Abstract
Candida spp. elicit cytokine production downstream of various pathogen recognition receptors, including C-type lectin-like receptors, TLRs, and nucleotide oligomerization domain (NOD)–like receptors. IL-12 family members IL-12p70 and IL-23 are important for host immunity against Candida spp. In this article, we show that IL-27, another IL-12 family member, is produced by myeloid cells in response to selected Candida spp. We demonstrate a novel mechanism for Candida parapsilosis–mediated induction of IL-27 in a TLR7-, MyD88-, and NOD2-dependent manner. Our data revealed that IFN-β is induced by C. parapsilosis, which in turn signals through the IFN-α/β receptor and STAT1/2 to induce IL-27. Moreover, IL-27R (WSX-1)–deficient mice systemically infected with C. parapsilosis displayed enhanced pathogen clearance compared with wild-type mice. This was associated with increased levels of proinflammatory cytokines in the serum and increased IFN-γ and IL-17 responses in the spleens of IL-27R–deficient mice. Thus, our data define a novel link between C. parapsilosis, TLR7, NOD2, IFN-β, and IL-27, and we have identified an important role for IL-27 in the immune response against C. parapsilosis. Overall, these findings demonstrate an important mechanism for the suppression of protective immune responses during infection with C. parapsilosis, which has potential relevance for infections with other fungal pathogens.
Introduction
Morbidity and mortality due to invasive fungal infections in hospitalized patients have increased in recent years, likely owing to medical advances resulting in more immunocompromised patients. Candida spp. are the most common cause of life-threatening invasive fungal infections in severely immunocompromised patients (1). From 2006 to 2007, Candida spp. were the fourth most common cause of health care–associated infections (2). An estimated 400,000 cases of life-threatening Candida infections occur per year, with mortality rates of 46–75%. Although several Candida spp. can cause disease, >95% of Candida infections are due to C. albicans (54%), C. glabrata (19%), C. parapsilosis (11%), and C. tropicalis (11%) (1, 3). In a direct comparison in mice, C. albicans and C. tropicalis were more pathogenic than C. glabrata and C. parapsilosis (4).
Colonization and development of candidiasis are determined by the interaction of Candida spp. with host immune cells. Myeloid cells such as monocytes, macrophages, and dendritic cells express pathogen recognition receptors that bind to the pathogen and initiate an immune response. Fungal cell wall components such as β-glucans and mannans are recognized by cell surface C-type lectin-like receptors, including Dectin-1, Dectin-2, and Mincle, and by TLRs such as TLR2 and TLR4 (5–9). More recently, it has been shown that nucleic acids and chitin from Candida spp. are sensed by the endosomal receptors TLR7 and TLR9 and the cytoplasmic receptor nucleotide oligomerization domain (NOD) 2 (10–12). Engagement of these receptors on myeloid cells results in the production of various cytokines, including TNF, IL-12, IL-23, IL-1β, IL-10, IL-6, and type I IFNs (IFN-α and IFN-β) (7, 10, 12–14). This activity in turn results in the induction of sustained Th1 and Th17 cell responses (6, 13, 15). Th1 cells produce IFN-γ, which has been shown to be critical in the control of candidiasis in mice (16, 17). In contrast, the protective role of Th17 cell–mediated cytokines, namely, IL-17 and IL-22, during infection with Candida spp. has been the subject of much debate. The Th17 cell response was reported to protect against disseminated, oropharyngeal, and mucocutaneous models of candidiasis (18–20) while increasing disease and susceptibility in a gastrointestinal model (21).
Dectin-1–, Dectin-2–, and Mincle-deficient mice display reduced myeloid/innate-derived cytokine/chemokine production and increased susceptibility to Candida infections (7, 13, 14). Consequently, Th1 and Th17 cell responses are severely attenuated with Dectin-2 blockade and Dectin-1 deficiency (6). The function of TLRs in in vivo models of candidiasis has been extensively studied, and mice lacking TLR2, TLR4, or TLR9 demonstrate varying levels of susceptibility to fungal infections, depending on the fungal species and the route of infection (22). Of interest, TLR7-deficient mice display increased susceptibility to low-dose systemic C. albicans infection; however, no differences were observed in susceptibility of TLR7 null mice to higher doses of C. albicans when compared with their wild-type (WT) counterparts (23). To our knowledge, no study has been reported on the role of NOD2 in the host defense to Candida spp. in mice. However, preliminary investigations in humans suggested no significant involvement of NOD2 in the recognition of C. albicans (24).
IL-12 family members (IL-12p70, IL-23, IL-27, and IL-35) are important regulators of T cell responses. Despite the structural similarities brought about through the sharing of common α and β subunit chains, this heterodimeric cytokine family has greatly differential effects on T cells (25). IL-12 and IL-23 are predominantly considered proinflammatory. IL-12 supports Th1 cell differentiation, whereas IL-23 enhances Th17 activities (25). In contrast, IL-35 is derived from regulatory T cells and suppresses effector T cell responses (26). IL-27 is a potent T cell immunomodulator that has both pro- and anti-inflammatory properties (27). IL-27 negatively regulates IL-2 signaling to inhibit effector T cell responses and limit host disease (28, 29). However, early studies suggested a proinflammatory role through enhancement of early Th1 cell differentiation (30). IL-27 also potently inhibits the differentiation of Th17 cells to protect against Th17-associated disease (31, 32). In agreement with this, patients with gain-of-function STAT1 mutations display enhanced responses to IL-27, IFN-γ, and IFN-α, and they demonstrate reduced IL-17 responses (33–35). In addition, IL-27 inhibits the development of inducible T regulatory cells (Tregs) (36), whereas other studies show that IL-27 promotes the growth and survival of Tregs at local sites of infection (37, 38).
IL-27 is a heterodimeric cytokine that is mainly produced by APCs [monocytes, macrophages, and dendritic cells (DCs)]. IL-27 consists of the p28 and EBI-3 (Epstein–Barr virus–induced gene 3) chains and it signals through the unique IL-27R subunit paired with gp130 (39). IL-27 is induced by TLR signaling via MyD88 and NF-κB (40) and via MyD88-independent Trif/IRF3 signaling (41). In addition, type I and type II IFNs induce IL-27 via the activation of several IRFs (IRF1, IRF3, IRF7, and IRF9) (41–44). TNF has also been shown to induce IL-27 (45). Of note, infections with various pathogenic agents, including Mycobacterium tuberculosis and Toxoplasma gondii, have been associated with increased expression of IL-27 (28, 29). However Aspergillus fumigatus–infected DCs did not produce significant amounts of IL-27 (46). Heat-killed C. albicans has been shown to enhance LPS-induced IL-27 production (47); however, it is currently unknown whether any Candida spp. directly induce IL-27 production and whether IL-27 is important for antifungal immunity.
In this article, we show for the first time, to our knowledge, that some Candida spp. induce IL-27 production in myeloid cells, whereas C. albicans does not. We show that C. parapsilosis–induced IL-27 is dependent on phagocytosis, TLR7/MyD88, and NOD2, and the resultant production of IFN-β, which signals through IFN-α/β receptor (IFNAR) and STAT1/2 to induce IL-27. Importantly, IL-27R–deficient mice displayed enhanced clearance of systemic infection with C. parapsilosis. This was associated with increased levels of proinflammatory cytokines in the serum and increased IFN-γ and IL-17 responses in the spleens of IL-27R–deficient mice. Thus, IL-27 plays an important role in immune response and pathogen clearance during infection with C. parapsilosis.
Materials and Methods
Mice
Il27ra−/− (48), Clec7a−/−, Tlr2−/−, Tlr4−/−, Tlr2/4−/−, Card9−/−, Il10−/−, and age- and gender matched control C57BL/6 mice were maintained and handled according to institutional and U.K. Home Office guidelines. Clec4e−/− and Fcre1g−/− mice were maintained at the University Hospital Erlangen. Nod2−/− mice were maintained according to German and European Union guidelines.
Ethics statement
This study was performed in strict accordance with the Project License and procedures that were approved by Cardiff University Animal Welfare and Ethical Review Body and the U.K. Home Office. The animal care and use protocol adhered to the Animals (Scientific Procedures) Act 1986.
Reagents
Ultrapure LPS, Pam3Csk4, and TDB were purchased from Invivogen. Curdlan (Wako Chemicals), a β-1,3-glucan preparation from Alcaligenes faecalis, was used in this study. GM-CSF, M-CSF, and TNF were purchased from PeproTech. Cytochalasin D (Sigma-Aldrich) and the TNF antagonist etanercept, a soluble TNFR:Fc fusion protein (Wyeth Europa) were used in this study. IFN-β, and ELISAs for IFN-β and IFN-α, were purchased from R&D. Anti-Ly6C, anti-CD11b, and anti-B220 were purchased from BioLegend. Anti–IL-27p28 was purchased from BD Biosciences. Anti-phosphoSTAT1, anti-STAT1, anti-IRF1, and anti-IRF3 were purchased from Cell Signaling Technology. Anti-IRF7 and anti–Lamin B1 (Abcam) were used in this study. Anti-NOD2 (clone H-300) and anti-TLR7 (clone V-20) were from Santa Cruz Biotechnology, and secondary Abs for immunofluorescence (Cy5 donkey anti-rat and Cy2 donkey anti-rabbit) were from Jackson ImmunoResearch. DAPI nuclei stain was from Life Technologies. Rhodamine Green-X was purchased from Invitrogen. C. albicans SC5314 from American Type Culture Collection was used in this study. C. albicans strains JIMS500019 (vaginal isolate), J981318 (vaginal isolate), and AM2005/0463 (blood isolate), C. glabrata strains SCS74761 and SCSB5311, C. tropicalis strains AM2007/0112 and SCS74663, and C. parapsilosis strains AM2005/0207 and SCSB5882 were a kind gift from Dr. Donna MacCallum (University of Aberdeen, Aberdeen, Scotland).
Preparation of Candida cultures
Candida spp. were plated on yeast extract/peptone/dextrose (YPD) agar, cultured for 20 h in YPD broth, washed three times with PBS, and resuspended at the required concentration in PBS. Candida spp. were heat killed by boiling for 30 min at 100°C.
Cell culture
Bone marrow cells were flushed out of the femurs and tibias of mice. Bone marrow–derived macrophages (BMDMs) were generated by culturing cells for 6–7 d in DMEM medium containing 10% FBS, 5% horse serum, 2 mM l-glutamine, penicillin/streptomycin, HEPES, and 10 ng/ml M-CSF. BM-derived DCs (BMDCs) were generated by culturing cells for 8 d in RPMI 1640 medium containing 10% FBS, 2 mM l-glutamine, penicillin/streptomycin, HEPES, NEAA, sodium pyruvate, 2-ME, and 10 ng/ml GM-CSF.
