Abstract
Hypoxia-induced retinal neovascularization is a major pathological condition in many vision-threatening diseases. In the present study, we determined whether interleukin (IL)-12, a cytokine that regulates angiogenesis, plays a role in the neovascularization in a mouse model of oxygen-induced retinopathy (OIR). We found that the expressions of the mRNAs of both IL-12p35 and IL-12p40 were significantly reduced in the OIR retinas compared to that of the room air-raised control. The sizes of the avascular areas and neovascular tufts were larger in IL-12p40 knock-out (KO) mice than that in wild type (WT) mice. In addition, an intravitreal injection of recombinant IL-12 reduced both avascular areas and neovascular tufts. IL-12 injection enhanced the expressions of interferon-gamma (IFN-γ) and other downstream chemokines. In an in vitro system, IL-12 had no significant effect on tube formation of human retinal microvascular endothelial cells (HRECs). Moreover, a blockade of IFN-γ suppressed the inhibitory effect of IL-12 on pathological neovascularization. These results suggest that IL-12 plays important roles in inhibiting pathological retinal neovascularization.
Hypoxia is a leading mechanism that induced angiogenesis, and retinal neovascularization is a major pathological condition in many vision-threatening diseases including proliferative diabetic retinopathy, retinal vein occlusion and retinopathy of prematurity1. These proliferative vascular diseases may lead to irreversible damage to the patients. Oxygen-induced retinopathy (OIR), a mouse model for retinopathy of prematurity and other hypoxia-induced retinal diseases, is widely used for the studies of retinal neovascularization2. In this model, two types of retinal neovascularization exist: pathological neovascularization and physiological revascularization. The previous one occurs with the sprouting of abnormal vessels that grow from the inner layer of the retina into the vitreous, and the other is against avascular areas with functional intraretinal vessels2,3.
We reported that the M2 macrophages, rather than the M1 macrophages, played essential roles in enhancing pathological retinal neovascularization in OIR model, and the majority of those macrophages had the M2 phenotype3. M1 macrophages express high levels of interleukin (IL)-12, IL-18, and IL-23, and low levels of IL-104,5. In contrast, M2 macrophages produce high levels of IL-10 and low levels of IL-12 and IL-236,7. This difference is significant as it has been reported that IL-10 promotes pathological neovascularization4, while IL-18 regulates pathological neovascularization negatively through the regression of blood vessels in the OIR model8.
IL-12 is an important cytokine that is naturally produced by antigen-presenting cells (APCs) including dendritic cells, monocytes, macrophages, and B cells after toll-like receptor engagement9,10. IL-12 is a heterodimeric cytokine encoded by two genes, p35 and p40 chain subunits, and these can combine to the active p70 heterodimer11,12,13. IL-12 induces the synthesis of IL-10, but IL-10 suppresses IL-12 production. This interaction is regarded as a negative feedback mechanism14,15,16. IL-12 is also a key factor of the immune responses of Th1 cells by inducing the production of IFN-γ which is considered to be a key cytokine in the Th1 response17,18,19. It has also been reported that CD4+ and CD8+ T cells are involved in the activation of IL-1220,21. On the other hand, IL-12 exposure leads to the infiltration of CD4+ and CD8+ T cells in antitumor studies22.
It is known that IL-12 inhibits angiogenesis in several systems including corneal neovascularization and tumor angiogenesis10,23,24,25,26,27,28. The concentrations of IL-12 in the aqueous humor is significantly higher in patients with neovascularization associated with diabetic retinopathy29, and higher concentrations of IFN-γ were detected in the vitreous of the patients with diabetic retinopathy30. Macrophages in IL-12p40-deficient mice have a bias to M2 activation from M1 phenotype31. In spite of all of these findings, the role of IL-12 in retinal neovascularization has not been fully understood.
From what we discussed above, we hypothesized that IL-12 might be involved in retinal neovascularization and could also be a potential therapeutic target. In the present study, we investigated the role played by IL-12 in retinal neovascularization of the OIR mouse model. The possible mechanism(s) of the IL-12 in retinal neovascularization were also presented.
Results
Down-regulation of mRNA of IL-12 after retinal ischemia
To investigate the expression of IL-12 during the development of retinal neovascularization, we determined the expressions of the mRNAs of IL-12p35 and IL-12p40 in the retinas of the OIR mouse model. Total mRNA was extracted from whole retinas of C57BL/6J mice pups with or without OIR at several selected time points. The results of real-time PCR showed that mRNA expression of IL-12p35 was significantly decreased at P14 and P17 compared to the control retinas (Fig. 1A). In addition, the expression of IL-12p40 was also significantly reduced at P17 (Fig. 1B). Similar to IL-12, the expression of IFN-γ was significantly decreased at P17 (Supplementary Fig. S1). These data indicate that the expression of the mRMA of IL-12 was reduced during pathological neovascularization.
Figure 1. Expression of the mRNAs of IL-12p35 and IL-12p40 determined by real-time RT-PCR in retinas with oxygen-induced retinopathy (OIR).
