley]
Distribution
Horseshoe Lake (Frequent): Howell HOLA−28, 40, 49 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−52, 56 (NCSC!)
Notes
Perennial herbs. Eulittoral zone and floating bogs (NLSS−C, NLSM−T, CPSI−CG, FB). Jul–Sep. Fig. 134
Drosera intermedia (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 134a.
Specimen: Howell HOLA-49 (NCSC)
Figure 134b.
Illustration
Figure 134c.
Habit
Figure 134d.
Inflorescences
Ebenaceae
Diospyros virginiana
L.
Diospyros virginiana Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−80 (NCSC!)
Notes
Trees. Juncture of eulittoral and supralittoral zones (NLSS−LW). May−Jun; Sep−Dec. Fig. 135
Diospyros virginiana (digital photographs taken by Nathan Howell [leaves and fruit] and Alexander Krings [twig and flowers]; illustration from Britton and Brown 1913)
Figure 135a.
Specimen: Howell LAWA-80 (NCSC)
Figure 135b.
Illustration
Figure 135c.
Twig, showing the typical dark bud and a single vascular bundle scar in the leaf scar
Figure 135d.
Leaves
Figure 135e.
Flowers
Figure 135f.
Fruit
Ericaceae
Chamaedaphne calyculata
(L.) Moench
Chamaedaphne calyculata Basionym: Andromeda calyculata L.
Chamaedaphne calyculata Taxon concept: [= Cassandra calyculata (L.) D. Don − RAB; = FNA, Weakley]
Distribution
Bakers Lake (Occasional): Howell BALA−1 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−1, 6, 42 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−15, 24 (NCSC!)
Singletary Lake: Fox, Wells, Sharp, Whitford, Fairchild s. n. (NCSC!)
Notes
Shrubs. Eulittoral zone, either in shallow water or in saturated organic soils at the high water mark (NLSS–C, NLSM-T, CPSI–CG, FB). Mar–Apr; Jun–Oct. Fig. 136
Chamaedaphne calyculata (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 136a.
Specimen: Howell HOLA-42 (NCSC)
Figure 136b.
Illustration
Figure 136c.
Inflorescences
Figure 136d.
Inflorescences
Eubotrys racemosa
(L.) Nutt.
Eubotrys racemosa Basionym: Andromeda racemosa L.
Eubotrys racemosa Taxon concept: [= Leucothoe racemosa (L.) A. Gray − RAB; = FNA, Weakley]
Distribution
Horseshoe Lake (Occasional): Howell HOLA−12 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−40, 151 (NCSC!); Matthews s.n. (NCU!)
Jones Lake (Occasional): Howell JOLA−30 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−1 (NCSC!)
Salters Lake (Occasional): Howell SALA−12 (NCSC!)
Singletary Lake (Occasional): Howell SILA−7 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones; sometimes on the bases of large Taxodium trunks (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Late Mar–early Jun; Sept–Oct. Fig. 137
Eubotrys racemosa (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 137a.
Specimen:Howell SALA-12 (NCSC)
Figure 137b.
Illustration
Figure 137c.
Inflorescence
Figure 137d.
Infructescence
Lyonia ligustrinavar.foliosiflora
(Michx.) Fernald
Lyonia ligustrinavar.foliosiflora Basionym: Andromeda paniculata var. foliosiflora Michx.
Lyonia ligustrinavar.foliosiflora Taxon concept: [< L. ligustrina (L.) DC. – RAB; = GW, FNA, Weakley]
Distribution
Bakers Lake (Infrequent): Howell BALA−17 (NCSC!)
Jones Lake (Infrequent): Howell JOLA−31 (NCSC!)
Salters Lake (Infrequent): Howell SALA−17 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones; sometimes growing from the bases of large Taxodium (NLSS–C). Late Apr–Jul; Sep–Oct. Two varieties of Lyonia ligustrina are commonly recognized: var. foliosiflora (Michx.) Fernald, with numerous and conspicuous leaf-like bracts in the inflorescence, and var. ligustrina, with no or few leaf-like bracts in the inflorescence. The material collected by the first author is var. foliosiflora, the more common variety found in the North Carolina Coastal Plain. Fig. 138
Lyonia ligustrina (digital photographs taken by Nathan Howell [leaves] and Alexander Krings [abaxial leaf surface, flower, infructescence]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 138a.
Specimen: Howell SALA-17 (NCSC)
Figure 138b.
Illustration
Figure 138c.
Leaves
Figure 138d.
Abaxial leaf surface
Figure 138e.
Flower
Figure 138f.
Fruits
Lyonia lucida
(Lam.) K. Koch
Lyonia lucida Basionym: Andromeda lucida Lam.
Lyonia lucida Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bakers Lake (Occasional): Howell BALA−8 (NCSC!)
Bay Tree Lake (Occasional): Howell BATR−17 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−7 (NCSC!)
Jones Lake (Frequent): Howell JOLA−11, 19 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−35 (NCSC!)
Little Singletary (Occasional): Howell LISI−5, 36 (NCSC!)
Salters Lake (Frequent): Howell SALA−5, 11 (NCSC!)
Singletary Lake (Frequent): Howell SILA−3 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones; sometimes growing from the bases of mature Taxodium (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Apr–early Jun; Sep–Oct. Fig. 139
Lyonia lucida (digital photographs taken by Nathan Howell [inflorescence] and Alexander Krings [leaf, flowers, fruits]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 139a.
Specimen: Howell SALA-11 (NCSC)
Figure 139b.
Illustration
Figure 139c.
Leaf (abaxial surface)
Figure 139d.
Inflorescence
Figure 139e.
Flowers
Figure 139f.
Fruits
Rhododendron viscosumvar.serrulatum
(Small) H.E. Ahles
Rhododendron viscosumvar.serrulatum Basionym: Azalea serrulata Small
Rhododendron viscosumvar.serrulatum Taxon concept: [= RAB; < R. viscosum – GW, FNA; = Weakley]
Distribution
Singletary Lake (Rare): Howell SILA−33, 34 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS–C). Late May–Jun; Jul–Oct. Fig. 140
Rhododendron viscosum (digital photographs taken by Nathan Howell [leaves, fruit] and Alexander Krings [flower, flower detail]; illustration from Britton and Brown 1913)
Figure 140a.
Specimen: Howell SILA-33 (NCSC)
Figure 140b.
Illustration
Figure 140c.
Leaves
Figure 140d.
Flower
Figure 140e.
Flower detail
Figure 140f.
Fruit
Vaccinium formosum
Andrews
Vaccinium formosum Taxon concept: [< V. corymbosum L. – RAB; = V. australe Small – GW; < V. corymbosum L. – FNA; = Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−33 (NCSC!)
Jones Lake (Occasional): Howell JOLA−13, 24, 26 (NCSC!)
Salters Lake (Occasional): Howell SALA−10 (NCSC!)
Singletary Lake (Occasional): Howell SILA−18 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSM−T, NLSS−LW). Late Feb−May; Jun−Aug. Fig. 141
Vaccinium formosum (digital photographs taken by Nathan Howell [leaves, infructescence] and Alexander Krings [inflorescence])
Figure 141a.
Specimen: Howell SALA-10 (NCSC)
Figure 141b.
Leaves
Figure 141c.

Inflorescence
Figure 141d.
Infructescence
Vaccinium fuscatum
Aiton
Vaccinium fuscatum Taxon concept: [= V. atrococcum (Gray) Heller – RAB; = GW; < V. corymbosum L. –FNA; Weakley]
Distribution
Bakers Lake (Occasional): Howell BALA−7 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−49 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−14, 39, 40 (NCSC!)
Salters Lake (Occasional): Howell SALA−16 (NCSC!)
Singletary Lake (Occasional): Howell SILA−21 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSM−T, NLSS−LW). Late Feb−May; Jun−Aug. Fig. 142
Vaccinium fuscatum (digital photographs taken by Nathan Howell [leaves, infructescence] and Alexander Krings [flower])
Figure 142a.
Specimen: Howell SALA-16 (NCSC)
Figure 142b.
Leaves
Figure 142c.
Flower
Figure 142d.
Infructescence
Zenobia pulverulenta
(W. Bartram ex Willd.) Pollard
Zenobia pulverulenta Basionym: Andromeda pulverulenta W. Bartram ex Willd.
Zenobia pulverulenta Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bakers Lake (Infrequent): Howell BALA−5 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−17, 22, 35 (NCSC!)
Jones Lake (Occasional): Howell JOLA−25 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−10, 23 (NCSC!)
Singletary Lake: Fox & Boyce 3781 (NCSC!); Fox, Wells, Sharp, Whitford, Fairchild 1708 (NCSC!)
Notes
Shrubs. Juncture of supralittoral and eulittoral zones; sometimes growing on the bases of mature Taxodium (NLSS–C, NLSM–T, CPSI–CG, FB). Apr–Jun; Sep–Oct. Fig. 143
Zenobia pulverulenta (digital photographs taken by Nathan Howell [inflorescence, flowers within] and Alexander Krings [leaves, flower]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 143a.
Specimen: Howell HOLA-35 (NCSC)
Figure 143b.
Illustration
Figure 143c.
Leaves
Figure 143d.
Inflorescence
Figure 143e.
Flowers within
Figure 143f.
Flower
Euphorbiaceae
Triadica sebifera
(L.) Small
Triadica sebifera Basionym: Croton sebifer L.
Triadica sebifera Taxon concept: [= Sapium sebiferum (L.) Roxb. – RAB, GW; = Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−92 (NCSC!)
Notes
Trees. Eulittoral zone (NLSS–LW). May–Jun; Aug–Nov. Fig. 144
Triadica sebifera (digital photograph taken by Nathan Howell)
Figure 144a.
Specimen: Howell LAWA-92 (NCSC)
Figure 144b.
Young tree
Fabaceae
Wisteria frutescens
(L.) Poir.
Wisteria frutescens Basionym: Glycine frutescens L.
Wisteria frutescens Taxon concept: [= RAB, GW, Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−37 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−99, 117 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−35 (NCSC!)
Notes
Lianas. Eulittoral zone (NLSS–LW). Apr–May; Jun–Sep. Fig. 145
Wisteria frutescens (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 145a.
Specimen: Howell BATR-37 (NCSC)
Figure 145b.
Illustration
Figure 145c.
Imparipinnate leaf
Figure 145d.
Fruit
Fagaceae
Quercus nigra
L.
Quercus nigra Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw: Godfrey 6320 (NCSC!)
Notes
Trees. Juncture of eulittoral and supralittoral zones (NLSS–LW). Apr; Sep–Nov. Fig. 146
Quercus nigra (digital photographs taken by Alexander Krings; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 146a.
Illustration
Figure 146b.

Bark
Figure 146c.

Leaves
Figure 146d.

Fruit
Gelsemiaceae
Gelsemium sempervirens
(L.) J. St.−Hil.
Gelsemium sempervirens Basionym: Bignonia sempervirens L.
Gelsemium sempervirens Taxon concept: [= RAB, GW, Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−10, 25 (NCSC!)
Horseshoe Lake (Rare): Howell HOLA−4 (NCSC!)
Jones Lake (Infrequent): Howell JOLA−34 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−41 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−34 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−36 (NCSC!)
Notes
Lianas. Eulittoral zone (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Mar–early May; Sept–Nov. Fig. 147
Gelsemium sempervirens (digital photographs taken by Alexander Krings; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 147a.
Specimen: Howell JOLA-34 (NCSC)
Figure 147b.
lllustration
Figure 147c.

Leaves
Figure 147d.

Flower
Figure 147e.

Fruit
Figure 147f.

Seed
Hydrangeaceae
Decumaria barbara
L.
Decumaria barbara Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Rare): Dumond 1621 (NCU!); Godfrey 52278 (NCSC!); Howell LAWA−86 (NCSC!)
Notes
Lianas. Eulittoral zone; climbing on trees and shrubs at or just below the maximum annual high water mark (NLSS−LW, NLSM−LWP). May−Jun; Jul−Oct. Fig. 148
Decumaria barbara (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 148a.
Specimen: Howell LAWA-86 (NCSC)
Figure 148b.
Illustration
Figure 148c.
Leaves
Figure 148d.
Fruits
Hypericaceae
Hypericum canadense
L.
Hypericum canadense Taxon concept: [= RAB, GW, Weakley]
Distribution
Horseshoe Lake (Rare): Howell HOLA−48 (NCSC!)
Notes
Annual or perennial herbs. Eulittoral zone; moist sandy soils at or just below the maximum annual high water mark (CPSI−CG). Jul−Sep. Fig. 149
Hypericum canadense (digital photographs taken by Nathan Howell)
Figure 149a.
Specimen: Howell HOLA-48 (NCSC)
Figure 149b.
Stem and leaves
Figure 149c.
Inflorescence
Figure 149d.
Flower
Hypericum mutilumvar.mutilum
Hypericum mutilumvar.mutilum Taxon concept: [< H. mutilum L. – RAB, GW; = Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−139 (NCSC!)
Notes
Annual or perennial herbs. Eulittoral zone; at or just below the maximum annual high water mark (NLSS−LW). Jun−Oct. Fig. 150
Hypericum mutilum (digital photographs taken by Nathan Howell)
Figure 150a.
Specimen: Howell LAWA-139 (NCSC)
Figure 150b.
Habit
Figure 150c.
Flower (front)
Figure 150d.
Flower (side)
Hypericum virginicum
L.
Hypericum virginicum Taxon concept: [= RAB; = Triadenum virginicum (L.) Raf. – GW; = Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−9, 56 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−33 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−54 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−C, NLSM−T, CPSI−CG, FB). Jul−Sep. Fig. 151
Hypericum virginicum (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 151a.
Specimen: Howell BATR-56 (NCSC)
Figure 151b.
Illustration
Figure 151c.
Leaves
Figure 151d.
Infructescence
Hypericum walteri
J.F. Gmel.
Hypericum walteri Taxon concept: [= RAB; = Triadenum walteri (J.F. Gmel.) Gleason – GW; = Weakley]
Distribution
Lake Waccamaw (Occasional): Howell LAWA−20, 134, 149 (NCSC!); Wilbur 9363 (DUKE!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Jul−Sep. Fig. 152
Hypericum walteri (digital photographs by Nathan Howell)
Figure 152a.
Specimen: Howell LAWA-20 (NCSC)
Figure 152b.
Habit
Figure 152c.
Leaves, showing distinct petioles
Figure 152d.
Infructescence
Iteaceae
Itea virginica
L.
Itea virginica Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bakers Lake (Infrequent): Howell BALA−6 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−9 (NCSC!)
Jones Lake (Occasional): Howell JOLA−27 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−85 (NCSC!)
Singletary Lake (Occasional): Howell SILA−2 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones; sometimes establishing itself on stumps, logs, and bases of trees in the eulittoral zone (NLSS−C, NLSS−LW, CPSI−CG). May−Jun. Fig. 153
Itea virginica (digital photographs taken by Nathan Howell [habit and inflorescence] and Alexander Krings [leaf and fruits]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 153a.
Specimen: Howell BALA-6 (NCSC)
Figure 153b.
Illustration
Figure 153c.
Habit
Figure 153d.
Leaf
Figure 153e.
Inflorescence
Figure 153f.
Fruits
Juglandaceae
Carya glabra
(Mill.) Sweet
Carya glabra Basionym: Juglans glabra Mill.
Carya glabra Taxon concept: [= RAB, GW; < C. glabra (Mill.) Sweet – FNA; = Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−96 (NCSC!); Matthews s.n. (DUKE!)
Notes
Trees. Juncture of eulittoral and supralittoral zones (NLSS−LW). Apr−May. Fig. 154
Carya glabra (digital photographs taken by Nathan Howell)
Figure 154a.
Specimen: Howell LAWA-96 (NCSC)
Figure 154b.
Stem and buds
Figure 154c.
Imparipinnate leaf
Figure 154d.
Fruits
Lamiaceae
Lycopus angustifolius
Elliott
Lycopus angustifolius Taxon concept: [< L. rubellus Moench var. angustifolius (Elliott) H.E. Ahles – RAB, GW; = Weakley]
Ecological interactions
Conservation status
SR−P; S1, G4?Q.
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−4, 156, 157 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS–LW). Jun–Sep. Fig. 155
Lycopus angustifolius (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 155a.
Specimen: Howell LAWA-4 (NCSC)
Figure 155b.
Illustration
Figure 155c.
Flowers
Figure 155d.
Fruits
Lauraceae
Persea palustris
(Raf.) Sarg.
Persea palustris Basionym: Tamala palustris Raf.
Persea palustris Taxon concept: [< P. borbonia – RAB; = GW, FNA, Weakley]
Distribution
Bakers Lake (Occasional): Howell BALA−12 (NCSC!)
Jones Lake (Occasional): Howell JOLA−6, 18 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−61, 69 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−6 (NCSC!)
Salters Lake (Occasional): Buell s.n. (DUKE!, NCSC!); Howell SALA−6, 20 (NCSC!)
Singletary Lake (Occasional): Howell SILA−10, 25, 27 (NCSC!)
Notes
Shrubs or small trees. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSS−LW). May−Jun; Sep−Oct. Fig. 156
Persea palustris (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 156a.
Specimen: Howell SALA-20 (NCSC)
Figure 156b.
Illustration
Figure 156c.
Twig (note pubescence)
Figure 156d.
Leaf abaxial surface
Figure 156e.
Flower
Figure 156f.
Fruits
Lentibulariaceae
Utricularia cornuta
Michx.
Utricularia cornuta Taxon concept: [= RAB, GW, Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−109 (NCSC!)
Notes
Annual or perennial herbs. Eulittoral zone; commonly in saturated sandy to peaty soils just above current water levels or in 1−4 inches of water (NLSS−LW). May−Sep. Fig. 157
Utricularia cornuta (digital photograph taken by Nathan Howell)
Figure 157a.
Specimen: Howell LAWA-109 (NCSC)
Figure 157b.
Habit
Utricularia gibba
L.
Utricularia gibba Taxon concept: [= RAB; = U. biflora Lam. – GW; = Weakley]
Distribution
Horseshoe Lake (Occasional): Howell HOLA−19 (NCSC!)
Notes
Annual or perennial herbs. Eulittoral and infralittoral zones; Godfrey and Wooten (1981) described the habit as “very much intertwined, forming large floating bunches or mats” (CPSI−CG). May−Sep. Fig. 158
Utricularia gibba (digital photograph taken by Nathan Howell)
Figure 158a.
Specimen: Howell HOLA-19 (NCSC)
Figure 158b.
Flower
Utricularia purpurea
Walter
Utricularia purpurea Taxon concept: [= RAB, GW, Weakley]
Distribution
Horseshoe Lake (Occasional): Howell HOLA−36 (NCSC!)
Notes
Annual or perennial herbs. Eulittoral and infralittoral zones; floating bogs (CPSI−CG). May−Sep. Fig. 159
Utricularia purpurea (digital photographs taken by Nathan Howell)
Figure 159a.
Specimen: Howell HOLA-36 (NCSC)
Figure 159b.
Habit
Figure 159c.
Flower
Figure 159d.
Flower
Utricularia resupinata
B.D. Greene ex Bigelow
Utricularia resupinata Taxon concept: [= GW, Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−123 (NCSC!)
Notes
Annual or perennial herbs. Eulittoral zone; commonly in saturated sandy to peaty soils above current lake levels or in 1−4 inches of water (NLSS−LW). Jun−Aug. Fig. 160
Utricularia resupinata (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 160a.
Specimen: Howell LAWA-123 (NCSC)
Figure 160b.

Illustration
Figure 160c.
Flower (front)
Figure 160d.
Flower (side)
Utricularia striata
Leconte ex Torr.
Utricularia striata Taxon concept: [= U. fibrosa Walter – RAB, GW; = Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−35, 42 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−27 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−14, 122 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; typically seen in shallow water or stranded on saturated organic soils (NLSS−LW, CPSI−CG, FB). May−Nov. Fig. 161
Utricularia striata (digital photographs taken by Nathan Howell)
Figure 161a.
Specimen: Howell BATR-42 (NCSC)
Figure 161b.
Habit
Figure 161c.
Flower
Figure 161d.
Flower
Utricularia subulata
L.
Utricularia subulata Taxon concept: [= RAB, GW, Weakley]
Distribution
Lake Waccamaw: ♦
Little Singletary Lake (Infrequent): Howell LISI−28, 49 (NCSC!)
Notes
Annual or perennial herbs. Eulittoral zone; typically found in saturated sands and peats (NLSS−C, NLSS−LW, NLSM−T). Mar−Aug. Fig. 162
Utricularia subulata (digital photographs taken by Nathan Howell [habit, flower front, flower back] and Alexander Krings [flower side]; illustration from Britton and Brown 1913)
Figure 162a.
Specimen: Howell LISI-28 (NCSC)
Figure 162b.
Illustration
Figure 162c.
Habit
Figure 162d.
Flower (front)
Figure 162e.
Flower (back)
Figure 162f.
Flower (side)
Linderniaceae
Lindernia dubiavar.dubia
Lindernia dubiavar.dubia Basionym: Gratiola dubia L.
Lindernia dubiavar.dubia Taxon concept: [= L. dubia (L.) Pennell – RAB, GW; = Weakley]
Distribution
Lake Waccamaw: Radford & Stewart 679 (NCU!)
Notes
Annual or biennial herbs. Eulittoral zone; saturated sandy soils (NLSS−LW). May−Nov. (Fig. 159). The first author did not encounter this taxon in the field, but a single voucher confirms its historic presence (see above). Fig. 163
Figure 163.
Lindernia dubia (illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Loganiaceae
Mitreola petiolata
(Walter ex J.F. Gmelin) Torr. & A. Gray
Mitreola petiolata Basionym: Cynoctonum petiolatum Walter ex J.F. Gmelin
Mitreola petiolata Taxon concept: [= Cynoctonum mitreola (L.) Britton – RAB; = GW, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−140, 154 (NCSC!)
Notes
Annual herbs. Eulittoral zone; shallow water (1−6 inches) or saturated soils above current lake levels (NLSS−LW). Jul−Sep; Sep−Nov. Fig. 164
Mitreola petiolata (digital photographs taken by Nathan Howell)
Figure 164a.
Specimen: Howell LAWA-140 (NCSC)
Figure 164b.
Stem and leaves
Figure 164c.
Inflorescence
Figure 164d.
Flower
Lythraceae
Decodon verticillatus
(L.) Elliott
Decodon verticillatus Basionym: Lythrum verticillatum L.
Decodon verticillatus Taxon concept: [= RAB, GW, Weakley]
Distribution
Bay Tree Lake (Infrequent): •
Horseshoe Lake (Occasional): Howell HOLA−31 (NCSC!)
Jones Lake (Infrequent): •
Salters Lake (Infrequent): •
Notes
Shrubs. Eulittoral zone (NLSS−C, NLSM−T, FB, CPSI−CG). Jul−Sep. Fig. 165
Decodon verticillatus (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 165a.
Specimen: Howell HOLA-31 (NCSC)
Figure 165b.
Illustration
Figure 165c.
Leaves
Figure 165d.
Fruits
Magnoliaceae
Magnolia virginianavar.virginiana
Magnolia virginianavar.virginiana Taxon concept: [< M. virginiana – RAB, GW, FNA; = Weakley]
Distribution
Bakers Lake (Occasional): Howell BALA−3 (NCSC!)
Jones Lake (Occasional): Howell JOLA−8, 29 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−62 (NCSC!)
Salters Lake (Occasional): Howell SALA−13 (NCSC!)
Singletary Lake (Occasional): Howell SILA−5, 19 (NCSC!)
Notes
Trees. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSS−LW, NLSM−T). Fig. 166
Magnolia virginiana (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 166a.
Specimen: Howell LAWA-62 (NCSC)
Figure 166b.
Illustration
Figure 166c.
Stem and terminal bud
Figure 166d.
Terminal bud (detail)
Figure 166e.
Leaves (note glaucescence below)
Figure 166f.
Flower
Melastomataceae
Rhexia aristosa
Britton
Rhexia aristosa Taxon concept: [= RAB, GW, Weakley]
Ecological interactions
Conservation status
SC–V, FSC; S3, G3G4.
Distribution
Horseshoe Lake: ►
Notes
Perennial herbs. floating bogs (CPSI–CG, FB). Jun–Sep. Fig. 167
Rhexia aristosa (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 167a.
Illustration
Figure 167b.
Calyx tube (lateral)
Figure 167c.
Calyx tube (latero-adaxial)
Figure 167d.
Flower (adaxial)
Rhexia cubensis
Griseb.
Rhexia cubensis Taxon concept: [= RAB, GW, Weakley]
Ecological interactions
Conservation status
W1; S3, G4G5.
Distribution
Lake Waccamaw (Occasional): Howell LAWA–113, 126, 129 (NCSC!); LeBlond 3990 (NCU!)
Notes
Perennial herbs. Eulittoral zone (NLS–LW). Jun–Sep. Fig. 168
Rhexia cubensis (digital photographs taken by Nathan Howell)
Figure 168a.
Specimen: Howell LAWA-113 (NCSC)
Figure 168b.
Leaves
Figure 168c.
Flower (unopened)
Figure 168d.
Flower (opened)
Rhexia marianavar.exalbida
Michx.
Rhexia marianavar.exalbida Taxon concept: [= RAB; < R. mariana var. mariana – GW; = Weakley]
Distribution
Horseshoe Lake (Rare): Howell HOLA−46 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (CPSI–CG). Jun–Sep. Fig. 169
Rhexia mariana var. exalbida (digital photographs taken by Nathan Howell)
Figure 169a.
Specimen: Howell HOLA-46 (NCSC)
Figure 169b.
Leaves
Figure 169c.
Flower (abaxial)
Figure 169d.
Flower (adaxial)
Rhexia nashii
Small
Rhexia nashii Taxon concept: [< R. mariana var. purpurea Michx. – RAB; = GW, Weakley]
Distribution
Bay Tree Lake (Occasional): Howell HOLA−39, 57 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−44 (NCSC!)
Jones Lake (Infrequent): Howell JOLA−38,39 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−45 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−26 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG, FB). May–Oct. Fig. 170
Rhexia nashii (digital photographs taken by Nathan Howell)
Figure 170a.
Specimen: Howell BATR-39 (NCSC)
Figure 170b.
Leaves
Figure 170c.
Calyx tube (and unopened corolla)
Figure 170d.
Flower
Rhexia virginica
L.
Rhexia virginica Taxon concept: [> R. virginica L. var. purshii – RAB; > R. virginica L. var. virginica; = GW, Weakley]
Distribution
Little Singletary Lake (Infrequent): Howell LISI – 47 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSM–T, NLSS–C). May–Oct. Fig. 171
Rhexia virginica (digital photographs taken by Nathan Howell)
Figure 171a.
Specimen: Howell LISI-47 (NCSC)
Figure 171b.
Stem and leaves
Figure 171c.
Flower (lateral)
Figure 171d.
Flower (adaxial)
Menyanthaceae
Nymphoides aquatica
(J.F. Gmel.) Kuntze
Nymphoides aquatica Basionym: Villarsia aquatica J.F. Gmel.
Nymphoides aquatica Taxon concept: [= RAB, GW, Weakley]
Distribution
Lake Waccamaw (Occasional): Harper 954 (NCU!); Howell LAWA−28, 54 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLSS−LW). Late Apr−Sep. Fig. 172
Nymphoides aquatica (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 172a.
Specimen: Howell LAWA-54 (NCSC)
Figure 172b.
Illustration
Figure 172c.
Leaf (abaxial surface)
Figure 172d.
Leaf (abaxial surface)
Figure 172e.
Flowers
Figure 172f.
Flowers
Myricaceae
Morella cerifera
(L.) Small
Morella cerifera Basionym: Myrica cerifera L.
Morella cerifera Taxon concept [< Myrica cerifera L. var. cerifera – RAB; < Myrica cerifera L. – GW, FNA; = Weakley]
Distribution
Bay Tree Lake (Infrequent): Howell BATR−11 (NCSC!)
Jones Lake (Infrequent): Howell JOLA−15 (NCSC!)
Lake Waccamaw (Occasional): Dennis 66-15 (DUKE!); Howell LAWA−36, 169 (NCSC!)
Salters Lake (Infrequent): Howell SALA−3 (NCSC!)
Notes
Shrubs or small trees. Juncture of eulittoral and supralittoral zones (NLSS−C, NLSS−LW). Apr; Aug–Oct. Fig. 173
Morella cerifera (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 173a.
Specimen: Howell BATR-11 (NCSC)
Figure 173b.
lllustration
Figure 173c.
Habit
Figure 173d.
Leaf
Figure 173e.
Staminate inflorescence
Figure 173f.
Fruits
Nelumbonaceae
Nelumbo lutea
Willd.
Nelumbo lutea Taxon concept: [= RAB, GW, FNA, Weakley]
Ecological interactions
Conservation status
W7; S2, G4.
Distribution
Lake Waccamaw: Bell 12836 (NCU!); Leonard, Burnham & Ripperton 1748 (NCU!); Radford 6078 (NCU!); Schallert 10662 (DUKE!)
Notes
Perennial herbs. Eulittoral and infralittoral zones (NLSS−LW, NLSM−LWP). Jun−Sep. Fig. 174
Figure 174.

