Abstract
A mixed species release of parasitoids is used to suppress outbreaks of tobacco whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae); however, this biocontrol may be inhibited by interspecific interactions. We investigated the effects of mixed releases of natural enemies of B. tabaci on predation rates, parasite performance and adult parasitoid emergence under greenhouse conditions. We tested the polyphagous predatory ladybird Harmonia axyridis (Coleoptera: Coccinellidae) and two whitefly-specific parasitoids, namely Encarsia formosa and Encarsia sophia (both, Hymenoptera: Aphelinidae). Harmonia axyridis exhibited the lowest rates of predation when released with each parasitoid than with both parasitoid species together and showed a significant preference for non-parasitized nymphs as prey. Both E. formosa and E. sophia parasitized more B. tabaci when released with the ladybird than when the wasps were released either alone or mixed with the other parasitoid. We also found that the presence of H. axyridis significantly reduced adult parasitoid emergence; the highest rate of adult emergence was obtained with parasitoids released alone. Our results indicate that different combinations of natural enemies can influence observed rates of predation, parasitism, and parasitoid emergence. Therefore, the combination of natural enemies to be used for a particular biological control program should depend on the specific objectives.
The introduction of predatory or parasitic insects via a ‘one biological control agent–one pest’ approach has produced effective suppression of exotic pests in several applications of classical or augmentative biological control1,2. However, an increasing number of laboratory and field studies have highlighted potential problems of this simple approach, such as impacts on non-target species and, in some cases, the limited effectiveness of imported biological control agents3,4,5. The mixed release of multiple natural enemies as a solution to these problems has been widely explored, because mixed releases may enhance pest control in some agroecosystems, including field crops, greenhouse vegetables and organic orchards6,7,8,9. One positive feature claimed for mixed releases is that employing multiple natural enemies with different feeding patterns could ensure continued suppression of a target pest throughout its lifecycle. However, natural enemy diversity could also result in limited or reduced pest control as a result of interactions including interspecific competition, guild predation, or super parasitism among multiple biological control agents10,11,12,13,14,15,16.
For the tobacco whitefly, Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae), particular attention has been paid to the use of parasitic or predatory natural enemies as biological controls17,18,19. This invasive whitefly, a species complex with over 30 species20,21, causes substantial damage to various crops and vegetables by direct feeding and by transmission of plant pathogens19,22,23,24,25. A series of outbreaks of B. tabaci in the Yangtze River basin and southern regions of China during the 1990s resulted in severe losses23,26,27,28,29. Due to conspicuous insecticide resistance30, many predatory or parasitic natural enemies have been introduced to suppress outbreaks of B. tabaci17,31,32,33,34. In some cases, the value measured in predation or parasitism efficiency of releasing multiple natural enemies in the management of B. tabaci has been found to be positive35,36. However, the successful application of mixed natural enemies can be constrained by interspecific competition among multiple biological control agents37,38 and the influence of various interactions38. Since no general rule has been established to predict the outcome of a mixed release of natural enemies, it is important to attempt to determine what outcome will result from the combined use of specific natural enemies to control a particular pest, prior to widespread implementation. This type of information can be very useful in the development of effective biological control programs.
In this study we were interested in determining how whiteflies are controlled by two species of parasitoids and a predator in various combinations. We studied the parasitoids Encarsia formosa Gahan and E. sophia (Girault and Dodd) (both Hymenoptera: Aphelinidae). These parasitoid species have been widely released to manage the outbreaks of whiteflies and are currently used on various greenhouse vegetables in China35,39. As heteronomus autoparasitoids, both E. formosa and E. sophia show high preference for the tobacco whitefly B. tabaci on various host plants, including tomato, eggplant, and poinsettia35,40,41. Previous studies show high competition between these two parasitoids42 or with other Encarsia species that share the same whitefly hosts43,44. Although the joint release of multiple whitefly parasitoids could improve the suppression of whiteflies, this has not always been observed (e.g. Collier & Hunter43). Competition between E. formosa and E. sophia and territorial colonization may modify the outcome45,46; hence the importance of testing their joint action.
As a predator we selected the Asian multicolored ladybird, Harmonia axyridis (Coleoptera: Coccinellidae), a generalist predator extensively employed as a biological control agent whose positive and negative effects have been reported previously47,48. Previous experiments have shown that this ladybird species exhibits significantly lower selectivity for B. tabaci and a reduced reproduction rate when fed B. tabaci whiteflies (SW, unpublished data). However, its generalist character could be helpful in controlling secondary pests and its release in combination with whitefly parasitoids might enhance whitefly control (see for example Pang et al.42, Zang et al.46). Before using this strategy it is essential to assess the possible effects of joint application of all of these control agents. Under semi-field greenhouse conditions, we assessed the parasitism and emergence rates of E. formosa and E. sophia and the predation rate of the ladybird H. axyridis when released alone and jointly. Our results allow us to predict outcomes were a management strategy for B. tabaci employing a mixture of these natural enemies to be implemented.
Materials and Methods
Insects and plants
Solanum lycopersicum plants
We used tomato plants var. Baofen-F1 (Changfeng Seed Co. Ltd., Xianyang City, Shaanxi, China) for B. tabaci rearing and the experiments. These plants were grown in plastic trays (55.0 × 25.0 × 20.0 cm, 10 plants per pot). The seedlings were transplanted individually into plastic flowerpots (height = 20.0 cm; diameter = 13.0 cm, one plant per pot) and maintained in artificial chambers (MH 351, Sanyo ◽, Japan) in conditions of 27 ± 1 °C, 60–65% relative humidity (RH) with a photoperiod of 14:10 (Light:Darkness). Tomato plants were used when they were approximately 30.0–35.0 cm in height with 5–7 true and fully expanded leaves.