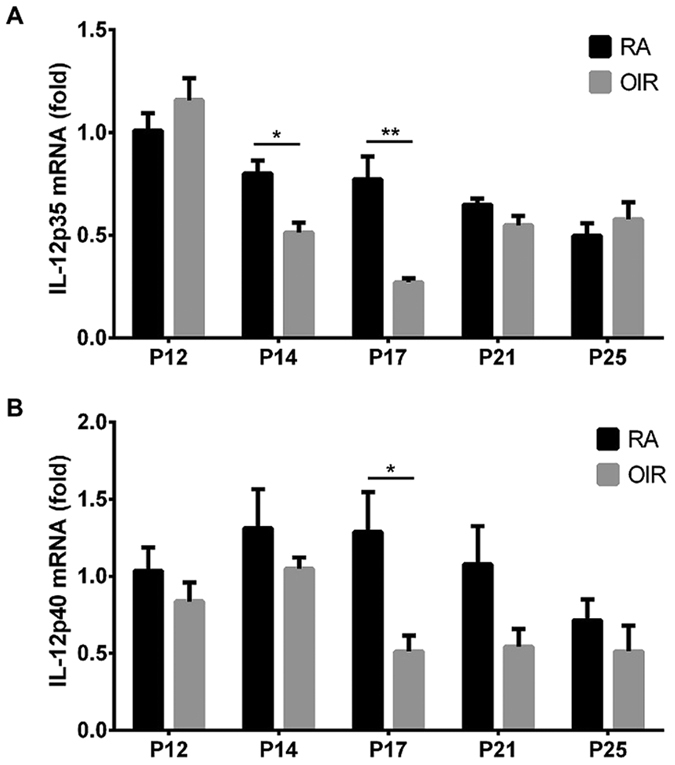
(A) Expression of IL-12p35 was significantly decreased at P14 and P17. (B) Expression of IL-12p40 was significantly decreased at P17. **P < 0.01, *P < 0.05 compared to the room air controls at the same time points (n = 4/group).
Deficiency of IL-12 enhances retinal neovascularization
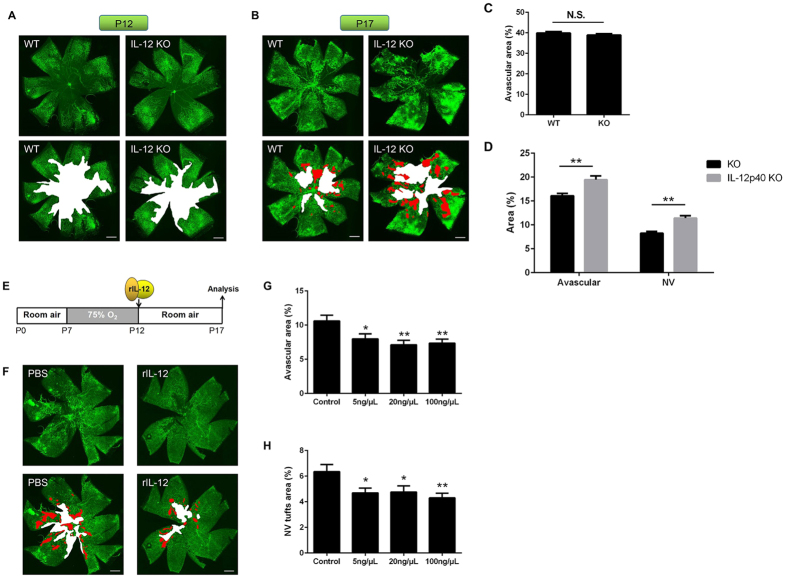
To determine if IL-12 affects retinal neovascularization, wild type (WT) and IL-12p40 knock-out (KO) mice were exposed to hyperoxygen from P7 to P12, and then returned to the room air. The retinal avascular areas (white color) in the IL-12p40 KO mice (38.91 ± 0.69%) were not significantly different from the WT (39.91 ± 0.76%) control at P12 (P > 0.05; n = 8, Fig. 2A,C). However, at P17 when pathological neovascularization was at its maximum, the IL-12p40 KO mice had significant larger pathological neovascular tufts (red color; 11.42 ± 0.48% vs 8.23 ± 0.38%; P < 0.01, n = 8) and avascular areas (white color; 19.48 ± 0.78% vs 16.10 ± 0.50%; P < 0.01, n = 8, Fig. 2B,D). Therefore, deficiency of IL-12p40 enhanced the pathological neovascularization in mice with OIR.
Figure 2. Suppressive effect of neovascularization by IL-12 in OIR retinas.
(A) Representative images of isolectin B4-stained retinal flat-mounts at P12 of WT (left) and IL-12p40 KO mice (right). (B) Representative images of isolectin B4-stained retinal flat-mounts at P17 of WT (left) and IL-12p40 KO mice (right). (C)Quantitative assessment of the avascular areas at P12 of WT and IL-12p40 KO mice (n = 8/group). (D) Quantitative assessment of avascular areas and neovascular tufts areas at P17 of WT and IL-12p40 KO mice (n = 8/group). Both avascular areas and neovascular tufts were more evident in the IL-12p40 KO mice than the WT control at P17. (E) Time course of the experiments with IL-12 injection. rIL-12 or PBS was injected into the vitreous at P12 immediately after returning the mice to room air. The eyes were analyzed at P17. (F) Representative images of isolectin B4-stained retinal flat-mounts at P17 in mice that were injected with PBS (left) and rIL-12 (right). (G) Quantitative assessment of avascular areas in the mice that were injected with PBS and rIL-12 (n = 12/group). (H) Quantitative assessment of neovascular tufts areas in the mice that were injected with PBS and rIL-12 (n = 12/group). Both avascular areas and neovascular tufts were smaller in the mice injected with rIL-12 than with PBS as control at P17. **P < 0.01, *P < 0.05 compared to the controls. Scale bars: 500 μm.
IL-12 regulation of retinal neovascularization
To confirm that IL-12 plays a role in retinal neovascularization, we injected recombinant IL-12 (rIL-12) into the vitreous immediately after returning the pups to room air on P12. Examination of the retinas collected on P17 showed that the avascular areas (white color) were reduced in the mice treated with rIL-12 at concentrations of 5 ng/μL (7.98 ± 0.74%), 20 ng/μL (7.12 ± 0.65%), and 100 ng/μL (7.36 ± 0.59%) compared to the control group (10.61 ± 0.83%; P < 0.05 and P < 0.01, n = 12/group, Fig. 2F,G).