Nelumbo lutea (illustration from Center for Aquatic and Invasive Plants, University of Florida, IFAS 2015)
Nymphaeaceae
Nuphar sagittifolia
(Walter) Pursh
Nuphar sagittifolia Basionym: Nymphaea sagittifolia Walter
Nuphar sagittifolia Taxon concept: [< N. luteum (L.) Sibth. & J.E. Smith ssp. sagittifolium (Walter) E.O. Beal – RAB, GW; = FNA, Weakley]
Ecological interactions
Conservation status
W1, FSC; S2, G5T2.
Distribution
Lake Waccamaw (Rare along south and southwest shorelines; frequent elsewhere): Buell & Godfrey 3505 (NCSC!); Fox 1878 (NCSC!); Godfrey & Buell 3505 (NCU!); Howell LAWA−83 (NCSC!); Leconte 1085 (DUKE!); Matthews s.n. (DUKE!, NCU!); Radford 681, 4348 (NCU!)
Notes
Perennial herbs. Infralittoral zone; encountered around dam, northern shorelines, and offshore (NLSS–LW, NLSM–LWP). Apr–Oct. Fig. 175
Nuphar sagittifolia (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 175a.
Specimen: Howell LAWA-83 (NCSC)
Figure 175b.

Illustration
Figure 175c.
Habit
Figure 175d.
Leaves
Figure 175e.
Flower
Figure 175f.
Flower
Nymphaea odoratavar.odorata
Nymphaea odoratavar.odorata Taxon concept: [< N. odorata – RAB, GW; = FNA, Weakley]
Distribution
Horseshoe Lake (Frequent): Beal 4349 (NCSC!); Buell & Whitford 1851 (DUKE!, NCSC!); Howell HOLA−18 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−17, 27, 76 (NCSC!)
Singletary Lake: Wilbur 60946 (DUKE!)
Notes
Perennial herbs. Eulittoral and infralittoral zones (NLSS–LW, NLSM–T, NLSM–LWP, CPSI–CG). Jun–Sep. Fig. 176
Nymphaea odorata var. odorata (digital photographs taken by Nathan Howell; illustration from Ball et al. 2002b, United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 176a.
Specimen: Howell LAWA-76 (NCSC)
Figure 176b.
Illustration
Figure 176c.
Leaves
Figure 176d.
Flower
Nyssaceae
Nyssa aquatica
L.
Nyssa aquatica Taxon concept: [= RAB, GW, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−56, 89 (NCSC!)
Notes
Trees. Eulittoral zone (NLSS−LW). Apr−May; Sep−Oct. Fig. 177
Nyssa aquatica (digital photographs taken by Nathan Howell [leaves, fruits] and Alexander Krings [bark, twig]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 177a.
Specimen: Howell LAWA-56 (NCSC)
Figure 177b.
Illustration
Figure 177c.

Bark
Figure 177d.

Twig
Figure 177e.
Leaves
Figure 177f.
Fruit
Nyssa biflora
Walter
Nyssa biflora Taxon concept: [= N. sylvatica Marshall var. biflora (Walter) Sarg. – RAB, GW; = Weakley]
Distribution
Bakers Lake (Frequent): Howell BALA−11 (NCSC!)
Bay Tree Lake (Occasional): Howell BATR−48 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−15 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−10 (NCSC!); Totten s.n. (NCU!)
Little Singletary Lake (Occasional): Howell LISI−12, 17 (NCSC!)
Salters Lake (Occasional): •
Singletary Lake (Occasional): Howell SILA−4 (NCSC!)
Notes
Trees. Eulittoral zone (NLSS–C, NLSS–LW, NLSM–T, CPSI–CG). Apr–Jun; Aug–Oct. Fig. 178
Nyssa biflora (digital photographs taken by Nathan Howell [leaves, fruits] and Alexander Krings [bark, twig]; illustration from Britton and Brown 1913)
Figure 178a.
Specimen: Howell BATR-48 (NCSC)
Figure 178b.
Illustration
Figure 178c.

Bark
Figure 178d.

Twig
Figure 178e.
Leaves
Figure 178f.
Fruits
Oleaceae
Fraxinus caroliniana
Mill.
Fraxinus caroliniana Taxon concept: [= RAB, GW, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−70, 75 (NCSC!)
Notes
Trees. Eulittoral zone (NLSS–LW). May; Jul–Oct. Fig. 179
Fraxinus caroliniana (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 179a.
Specimen: Howell LAWA-70 (NCSC)
Figure 179b.
Illustration
Figure 179c.
Stem
Figure 179d.
Imparipinnate leaf
Figure 179e.
Leaflet
Figure 179f.
Fruits
Onagraceae
Ludwigia brevipes
(Long) Eames
Ludwigia brevipes Basionym: Ludwigiantha brevipes Long
Ludwigia brevipes Taxon concept: [= RAB, GW, Weakley]
Ecological interactions
Conservation status
SR–T, FSC; S1S2, G2G3.
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−102, 118 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLS–LW). Jul–Oct. Fig. 180
Ludwigia brevipes (digital photographs by Nathan Howell; illustration from Britton and Brown 1913)
Figure 180a.
Specimen: Howell LAWA-118 (NCSC)
Figure 180b.

Illustration
Figure 180c.
Habit
Figure 180d.
Flower
Ludwigia sphaerocarpa
Elliott
Ludwigia sphaerocarpa Taxon concept: [= RAB, GW, Weakley]
Ecological interactions
Conservation status
E; S1, G5.
Distribution
Lake Waccamaw (Occasional): Howell LAWA−33, 143 (NCSC!)
Notes
Perennial herbs. Eulittoral zone (NLS–LW). Jun–Sep. Fig. 181
Ludwigia sphaerocarpa (digital photographs taken by Nathan Howell)
Figure 181a.
Specimen: Howell LAWA-143 (NCSC)
Figure 181b.
Submerged leaves
Figure 181c.
Flowers
Figure 181d.
Fruits
Plantaginaceae
Bacopa caroliniana
(Walter) B.L. Rob.
Bacopa caroliniana Basionym: Obolaria caroliniana Walter
Bacopa caroliniana Taxon concept: [= RAB, GW, Weakley]
Ecological interactions
Conservation status
T; S1, G4G5.
Distribution
Lake Waccamaw (Rare): Howell LAWA−66, 120 (NCSC!); LeBlond 3984 (NCU!)
Notes
Perennial herbs. Eulittoral zone; calm, quiet waters (NLSS–LW). May–Sep. Fig. 182
Bacopa caroliniana (digital photographs by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 182a.
Specimen: Howell LAWA-120 (NCSC)
Figure 182b.
Illustration
Figure 182c.
Habit
Figure 182d.
Flower
Nuttallanthus canadensis
(L.) D.A. Sutton
Nuttallanthus canadensis Basionym: Antirrhhinum canadense L.
Nuttallanthus canadensis Taxon concept: [< Linaria canadensis (L.) Dum. Cours.; = Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR–28 (NCSC!)
Notes
Annual or biennial herbs. Juncture of eulittoral and supralittoral zones. Mar−Jul. Fig. 183
Nuttallanthus canadensis (digital photographs taken by Nathan Howell [inflorescence] and Alexander Krings [flower]; illustration from Britton and Brown 1913)
Figure 183a.
Specimen: Howell BATR-28 (NCSC)
Figure 183b.
Illustration
Figure 183c.
Inflorescence
Figure 183d.
Flower
Platanaceae
Platanus occidentalis
L.
Platanus occidentalis Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−68 (NCSC!)
Notes
Trees. Eulittoral zone; on saturated soils of sandbars and shorelines (NLSS−LW). Apr−May; Sep−Nov. Fig. 184
Platanus occidentalis (digital photographs taken by Nathan Howell [twigs and leaves] and Alexander Krings [bark])
Figure 184a.
Specimen: Howell LAWA-68 (NCSC)
Figure 184b.

Bark
Figure 184c.
Twigs and leaves
Figure 184d.
Leaves
Polygalaceae
Polygala lutea
L.
Polygala lutea Taxon concept: [= RAB, GW, Weakley]
Distribution
Horseshoe Lake (Rare): Howell HOLA−50 (NCSC!)
Lake Waccamaw (Rare): Howell LAWA−127 (NCSC!)
Notes
Biennial herbs. Eulittoral zone; moist sandy soils at or below the maximum annual high water mark (NLSS−LW, CPSI−CG). Apr−Oct. Fig. 185
Polygala lutea (digital photographs taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 185a.
Specimen: Howell LAWA-128 (NCSC)
Figure 185b.
Illustration
Figure 185c.
Basal leaf
Figure 185d.
Inflorescence
Polygonaceae
Rumex hastatulus
Baldwin
Rumex hastatulus Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake (Infrequent): Howell BATR−14, 23, 30 (NCSC!)
Notes
Annual or short-lived perennial herbs. Eulittoral zone; moist sandy to peaty shores. Mar−May; May−Jul. Fig. 186
Rumex hastatulatus (digital photograph taken by Nathan Howell)
Figure 186a.
Specimen: Howell BATR-14 (NCSC)
Figure 186b.
Infructescence
Ranunculaceae
Clematis crispa
L.
Clematis crispa Taxon concept: [= RAB, GW, FNA. Weakley]
Distribution
Lake Waccamaw: Matthews s.n. (NCU!)
Notes
Perennial, sometimes lianescent, vines. Eulittoral zone (NLSS–LW). Apr–Aug. Fig. 187
Clematis crispa (digital photograph taken by Alexander Krings; illustration from Britton and Brown 1913)
Figure 187a.
Illustration
Figure 187b.

Flower
Rhamnaceae
Berchemia scandens
(Hill) K. Koch
Berchemia scandens Basionym: Rhamnus scandens Hill
Berchemia scandens Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Occasional): Howell LAWA−38 (NCSC!)
Notes
Lianas. Eulittoral zone (NLSS–LW). Apr–May; Aug–Oct. Fig. 188
Berchemia scandens (digital photographs taken by; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 188a.
Specimen: Howell LAWA-38 (NCSC)
Figure 188b.
Illustration
Figure 188c.
Habit
Figure 188d.
Stem and leaves
Rosaceae
Amelanchier canadensis
(L.) Medik.
Amelanchier canadensis Basionym: Mespilus canadensis L.
Amelanchier canadensis Taxon concept: [=RAB, GW, FNA, Weakley]
Distribution
Bay Tree Lake: Radford 1354 (NCU!)
Notes
Shrubs or small trees. Juncture of eulittoral and supralittoral zones. Mar−Apr; May−Jun. Fig. 189
Figure 189.
Amelanchier canadensis (illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Amelanchier obovalis
(Michx.) Ashe
Amelanchier obovalis Basionym: Mespilus canadensis L. var. obovalis Michx.
Amelanchier obovalis Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−48 (NCSC!)
Notes
Shrubs. Eulittoral zone (NLSS−LW). Mar−Apr; May−Jun. The only specimen encountered by the current author was found in a shallow concave depression in the middle of two boles of Taxodium ascendens arising from the same stump. The shrub established itself in the small amount of soil that had accumulated in the depression through the years. Fig. 190
Amelanchier obovalis (digital photograph taken by Nathan Howell)
Figure 190a.
Specimen: Howell LAWA-48 (NCSC)
Figure 190b.
Fruits (immature)
Aronia arbutifolia
(L.) Pers.
Aronia arbutifolia Basionym: Mespilus arbutifolia L.
Aronia arbutifolia Taxon concept: [= Sorbus arbutifolia (L.) Hyenh. var. arbutifolia; = RAB; = GW, FNA, Weakley]
Distribution
Salters Lake: Buell s.n. (NCSC!)
Singletary Lake (Rare): Howell SILA−24 (NCSC!)
Notes
Shrubs. Juncture of eulittoral and supralittoral zones (NLSS−C). Mar−May; Sep−Nov. Fig. 191
Aronia arbutifolia (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 191a.
Specimen: Howell SILA-24 (NCSC)
Figure 191b.
Illustration
Figure 191c.
Inflorescence
Figure 191d.
Infructescence
Rosa palustris
Marshall
Rosa palustris Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): LAWA−74, 112 (NCSC!)
Singletary Lake: Fox, Wells, Sharp, Whitford, Fairchild s. n. (NCSC!)
Notes
Shrubs. Eulittoral zone; sandy to peaty soils at or just below the maximum annual high water mark (NLSS−C, NLSS−LW). May−Jul; Sep−Oct. Rosa palustris can be distinguished from R. multiflora, a common exotic in the North Carolina Coastal Plain, by its large (adnate portion 13–30 mm long), entire, stipules. Those of R. multiflora are up to 21 mm long (adnate portion 3–15 mm long) and pectinate- fringed. Fig. 192
Rosa palustris (digital photographs taken by Nathan Howell)
Figure 192a.
Specimen: Howell LAWA-112 (NCSC)
Figure 192b.
Leaf
Figure 192c.
Flower
Figure 192d.
Fruit
Rubus pensilvanicus
Poir.
Rubus pensilvanicus Taxon concept: [> R. argutus Link – RAB, GW; > R. betulifolius Small – RAB; = Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−73, 97 (NCSC!)
Notes
Shrubs. Eulittoral zone; sandy to peaty soils at or just below the maximum annual high water mark (NLSS−LW). Apr−May; Late May−Jul. Fig. 193
Rubus pensilvanicus (digital photograph taken by Nathan Howell)
Figure 193a.
Specimen: Howell LAWA-73 (NCSC)
Figure 193b.
Leaf
Rubiaceae
Cephalanthus occidentalis
L.
Cephalanthus occidentalis Taxon concept: [= RAB; < C. occidentalis L. var. occidentalis – GW; = Weakley]
Distribution
Lake Waccamaw (Occasional): Howell LAWA−104, 119, 165 (NCSC!)
Notes
Shrubs. Eulittoral zone (NLSS–LW). Jun–Jul. Fig. 194
Cephalanthus occidentalis (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 194a.
Specimen: Howell LAWA-104 (NCSC)
Figure 194b.
Illustration
Figure 194c.
Leaves
Figure 194d.
Inflorescence
Diodia virginiana
L.
Diodia virginiana Taxon concept: [= RAB, GW, Weakley]
Distribution
Bay Tree Lake (Rare): Howell BATR−22 (NCSC!)
Notes
Annual or perennial herbs. Eulittoral zone; sandy soils at or just below the maximum annual high water mark. Jun–Dec. Fig. 195
Diodia virginiana (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 195a.
Specimen: Howell BATR-22 (NCSC)
Figure 195b.
Illustration
Figure 195c.
Flower (adaxial)
Figure 195d.
Flower (lateral)
Galium obtusumvar.obtusum
Galium obtusumvar.obtusum Taxon concept: [= RAB; < G. obtusum – GW; = Weakley]
Distribution
Lake Waccamaw (Rare): Howell LAWA−138 (NCSC!)
Notes
Perennial herbs. Eulittoral zone; at or just below maximum annual high water mark (NLSS–LW). Apr–May. Fig. 196
Galium obtusum var. obtusum (digital photographs taken by Nathan Howell)
Figure 196a.
Specimen: Howell LAWA-138 (NCSC)
Figure 196b.
Leaves
Figure 196c.
Flowers
Figure 196d.
Fruits
Salicaceae
Populus heterophylla
L.
Populus heterophylla Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−91 (NCSC!)
Notes
Trees. Eulittoral zone; saturated soils at or just below the maximum annual high water mark (NLSS−LW). Mar−Apr. Fig. 197
Populus heterophylla (digital photographs taken by Nathan Howell [young tree, leaves, inflorescences] and Alexander Krings [abaxial leaf surface]; illustration from Britton and Brown 1913)
Figure 197a.
Specimen: Howell LAWA-91 (NCSC)
Figure 197b.
Illustration
Figure 197c.
Young tree
Figure 197d.
Leaves
Figure 197e.
Abaxial leaf surface
Figure 197f.
Inflorescences
Salix caroliniana
Michx.
Salix caroliniana Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw: Harper 970 (NCU!); Matthews s.n. (DUKE!, NCU!)
Notes
Trees. Eulittoral zone; sandbars and sandy shorelines (NLSS−LW). Mar−Apr. This taxon was not encountered by the first author, but voucher specimens confirm its historical presence. Fig. 198
Salix caroliniana (digital photograph taken by Alexander Krings; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 198a.
Illustration
Figure 198b.
Leaves (abaxial surface [top], adaxial surface [bottom])
Salix nigra
Marshall
Salix nigra Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−72 (NCSC!)
Notes
Trees. Eulittoral zone; sandbars and sandy shorelines (NLSS−LW). Mar−Apr. Fig. 199
Salix nigra (digital photograph taken by Alexander Krings)
Figure 199a.
Specimen: Howell LAWA-72 (NCSC)
Figure 199b.
Leaves (abaxial surface [top], adaxial surface [bottom])
Santalaceae
Phoradendron leucarpumvar.leucarpum
Phoradendron leucarpumvar.leucarpum Basionym: Viscum leucarpum Raf.
Phoradendron leucarpumvar.leucarpum Taxon concept: [< P. serotinum (Raf.) M.C. Johnst. – RAB; = Weakley]
Distribution
Jones Lake (Infrequent): Howell JOLA−12 (NCSC!)
Salters Lake (Infrequent): Howell SALA−7, 19 (NCSC!)
Notes
Epiphytic shrubs. Eulittoral zone; typically on limbs of Acer or Nyssa (NLSS–C). Oct–Nov; Nov–Jan. Fig. 200
Phoradendron leucocarpum (digital photographs taken by Nathan Howell; illustraton from Britton and Brown 1913)
Figure 200a.
Specimen: Howell SALA-7 (NCSC)
Figure 200b.
Illustration
Figure 200c.
Habit
Figure 200d.
Fruits
Sapindaceae
Acer rubrumvar.rubrum
Acer rubrumvar.rubrum Taxon concept: [< A. rubrum L. – RAB, GW; = Weakley]
Distribution
Bakers Lake (Rare): Howell BALA−4 (NCSC!)
Notes
Trees. Eulittoral zone; typically in saturated organic to sandy soils at or just below the maximum annual high water mark (NLSS−C). Jan−Mar; Apr−July. Fig. 201
Acer rubrum var. rubrum (digital photograph taken by Nathan Howell; illustration from Britton and Brown 1913)
Figure 201a.
Illustration
Figure 201b.
Leaves
Acer rubrumvar.trilobum
Torr. & A. Gray ex K. Koch
Acer rubrumvar.trilobum Taxon concept: [< A. rubrum L. – RAB, GW; = Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−1 (NCSC!)
Horseshoe Lake (Occasional): Howell HOLA−5, 20 (NCSC!)
Jones Lake (Occasional): Howell JOLA−9, 21 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−22, 43, 44 (NCSC!)
Little Singletary Lake (Occasional): Howell LISI−11, 21 (NCSC!)
Salters Lake (Occasional): Beckman & Linnenburger 27 (DUKE!); Howell SALA−2, 18 (NCSC!)
Singletary Lake (Occasional): Howell SILA−8 (NCSC!)
Notes
Trees. Eulittoral zone; typically in saturated organic to sandy soils at or just below the maximum annual high water mark (NLSS−C, NLSS−LW, NLSM−T, CPSI−CG). Jan−Mar; Apr−Jun. Fig. 202
Acer rubrum var. trilobum (digital photograph taken by Nathan Howell)
Figure 202a.
Specimen: Howell BATR-1 (NCSC)
Figure 202b.
Leaves
Aesculus paviavar.pavia
Aesculus paviavar.pavia Taxon concept: [< A. pavia L. − RAB; = Weakley]
Distribution
Lake Waccamaw: Harbison 6084 (NCU!); Harper s. n., 955, 965 (NCU!); Matthews s.n. (NCU!); Oosting 3498 (DUKE!); Reed & Stites 275 (NCU!)
Notes
Shrubs or trees. Eulittoral zone (NLSS−LW). Apr−early May; Jul−Aug. Fig. 203
Figure 203.
Aesculus pavia (illustration from Britton and Brown 1913)
Sarraceniaceae
Sarracenia flava
L.
Sarracenia flava Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Horseshoe Lake (Abundant): Buell & Whitford s.n. (NCSC!); Howell HOLA−16, 41 (NCSC!)
Notes
Perennial herbs. floating bogs (CPSI-CG, FB). Mar–Apr; May–Jun. Fig. 204
Sarracenia flava (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 204a.
Specimen: Howell HOLA-41 (NCSC)
Figure 204b.
Illustration
Figure 204c.
Habit
Figure 204d.
Flower (petals removed)
Theaceae
Gordonia lasianthus
(L.) J. Ellis
Gordonia lasianthus Basionym: Hypericum lasianthus L.
Gordonia lasianthus Taxon concept: [= RAB, GW, FNA, Weakley]
Distribution
Bakers Lake (Infrequent): Howell BALA−16 (NCSC!)
Horseshoe Lake: Buell 2262 (NCSC!)
Jones Lake (Occasional): Howell JOLA−2 (NCSC!)
Little Singletary Lake (Infrequent): Howell LISI−30, 48 (NCSC!)
Singletary Lake (Infrequent): Howell SILA−30 (NCSC!)
Notes
Trees. Juncture of eulittoral and supralittoral zones (NLSS−C). Jul−Sep; Sep−Oct. Fig. 205
Gordonia lasianthus (digital photographs taken by Nathan Howell; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 205a.
Specimen: Howell BALA-16 (NCSC)
Figure 205b.
Illustration
Figure 205c.
Twig and leaves
Figure 205d.
Leaves
Figure 205e.
Flower
Figure 205f.
Fruit
Ulmaceae
Ulmus americanavar.americana
Ulmus americanavar.americana Taxon concept: [< U. americana L. – RAB, GW, FNA; = Weakley]
Distribution
Lake Waccamaw (Rare): Bell 12839 (NCU!); Godfrey 6318 (NCSC!); Howell LAWA−95 (NCSC!)
Notes
Trees. Juncture of the eulittoral and supralittoral zones (NLSS−LW). Feb−Mar; Mar−Apr. Fig. 206
Ulmus americana (digital photograph taken by Nathan Howell)
Figure 206a.
Specimen: Howell LAWA-95 (NCSC)
Figure 206b.
Leaves
Vitaceae
Muscadinia rotundifoliavar.rotundifolia
Muscadinia rotundifoliavar.rotundifolia Basionym: Vitis rotundifolia Michx.
Muscadinia rotundifoliavar.rotundifolia Taxon concept: [< Vitis rotundifolia Michx. – RAB, GW; = Weakley]
Distribution
Bay Tree Lake (Occasional): Howell BATR−46 (NCSC!)
Lake Waccamaw (Occasional): Howell LAWA−64, 137 (NCSC!)
Salters Lake (Infrequent): Howell SALA−21 (NCSC!)
Notes
Lianas. Upper eulittoral zone; typically at the high water mark forming dense tangles along the waters edge (NLSS–C, NLSS–LW, NLSM–T). Late Apr–May; late Jul–Sep. Fig. 207
Muscadinia rotundifolia (digital photographs taken by Nathan Howell [all, except flowers] and Alexander Krings [flowers]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 207a.
Specimen: Howell BATR-46 (NCSC)
Figure 207b.
Illustration
Figure 207c.
Habit
Figure 207d.
Leaf
Figure 207e.
Flowers
Figure 207f.
Fruits
Parthenocissus quinquefolia
(L.) Planch.
Parthenocissus quinquefolia Basionym: Hedera quinquefolia L.
Parthenocissus quinquefolia Taxon concept: [= RAB, Weakley]
Distribution
Lake Waccamaw (Infrequent): Howell LAWA−94 (NCSC!)
Notes
Lianas. Eulittoral zone; growing on fallen trees, shrubs, and erect trees at or just below the maximum annual high water mark (NLSS−LW). May−Jul; Jul−Aug. Fig. 208
Parthenocissus quinquefolia (digital photographs taken by Nathan Howell [fruits] and Alexander Krings [flowers]; illustration from United States Department of Agriculture, Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service 2015)
Figure 208a.
Specimen: Howell LAWA-94 (NCSC)
Figure 208b.
Illustration
Figure 208c.