Cell stimulations
BMDMs were harvested, resuspended in RPMI 1640 and 10% FBS, plated at 1 × 107 cells/10 cm2, and then left overnight at 37°C. Media were removed, and 1 × 107 Candida CFU were added per plate for the indicated times. Amphotericin B (Fungizone) (2.5 μg/ml) was added 2 h after stimulation. Cytosol and nuclear extracts were prepared using Novagen NucBuster Protein Extraction Kit (Merck Millipore). Lysates were clarified by centrifugation, and nonreducing sample buffer was added to lysates and heated for 5 min at 95°C. Lysates were separated by SDS-PAGE (Bio-Rad), transferred to polyvinylidene difluoride membrane (Merck Millipore), and analyzed by Western blot.
Cytokine assays
BMDMs and BMDCs were plated at a density of 1 × 105 cells per well in a 96-well plate in RMPI 1640 containing 10% FBS. BMDMs and BMDCs were stimulated with Candida spp. yeast, heat-killed yeast, LPS, Pam3Csk4, curdlan, TDB, or IFN-β for 24 h. Inhibitors or blocking Abs were added to cells 30 min to 1 h prior to stimulation. Amphotericin B (Fungizone) was added 2 h after stimulation for all experiments except for the cytochalasin D experiment, in which amphotericin B (Fungizone) was added 4 h after stimulation. Cell culture supernatants were recovered and assayed for cytokine by ELISA (Affymetrix eBioscience), according to the manufacturer’s protocol. The IL-27 ELISA detects the heterodimer (IL-27p28 and EBI3).
RNA, cDNA, and real-time quantitative PCR
RNA was extracted using TRIzol (Life Technologies) and further purified using the RNeasy Mini Kit with on-column DNase treatment (QIAGEN). cDNA was synthesized from total RNA using the TaqMan Reverse Transcription Kit (Invitrogen). Gene expression was determined on the QuantStudio 12K Flex Real-Time PCR System (Life Technologies) using ABI TaqMan Primer and Probe Sets (Life Technologies). Gene expression was normalized against Hprt1.
In vivo Candida spp. infections
Mice were matched by gender and age (8–12 wk old), and 100 μl C. parapsilosis or C. albicans in PBS was injected i.v. Mice were monitored and weighed daily. Experiments were continued for ≤42 d for C. parapsilosis or ≤30 d for C. albicans. For both in vivo models, mice were bled by cardiac puncture after sacrifice, and kidneys, brains, and spleens were harvested. The left kidney was placed in PBS and homogenized, and serial dilutions were plated on YPD agar containing 50 μg/ml chloramphenicol. The plates were cultured for 48 h, and CFU were calculated per gram of organ. For the C. parapsilosis model, the right kidney was placed in 10% formalin, embedded in paraffin wax blocks, processed using an automated tissue processor, sectioned at 4 μm, deparaffinized, and stained with H&E and periodic acid–Schiff according to standard protocols. The spleens were homogenized, RBCs were lysed with ACK lysis buffer, and the cells were washed with PBS. The cells were resuspended in IMDM containing 10% FBS, 2 mM l-glutamine, penicillin/streptomycin, and 2-ME; plated; and restimulated with C. parapsilosis for 48 h or with PMA (50 ng/ml) and ionomycin (0.5 μg/ml) in the presence of 0.2% brefeldin A for 4 h. IFN-γ and IL-17 levels were measured by ELISA, or IFN-γ– and IL-17A–producing cells were analyzed by flow cytometry.
Histology scoring
Kidneys were bisected and examined at 4 μm, and the cortex and medulla were individually assessed for the presence of neutrophils (acute inflammation) and lymphocytes/plasma cells (chronic inflammation) and scored as follows: score 0 = no inflammation; score 1 = <3 foci of inflammation; score 2 = 4–6 foci of inflammation; score 3 = >6 foci of inflammation, but less than 25% of the kidney affected; score 4 = >25% of the kidney affected. Each individual focus was subsequently assessed to determine the area of inflammation (in square micrometers) using cellSens Software (Olympus Corporation, Tokyo, Japan), and the total percentage of the affected kidney was determined using the cumulative area of inflammation/total area of the kidney section. Scoring was performed by a pathologist blinded to the experimental groups.
Flow cytometry
Bone marrow or spleen cells from naive mice were stimulated with Candida spp. for 22 h in the presence of brefeldin A for the last 12 h. IL-27p28–producing cells were determined by flow cytometry.
Immunofluorescence
Intracellular localization of NOD2 and TLR7 was assessed by immunofluorescence, as described previously (12). Briefly, BMDMs were harvested, resuspended in RPMI 1640 supplemented with 10% FBS, plated into 24-well plates containing coverslips at a density of 1 × 105 per well, and cultured overnight. Media were removed from BMDMs, and 1 × 105 Candida spp. in fresh media was added to BMDMs for 1 h at 37°C. Cells were fixed with 4% paraformaldehyde in PBS for 30 min and permeabilized with 0.5% Triton-X 100 in PBS for 10 min. Cells were blocked with 5% donkey serum in PBS for 30 min prior to staining with anti-NOD2 or anti-TLR7 for 1 h. Cells were then incubated with Cy5 anti-rat and Cy2 anti-rabbit for 1 h, and coverslips were mounted onto glass microscopic slides using mounting medium containing DAPI. Immunofluorescence staining was visualized using a Zeiss Apotome microscope fitted with a ×63 oil immersion lens. For the Candida counts, 3.2 × 108 Candida CFU were incubated with Rhodamine Green-X for 30 min and washed extensively with PBS to remove any unbound Rhodamine Green-X before use. Media were removed from BMDMs, and 1 × 105 Rhodamine Green-X–labeled Candida spp. in fresh media was added to BMDMs for 1 h at 37°C before fixing with 4% paraformaldehyde and mounting coverslips onto glass microscopic slides using mounting medium containing DAPI. Images were taken at ×20 magnification from five different slide sections per experiment, and the number of Candida cells per infected macrophage was counted.
Statistical methods
Data are presented as means ± SEM and are representative of two to four independent experiments. One-way ANOVA followed by Bonferroni’s posttest was used for statistical analysis when multiple groups were analyzed. Student t test or Mann–Whitney U test was used for statistical analysis when two groups were analyzed. When data did not follow a Gaussian distribution, it was transformed by Y = sqrt(Y + 0.5) (49) and analyzed by Student t test. Statistical significance was set at *p < 0.05, **p < 0.005, and ***p < 0.001.
Results
IL-27 production is induced by C. parapsilosis in myeloid cells
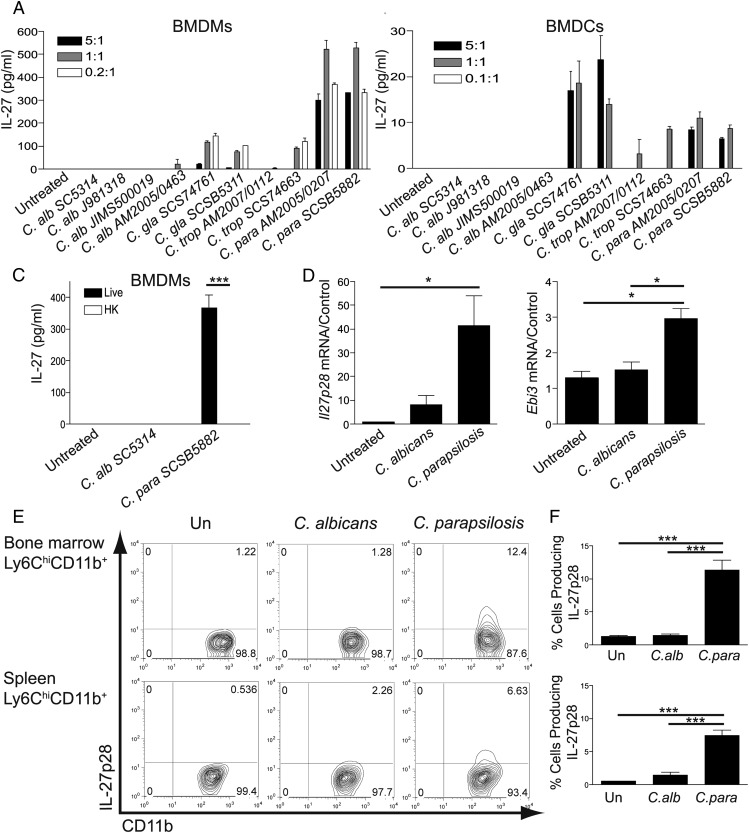
As various Candida spp. induce IL-12 and IL-23 and both of these IL-12 family members are involved in the antifungal immune response (17, 18, 21), we postulated that an additional IL-12 family member, IL-27, may play an important role in the antifungal response to Candida. We first aimed to determine whether any Candida spp. induce IL-27 production. To investigate this possibility, we stimulated BMDMs and BMDCs with two to four strains each of C. albicans, C. glabrata, C. tropicalis, and C. parapsilosis. Of interest, although all four strains of C. albicans failed to induce production of IL-27, C. parapsilosis, C. glabrata, and one strain of C. tropicalis induced varying levels of IL-27 (Fig. 1A, 1B). BMDCs produced very low levels of IL-27, and these were considerably lower than those produced by BMDMs. As C. parapsilosis induced the highest level of IL-27, the following experiments focused on the commonly used laboratory strain of C. albicans (SC5314) versus a clinically isolated strain of C. parapsilosis (SCSB5882). Of note, heat-killed C. parapsilosis failed to elicit IL-27 production (Fig. 1C), indicating that live C. parapsilosis is necessary to induce robust IL-27 levels. Both subunits of IL-27 (Il27p28 and Ebi3) were induced at the RNA level in response to C. parapsilosis and, to a much lesser extent, by C. albicans (Fig. 1D). As BMDMs and BMDCs are differentiated in vitro, we wanted to confirm whether C. parapsilosis could induce IL-27 in primary cells stimulated ex vivo. To this end, bone marrow and splenic cells were stimulated with C. albicans or C. parapsilosis for 22 h. As with the BMDMs and BMDCs, we observed that C. parapsilosis induced IL-27p28 in both bone marrow and splenic B220−Ly6ChiCD11b+ cells, whereas C. albicans did not (Fig. 1E, 1F). These data demonstrate interstrain/interspecies variability in the ability of Candida to induce IL-27 in myeloid cells.
FIGURE 1.