The size of the pathological neovascular tufts (red color) were also reduced in the mice treated with rIL-12 at a concentration of 5 ng/μL (4.70 ± 0.37%), 20 ng/μL (4.76 ± 0.48%), and 100 ng/μL (4.30 ± 0.37%) compared to the control group (6.35 ± 0.56%; P < 0.05 and P < 0.01, n = 12/group, Fig. 2F,H).
These results indicate that IL-12 had anti-angiogenic effects on pathological neovascularization and promoted physiological revascularization.
Effects of IL-12 on expression of cytokines and growth factors in retinas of OIR mice
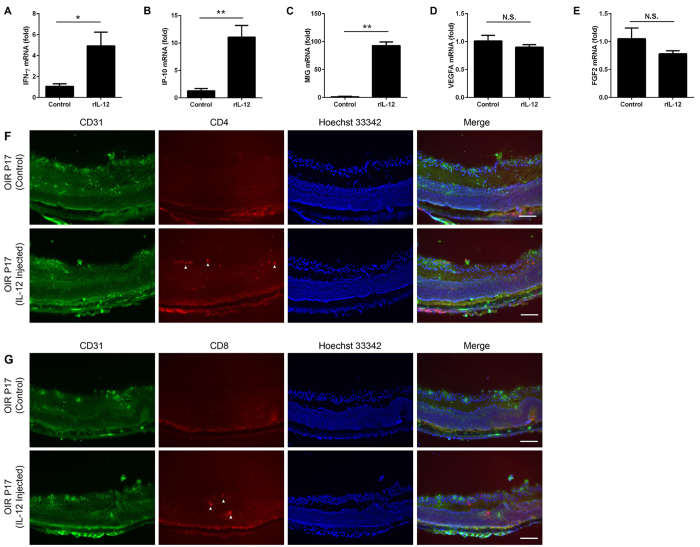
To further investigate the mechanisms that caused by IL-12 on retinal neovascularization, we injected rIL-12 or PBS intravitreally in pups at day 12 when they were returned to room air. We determined the level of expression of the downstream cytokines and growth factors in the whole retinas at P17. After the intravitreal injection of rIL-12, the mRNA expressions of IFN-γ, monokine-induced by gamma interferon (CXCL9/MIG), and interferon-inducible protein 10 (CXCL10/IP-10) were markedly increased compared to that of the control group (P < 0.05, P < 0.01, n = 4; Fig. 3A–C). The expressions of VEGFA and FGF2 were not significantly reduced after injection of rIL-12 (P = 0.341 and 0.223 respectively, n = 4, Fig. 3D,E). These findings indicate that IL-12 stimulated the synthesis of its downstream cytokines which probably are the factors inhibiting the neovascularization in the OIR retinas.
Figure 3. Effect of intravitreal IL-12 on the expressions of cytokines and the infiltration of CD4+ and CD8+ T cells in OIR retinas.
Expressions of IFN-γ (A), IP-10 (B), and MIG (C) were significantly increased in the eyes that were injected with rIL-12. However, the expressions of VEGFA (D) and FGF2 (E) are not significantly changed in the rIL-12 injected group. Double staining for CD31 (green) and CD4 or CD8 (red) in cryostat sections of OIR eyes that were injected with PBS or rIL-12. Some CD4+ (F) and CD8+ cells (G) infiltrated in the retina (arrowheads) of the rIL-12 injected group. **P < 0.01, *P < 0.05 compared to the controls that were injected with PBS (n = 4/group). Scale bars: 100 μm.
Infiltration of T cells in IL-12 treated retinas
To determine whether CD4− and CD8− positive T cells had infiltrated the retina of IL-12-injected mice, we injected rIL-12 into the vitreous, and then performed immunofluorescence staining using antibodies for CD4 and CD8 together with the endothelial cell marker CD31. The results showed that CD4+ and CD8+ T cells had infiltrated into the IL-12-treated retinas, and some of the T cells were associated with the neovascular tufts (Fig. 3F,G). In addition, fewer CD4+ and CD8+ T cells were found in the retina of PBS-injected eyes (Fig. 3F,G). These results indicated that IL-12 might stimulate the infiltration of T cells in the OIR retinas.
No direct effect of IL-12 on tube formation by retinal endothelial cells (HRECs) in culture
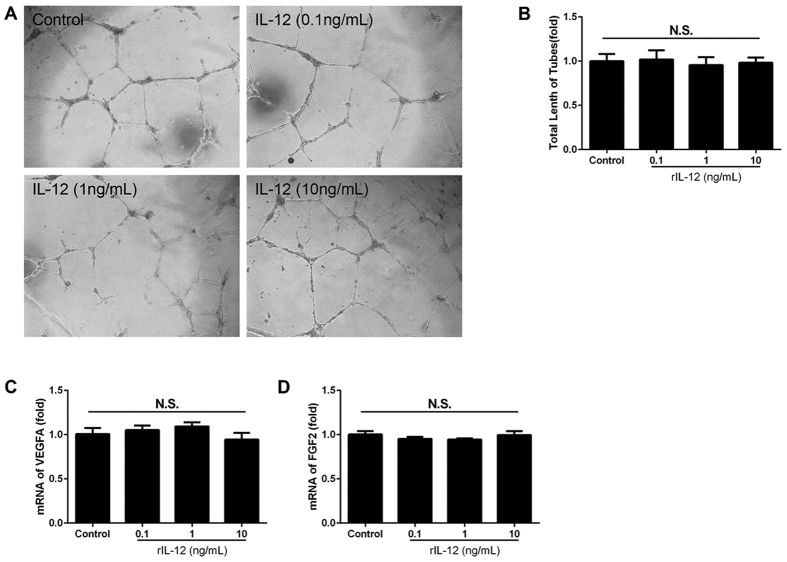
To examine whether IL-12 has direct effects on HRECs, we cultured HRECs in a tube formation assay with or without human rIL-12. The total length of the tubes in the groups that were cultured with rIL-12 had no significant difference compare to the control group (P = 0.960, n = 12, Fig. 4A,B). However, both MIG and IP-10 inhibited tube formation by HRECs (Supplementary Fig. S2).