Bemisia tabaci
Over 3000 pairs of B. tabaci were collected from greenhouse eggplants at the NOYA® organic vegetable production station (40°10′38.18″N and 116°59′53.80″E), in Ping’gu district, Beijing during April 2012. The B. tabaci samples were classified as the Q1 biotype (MED – Q1 cryptic species) by employing a random amplification of polymorphic DNA (RAPD)-PCR (Qiu et al. 2003). The collected whiteflies were reared on tomato plants placed in aluminum rearing cages (50.0 × 60.0 × 45.0 cm) whose sides were covered with mesh net. Each rearing cage contained 2–3 tomato plants and 500–700 whitefly individuals. Whitefly rearing was performed at the Institute of Plant & Environment Protection, Beijing Academy and Forestry Sciences. Rearing conditions were 27 ± 1 °C, 60–65% relative humidity (RH), and a photoperiod of 14:10 (Light: Darkness). The conditions were maintained using an automatic environment management system (Suntech®, L105, Beijing, China).
Parasitoid wasps
Individuals of E. formosa (over 350 parthenogenetic female adults) and E. sophia (317 females and 103 males) were collected from a tobacco field at the Wang’jia’yuan Biodiversity Research Station (40°10′45.30″N and 116°2′38.27″E), Chang’ping district, Beijing during April 2012. E. sophia is known as a complex including multiple cryptic species49. We classified E. sophia as the Pakistan and Spanish genotype by using the mitochondrial cytochrome oxidase subunit I gene50.The wasp populations were maintained under the same laboratory conditions as the whiteflies. Each rearing cage contained 4–6 tomato plants infested with whitefly nymphs, and was maintained with 200–220 females and 30–50 males of E. sophia or 150–200 E. formosa individuals, each species kept separately. Tomato plants harboring whitefly nymphs were replaced every three days to enable reproduction of the wasps. Both wasp species were employed in the experiments after living for three generations under our laboratory conditions in order to standardize host preference. Five day old wasps were used in experiments to ensure they had reached sexual maturity41,51. For pre-mating, a pair of 5 day old E. sophia adults were introduced into a plastic petri dish (D = 4.5 cm). After 24 h, the mated female adults were collected for following tests. We used unmated (parthenogenetic) E. formosa females and pre-mated E. sophia females. Prior to the experiments, the wasps were provided with honey droplets as a food supplement.
Harmonia axyridis
A total of 110 pairs of H. axyridis adults were captured using a rape-pollen trapping chamber in Beijing Botanic Garden (39°59′29.65″N and 116°12′34.33″E), Haidian district, Beijing during May 2012. The ladybirds were transported and maintained under the same environmental conditions as the wasps in a rearing chamber at the Wang’jia’yuan Biodiversity Research Station. The ladybirds were reared in custom-made cages (50.0 × 50.0 × 60.0 cm, employing a 60-mesh fabric net and aluminum frames, with a ladybird density of 50–70 pairs of adults or 100–140 larvae per cage) and provided with an abundant daily supply of nymphs of the aphid Megoura japonica (Hemiptera: Aphididae) Matsumura on house bean Vicia faba var. Liying (Xinfeng seed Co. ltd, Beijing) sprouts. Ladybirds were raised for 3 generations under laboratory conditions to standardize food preference before use in experiments. Ladybirds were used in experiments at the 4th instar stage; at this stage ladybird larvae consume a substantial amount of prey52.
Semi-field experiments
Our experiments were performed in a greenhouse (under natural environmental conditions) located at the NOYA® organic vegetable production station (40°10′38.18″N and 116°59′53.80″E). This greenhouse was divided into 6 isolated plots (Length: 12 m, Width: 7.0 m); limited by 80-mesh fabric net, 120 evenly spaced tomato plants were transplanted into each plot (12 plant per row × 10 rows). The greenhouse experiments were replicated three times during the course of the study: 9–27 July; 1–17 August; and 21 August–8 September.
Based on pilot study observations, six natural enemy combination treatments were established at each plot: (i) 30 E. formosa females (thereafter F), (ii) 30 E. sophia females (thereafter S), (iii) 30 E. formosa females and 10 H. axyridis (thereafter F + H), (iv) 30 E. sophia females and 10 H. axyridis (thereafter S + H), (v) 15 E. formosa females and 15 E. sophia females (thereafter F + S)] and, (vi) 15 E. formosa females, 15 E. sophia females and 10 H. axyridis (thereafter F + S + H).
The observations were conducted in different greenhouse plots, each employing one natural enemy combination. In each plot, 30 tomato plants with 9–10 true and fully expanded leaves were randomly selected. Each selected plant was covered with a cylindrical net cage (height = 50.0 cm; diameter = 30.0 cm, composed of 80-mesh fabric net and aluminum frames). Then, 250 pairs of B. tabaci adults were released onto each caged tomato plant and allowed to oviposit. After 48 h, the B. tabaci adults were removed from the cages. Then, after 14 days, all B. tabaci except third-instar nymphs (identified as light green or yellow elliptic body covered with wax, and body size of approximately 0.5 mm), the most suitable instar for parasitism, were removed from the plants by using a dissecting needle and smooth brush. Natural enemies were then introduced into the cages. At this point, six leaves of each plant were randomly selected and marked with cardboard labels (three with black labels and three with red labels). The number of nymphs on the six leaves was recorded at the time of labeling (i.e. the initial number of nymphs).
Forty-eight hours after the introduction of natural enemies, the three leaves with black labels from each tomato plant were removed and transferred to the laboratory. All nymphs on the leaves were assessed to determine if they had been preyed upon or not. To assess predation events, we looked for the remains of the body of the nymphs using a stereoscope (SteREO Discovery V20, Zeiss, Germany). We recorded the number of preyed upon nymphs and calculated percentages of predation (number of preyed upon nymphs/number of initial nymphs). All individual nymphs and nymph remains on the leaves were kept separate to assess parasitism using molecular analyses53. This method is based on the cytochrome oxidase subunit I (COI) gene of mitochondrial DNA (mtDNA) and enables the detection and identification of E. formosa and E. sophia in B. tabaci nymphs at very early developmental stages53. By this method the total numbers of non-parasitized and parasitized whitefly nymphs (by E. sophia, E. formosa, or both) on the black labeled leaves were determined and the percentage (=number of parasitized nymphs/initial number of nymphs) of B. tabaci nymphs parasitized by E. sophia, E. formosa, or both was calculated.