IL-27 Production is induced by C. parapsilosis in myeloid cells. BMDMs (A) and BMDCs (B) from WT mice were stimulated with 10 species and strains of Candida at the indicated ratios of Candida/cells. Cytokine levels in the supernatants were measured after 24-h incubation. (C) BMDMs from WT mice were stimulated with live or heat-killed Candida spp. Cytokine levels in the supernatants were measured after 24-h incubation. (A–C) Results are presented as means ± SEM of three replicates, and data are representative of three independent experiments. ***p < 0.001. (D) BMDMs were stimulated with Candida spp. for 24 h. RNA was isolated, cDNA was prepared, and Il27p28 and Ebi3 mRNA transcripts were detected by real-time quantitative PCR. mRNA levels were normalized to Hprt1. Graph displays mean ± SEM of four biological replicates from three independent experiments. *p < 0.05. (E and F) Splenic and BM cells were cultured with Candida spp. at a ratio of 1:1 Candida/cells for 22 h. IL-27p28 levels were analyzed by flow cytometry. Flow cytometry plots were gated on singlet, autofluorescent−, B220−, and Ly6ChiCD11b+ cells. Data are representative of three independent experiments. (F) Graphs display mean ± SEM percentage of cells expressing IL-27p28 from three mice analyzed by flow cytometry. Graphs are representative of three independent experiments. **p < 0.005 (one-way ANOVA, Bonferroni’s posttest).
C. parapsilosis–induced IL-27 production is dependent on phagocytosis, MyD88, TLR7, and NOD2
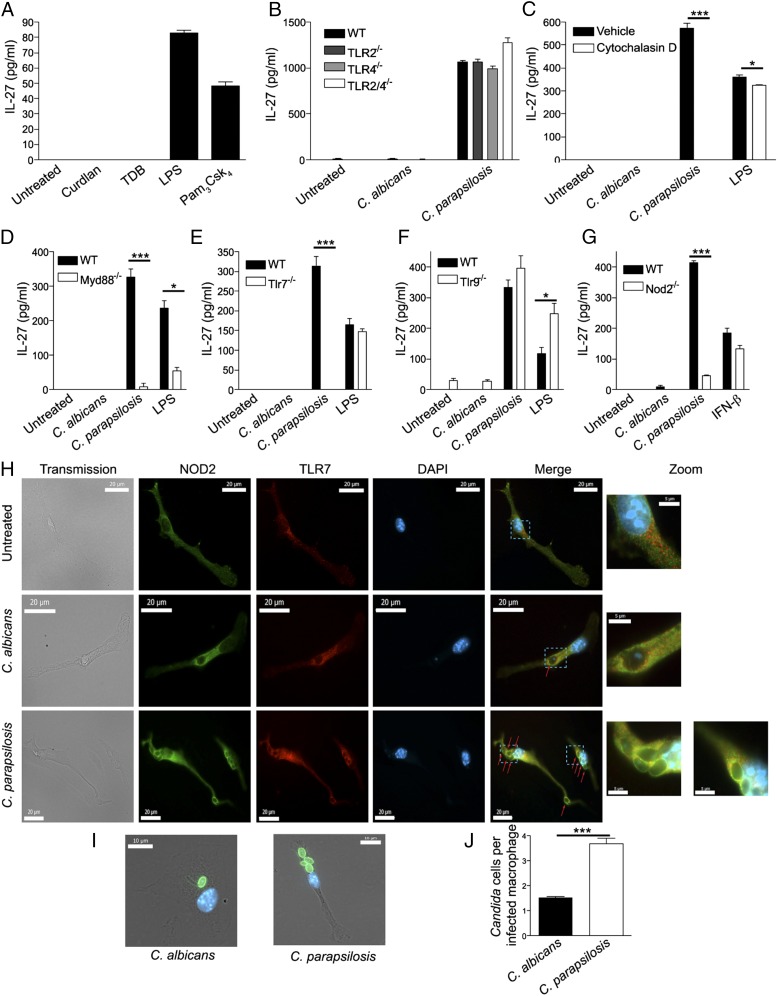
TLR ligands have been shown to induce IL-27 (40, 41). Given that C. parapsilosis induced robust IL-27 production in BMDMs, we next sought to determine which receptors were involved in this response. BMDMs were stimulated with a Dectin-1 ligand (curdlan), a Mincle ligand (TDB), and ligands for TLR4 (LPS) and TLR2 (Pam3Csk4). Only the TLR2 and TLR4 ligands induced IL-27 (Fig. 2A). As Candida spp. contain TLR2/4 ligands in their cell wall, we hypothesized that the IL-27 production was TLR2/4 dependent. Surprisingly, C. parapsilosis–induced IL-27 production was TLR2/4 independent (Fig. 2B). We therefore investigated whether some C-type lectin-like receptors and their associated signaling pathways were required for IL-27 secretion. In agreement with the ligand data from Fig. 2A, BMDMs from Dectin-1 (Clec7a) null mice (Supplemental Fig. 1A) and Mincle (Clec4e) null mice (Supplemental Fig. 1B) confirmed that these receptors were not required for IL-27 induction. As both Mincle and Dectin-2 signal through the FcεRIγ adaptor and several fungal-associated receptors signal through CARD9, we also stimulated FcεRIγ and CARD9 null BMDMs with Candida spp. to find that these signaling components were not required for IL-27 production (Supplemental Fig. 1C, 1D). As none of the expected cell surface receptors were responsible for C. parapsilosis–induced IL-27 production, we next investigated whether phagocytosis was involved. To this end, BMDMs were incubated with cytochalasin D. This resulted in ablation of C. parapsilosis–induced IL-27 production, whereas the effect on LPS was much less pronounced (Fig. 2C). This finding suggested the involvement of endosomal/intracellular receptors. Biondo et al. (23, 50) demonstrated that fungal RNA and fungal DNA signal through the endosomal receptors TLR7 and TLR9, respectively. In addition, NOD2 has recently been shown to respond to the fungal ligand chitin in collaboration with TLR9 (12), and NOD2 responds to viral RNA (51). In line with these data, we next examined whether TLR7/9-MyD88 or NOD2 was involved in mediating C. parapsilosis–induced IL-27 production. Stimulation of MyD88 (Fig. 2D) and TLR7 (Fig. 2E) null BMDMs demonstrated almost complete ablation of C. parapsilosis–induced IL-27 production. However, C. parapsilosis–induced IL-27 secretion was independent of TLR9 (Fig. 2F). Of interest, NOD2 (Fig. 2G) null cells showed a significant reduction in IL-27 production. These data indicate that C. parapsilosis–induced IL-27 production is dependent on phagocytosis, TLR7/MyD88, and NOD2.
FIGURE 2.
Candida-induced IL-27 production is dependent on phagocytosis, MyD88, TLR7, and NOD2. (A) BMDMs from WT mice were stimulated with 10 μg/ml curdlan, 10 μg/ml TDB, 10 ng/ml LPS, and 1 μg/ml Pam3Csk4. Cytokine levels in the supernatants were measured after 24-h incubation. (B) BMDMs from WT, Tlr2−/−, Tlr4−/−, and Tlr2/4−/− mice were stimulated with Candida spp. Cytokine levels in the supernatants were measured after 24-h incubation. (C) BMDMs from WT mice were stimulated with Candida spp. in the presence of vehicle control or 10 μM cytochalasin D. Cytokine levels in the supernatants were measured after 24-h incubation. *p < 0.05, ***p < 0.001. (D–G) BMDMs from WT and Myd88−/− mice (D), WT and Tlr7−/− mice (E), WT and Tlr9−/− mice (F), and WT and Nod2−/− mice (G) were stimulated with Candida spp. and 200 ng/ml LPS or 200 U/ml IFN-β. Cytokine levels in the supernatants were measured after 24-h incubation. For all graphical data, results are presented as means ± SEM of three replicates, and data are representative of two to four independent experiments. *p < 0.05 (D and F), ***p < 0.001 (D, E, and G) (one-way ANOVA, Bonferroni’s posttest). (H) BMDMs from WT mice were unstimulated or stimulated with C. albicans or C. parapsilosis (red arrows) for 1 h. Cells were stained with anti-NOD2 (green), anti-TLR7 (red), and DAPI (blue). Scale bars, 20 μm. Images on the far right are zoomed sections (blue dashed boxes) from the merged images; scale bars, 5 μm. Data are representative of two to four independent experiments. (I and J) BMDMs from WT mice were stimulated with Rhodamine Green-X–labeled C. albicans or C. parapsilosis (green) for 1 h. Cells were stained with DAPI (blue). (I) Images displayed are overlaid on transmission images. Scale bars, 10 μm. Data are representative of three independent experiments. (J) Graph displays mean number of Candida cells per infected BMDM. Data are representative of three independent experiments. ***p < 0.001 (Mann–Whitney U test).
As Wagener et al. (12) observed that TLR9 and NOD2 colocalized in response to the fungal ligand chitin, we aimed to determine whether TLR7 and NOD2 colocalized in a similar manner in response to C. parapsilosis. To this end, WT BMDMs were left unstimulated or stimulated with C. albicans or C. parapsilosis for 1 h. Although some colocalization of TLR7 and NOD2 was observed in unstimulated cells, colocalization of TLR7 and NOD2 increased in response to Candida spp. (Fig. 2H). Colocalization of TLR7 and NOD2 surrounding yeast cells was particularly evident in C. parapsilosis–treated BMDMs. Of note, Candida-infected BMDMs appeared to contain more C. parapsilosis cells than C. albicans cells. To confirm this observation, C. albicans and C. parapsilosis were labeled with Rhodamine Green-X, WT BMDMs were stimulated with the labeled Candida spp. for 1 h, and Candida cells per infected BMDM were counted. In agreement with previous findings (52), we observed that on a per cell basis, infected BMDMs ingested more C. parapsilosis cells than C. albicans cells (Fig. 2I, 2J). These data indicate that TLR7 and NOD2 colocalize in response to Candida spp. in a manner similar to TLR9 and NOD2 colocalization.