Figure 4. IL-12 has no direct effect on the growth of human microvascular retinal epithelial cells (HRECs) in vitro.
Photographs of tube formation were taken after cultured alone or stimulated by rIL-12 (A). The lengths of the tubes were quantitative assessed in each group. The total lengths were not significantly different among the groups (B, n = 12/group). The mRNA expressions of VEGFA (C) and FGF2 (D) were not changed after exposure to rIL-12 (n = 4/group). P > 0.05 compared to the controls without rIL-12.
To confirm the effects of IL-12 on the change in the expression of its downstream cytokine IFN-γ and growth factors, we cultured HRECs with or without human rIL-12. Then, the cells were collected and mRNA expressions of cytokines were determined by real-time RT-PCR. The results showed that the expressions of both VEGFA and FGF2 were not affected 24 h after exposure to rIL-12 (P = 0.397 and 0.495 respectively, n = 4, Fig. 4C,D). In addition, IFN-γ was not detected in all of the groups. These findings indicate that IL-12 has no direct effect on tube formation by HRECs in vitro.
Suppression of IFN-γ blocks the inhibitory effect of IL-12 on pathological neovascularization
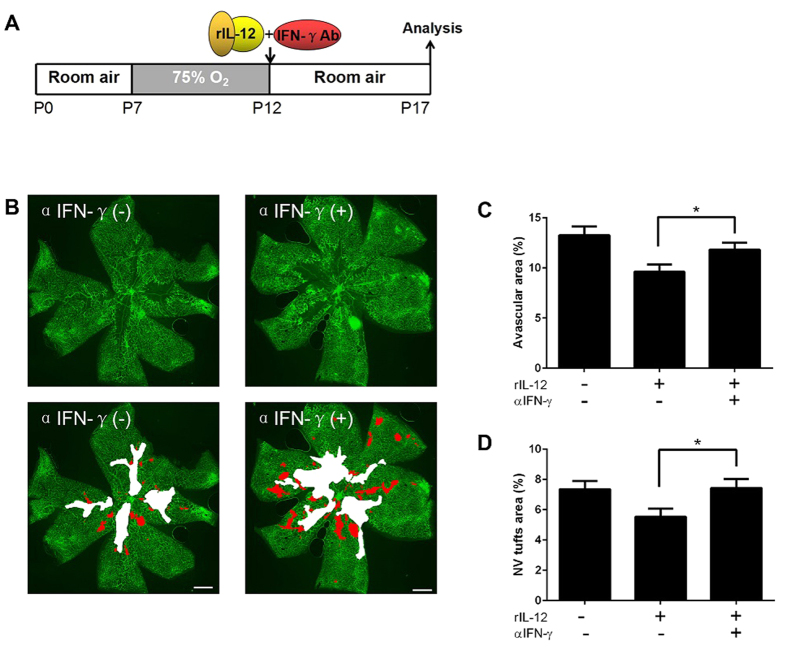
To confirm whether IFN-γ mediated the anti-angiogenic effects of IL-12 in the retina, we injected rIL-12 mixed with or without anti-IFN-γ neutralizing antibody into the vitreous at P12. The results showed that the group injected with anti-IFN-γ had larger areas of neovascular tufts (red color) compared to the non-immune IgG control group (7.62 ± 0.63% and 5.53 ± 0.53% respectively; P < 0.05, n = 12 and 14, Fig. 5B,D). Moreover, the size of the avascular areas (white color) was significantly larger than that of the non-immune IgG control group after the injection of anti-IFN-γ antibody (11.74 ± 0.72% and 9.62 ± 0.71% respectively; P < 0.05, n = 12 and 14, Fig. 5B,C). These results indicated that an inhibition of IFN-γ blocked both the reduction of pathological neovascularization and the enhanced physiological revascularization that caused by IL-12. Thus, IFN-γ might be a key mediator of the anti-angiogenic effects induced by IL-12 in OIR retinas.
Figure 5. Intravitreal injection of anti-IFN-γ rescues the anti-angiogenic activity of IL-12.
(A) Time course of the experiments. rIL-12 mixed with anti-IFN-γ antibody or non-immune IgG was injected into the vitreous at P12. The eyes were analyzed at P17. (B) Representative images of flat-mounted retinas in the OIR mice that were injected of rIL-12 with non-immune IgG or anti-IFN-γ antibody at P12. (C) The size of the avascular areas was significantly larger than that of the controls. (D) The size of neovascular tufts was significantly larger than that of the controls. *P < 0.05 compared to the controls injected with rIL-12 and non-immune IgG (n = 12 or 14/group). Scale bars: 500 μm.
Discussion
The anti-angiogenic activity of IL-12 is achieved by regulating the formation of new blood vessels. As far as we know, this study is the first to show the role of IL-12 in retinal neovascularization. The expressions of the mRNAs of IL-12p35 and IL-12p40 were significantly decreased in OIR retinas (Fig. 1), which was most likely because IL-12 is mainly produced by M1 activation. In our previous studies, we showed that the activation of M2 rather than M1 macrophages played a more important role in retinal neovascularization3. This might be one of the reasons for these findings.