Flowers
Figure 208d.
Fruits
Identification Keys
Keys to the major vascular plant groups
| 1 | Plant reproducing by spores | Pteridophytes |
| – | Plant reproducing by seeds | 2 |
| 2 | Seeds borne in woody cones; leaves needle-like or scale-like, < 3 mm wide | Gymnosperms |
| – | Seeds borne in fruits; leaves various | 3 |
| 3 | Plant exhibiting ≥ 2 of the following characters: Cotyledon 1; stem vascular bundles scattered; leaves parallel veined; floral parts in 3s | Monocotyledons |
| – | Plant exhibiting ≥ 2 of the following characters: Cotyledons 2; stem vascular bundles in a ring; leaves without parallel venation; floral parts in 4s and 5s | Basal Angiosperms, Magnoliids, and Eudicotyledons |
PTERIDOPHYTES
| 1 | Leaves simple, scale-like, < 2 cm long, each leaf with 1, unbranched vein; sporangia borne in strobili at the tips of shoots | Lycopodiaceae [Lycopodiella appressa] Fig. 21 |
| – | Leaves pinnatifid to 2-pinnate, “ferny”, > 2 cm long, each leaf bearing numerous pinately-branched veins; sporangia borne in sori on the undersides of modified or unmodified pinnae | 2 |
| 2 | Plant epiphytic, growing on large limbs and tree trunks along shorelines; leaves (not including the petiole) 3−25 × 2.5−5 cm, evergreen, undersides with peltate, gray scales | Polypodiaceae [Pleopeltis polypodioides ssp. michauxiana]Fig. 24 |
| – | Plant not epiphytic, growing in inundated, saturated, or moist soils of shorelines; leaves (not including the petiole) > 25 cm × 5 cm, deciduous or evergreen, undersides lacking peltate, gray scales | 3 |
| 3 | Stipules present, wing-like; leaves 2-pinnate or more divided, pinnae divided to their midribs; sori and indusia lacking | Osmundaceae [Osmunda spectabilis] Fig. 23 |
| – | Stipules absent; leaves 1-pinnate-pinatifid or less divided; sori and indusia present | 4 |
| 4 | Leaves 1-pinnatifid, the rachis winged by leaf tissue throughout most or allof its length | 5 |
| – | Leaves 1-pinnate-pinnatifid, the pinnae fully divided from one another (the rachis not winged by leaf tissue throughout most or all of its length) | 6 |
| 5 | Fertile leaf woody, with bead-like segments; margins of sterile leaves entire, wavy, the lowermost pinnae sometimes becoming slightly lobed; pinnae with obtuse apices | Onocleaceae [Onoclea sensibilis] Fig. 22 |
| – | Fertile leaf herbaceous, not woody or with bead-like segments; margins of sterile pinnae finely serrulate; pinnae mostly with acute apices | Blechnaceae [Lorinseria areolata] Fig. 19 |
| 6 | Rhizomes long-creeping; leaves deciduous, monomorphic, 28–60 cm long, scattered along the rhizome, forming clonal patches; petiole dark purple to black proximally; sori elongate, borne end to end along both sides of main veins, pinnae lobes of sterile leaves with reticulate, chain-like venation on either side of the central vein | Blechnaceae [Anchistea virginica] Fig. 18 |
| – | Rhizomes short-creeping; leaves evergreen, somewhat dimorphic (fertile pinnae in distal half of leaves), 35–120 cm long, clustered on the rhizome, not forming clonal patches; petiole not purple to black proximally; sori circular, not borne end to end along the main veins, located midway between main vein and pinnae lobe margins; pinnae lobes of sterile portions of leaves lacking a chain-like venation pattern on either side of the central vein | Dryopteridaceae [Dryopteris ludoviciana] Fig. 20 |
Key adapted from Radford et al. (1968), Smith (1993a), and Weakley (2012).
Note: Successful keying of ferns is greatly facilitated by a basic understanding of fern morphology. Thornhill et al. (2014) provided a useful summary of important morphological terms: “Pinnate indicates lobing of leaves, leaflets, or pinnules entirely to the rachis or midrib. Pinnatifid indicates lobing of leaves, leaflets, or pinnules to near the midrib (i.e., not all the way to the rachis or midrib, as in the leaflets of Anchistea virginica). Pinnate- pinnatifid refers to a leaf blade that is once-pinnate and whose segments (pinnae) are themselves pinnatifid. Sori are the spore-producing structures found on many species of ferns; these may be either exposed or covered by the margin of the leaves (a false indusium) or a separate structure altogether (a true indusium). Leaf-like structures that bear sporangia are called sporophylls; these may be similar to the sterile leaves or be highly modified (e.g., the compact, cone-like structures, or strobili of the Lycopodiaceae)”.
GYMNOSPERMS
| 1 | Leaves scale-like or needle-like, < 1.5 cm long, not in fascicles; seed cone scales valvate or imbricate, if imbricate then leaves opposite and scale-like; seeds 1−3 per scale | Cupressaceae |
| – | Leaves needle-like, (10−) 12−45 cm long, in fascicles of 2−3 leaves; seed cone scales imbricate; seeds 2 per scale | Pinaceae [Pinus] |
Key adapted from Eckenwalder and Thieret (1993).
Cupressaceae
| 1 | Leaves scale-like, 1−3 mm long, opposite or whorled, evergreen; mature seed cones woody, 4−9 mm broad, scales imbricate; seeds 1–2 (−3) per scale | Chamaecyparis thyoides Fig. 25 |
| – | Leaves linear, 3−17 mm long, alternate, deciduous; mature seed cones woody, 1.3–3.6 cm broad, scales valvate; seeds (1−) 2 per scale | Taxodium |
Key adapted from Watson and Eckenwalder (1993) and Weakley (2012).
Taxodium Rich.
| 1 | Leaves mostly vertically ascending, appressed and overlapping, spirally arranged; branchlets ascending from twigs, secundly erect; bark 1–2.5 cm thick, furrowed, dark- brown, not exfoliating; larger knees short, rarely > 4 dm tall, with thick, compact bark on top; trees of isolated depressions, natural lakes, wet savannas, pocosins, other wet peaty habitats, and, less commonly, blackwater swamps | Taxodium ascendens Fig. 26 |
| – | Leaves pendent to horizontally spreading to laterally divergent, spirally arranged but generally appearing distichous (“featherlike”); branchlets not ascending from twigs; bark < 1 cm thick, exfoliating in shreddy, orange-brown strips; larger knees often tall, frequently > 4 dm tall, with thin, shreddy bark on top; trees of blackwater swamps, brownwater swamps, natural lakes, and millponds; usually in riverine situations | Taxodium distichum Fig. 27 |
Key adapted from Watson (1993), Weakley (2012), and Thornhill et al. (2014).
Note: “In the following key, leaf and branchlet characters of T. ascendens refer to mature trees; foliage of juvenile trees often mimics that of T. distichum. Leaf and branchlet characters of T. distichum refer to both mature and juvenile trees; however, in the crowns of mature T. distichum, leaf and branchlet characters sometimes mimic those of T. ascendens. For these reasons, accurate identification of the two species often requires observation of other, non-foliage features, including the stature of the “knees”, the thickness and texture of the bark, and the habitat in which the trees grow” (Thornhill et al. 2014).
Pinaceae
| 1 | Open seed cones about as broad as long, “top-shaped”, 3–6 cm long, serotinous; trunks typically producing epicormic branches, especially in response to fire | Pinus serotina Fig. 28 |
| – | Open seed cones distinctly longer than broad, not top-shaped, 6–18(−20) cm long, not serotinous; trunk not producing epicormic branches | Pinus taeda Fig. 29 |
Key adapted from Radford et al. (1968), Kral (1993), and Weakley (2012).
MONOCOTYLEDONS
| 1 | Plant an epiphyte, growing on the trunks and limbs of trees in the littoral zone | 2 |
| – | Plant not epiphytic, rooted in soil or freely floating | 3 |
| 2 | Plant green, erect, not scurfy; leaves lanceolate; roots present, fibrous; flowers in racemes, petals dimorphic (two similar in size, the third differentiated into a broad lip) | Orchidaceae [Epidendrum magnoliae] |
| – | Plants gray, pendent (often in masses), scurfy; leaves filiform; roots absent; flowers solitary, petals monomorphic | Bromeliaceae [Tillandsia usneoides Fig. 34] |
| 3 | Plant diminutive ≤ 1.5 mm long in any dimension, floating or submersed in water, sometimes left stranded on mud or debris by receding water levels, plants thallus-like, not differentiated into stems and leaves, rootless or with few simple roots | Araceae [Wolffia] |
| – | Plant not diminutive or thallus-like, > 2 mm in any dimension, differentiated into stems and leaves, rooted in soil or floating on water surface | 4 |
| 4 | Stems woody | 5 |
| – | Stems herbaceous | 6 |
| 5 | Leafy stem erect, smooth, lacking prickles; internodes hollow | Poaceae [Arundinaria tecta Fig. 80] |
| – | Leafy stems climbing by stipular tendrils, armed with prickles; internodes solid | Smilacaceae [Smilax] |
| 6 | Flowers borne in a single compact head terminating an elongate scape | 7 |
| – | Flowers not borne in single compact heads atop elongated scapes | 8 |
| 7 | Flowering head involucrate, white to gray, hemispheric, “button-like”, < 1 cm tall; flowers 2−3-merous, unisexual, 1.5−4 mm long, pale to grayish, not subtended by a scale- like bract, sepals and petals partially coated with club-shaped hairs; anthers black, 2-locular | Eriocaulaceae |
| – | Flowering head not involucrate, brown, globose to cylindrical, “cone-like”, 0.5−3.5 cm tall; flowers 3-merous, bi-sexual, individual petals 3−6 mm long, yellow, subtended by a conspicuous scale-like bract, sepals and petals not coated with white club-shaped hairs; anthers yellow, 2−4-locular | Xyridaceae |
| 8 | Flowers and fruits subtended by imbricate or distichous bracts or scales and for the most part hidden by them, usually only the stamens and styles protruding at anthesis; fruit 1-seeded | 9 |
| – | Flowers and fruits not subtended by imbricate or distichous scales, or if so, then the flowers exceeding or equalling the bracts or scales and not hidden; fruit > 1- seeded | 10 |
| 9 | Leaves usually 3-ranked, sheaths typically closed; culms typically triangular in cross- section and solid; fruit an achene | Cyperaceae |
| – | Leaves usually 2-ranked, sheaths open (split lengthwise on the side opposite the blade); culms terete in cross-section, usually hollow; fruit a caryopsis | Poaceae |
| 10 | Plants aquatic, wholly submersed (except for Mayaca fluviatilis, which may be found wholly submersed or growing erect in saturated soils along shorelines); inflorescences submersed, floating, or just above the water surface | 11 |
| – | Plants terrestrial, or if growing in shallow water then the inflorescences well above the water surface (except during infrequent flooding events) | 13 |
| 11 | Leaves opposite or whorled (if opposite but appearing whorled, then leaf bases dilated and sheathlike); flowers either lacking perianth parts as in Najas or inconspicuous as in Hydrilla | Hydrocharitaceae |
| – | Leaves alternate; perianth parts present or not, if so, then conspicuous | 12 |
| 12 | Plant moss-like, habit ranging from wholly submersed to completely emersed; not heterophyllous; leaves 20−200 (−300) × 0.5−1 mm, very numerous and tightly spaced, spirally arranged, apices sometimes slightly bifid; flowers solitary in the leaf axils, petals rose to maroon to lilac, sometimes white basally, obovate | Mayacaceae [Mayaca fluviatilis Fig. 73] |
| – | Plant not moss-like, habit restricted to wholly submersed; heterophyllous or not, if heterophyllous, then the submersed leaves transluscent and with a soft, fragile, texture, the floating leaves coriaceous; leaves 10−160 × 0.5−85 mm, diffusely spaced, somewhat spirally arranged in P. pusillus, no so in P. pulcher, apices entire; flowers in axillary spikes, perianth lacking | Potamogetonaceae |
| 13 | Inflorescence a spadix surrounded by a yellow spathe; leaves 17−70 × 10−40 cm, peltate, bases cordate to sagittate to hastate, adaxial surface glaucous blue-green, typically with a red or purple spot where the petiole attaches to the blade | Araceae [Colocasia esculenta] |
| – | Plant not with the above combination of characters | 14 |
| 14 | Perianth segments densely pubescent abaxially | 15 |
| – | Perianth segments not densely pubescent abaxially | 16 |
| 15 | Leaves linear, equitant; corolla yellow; ovary inferior | Haemodoraceae [Lachnanthes caroliniana Fig. 61] |
| – | Leaves cordate to lanceolate, not equitant; corolla blue to purple; ovary superior | Pontederiaceae [Pontederia cordata] |
| 16 | Corolla stellate, petals white, female flowers exhibiting an apocarpous gynoecium, each pistil ripening into an achene; phyllodia present | Alismataceae [Sagittaria] |
| – | Corolla not stellate (or, if so, then petals not white), female flowers not exhibiting an apocarpous gynoecium, 1 pistil restricted to each flower, ripening into a capsule; phyllodia absent | 17 |
| 17 | Plant annual, diminutive, 5−20 cm tall, stems filiform; leaves minutely scale-like | Burmanniaceae [Burmannia capitata Fig. 35] |
| – | Plant perennial, not diminutive, > 20 cm tall, stems not filiform; leaves not scale-like (though blades not well-developed in Juncus effusus) | 18 |
| 18 | Ovary superior; perianth parts bract-like, dry, scarious, persistent, not petal-like; leaves septate or not, terete, or flat and blade-like | Juncaceae |
| – | Ovary inferior, perianth parts petal-like, neither bract-like, hard, nor scarious, not persistent; leaves flat and blade-like, never septate | 19 |
| 19 | Flowers radially symmetric; androecium and gynoecium in separate whorls, not borne in a column; pollen free | Hypoxidaceae [Hypoxis curtisii Fig. 64] |
| – | Flowers strongly bilaterally symmetric; androecium and gynoecium borne in a column; pollen in pollinia (pollen sacs) | Orchidaceae |
Key adapted from Radford et al. (1968), Godfrey and Wooten (1979), and Weakley (2012).
Alismataceae
| 1 | Leaf blades floating, cordate basally | Sagittaria filiformis Fig. 30 |
| – | Leaf blades not floating, without basal lobes, linear to lanceolate, or modified asbladeless phyllodia, these with a spongy texture | 2 |
| 2 | Stalks of the pistillate flowering heads stout and reflexed in fruit; stamen filaments glabrous | Sagittaria filiformis |
| – | Stalks of the pistillate flowering heads not overly stout and either spreading or ascending in fruit; stamen filaments roughened with minute scales | 3 |
| 3 | Mature leaves all phyllodial, phyllodia terete or very nearly so | Sagittaria isoetiformis Fig. 32 |
| – | Mature leaves with blades and petioles, or phyllodia flattened on the adaxial surface or triangular in cross-section | 4 |
| 4 | Plant with corms or stolons, coarse rhizomes lacking; blades of emersed leaves < 3 (−4) mm wide; flowers ≤ 1.3 cm in diam. | Sagittaria isoetiformis |
| – | Coarse rhizomes present, stolons and corms absent; blades of emersed leaves > 1 cm wide; flowers ≤ 2.3 cm in diameter | 5 |
| 5 | Larger phyllodes ≤ 1 cm wide, apices acute; pistillate pedicels 1−4 cm long; median resin duct of mature achene club-shaped, 2× the width of the posterior duct | Sagittaria graminea Fig. 31 |
| – | Larger phyllodes 0.8−2.5 cm wide, apices blunt; pistillate pedicels 2−5 (−6.5) cm long; median resin duct of mature achene linear, about as wide as the posterior duct (or ducts absent) | Sagittaria weatherbiana |
Key adapted from Durand (2000), Godfrey and Wooten (1979), and Weakley (2012).
Araceae
| 1 | Plant terrestrial, stems present, rooted in moist to saturated soils; leaf blades to 70 cm long | Colocasia esculenta Fig. 33 |
| – | Plant floating, diminutive, thallus-like, stems absent, dropping water levels sometimes leaving some plants stranded; leaf blades < 0.2 cm long | Wolffia spp. |
Key adapted from Thompson (2000) and Weakley (2012).
Wolffia Horkel ex Schleid.
| 1 | Fronds nutshell-like, upper surface flattened, 0.5−1 × as deep as wide, a small portion not flattened and with minute central papillae, fronds brownish punctate above (best seen in dead fronds), cells of fronds inflated in the lower portions and becoming progressively smaller and more compact toward the upper surface | Wolffia brasiliensis Fig. 209 |
| – | Fronds globoid to ovoid, upper surface convex, 1−1.5 × as deep as wide, a small portion slightly flattened and roughened with minute central papillae, fronds not brownish punctate above, cells of frond uniformly inflated throughout | Wolffia columbiana |
Key adapted from Weakley (2012).
Note: The first author did not encountered taxa within this genus in the field; however, the Carolina Vegetation Survey reported “Wolffia spp.” from the southwest side of Lake Waccamaw. Although a species-level identification has not been made, a key to the two species most likely to inhabit this location is provided below.
Figure 209.