To determine the rates of emergence of E. sophia and E. formosa in the various treatments, the three leaves marked with red labels were isolated in 600-ml plastic bags with over 400 holes drilled for ventilation after some parasitized whitefly nymphs had turned black, indicating that the parasitoids had developed to the pupal stage. The number of black nymphs was counted and recorded as the total number of parasitized nymphs. The marked leaves were then checked daily and the number of newly emerged E. sophia and E. formosa were recorded. The number of emerged parasitoids (E. sophia, E. formosa, or both) from all three leaves was recorded and used to calculate the percentage of adult emergence (=total number of emerged adults/total number of parasitized nymphs).
Statistical analysis
The percentages of total B. tabaci nymphs preyed upon by H. axyridis and percentages of adult parasitoid emergence were compared using multiple factorials ANOVA across multiple natural enemy combinations as independent factors and 3 different temporal replications as partial factors. Multiple comparisons were performed via a Tukey HSD test (P = 0.05). The predation rate on non-parasitized whitefly nymphs was compared against the predation rate on parasitized nymphs using a Chi-square test (P = 0.05). Arcsine square root transformation was applied to the percentage data before statistical analysis. ANOVA and Tukey analyses were processed using the statistical analysis software SPSS 18.054.
Results
Predation of Harmonia axyridis
The rates of predation of H. axyridis on whitefly nymphs were significantly different depending upon the natural enemy combination present (Fig. 1). Significantly more whitefly nymphs were preyed upon by the ladybird when released with both parasitoids simultaneously than when released with either E. formosa or E. sophia individually (Fig. 1A, F = 12.36, P < 0.01). The temporal replications did not show any significant influences on predation (F = 0.76, P = 0.55). Harmonia axyridis also showed a significant preference for non-parasitized whitefly nymphs in all treatments where parasitoids were present (Fig. 1B, H + F: X2 = 81.03; H + S: X2 = 84.61; H + F + S: X2 = 86.64; all P < 0.01). No significant difference was found between the predation rates on non parasitized nymphs across treatments (Fig. 1B) (F = 1.113, P = 0.247), and no significant differences were found between temporal replications (F = 0.86, P = 0.36).
Figure 1.
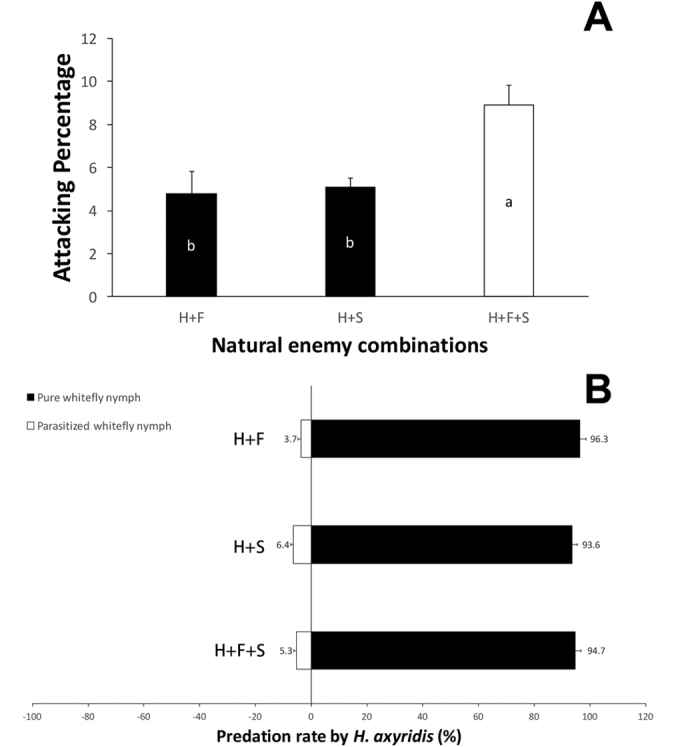
The Attacking percentage (A) and predation rate by Harmonia axyridis which released with Encarsia formosa, E. sophia or both of them respectively (B). The error bars showed in the charts are standard error. The different letters with the columns in chart A indicate the significant differences among different natural enemy combinations in P = 0.05 by Tukey HSD test.
Parasitic proportion of parasitoids
When each was released alone, E. formosa parasitized significantly more B. tabaci than E. sophia did (Fig. 2A) (t = 101.47, P < 0.01), a difference invariant over temporal replications (F = 1.31, P = 1.23). The percentage of parasitism by E. formosa was significantly higher when this wasp was released alone relative to treatments where it was released with E. sophia and with both E. sophia and H. axyridis (Fig. 2B) (F = 76.87, d.f. = P < 0.01) and did not vary across temporal replications (F = 0.98, P = 0.44). However, the parasitism rate of E. formosa was significantly higher when released in combination with H. axyridis than when alone(Fig. 2B). Similarly, the percentage of parasitism by E. sophia alone was significantly higher than parasitism observed when E. sophia was released along with E. formosa or along with E. formosa and H. axyridis together (Fig. 2C). The parasitism rate shown by E. sophia when released along with H. axyridis was also significantly higher than E. sophia released alone. (Fig. 2C) (F = 85.13, P < 0.01) this difference was also unchanged over temporal replications (F = 1.17, P = 0.28). An overall view of the parasitism outcome across different combinations is provided by Fig. 2D. We can identify three main outcomes depending upon the natural enemy combination. First, the highest rate of parasitism was exhibited by each parasitoid released with only the predator (Fig. 2D). In a second group we found lower rates of parasitism for each wasp species acting alone (Fig. 2D). A third group showing the lowest rates of parasitism ocurred for those treatments where both parasitoids were present, with or without the predator (Fig. 2D).
Figure 2. The percentage of parasitism of two parasitoids in different natural enemy combination treatments.