IL-27 is a late-induced protein
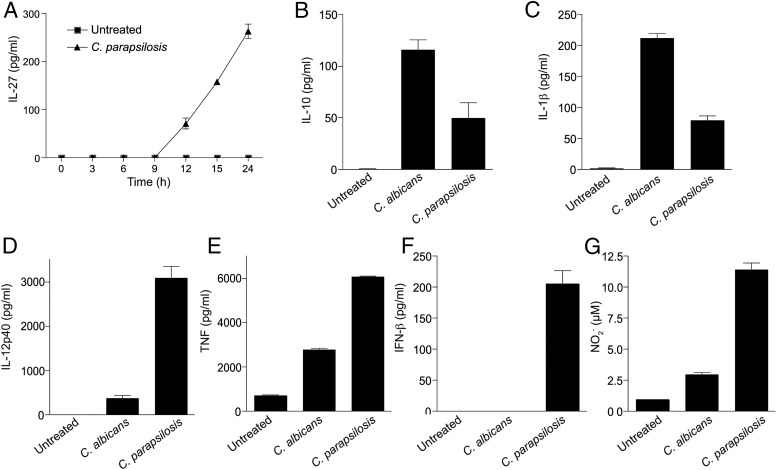
We next sought to examine the kinetics of IL-27 production to determine whether it is directly induced by C. parapsilosis or whether an intermediate is involved. Interestingly, IL-27 production was only detected after 9- to 12-h stimulation with C. parapsilosis (Fig. 3A). As this is a late-induced protein, this suggested that an intermediate is involved in the induction of IL-27 by C. parapsilosis. To identify potential intermediates, we examined the expression profile of various other cytokines in response to the two Candida spp. IL-27 promotes the induction of IL-10 (53–55), and IL-10 has recently been shown to inhibit MyD88-dependent IL-27 release in macrophages (56), suggesting that IL-10 would not likely be responsible for the induction of IL-27 in our experiments. In agreement with this, IL-10 showed a differential expression profile to IL-27 in response to Candida spp. (Fig. 3B). IL-1β also showed a differential expression profile to IL-27 in response to Candida spp. (Fig. 3C). In addition, induction of IFN-α was absent in response to Candida spp. despite significant induction of Ifna4 at the RNA level (data not shown). In contrast, IL-12p40, TNF, and, in particular, IFN-β showed similar response patterns to IL-27 (Fig. 3D–F). Notably, TNF has previously been shown to be an early-induced gene in response to β-glucan, whereas IL-10 and IL-12p40 were late-induced genes (57). Both TNF and IFN-β have previously been shown to induce IL-27 (43, 45). We therefore postulated that TNF and IFN-β may function as intermediates in C. parapsilosis–induced IL-27 production. In support of a role for IFN-β, inducible NO synthase is an IFN-β–inducible gene and NO2− production displayed a similar response to that of IL-27 (Fig. 3G).
FIGURE 3.
IL-27 is a late-induced protein. (A) BMDMs from WT mice were stimulated with C. parapsilosis, and IL-27 levels in the supernatants were measured after the indicated timepoints. (B–F) BMDMs from WT mice were stimulated with Candida spp., and cytokine levels in the supernatants were measured after 24-h incubation. (G) BMDMs from WT mice were stimulated with Candida spp., and NO2− levels in the supernatants were measured after 24-h incubation. For all graphical data, results are presented as means ± SEM of three replicates and data are representative of three to four independent experiments.
C. parapsilosis–induced IL-27 production is IFN-β dependent
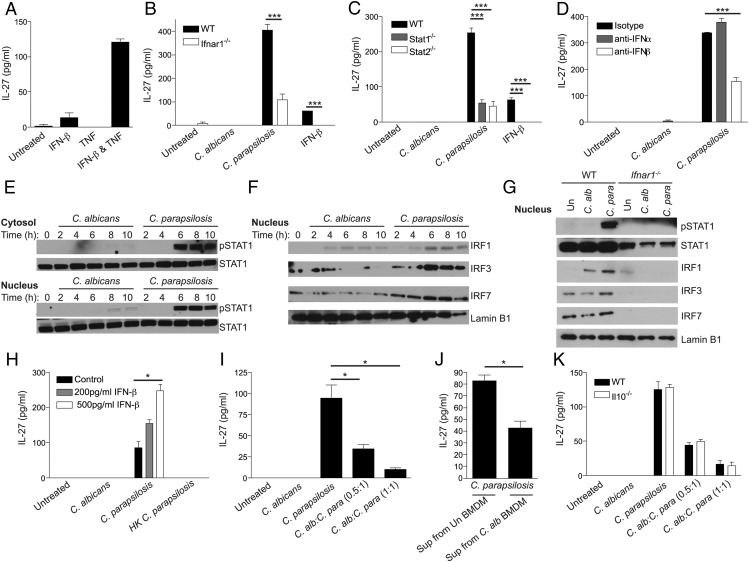
In agreement with previous findings, we observed that IFN-β can induce IL-27 in BMDMs (Fig. 4A); however, we saw no IL-27 production in response to TNF at the concentration used (10 ng/ml). Of interest, though, IFN-β and TNF synergistically induced robust IL-27 production (Fig. 4A). To dissect the contribution of TNF to C. parapsilosis–induced IL-27 production, WT BMDMs were stimulated with Candida spp. in the presence of the TNF inhibitor etanercept. This showed only a minor effect on IL-27 production (Supplemental Fig. 1E), despite a significant reduction in TNF levels (Supplemental Fig. 1F) in the presence of etanercept. We next evaluated IFN-β as the key intermediate in C. parapsilosis–induced IL-27 production. When IFNAR1 (Fig 4B) and STAT1/2 (Fig. 4C) null BMDMs were stimulated with Candida spp., we observed that the IFN pathway was critical in driving the IL-27 response. Furthermore, when BMDMs were stimulated with C. parapsilosis in the presence of blocking Abs to IFN-α and IFN-β, a substantial reduction in IL-27 was detected only in the presence of the anti–IFN-β Ab (Fig. 4D).
FIGURE 4.
Candida-induced IL-27 production is IFN-β dependent. (A) BMDMs from WT mice were stimulated with IFN-β (200 U/ml) or TNF (10 ng/ml), and cytokine levels in the supernatants were measured after 24-h incubation. (B and C) BMDMs from WT and Ifnar1−/− mice (B) and WT, Stat1−/−, and Stat2−/− mice (C) were stimulated with Candida spp., and cytokine levels in the supernatants were measured after 24-h incubation. ***p < 0.001 (one-way ANOVA, Bonferroni’s posttest). (D) BMDMs from WT mice were stimulated with Candida spp. in the presence of isotype control or blocking Abs to IFN-α or IFN-β (10 μg/ml). Cytokine levels in the supernatants were measured after 24-h incubation. For all graphical data, results are presented as means ± SEM of three replicates and data are representative of three to four independent experiments. ***p < 0.001 (one-way ANOVA, Bonferroni’s posttest). (E–G) BMDMs from WT mice (E and F) or WT and Ifnar1−/− mice (G) were stimulated for the indicated times (E and F) or 6 h (G) with 1:1 Candida/cells. (E) Cytosolic and nuclear fractions were immunoblotted with anti-pSTAT1 and anti–STAT-1. (F) Nuclear fractions were immunoblotted with anti-IRF1, anti-IRF3, anti-IRF7, and anti-Lamin B1. (G) Nuclear fractions were immunoblotted with anti-pSTAT1, anti-STAT1, anti-IRF1, anti-IRF3, anti-IRF7, and anti–Lamin B1. (E–G) Data are representative of two independent experiments. (H) BMDMs from WT mice were stimulated with live or heat-killed Candida spp. in the presence or absence of IFN-β, and Il-27 levels in the supernatants were measured after 24-h incubation. (I) BMDMs from WT mice were stimulated with C. albicans, C. parapsilosis, or both spp., and IL-27 levels in the supernatants were measured after 24-h incubation. (J) BMDMs from WT mice were unstimulated or stimulated with C. albicans for 24 h. The supernatants from these cells were added to WT BMDMs stimulated with C. parapsilosis, and IL-27 levels in the supernatants were measured after 24-h incubation. (K) BMDMs from WT and Il10−/− mice were stimulated with C. albicans, C. parapsilosis, or both spp., and IL-27 levels in the supernatants were measured after 24-h incubation. (H–K) For all graphical data, results are presented as means ± SEM of three replicates, and data are representative of two to four independent experiments. *p < 0.05 [one-way ANOVA, Bonferroni’s posttest (H and I), Student t test (J)].
We have demonstrated differential induction of IL-27 in response to C. parapsilosis versus C. albicans (see Fig. 1A). To further determine whether this differential control of IL-27 was linked to the IFN response, we investigated downstream signaling pathways. In this context, the robust IL-27 response to C. parapsilosis was associated with strong and prolonged phosphorylation of STAT1 in both the cytosol and the nucleus (Fig. 4E) and with translocation of IRF1, IRF3, and IRF7 to the nucleus (Fig. 4F). Conversely, the absence of an IL-27 response to C. albicans was associated with weak and transient phosphorylation of STAT1 and translocation of IRF1, IRF3, and IRF7 (Fig. 4E, 4F). In agreement with these data, STAT1 phosphorylation and translocation of IRF1, IRF3, and IRF7 were lost in Ifnar1−/− cells (Fig. 4G). These data indicate a strong dependence of C. parapsilosis–induced IL-27 production on IFN-β.
As we have shown that C. parapsilosis induced IL-27 via IFN-β, we next sought to determine whether IFN-β could synergize with C. albicans to induce IL-27. We observed that low levels of IFN-β increased the production of IL-27 in response to C. parapsilosis; however, IFN-β did not synergize with C. albicans or heat-killed C. parapsilosis (Fig. 4H). In addition to C. albicans failing to induce IFN-β production (Fig. 3F), we next asked whether C. albicans could actively suppress IL-27 production. To this end, C. albicans and C. parapsilosis were added to BMDMs at the given ratios. We demonstrated that addition of C. albicans actively blocked IL-27 production in response to C. parapsilosis (Fig. 4I). In addition, we found that supernatants from BMDMs stimulated with C. albicans also blocked C. parapsilosis–induced IL-27 production (Fig. 4J), indicating that although C. parapsilosis induces IL-27 via IFN-β production, C. albicans inhibits IL-27 production via a soluble mediator. As C. albicans induces more IL-10 than C. parapsilosis (Fig. 3B), we further determined whether IL-10 was responsible for blocking C. parapsilosis–induced IL-27. Notably, C. albicans continued to block C. parapsilosis–induced IL-27 production in the absence of IL-10 (Fig. 4K), indicating the involvement of an as yet unidentified soluble mediator.