The knockout of IL-12p40 enhanced the pathological neovascularization and interfered with the physiological revascularization at P17, but it had no effects on the size of the avascular areas at P12 (Fig. 2). In this model, IL-12 probably regulates the physiological revascularization and the subsequent pathological neovascularization. In addition, injection of rIL-12 inhibited the pathological neovascularization and promoted physiological revascularization (Fig. 2). However, in the in vitro studies using HRECs, IL-12 had neither a direct effect on the tube formation nor on the production of growth factors VEGFA and FGF2 (Fig. 3). These observations demonstrated that no direct anti-angiogenic action of IL-12 on HRECs in culture.
The anti-angiogenetic activity of IL-12 is probably indirect and is mediated by IFN-γ production by several kinds of leukocytes32,33,34,35. IL-12 and IFN-γ are strongly related to Th1 differentiation and development19,36,37. IP-10 (CXCL10) and MIG (CXCL9) are CXC chemokines which are mainly induced by IFN-γ and both contribute to the anti-tumor effects of IL-1227,28. An earlier study showed that IL-12 inhibits angiogenesis through the activation of IP-1038. In our study, IL-12 injection enhanced the production of IFN-γ as well as the secretion of cytokines MIG and IP-10 in the OIR retinas (Fig. 3). These results indicate that IFN-γ and/or its induced cytokines might mediate the inhibitory effects of IL-12 on pathological retinal neovascularization. In addition, an injection of IL-12 did not reduce the level of VEGFA and FGF2 significantly. Thus, the anti-angiogenic effects induced by IL-12 might through mechanism(s) independent from VEGFA and FGF2. We also noted that IL-12 did not have a direct effect on the tube formation of HRECs (Fig. 4). Instead, MIG and IP-10 inhibited the growth of tube formation of HRECs (Supplementary Fig. 2). It is possible that leukocytes and/or other cells, rather than HRECs, might be necessary for the activation of IFN-γ and its downstream chemokines MIG and IP-10.
IL-12 stimulates the production of IFN-γ by leukocytes including NK cells and T cells39,40. We injected rIL-12 into the vitreous at P12 and noted that CD4+ and CD8+ T cells infiltrated the IL-12-treated retinas (Fig. 3). This suggests that the IL-12 may enhanced the differentiation and/or recruitment of T cells which then resulted in a secretion of IFN-γ in the OIR retinas.
The role played by IFN-γ in angiogenesis is controversial. Nagineni et al. reported that IFN-γ promotes pathological neovascularization in eyes with AMD by enhancing the secretion of VEGF by the HRPE cells41. High expression of IFN-γ in ocular tissues in patients with PDR was reported to be an indirect inducer of angiogenesis through the activation of VEGF42. However, an intraocular injection of IFN-γ stimulated macrophages and injection of recombinant IFN-γ significantly reduced pathological choroidal neovascularization43,44. Consistent with these reports, the results of the present study indicated that the inhibitory effect of IL-12 on pathological neovascularization was mediated by IFN-γ. This was found by demonstrating that anti-IFN-γ exposure could not only block the IL-12-induced suppression of pathological neovascularization, but also block the enhanced physiological revascularization in OIR retinas (Fig. 5). This confirmed that IFN-γ is required for the inhibition of IL-12 in retinal neovascularization. Further studies investigating the mechanisms of IL-12-IFN-γ axis might provide important information on pathological neovascularization.
The IL-12 family of cytokines includes IL-12, IL-23, IL-27, and IL-3545,46. IL-12 shares p35 with IL-35 and p40 with IL-2346. Interestingly, it has been reported that IL-35 and IL-23 promote angiogenesis in tumors which might be involved in the Th17 pathway47,48. Further studies are required to determine the role of IL-12, IL-23, IL-27, and IL-35 in the formation of retinal neovascularization.
We recently reported that the M2 macrophages and their related cytokines were highly expressed in fibrovascular membranes of the patients with proliferative diabetic retinopathy49,50. M2 rather than M1 macrophages enhanced the pathological neovascularization in the OIR model3. M1 and M2 macrophages promote Th1 and Th2 responses respectively, and they produced cytokines of the Th1 and Th2 responses, e.g., IFN-γ and IL-4, which also play some roles in the regulation of M2 and M1 activities respectively51. As M2 macrophages play a critical role in retinal neovascularization in this model3, the majority of the macrophages should be M2 macrophages polarized from M1 which may lead to the decrease of IL-12 production in the OIR retinas (Fig. 1). It is known that IFN-γ is an essential cytokine that biases the M1 polarization52, and it is reasonable that IL-12 stimulation biases the M1 activation. Above all, IL-12 might regulate and also be regulated by M1-M2 activations of macrophages during its inhibition of pathological neovascularization in OIR retinas. This should be examined in more detail in future investigations.
In conclusion, IL-12 inhibits the pathological retinal neovascularization that is mediated by IFN-γ in the mouse model of OIR. Therefore, IL-12 should be considered as a potential therapeutic agent for inhibiting pathological neovascularization.
Methods
Mouse Model of oxygen-induced retinopathy (OIR)
C57BL/6 J (WT) mice (Kyudo Company, Tosu, Saga, Japan) and IL-12p40 knock-out (KO) mice (Jackson Laboratory, Bar Harbor, ME, USA) were used for the animal experiments in the study. The animal experiments were handled according to the guidelines of Association for Research in Vision and Ophthalmology (ARVO) Statement on the Use of Animals in Ophthalmic and Vision Research. The experimental procedures were approved by the Institutional Animal Care and Use Committee of Kyushu University.
OIR was induced in mice as described in detail2,53,54. Briefly, postnatal day 7 (P7) pups were exposed to 75% oxygen for 5 days, and then returned to room air at P12. After the mice were sacrificed by cervical dislocation, the eyes were enucleated and used for the following experiments.