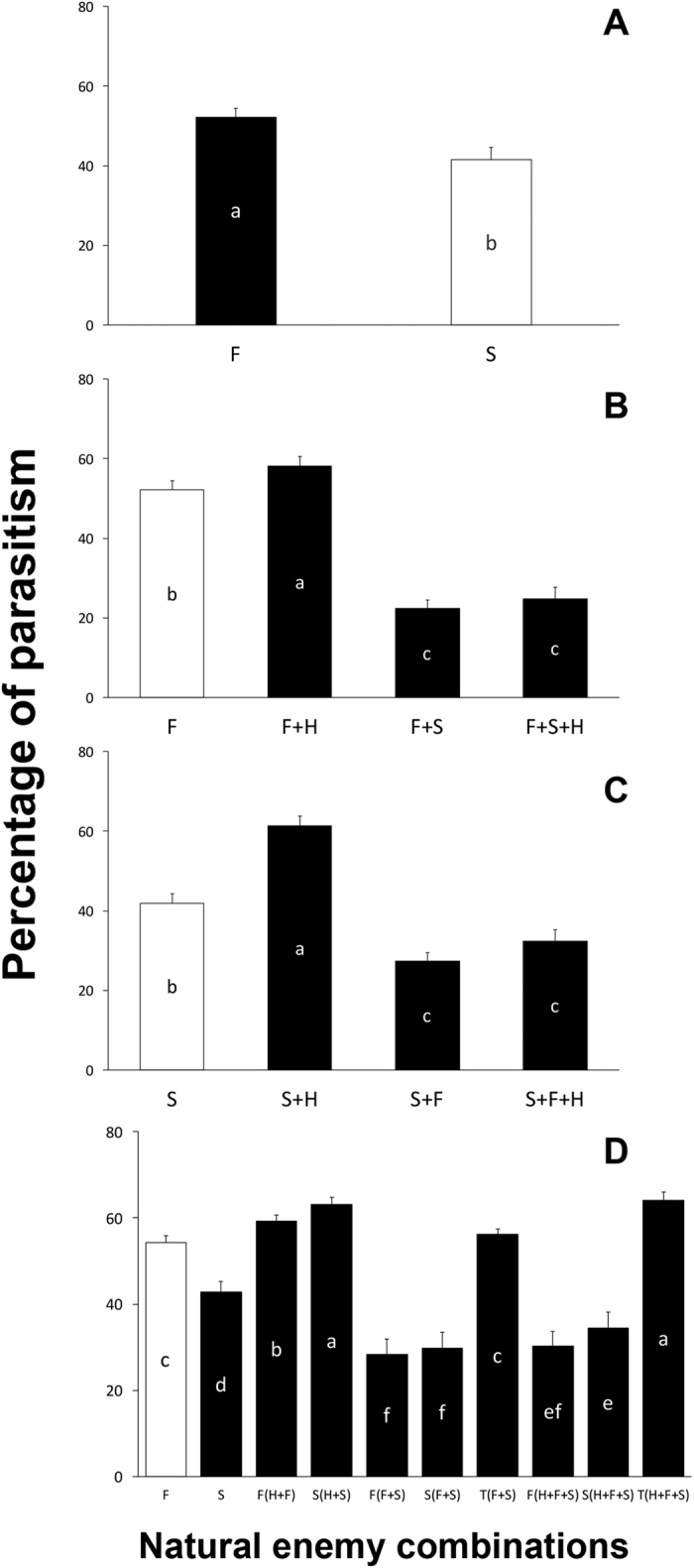
(A) The percentage of parasitism of Encarsia formosa and E. sophia when t released independently; (B) the percentage of parasitism of E. formosa when released with different natural enemies; (C) the percentage of parasitism of E. sophia when released with different natural enemies; (D) the the percentage of parasitism of two parasitoids when released with different natural enemies. The abbreviated letters in the figure means as: F = E. formosa; S = E. sophia; H = Harmonia axyridis; T = total parasitic percentage. The error bars on the top of the columns are standard errors. The different letters within the columns indicate the significant differences among different natural enemy combinations in P = 0.05 by Tukey HSD test.
Adult parasitoid emergence
Both parasitoid species showed similar percentages of parasitoid emergence (Fig. 3A) (t = 0.157, P = 0.0876) this was not influenced by temporal replications (F = 1.04, P = 0.19). E. formosa released alone exhibited an emergence rate similar to that which occurred when released along with E. sophia (Fig. 3B). However, the emergence rate of E. formosa alone was significantly higher that the rates exhibited by this species when released with H. axyridis or with both H. axyridis and E. sophia (Fig. 3B) (F = 93.13, P < 0.01) and not influenced by temporal replications (F = 1.26, P = 1.14). E. sophia rates of adult emergence when released alone were similar to those displayed when released along with E. formosa or with both E. formosa and H. axyridis (Fig. 3C). However, significantly more E. sophia adults emerged when E. sophia was released alone than when released with the predator (Fig. 3C) (F = 64.13, P < 0.01)this difference was also not influenced by temporal replication variation (F = 1.06, P = 0.19). The percentages of adult emergence divide into two principal groups. The group with the highest rates of adult emergence includes those treatments where the wasps were released alone or in combination with the other parasitoid wasp (Fig. 3D). The lowest levels of adult emergence were obtained when each wasp was released in combination with the predator, or when both wasps were released with the predator (Fig. 3D) (F = 101.47, P < 0.01). This was not influenced by the temporal replications (F = 1.01, P = 0.17).
Figure 3. The percentage of adults emergence of two parasitoids in different natural enemy combination treatments.