Il27ra−/− mice display enhanced clearance of C. parapsilosis
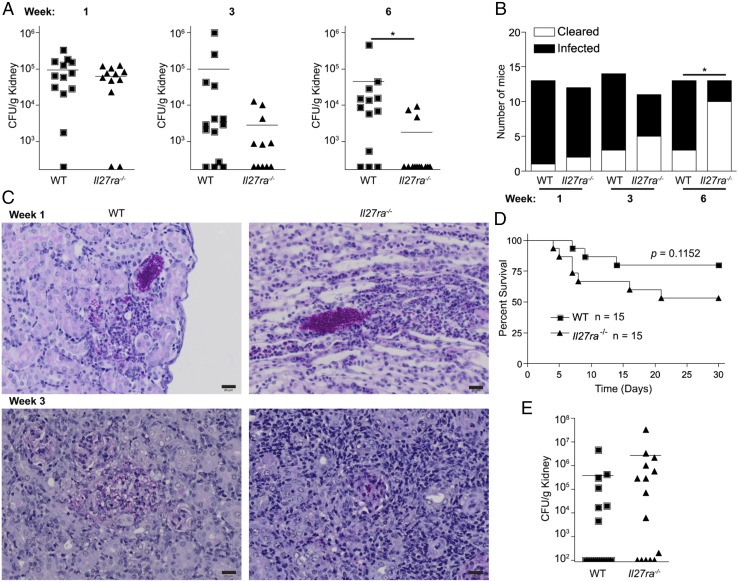
C. parapsilosis induced significant levels of IL-27 from myeloid cells in vitro and ex vivo (see Fig. 1). Systemic infection (i.v.) of WT mice with C. parapsilosis similarly demonstrated in vivo production of IL-27p28 by F4/80+CD11b+Ly6c+ cells and CD11c+MHCII+ cells (Supplemental Fig. 2). To determine whether IL-27 was important for host pathogen clearance, we next infected WT and Il27ra−/− mice with C. parapsilosis, and Il27ra−/− mice, compared with WT mice, displayed enhanced fungal clearance, as shown by the progressive reduction in fungal burden in the kidneys by week 6 (Fig. 5A–C). WT and Il27ra−/− mice displayed comparable fungal burden in the brains at 1 wk post infection, which was followed by complete clearance by week 3 (data not shown). Although C. albicans does not induce IL-27 in vitro, we next examined whether the increased clearance was specific for C. parapsilosis or whether C. albicans induced a similar effect in vivo. C. albicans is considerably more pathogenic than C. parapsilosis, and as a result infected mice will succumb to the infection. Although Il27ra−/− mice displayed improved clearance of C. parapsilosis (Fig. 5A–C), they did not show improved clearance of C. albicans or improved survival (Fig. 5D, 5E). Overall, these data demonstrate that C. parapsilosis–induced IL-27 hinders fungal clearance in a systemic C. parapsilosis infection model.
FIGURE 5.
Il27ra−/− mice display enhanced clearance of C. parapsilosis. (A) CFU in the kidneys of WT (■) and Il27ra−/−(▲) mice 1, 3, and 6 wk after i.v. infection with 1.5 × 107 CFU C. parapsilosis. Graphs are the cumulative result of two independent experiments. *p < 0.05 (Student t test on transformed data). Each symbol represents an individual mouse. (B) Number of mice that have cleared the infection (<200 CFU/g kidney) (white bar) or remain infected (black bar) 1, 3, or 6 wk after i.v. infection with 1.5 × 107 CFU C. parapsilosis. *p < 0.05 (Student t test on transformed data). (C) Fungal growth in representative WT (left panels [×20 magnification]) or Il27ra−/− (right panels [×20 magnification]) kidneys 1 and 3 wk after i.v. infection with 1.5 × 107 CFU C. parapsilosis. Kidney sections were stained with periodic acid–Schiff. Scale bars, 20 μm. (D and E) Survival curves (D) and CFU in the kidneys (E) of WT (■) and Il27ra−/−(▲) mice infected i.v. with 1.5 × 105 CFU C. albicans for 30 d. Each symbol represents an individual mouse. Graphs are the cumulative result of two independent experiments. (D) p = 0.1152 (log-rank test), n = 15.
Inflammatory infiltrates are minimally elevated in Il27ra−/− mice
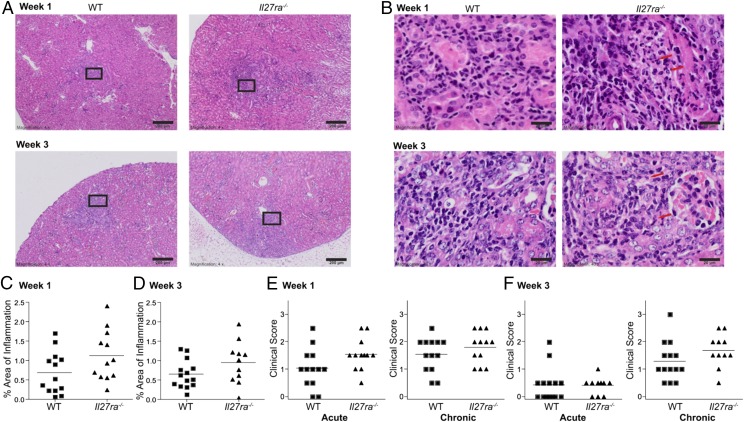
Previous studies have demonstrated that Il27ra−/− mice display increased organ pathological changes in response to various pathogens, including T. gondii, malaria-causing parasites (Plasmodium), and Leishmania donovani (29, 58, 59). Therefore, we next examined whether Il27ra−/− mice displayed increased inflammatory infiltrates in the kidneys following infection with C. parapsilosis (Fig. 6A, 6B). In general, Il27ra−/− mice displayed a trend toward increased inflammation when compared with WT mice; however, this was not statistically significant (Fig. 6C–F). At 1 wk and 3 wk post infection, the percentage area of inflammation in the kidneys from Il27ra−/− mice was slightly elevated (Fig. 6C, 6D) compared with that in WT mice. In addition, when compared with WT mice, Il27ra−/− mice with patchy tubulointerstitial suppurative nephritis showed only slightly elevated foci of neutrophils (acute inflammation) at 1 wk post infection (Fig. 6E) and slightly elevated chronic inflammation (lymphocytes) 3 wk post infection (Fig. 6F). These data indicate that IL-27 delays clearance of C. parapsilosis but does not have a significant effect on low-level nephritis caused by C. parapsilosis.
FIGURE 6.
Inflammatory infiltrates are minimally elevated in Il27ra−/− mice. (A) Representative WT (left panels [×4 magnification]) or Il27ra−/− (right panels [×4 magnification]) kidneys, 1 and 3 wk after i.v. infection with 1.5 × 107 CFU C. parapsilosis. Kidney sections were stained with H&E. Scale bars, 200 μm. (B) Higher magnification (×40 magnification) of boxed areas from (A). Scale bars represent 20 μm. Red arrows indicate neutrophil infiltration. (C and D) Graphs display percentage of area of inflammation in the kidneys from mice 1 wk (C) or 3 wk (D) after i.v. infection with 1.5 × 107 CFU C. parapsilosis. ■, WT mice; ▲, Il27ra−/− mice. (E and F) Graphs display average acute (neutrophilic) and chronic (lymphocytic) clinical scores from the cortex and medulla of kidneys from mice 1 wk (E) or 3 wk (F) after i.v. infection with 1.5 × 107 CFU C. parapsilosis stained with H&E. Graphs are the cumulative result of two independent experiments. Each symbol represents the average score from cortex and medulla for an individual mouse.
Il27ra−/− mice display increased proinflammatory responses to C. parapsilosis
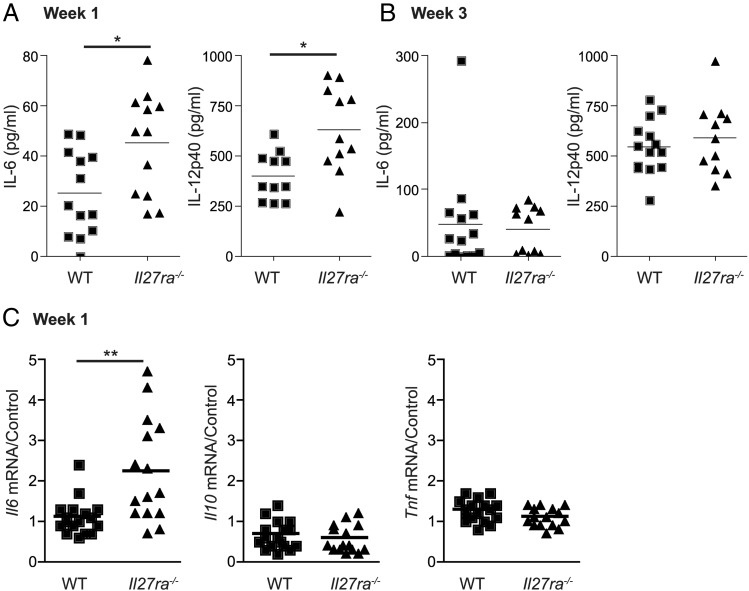
As Il27ra−/− mice displayed improved clearance of C. parapsilosis, we wanted to determine whether IL-27 affects the host inflammatory response to C. parapsilosis. Of note, Il27ra−/− mice demonstrated increased serum levels of some proinflammatory cytokines (IL-12p40, IL-6) 1 wk post infection with C. parapsilosis (Fig. 7A). However, by week 3, serum cytokine levels were similar between WT and Il27ra−/− mice (Fig. 7B). Serum levels of other cytokines, including IL-10 and TNF, were very low or undetected (data not shown). At 1 wk post infection, mRNA levels of Il6 in the spleen were increased in Il27ra−/− mice, similar to the serum; however, Il10 and Tnf mRNA levels were similar between WT and Il27ra−/− mice (Fig. 7C). These data indicate that selective proinflammatory cytokines (IL-12p40, IL-6) are increased in Il27ra−/− mice at early stages of infection with C. parapsilosis.
FIGURE 7.
Il27ra−/− mice display increased proinflammatory responses. (A and B) Cytokine levels in the serum of WT (■) and Il27ra−/− (▲) mice 1 wk (A) and 3 wk (B) post infection with 1.5 × 107 CFU C. parapsilosis. Graphs are the cumulative result of two independent experiments. *p < 0.05 (Student t test). Each symbol represents an individual mouse. (C) RNA was isolated from spleen cells 1 wk post infection with 1.5 × 107 CFU C. parapsilosis; cDNA was prepared; and Il6, Il10, and Tnf mRNA transcripts were detected by real-time quantitative PCR. mRNA levels were normalized to Hprt1. Graphs are the cumulative result of three independent experiments. Each symbol represents an individual mouse. **p < 0.005 (Student t test).