Quantitative Real-time RT-PCR
Total RNA was extracted from the homogenized retinas or the cells by a MagDEA RNA kit (Precision System Science, Pleasanton, CA, USA)55. After quantifying the concentrations of the RNAs, complementary DNAs were synthesized by a First Strand cDNA Synthesis Kit (Roche, Mannheim, Germany). Quantitative real-time RT-PCR was performed and analyzed by FastStart Essential DNA Probes Master (Roche) and Taqman® gene expression assays (Applied Biosystems, Foster City, CA, USA) in a LightCycler® 96 Real-time PCR System (Roche) as described3. GAPDH was used as the endogenous control. The reference numbers for the assays were: Mm99999915_g1 (GAPDH), Mm00434165_m1 (IL-12α), Mm00434174_m1 (IL-12β), Mm01168134_m1 (IFN-γ), Mm00445235_m1 (CXCL10), Mm00434946_m1 (CXCL9), Mm01281449_m1 (VEGFA), Mm00433287_m1 (bFGF), Hs02758991_g1 (GAPDH), Hs00900055_m1 (VEGFA), Hs00266645_m1 (FGF2), and Hs00989291_m1 (IFN-γ).
Immunofluorescent Staining
Retinas were isolated from the eyecups as described3, which used for flat-mounts. For other eyes, sections (20 μm) were cut using a cryostat (Leica CM 1800, Bannockburn, IL, USA). Phosphate buffered saline and Tween (PBST) was used for rinsing. After blocking by 1% BSA, the samples were incubated with the primary antibodies or conjugated antibodies overnight at 4 °C. Some samples were incubated with the second antibodies on the next day. Finally, the flat-mounted retinas and cryostat sections were covered with aqueous mounting medium (Thermo Scientific, Fremont, CA, USA).
The flat-mounts were incubated with fluorescein-labeled isolectin B4 (1:150 dilution; Vector Laboratories, Burlingame, CA). Then, we measured the sizes of the neovascular tufts and avascular areas according to a reported protocol2,54. The following antibodies were used for incubating cryostat sections: Alexa Fluor® 647 anti-mouse CD4 (1:50 dilution, Biolegend, SD, USA), Alexa Fluor® 647 anti-mouse CD8a (1:50 dilution, Biolegend), CD31 antibody (1:20 dilution, R&D System, MN, USA) and Alexa Fluor® 488 chicken anti-goat IgG (1:100 dilution, Molecular Probes, Eugene, OR, USA). Hoechst 33342 (1:1000 dilution, Molecular Probes) was used to counterstain the nuclei. The retinas or cryostat sections were photographed by a fluorescent microscope (BZ-9000; KEYENCE, Osaka, Japan) and merged using the image-joint software BZ-Analyzer (KEYENCE)55,56,57.
Intravitreal injections of Recombinant IL-12 and Antibody against IFN-γ
Recombinant mouse IL-12 (rIL-12) was diluted in PBS to 0, 5, 20, and 100 ng/μL (R&D Systems) and then injected intravitreally on P12 (0.5 μL/eye). In another experiment, anti-IFN-γ neutralizing antibody (R&D Systems) was used. Briefly, rIL-12 (5 ng/μL) was mixed with anti-IFN-γ antibody (0.5 μg/μL) in the same proportion, and then injected intravitreally on P12 (1 μL/eye). Non-immune IgG (R&D Systems) was used as the control. A 33-gauge needle on a Hamilton syringe was used for the intravitreal injections. Eyes were enucleated and analyzed at P17.
Culture of HRECs
Human retinal microvascular endothelial cells (HRECs; Cell Systems Corporation, Kirkland, WA, USA) were maintained with CS-C complete medium (Cell Systems Corporation) in 6% CO2 at 37 °C. Cells at passages 5–7 were used for the studies.
HRECs suspensions (2 × 104 cells/500 μL) were seeded in 24 wells plates, cultured in C-SC medium with 10% FBS, and allowed the attachment. After starvation in C-SC medium with 1% FBS for 24 h, the medium was replaced with or without recombinant human IL-12 (rIL-12) at 0.1 ng, 1 ng, or 10 ng/mL (R&D System) for 24 h. Then the cells were collected and examined for the mRNA expressions by real-time RT-PCR.
Tube Formation Assays for HRECs
Tube formation studies were performed as described in detail3,58,59,60. Briefly, growth factor-reduced Matrigel matrix (BD Biosciences, Franklin Lakes, NJ, USA) was aliquoted into each well of a 96-wells plate for 30 min at 37 °C. After gel formation has occurred, serum starved HRECs were suspended in C-SC medium, and seeded at a final density of 2 × 104 cells/100 μL. The cells were cultured with or without recombinant human IL-12 (rIL-12) at 0.1 ng, 1 ng, and 10 ng/mL (R&D System). In another experiment, recombinant human MIG/CXCL9 (100 ng/mL, R&D System) and IP-10/CXCL10 (100 ng/mL, R&D System) were added to the medium of the cells. We examined six independent wells for each group. After culturing for 24 h, two representative images per well were photographed randomly by a digital camera with a phase contrast microscope (Olympus CK2, Olympus, Tokyo, Japan). Angiogenesis Analyzer for NIH ImageJ software (http://rsb.info.nih.gov/ij) was used for the final analyses.
Statistical Analyses
In the present study, the results are presented as the means ± standard error (SEMs). The statistical analyses of the differences between each group were determined by Student’s t tests or one-way ANOVA that followed by Dunnett’s tests. P < 0.05 was considered statistically significant. Statistical analyses were performed using JMP 10.0.2 (SAS Institute, Cary, NC, USA).