Wolffia brasiliensis (from Britton and Brown 1913)
Cyperaceae
| 1 | Achenes enclosed in a perigynium; flowers unisexual | Carex |
| – | Achenes not enclosed within a perigynium; flowers unisexual or bisexual | 2 |
| 2 | Leaves absent; spikelets 1 per culm, terminal | Eleocharis |
| – | Leaves present; spikelets ≥ 1 per culm, terminal or axillary | 3 |
| 3 | Spikelet scales distichous (two−ranked) | 4 |
| – | Spikelet scales spirally arranged, imbricate | 5 |
| 4 | Leaves not 3−ranked, predominantly basal; inflorescence terminal; perianth bristles lacking | Cyperus |
| – | Leaves prominently 3−ranked, cauline; inflorescence axillary; perianth bristles 6−9 | Dulichium arundinaceum Fig. 44 |
| 5 | Base of style hardened, differentiated from achene body, persistent as a tubercle at apex of achene | Rhynchospora |
| – | Base of style not hardened; tubercle absent from apex of achene | 6 |
| 6 | Perianth bristles present | 7 |
| – | Perianth bristles absent | 8 |
| 7 | Perianth scales 3, stalked, paddle−shaped; perianth bristles 3 | Fuirena pumila Fig. 50 |
| – | Perianth scales lacking; perianth bristles typically 4−8 | Scirpus cyperinus Fig. 59 |
| 8 | Style entire along margins; culms obtusely angled, 50−80 cm tall; leaf blade margins scaberulous; perennial | Cladium mariscoides Fig. 40 |
| – | Style fringed along margins; culms flattened, to 40 cm tall; leaf blade margins glabrous; annual | Fimbristylis autumnalis Fig. 49 |
Key adapted from Radford et al. (1968), Ball et al. (2002a), and Weakley (2012).
Carex L.
| 1 | Achene lenticular (biconvex); stigmas 2; perigynia wing-margined | 2 |
| – | Achene trigonous (three-sided); stigmas 3; perigynia not wing-margined | 3 |
| 2 | Pistillate scales in middle to lower portions of spike 2.8−3.5 (3.8) mm long, apices short-aristate; leaf blades 3−7 per fertile culm, 11−50 × 0.25−0.6 cm; spikes 6−20 × 4−9 mm; perigynia faintly 3−8 nerved on each face, obovate, 4−5.5 × 2.5−3.8 mm; achenes oblong, 1.7−2 × 0.9−1.1 mm, 0.3−0.4 mm thick | Carex alata Fig. 36 |
| – | Pistillate scales in middle to lower portions of spike 2.2−3.7 mm long, apices mostly obtuse, not short-aristate; leaf blades 2−4 (−6) per fertile culm, 8−30 × 0.25−0.4 cm; spikes 6−13 (−17) × 3.8−7 mm; perigynia conspicuously 5−many- nerved on each face, obovate, 3−4.6 × 1.6−2.6 (2.8) mm; achenes oblong, 1.3−1.7 × 0.7−1 mm, 0.4−0.5 mm thick | Carex longii Fig. 37 |
| 3 | Style jointed near the base, disarticulating at the joint; culms erect 20−100 (−130) cm; pistillate spikes 1.5−6.5 × 1.3−3 cm; perigynia 11−19 × 3−6 mm; pistillate scales about as long as the body of the perigynia; achenes 3−4 (−4.5) × 1.7−2.6 (−2.8) mm | Carex lupulina Fig. 38 |
| – | Style not jointed near the base, hardened and persistent, remaining attached to the mature achene; culms erect 40−90 cm; pistillate spikes 2−4 × 0.7−0.8 cm; perigynia 3.9−7 × 2−3.3 mm; lower pistillate scales about as long as the body of the perigynia, upper about 1⁄2 as long; achenes 2−2.5 ×1.5−2 mm | Carex striata Fig. 39 |
Key adapted from Radford et al. (1968), Godfrey and Wooten (1979), and Ball and Reznicek (2002).
Cyperus L.
| 1 | Stigmas 2; achenes lenticular | Cyperus polystachyos Fig. 43 |
| – | Stigmas 3; achenes trigonous | 2 |
| 2 | Mature spikelets shedding scales and achenes individually, leaving the rachilla intact (for at least a short while); roots and lower sheaths conspicuously reddish-purple; culms trigonous to roundly trigonous, (0.5−) 5−25 (−105) cm × 0.1−0.25 (0.75) cm; spikelets 3−8 (−11) × 1−1.5 mm; pistillate scales deciduous, laterally light brown with red speckles and ribless, medially greennish and 3-ribbed, 1.3−1.5 × 0.8−1.2 mm, apex obtuse, mucronulate; achenes sessile, ovoid, (0.4−) 0.7−1 × 0.4−0.6 mm, surface glabrous | Cyperus erythrorhizos Fig. 41 |
| – | Mature spikelets disarticulating into segemets, each comprised of a scale, an achene, and a cartilaginously thickened section of the rachilla; roots and lower sheaths not conspicuously reddish-purple; culms trigonous (4−) 10−50 (−130) × (0.05−) 0.1−0.4 cm; spikelets (5−) 8−15 (−38) × 0.8−1.3 (−1.9) mm; floral scales medially green and 2−5 ribbed, laterally straw-colored to reddish and 1−3 ribbed, (2−) 2.2−2.8 (−3.2) × (1.2−) 1.4−1.6 (−1.8) mm, apex entire or emarginate; achene stipitate, narrowly ellipsoid to oblong, (1−) 1.2−1.5 (−1.9) × 0.5−0.6 (−0.75) mm, surface finely papillose | Cyperus odoratus Fig. 42 |
Key adapted from Radford et al. (1968), Godfrey and Wooten (1979), Tucker et al. (2002), and Weakley (2012).
Eleocharis R.Br.
| 1 | Culm as broad or broader than width of terminal spike, nodose-septate | Eleocharis equisetoides Fig. 46 |
| – | Culm narrower than width of terminal spike, not nodose-septate | 2 |
| 2 | Culms strictly producing fertile spikelets, vegetative proliferations absent; achenes lenticular or biconvex; styles 2−branched | Eleocharis olivacea var. olivacea Fig. 47 |
| – | Culms producing vegetative proliferations or fertile spikelets; achenes trigonous or nearly terete; styles 3−branched | 3 |
| 3 | Upper portion of sheath thin and scarious, lacking a noticeable red-dotted band encircling the apex of sheath (i.e, the apex of the sheath is not differently colored than the lower potions of sheath); sheath tips 1−2 mm long; culms usually more thin and capillary; scales of spikes 2-ranked (distichous); spike usually 2−4 flowered; achenes trigonous, smooth, grayish-olive, 0.6−0.9 × 0.4−0.6 mm, apex constricted proximal to tubercle; tubercle pyramidal, trigonous, 0.2−0.3 (−0.4) × 0.2−0.5 mm | Eleocharis baldwinii Fig. 45 |
| – | Upper portion of sheath firm, a noticeable red-dotted band encircling the sheath apex present (i.e., the sheath apex a different color than the lower sheath); sheath tips <1 mm long; culms usually more robust and less capillary than E. baldwinii; scales of spike spirally imbricate, not 2-ranked; spike with > 4 flowers; achenes trigonous, finely reticulate, gray to greenish, 0.6−0.9 × 0.55−0.8 mm, apex constricted proximal to tubercle; tubercle pyramidal, trigonous, 0.2−0.5 × 0.4−0.5 mm | Eleocharis vivipara Fig. 48 |
Key adapted from Smith et al. (2002) and Weakley (2012).
Note: Achene measurements in this key do not include the tubercle. Eleocharis baldwinii and E. vivipara can be difficult to distinguish in the field when they are both in their vegetative forms. One should pay particular attention to the sheaths encircling the culms; the differences are highlighted in the key below.
Rhynchospora Vahl
| 1 | Tubercle 3−23 mm long; style simple or bifid only at tip | 2 |
| – | Tubercle < 3 mm long; style divided into 2 slender branches | 4 |
| 2 | Longest perianth bristles shorter than the achene body | Rhynchospora corniculata Fig. 52 |
| – | Longest perianth bristles equaling or exceeding the achene body | 3 |
| 3 | Plants rhizomatous; primary clusters with 1−6 loosely clustered spikelets; achene (3.5−) 4.0−4.8 mm long | Rhynchospora inundata Fig. 55 |
| – | Plants cespitose; primary clusters with 10−50 densely clustered spikelets; achene (4.5−) 5−6 mm long | Rhynchospora macrostachya Fig. 57 |
| 4 | Inflorescence bracts several, bright white basally | Rhynchospora latifolia Fig. 56 |
| – | Inflorescence bracts 0−several, not white basally | 5 |
| 5 | Perianth bristles retrorsely barbellate (at least distally) | Rhynchospora alba Fig. 51 |
| – | Perianth bristles antrorsely barbellate | 6 |
| 6 | Surface of achene smooth, minutely pitted, or finely striate | 7 |
| – | Surface of achene transversely ridged, rugose, or honeycomb-reticulate | 8 |
| 7 | Bristles > 1⁄2 as long or exceeding the achene body; larger basal leaves 1.3−2.5 mm wide, achene elliptic, 1.1−1.3 mm wide, tubercle triangular−attenuate | Rhynchospora distans Fig. 53 |
| – | Bristles virtually non-existent to 1⁄2 as long as the achene body (rarely > 1⁄2 as long as the achene body); larger basal leaves 2−4 mm wide; achene suborbicular, 1.2−1.5 mm wide, tubercle triangular | [Rhynchospora fascicularis] |
| 8 | Achenes biconvex, not flat or concave on one side | Rhynchospora nitens Fig. 58 |
| – | Achene faces flat or concave, when one face is concave, the other slightly convex | 9 |
| 9 | Achene < 2× as long as wide, obovate, tubercle triangular, 0.2−0.9 mm long | Rhynchospora elliottii |
| – | Achene at least 2× as long as wide, elliptic−oblong, tubercle subulate, 0.8−1.2 mm long | Rhynchospora inexpansa Fig. 54 |
Key adapted from Kral (2002a) and Weakley (2012).
Note: A voucher (Wilbur 49814, DUKE) for Rhynchospora fascicularis (Michx.) Vahl was collected from the shoreline of Lake Waccamaw; however, this specimen appears referable to R. distans (Michx.) Vahl. Nonetheless, though not otherwise reported from the littoral zone of Carolina bay lakes, R. fascicularis has the potential to occur in these sites and is therefore included in the key below. Achene measurements in this key do not include the tubercle (i.e., the tubercle and achene should be measured as two separate entities).
Eriocaulaceae
| 1 | Plant 4−21 cm tall (−100 cm when submersed); receptacle/base of flowers glabrous or sparingly hairy; heads overall appearing dark gray to white, 4−10 mm in diam. when in full flower and fruit; seeds light-brown or red-brown, ovoid to broadly ellipsoid, faintly reticulate, not papillate; of sandy to peaty shorelines, bogs, and streams | Eriocaulon aquaticum Fig. 60 |
| – | Plant 20−70 cm tall; receptacle/base of flowers copiously hairy; heads overall appearing white, 10−20 mm in diam. when in full flower or fruit; seeds dark lustrous brown, broadly ovoid to round but asymmetric, minutely spiny papillate; of seasonally floooded depression ponds, savannas, flatwoods, ditches | [Eriocaulon compressum] |
Key adapted from Kral (2000a) and Weakley (2012).
Note: Although the first author has only encountered E. aquaticum in the field, E. compressum was reported from the NCSU Crop Science Department (Rob Richardson and Justin Nawrocki, pers. comm., April 9, 2015) and is therefore included in the key below.
Hydrocharitaceae
| 1 | Leaves noticeably rough to the touch, in whorls of (3−) 4−8, 1.2−4 mm wide, lacking sheaths, margins conspicuously serrulate, each serration tipped with 1-celled sharp teeth, 1- nerved, mid-vein keeled below, keels bearing conical protrusions, each armed with sharp teeth; plants dioecious, flowers unisexual (only female plants found in the southeastern United States) | Hydrilla verticillata Fig. 62 |
| – | Leaves not rough, opposite or sometimes crowded and appearing whorled, 0.2−2.1 mm wide, sheaths present, margins minutely serrulate, 1-nerved, midvein lacking an abaxial keel and conical protrusions; plants monoecious, flowers unisexual | Najas guadalupensis var. guadalupensis Fig. 63 |
Key adapted from Godfrey and Wooten (1979), Haynes (2000b), and Weakley (2012).
Juncaceae
| 1 | Inflorescence bract exceeding the inflorescence, inflorescence thus appearing lateral | 2 |
| – | Inflorescence bract not exceeding the inflorescence, inflorescence appearing terminal | 3 |
| 2 | Basal sheaths (or at least a few) producing elongate well-developed blades; inflorescence bract channeled on one side; capsules subglobose | Juncus coriaceus Fig. 68 |
| – | Basal sheaths not producing elongate blades; inflorescence bract not channeled on one side; capsules more or less oblong, 3-sided | Juncus effusus ssp. solutus Fig. 69 |
| 3 | Leaf blades not septate | 4 |
| – | Leaf blades septate | 5 |
| 4 | Stems erect and with a hardened base, never creeping or forming mats; perianth < 6 mm long; plant not cinfined to aquatic settings, may occur in uplands as well as wetland margins, never submersed | Juncus biflorus Fig. 66 |
| – | Stems soft, weak, creeping and rooting at the nodes, often forming homogeneous mats or stands in shallow water or saturated soils above current water level; perianth 6−10 mm long; plant strictly aquatic, submersed and sterile or emersed/stranded and fertile | Juncus repens Fig. 71 |
| 5 | Flowers or fruits borne singly (solitary) on the branches of the inflorescence; inflorescence diffuse, with slender flexuous branches; flowers often aborted; seeds without tail-like appendages | Juncus pelocarpus Fig. 70 |
| – | Flowers or fruits borne in heads of 3 or more, heads often spherical; inflorescence not diffuse, branches not slender and flexuous; flowers seldom aborted; seeds with or without tail-like appendages | 6 |
| 6 | Mature seeds with elongate tail-like appendages, body of seeds 1.2−2.2 mm long | Juncus canadensis Fig. 67 |
| – | Mature seeds lacking elongate tail-like appendages; body of seeds < 0.7 mm long | 7 |
| 7 | Heads turbinate to hemispherical, 3−15-flowered; capsules 2.8−3.5 (−4) mm long, straw-colored, exerted, abruptly contracting at the summit, apex acute, valves separating (dehiscing) at maturity, equaling or just exceeding the perianth; stamens 3 or 6; seeds ellipsoid, clear amber | Juncus acuminatus Fig. 65 |
| – | Heads spherical, 15−60-flowered; capsules 2−3 mm long, straw-colored, exerted, apex gradually tapering to the summit, remaining attached at the tip, valves not separating (dehiscing) at maturity, subulate tips of the capsules exceeding the perianth when fully mature; stamens 3; seeds oblong, dark to clear yellow amber | Juncus scirpoides var. compositus Fig. 72 |
Key adapted from Godfrey and Wooten (1979), Brooks and Clemants (2000), and Weakley (2012).
Orchidaceae
| 1 | Plant an epiphyte, typically found on bases, boles, and large limbs of Taxodium, Nyssa, Liquidambar, and other deciduous hardwoods | Epidendrum magnoliae |
| – | Plant not epiphytic, found in the littoral zone and on floating bogs | 2 |
| 2 | Corolla greenish-colored, lip with a spur, spur deeply divided into 3 linear segments; leaves 3−5, basally disposed | Habenaria repens |
| – | Corolla white, pink, purple or magenta, lip not spurred; leaves basally disposed or cauline | 3 |
| 3 | Flowers arranged in distinct spirals (often appearing 3–4 ranked if spiral is “tight”, white, relatively small, 3–5 mm wide | Spiranthes laciniata Fig. 76 |
| – | Flowers not in distinct spirals, pink, magenta, purple, larger, typically ≥ 1 cm wide | 4 |
| 4 | Flowers not resupinate, lip oriented upwards, bearing numerous orange or yellow clavellate trichomes reminiscent of stamens | Calopogon tuberosus var. tuberosus Fig. 74 |
| – | Flowers resupinate, lip oriented downwards, not bearing numerous stamen-like trichomes | Pogonia ophioglossoides Fig. 75 |
Key adapted from Romero-Gonzáles et al. (2002) and Weakley (2012).
Poaceae
| 1 | Culm perennial, woody, developing complex branching systems from upper culm nodes; [Bambuseae] | Arundinaria tecta Fig. 80 |
| – | Culm annual or facultatively perennial, herbaceous, not developing complex branching systems from upper culm nodes | 2 |
| 2 | Spikelets almost always with 2 florets, lower floret in spikelet always sterile or staminate, frequently absent or reduced to lemma, upper floret bisexual, staminate, or sterile, unawned or awned from the lemma apices; [Andropogoneae and Paniceae] | 3 |
| – | Spikelets either not with 2 florets or with two florets and the lower bisexual or upper floret awned from lemma backs or bases [various tribes] | 10 |
| 3 | Spikelets in sessile-pedicellate pairs, not arranged in conspicuous rows on one side of the rachis; glumes stiff, indurate; usually subequal in length, one or usually both exceeding the floret (excluding the lemma awn); lemmas hyaline; paleas hyaline or absent; [Andropogoneae] | 4 |
| – | Spikelets solitary, or if paired, then forming 2–4 obvious rows on one side of rachis; glumes membranous, lower usually shorter than upper or absent entirely, upper glumes shorter than or nearly equaling upper floret; lower lemmas membranous, upper lemmas typically stiff and indurate, occasionally membranous; upper paleas of similar texture to upper lemmeas; [Paniceae] | 5 |
| 4 | Plant to 1 m tall; spikelets of the pair unalike, sessile bisexual, pedicellate sterile, vestigial, or absent | Andropogon |
| – | Plant to 3 m tall; spikelets of the pair alike, pedicellate spikelet perfect | Saccharum giganteum Fig. 91 |
| 5 | Base of spikelets with rounded, distended, swellings (gibbous) | Sacciolepis striata Fig. 92 |
| – | Spikelets not gibbous | 6 |
| 6 | Plant producing simple culms with terminal “spring” paniculate inflorescences before mid-summer, the culms branching and producing lateral “autumnal” inflorescences from mid to lower culm nodes in the summer and autumn, these often his by the fascicles of smaller “autumnal” leaves; upper florets not disarticulating at maturity | Dichanthelium |
| – | Plant producing terminal panicles in late summer and fall; culms usually not branching from mid to lower culm nodes, or, if so, the branches seldom further branched; upper florets disarticulating or not at maturity | 7 |
| 7 | Plant annual, lacking rhizomes or hard knotty crowns; spikelets verrucose | Panicum [in part] |
| – | Plant a perennial, with rhizomes or hard knotty crowns; spikelets not verrucose | 8 |
| 8 | Plant with hard, knotty crowns, lacking rhizomes; upper lemmas 1.2−1.6 mm long | Coleataenia [in part] |
| – | Plant with rhizomes; upper lemmas 1.6−4 mm long | 9 |
| 9 | Culms slightly compressed below; ligules ≤ 0.5 mm tall; spikelets subsecund, usually obliquely bent above the first glume, pedicels appressed; upper lemma apices lacking papillae, with minute tuft of hair | Coleataenia [in part] |
| – | Culms terete, not slightly compressed below; ligules 2−6 mm tall; spikelets not secund, not obliquely bent above first glume, pedicels spreading; upper lamma apices with simple or compound papillae, glabrous | Panicum [in part] |
| 10 | Plant seldom seen in flower; spikelets composed of a single floret, florets imperfect; culms ≤ 2 mm wide, slender, flexuous, prostrate; leaves conspicuously clustered at the culm apices, floating (lentic system) or streaming (lotic system) on the water surface, or emergent after receding water levels; glumes absent; [Oryzeae] | Luziola fluitans Fig. 87 |
| – | Plants regularly seen in flower; spikelets composed of ≥ 1 floret, florets imperfect or perfect; culms > 2 mm wide, slender, flexuous, or prostrate; leaves not conspicuously clustered at the culm apices, not floating or emergent after receding water levels; glumes present | 11 |
| 11 | Spikelets with (4−) 6−30 florets; [Cynodonteae] | Eragrostis |
| – | Spikelets with ≤ 3 florets; [Poaeae] | 12 |
| 12 | Culm 1.5-8.2 dm; sheaths glabrous; ligules (0.7−) 1.2−4 mm tall; blades 3−10 × 0.1−0.2 cm; panicles (5−) 10−25 (36) × (3) 4−24 cm, diffuse, the whole panicle detaching at the base at maturity, the resulting detached panicle resemblig a “tumbleweed” | Agrostis hyemalis Fig. 77 |
| – | Culm (0.9−) 2−13 dm; sheaths glabrous, hairy, or scabridulous; ligules (1−) 1.5−2.5 mm tall; blades 5−14 × (0.1-) 0.2-0.8 cm; panicles (2−) 5−15 (−25) × 0.5−2 cm, compact, spike-like, the panicle not detaching at the base at maturity | Sphenopholis obtusata Fig. 93 |
Key adapted from Radford et al. (1968), Godfrey and Wooten (1979), Barkworth (2003a), Barkworth (2003b), Barkworth (2007), and Weakley (2012).
Andropogon L.
| 1 | Leaves strongly glaucous (appearing powdery-white and leaving white residue on fingers when rubbed), glabrous; ligules (0.9−) 1.5 (−2) mm tall | Andropogon glaucopsis Fig. 78 |
| – | Leaves green, not glaucous (never powdery-white), pubescent (at least on the margin near the collar; ligules 0.2−1 mm tall | Andropogon virginicus var. virginicus Fig. 79 |
Key adapted from Weakley (2012).
Coleataenia Griseb.
| 1 | Cauline leaf blades 2−8 mm wide; glumes and sterile lemmas keeled along midvein; apices of fertile lemmas with a minute tuft of hairs | Coleataenia longifolia var. longifolia Fig. 81 |
| – | Cauline leaf blades 1−4 mm wide; glumes and sterile lemmas not keeled along midvein; apices of fertile lemmas lacking a minute tuft of stiff hairs | Coleataenia tenera |
Key adapted from Weakley (2012).
Dichanthelium (Hitchc. et Chase) Gould
| 1 | Spikelets 0.8−2.0 mm long | 2 |
| – | Spikelets 2.1−3.2 mm long | 5 |
| 2 | Internodes glabrous | 3 |
| – | Internodes crisp-puberulent | 4 |
| 3 | Plants with hard knotty crowns; culms to 100 cm; nodes without a distinct constricted yellow ring; vernal cauline leaves 15−20× as long as wide (5−12 cm long); ligules < 1 mm tall; spikelets 1.7−2.3 mm, glabrous | Dichanthelium dichotomum var. roanokense |
| – | Plants cespitose; culms 30−75 cm; nodes with a distinct constricted yellow ring; vernal cauline leaves < 15× as long as wide (5−10 cm long); ligules 0.2−0.5 mm tall; ligules 0.2−0.5 mm; spikelets 0.9−1.2 mm, puberulent to subglabrous | Dichanthelium erectifolium Fig. 83 |
| 4 | Spikelets 1.5−1.8 mm; first glume 0.5−0.8 mm; lower culm blades 2−5 mm wide | Dichanthelium portoricense Fig. 85 |
| – | Spikelets (1.8−) 1.9−2.2 (−2.3) mm; first glume 0.8−1.2 mm; lower culm blades 4−8 mm wide | Dichanthelium species 3 (=lancearium) |
| 5 | Larger culm blades usually 6−15 mm wide; spikelets 2−3 mm, pubescent; internodes glabrous | 6 |
| – | Larger culm blades usually 3.5−8 mm wide; spikelets 1.7−2.3 mm, pubescent or glabrous; internodes crisp-puberulent or glabrous | 7 |
| 6 | Larger culm blades 6−12 mm wide; lower culm nodes not bearded; spikelets 2−3 mm long; first glumes 0.5–1 mm long | Dichanthelium boreale Fig. 82 |
| – | Larger culm blades 13−25 mm wide; lower culm nodes bearded (often retrorsely); spikelets (2−) 2.2−2.8 mm long; first glumes 0.5–1.3 mm long | Dichanthelium mattamuskeetense Fig. 84 |
| 7 | Spikelets 1.7−2.3 mm long, glabrous; first glume 0.6−1.1 mm; largest vernal blades 15−20× as long as wide; internodes glabrous | Dichanthelium dichotomum var. roanokense |
| – | Spikelets (1.8) 1.9−2.2 (2.3) mm long, pubescent; first glume 0.8−1.2 mm; largest vernal blades < 15× as long as wide; internodes crisp-puberulent | Dichanthelium species 3 (=lancearium) |
Key adapted from Freckmann and Lelong (2003b) and Weakley (2012).
Eragrostis Wolf
| 1 | Lateral spikelets with widely spreading pedicels; lower pedicles longer than spikelets; disarticulation of the lemmas only, paleas are persistent | Eragrostis elliottii Fig. 86 |
| – | Lateral spikelets with appressed pedicels, lower pedicels shorter than spikelets; disarticulation usually of the whole floret | Eragrostis refracta |
Key adapted from Godfrey and Wooten (1979) and Weakley (2012).
Panicum L.
| 1 | Glumes and lower lemmas verrucose; ligules 0.2−0.5 mm tall | Panicum verrucosum Fig. 89 |
| – | Glumes and lower lemmas smooth, not verrucose; ligules 0.5−6 mm tall | 2 |
| 2 | Panicle < 1 cm wide at maturity; upper glume and lower lemma 3−5 veined; ligule <1 mm tall | Panicum hemitomon Fig. 88 |
| – | Panicle 4−20 cm wide at maturity; upper glume and lower lemma 7−11 veined; ligule 2−6 mm tall | Panicum virgatum Fig. 90 |
Key adapted from Freckmann and Lelong (2003a) and Weakley (2012).
Pontederiaceae
| 1 | Outside of floral tube villous when young, essentially glabrous to sparsely glandular at maturity; leaves ovate to triangular−lanceolate, 2.2−21 cm wide, base usually cordate or truncate | Pontederia cordata var. cordata Fig. 94 |
| – | Outside of floral tube persistently pubescent with glandular hairs; leaves lanceolate, 0.4−8.3 cm wide, base usually cuneate to truncate | Pontederia cordata var. lancifolia |
Key adapted from Godfrey and Wooten (1979) and Weakley (2012).
Potamogetonaceae
| 1 | Plant with floating and submersed leaves; submersed to 30 mm wide, linear to narrowly- lanceolate to lanceolate, mid to upper stem leaves translucent, with 4−8 rows of lacunae aon either side of midvein, floating to 85 mm wide, coriaceous, ovate to oblong-elliptic, bases rounded or slightly cordate | Potamogeton pulcher Fig. 95 |
| – | Plant with submersed leaves only, leaves linear, thread-like, or ribbonlike, to 3 mm wide, obvious lacunae absent on either side of midvein | Potamogeton pusillus |
Key adapted from Weakley (2012).
Note: The first author has not encountered Potamogeton in the field, but Potamogeton pusillus L. and Potamogeton pulcher were reported from Lake Waccamaw by the NCSU Crop Science Department (Rob Richardson and Justin Nawrocki, pers. comm., April 9, 2015) and Richard LeBlond with the North Carolia Natural Heritage Program (see specimen label of LeBlond 3382, NCU!). A key to these reported taxa is provided below.
Smilacaceae
| 1 | Leaves evergreen, blades more or less oblong to linear or narrowly lanceolate, thick, coriaceous, midvein (as seen from the abaxial leaf surface) much more pronounced than the secondary veins, which are not noticeably evident (except perhaps at base of leaf blade) | Smilax laurifolia Fig. 97 |
| – | Leaves deciduous or evergreen, blades ovate to suborbicular, membraneous, midvein (as seen from the abaxial leaf surface) little if any more pronounced than the secondary veins, which are noticeably evident | 2 |
| 2 | Abaxial surface of mature leaves strongly glaucous | Smilax glauca Fig. 96 |
| – | Abaxial surface of mature leaves not glaucous, usually paler green than the adaxial surface | 3 |
| 3 | Mature berries blue-black, seeds (1−) 2−3 per berry; perianth green; leaves semi- evergreen to evergreen, margins of mature leaf blades usually not revolute, typically with small, flat, tooth-like projections near the base; of various upland and wetland habitats, typically not restricted to sites that are inundated for much of the year | Smilax rotundifolia Fig. 98 |
| – | Mature berries bright red, seeds 2−4 per berry; perianth brownish-yellow; leaves deciduous; margins of mature leaf blades usually revolute, lacking small, flat, tooth-like projections near the base; restricted to sites with long hydroperiods | Smilax walteri Fig. 99 |
Key adapted from Godfrey and Wooten (1979), Holmes (2002), and Weakley (2012).
Xyridaceae
| 1 | Keel of lateral sepals long–fimbriate towards apex, fimbriate tip conspicuously protruding beyond the subtending bract (sometimes eroded and less evident in older spikes) | Xyris fimbriata Fig. 100 |
| – | Keel of lateral sepals lacerate, not ciliate or long-fimbriate | 2 |
| 2 | Lateral sepals longer than and protruding from the subtending bracts; scapes 5−15 dm tall | Xyris smalliana Fig. 102 |
| – | Lateral sepals shorter than subtending bracts, hidden (except when spikes open during maturity); scapes 1.5−12 dm tall | 3 |
| 3 | Summit of the scape distinctly flattened and broad relative to the spike; scape ridges 2−3, the two more prominent ridges comprising the flattened edge of the scape, therefore the upper scape ellipsoidal or fusiform in cross-section | Xyris iridifolia |