Il27ra−/− mice display increased IFN-γ production in response to C. parapsilosis
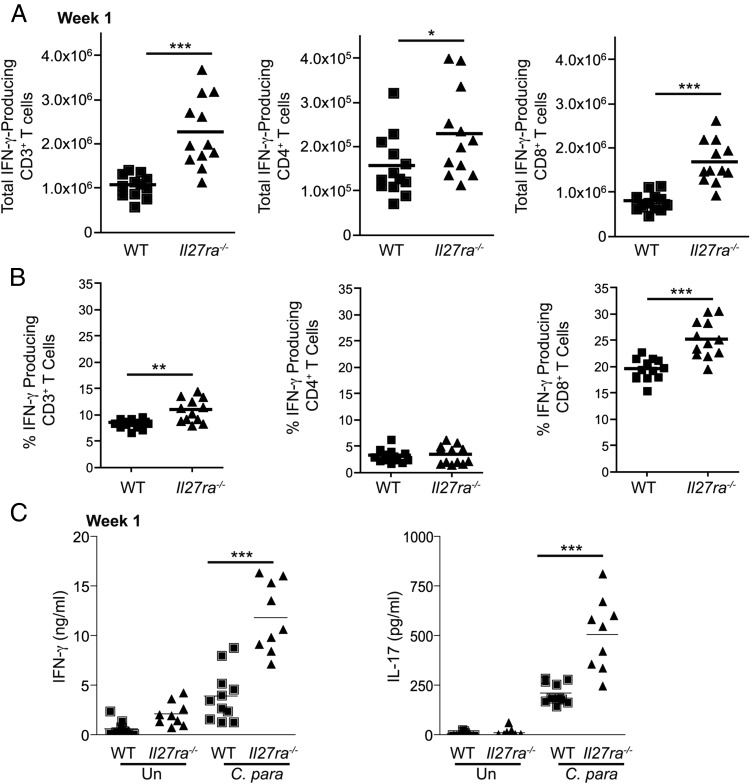
To examine IFN-γ and IL-17 responses, splenocytes from C. parapsilosis–infected mice were restimulated with PMA/ionomycin for 4 h. We observed an increased number (Fig. 8A) and percentage (Fig. 8B, Supplemental Fig. 3) of splenic IFN-γ–producing T cells in C. parapsilosis–infected Il27ra −/− mice, whereas IL-17–producing cells were very low (Supplemental Fig. 3). Of interest, the majority of these IFN-γ–producing T cells were CD8+ rather than CD4+ Th cells (Fig. 8A, 8B, Supplemental Fig. 3). In addition, splenocytes from WT and Il27ra−/− mice 1 wk post infection were restimulated with C. parapsilosis for 48 h (Fig. 8C), and IFN-γ and IL-17 levels were analyzed. Cells from Il27ra−/− mice, compared with cells from WT mice, demonstrated increased robust production of IFN-γ and IL-17 in response to C. parapsilosis (Fig. 8C). In contrast, splenic cells from naive mice stimulated with C. parapsilosis for 48 h produced little IFN-γ or IL-17 (Supplemental Fig. 3). In addition, we demonstrated that Foxp3 mRNA levels in the spleen are reduced in Il27ra−/− mice (Supplemental Fig. 3), suggesting that Treg numbers are reduced in these mice.
FIGURE 8.
Il27ra−/− mice display increased IFN-γ production in response to C. parapsilosis. (A and B) WT (■) and Il27ra−/− (▲) mice were injected i.v. with C. parapsilosis. At 1 wk post infection, splenic cells were restimulated with PMA/ionomycin for 4 h. IFN-γ– and IL-17–producing NK1.1−CD3+ T cells, NK1.1−CD3+CD4+CD8− T cells, and NK1.1−CD3+CD4−CD8+ T cells were measured by flow cytometry. Graph displays total number (A) and percentage (B) of CD3+NK1.1− T cells, or CD8+ or CD4+ T cells producing IFN-γ. Each symbol represents an individual mouse. Graphs are the cumulative result of two independent experiments. *p < 0.05, **p < 0.005, ***p < 0.001 (Student t test). (C) WT and Il27ra−/− splenic cells 1 wk after i.v. injection of C. parapsilosis were left unstimulated or stimulated with C. parapsilosis for 48 h. IFN-γ and IL-17 levels in the supernatants were measured by ELISA. Graphs are the cumulative result of two independent experiments. Each symbol represents an individual mouse. ***p < 0.001 (one-way ANOVA, Bonferroni’s posttest).
Thus, these data indicate that IL-27 inhibits T cell effector cytokine production in response to C. parapsilosis.
Discussion
In this article, for the first time, to our knowledge, we have demonstrated that specific Candida spp. induce IL-27 production in myeloid cells and we have identified an important role for IL-27 in the immune response to C. parapsilosis. We have shown that C. parapsilosis–induced IL-27 production was dependent on TLR7/MyD88 and NOD2 signaling. In addition, IL-27 induction was downstream of IFN-β production, followed by signaling through IFNAR1; STAT1/2 (Supplemental Fig. 4); and the activation of IRF1, IRF3, and IRF7. Similar to findings with other infectious agents, IL-27 inhibits IFN-γ and IL-17 responses (28, 58) and Il27ra−/− mice demonstrate enhanced clearance of C. parapsilosis, compared with WT mice. However, in contrast to findings with other infectious agents, the enhanced IFN-γ responses did not result in severely increased disease in Il27ra−/− mice. Taken together, these data indicate that blocking IL-27 during C. parapsilosis infections could expedite clearance of the pathogen.
On the basis of our data, we believe that various factors control the ability of C. parapsilosis to induce IL-27 and the inability of C. albicans to induce IL-27. First, we propose that after initial recognition, phagocytosis of C. parapsilosis is required to facilitate activation of a pathway involving TLR7 and NOD2 that culminates in the production of IL-27. In agreement with previous findings (52), we show that on a per cell basis, macrophages ingest more C. parapsilosis cells than C. albicans cells (Fig. 2I, 2J). Our data also indicate that phagocytosed C. parapsilosis signals through TLR7 and NOD2. Candida RNA has been shown to signal through TLR7 (10, 23), and NOD2 was recently shown to recognize viral RNA (51). Although chitin was also recently shown to signal through NOD2, TLR9, and CARD9 (12), we have demonstrated that C. parapsilosis–induced IL-27 is independent of TLR9 and CARD9. Interestingly, TLR9 and NOD2 were shown to colocalize in response to chitin (12). In our studies, we have observed that although some basal TLR7 and NOD2 colocalization occurs, Candida spp. induce TLR7 and NOD2 colocalization surrounding the yeast cells, which was particularly robust around C. parapsilosis cells. However, further studies are required to determine what signals cause the colocalization of TLR7 and NOD2 and what ligand or ligands signal through TLR7 and NOD2.
Second, we have demonstrated that C. parapsilosis induces IFN-β and subsequently IL-27; however, C. albicans does not induce IFN-β or the resulting IL-27. Candida spp. have recently been shown to induce IFN-β, although the mechanism remains controversial. Bourgeois et al. (10) reported that C. glabrata induced significant levels of IFN-β from BMDCs, but not BMDMs, whereas C. albicans– and C. dubliniensis–induced IFN-β levels from BMDCs were considerably lower. C. glabrata–induced IFN-β was produced in a TLR2-, TLR4-, TLR9-, Dectin-1–, and CD11b-independent manner. IFN-β was produced in a phagocytosis-, TLR7/MyD88-, and IRF1-dependent manner, and the resulting IFN-β subsequently signaled through IFNAR1 to induce IRF7 activation, producing a feedback loop resulting in further IFN-β production. Biondo et al. (50) showed that C. albicans and S. cerevisae induced IFN-β from DCs in a TLR7/TLR9-MyD88– and IRF1/3/7-dependent manner. In contrast to the previous two studies, del Fresno et al. (60) reported that heat-killed C. albicans or curdlan-induced IFN-β from DCs was Dectin-1, Dectin-2, Syk, and Card9 dependent. They also showed the Dectin-1–induced IFN-β was IRF5 dependent and IRF3/7 independent. The differences in these studies could be due to the different ligands or species/strains of Candida used. In our study, we demonstrate a phagocytosis-, TLR7/MyD88-, IFNAR1-, and STAT1/2-dependent induction of IL-27 downstream of C. parapsilosis that is reminiscent of the pathway reported by Bourgeois et al. to induce IFN-β in response to C. glabrata (10). Of note, the previous three studies observed either no or very little IFN-β production from BMDMs (10, 50, 60). In contrast, we observed IFN-β production from BMDMs in response to C. parapsilosis (Fig. 3F). C. parapsilosis was not used in the previous studies, which could explain this discrepancy. In addition, we have observed the dependence of IL-27 production on NOD2. NOD2 has previously been linked to the induction of IFN-β production in response to Listeria monocytogenes, which involved synergy with other cytosolic microbial sensors (61). TLR7 and NOD2 are both required for C. parapsilosis–induced IL-27, although whether this involves synergy between these two receptors remains to be determined.
Third, we found that in addition to not inducing IL-27, C. albicans actively blocks C. parapsilosis–induced IL-27 production via a soluble mediator. C. albicans is much more virulent than C. parapsilosis, and mice infected with C. albicans display increased organ pathological changes compared with mice infected with C. parapsilosis (data not shown). As IL-27 limits host disease (28, 29, 31, 32), it is possible that the ability of C. albicans to block IL-27 production may contribute to the increased pathological changes observed in mice infected with C. albicans versus those infected with C. parapsilosis. In addition, although we have not investigated this further, it is possible that differences in the intracellular fates of C. albicans and C. parapsilosis could affect their ability to induce IL-27. As mentioned previously, C. parapsilosis is less pathogenic than C. albicans (4). Although various Candida spp. can promote intracellular survival through modulation of phagosome maturation (62, 63), C. albicans can also escape from the phagosome and cause host cell lysis (63–65). It is possible that the increased length of time spent by C. parapsilosis in the phagosome through modulation of phagosome maturation (52) may promote sustained activation compared with that in C. albicans. Taken together, these data suggest that following phagocytosis, C. parapsilosis signals through TLR7 and NOD2, resulting in IFN-β production, which subsequently leads to IL-27 production.