Additional Information
How to cite this article: Zhou, Y. et al. Interleukin-12 inhibits pathological neovascularization in mouse model of oxygen-induced retinopathy. Sci. Rep. 6, 28140; doi: 10.1038/srep28140 (2016).
Supplementary Material
Acknowledgments
This work was supported in part by Japan Society for the Promotion of Science (JSPS) (Grant Numbers 26293374, 26670757 and 16K15734) and Takeda Science Foundation. Yedi Zhou thanks to China Scholarship Council (CSC) for their support. We thank Ms. Masayo Eto, Kinuko Sasada, and Hiroko Miura for their excellent technical assistance.
Footnotes
Author Contributions Y.Z. and S.Y. proposed and designed the experiments, Y.Z., Y. Kubo, Y. Kobayashi, T.N. and M.Y. conducted the experiments. Y.Z., Y. Kobayashi, K.I., S.N. and Y.I. analyzed the data. Y.Z., S.Y., K.-H.S. and T.I. wrote the manuscript. All authors approved the final version of the manuscript.
References
- Yoshida A., Yoshida S., Ishibashi T. & Inomata H. Intraocular neovascularization. Histol Histopathol 14, 1287–1294 (1999). [DOI] [PubMed] [Google Scholar]
- Connor K. M. et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 4, 1565–1573, doi: 10.1038/nprot.2009.187 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. et al. M2 Macrophages Enhance Pathological Neovascularization in the Mouse Model of Oxygen-Induced Retinopathy. Invest Ophthalmol Vis Sci. 56, 4767–4777, doi: 10.1167/iovs.14-16012 (2015). [DOI] [PubMed] [Google Scholar]
- Dace D. S., Khan A. A., Kelly J. & Apte R. S. Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PLoS One 3, e3381, doi: 10.1371/journal.pone.0003381 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer M. et al. High-resolution transcriptome of human macrophages. PLoS One 7, e45466, doi: 10.1371/journal.pone.0045466 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao N. B. et al. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012, 948098, doi: 10.1155/2012/948098 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. Macrophage diversity and polarization: in vivo veritas. Blood 108, 408–409, doi: 10.1182/blood-2006-05-019430 (2006). [DOI] [Google Scholar]
- Qiao H. et al. Interleukin-18 regulates pathological intraocular neovascularization. J Leukoc Bio.l 81, 1012–1021, doi: 10.1189/jlb.0506342 (2007). [DOI] [PubMed] [Google Scholar]
- Trinchieri G. et al. Producer cells of interleukin 12. Parasitol Today 9, 97 (1993). [DOI] [PubMed] [Google Scholar]
- Tugues S. et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ 22, 237–246, doi: 10.1038/cdd.2014.134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhaut D. S. et al. Cloning and expression of murine IL-12. J Immunol 148, 3433–3440 (1992). [PubMed] [Google Scholar]
- Gubler U. et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA 88, 4143–4147 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babik J. M. et al. Expression of murine IL-12 is regulated by translational control of the p35 subunit. J Immunol 162, 4069–4078 (1999). [PubMed] [Google Scholar]
- Du C. & Sriram S. Mechanism of inhibition of LPS-induced IL-12p40 production by IL-10 and TGF-beta in ANA-1 cells. J Leukoc Biol. 64, 92–97 (1998). [DOI] [PubMed] [Google Scholar]
- D’Andrea A. et al. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 178, 1041–1048 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyaard L., Hovenkamp E., Otto S. A. & Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol 156, 2776–2782 (1996). [PubMed] [Google Scholar]
- Decken K. et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun 66, 4994–5000 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S. et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260, 547–549 (1993). [DOI] [PubMed] [Google Scholar]
- Athie-Morales V., Smits H. H., Cantrell D. A. & Hilkens C. M. U. Sustained IL-12 signaling is required for Th1 development. J Immunol 172, 61–69 (2004). [DOI] [PubMed] [Google Scholar]
- Nastala C. L. et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol 153, 1697–1706 (1994). [PubMed] [Google Scholar]
- Brunda M. J. et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med 178, 1223–1230 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertl U. et al. IFN-gamma-inducible protein-10 is essential for the generation of a protective tumor-specific CD8 T cell response induced by single-chain IL-12 gene therapy. J Immunol 166, 6944–6951 (2001). [DOI] [PubMed] [Google Scholar]
- Wong R. J. et al. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res. 10, 4509–4516, doi: 10.1158/1078-0432.CCR-04-0081 (2004). [DOI] [PubMed] [Google Scholar]
- Yao L. et al. Contribution of natural killer cells to inhibition of angiogenesis by interleukin-12. Blood 93, 1612–1621 (1999). [PubMed] [Google Scholar]
- Sgadari C., Angiolillo A. L. & Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 87, 3877–3882 (1996). [PubMed] [Google Scholar]
- Voest E. E. et al. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 87, 581–586 (1995). [DOI] [PubMed] [Google Scholar]
- Tannenbaum C. S. et al. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol 161, 927–932 (1998). [PubMed] [Google Scholar]
- Kanegane C. et al. Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12. J Leukoc Biol. 64, 384–392 (1998). [DOI] [PubMed] [Google Scholar]
- Gverovic Antunica A. et al. IL-12 concentrations in the aqueous humor and serum of diabetic retinopathy patients. Graefes Arch Clin Exp Ophthalmol 250, 815–821, doi: 10.1007/s00417-011-1905-4 (2012). [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nakazawa M., Suzuki K., Yamazaki H. & Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol 55, 256–263, doi: 10.1007/s10384-011-0004-8 (2011). [DOI] [PubMed] [Google Scholar]