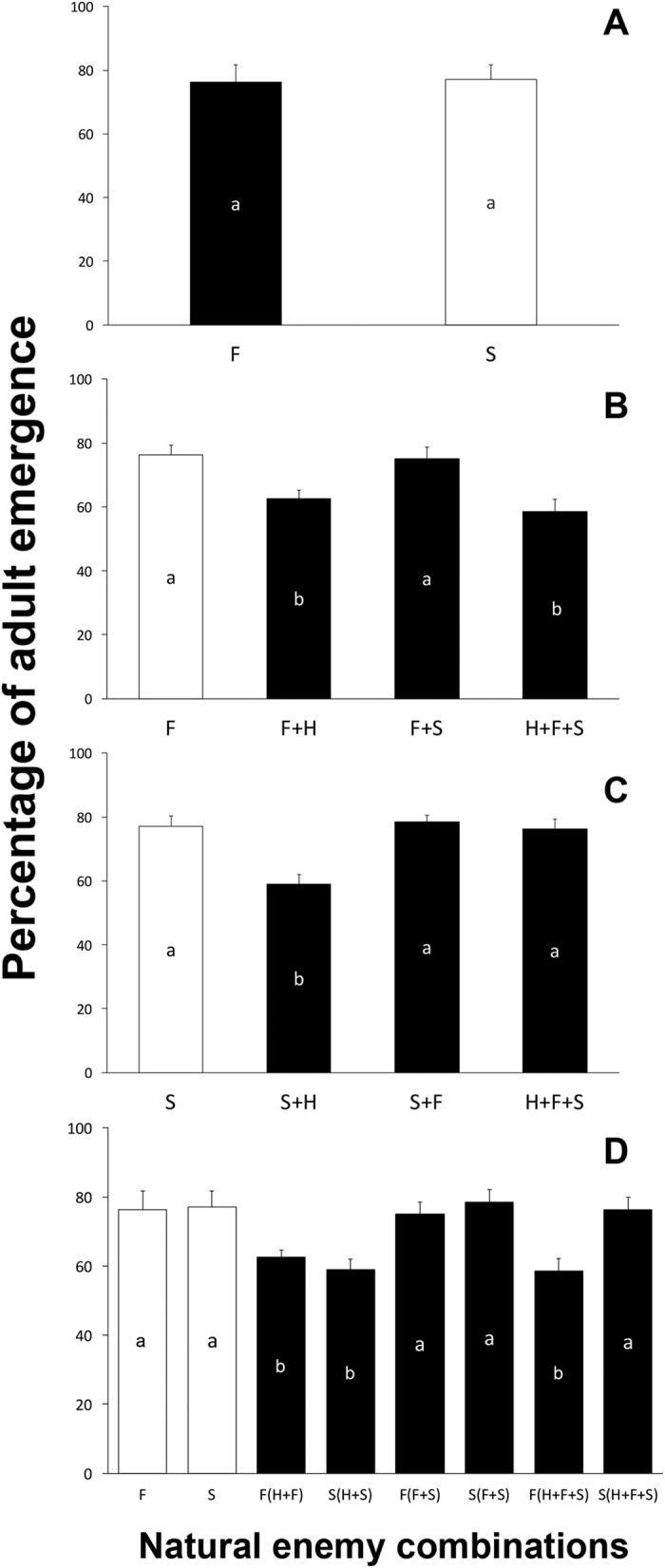
(A) the percentage of adults emergence of Encarsia formosa and E. sophia when released independently; (B) the percentage of adults emergence of E. formosa when released with different natural enemies; (C) the percentage of adults emergence of E. sophia when released with different natural enemies; (D) the the percentage of adults emergence of two parasitoids when released with different natural enemies. The abbreviated letters in the figure means as: F = E. formosa; S = E. sophia; H = Harmonia axyridis. The error bars on the top of the columns are standard errors. The different letters within the columns indicate the significant differences among different natural enemy combinations in P = 0.05 by Tukey HSD test.
Discussion
Our results showed that the release of the predator along with both parasitoid species resulted in a higher rate of whitefly predation relative to the other treatments. Thus, more whitefly nymphs will be preyed upon when the predator is released with both parasitoids, a positive outcome for pest control. These results indicate that the presence of both parasitoids induces a higher rate of predation. A possible explanation for the increased rate of predation is some cue provided by parasitoids, a cue which might be physical (e.g. motion detection of the wasps) or biochemical (e.g. semiochemical traces related to the wounds produced by parasitoids when host-feeding). We know that some predators are able to detect biochemical information or fingerprints of conspecifics55,56. The role of physical characteristics of prey (e.g. color and size) in detection and preference by a predator has also been discussed14,57. The finding of more prey consumed contrasts with previous findings that increasing the number of biological control agents does not necessarily produce positive effects on pest suppression58. It seems important to evaluate specific combinations of natural enemies before use on biological control programs due to the observed heterogeneity of effects on pest suppression59.
We found that the predator significantly preferred non-parasitized nymphs. Our study used third-instar nymphs exposed at the same time to parasitoids and predators, requiring predators to choose between preys very similar in appearance. Multiple factors have been proposed to explain a predator’s preference for parasitized over non-parasitized nymphs. Examples are size and color of prey57 and mechanical and physiological changes related to parasitism14. Our results indicate that factors related to physiological changes following parasitism may play an important role in the predator’s preference. Other predators have been reported to show similar a preference for non-parasitized over parasitized hosts (e.g. Colfer and Rosenheim12; Velasco-Hernandez et al.14).
Our results show that when released alone, E. formosa exhibited higher rates of parasitism than E. sophia (Fig. 2A). These results are in agreement with those reported by Pang et al.42 under laboratory conditions. Both experiments indicate that the wasp E. formosa will be able to parasitize more nymphs than E. sophia when each is released alone. However, the rate of parasitism of each parasitoid when alone was surpassed by the rates obtained when each parasitoid was released along with the predator (Fig. 2B,C). Thus, the parasitoids increased their rates of parasitism in the presence of the predator. Similar results have been reported for other parasitoid species in the presence of other predators. For example, the parasitoid Aphidius ervi (Braconidae, Aphidiinae) attacking the pea aphid Acyrthosiphon pisum (aphididae, Macrosiphoninae) in the presence of the predator Coccinella septempunctata, exhibited a higher rate of ovipositions60. In a similar effect, the parasitoid Eretmocerus eremicus (Hymenoptera: Aphelinidae) increases its number of ovipositions in the presence of the predator Geocoris punctipes (Hemiptera: Lygaeidae)15. The later authors suggest that this increase in ovipositions may be a response to wasp predation (i.e. intraguild predation or IGP). Although in the current study IGP was low (Fig. 1B), the parasitoids could be increasing their rate of parasitism to counteract the effect of competition for the shared whitefly nymph prey. This type of response to competitive situations has been previously documented; the introduction of a stronger competitor influencing a weaker competitor to become more efficient (Griffen & Williamson61; Mullan et al.62, but see Chailleux et al.9).
We note that the lowest rates of parasitism were found when parasitoids were released together, regardless of the presence of the predator. The joint presence of the two parasitoid species inhibits their performance. It is possible that competition between these species as previously documented42,46 explains this reduction in performance. Conditions such as heterospecific host-feeding42 or the sex-ratio of E. sophia46 could play an important role in the parasitism performance of these species when released together.