The role of the IL-27R during infectious diseases is complex and has been the subject of numerous investigations in recent years. IL-27R was reported to be critical for resistance to Trypanosoma cruzi and Leishmania major infections in mice (48, 66); however, other studies reported enhanced clearance of infections and subsequent development of lethal disease in Il27ra−/− mice, which highlights the complex roles of IL-27 during infection. During the L. major infection model Il27ra−/− mice displayed increased Th2 cell cytokines and reduced Th1 cell responses, whereas during the T. cruzi infection model Il27ra−/− mice displayed increased Th2 and Th1 cytokines (48, 66). The elevated Th2 cell response to T. cruzi in Il27ra−/− mice was responsible for prolonged parasitemia in these mice, and the liver disease in Il27ra−/− mice was due to the enhanced Th1 cell responses (66). Of interest, reduced bacterial loads were reported in organs of Il27ra−/− mice infected with M. tuberculosis when compared with organs in WT mice. This finding was accompanied by increased production of proinflammatory cytokines (TNF and IL-12p40), CD4+ T cell activation, IFN-γ production, and accelerated death due to chronic hyperinflammation (28). In a more recent study, Findlay et al. (58) demonstrated enhanced parasite clearance in a model of Plasmodium berghei infection associated with elevated accumulation of IFN-γ–producing CD4+ T cells. Il27ra−/− mice developed severe liver disease that was prevented by depleting CD4+ T cells, but not CD8+ T cells. This group recently showed that IL-27R signaling inhibits the generation of terminally differentiated KLRG-1+ Th1 cells, thereby limiting IFN-γ production from T cells (67). Similarly, during a model of T. gondii infection, Il27ra−/− mice displayed enhanced Th1 cell responses and they developed a lethal inflammatory disease that was rescued by the depletion of CD4+ T cells, but not CD8+ T cells (29). This group also demonstrated that IL-27 is important for the development of specialized Tregs that control Th1 cell responses at local sites of inflammation (37). In our study, we demonstrated enhanced clearance of C. parapsilosis in Il27ra−/− mice that is associated with increased production of proinflammatory cytokines in the serum and increased IFN-γ and IL-17 production in the spleens. The adaptive T cell response to C. parapsilosis has not been widely characterized to date; however, one study in PBMCs demonstrated that C. parapsilosis induces both Th1 and Th17 responses (68). Our results clearly demonstrate that Il27ra−/− mice display an enhanced inflammatory response, which promotes C. parapsilosis eradication. Although we have observed enhanced inflammatory responses and slightly elevated inflammation in the kidneys, these changes have not resulted in severe or lethal disease. This may be because C. parapsilosis is mainly nonpathogenic in healthy individuals and because in our model it induces a CD8+ T cell–biased response with a much lower CD4+ T cell response.
Taken together, our results identify a previously unrecognized role for IL-27 in the regulation of C. parapsilosis infections. This is the first description of IL-27 production in response to any Candida spp. alone. IL-27 is produced through a complex phagocytosis, TLR7/NOD2, IFN-β, IFNAR1-STAT1/2 pathway (Supplemental Fig. 4). The absence of IL-27 signaling promotes enhanced IFN-γ and IL-17 responses that correlate with enhanced clearance of the pathogen. Therefore, blockade of IL-27 signaling during C. parapsilosis infections could be considered as a potential therapy; however, further studies are required to determine whether this would be beneficial.
Supplementary Material
Acknowledgments
We thank Donna MacCallum (University of Aberdeen, Aberdeen, U.K.) for providing Candida spp.; Giorgio Trinchieri (National Institutes of Health, Bethesda, MD) and Daniel McVicar (National Cancer Institute–Frederick, Frederick, MD) for providing bone marrow from Myd88−/−, Tlr9−/−, Ifnar1−/−, Stat1−/−, and Stat2−/− mice; Caetano Reis e Sousa (Cancer Research UK) for providing bones from Tlr7−/− mice; Laura Quigley, Pani Tourlomousis, Neil Rogers, Barbara Bodendorfer, and Simone Lipinski for preparing bones/bone marrow from the Tlr2−/−, Tlr4−/−, Tlr2/4−/−, Card9−/−, Tlr7−/−, Myd88−/−, Tlr9−/−, Ifnar1−/−, Stat1−/−, Stat2−/−, Clec4e−/−, Fcre1g−/−, and Nod2−/− mice used in this study; Geraint Millar and Kathy Allsopp for histology preparations; and Scott Durum for critically reviewing this manuscript.
This work was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant 099953/Z/12/Z) (to S.J.O.) and an Arthritis Research UK Fellowship (Ref. 20305) (to G.W.J.). P.R. was supported by Deutsche Forschungsgemeinschaft Cluster of Excellence Inflammation at Interfaces and Deutsche Forschungsgemeinschaft Grant SFB 877, TP B9; P.R.T. is supported by Medical Research Council UK Grants MR/J002151/1 and MR/K02003X/1; R.L. is supported by Deutsche Forschungsgemeinschaft Grant SFB 796, TP B6; C.E.B. is supported by Biotechnology and Biological Sciences Research Council Grant BB/K006436/1; and I.R.H. is supported by a Wellcome Trust Senior Research Fellowship in Basic Biomedical Sciences (Ref. 098026).
The online version of this article contains supplemental material.
- BMDC
- BM-derived DC
- BMDM
- bone marrow–derived macrophage
- DC
- dendritic cell
- IFNAR
- IFN-α/β receptor
- NOD
- nucleotide oligomerization domain
- Treg
- T regulatory cell
- WT
- wild-type
- YPD
- yeast extract/peptone/dextrose.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Alangaden G. J. 2011. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect. Dis. Clin. North Am. 25: 201–225. [DOI] [PubMed] [Google Scholar]
- 2.Hidron A. I., Edwards J. R., Patel J., Horan T. C., Sievert D. M., Pollock D. A., Fridkin S. K., National Healthcare Safety Network Team. Participating National Healthcare Safety Network Facilities 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29: 996–1011. [DOI] [PubMed] [Google Scholar]
- 3.Brown G. D., Denning D. W., Gow N. A., Levitz S. M., Netea M. G., White T. C. 2012. Hidden killers: human fungal infections. Sci. Transl. Med. 4: 165rv13. [DOI] [PubMed] [Google Scholar]
- 4.Arendrup M., Horn T., Frimodt-Møller N. 2002. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30: 286–291. [DOI] [PubMed] [Google Scholar]
- 5.Brown G. D., Taylor P. R., Reid D. M., Willment J. A., Williams D. L., Martinez-Pomares L., Wong S. Y., Gordon S. 2002. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 196: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson M. J., Osorio F., Rosas M., Freitas R. P., Schweighoffer E., Gross O., Verbeek J. S., Ruland J., Tybulewicz V., Brown G. D., et al. 2009. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 206: 2037–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells C. A., Salvage-Jones J. A., Li X., Hitchens K., Butcher S., Murray R. Z., Beckhouse A. G., Lo Y. L., Manzanero S., Cobbold C., et al. 2008. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 180: 7404–7413. [DOI] [PubMed] [Google Scholar]
- 8.Jouault T., Ibata-Ombetta S., Takeuchi O., Trinel P. A., Sacchetti P., Lefebvre P., Akira S., Poulain D. 2003. Candida albicans phospholipomannan is sensed through toll-like receptors. J. Infect. Dis. 188: 165–172. [DOI] [PubMed] [Google Scholar]
- 9.Netea M. G., Gow N. A., Munro C. A., Bates S., Collins C., Ferwerda G., Hobson R. P., Bertram G., Hughes H. B., Jansen T., et al. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116: 1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourgeois C., Majer O., Frohner I. E., Lesiak-Markowicz I., Hildering K. S., Glaser W., Stockinger S., Decker T., Akira S., Müller M., Kuchler K. 2011. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-β signaling. J. Immunol. 186: 3104–3112. [DOI] [PubMed] [Google Scholar]
- 11.Miyazato A., Nakamura K., Yamamoto N., Mora-Montes H. M., Tanaka M., Abe Y., Tanno D., Inden K., Gang X., Ishii K., et al. 2009. Toll-like receptor 9-dependent activation of myeloid dendritic cells by deoxynucleic acids from Candida albicans. Infect. Immun. 77: 3056–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagener J., Malireddi R. K., Lenardon M. D., Köberle M., Vautier S., MacCallum D. M., Biedermann T., Schaller M., Netea M. G., Kanneganti T. D., et al. 2014. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 10: e1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., Fujikado N., Kusaka T., Kubo S., Chung S. H., et al. 2010. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32: 681–691. [DOI] [PubMed] [Google Scholar]
- 14.Taylor P. R., Tsoni S. V., Willment J. A., Dennehy K. M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., Brown G. D. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 8: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeibundGut-Landmann S., Gross O., Robinson M. J., Osorio F., Slack E. C., Tsoni S. V., Schweighoffer E., Tybulewicz V., Brown G. D., Ruland J., Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8: 630–638. [DOI] [PubMed] [Google Scholar]
- 16.Gozalbo D., Gil M. L. 2009. IFN-gamma in Candida albicans infections. Front. Biosci. (Landmark Ed.) 14: 1970–1978. [DOI] [PubMed] [Google Scholar]
- 17.Romani L., Mencacci A., Tonnetti L., Spaccapelo R., Cenci E., Puccetti P., Wolf S. F., Bistoni F. 1994. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J. Immunol. 153: 5167–5175. [PubMed] [Google Scholar]
- 18.Conti H. R., Shen F., Nayyar N., Stocum E., Sun J. N., Lindemann M. J., Ho A. W., Hai J. H., Yu J. J., Jung J. W., et al. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W., Na L., Fidel P. L., Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 190: 624–631. [DOI] [PubMed] [Google Scholar]
- 20.Eyerich K., Foerster S., Rombold S., Seidl H. P., Behrendt H., Hofmann H., Ring J., Traidl-Hoffmann C. 2008. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J. Invest. Dermatol. 128: 2640–2645. [DOI] [PubMed] [Google Scholar]
- 21.Zelante T., De Luca A., Bonifazi P., Montagnoli C., Bozza S., Moretti S., Belladonna M. L., Vacca C., Conte C., Mosci P., et al. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37: 2695–2706. [DOI] [PubMed] [Google Scholar]
- 22.Bourgeois C., Kuchler K. 2012. Fungal pathogens—a sweet and sour treat for toll-like receptors. Front. Cell. Infect. Microbiol. 2: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biondo C., Malara A., Costa A., Signorino G., Cardile F., Midiri A., Galbo R., Papasergi S., Domina M., Pugliese M., et al. 2012. Recognition of fungal RNA by TLR7 has a nonredundant role in host defense against experimental candidiasis. Eur. J. Immunol. 42: 2632–2643. [DOI] [PubMed] [Google Scholar]