- Bastos K. R., Alvarez J. M., Marinho C. R., Rizzo L. V. & Lima M. R. Macrophages from IL-12p40-deficient mice have a bias toward the M2 activation profile. J Leukoc Biol. 71, 271–278 (2002). [PubMed] [Google Scholar]
- Tominaga K. et al. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol 12, 151–160 (2000). [DOI] [PubMed] [Google Scholar]
- Magram J. et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4, 471–481 (1996). [DOI] [PubMed] [Google Scholar]
- Duda D. G. et al. Direct in vitro evidence and in vivo analysis of the antiangiogenesis effects of interleukin 12. Cancer Res. 60, 1111–1116 (2000). [PubMed] [Google Scholar]
- Strasly M. et al. IL-12 inhibition of endothelial cell functions and angiogenesis depends on lymphocyte-endothelial cell cross-talk. J Immunol 166, 3890–3899 (2001). [DOI] [PubMed] [Google Scholar]
- Viallard J. F. et al. Th1 (IL-2, interferon-gamma (IFN-gamma)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE). Clin Exp Immunol 115, 189–195 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T. et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: Synergism with IL-18 for IFN-gamma production. J Immunol 161, 3400–3407 (1998). [PubMed] [Google Scholar]
- Sgadari C., Angiolillo A. L. & Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 87, 3877–3882 (1996). [PubMed] [Google Scholar]
- Thierfelder W. E. et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382, 171–174, doi: 10.1038/382171a0 (1996). [DOI] [PubMed] [Google Scholar]
- Del Vecchio M. et al. Interleukin-12: Biological properties and clinical application. Clin Cancer Res. 13, 4677–4685 (2007). [DOI] [PubMed] [Google Scholar]
- Nagineni C. N., Kommineni V. K., William A., Detrick B. & Hooks J. J. Regulation of VEGF expression in human retinal cells by cytokines: implications for the role of inflammation in age-related macular degeneration. J Cell Physiol 227, 116–126, doi: 10.1002/jcp.22708 (2012). [DOI] [PubMed] [Google Scholar]
- Paine S. K. et al. Association of vascular endothelial growth factor, transforming growth factor beta, and interferon gamma gene polymorphisms with proliferative diabetic retinopathy in patients with type 2 diabetes. Mol Vis. 18, 2749–2757 (2012). [PMC free article] [PubMed] [Google Scholar]
- Zandi S. et al. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep. 10, 1173–1186, doi: 10.1016/j.celrep.2015.01.050 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman M. E. et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452, 591–597, doi: 10.1038/nature06765 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee K., Guzzo C., Che Mat N. F., Ma W. & Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets 8, 40–52 (2009). [DOI] [PubMed] [Google Scholar]
- Vignali D. A. & Kuchroo V. K. IL-12 family cytokines: immunological playmakers. Nat Immunol 13, 722–728, doi: 10.1038/ni.2366 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langowski J. L. et al. IL-23 promotes tumour incidence and growth. Nature 442, 461–465, doi: 10.1038/nature04808 (2006). [DOI] [PubMed] [Google Scholar]
- Wang Z. et al. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol 190, 2415–2423, doi: 10.4049/jimmunol.1202535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y. et al. Overexpression of CD163 in vitreous and fibrovascular membranes of patients with proliferative diabetic retinopathy: possible involvement of periostin. Br J Ophthalmol 99, 451–456, doi: 10.1136/bjophthalmol-2014-305321 (2015). [DOI] [PubMed] [Google Scholar]
- Yoshida S. et al. Increased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: implications for M2 macrophage-involving fibrovascular membrane formation. Br J Ophthalmol 99, 629–634, doi: 10.1136/bjophthalmol-2014-305860 (2015). [DOI] [PubMed] [Google Scholar]
- Mills C. D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol 32, 463–488 (2012). [DOI] [PubMed] [Google Scholar]
- Mantovani A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25, 677–686, doi: 10.1016/j.it.2004.09.015 (2004). [DOI] [PubMed] [Google Scholar]
- Yamaji Y. et al. TEM7 (PLXDC1) in neovascular endothelial cells of fibrovascular membranes from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 49, 3151–3157, doi: 10.1167/iovs.07-1249 (2008). [DOI] [PubMed] [Google Scholar]
- Ishikawa K. et al. Bone marrow-derived monocyte lineage cells recruited by MIP-1beta promote physiological revascularization in mouse model of oxygen-induced retinopathy. Lab Invest 92, 91–101, doi: 10.1038/labinvest.2011.141 (2012). [DOI] [PubMed] [Google Scholar]
- Ishikawa K. et al. Gene expression profile of hyperoxic and hypoxic retinas in a mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 51, 4307–4319, doi: 10.1167/iovs.09-4605 (2010). [DOI] [PubMed] [Google Scholar]
- Arima M. et al. Involvement of periostin in regression of hyaloidvascular system during ocular development. Invest Ophthalmol Vis Sci. 53, 6495–6503, doi: 10.1167/iovs.12-9684 (2012). [DOI] [PubMed] [Google Scholar]
- Ishikawa K. et al. Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB J. 28, 131–142, doi: 10.1096/fj.13-229740 (2014). [DOI] [PubMed] [Google Scholar]
- Im E., Venkatakrishnan A. & Kazlauskas A. Cathepsin B regulates the intrinsic angiogenic threshold of endothelial cells. Mol Biol Cell 16, 3488–3500, doi: 10.1091/mbc.E04-11-1029 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaczuk A., Selmi A. & Bednarek R. Bacterial expression, purification and angiogenesis-promoting activity of human thymosin beta4. Protein Expr Purif 90, 142–152, doi: 10.1016/j.pep.2013.06.003 (2013). [DOI] [PubMed] [Google Scholar]
- Dallaglio K. et al. Paradoxic effects of metformin on endothelial cells and angiogenesis. Carcinogenesis 35, 1055–1066, doi: 10.1093/carcin/bgu001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.