Contrasting results were found when we analyzed the rates of adult parasitoid emergence (Fig. 3). Higher rates of adult emergence were obtained when parasitoids were released without predators, whether alone or mixed (Fig. 3B,C). Predator presence has a negative effect on the adult emergence of both parasitoid species. In our experimental design the adult emergence rate was calculated by dividing the number of emerged wasps by the number of nymphs turned dark by the presence of the parasitoid. Thus, the measured reduction in the rate of adult emergence is due to mortality during the immature stage and not to predation. However, the predator had access to the parasitized nymphs for 48 hours and could have hurt the immature wasps, increasing mortality prior to the adult stage14,63. This is the best explanation we have now, as predator absence is the only distinctive factor in treatments displaying higher adult wasp emergence. Of course, this hypothesis remains to be tested.
It appears that the effectiveness of mixed releasing of these biological control agents is not influenced by the combination of agents, but by the ratio between predators and parasitoids. Regulation of the complex structure, especially the population scales of primary species, may direct influence the changing of the food web around them64,65. An imbalanced ratio between intra guild predators and parasitoids may increase the risks of interspecific cannibalism and decrease the efficiency of pest management66. The best way to avoid this problem is to identify the most effective ratio of these biological control agents. Here, we did not attempt to optimize the ratio of H. axyridis to E. formosa and E. sophia for the best B. tabaci suppression. A future experiment will be designed to explore the optimal predators/parasitoid ratio for mixed natural enemy releases. Our results seem help useful for the design of biological pest control plans because they can assist in exploring scenarios that employ different natural enemy combinations. For example, if the aim is simply to reduce the population of whiteflies, it is possible that the combination of one wasp and the predator is the best choice. This is due to the high rate of parasitism obtained with this combination, and the predator’s high preference for non-parasitized nymphs. However, if establishment of either of the parasitoids is most important, our results indicate that the best option would be to release the parasitoids individually or together, absent the predator. This is because predator presence reduces the rate of emergence of parasitoids. Of course, we only assessed the interaction between parasitoids and predatory ladybirds in terms of a single generation of parasitoid progeny and predation measured during a very limited time span. The actual consequences of mixed releasing of natural enemies according to our suggestion may prove different in the field where consecutive and overlapping generations of natural enemies occur. The combination of natural enemies studied here needs to be tested in additional experiments in more natural conditions, taking into account the effects of multiple overlapping generations of natural enemies.
Additional Information
How to cite this article: Tan, X. et al. Mixed release of two parasitoids and a polyphagous ladybird as a potential strategy to control the tobacco whitefly Bemisia tabaci. Sci. Rep. 6, 28245; doi: 10.1038/srep28245 (2016).
Acknowledgments
This study was funded through the Major State Basic Research Development Program of China (973 Program) (No. 2013CB127605), Special Fund for Agro-scientific Research in the Public Interest (No. 201303024 and No. 201303108) , Beijing NOVA Program (No. Z121105002512039) and Youth Fund of Scientific Research of Beijing Academy of Agriculture and Forestry Sciences (No. QNJJ201008). The authors also thank for support from the project EUCLID (H2020-SFS-2014, grant number: 633999).
Footnotes
Author Contributions X.T., F.Z., S.W., N.D. and F.G. designed experiments; X.T. and N.H. performed experiments; X.T., N.H. and S.W. contributed materials and analytic tools; X.T., R.R., S.W. and N.D. analyzed data; X.T., N.H., F.Z., R.R., S.W., N.D. and F.G. wrote the paper.
References
- Clausen C. P. Biological Control of Insect Pests. Annu. Rev. of Entomol. 3, 291–310 (2003). [Google Scholar]
- Ehlers R. U. Regulation of Biological Control Agents. Regulation of Bio. Control. Age. 3–23 (2011). [Google Scholar]
- Howarth F. Environmental impacts of classic biologiacl control. Annu. Rev. of Entomol. 36, 485–509 (1991). [Google Scholar]
- Louda S. M., Pemberton RWJohnson M. T. & Follett P. A. Nontarget effects–the Achilles’ heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Annu. Rev. of Entomol. 48, 365–396 (2003). [DOI] [PubMed] [Google Scholar]
- Van Lenteren J., Bale J., Bigler E., Hokkanen H. & Loomans A. Assessing risks of releasing exotic biological control agents of arthropod pests. Annu. Rev. of Entomol. 51, 609–634 (2006). [DOI] [PubMed] [Google Scholar]
- Guetsky R., Shtienberg D., Elad Y. & Dinoor A. Combining Biocontrol Agents to Reduce the Variability of Biological Control. Phytopath. 91, 621–627 (2001). [DOI] [PubMed] [Google Scholar]
- Chailleux A. et al. Potential for combined use of parasitoids and generalist predators for biological control of the key invasive tomato pest Tuta absoluta. J. of Pest. Sci. 86, 533–541 (2013). [Google Scholar]
- Ana S., Eric C., Yuxiang W., Z., Edwige A. D. & Deneux N. New parasitoid-predator associations: female parasitoids do not avoid competition with generalist predators when sharing invasive prey. Sci. of Nat. 101, 1075–1083 (2014). [DOI] [PubMed] [Google Scholar]
- Chailleux A., Mohl E., Teixeira-Alves M., Messelink G. & Desneux N. Indirect interactions in agro-ecosystems: potential for optimizing Integrated Pest Management. Pest Man. Sci. 70, 1769–1779 (2014). [DOI] [PubMed] [Google Scholar]
- Boivin G. & Brodeur J. Intra- and Interspecific Interactions among Parasitoids: Mechanisms, Outcome and Bio.logical Control. (Springer Netherlands, 2007). [Google Scholar]
- Lucas É., Coderre D. & Brodeur J. Intraguild predtion among aphid predators: charaterization and influence of extraguild prey density. Ecology 79, 1084–1092 (1998). [Google Scholar]
- Rosenheim J. A., Wilhoit L. R. & Armer C. A. Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia 96, 439–449 (1993). [DOI] [PubMed] [Google Scholar]
- Sher R. B., Parrella M. P. & Kaya H. K. Biological control of the leafminer Liriomyza trifolii (Burgess): implications for intraguild predation between Diglyphus begini Ashmead and Steinernema carpocapsae (Weiser). Bio. Control 17, 155–163 (2000). [Google Scholar]