- 24.van der Graaf C. A., Netea M. G., Franke B., Girardin S. E., van der Meer J. W., Kullberg B. J. 2006. Nucleotide oligomerization domain 2 (Nod2) is not involved in the pattern recognition of Candida albicans. Clin. Vaccine Immunol. 13: 423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignali D. A., Kuchroo V. K. 2012. IL-12 family cytokines: immunological playmakers. Nat. Immunol. 13: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson B. M., Sullivan J. A., Burlingham W. J. 2013. Interleukin 35: a key mediator of suppression and the propagation of infectious tolerance. Front. Immunol. 4: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goriely S., Neurath M. F., Goldman M. 2008. How microorganisms tip the balance between interleukin-12 family members. Nat. Rev. Immunol. 8: 81–86. [DOI] [PubMed] [Google Scholar]
- 28.Hölscher C., Hölscher A., Rückerl D., Yoshimoto T., Yoshida H., Mak T., Saris C., Ehlers S. 2005. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 174: 3534–3544. [DOI] [PubMed] [Google Scholar]
- 29.Villarino A., Hibbert L., Lieberman L., Wilson E., Mak T., Yoshida H., Kastelein R. A., Saris C., Hunter C. A. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19: 645–655. [DOI] [PubMed] [Google Scholar]
- 30.Owaki T., Asakawa M., Morishima N., Hata K., Fukai F., Matsui M., Mizuguchi J., Yoshimoto T. 2005. A role for IL-27 in early regulation of Th1 differentiation. J. Immunol. 175: 2191–2200. [DOI] [PubMed] [Google Scholar]
- 31.Batten M., Li J., Yi S., Kljavin N. M., Danilenko D. M., Lucas S., Lee J., de Sauvage F. J., Ghilardi N. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 7: 929–936. [DOI] [PubMed] [Google Scholar]
- 32.Stumhofer J. S., Laurence A., Wilson E. H., Huang E., Tato C. M., Johnson L. M., Villarino A. V., Huang Q., Yoshimura A., Sehy D., et al. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7: 937–945. [DOI] [PubMed] [Google Scholar]
- 33.Liu L., Okada S., Kong X. F., Kreins A. Y., Cypowyj S., Abhyankar A., Toubiana J., Itan Y., Audry M., Nitschke P., et al. 2011. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 208: 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Depner M., Fuchs S., Raabe J., Frede N., Glocker C., Doffinger R., Gkrania-Klotsas E., Kumararatne D., Atkinson T. P., Schroeder H. W., Jr., et al. 2016. The extended clinical phenotype of 26 patients with chronic mucocutaneous candidiasis due to gain-of-function mutations in STAT1. J. Clin. Immunol. 36: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirahara K., Onodera A., Villarino A. V., Bonelli M., Sciumè G., Laurence A., Sun H. W., Brooks S. R., Vahedi G., Shih H. Y., et al. 2015. Asymmetric action of STAT transcription factors drives transcriptional outputs and cytokine specificity. Immunity 42: 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neufert C., Becker C., Wirtz S., Fantini M. C., Weigmann B., Galle P. R., Neurath M. F. 2007. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur. J. Immunol. 37: 1809–1816. [DOI] [PubMed] [Google Scholar]
- 37.Hall A. O., Beiting D. P., Tato C., John B., Oldenhove G., Lombana C. G., Pritchard G. H., Silver J. S., Bouladoux N., Stumhofer J. S., et al. 2012. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 37: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter C. A., Kastelein R. 2012. Interleukin-27: balancing protective and pathological immunity. Immunity 37: 960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stumhofer J. S., Hunter C. A. 2008. Advances in understanding the anti-inflammatory properties of IL-27. Immunol. Lett. 117: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wirtz S., Becker C., Fantini M. C., Nieuwenhuis E. E., Tubbe I., Galle P. R., Schild H. J., Birkenbach M., Blumberg R. S., Neurath M. F. 2005. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. J. Immunol. 174: 2814–2824. [DOI] [PubMed] [Google Scholar]
- 41.Molle C., Nguyen M., Flamand V., Renneson J., Trottein F., De Wit D., Willems F., Goldman M., Goriely S. 2007. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J. Immunol. 178: 7607–7615. [DOI] [PubMed] [Google Scholar]
- 42.Liu J., Guan X., Ma X. 2007. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J. Exp. Med. 204: 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molle C., Goldman M., Goriely S. 2010. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J. Immunol. 184: 1784–1792. [DOI] [PubMed] [Google Scholar]
- 44.Pirhonen J., Sirén J., Julkunen I., Matikainen S. 2007. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J. Leukoc. Biol. 82: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 45.Kalliolias G. D., Gordon R. A., Ivashkiv L. B. 2010. Suppression of TNF-α and IL-1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL-27. J. Immunol. 185: 7047–7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gafa V., Lande R., Gagliardi M. C., Severa M., Giacomini E., Remoli M. E., Nisini R., Ramoni C., Di Francesco P., Aldebert D., et al. 2006. Human dendritic cells following Aspergillus fumigatus infection express the CCR7 receptor and a differential pattern of interleukin-12 (IL-12), IL-23, and IL-27 cytokines, which lead to a Th1 response. Infect. Immun. 74: 1480–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei X. Q., Rogers H., Lewis M. A., Williams D. W. 2011. The role of the IL-12 cytokine family in directing T-cell responses in oral candidosis. Clin. Dev. Immunol. 2011: 697340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida H., Hamano S., Senaldi G., Covey T., Faggioni R., Mu S., Xia M., Wakeham A. C., Nishina H., Potter J., et al. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 15: 569–578. [DOI] [PubMed] [Google Scholar]
- 49.McDonald J. H. 2014. Handbook of Biological Statistics. Sparky House Publishing, Baltimore, Maryland. [Google Scholar]
- 50.Biondo C., Signorino G., Costa A., Midiri A., Gerace E., Galbo R., Bellantoni A., Malara A., Beninati C., Teti G., Mancuso G. 2011. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur. J. Immunol. 41: 1969–1979. [DOI] [PubMed] [Google Scholar]
- 51.Sabbah A., Chang T. H., Harnack R., Frohlich V., Tominaga K., Dube P. H., Xiang Y., Bose S. 2009. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10: 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tóth R., Tóth A., Papp C., Jankovics F., Vágvölgyi C., Alonso M. F., Bain J. M., Erwig L. P., Gácser A. 2014. Kinetic studies of Candida parapsilosis phagocytosis by macrophages and detection of intracellular survival mechanisms. Front. Microbiol. 5: 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Awasthi A., Carrier Y., Peron J. P., Bettelli E., Kamanaka M., Flavell R. A., Kuchroo V. K., Oukka M., Weiner H. L. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8: 1380–1389. [DOI] [PubMed] [Google Scholar]
- 54.Fitzgerald D. C., Ciric B., Touil T., Harle H., Grammatikopolou J., Das Sarma J., Gran B., Zhang G. X., Rostami A. 2007. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 179: 3268–3275. [DOI] [PubMed] [Google Scholar]
- 55.Stumhofer J. S., Silver J. S., Laurence A., Porrett P. M., Harris T. H., Turka L. A., Ernst M., Saris C. J., O’Shea J. J., Hunter C. A. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8: 1363–1371. [DOI] [PubMed] [Google Scholar]
- 56.Bosmann M., Russkamp N. F., Strobl B., Roewe J., Balouzian L., Pache F., Radsak M. P., van Rooijen N., Zetoune F. S., Sarma J. V., et al. 2014. Interruption of macrophage-derived IL-27(p28) production by IL-10 during sepsis requires STAT3 but not SOCS3. J. Immunol. 193: 5668–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardone M., Dzutsev A. K., Li H., Riteau N., Gerosa F., Shenderov K., Winkler-Pickett R., Provezza L., Riboldi E., Leighty R. M., et al. 2014. Interleukin-1 and interferon-γ orchestrate β-glucan-activated human dendritic cell programming via IκB-ζ modulation. PLoS One 9: e114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Findlay E. G., Greig R., Stumhofer J. S., Hafalla J. C., de Souza J. B., Saris C. J., Hunter C. A., Riley E. M., Couper K. N. 2010. Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. J. Immunol. 185: 2482–2492. [DOI] [PubMed] [Google Scholar]
- 59.Rosas L. E., Satoskar A. A., Roth K. M., Keiser T. L., Barbi J., Hunter C., de Sauvage F. J., Satoskar A. R. 2006. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to Leishmania donovani infection but develop severe liver immunopathology. Am. J. Pathol. 168: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.del Fresno C., Soulat D., Roth S., Blazek K., Udalova I., Sancho D., Ruland J., Ardavín C. 2013. Interferon-β production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity 38: 1176–1186. [DOI] [PubMed] [Google Scholar]
- 61.Herskovits A. A., Auerbuch V., Portnoy D. A. 2007. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 3: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seider K., Brunke S., Schild L., Jablonowski N., Wilson D., Majer O., Barz D., Haas A., Kuchler K., Schaller M., Hube B. 2011. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J. Immunol. 187: 3072–3086. [DOI] [PubMed] [Google Scholar]
- 63.Bain J. M., Louw J., Lewis L. E., Okai B., Walls C. A., Ballou E. R., Walker L. A., Reid D., Munro C. A., Brown A. J., et al. 2014. Candida albicans hypha formation and mannan masking of β-glucan inhibit macrophage phagosome maturation. MBio 5: e01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marcil A., Gadoury C., Ash J., Zhang J., Nantel A., Whiteway M. 2008. Analysis of PRA1 and its relationship to Candida albicans–macrophage interactions. Infect. Immun. 76: 4345–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández-Arenas E., Bleck C. K., Nombela C., Gil C., Griffiths G., Diez-Orejas R. 2009. Candida albicans actively modulates intracellular membrane trafficking in mouse macrophage phagosomes. Cell. Microbiol. 11: 560–589. [DOI] [PubMed] [Google Scholar]
- 66.Hamano S., Himeno K., Miyazaki Y., Ishii K., Yamanaka A., Takeda A., Zhang M., Hisaeda H., Mak T. W., Yoshimura A., Yoshida H. 2003. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity 19: 657–667. [DOI] [PubMed] [Google Scholar]
- 67.Villegas-Mendez A., de Souza J. B., Lavelle S. W., Gwyer Findlay E., Shaw T. N., van Rooijen N., Saris C. J., Hunter C. A., Riley E. M., Couper K. N. 2013. IL-27 receptor signalling restricts the formation of pathogenic, terminally differentiated Th1 cells during malaria infection by repressing IL-12 dependent signals. PLoS Pathog. 9: e1003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tóth A., Csonka K., Jacobs C., Vágvölgyi C., Nosanchuk J. D., Netea M. G., Gácser A. 2013. Candida albicans and Candida parapsilosis induce different T-cell responses in human peripheral blood mononuclear cells. J. Infect. Dis. 208: 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.