| – | Summit of the scape not flattened and broad relative to the spike; scape ridges > 3 (at least on the mid to lower portions of the scape), scape much narrower than the spike, terete or slightly flattened in cross-section | Xyris jupicai Fig. 101 |
Key adapted from Godfrey and Wooten (1979), Kral (2000b), and Weakley (2012).
BASAL ANGIOSPERMS, MAGNOLIIDS, and EUDICOTYLEDONS
Key 1: Woody plants (trees, shrubs, and lianas)
| 1 | Plant a liana, climbing by means of tendrils, adventitious roots, or twining stems | 2 |
| – | Plant a tree or shrub, not climbing | 10 |
| 2 | Leaves compound | 3 |
| – | Leaves simple | 7 |
| 3 | Leaves opposite | 4 |
| – | Leaves alternate | 5 |
| 4 | Stems not ribbed; leaves 1-pinnate, leaflets (5−) 7−11 (−15), 4−8 cm long, ovate, unlobed, margins coarsely serrate, rounded at base, apices acute; inflorescence > 1-flowered, flowers erect or spreading, pedicels shorter to slightly longer than the calyx tube; calyx greenish-yellow or orange, campanulate to funnel-shaped, 1.5−2 cm long, lobes ascending, margins entire; corolla showy, orange to scarlet, funnelform, 6−8 cm long; fruit a fusiform, falcate, capsule | Bignoniaceae [Campsis radicans; Fig. 127] |
| – | Stems ribbed; leaves 1−2-pinnate or sometimes simple or trifoliolate, leaflets 4−10 plus a ± tendrilate terminal leaflet, (1.5−) 3−10 cm long, linear to ovate, unlobed or proximally 3−5 lobed, margins entire, bases broadly to narrowly cuneate, truncate, occasionally subcordate, apices acute, obtuse, or acuminate; inflorescence 1-flowered, flower pendent, pedicel > 2x length of the calyx tube; calyx violet-blue, campanulate, 2.5−5 cm long, lobes strongly recurved, margins crisped; corolla lacking; fruit an aggregate of achenes | Ranunculaceae [Clematis crispa; Fig. 187] |
| 5 | Leaves trifoliolate; plant climbing by adventitious roots, tendrils absent; fruit a drupe, white; plant containing contact poisons | Anacardiaceae [Toxicodendron radicans; Fig. 106] |
| – | Leaves palmately or pinnately compound but not trifoliolate; plant twining or tendrillate; fruit a legume and brown or a berry and blue; plant not containing contact poisons | 6 |
| 6 | Plant twining, tendrils absent; leaves 1-pinnate, leaflets 9−15, entire; fruit a legume | Fabaceae [Wisteria frutescens; Fig. 145] |
| – | Plant tendrillate; leaves palmately compound, leaflets (3−) 5 (−7), coarsely serrate on their distal margins; fruit a berry | Vitaceae [Parthenocissus quinquefolia; Fig. 208] |
| 7 | Leaves opposite | 8 |
| – | Leaves alternate | 9 |
| 8 | Plant twining; leaves evergreen, lanceolate to ovate, 3−9 × 1−2.5 cm, apices acute to acuminate, margins entire; flowers solitary or sometimes in 2−3-flowered axillary cymes; petals lemon yellow, connate; ovary superior | Gelsemiaceae [Gelsemium sempervirens; Fig. 147] |
| – | Plant climbing by means of aerial adventitous roots; leaves deciduous, ovate to orbicular 3−12 cm × 1−8 cm, apices abruptly short acuminate, acute, or obtuse, margins distally serrate; flowers numerous, borne in terminal compound cymes; petals white, not connate; ovary inferior | Hydrangeaceae [Decumaria barbara; Fig. 148] |
| 9 | Tendrils lacking; leaves 3−8 × 1.5−4 cm, elliptic to ovate, margins slightly wavy to entire; fruit a blue-black drupe, 5−7 mm long | Rhamnaceae [Berchemia scandens; Fig. 188] |
| – | Tendrils present, borne opposite the leaves; leaves 5−12 × 5−12 cm, orbicular to ovate, margins prominently dentate-serrate; fruit a blue-black berry, 1−2.5 cm long | Vitaceae [Muscadinia rotundifolia; Fig. 207] |
| 10 | Leaves opposite or whorled | 11 |
| – | Leaves alternate | 15 |
| 11 | Plant exhibiting varying degrees of both opposite and whorled leaf arrangement | 12 |
| – | Plant exhibiting strictly opposite leaf arrangement | 13 |
| 12 | Plant woody proximally and herbaceous distally, stem with a soft corky texture when under water, young stems strongly pubescent, green; petioles not connected by a central stipule or stipular scars; leaves lanceolate to elliptic, to 20 × 5 cm, bases and apices acute, glabrous adaxially and pubescent with branched hairs abaxially; flowers in cymose inflorescences; corolla majenta; stamens of 3 possible lengths, 2 of the 3 occuring in any one flower | Lythraceae [Decodon verticillatus; Fig. 165] |
| – | Plants woody entirely, stem not soft and corky when submersed, young stems sometimes short-pilose initially but becoming glabrous with age, reddish-brown; petioles connected by a central stipule or stipular scar; leaves oval, oblong oval, elliptic or ovate, to 15 × 10 cm, bases broadly rounded to cuneate, apices acute or acuminate, glabrous adaxially and short-pilose abaxially (at least on the principal veins); flowers in dense globose heads, corolla white; stamens of one length | Rubiaceae [Cephalanthus occidentalis; Fig. 194] |
| 13 | Leaves simple | Sapindaceae [Acer] |
| – | Leaves compound | 14 |
| 14 | Leaves 1-pinnate, imparipinnate, leaflets 5−7 (−9); inflorescences borne on old wood of previous growing seasons before the development of new shoots; corolla not scarlet red; fruit a samara | Oleaceae [Fraxinus caroliniana; Fig. 179] |
| – | Leaves palmately compound, leaflets 5−7; inflorescences borne on new shoots of current year; corolla scarlet red; fruit a capsule | Sapindaceae [Aesculus pavia; Fig. 203] |
| 15 | Leaves compound | 16 |
| – | Leaves simple | 18 |
| 16 | Stems arching, trailing, or erect to 2 m tall, armed with numerous prickles; leaves 1-pinnately or 1-palmately compound, leaflets 3−9; fruit an aggregate of drupes or an aggregate of achenes enclosed in a hip | Rosaceae [Rosa & Rubus] |
| – | Stems erect, > 2m in height, lacking prickles; leaves 1-pinnately compound, leaflets 5−23; fruit a nut or drupe | 17 |
| 17 | Plant a shrub to small tree, to 7 m tall; stems densely short pubescent; leaflets 9−11 (−23), 3−8 ×1−4 cm, rachis winged; fruit a drupe | Anacardiaceae [Rhus copallinum var. copallinum; Fig. 105] |
| – | Plant a medium to large tree (unless in juvenile stage of development), to 30 m tall; stems not densely pubescent; leaflets (3−) 5 (−7), 3−22.5 × 1.8−13 cm; rachis not winged; fruit a nut enclosed within a husk | Juglandaceae [Carya glabra; Fig. 154] |
| 18 | Flowers borne in heads subtended by an involucre of bracts | Asteraceae [Baccharis halimifolia; Fig. 112] |
| – | Flowers not borne in heads subtended by an involucre of bracts | 19 |
| 19 | Leaves palmately lobed, margins glandular-serrate; fruit a multiple of capsules | Altingiaceae [Liquidambar styraciflua; Fig. 103] |
| – | Leaves pinnately or palmately lobed, margins otherwise; fruit otherwise | 20 |
| 20 | Fruit a nut (acorn) bearing a basal cupule (“cap”); buds conspicuously clustered at twig tips, scales imbricate | Fagaceae [Quercus nigra; Fig. 146] |
| – | Fruit otherwise; axillary buds not clusted at twigs tips with scales imbricate | 21 |
| 21 | Stipular scars conspicuous, completely encircling the twig stems or nodes | 22 |
| – | Stipular scars (if present) not encircling the twig stems or nodes completely | 23 |
| 22 | Bark of mature specimens gray, intact; leaves 6−15 × 2−6 cm, tardily deciduous, long-elliptic to oblong, adaxial surface light green and glabrous, abaxial surface strongly glaucous, petiole base solid, not covering axillary bud; flowers very showy and fragrant, “magnolia-like”; fruit an aggregate of follicles, elongate, seeds red-arillate | Magnoliaceae [Magnolia virginiana var. virginiana; Fig. 166] |
| – | Bark of mature specimens brown, furrowed, and scaly proximally, sloughing away to reveal a bright white smooth inner bark distally; leaves to 35 cm long and wide, deciduous, as long as broad, both adaxial and abaxial surfaces green, petiole base hollow, covering axillary bud; flowers neither showy nor fragrant; fruit a multiple of achenes, spherical, seeds brown, not arillate | Platanaceae [Platanus occidentalis; Fig. 184] |
| 23 | Flowers unisexual and borne in catkins | 24 |
| – | Flowers bisexual and not borne in catkins | 26 |
| 24 | Leaves pleasantly aromatic when crushed, glandular punctate abaxially | Myricaceae [Morella cerifera; Fig. 173] |
| – | Leaves not aromatic when crushed; not glandular punctate abaxially | 25 |
| 25 | Leaves < 3× as long as broad, very “neatly” pinnately veined, lateral veins consistently parallel to one another, ovate-triangular, sub-rhombic, elliptic, obovate, or oblong, margins either doubly serrate or slightly wavy; fruit a nutlet, 1-seeded | Betulaceae |
| – | Leaves > 3× as long as broad, not so neatly pinnately veined, lanceolate, margins serrate; fruit a capsule, 2-valved, many-seeded | Salicaceae [Salix] |
| 26 | Leaves broadly ovate to rhombic-ovate | 27 |
| – | Leaves longer than broad | 28 |
| 27 | Plant exuding milky sap when injured; leaves to 7 (−9) cm long; fruit a 3-valved capsule, maturing after leaf maturation in late summer to fall | Euphorbiaceae [Triadica sebiferum; Fig. 144] |
| – | Plant lacking milky sap; larger leaves ≥ 10 cm long; fruit a 2−4-valved capsule, maturing prior to leaf emergence in the spring | Salicaceae [Populus heterophylla; Fig. 197] |
| 28 | Fruits dry (capsules, berry-like, samaras) | 29 |
| – | Fruits fleshy (berries, drupes, pomes) | 34 |
| 29 | Leaves 2-ranked on the twigs, bases markedly oblique; fruit a samara | Ulmaceae [Ulmus americana; Fig. 206] |
| – | Leaves ≥ 3-ranked on the twigs, bases not oblique; fruit not a samara | 30 |
| 30 | Fruit indehiscent, berry-like; stems typically sharply longitudinally ridged below point of attachment of leaf petioles; leaves spatulate to oblanceolate, margins entire | Cyrillaceae [Cyrilla racemiflora; Fig. 133] |
| – | Fruit a dehiscent capsule; stems not longitudinally ridged below point of attachment of leaf petioles; leaves obovate, elliptic, oblong, or lanceolate, margins toothed (if entire, then blades with a perimarginal vein, lepidote, or with margins ciliate) | 31 |
| 31 | Plant a tree, to 26 m tall; flowers solitary, axillary; stamens > 50 | Theaceae [Gordonia lasianthus; Fig. 205] |
| – | Plant a shrub, < 6 m tall; flowers numerous, borne in racemes or spikes; stamens ≤ 10 | 32 |
| 32 | Pith chambered; ovary 2-locular | Iteaceae [Itea virginica; Fig. 153] |
| – | Pith solid; ovary ≥ 3-locular | 33 |
| 33 | Young twigs, inflorescence rachises, pedicels, and calyces stellate-pubescent; leaves oblanceolate, widest above middle, margins serrate distally; corolla rotate, petals connate ≤ ½ their length, lobes 5−8 mm long; ovary 3-locular | Clethraceae [Clethra alnifolia; Fig. 132] |
| – | Young twigs, inflorescence rachises, pedicels, and calyces glabrous or pubescent, but not stellate-pubescent; leaves lanceolate, ovate, elliptic, oblanceolate, or narrowly obovate, widest at or below the middle (widest above middle in Rhododendron viscosum, but margins finely bristly-ciliate); corolla urceolate, campanulate, globose, rotate, or funnelform, petals connate ≥ ½ their length, lobes either < 4 mm long or 7−24 mm long; ovary 5-locular | Ericaceae [in part] |
| 34 | Fruit a pome | Rosaceae [Amelanchier and Aronia] |
| – | Fruit a drupe or berry | 35 |
| 35 | Leaves evergreen | 36 |
| – | Leaves deciduous or tardily deciduous | 37 |
| 36 | Leaves not aromatic when crushed; margins spinose, crenate, or sometimes entire, lacking deforming galls; drupes containing 4−8 seeds | Aquifoliaceae [Ilex] |
| – | Leaves with a spicy aromatic scent when crushed; margins entire, often with numerous deforming galls (galls a result of red bay psyllid activity); drupes containing 1 seed | Lauraceae [Persea palustris; Fig. 156] |
| 37 | Plant a shrub, typically < 4 m in height; flowers perfect; fruit a blue, purple, or black berry; seeds ≥ 10, ca. 1.2 mm long | Ericaceae [Vaccinium] |
| – | Plant a small to full sized tree, > 4 m in height; flowers imperfect or perfect; fruit a drupe or berry, if berry then yellow to orange (2−) 3−5 (−7.7) cm in diam, seeds 3−8, > 5 mm long | 38 |
| 38 | Vascular bundle scars 1 per leaf scar; leaves generally widest at or below the middle, margins lacking teeth, fruit a berry, orange at maturity, (2−) 3−5 (−7.7) cm in diam, subtended by a thick leathery calyx | Ebenaceae [Diospyros virginiana; Fig. 135] |
| – | Vascular bundle scars 3 per leaf scar; leaves generally widest at or above the middle, sometimes toothed (as in Nyssa aquatica); fruit a drupe, blue-black at maturity 0.7−1.2 cm in diam., a thick leathery calyx lacking | Nyssaceae [Nyssa] |
Key adapted from Radford et al. (1968), Godfrey and Wooten (1981), and Weakley (2012).
Key 2: Herbaceous plants (herbs and vines)
| 1 | Flowers borne in ligulate, radiate, or discoid heads subtended by an involucre of bracts | Asteraceae |
| – | Flowers various but not borne in heads subtended by an involucre of bracts | 2 |
| 2 | Plant carnivorous, leaves modified into tube-like pitchers (Sarraceniaceae) or containing small inconspicuous “bladders” (Lentibulariaceae) or with obvious glandular trichomes (Droseraceae) | 3 |
| – | Plant not carnivorous, lacking the above carnivorous characters | 5 |
| 3 | Leaves modified into conspicuous water-storing, tubular pitchers; flowers with a conspicuous style disk, a strong odor of ammonia (somewhat like cat urine) present; stamens 50−100 | Sarraceniaceae [Sarracenia flava; Fig. 204] |
| – | Leaves not modified into water-storing, tubular pitchers; flowers lacking a style disk, lacking a strong odor of ammonia; stamens < 50 | 4 |
| 4 | Plants terrestrial (occuring in moist to saturated soils), leaves lacking bladder-like traps, instead exhibiting glandular trichomes; corolla actinomorphic, not 2-lipped, white | Droseraceae [Drosera intermedia; Fig. 134] |
| – | Plant terrestrial (occuring in moist to saturated soils) or aquatic (typically found floating on the water surface), leaves bearing small, subterranean, urn-like or bladder-like traps; corolla zygomorphic, 2-lipped, corolla yellow or purple-lavender | Lentibulariaceae [Utricularia] |
| 5 | Plant a rooted aquatic, having either submersed, floating, or both submersed and floating leaves [Plants included in this section are the “prototypical” truly aquatic plants, exhibiting submersed or floating leaves. However, fluctuating water levels can cause a small degree of ambiguity. Increasing water levels may flood emergent wetland plants and give them the appearance of having submersed or floating leaves. Similarly, receding water levels may leave “prototypical” aquatic plants stranded and give them the appearance of emergents. Taking this into consideration, certain families and genera are included both in this lead and the next to ensure a broad range of environmental conditions are covered] | 6 |
| – | Plant terrestrial, emergent, with only roots and/or basal leaves inundated | 11 |
| 6 | Leaves of two types: submersed cauline, opposite, and comprised of dichotomously dissected linear segments, floating alternate, simple, and peltate, blades elongate-rhombic | Cabombaceae [Cabomba caroliniana; Fig. 129] |
| – | Leaves of one type (two in Nuphar sagittifolia, but then submersed leaves not cauline and not dichotomously divided): floating (submersed, floating, or erect in Hydrocotyle umbellata), peltate or not, blades oval, orbicular, cordate, ovate, reniform, lanceolate, or oblong-lanceolate | 7 |
| 7 | Leaves peltate | 8 |
| – | Leaves not peltate, petiole attached to a cuneate, sagittate, or cordate base | 10 |
| 8 | Underwater portions of plant coated with transparent mucilaginous jelly; leaves elliptic | Cabombaceae [Brasenia schreberi; Fig. 128] |
| – | Underwater portions of plant lacking mucilaginous jelly; leaves orbicular | 9 |
| 9 | Leaves < 8 cm in diam., submersed, floating, or emersed at maturity, margins crenate; peduncle (inflorescence stalk) equaling or just exceeding the leaves | Araliaceae [Hydrocotyle umbellata; Fig. 111] |
| – | Leaves > 20 cm in diam., floating (sometimes emersed during falling water levels), margins entire; peduncle (inflorescence stalk) tall, commonly overtopping the leaves | Nelumbonaceae [Nelumbo lutea; Fig. 174] |
| 10 | Leaf 5−15 cm long, ovate to reniform; petiole often reddish purple-punctate; inflorescence borne amongst or immediately subtended by a cluster of stout, fleshy, tuber-like, bannana-shaped roots; flowers 4−5-merous (eudicot) | Menyanthaceae [Nymphoides aquatica; Fig. 172] |
| – | Leaf (5−) 10−50 cm long, orbicular or lanceolate to oblong-lanceolate; petiole not reddish purple-punctate; inflorescence not amongst or subtended by fleshy tuber-like roots; flowers > 5-merous (basal angiosperm) | Nymphaeaceae |
| 11 | Leaves peltate | Araliaceae [Hydrocotyle umbellata; Fig. 111] |
| – | Leaves not peltate | 12 |
| 12 | Plant exuding milky sap when injured | 13 |
| – | Plant exuding clear sap when injured | 14 |
| 13 | Plant 2−10 dm tall; cauline leaves .1−15 × .1−.8 cm, linear, narrowly elliptic, or oblanceolate, margins callose toothed; flowers single and relatively distant from one another on the racemes or raceme-like branches; sepals not composed of an inner two (enlarged and wing-like) and an outer three (reduced); corolla blue to bluish-white | Campanulaceae |
| – | Plant 0.5−4 dm tall; leaves 1.5−6 × 0.5−2 cm, spatulate to obovate, margins lacking callose teeth; flowers in dense racemose terminal heads; sepals 5, composed of an inner two (enlarged and wing-like) and on outer three (reduced); corolla orange | Polygalaceae [Polygala lutea Fig. 185] |
| 14 | Leaves basal (sprouting form the nodes of a stolon) and simple or cauline and 1−3-pinnately compound (Cicuta maculata; this plant is extremely poisonous and care should be taken when handling plant parts); inflorescence a single or compound umbel; fruit a schizocarp | Apiaceae |
| – | Leaves various, if basal then not sprouting from nodes of a stolon and if cauline then not compound; inflorescence not umbellate; fruits various but not a schizocarp | 15 |
| 15 | Cauline leaves alternate | 16 |
| – | Cauline leaves opposite | 18 |
| 16 | Perianth differentiated into sepals and petals; corolla zygomorphic, bluish-purple and white | Plantaginaceae [Nuttallanthus canadensis Fig. 183] |
| – | Perianth undifferentiated and comprised of green, pinkish, or red tepals, or comprised solely of sepals (petals lacking) | 17 |
| 17 | Mature stems to 8 dm tall, relatively dainty (herb-like), if submersed then not spongy and thickened, ocrea present; leaves primarily basal, those along stem stem much reduced and distant from one another, bases mostly hastate (sometimes cuneate due to relatively frequent wave disturbance); inflorescence composed of terminal paniculate racemes; sepals 6; fruit a single achene kept inside the inner calyces | Polygonaceae [Rumex hastatulus Fig. 186] |
| – | Mature stems to 10 dm tall, robust (shrub-like), submersed stems becoming very spongy and enlarged, apparently “splitting” in place, ocrea absent; leaves evenly distributed on the stem, not reduced and distant on the stem; inflorescence composed of a solitary flower in the leaf axils; sepals 4; fruit a capsule | Onagraceae [Ludwigia sphaerocarpa Fig. 181] |
| 18 | Stems dichotomously branched, “wiry” in overall appearance; cauline leaves ≤ 2 mm long, subulate, bases pectinately-fringed | Caryophyllaceae [Stipulicida setacea Fig. 131] |
| – | Stems not dichotomously branched, not “wiry” in overall appearance; cauline leaves > 2 mm long, not subulate, bases not pectinately-fringed | 19 |
| 19 | Stems 4-angled; flowers in dense axillary clusters; corolla < 7 mm long, connate most of length, white, somewhat bi-labiate, 5-lobed, pubescent within; fruit a nutlet | Lamiaceae [Lycopus angustifolius Fig. 155] |
| – | Stems not 4-angled (4-angled in Melastomataceae, but not with the above combination of floral characters); flowers not in dense axilary clusters; corolla not as above; fruits various but not a nutlet | 20 |
| 20 | Corolla ≤ 5 mm long; flowers secund on small branchlets; capsule swollen at the base with two incurving appendages distally, thus having the appearance of “horns” | Loganiaceae [Mitreola petiolata Fig. 164] |
| – | Corolla ≥ 5 mm long; flowers not secund on small branchlets; capsule not swollen at the base with horn-like appendages distally | 21 |
| 21 | Plants commonly creeping and forming small to large mats in shallow water; flowers solitary or in head-like inflorescences arising from leaf axils | 22 |
| – | Plants not creeping or forming small to large mats in shallow water; flowers and fruits not borne in leaf axils (except for Linderniaceae and Rubiaceae) | 24 |
| 22 | Leaves to 9 cm long, linear-elliptic, apices acute and tipped with a tiny spine; inflorescence a multi-flowered axillary or terminal white head; fruit an utricle | Amaranthaceae [Alternanthera philoxeroides Fig. 104] |
| – | Leaves to 2 cm long, ovate, oblanceolate, or sometimes elliptic, apices acute to rounded, lacking a tiny spine; inflorescence composed of a single axillary flower, corolla pale or bright blue to violet-blue or yellow; fruit a capsule | 23 |
| 23 | Plant lacking a pleasant citrus-spicy aroma when crushed; stems without spongy or succulent texture; leaves to 2.5 ×0.6 cm, oblanceolate, apices acute to obtuse, petals separate, ≤ 9 mm long yellow | Onagraceae [Ludwigia brevipes Fig. 180] |
| – | Plant with a very pleasant citrus-spicy aroma when crushed; stems with a spongy and succulent texture; leaves to 2 × 1.5 cm, ovate, apices obtuse to rounded; petals connate, 9−13 mm long, pale or bright blue to violet-blue | Plantaginaceae [Bacopa caroliniana Fig. 182] |
| 24 | Flowers axillary, solitary, usually in the axils of one of a given pair of leaves (sometimes one in each axil of the pair); sepals linear-attenuate scabrous; corolla funnelform, 5-lobed, upper lip erect and shallowly 2-lobed, lower lip deflexed and 3-lobed, lavender | Linderniaceae [Lindernia dubia Fig. 163] |
| – | Flowers axillary or not, if so, then sometimes having more than 1 flower per axil and always in the axils of both of a given pair of leaves; sepals various, not scabrous; corolla various but if connate then not with the above floral characters, white, lavender, rose, pink, purple, or yellow | 25 |
| 25 | Leaves connected by interposed stipules or foliaceous stipules, if foliaceous, then indistinguishable from the leaves, thus the leaves appearing whorled; corollas white, connate basally to form a tube, or separated into 3–4 distinct petals | Rubiaceae [in part] |
| – | Leaves not connected by interposed or foliaceous stipules, not appearing whorled; corolla yellow, purple, pink, rose, or lavendar, never connate basally to form a tube, always separated into 4−5 distinct petals | 26 |
| 26 | Leaves glabrous, punctate-dotted, entire, not decussate; petals 4−5, pink (flesh-colored) or yellow; stamens sometimes grouped into fascicles, staminodia sometimes present; ovary superior, fruit a septicidal capsule not enclosed within a hypanthium | Hypericaceae |
| – | Leaves often pubescent or sparingly pubescent, not punctate-dotted, usually serrated or coarsely toothed, decussate; petals 4, pink, rose, or lavendar; stamens never grouped into fascicles, staminodia lacking; ovary inferior; fruit a loculicidal capsuse enclosed within an urceolate-shaped hypanthium | Melastomataceae |
Key adapted from Radford et al. (1968), Godfrey and Wooten (1981), and Weakley (2012).
Anacardiaceae
| 1 | Leaves imparipinnate, leaflets ≥ 7, rachis winged; fruits red, glandular pubescent; plant lacking contact poisons; inflorescences terminal | Rhus copallinum var. copallinum Fig. 105 |
| – | Leaves pinnately trifoliolate; fruits white to yellow, glabrous or puberulant, hairs eglandular; plant containing contact poisons; inflorescences axillary | Toxicodendron radicans var. radicans Fig. 106 |
Key adapted from Radford et al. (1968) and Weakley (2012).
Apiaceae
| 1 | Stems elongate-rhizomatous, horizontal, low-growing; leaves simple, blades ovate to oblong, 1.5–5 (−10) × 1.5 −3.5 (−8) cm, apices rounded, base cordate to truncate, margins denticulate; umbels simple, 1−4 (−9) flowers per umbel, pedicels 0.5−3 mm long; fruit strongly flattened laterally, prominently nerved with raised reticulate venation between nerves, corky ribs lacking | Centella asiatica Fig. 107 |
| – | Stems erect, not horizontal or low-growing; leaves 1–3 times pinnately compound, blades to 30 × 25 cm, leaflets lanceolate to lance-oblong, 4−7 (−14) cm ×0.6−3 (−5) cm, apices acute, bases cuneate to rounded, frequently asymmetrically so, margins serrate; umbels compound, > 9 flowers per umbel, pedicels 2−10 mm long; fruit somewhat flattened laterally with strong, flattish corky ribs | Cicuta maculata Fig. 108 |
Key adapted from Radford et al. (1968), Godfrey and Wooten (1981), and Weakley (2012).
Aquifoliaceae
| 1 | Leaves 1.5−3× as long as wide, ca. 2−3 cm wide, with a few, irregularly spaced, marginal spinose teeth, if present, spreading away from the leaf apex | Ilex coriacea Fig. 109 |
| – | Leaves 3−4× as long as wide, ca. 1 cm wide (almost never > 2 cm wide), crenate in the apical 1/2−1/3 of the leaf, marginal teeth pointing toward the leaf apex | Ilex glabra Fig. 110 |
Key adapted from Godfrey and Wooten (1981) and Weakley (2012).
Araliaceae
| 1 | Leaves not peltate | Hydrocotyle ranunculoides |
| – | Leaves peltate | 2 |
| 2 | Inflorescence umbellate, leaf blades 1–4 (–7) cm wide | Hydrocotyle umbellata Fig. 111 |
| – | Inflorescence verticillate, all flowers borne sessile to subsessile on the unbranched inflorescence axis; leaf blades 1–6 cm wide | Hydrocotyle verticillata Fig. 210 |
Key adapted from Godfrey and Wooten (1981) and Weakley (2012).
Note: The North Carolina Natural Heritage Program (North Carolina Natural Heritage Program 2014) and Carolina Vegetation Survey (Peet et al. 2013a, Peet et al. 2013b) reported Hydrocotyle umbellata from Lake Waccamaw; however, reproductive specimens were not encountered by the first author. Three species of Hydrocotyle are likely to occur in North Carolina Coastal Plain littoral communities. Two of the three posses peltate leaves. All material collected by the current author possessed peltate leaves and thus can be either H. umbellata or H. verticillata. Of these, H. umbellata is most likely to occur in this habitat, but as a precautionary measure, all three are included in the key below.
Hydrocotyle verticillata (digital photographs taken by Alexander Krings)
Figure 210a.