- Velasco-Hernández M. C., Ramirez‐Romero R., Lizette C., Claudia M. R. & Desneux N. Intraguild Predation on the Whitefly Parasitoid Eretmocerus eremicus by the Generalist Predator Geocoris punctipes: A Behavioral Approach. Plos One 8, e80679–e80679 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Hernández M. C. et al. Foraging behaviour of the parasitoid Eretmocerus eremicus under intraguild predation risk by Macrolophus pygmaeus. Pest Man. Sci. 71, 1346–1353 (2014). [DOI] [PubMed] [Google Scholar]
- Mirande L., Desneux N., Haramboure M. & Schneider M. I. Intraguild predation between an exotic and a native coccinellid in Argentina: the role of prey density. J. of Pest Sci. 88, 155–162 (2014). [Google Scholar]
- Gerling D. Natural enemies of Bemisia tabaci, biological characteristics and potential as biological control agents: A review. Agri., Ecosyst. & Environ. 17, 99–110 (1986). [Google Scholar]
- Bompard A., Jaworski C. C., Bearez P. & Desneux N. Sharing a predator: can an invasive alien pest affect the predation on a local pest? Pop. Eco. 55, 433–440 (2013). [Google Scholar]
- Jaworski C. C., Chailleux A., Bearez P. & Desneux N. Predator-mediated apparent competition between pests fails to prevent yield loss despite actual pest populations decrease. J. of Pest Sci. 88, 793–803 (2015). [Google Scholar]
- Boykin L. M., Bell C. D., Evans G., Small I. & De Barro P. J. Is agriculture driving the diversification of the Bemisia tabaci species complex (Hemiptera: Sternorrhyncha: Aleyrodidae)?: Dating, diversification and biogeographic evidence revealed. BMC Evo. Bio. 13, 1–10, doi: 10.1186/1471-2148-13-228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykin L. M. Bemisia tabaci nomenclature: lessons learned. Pest Man. Sci. 70, 1454–1459 (2014). [DOI] [PubMed] [Google Scholar]
- Akad F., Dotan N. & Czosnek H. Trapping of Tomato yellow leaf curl virus (TYLCV) and other plant viruses with a GroEL homologue from the whitefly Bemisia tabaci. Arch. of Virol. 149, 1481–1497, doi: 10.1007/s00705-004-0317-8 (2004). [DOI] [PubMed] [Google Scholar]
- Xu R. In Bemisia: 1995. Taxonomy, Biology, Damage, Control and Management. (eds Gerling D. & Mayer T.) (Intercept, 1996). [Google Scholar]
- Baldin E. L. L. et al. Bioactivity of Pelargonium graveolens essential oil and related monoterpenoids against sweet potato whitefly, Bemisia tabaci biotype B. J. of Pest Sci. 88, 1–9 (2014). [Google Scholar]
- Jiao X. et al. Host preference and nymph performance of B and Q putative species of Bemisia tabaci on three host plants. J. of Pest Sci. 85, 423–430 (2012). [Google Scholar]
- Barro P. J. D., Liu S. S., Boykin L. M. & Dinsdale A. B. Bemisia tabaci: A Statement of Species Status. Annu. Rev. of Entomol. 56, 1–19, doi: 10.1146/annurev-ento-112408-085504 (2011). [DOI] [PubMed] [Google Scholar]
- Oliveira M. R. V., Henneberry T. J. & Anderson P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 20, 709–723 (2001). [Google Scholar]
- He Y., Zhao J., Zheng Y., Desneux N. & Wu K. Lethal effect of imidacloprid on the coccinellid predator Serangium japonicum and sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicology 21, 1291–1300 (2012). [DOI] [PubMed] [Google Scholar]
- He Y. et al. Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly, Bemisia tabaci. Diabet. Croatica 8, 65–89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Tian Y. A., Biondi A., Desneux N. & Gao X. W. Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicology 21, 1889–1898 (2012). [DOI] [PubMed] [Google Scholar]
- Breene R. G., Meagher R. L., Nordlund D. A. & Wang Y. T. Biological control of Bemisia tabaci (Homoptera: Aleyrodidae) in a greenhouse using Chrysoperla rufilabris (Neuroptera: Chrysopidae). Bio. Control 2, 9–14 (1992). [Google Scholar]
- Goolsby J. A., Ciomperlik M. A., Legaspi B. C., Legaspi J. C. & Wendel L. E. Laboratory and Field Evaluation of Exotic Parasitoids of Bemisia tabaci (Gennadius) (Biotype “B”) (Homoptera: Aleyrodidae) in the Lower Rio Grande Valley of Texas. Bio. Control 12, 127–135 (1998). [Google Scholar]
- Zolnerowich G. & Rose M. Eretmocerus haldeman (Hymenoptera: Aphelinidae) imported and released in the United States for control of Bemisia (Tabaci complex) (Homoptera: Aleyrodidae). Proc. Entomol. Soc. Wash. 100, 310–323 (1998). [Google Scholar]
- Moreno-Ripoll R., Gabarra R., Symondson W. O. C., King R. A. & Agustí N. Do the interactions among natural enemies compromise the biological control of the whitefly Bemisia tabaci? J. of Pest Sci. 87, 133–141 (2014). [Google Scholar]
- Gerling D., Alomar Ò. & Arnò J. Biological control of Bemisia tabaci using predators and parasitoids. Crop Prot. 20, 779–799, (2001). [Google Scholar]
- Stansly P. A., Calvo F. J. & Urbaneja A. Augmentative biological control of Bemisia tabaci biotype “Q” in Spanish greenhouse pepper production using Eretmocerus spp. Crop Prot. 24, 829–835 (2005). [Google Scholar]
- Bográn C. E., Heinz K. M. & Ciomperlik M. A. Interspecific competition among Insect parasitoids: field experiments with whiteflies as hosts in cotton. Ecology 83, 653–668 (2002). [Google Scholar]
- Heinz K. M. & Nelson J. M. Interspecific interactions among natural enemies of Bemisia in an inundative biological control program. Bio. Control 6, 384–393 (1996). [Google Scholar]
- Meng X. F., HE J. H., Liu S. S. & Chen X. X. Parasitoids of Bemisia tabaci and their use as biological control agents. Chinese J. of Bio. Control 22, 174–179 (2006). [Google Scholar]
- Enkegaard A. Temperature dependent functional response of Encarsia formosa parasitizing the Poinsettia-strain of the cotton whitefly, Bemisia tabaci, on Poinsettia. Entomol. Exp. Et Appl. 69, 251–261 (1993). [Google Scholar]
- Hoddle M. S., Driesche R. G. V. & Sanderson J. P. Biology and use of the whitefly parasitoid Encarsia formosa. Annu. Rev. of Entomol. 43, 645–669 (1998). [DOI] [PubMed] [Google Scholar]
- Pang S. T., Wang L., Hou Y. H. & Shi Z. H. Interspecific interference competition between Encarsia formosa and Encarsia sophia (Hymenoptera: Aphelinidae) in parasitizing Bemisia tabaci (Hemiptera: Aleyrodidae) on five tomato varieties. Insect Sci. 18, 92–100 (2011). [Google Scholar]
- Collier T. R. & Hunter M. S. Lethal interference competition in the whitefly parasitoids Eretmocerus eremicus and Encarsia sophia. Oecologia 129, 147–154 (2001). [DOI] [PubMed] [Google Scholar]
- Pedata P. A. & Giorgini M. E. Interspecific host discrimination and within-host competition between Encarsia formosa and E-pergandiella (Hymenoptera: Aphelinidae), two endoparasitoids of whiteflies (Hemiptera: Aleyrodidae). Bull. of Entomol. Res. 92, 521–528 (2002). [DOI] [PubMed] [Google Scholar]
- Xu D. The Study of Interspecific Competition between Encarsia Formosa and Encarsia Sophia Master thesis, Zhejiang University, (2013).
- Zang L., Liu T. & Wan F. Reevaluation of the value of autoparasitoids in biological control. Plos One 6, e20324 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facon B. et al. Can things get worse when an invasive species hybridizes? The harlequin ladybird Harmonia axyridis in France as a case study. Evol. Appl. 4, 71–88 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. L. The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non-target impacts. J. of Insect Sci. 3, 32 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgini M. & Baldanza F. Species status of two populations of Encarsia sophia (Girault & Dodd) (Hymenoptera: Aphelinidae) native to different geographic areas. Biol. Control 30, 25–35 (2004). [Google Scholar]
- Monti M., Nappo A. & Giorgini M. Molecular characterization of closely related species in the parasitic genus Encarsia (Hymenoptera: Aphelinidae) based on the mitochondrial cytochrome oxidase subunit I gene. Bull. of Entomol. Res. 95, 401–408 (2005). [DOI] [PubMed] [Google Scholar]
- Zhou C. Q., Yuan-Xi L. I., Liu T. X., Zhang F. & Luo C. Development and morphology of female immature of Encarsia sophia and their longevity and fecundity. Chinese J. of Bio. Control 26, 113–118 (2010). [Google Scholar]
- Lee J. H. & Kang T. J. Functional response of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) to Aphis gossypii Glover (Homoptera: Aphididae) in the Laboratory. Bio. Control 31, 306–310 (2004). [Google Scholar]
- Zhang X. M., Hong-Xing X. U., Wang S., Ali A. & Zhang F. Molecular and iso-enzymatic identification of the nymphal parasitoid,Encarsia sophia (Hymenoptera: Aphelinidae)of the whitefly,Bemisia tabaci Genn. (Homoptera: Aleyrodidae). J. of Environ. Entomol. 35, 196–203 (2013). [Google Scholar]
- Allen P. & Bennett K. PASW statistics by SPSS: A practical guide: Version 18.0. South Melbourne: Cengage Learning (South Melbourne, 2010). [Google Scholar]
- Marques F. D. A., Mcelfresh J. S. & Millar J. G. Female-produced sex pheromone of the predatory bug Geocoris punctipes. J. of Chem. Eco. 26, 2843–2855 (2000). [Google Scholar]
- Aldrich J. R., Oliver J. E., Shifflet T., Smith C. L. & Dively G. P. Semiochemical investigations of the insidious flower bug, Orius insidiosus (Say). J. of Chemi. Eco. 33, 1477–1493 (2007). [DOI] [PubMed] [Google Scholar]
- Naranjo S. E. Intraguild predation on Eretmocerus sp. nr. emiratus, a parasitoid of Bemisia tabaci, by three generalist predators with implications for estimating the level and impact of parasitism. Biocontrol Sci. & Tech. 17, 605–622 (2007). [Google Scholar]
- Denoth M., Frid L. & Myers J. H. Multiple agents in biological control: improving the odds? Bio. Control 24, 20–30 (2002). [Google Scholar]
- Rosenheim J. A., Kaya HKEhler L. E., Marois J. J. & Jaffee B. A. Intraguild predation among biological -control agents- theory and evidence Biological Control 5, 303–335 (1995). [Google Scholar]
- Taylor A. D. Environmental variability and the persistence of Parasitoid–host metapopulation models. Theo. Pop. Bio. 53, 98–107 (1998). [DOI] [PubMed] [Google Scholar]
- Griffen B. D. & Tucker W. Influence of predator density on nonindependent effects of multiple predator species. Oecologia 155, 151–159 (2008). [DOI] [PubMed] [Google Scholar]
- Mullan R., Glass D. H. & Mccartney M. Species diversity and predation strategies in a multiple species predator–prey model. Comm. in Nonlinear Sci. & Num. Simul. 25, 118–135 (2015). [Google Scholar]
- Muştu M., Kilinçer N., Ülgentürk S. & Kaydan M. B. Feeding behavior of Cryptolaemus montrouzieri on mealybugs parasitized by Anagyrus pseudococci. Phytoparasitica 36(4), 360–367 (2008). [Google Scholar]
- Polis G. A. & Strong D. R. Food web complexity and community dynamics. Am. Nat. t 147, 813–846 (1996). [Google Scholar]
- Neil R. & Mccann K. S. Integrating food web diversity, structure and stability. Trends in Eco. & Evo. 27, 40–46 (2011). [DOI] [PubMed] [Google Scholar]
- Traugott M., Bell J. R., Raso L., Sint D. & Symondson W. O. C. Generalist predators disrupt parasitoid aphid control by direct and coincidental intraguild predation. Bull. of Entomol. Res. 102, 239–247 (2012). [DOI] [PubMed] [Google Scholar]