Peltate leaf
Figure 210b.

Infructescence
Figure 210c.

Infructescence
Figure 210d.

Infructescence
Asteraceae
| 1 | Plant a woody shrub, with obvious woody growth well above ground level | Baccharis halimifolia Fig. 112 |
| – | Plant an herb or twining vine, lacking obvious woody growth above ground level | 2 |
| 2 | Plant a twining vine; leaves opposite, bases cordate, margins coarsely toothed | Mikania scandens Fig. 121 |
| – | Plant an herb; leaves opposite, alternate, whorled, or basally disposed, bases and margins various | 3 |
| 3 | Plant exuding milky sap when cut or damaged; heads ligulate (containing only ligulate [ray] flowers) | 4 |
| – | Plant exuding clear sap when cut or damaged; heads discoid (only containing disc flowers) or radiate (with both ligulate [ray] and disc flowers in the same head) | 5 |
| 4 | Leaves completely basally disposed in a rosette, the flowering stem therefore being scapose (lacking leaves); involucre of 2 or more series of bracts; rays 1–1.5 cm long; cypselas beaked; pappus composed strictly of bristles | Hypochaeris radicata Fig. 119 |
| – | Leaves primarily basally disposed, sometimes a few leaves extending up the stem, these alternate; involucre of 1 series of bracts; rays 0.6–1 cm long; cypselas beakless, pappus composed of 5 bristles and 5 scales | |
| 5 | Leaves opposite or whorled (at least on the lower stem nodes) | 6 |
| – | Leaves alternate | 8 |
| 6 | Leaves whorled, 8−20 × 0.3−2 mm; inflorescence composed of a single, terminal, pink, discoid head; plants no more than 45 cm tall, mat-forming | Sclerolepis uniflora Fig. 123 |
| – | Leaves opposite (at least on lower stem nodes, sometimes becoming alternate distally), leaves > 20 × > 2 mm; inflorescence composed of more than one head, heads discoid or radiate, not pink; mature plants > 45 cm tall, erect, not mat-forming | 7 |
| 7 | Plant an annual; heads radiate, borne singly or in ± corymbiform arrays, rays yellow; leaves simple, (20−) 50−100 (−160+) × (5−) 10−25 (−40+) mm, sessile; phyllaries 8−12, ovate to obovate to lance-oblong, (4−) 6−8 (−10+) mm, tips orange to purplish; disc florets (25−) 60−100 (−150+); cypselae 6−10 mm, pappi of 2−4 retrorsely barbed awns, 3−5 mm long | Bidens laevis Fig. 113 |
| – | Plant a perennial; heads discoid, corymbiform or paniculiform arrays, corollas white; leaves simple or pinnate/pinnatifid, 5−100 × 0.2−10 (−15) mm, sessile; phyllaries 8−10, narrowly elliptic, 0.5−8 × 0.2−1.2 mm, tips green; disc florets 5; cypselae 1−3 mm, pappi of 20−40 antrorsely barbed bristles, 2−5 mm long | Eupatorium |
| 8 | Heads discoid, phyllaries pink, disc corollas rose-pink; stems, leaves, and phyllaries stipitate to sessile glandular (sometimes viscid) | Pluchea baccharis Fig. 122 |
| – | Heads radiate, phyllaries green, disc corollas yellow; stems, leaves, and phyllaries eglandular | 9 |
| 9 | Ray florets yellow | 10 |
| – | Ray florets white | 11 |
| 10 | Leaves on the middle to distal portions of the stem linear, 24−70 × 1−3 mm, bases attenuate, if sessile, not clasping the stem, abundantly gland-dotted, scabro-villous on mid-nerves; heads corymbose, ray florets 7−17 (−25), disc florets 3−22, corollas 3.3−4.8 mm long; stems sparsely pubescent, 2.5−10 dm tall | Euthamia caroliniana Fig. 118 |
| – | Leaves on the middle to distal portions of the stem lanceolate-ovate to ovate-oblong, larger leaves 35−120 × 8−35 mm, bases auriculate, broad and more or less clasping, hirsuto-villous on the midnerves, not gland-dotted; heads paniculate, ray florets (2−) 4−10, disc florets (2−) 4−7, corollas 4−5 mm long; stems conspicuously hirsute, 5−15 dm tall | Solidago fistulosa Fig. 124 |
| 11 | Leaves cauline, linear to lanceolate, 2−22 × 0.2−3 cm, not fleshy thickened; heads 50−100; ray florets 8−20 mm long; involucres 2.4−3.8 × 3.7−8.7 mm; cypselae obovoid, 1−3 mm, pappi comprised of 9 or 18 awns, (0−) 0.4−1.2 mm long; plants 3−20 dm tall, stoloniferous | Boltonia asteroides var. glastifolia Fig. 114 |
| – | Leaves mostly basal, narrowly to broadly oblanceolate to spatulate, 2−10 (−15+) × 0.4−2.5 cm, more or less fleshy thickened; heads (1−) 4−20 (−25); ray florets 5−10 mm long; involucres 3−4 ×5−11 mm; cypselae subterete, 1.2−1.6 mm, pappi comprised of setae (outer) and 16−25 bristles (inner), bristles 2.5−3.3 mm long; plants 1.5−5 dm tall, rhizomatous or fibrous-rooted | Erigeron vernus Fig. 115 |
Key adapted from Radford et al. (1968), Godfrey and Wooten (1981), Barkley et al. (2006), and Weakley (2012).
Betulaceae
| 1 | Buds stalked; pistillate catkins becoming hard and woody, forming a persisting cone-like catkin that persists through the winter and into the next growing season; plant a shrub, < 4 m tall; bark tight, not sloughing away from trunk; leaves 3-ranked, blades 5−10 cm × 2.5−5 cm, obovate, elliptic, or oblong, margins entire to serrulate | Alnus serrulata Fig. 125 |
| – | Buds sessile; pistillate catkins not becoming woody or hard and not persisting through the winter and into the next season; plant a tree, > 10 m tall; bark loose, sloughing away from trunk, usually with the consistency of paper; leaves 2-ranked, blades 3−10 cm × 1.5−3 cm, ovate-triangular or sub-rhombic, margins coarsely doubly serrate to dentate | Betula nigra Fig. 126 |
Key adapted from Radford et al. (1968), Godfrey and Wooten (1981), and Weakley (2012).
Cabombaceae
| 1 | Leaves floating only, blades elliptic, 3.5–11 × 2–6.5 cm, peltate; submersed plant parts coated with a layer of transparent mucilage | Brasenia schreberi Fig. 128 |
| – | Leaves floating and submersed, blades of floating leaves elliptic, 0.6–3 × 0.1–0.4 cm, peltate, blades of submersed leaves dichotomously divided into linear segments; submersed plant parts not coated with mucilage | Cabomba caroliniana Fig. 129 |
Key adapted from Godfrey and Wooten (1981) and Weakley (2012).
Campanulaceae
| 1 | Plant perennial; stems 7−10 dm tall; stem leaves linear to narrowly oblanceolate, 8−15 × 0.5−0.8 cm, margins callose glandular (sometimes not), often with short translucent trichomes on or near the margins; subtending bracts shorter than or exceeding the pedicels in length; corollas (including hypanthium) 18−33 mm long, fenestrate (with a slit or window on each side of the corolla tube at the base); plant of seasonally wet to inundated soils | Lobelia glandulosa |
| – | Plant annual; stems 2−7.5 dm tall, stem leaves lanceolate to linear, 1−3.5 × 0.1−0.4 cm, margins callose (not glandular), lacking short transluscent trichomes on or near the margins; subtending bracts shorter than or rarely equaling the pedicels in length; corollas (including the hypanthium) 8−14 mm long, not fenestrate; plant of various savnna-like habitats, and occassionally in wetter soils, but never found in inundated soils or wetlands | Lobelia nuttallii Fig. 130 |
Key adapted from Godfrey and Wooten (1981) and Weakley (2012).
Ericaceae
| 1 | Ovary inferior; fruit a berry | Vaccinium |
| – | Ovary superior; fruit a capsule | 2 |
| 2 | Leaves evergreen, blades coriaceous, adaxial surface either dark green and shiny or dull olive green and lepidote (covered with small, white or yellowish scurfy scales) | 3 |
| – | Leaves deciduous, blades membranous or subcoriaceous, deciduous, adaxial surface light to dark green, dull, not lepidote | 4 |
| 3 | Twig and leaf blade surfaces prominently lepidote, adaxial leaf surface dull olive green, lacking a prominent perimarginal vein | Chamaedaphne calyculata Fig. 136 |
| – | Twig and adaxial leaf blade surfaces glabrous, not lepidote, adaxial leaf surface dark green and shiny, larger leaves with a prominent perimarginal vein ca. 1 mm from blade margin | Lyonia [Lyonia lucida Fig. 139] |
| 4 | Leaves predominantly obovate or oblanceolate, margins distinctly long-ciliate; corolla funnelform, lobes > 10 mm long; capsule elongate, > 2 × as long as broad, 7–23 mm long | Rhododendron viscosum var. serrulatum Fig. 140 |
| – | Leaves various, margins not long-ciliate; corolla urceolate, campanulate, or globose, lobes < 5 mm long, capsule oblate (spheroidal, but flattened apically and basally), ovoid, globose, or subglobose, nearly as broad as long or broader, 2–6.5 mm long | 5 |
| 5 | Leaf margins crenate; corolla campanulate; capsule oblate | Zenobia pulverulenta Fig. 143 |
| – | Leaf margins spinulose-serrate, serrulate, or entire; corolla urceolate or globose; capsule ovoid, globose, or subglobose | 6 |
| 6 | Leaf margins spinulose-serrate; inflorescence of racemes produced along stems of previous year; capsules not thickened and whitish along sutures; seeds 5–10 per capsule | Eubotrys racemosa Fig. 137 |
| – | Leaf margins entire to minutely serrulate; inflorescence of terminal panicles produced on stems of current year, proximal inflorescences often with conspicuous leaf-like bracts; capsules thickened and whitish along sutures; seeds 100–300+ per capsule | Lyonia ligustrina Fig. 138 |
Key adapted from Tucker (2009b) and Weakley (2012).
Lyonia Nutt.
| 1 | Leaves deciduous, blades subcoriaceous, dull, lacking a prominent perimarginal vein, margins serrulate; corollas urceolate 2–4(−4.5) mm long; calyx lobes 0.5–1.5 mm long | Lyonia ligustrina var. foliosiflora Fig. 138 |
| – | Leaves evergreen, blades coriaceous, shiny, with a prominent perimarginal vein, leaf margins entire; corollas cylindric 5–14 mm long; calyx lobes 2–9.5 mm long | Lyonia lucida Fig. 139 |
Key adapted from Judd (2009) and Weakley (2012).
Vaccinium L.
| 1 | Twigs of the year glabrous; leaves glabrous below, margins eciliate; berries blue | Vaccinium formosum Fig. 141 |
| – | Twigs of the year pubescent; leaves pubescent below, margins ciliate; berries black | Vaccinium fuscatum Fig. 142 |
Key adapted from Weakley (2012).
Hypericaceae
| 1 | Petals flesh-colored to pink; stamens in 3 fascicles, each fascicle containing 3 stamen; 3 orange staminodial glands alternating with the 3 fascicles of stamen | 2 |
| – | Petals yellow, stamens few, not in fascicles; orange staminodial glands lacking | 3 |
| 2 | Leaves sessile, clasping the stem, cordate or subcordate at the base, mostly 2−7 × 1−3 cm; sepals 5−8 mm long at maturity, acute to acuminate; filaments united basally; styles 1.8−3 mm long | Hypericum virginicum Fig. 151 |
| – | Leaves petiolate (at least the lower), not clasping the stem, cuneate at the base, up to 15 × 3.5 cm; sepals 3−5 mm long at maturity, apices obtuse; filaments united to above the middle; styles 1.5−3 mm long | Hypericum walteri Fig. 152 |
| 3 | Leaf blades lanceolate to linear, 1−3-nerved, 6−30 mm long, bases attenuate to cuneate, not clasping, apices blunt to acute; petals 5, 6−8 mm long; capsules purplish, slightly exceeding the calyx | Hypericum canadense Fig. 149 |
| – | Leaf blades ovate, elliptic, lanceolate, 5-nerved, 10−50 mm long, bases broad, sometimes clasping, apices rounded to blunt; petals 5, 2−3 mm long; capsules not purplish, equaling the calyx | Hypericum mutilum var. mutilum Fig. 150 |
Key adapted from Godfrey and Wooten (1981) and Weakley (2012).
Lentibulariaceae
| 1 | Plants aquatic, floating unattached in water (sometimes stranded on top of soil by receding water levels); bladders 0.7–5 mm long, mostly > than 1.0 mm long; seeds 0.5–2 mm long | 2 |
| – | Plants terrestrial, attached to soil (principal branches within the soil); bladders 0.2–1.1 mm long, mostly < 1.0 mm long; seeds 0.2−0.25 mm long | 5 |
| 2 | Flowers purple; leaves divided into verticillate segments with terminal traps | Utricularia purpurea Fig. 159 |
| – | Flowers yellow; leaves divided into alternate segments with lateral traps; upper corolla lip larger than the lower, obscurely 3–lobed | 3 |
| 3 | Plant exhibiting vegetative shoots of two types, some bearing leafy segments with few or no traps, others bearing reduced segments and many traps; seeds 1.0–2.5 mm long, with an irregularly deeply lobed or partial wing (plant of shallow water or left stranded on soil surface after receding water levels) | Utricularia striata Fig. 161 |
| – | Plant exhibiting uniform vegetative shoots, all bearing sparsely divided leaf segments with traps; seeds 0.8–1.1 mm long, with a continuous, circumferential wing, slightly to irregularly lobed | 4 |
| 4 | Lower corolla lip 8–10 mm long, equaling or slightly shorter than the conical, 5–9 mm long spur; leaves usually forked twice | [Utricularia biflora Fig. 211] |
| – | Lower corolla lip 5–6 mm long exceeding the blunt 3.5–4.5 mm long spur; leaves usually forked once | Utricularia gibba Fig. 158 |
| 5 | Corolla rose pink; inflorescence 1 (−2)-flowered; bract at base of pedicel tubular, attached circumferentially around stem; aerial leaves (when present) terete, septate | Utricularia resupinata Fig. 160 |
| – | Corolla yellow (sometimes fading white); inflorescence (1−) 2−15-flowered; bract at base of the pedicel peltate or ovate, attached on one side of the stem; aerial leaves (when present) flattened, not septate | 6 |
| 6 | Pedicels subtended by a single ovate (attached at base) bract; pair of bracteoles present, bracteoles linear to lanceolate, a little longer than the bract; corolla spur oriented downward or backward, at right angle to lower corolla lip | Utricularia cornuta Fig. 157 |
| – | Pedicels subtended by a single peltate (attached in middle) bract, unattached at either end; pair of bracteoles absent; corolla spur oriented forward, essentially appressed to lower corolla lip; aerial leaves (when present) with subacute or obtuse apices | Utricularia subulata Fig. 162 |
Key adapted from Godfrey and Wooten (1981), Taylor (1989), and Weakley (2012).
Note: Traditionally, in the southeastern United States, U. biflora and U. gibba have been recognized as distinct species (Radford et al. 1968). However, Radford et al. (1968) described the two as “doubtfully distinct” and neither Godfrey and Wooten (1981), nor Taylor (1989), recognized a distinction. Here, we follow Weakley (2012) in provisionally recognizing the two species as distinct, pending a world-wide revision. During the present work, only U. gibba was encountered in the field, but U. biflora (bracketed in key below) is keyed here due to its morphological similarity, overlapping range, and similar habitat requirements.
Utricularia biflora (digital photographs taken by Alexander Krings)
Figure 211a.
Flower
Figure 211b.
Flower, showing petal exceeding spur
Melastomataceae
| 1 | Sepal lobes aristate, awn tip 0.5–1.5 mm long, hairs 3–5 mm long, yellow, stiff | Rhexia aristosa Fig. 167 |
| – | Sepal lobes obtuse to acuminate, not aristate, hairs < 3 mm long, neither yellow nor stiff | 2 |
| 2 | Leaves linear or linear-elliptic, 1– 5(−8) mm wide | 3 |
| – | Leaves lanceolate, elliptic, or ovate, (5−) 7–20 (−35) mm wide | 4 |
| 3 | Petals lavender–rose, (1−) 1.5−2 (−2.5) cm long; mature hypanthium 10−14 mm long, hairs glandular; marginal nerves of leaf abaxial surface absent or obscure and discontinuous; anthers 7−10 mm long | Rhexia cubensis Fig. 168 |
| – | Petals white to pink, (0.7−) 0.9−1.4 cm long; mature hypanthium 6−10 mm long, glabrous or hairs glandular; marginal nerves of leaf abaxial surface prominent; anthers 5−8 mm long | Rhexia mariana var. exalbida Fig. 169 |
| 4 | Four stem faces at mid–stem noticeably unequal, one pair of opposite faces broader, convex, darker green, the narrower pair concave or flat, pale, arrangement of broader and narrower faces alternating at each internode up the stem, angles at midstem not winged | Rhexia nashii Fig. 170 |
| – | Four stem faces at mid–stem about equal, almost flat, angles at midstem conspicuously winged | Rhexia virginica Fig. 171 |
Key adapted from Radford et al. (1968) and Weakley (2012).
Nymphaeaceae
| 1 | Perianth globose at anthesis, 2–5 cm diam.; margin of stigmatic disk crenate to dentate; leaves linear to lanceolate 15−30 (−50) × 5−10 (−11.5) cm, green both adaxially and abaxially, venation essentially pinnate, often of two types, submersed leaves (when present) thinner in texture than floating or emersed leaves, 60–90% of surface area of floating or emersed leaves with vasculature derived from the midrib; sepals 6, green to yellow, petaloid; petals inconspicuous, yellow, stamen-like, shorter than the sepals; rhizome with triangular or winged leaf scars | Nuphar sagittifolia Fig. 175 |
| – | Perianth spreading at anthesis, 4–20 cm diam.; margin of stigmatic disk with prominent, distinct, upwardly incurved appendages; leaves ovate to orbiculate (5−) 10−40 × (5−) 10−40 cm, green adaxially and deep reddish-purple abaxially, venation essentially palmate, of one type, floating, 25–40% of surface area with vasculature derived from the midrib; sepals 4, greenish or reddish tinged, not petaloid; petals showy, white to pink, distinctly longer than the sepals; rhizomes with circular leaf scars | Nymphaea odorata var. odorata Fig. 176 |
Key adapted from Godfrey and Wooten (1981) and Weakley (2012).
Nyssaceae
| 1 | Petioles of mature leaves 3−6 cm long; mature leaf blades exceeding 10 cm long, margins with a few irregular teeth; drupes ≥ 20 mm long | Nyssa aquatica Fig. 177 |
| – | Petioles of mature leaves < 3 cm long; mature leaves ≤ 10 cm long, margins lacking irregular teeth; drupes 10−15 mm long | Nyssa biflora Fig. 178 |
Key adapted from Godfrey and Wooten (1981) and Weakley (2012).
Onagraceae
| 1 | Leaves opposite, oblanceolate to elliptic, 8−20 mm; pedicels conspicuous, 5−16 mm long; petals 4, 4−5 mm long, slightly larger than the calyx segments; capsule obconical, 6−8 mm long, slightly quadrangular in cross-section, curved; seed coat with rectangular reticulations; plants pubescent with short hooked hairs; plant creeping and rooting at the nodes | Ludwigia brevipes Fig. 180 |
| – | Leaves alternate, lanceolate to linear-lanceolate, 3−10 cm; pedicels 0−1 mm long; petals lacking; capsules subglobose, 2.5−4.5 mm long, terete in cross-section or with broadly rounded lobes; seed coat with square reticulations, pentagonal or circular; plants glabrous to slightly pubescent, if pubescent, then hairs not hooked; plant erect and ascending, not rooting at the nodes | Ludwigia sphaerocarpa Fig. 181 |
Key adapted from Weakley (2012).
Plantaginaceae
| 1 | Plant a true aquatic, forming extensive mats in shallow water; plant parts spicy aromatic when crushed; stems lax, fleshy, semi-succulent, pubescent; leaves of the flowering stem 55−28 × 7–15 mm, opposite, ovate to widely elliptic, with 3−7 palmate veins; inflorescence composed of a single axillary flower; corolla bluish-purple, 9–11 mm long, not spurred, orifice distinct | Bacopa caroliniana Fig. 182 |
| – | Plant terrestrial, usually found at the upper margins of the high water mark in moist sandy soil; plant parts not aromatic when crushed; stems not lax, fleshy or semi-succulent, nor pubescent; leaves of the flowering stem 5–20 × 1−3.5 mm, alternate, linear < 3 veins; inflorescence a terminal raceme; corolla bluish-purple and white, 5–15 mm long (including the spur), spurred, orifice obscured | Nuttallanthus canadensis Fig. 183 |
Key adapted from Radford et al. (1968) and Weakley (2012).
Rosaceae
| 1 | Leaves simple; fruit a pome | 2 |
| – | Leaves compound; fruit achenes enclosed within a hip or an aggregate of drupelets | 4 |
| 2 | Inflorescence corymbose; adaxial surface of leaves with dark glandular trichomes along the midrib, leaf margins finely serrate, teeth tipped with red glands; mature fruit red | Aronia arbutifolia Fig. 191 |
| – | Inflorescence racemose; adaxial surface of leaves lacking glandular trichomes, leaf margins serrate, teeth tips lacking red glands; mature fruit blue to purple [Amelanchier] | 3 |
| 3 | Plant a small shrub or tree, 8–20 m tall, not rhizomatous; pedicels of varying lengths, the longest > 1 cm long; petals 6–12 mm long | Amelanchier canadensis Fig. 189 |
| – | Plant a small shrub, 0.2–2.5 m tall, rhizomatous; pedicels nearly uniform in length, usually < 1 cm long; petals 5.9–7.7 mm long | Amelanchier obovalis Fig. 190 |
| 4 | Leaves odd-pinnately compound, leaflet margins usually crenulate to serrulate; fruit a hip, developing from an urceolate hypanthium, enclosing the ovaries and achenes except for the apical orifice | Rosa palustris Fig. 192 |
| – | Leaves palmately compound, leaflet margins usually serrate to doubly serrate; fruit an aggregate of drupelets, developing from a flatish to hemispheric hypanthium, ovaries and druplets exposed, not borne inside a hypanthium | Rubus pensilvanicus Fig. 193 |
Key adapted from Godfrey and Wooten (1981) and Weakley (2012).
Rubiaceae
| 1 | Plant woody, a shrub or small tree; inflorescence in dense globose heads; corolla narrowly infundibuliform, white, lobes 4, shorter than the tube; length of exerted style ca. 3× or more the length of a corolla lobe | Cephalanthus occidentalis Fig. 194 |
| – | Plant herbaceous; inflorescence of solitary or few-flowered, axillary cymes; corolla salverform to subcampanulate, white, lobes 3−4; length of exerted style ca. 1× the length of a corolla lobe or less | 2 |
| 2 | Plant pubescent to glabrous, erect or spreading; stem often with a reddish tinge; leaves opposite, elliptic-lanceolate to oblanceolate, 2−7 cm × 4−12 mm; flowers sessile, borne in leaf axils, 1 (−2) per axil; corolla salverform, 7−9 mm long, lobes 4, 3−4 mm long, inner surface pubescent; fruit oblong-ellipsoid, pubescent, 3−5 mm wide, prominently ridged | Diodia virginiana Fig. 195 |
| – | Plant glabrous, erect or spreading; stem lacking a reddish tinge; leaves whorled, 4 per node, linear-obovate, 8−20 mm × 1.5−4 mm; flowers in branched terminal and axillary cymes, 1−3-flowered; corolla subcampanulate, corolla lobes 3−4, < 3 mm long, inner surface glabrous; fruit orbicular, glabrous, 2.5−4 mm wide, smooth, not ridged | Galium obtusum var. obtusum Fig. 196 |
Key adapted from Godfrey and Wooten (1981) and Weakley (2012).
Salicaceae
| 1 | Buds scales imbricate; leaf blades ovate, < 3 × as long as broad, bases truncate to broadly rounded, slightly cordate; inflorescences pendulous; stamens 5−80 | Populus heterophylla Fig. 197 |
| – | Bud scales 1; leaf blades lanceolate, > 3 × as long as broad, bases cuneate, not cordate; inflorescences erect or spreading; stamens 1−9 | 2 |
| 2 | Mature leaf undersides glaucous, glabrous to sparsely pubescent, blades (4−) average 7.5 (−13) × as long as wide; stipules usually prominent and persisting, to 15 mm long | Salix caroliniana Fig. 198 |
| – | Mature leaf undersides green, not glaucous, glabrous, blades (4−) average 9 (−16) × as long as wide; stipules not persisting, to 12 mm long | Salix nigra Fig. 199 |
Key adapted from Godfrey and Wooten (1981), Argus et al. (2010), and Weakley (2012).
Sapindaceae
| 1 | Leaves palmately compound; fruit a capsule | Aesculus pavia var. pavia Fig. 203 |
| – | Leaves simple; fruit a schizocarp composed of two 1-seeded samaras | 2 |
| 2 | Leaves (3−) 5 (−9) lobed, central lobe 4−8 cm long, upper two lateral lobes 2−5 cm long, bases generally cordate | Acer rubrum var. rubrum Fig. 201 |
| – | Leaves (0−) 3 (−5) lobed, central lobe 1−5 cm long, upper two lateral lobes (if leaves more than 3-lobed) 0.5−2 (−3) cm long, bases cuneate to rounded to subcordate | Acer rubrum var. trilobum Fig. 202 |
Key adapted from Radford et al. (1968) and Weakley (2012).
Vitaceae
| 1 | Leaves simple, leaf margins prominently dentate-serrate throughout, bases cordate; tendrils unbranched, lacking adhesive pads | Muscadinia rotundifolia Fig. 207 |
| – | Leaves palmately compound, leaflet margins coarsely serrate above the middle, entire below middle, bases cuneate; tendrils branched, bearing adhesive pads at the tips | Parthenocissus quinquefolia Fig. 208 |
Key adapted from Radford et al. (1968) and Weakley (2012).
Supplementary Material
Carolina bay lakes literature
Nathan Howell
Data type: references
Brief description: List of citations regarding Carolina bay lakes
File: oo_72560.doc
Floras, manuals, guides, and broader floristic works on site-specific and broad-scale aquatic/wetland habitats of the eastern United States.
Nathan Howell
Data type: references
Brief description: List of some floras, manuals, guides, and broader floristic works aquatic/wetland habitats of the eastern United States that may be of interest to readers.
File: oo_72561.doc
Sample taxon entry with brief descriptions of working parts.
Nathan Howell
Data type: taxon entry components and definitions
File: oo_72562.doc
Literature highlighting the ecological, biological, and cultural importance of Carolina bays.
Nathan Howell
Data type: references
File: oo_72563.doc
Suggested collection methods for problematic aquatic taxa and sampling methods of floating bog communities.
Nathan Howell
Data type: collection suggestions
File: oo_72564.doc
Checklist of the littoral zone vascular flora of unaltered Carolina bay lake shorelines (i.e., Bakers Lake, Bay Tree Lake, Horseshoe Lake, Jones Lake, Lake Waccamaw, Little Singletary Lake, Salters Lake, Singletary Lake).
Nathan Howell
Data type: occurrences
Brief description: Taxa are organized by major plant groups (i.e., pteridophytes, gymnosperms, basal angiosperms, magnoliids, monocotyledons, and eudicotyledons), then alphabetically by family, genus, and species. Parentheses around a taxon indicate that it is not vouchered (i.e., it has been reported by state agencies or has been observed by the first author, but has not been collected as a voucher specimen; see text for details). For taxa collected from Carolina bay lake littoral zones by the present author, abundance estimates sensu Palmer et al. (1995) are provided. Abundance estimates in this checklist reflect the abundance in which the taxa occur within each lake. Status and rank designations are also provided for rare taxa monitored by the NC Natural Heritage Program (Robinson and Finnegan 2014). The term “restricted” is used here only to indicate the presence of a taxon within a particular lake among all those surveyed and not in a global sense (e.g., a taxon here considered restricted to Lake Waccamaw has not been found in the other lakes surveyed, but may exist in other localities in the state or country). A = Abundant; F= Frequent; I=Infrequent; O = Occasional; R = Rare; = þ restricted to lake indicated; () = not vouchered (i.e., reported by state agencies or observed by the present author, but not collected as a voucher specimen; see text for details); H = taxon has been collected and vouchered in the past but not by the present author. BALA = Bakers Lake; BATR = Bay Tree Lake; HOLA = Horseshoe Lake; JOLA = Jones Lake; LAWA = Lake Waccamaw; LISI = Little Singletary; SALA = Salters Lake; SILA = Singletary Lake.
File: oo_72565.doc
Provisional checklist of the littoral zone vascular flora from White Lake based on historical vouchers, personal observations, and literature reviews.
Nathan Howell
Data type: occurrences
Brief description: This checklist does not represent a complete inventory of this locality, but rather serves as a baseline for future research. Taxa are arranged by major groups (i.e., gymnosperms, magnoliids, monocotyledons, and eudicotyledons), then alphabetically by family, genus, and species. Basal angiosperms and pteridophytes were not represented by vouchers, observations, or reports and are therefore not included in the following checklist. Brackets around a taxon indicate that it is unvouchered (i.e., it has been reported by outside agencies or has been observed by the present author, but has not been collected). Status and rank designations are also provided for rare taxa monitored by the NC Natural Heritage Program (Robinson and Finnegan 2014).
File: oo_82955.doc
Climate data supporting Fig 15 (Walter climate diagrams)
Nathan Howell
Data type: climate
Brief description: Monthly mean temperature and precipitation data for Bladen and Columbus County.
File: oo_75216.xlsx
Data supporting Fig 16 (Distribution of plant habit across all Carolina bay lakes)
Nathan Howell
Data type: morphological
Brief description: Counts of the number of taxa in the categories of herb, tree/shrub, and vine for each Carolina bay lake flora.
File: oo_75217.xlsx
Data supporting Fig 17 (The thirteen most species-rich vascular plant families across all Carolina bay lakes)
Nathan Howell
Data type: taxonomic
Brief description: Counts of the number of taxa in each of the thirteen most species-rich vascular plant families in each Carolina bay lake flora.
File: oo_75218.xlsx
Howell specimen collections
Nathan Howell
Data type: occurrences
Brief description: Comma delimited file of occurrence data (DwC) for the specimens collected by the first author from Carolina Bay Lakes. Precise locality data has been redacted for species of conservation concern. Specimens are deposited at NCSC. Images are available through http://sernecportal.org.
File: oo_83035.csv
Acknowledgements
NH, AK, RB: We thank D. Cicuzza, W. Hoffmann, and T.R. Wentworth for their critical review of a previous version of this manuscript. We also thank D. Gamble (UNCW) for permission to re-use Fig. 1 from T.E. Ross's article "Pocosins and Carolina Bays Compared" (The North Carolina Geographer 11: 22−32, 2003) and J. Mickel (NY) for permission to re-use an illustration of Dryopteris ludoviciana.
NH: I thank the individuals and organizations that directly had a hand in the completion of this project; without their expertise, opinions, edits, financial assistance, and permission to access private property, this work would not have been possible.
The North Carolina State Parks graciously allowed me to collect plants from the shorelines of five Carolina bay lakes in Bladen County, North Carolina. The North Carolina Wildlife Resources Commission was kind enough to grant access to Horseshoe Lake and Little Singletary Lake. I thank Glenn and Carol Lewis for offering their land as an easement to Little Singletary Lake; they were very gracious, and I thoroughly enjoyed listening to Glenn’s stories of Native American artifacts, lake history, black bears, and wildfires.
Dr. Clemuel Johnson graciously gave permission to survey Bakers Lake Natural Area and I am very thankful for his generosity. Stephen Clark, son-in-law of Dr. Clemuel Johnson, also provided valuable information regarding wildlife use of Bakers Lake and surrounding natural areas. “Chick” Gaddy provided valuable information concerning Carolina bays and associated South Carolina natural community types. Mr. Gaddy’s enthusiastic disposition and knowledge of South Carolina ecosystems was very beneficial to this study and I am truly grateful for his time. Garrett German provided a wealth of information concerning waterfowl use of Carolina bay lakes. Rob Richardson and Justin Nawrocki of the NCSU Crop Science Department provided a list of several plant species found from Lake Waccamaw that added greatly to this work.
I am deeply indebted to the North Carolina Native Plant Society and the Society of Herbarium Curators. These two organizations were kind enough to provide funding for this research. Without their financial assistance, my wallet would surely be a little lighter. Ed Corey has helped me immeasurably through the years and I am deeply indebted to him. Dr. Jon Stucky has been a true pal and never once hesitated to reply to my numerous – sometimes assuredly annoying − emails concerning plant identifications. My girlfriend Morgan Kirby has stuck by my side through this project and on several occassions has been swindled into mounting plant specimens; for that, she deserves an award for her patience and understanding.
Colter Chitwood has been a loyal friend, editor, and dog sitter. I don’t know what I would have done without him. I wish him the very best in his future travels and research. Maybe we can meet on the Madison one of these days. I would also like to thank past and present floristics students at North Carolina State University. Robert Thornhill captivated me with his exuberant passion for North Carolina’s diverse Coastal Plain flora and encouraged me to pursue a flora of my own. To the kind gentleman who gave me a ride to Bay Tree Resorts after my boat was taken by devilish winds of Bay Tree Lake on the morning of July 9, 2014, THANK YOU! Drs. Layne Huiet and Bob Wilbur of DUKE and CarolAnn McCormick of NCU helped tremendously with herbarium crawls. Those herbaria can get quite lonely, and having a conversation with someone is worth its weight in gold.
References
- Adanson N., Rafinesque U., Rafinesque U. Pontederia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Allen D., Carpenter J., Clark R., Fisk M., Hall J., Hamlett M., Harrold I., Hughes T., Johnstone B., McConnell J., Olfenbuttal C., Padgett T., Phillips C., Shughart K., Stanford E., Strope B., Turner D., Ward C., Wilson B. Suggs Mill Pond Game Land Management Plan. North Carolina Wildlife Resources Commission; Raleigh: 2015. 255 [Google Scholar]
- Andrews E. G., Windham M. D. Pleopeltis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Argus G. W., Eckenwalder J. E., Kiger R. W. Salicaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2010. [Google Scholar]
- Arrhenius O. Species and area. Journal of Ecology. 1921;9(1):95–99. doi: 10.2307/2255763. [DOI] [Google Scholar]
- Ball P. W., Reznicek A. A. Carex . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Ball P. W., Reznicek A. A., Murray D. F. Cyperaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Ball P. W., Reznicek A. A., Murray D. F. Salicaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Barkley T. M., Brouillet L., Strother J. L. Key to Asteraceae tribes. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Barkworth M. E. Paniceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2003. [Google Scholar]
- Barkworth M. E. Andropogoneae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2003. [Google Scholar]
- Barkworth M. E. Poaceae: Key to tribes. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2007. [Google Scholar]
- Bartram J., Harper F. Diary of a journey through the Carolinas, Georgia, and Florida from July 1, 1765, to April 10, 1766. Transactions of the American Philosophical Society. 1942;33(1):1–120. doi: 10.2307/1005551. [DOI] [Google Scholar]
- Bennett S. H., Nelson J. B. Distribution and status of Carolina Bays in South Carolina. South Carolina Wildlife and Marine Resources Department, Nongame and Heritage Trust Publications.; Columbia: 1991. 98 [Google Scholar]
- Biggs J, Williams P., Whitfield M., Nicolet P., Weatherby A. 15 years of pond assessment in Britain: results and lessons learned from the work of pond conservation. Aquatic Conservation: Marine and Freshwater Ecosystems. 2005;15(6):693–714. doi: 10.1002/aqc.745. [DOI] [Google Scholar]
- Bogler DJ. Hypochaeris . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Bornstein AJ. Myricaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Brinson MM. Landscape properties of pocosins and associated wetlands. Wetlands. 1991;11(1):441–465. doi: 10.1007/bf03160761. [DOI] [Google Scholar]
- Britton NL, Brown A. An illustrated flora of the northern United States, Canada, and the British Possessions, 3 vols. Scribner; New York: 1913. 1726 [Google Scholar]
- Brönmark C., Hansson LA. The biology of lakes and ponds. Oxford University Press; New York: 2005. 304 [Google Scholar]
- Brooks RE, Clemants SE. Juncus . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2000. [Google Scholar]
- Brusnyk LM, Gilbert FF. Use of shoreline timber reserves by moose. Journal of Wildlife Management. 1983;47(3):673–685. doi: 10.2307/3808603. [DOI] [Google Scholar]
- Campbell CS. Andropogon . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2003. [Google Scholar]
- Casterlin ME, Rynolds WW, Lindquist DG, Yarborough CG. Algal and physiochemical indicators of eutrophication in a lake harboring endemic species: Lake Waccamaw, North Carolina. Journal of the Elisha Mitchell Scientific Society. 1984;100:83–103. [Google Scholar]
- Center for Aquatic and Invasive Plants University of Florida, IFAS. Line drawings. http://plants.ifas.ufl.edu/linedrawings
- Chambers KL, O'Kennon RJ. Krigia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Christensen NL. Vegetation of the southeastern Coastal Plain. In: Barbour MG, Billings WD, editors. North American terrestrial vegetation. Cambridge University Press; New York: 1999. [Google Scholar]
- Clark LG, Triplett JK. Arundinaria . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2007. [Google Scholar]
- Clemants SE. Amaranthaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2003. [Google Scholar]
- Connor EF, McCoy ED. The statistics and biology of the species-area relationship. American Midland Naturalist. 1979;113(6):791–833. doi: 10.1086/283438. [DOI] [Google Scholar]
- Covington AK, Bates RG, Durst RA. Definition of pH scales, standard reference values, measurement of pH and related terminology (Recommendations 1984). Pure and Applied Chemistry. 1985;57(3):531–542. doi: 10.1351/pac198557030531. [DOI] [Google Scholar]
- Cowardin LM, Carter V, Golet FC, LaRoe ET. Classification of wetlands and deepwater habitats of the United States. US Department of the Interior, Fish and Wildlife Service; Washington, DC: 1979. 90 [Google Scholar]
- Cranfill RB. Woodwardia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Crow GE. Species diversity in aquatic angiosperms: Latitudinal patterns. Aquatic Botany. 1993;44:229–258. doi: 10.1016/0304-3770(93)90072-5. [DOI] [Google Scholar]
- Daniels RB, Kleiss HJ, Buol SW, Byrd HJ, Phillips JA. Soil systems in North Carolina. Technical Bulletin 314. North Carolina Agricultural Research Service; Raleigh: 1984. 118 [Google Scholar]
- Daniel TF. Sphenopholis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2007. [Google Scholar]
- Denny P. Permanent swamp vegetation of the Upper Nile. In: Dumont HJ, Moghraby AI el, Desougi LA, editors. Limnology and Marine Biology in the Sudan. Dr W Junk Publishers; The Hague: 1984. [Google Scholar]
- Denslow M. W., Palmer M. W., Murrell Z. E. A bibliography of North Carolina local floras. Castanea. 2010;75(4):475–483. doi: 10.2179/10-015.1. [DOI] [Google Scholar]
- Dodson SI, Arnott SE, Cottingham KL. The relationship in lake communities between primary productivity and species richness. Ecology. 2000;81(10):2662–2679. doi: 10.1890/0012-9658(2000)081[2662:trilcb]2.0.co;2. [DOI] [Google Scholar]
- Dorr L. J. Zenobia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Dudgeon D., Arthington A. H., Gessner M. O., Kawabata Z. I., Knowler D. J., Lévêque C., Naiman R. J., Prieur-Richard A., Soto D., Stiassny M. L.J., Sullivan C. A. Freshwater Biodiversity: importance, threats, status and conservation challenges. Biological Reviews. 2006;81(2):163–182. doi: 10.1017/s1464793105006950. [DOI] [PubMed] [Google Scholar]
- Durand T. Sagittaria . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2000. [Google Scholar]
- Eckenwalder J. E. Diospyros . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Eckenwalder J. E., Thieret J. W. Key to families of Gymnosperms. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Edmonds J. Oxford atlas of exploration. Oxford University Press; New York: 1997. 248 [Google Scholar]
- (ESRI) Environmental Systems Research Institute. ArcGis Desktop: Release 10.2.2. [2014-02-27T00:00:00+02:00];2014
- Ewert D. N., Hamas M. J., Smith R. J., Dallman M. E., Jorgensen S. W. Distribution of migratory landbirds along the northern Lake Huron shoreline. Wilson Journal of Ornithology. 2011;123(3):536–547. doi: 10.1676/09-122.1. [DOI] [Google Scholar]
- Eyles D. E. A phytosociological study of the Castalia-Myriophyllum community of Georgia Coastal Plain Boggy Ponds. American Midland Naturalist. 1941;26(2):421–438. doi: 10.2307/2420968. [DOI] [Google Scholar]
- Fabijan D. M. Chamaedaphne . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Faden R. B. Mayacaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2000. [Google Scholar]
- Findlay S. C., Houlahan J. Anthropogenic correlates of biodiversity in southeastern Ontario wetlands. Conservation Biology. 1997;11(4):1000–1009. [Google Scholar]
- Ford M. T., Flaspohler D. J. Scale-dependent response by breeding songbirds to residential development along Lake Superior. Wilson Journal of Ornithology. 2010;122(2):296–306. [Google Scholar]
- Freckmann R. W., Lelong M. G. Panicum . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2003. [Google Scholar]
- Freckmann R. W., Lelong M. G. Dichanthelium . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2003. [Google Scholar]
- Frey D. G. Morphometry and hydrography of some natural lakes of the North Carolina coastal plain: the bay lake as a morphometric type. Journal of the Elisha Mitchell Scientific Society. 1949;65(1):1–37. [Google Scholar]
- Frey D. G. Pollen succession in the sediments of Singletary Lake, North Carolina. Ecology. 1951;32(3):518–533. [Google Scholar]
- Frey D. G. The fishes of North Carolina’s bay lakes and their intraspecific variation. Journal of the Elisha Mitchell Scientific Society. 1951;67(1):1–44. [Google Scholar]
- Frey D. G. Evidence for the recent enlargement of the" Bay" lakes of North Carolina. Ecology. 1954;35(1):78–88. doi: 10.2307/1931408. [DOI] [Google Scholar]
- Frost P. C., Hicks A. L. Human shoreline development and the nutrient stoichiometry of aquatic plant communities in Canadian shield lakes. Journal of Fisheries and Aquatic Sciences. 2012;69(10):1642–1650. [Google Scholar]
- Furlow J. J. Betulaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Garren K. H. Effects of fire on vegetation of the southeastern United States. The Botanical Review. 1943;9(9):617–654. doi: 10.1007/bf02872506. [DOI] [Google Scholar]
- Gentleman Y. A new voyage to Georgia: By a young gentleman. Giving an account of his travels to South Carolina, and part of North Carolina. To which is added, a curious account of the Indians. By an honourable person. And a poem to James Oglethorpe, Esq; on his arrival from Georgia. Printed for J. Wilford, at the Three Flower de Luces, behind the Chapter-House, in St. Paul's Church-Yard; London: 1737. 62 [Google Scholar]
- Glaser P. H., Janssens J. A., Siegel D. I. The response of vegetation to chemical and hydrological gradients in the Lost River Peatland, Northern Minnesota. Journal of Ecology. 1990;78(4):1021–1048. doi: 10.2307/2260950. [DOI] [Google Scholar]
- Glenn L. C. Some notes on Darlington (SC), 'Bays'. Science. 1895;2(41):472. doi: 10.1126/science.2.41.472. [DOI] [PubMed] [Google Scholar]
- Godfrey R. K., Wooten J. W. Aquatic and wetland plants of southeastern United States. Monocotyledons. Vol. 1. University of Georgia Press; Athens: 1979. 712 [Google Scholar]
- Godfrey R. K., Wooten J. W. Aquatic and wetland plants of southeastern United States. Dicotyledons. Vol. 2. University of Georgia Press; Athens: 1981. 933 [Google Scholar]
- Goldman D. H., Magrath L. K., Catling P. M. Calopogon . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Hágsater E., Salisbury A., Rafinesque L., Rafinesque N., Small S., Rafinesque T. Epidendrum . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Haines A. Euthamia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Harvey M. J. Agrostis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2007. [Google Scholar]
- Haynes R. R. Najadaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2000. [Google Scholar]
- Haynes R. R. Hydrocharitaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2000. [Google Scholar]
- Heinonen P., Ziglio G., Beken A. Van der. Hydrological and limnological aspects of lake monitoring. Vol. 15. John Wiley & Sons; New York: 2008. 392 [Google Scholar]
- Henry R. D., Scott A. R. The wetland vascular flora of four seeps in McDonough County, Illinois. Phytologia. 1984;56(1):1–15. [Google Scholar]
- Herndon A. Hypoxis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Hitchcock A. S., Chase A. Manual of the grasses of the United States. United State Department of Agriculture; Washington: 1951. 1051 [Google Scholar]
- Holmes W. C. Smilax . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Holmes W. C. Mikania . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Hubbs C. L., Raney E. C. Endemic fish fauna of Lake Waccamaw, North Carolina. University of Michigan Press; Ann Arbor: 1946. 30 [Google Scholar]
- Hunt K. W. Mats on a southeastern Coastal Plain reservoir. Bulletin of the Torrey Botanical Club. 1943;70(5):481–488. doi: 10.2307/2481394. [DOI] [Google Scholar]
- Jensen R. J. Quercus . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Juday C., Birge E. A. The transparency, the color and the specific conductance of the lake waters of northeastern Wisconsin. Transactions of the Wisconsin Academy of Sciences, Arts, and Letters. 1933;28:205–259. [Google Scholar]
- Judd W. S. Lyonia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Judd W. S., Kron K. A. Rhododendron . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Karaman-Castro V., Urbatsch L. E. Boltonia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Kaul R. B. Platanaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Keddy P. A. Wetland Ecology: principles and conservation. Cambridge University Press; New York: 2010. 614 [Google Scholar]
- Keddy P. A., Fraser L. H. Four general principles for the management and conservation of wetlands in large lakes: the role of water levels, nutrients, competitive hierarchies and centrifugal organization. Lakes & Reservoirs: Research & Management. 2000;5(3):177–185. [Google Scholar]
- Kelley W. R., Batson W. T. An ecological study of the land plants and cold- blooded vertebrates of the Savannah River project area. PartVI. Conspicuous vegetational zonation in a “Carolina bay”. University of South Carolina Publication Series III. Biology. 1955;1:244–248. [Google Scholar]
- Kirkman L. K., Lide R. F., Wein G., Sharitz R. R. Vegetation changes and land-use legacies of depression wetlands of the western coastal plain of South Carolina: 1951–1992. Wetlands. 1996;16(4):564–576. doi: 10.1007/bf03161347. [DOI] [Google Scholar]
- Kral R. Pinus . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Kral R. Xyridaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2000. [Google Scholar]
- Kral R. Eriocaulon . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2000. [Google Scholar]
- Kral R. Rhynchospora . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Kral R. Fimbristylis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Kral R., Persoon V. Fuirena . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Lacoul P., Freedman B. Environmental influences on aquatic plants in freshwater ecosystems. Environmental Reviews. 2006;14(2):89–136. doi: 10.1139/a06-001. [DOI] [Google Scholar]
- Lamont E. E. Sclerolepis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Leab R. J. Soil survey of Bladen County, North Carolina. United States Soil Conservation Service; Washington, DC: 1990. 179 [Google Scholar]
- LeBlond R. J. Inventory of the natural areas and rare species of Columbus County, North Carolina. North Carolina Department of Environment, Health, and Natural Resources, Division of Parks and Recreation, Natural Heritage Program; Raleigh: 1995. 162 [Google Scholar]
- LeBlond R. J., Grant G. S. Natural area inventory of Bladen County, North Carolina. North Carolina Department of Environment and Natural Resources, Office of Conservation and Community Affairs, Natural Heritage Program; Raleigh: 2005. 212 [Google Scholar]
- LeGrand H. E., Ratcliffe J. A., Finnegan J. T. Natural heritage program list of the rare animal species of North Carolina. North Carolina Department of Environment and Natural Resources, Office of Land and Water Stewardship, Natural Heritage Program; Raleigh: 2014. 172 [Google Scholar]
- Lemke D. E. Cyrilla . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Lewis D. Q. Burmanniaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Louder D. E. An annotated check list of the North Carolina bay lakes fishes. Journal of the Elisha Mitchell Scientific Society. 1962;78:68–73. [Google Scholar]
- Luther H. E., Brown G. K. Tillandsia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2000. [Google Scholar]
- Lynch J. M., Peacock S. L. Natural areas inventory of Washington County, North Carolina. North Carolina Department of Natural Resources and Community Development, Office of Coastal Management, Coastal Energy Impact Program; Raleigh: 1982. 173 [Google Scholar]
- Martínez-y-Pérez José L., Mejía-Saulés Teresa, Sosa Victoria. A Taxonomic Revision of Luziola (Poaceae: Oryzeae) Systematic Botany. 2008;33(4):702–718. doi: 10.1600/036364408786500226. [DOI] [Google Scholar]
- Mastrogiuseppe J. Dulichium . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Mastrogiuseppe J., Rothrock P. E., Dibble A. C., Reznicek A. A. Carex sect. Ovales. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Mellichamp T. L., Chase F. W. Sarracenia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Melton F. A., Schriever W. The Carolina" Bays": Are they meteorite scars? Journal of Geology. 1933;41(1):52–66. [Google Scholar]
- Meyer F. G. Hamamelidaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Meyer F. G. Magnoliaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Michener D. C. Chamaecyparis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Mickel J. T. How to know the ferns and fern allies. William C. Brown Co.; Dubuque: 1979. 229 [Google Scholar]
- Mitsch W. J., Gosselink J. G. Wetlands, 2nd ed. Van Nostrand Reinhold and John Wiley & Sons; New York: 1993. 722 [Google Scholar]
- Morin N. R. Iteaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Moss B., Johnes P., Phillips G. The monitoring of ecological quality and the classification of standing waters in temperate regions: A review and proposal based on a worked scheme for British waters. Biological Reviews. 1996;71(2):301–339. doi: 10.1111/j.1469-185x.1996.tb00750.x. [DOI] [Google Scholar]
- Mosyaking S. L. Rumex . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2005. [Google Scholar]
- NatureServe NatureServe Explorer: An online encyclopedia of life. http://www.natureserve.org
- Nesom G. L. Erigeron . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Nesom G. L. Pluchea . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Lands New Hampshire Division of Forests and. Natural communities of New Hampshire – Photo guide. floating marshy peat mat. http://www.nhdfl.org/about-forests-and-lands/bureaus/natural-heritage-bureau/photo-index/floating-marshy-peat-mat.aspx. [2015-07-10T00:00:00+03:00];
- Newman M. C., Schalles J. F. The water chemistry of Carolina bays: A regional survey. Archiv für Hydrobiologie. 1990;118(2):147–168. [Google Scholar]
- Nifong T. D. The “clay subsoil” bays of North Carolina. Report submitted to the North Carolina Nature Conservancy; Chapel Hill: 1982. 90 [Google Scholar]
- Nifong T. D. An ecosystematic analysis of Carolina bays in the coastal plain of the Carolinas. University of North Carolina; Chapel Hill: 1998. 794 [Google Scholar]
- Development North Carolina Department of Conservation and. Eighth biennial report of the department of conservation and development of the state of North Carolina. Biennium ending June 30, 1940. North Carolina Department of Conservation and Development; Raleigh: 1940. 172 [Google Scholar]
- Management North Carolina Division of Environmental. Water quality progress in North Carolina: 1992−1993 305(b) report. Report Number 94-06. North Carolina Department of Environment, Health, and Natural Resources; Raleigh: 1994. various [Google Scholar]
- Management North Carolina Division of Environmental. A field guide to North Carolina wetlands. Report Number 96-01. North Carolina Department of Environment, Health, and Natural Resources; Raleigh: 1996. 130 [Google Scholar]
- North Carolina Division of Parks and Recreation Planning and Development Section. Singletary Lake State Park General Management Plan. North Carolina Department of Environment and Natural Resources; Raleigh: 1996. 69 [Google Scholar]
- North Carolina Division of Parks and Recreation Planning and Development Section. Bay Tree Lake State Park General Management Plan. North Carolina Department of Environment, Health, and Natural Resources; Raleigh: 1996. 53 [Google Scholar]
- North Carolina Division of Parks and Recreation Planning and Development Section. Jones Lake State Park general management plan. North Carolina Department of Environment and Natural Resources; Raleigh: 2006. 55 [Google Scholar]
- North Carolina Division of Parks and Recreation Planning and Development Section. Lake Waccamaw State Park general management plan. North Carolina Department of Environment and Natural Resources; Raleigh: 2006. 61 [Google Scholar]
- North Carolina Division of Water Quality Environmental Sciences Section, Intensive Survey Unit. Lake and reservoir assessments Cape Fear River basin: 4 June 2009. North Carolina Department of Environment, Health, and Natural Resources; Raleigh: 2009. 45 [Google Scholar]
- North Carolina Division of Water Quality Environmental Sciences Section, Intensive Survey Unit. Lake and Reservoir Assessments Lumber River Basin: 13 March 2012. North Carolina Department of Environment, Health, and Natural Resources; Raleigh: 2012. 15 [Google Scholar]
- Program North Carolina Natural Heritage. Element occurrence records. North Carolina Department of Environment and Natural Resources; Raleigh: 2014. various [Google Scholar]
- Program North Carolina Plant Conservation. Protected plant species list. http://www.ncagr.gov/plantindustry/plant/plantconserve/plist.htm
- Nowacki G. J., Abrams M. D. The demise of fire and the “mesophication” of forests in the eastern United States. Bioscience. 2008;58(2):123–138. [Google Scholar]
- Palmer M. W., Wade G. L., Neal P. Standards for the writing of floras. Bioscience. 1995;45(5):339–345. doi: 10.2307/1312495. [DOI] [Google Scholar]
- Palmquist K. A., Peet R. K., Weakley A. S. Changes in plant species richness following reduced fire frequency in one of the most species-rich savannas in North America. Journal of Vegetation Science. 2014;25(6):1426–1437. doi: 10.1111/jvs.12186. [DOI] [Google Scholar]
- Peet R. K., Allard D. J. Longleaf pine vegetation of the southern Atlantic and eastern gulf coast regions: a preliminary classification. In: The Longleaf Pine Ecosystem: ecology, restoration, and management. In: Hermann S. M., editor. The Longleaf Pine Ecosystem: ecology, restoration, and management; 18th Tall Timbers Fire Ecology Conference.; Tall Timbers Research Station. Tallahassee: 1993. [Google Scholar]
- Peet R. K., Lee M. T., Jennings M. D., Faber-Langendoen D. VegBank: The vegetation plot archive of the Ecological Society of America. http://vegbank.org. [2015-06-25T00:00:00+03:00];
- Peet R. K., Wentworth T. R., Schafale M. P., Weakley A. S., Lee M. T. Carolina Vegetation Survey database. Version 3.0. http://cvs.bio.unc.edu/
- Peterson P. M. Eragrostis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2003. [Google Scholar]
- Pieczynska E. The lentic aquatic-terrestrial ecotones: Their structure, function, and importance. In: Decamps H., Naiman R. J., editors. The ecology and management of aquatic-terrestrial ecotones. United Nations Educational, Scientific, and Cultural Organization; Paris: 1990. [Google Scholar]
- Pimentel D., Zuniga R., Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics. 2005;52(3):273–288. doi: 10.1016/j.ecolecon.2004.10.002. [DOI] [Google Scholar]
- Prince L. M. Theaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Pringle J. S. Clematis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Prouty W. F. "Carolina bays" and elliptical lake basins. Journal of Geology. 1935;43(2):200–207. doi: 10.1086/624288. [DOI] [Google Scholar]
- Prouty W. F. Carolina bays and their origin. Geological Society of America Bulletin. 1952;63(2):167–224. doi: 10.1130/0016-7606(1952)63[167:cbato]2.0.co;2. [DOI] [Google Scholar]
- Radford A. E., Ahles H. E., Bell C. R. Manual of the vascular flora of the Carolinas. University of North Carolina Press; Chapel Hill: 1968. 1183 [Google Scholar]
- Radomski P., Goeman T. J. Consequences of human lakeshore development on emergent and floating-leaf aquatic vegetation abundance. North American Journal of Fisheries Management. 2001;21(1):46–61. [Google Scholar]
- Ramey V., Peichel B. Hydrilla verticillata . http://plants.ifas.ufl.edu/183
- Rasmussen J. B., Godbout L., Schallenberg M. The humic content of lake water and its relationship to watershed and lake morphometry. Limnology and Oceanography. 1989;34(7):1336–1343. doi: 10.4319/lo.1989.34.7.1336. [DOI] [Google Scholar]
- Reznicek A. A. Carex sect. Lupulinae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Reznicek A. A., Catling P. A. Carex sect. Paludosae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Richardson C. J. Pocosin wetlands: An integrated analysis of Coastal Plain freshwater bogs in North Carolina. Hutchinson Ross Publication Company; Stroudsburg: 1981. 364 [Google Scholar]
- Richardson C. J. Pocosins: Vanishing wastelands or valuable wetlands? Bioscience. 1983;33(10):626–633. [Google Scholar]
- Richardson C. J., Gibbons J. W. Pocosins, Carolina bays, and mountain bogs. In: Martin W. H., Boyce S. G., Echternacht A. C., editors. Biodiversity of the southeastern United States/lowland terrestrial communities. John Wiley & Sons; New York: 1993. 502 [Google Scholar]
- Roberts D. A., Singer R., Boylen C. W. The submersed macrophyte communities of Adirondack lakes (New York, USA) of varying degrees of acidity. Aquatic Botany. 1985;21(3):219–235. [Google Scholar]
- Robertson K. R. Lachnanthes . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Robinson L. G., Finnegan J. T. Natural heritage program list of rare plant species of North Carolina. North Carolina Department of Environment and Natural Resources, Office of Land and Water Stewardship, Natural Heritage Program; Raleigh: 2014. 138 [Google Scholar]
- Roelofs J. G.M. Impact of acidification and eutrophication on macrophyte communities in soft waters in the Nehterlands 1. Field observations. Aquatic Botany. 1983;17(2):139–155. [Google Scholar]
- Romero-Gonzáles G. A., Fernández-Concha G. C., Dressler R. L., McGrath L. K., Argus G. W. Orchidaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Rørslett B. Principal determinants of aquatic macrophyte richness in northern European lakes. Aquatic Botany. 1991;39(1):173–193. doi: 10.1016/0304-3770(91)90031-y. [DOI] [Google Scholar]
- Rosenzweig, M.L. Species diversity in space and time. Cambridge University Press; New York: 1995. 436 [Google Scholar]
- Ross T. E. Carolina Bays: An annotated and comprehensive bibliography, 1844-2000. Carolinas Press; Southern Pines: 2000. 113 [Google Scholar]
- Ross T. E. Pocosins and Carolina bays compared. The North Carolina Geographer. 2003;11:22–32. [Google Scholar]
- Ruch D. G., Torke B. G., Hess B. R., Badger K. S., Rothrock P. E. The vascular flora and plant communities of the Bennett Wetland Complex in Henry County, Indiana. Proceedings of the Indiana Academy of Science. 2009;118(1):39–54. [Google Scholar]
- Savage H. The mysterious Carolina bays. University of South Carolina Press; Columbia: 1982. 121 [Google Scholar]
- Schafale M. P. Guide to the natural communities of North Carolina. Fourth approximation. North Carolina Department of Environment and Natural Resources, Division of Parks and Recreation, Natural Heritage Program; Raleigh: 2012. 217 [Google Scholar]
- Schindler D. W. Effects of acid rain on freshwater ecosystems. Science. 1988;239(4836):149–157. doi: 10.1126/science.239.4836.149. [DOI] [PubMed] [Google Scholar]
- Sculthorpe C. D. Biology of aquatic vascular plants. Edward Arnold Publishing; London: 1967. 610 [Google Scholar]
- Semple J. C., Cook R. E. Solidago . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Sharitz R. R. Carolina bay wetlands: Unique habitats of the southeastern United States. Wetlands. 2003;23(3):550–562. doi: 10.1672/0277-5212(2003)023[0550:cbwuho]2.0.co;2. [DOI] [Google Scholar]
- Sharitz R. R., Gibbons J. W. Ecology of southeastern shrub bogs (pocosins) and Carolina bays: A community profile. U.S. Fish and Wildlife Service; Washington, DC: 1982. 93 [Google Scholar]
- Sherman-Broyles S. L. Ulmus . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Sheviak C. J. Habenaria . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Sheviak C. J., Brown P. M. Spiranthes . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Sheviak C. J., Catling P. M. Pogonia . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Sieren D. J., Warr K. R. The flora of limesink depressions in Carolina Beach State Park (North Carolina) Rhodora. 1992;94(878):156–166. [Google Scholar]
- Siripun K. C., Schilling E. E. Eupatorium . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Smith A. R. Key to pteridophyte families. In: Flora of North America Flora of North America Editorial Committee (Eds), editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Smith A. R. Dryopteridaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Smith J. B., Tirpak D. A. The potential effects of global climate change on the United States. Report to Congress. US Environmental Protection Agency, Office of Planning, Policy, and Evaluation, Office of Research and Development; Washington, DC: 1989. 471 [Google Scholar]
- Smith S. G., Bruhl J. J., Gonzáles-Elizondo M. S., Menapace F. J. Eleocharis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Soller D. R. Geology and tectonic history of the lower Cape Fear River valley, southeastern North Carolina. U.S. Geological Survey Professional Paper 1466-A. United States Government Printing Office; Washington, DC: 1988. 60 [Google Scholar]
- Søndergaard M., Jeppesen E., Jensen J. P. Pond or lake: does it make any difference? Archiv für Hydrobiologie. 2005;162(2):143–165. [Google Scholar]
- Spence D. H.N. Zonation of plants in freshwater lakes. Vol. 12.Advances in ecological research. Academic Press; New York: 1982. 37-126 [Google Scholar]
- Spruill W. E. Soil survey of Columbus County, North Carolina. United States Soil Conservation Service; Washington, DC: 1990. 138 [Google Scholar]
- Stager J. C., Cahoon L. B. The age and trophic history of Lake Waccamaw, North Carolina. Journal of the Elisha Mitchell Scientific Society. 1987;103(1):1–13. [Google Scholar]
- Carolina State Climate Office of North. 1971-Climate Normals. http://www.nc-climate.ncsu.edu/cronos/normals.php. [2014-07-23T00:00:00+03:00];
- Stone D. E. Carya . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Strother J. L., Weedon R. R. Bidens . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Stuckey R. L. A floristic analysis of the vascular plants of a marsh at Perry’s Victory Monument, Lake Erie. Michigan Botanist. 1975;14:144–166. [Google Scholar]
- Sundberg S. D., Bogler D. J. Baccharis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2006. [Google Scholar]
- Sutter R. D., Kral R. The ecology, status, and conservation of two non-alluvial wetland communities in the south Atlantic and eastern Gulf coastal plain, USA. Biological Conservation. 1994;68(3):235–243. [Google Scholar]
- Swanson A., Rabeler R. K. Stipulicida . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2005. [Google Scholar]
- Taylor P. The genus Utricularia: a taxonomic monograph. Volume 14. Kew Bulletin Additional Series. Royal Botanic Gardens; Kew: 1989. 724 [Google Scholar]
- Terando A. J., Constanza J., Belyea C., Dunn R. R., McKerrow A., Collazo J. A. The southern megapolis: Using the past to predict the future of urban sprawl in the southeast US. PLOS ONE. 2014;9(7):e102261. doi: 10.1371/journal.pone.0102261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell E. E. Luziola . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2007. [Google Scholar]
- Thom B. G. Carolina Bays in Horry and Marion Counties, South Carolina. Geological Society of America Bulletin. 1970;81(3):783–814. doi: 10.1130/0016-7606(1970)81[783:cbiham]2.0.co;2. [DOI] [Google Scholar]
- Thompson S. A. Araceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2000. [Google Scholar]
- Thornhill R., Krings A., Lindbo D., Stucky J. Guide to the vascular flora of the savannas and flatwoods of Shaken Creek Preserve and vicinity (Pender & Onslow counties, North Carolina, U.S.A.) Biodiversity Data Journal. 2014;2:1–422. doi: 10.3897/bdj.2.e1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D., Cassman K. G., Matson P. A., Naylor R., Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418(6898):671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Tiner R. W. Geographically isolated wetlands of the United States. Wetlands. 2003;23(3):494–516. doi: 10.1672/0277-5212(2003)023[0494:giwotu]2.0.co;2. [DOI] [Google Scholar]
- Tucker G. C. Cladium . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Tucker G. C. Ericaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Tucker G. C. Eubotrys . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Tucker G. C., Jones S. C. Clethra . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Tucker G. C., Marcks B. G., Carter J. R. Cyperus . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2002. [Google Scholar]
- Tuomey M. Report on the geology of South Carolina. AS Johnston; Columbia: 1848. 293 [Google Scholar]
- United States Department of Agriculture Natural Resource Conservation Service PLANTS Database / United States Department of Agriculture, Natural Resource Conservation Service. Wetland flora: Field office illustrated guide to plant species. http://plants.usda.gov. [2015-06-02T00:00:00+03:00];
- United States Department of Agriculture Natural Resources Conservation Service (USDA- NRCS) The PLANTS database. http://plants.usda.gov. [2015-06-02T00:00:00+03:00];
- Vander Kloet S. P. Vaccinium . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2009. [Google Scholar]
- Vepraskas M. V., Richardson J. L. Wetland soils: genesis, hydrology, landscapes, and classification. CRC Press; Boca Raton: 2001. 417 [Google Scholar]
- Vestergaard O., Sand-Jensen K. Aquatic macrophyte richness in Danish lakes in relation to alkalinity, transparency, and lake area. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57(10):2022–2031. doi: 10.1139/f00-156. [DOI] [Google Scholar]
- Vitt D. H., Chee W. The relationships of vegetation to surface water chemistry and peat chemistry in fens of Alberta, Canada. Vegetatio. 1990;89(2):87–106. [Google Scholar]
- Wagner W. H., Beitel J. M. Lycopodiella . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Wagner W. H., Montgomery J. D. Dryopteris . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Warren R. J., Pittillo J. D., Rossell I. M. Vascular flora of a southern Appalachain fen and floodplain complex. Castanea. 2004;69(2):116–124. [Google Scholar]
- Watson F. D. Taxodium . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Watson F. D., Eckenwalder J. E. Cupressaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Weakley A. S. Flora of the southern and mid-Atlantic states. Courtesy of the author, University of North Carolina; Chapel Hill: 2012. 1225 [Google Scholar]
- Weiss C. M., Kuenzler E. J. The trophic status of North Carolina lakes. Rpt. 119. Water Resources Research Institute, Department of Environmental Sciences and Engineering, School of Public Health, University of North Carolina; Chapel Hill: 1976. 224 [Google Scholar]
- Wells B. W., Boyce S. G. Carolina bays: additional data on their origin, age and history. Journal of the Elisha Mitchell Scientific Society. 1953;69(2):119–141. [Google Scholar]
- Wetzel R. G. Limnology: Lake and River Ecosystems. 3. Academic Press; San Diego: 2001. 1006 [Google Scholar]
- Whetstone D. R., Atkinson T. A. Osmundaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
- Whitehead D. R. Developmental and environmental history of the Dismal Swamp. Ecological Monographs. 1972;42:301–315. doi: 10.2307/1942212. [DOI] [Google Scholar]
- Whitehead D. R. Late Pleistocene vegetational changes in northeastern North Carolina. Ecological Monographs. 1981;51:451–471. doi: 10.2307/2937324. [DOI] [Google Scholar]
- Wiersema J. H. Nelumbonaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Wiersema J. H. Cabombaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Wiersema J. H., Hellquist C. H. Nymphaeaceae. In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1997. [Google Scholar]
- Williams C. B. Patterns in the balance of nature. Academic Press; London: 1964. 324 [Google Scholar]
- Williams P., Whitfield M., Biggs J., Bray S., Fox G., Nicolet P., Sear D. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biological Conservation. 2004;115(2):329–341. doi: 10.1016/s0006-3207(03)00153-8. [DOI] [Google Scholar]
- Wilson K. A. North Carolina wetlands, their distribution and management. Federal aid in wildlife restoration project W-6-R. North Carolina Wildlife Resources Commission; Raleigh: 1962. 169 [Google Scholar]
- Wipff J. K. Sacciolepis . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 2003. [Google Scholar]
- Wofford B. E. Persea . In: (Eds) Flora of North America Editorial Committee., editor. Flora of North America. Oxford University Press; New York: 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Carolina bay lakes literature
Nathan Howell
Data type: references
Brief description: List of citations regarding Carolina bay lakes
File: oo_72560.doc
Floras, manuals, guides, and broader floristic works on site-specific and broad-scale aquatic/wetland habitats of the eastern United States.
Nathan Howell
Data type: references
Brief description: List of some floras, manuals, guides, and broader floristic works aquatic/wetland habitats of the eastern United States that may be of interest to readers.
File: oo_72561.doc
Sample taxon entry with brief descriptions of working parts.
Nathan Howell
Data type: taxon entry components and definitions
File: oo_72562.doc
Literature highlighting the ecological, biological, and cultural importance of Carolina bays.
Nathan Howell
Data type: references
File: oo_72563.doc
Suggested collection methods for problematic aquatic taxa and sampling methods of floating bog communities.
Nathan Howell
Data type: collection suggestions
File: oo_72564.doc
Checklist of the littoral zone vascular flora of unaltered Carolina bay lake shorelines (i.e., Bakers Lake, Bay Tree Lake, Horseshoe Lake, Jones Lake, Lake Waccamaw, Little Singletary Lake, Salters Lake, Singletary Lake).
Nathan Howell
Data type: occurrences
Brief description: Taxa are organized by major plant groups (i.e., pteridophytes, gymnosperms, basal angiosperms, magnoliids, monocotyledons, and eudicotyledons), then alphabetically by family, genus, and species. Parentheses around a taxon indicate that it is not vouchered (i.e., it has been reported by state agencies or has been observed by the first author, but has not been collected as a voucher specimen; see text for details). For taxa collected from Carolina bay lake littoral zones by the present author, abundance estimates sensu Palmer et al. (1995) are provided. Abundance estimates in this checklist reflect the abundance in which the taxa occur within each lake. Status and rank designations are also provided for rare taxa monitored by the NC Natural Heritage Program (Robinson and Finnegan 2014). The term “restricted” is used here only to indicate the presence of a taxon within a particular lake among all those surveyed and not in a global sense (e.g., a taxon here considered restricted to Lake Waccamaw has not been found in the other lakes surveyed, but may exist in other localities in the state or country). A = Abundant; F= Frequent; I=Infrequent; O = Occasional; R = Rare; = þ restricted to lake indicated; () = not vouchered (i.e., reported by state agencies or observed by the present author, but not collected as a voucher specimen; see text for details); H = taxon has been collected and vouchered in the past but not by the present author. BALA = Bakers Lake; BATR = Bay Tree Lake; HOLA = Horseshoe Lake; JOLA = Jones Lake; LAWA = Lake Waccamaw; LISI = Little Singletary; SALA = Salters Lake; SILA = Singletary Lake.
File: oo_72565.doc
Provisional checklist of the littoral zone vascular flora from White Lake based on historical vouchers, personal observations, and literature reviews.
Nathan Howell
Data type: occurrences
Brief description: This checklist does not represent a complete inventory of this locality, but rather serves as a baseline for future research. Taxa are arranged by major groups (i.e., gymnosperms, magnoliids, monocotyledons, and eudicotyledons), then alphabetically by family, genus, and species. Basal angiosperms and pteridophytes were not represented by vouchers, observations, or reports and are therefore not included in the following checklist. Brackets around a taxon indicate that it is unvouchered (i.e., it has been reported by outside agencies or has been observed by the present author, but has not been collected). Status and rank designations are also provided for rare taxa monitored by the NC Natural Heritage Program (Robinson and Finnegan 2014).
File: oo_82955.doc
Climate data supporting Fig 15 (Walter climate diagrams)
Nathan Howell
Data type: climate
Brief description: Monthly mean temperature and precipitation data for Bladen and Columbus County.
File: oo_75216.xlsx
Data supporting Fig 16 (Distribution of plant habit across all Carolina bay lakes)
Nathan Howell
Data type: morphological
Brief description: Counts of the number of taxa in the categories of herb, tree/shrub, and vine for each Carolina bay lake flora.
File: oo_75217.xlsx
Data supporting Fig 17 (The thirteen most species-rich vascular plant families across all Carolina bay lakes)
Nathan Howell
Data type: taxonomic
Brief description: Counts of the number of taxa in each of the thirteen most species-rich vascular plant families in each Carolina bay lake flora.
File: oo_75218.xlsx
Howell specimen collections
Nathan Howell
Data type: occurrences
Brief description: Comma delimited file of occurrence data (DwC) for the specimens collected by the first author from Carolina Bay Lakes. Precise locality data has been redacted for species of conservation concern. Specimens are deposited at NCSC. Images are available through http://sernecportal.org.
File: oo_83035.csv