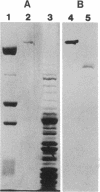
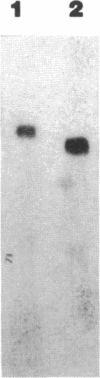
Abstract
We have isolated and determined the nucleotide sequence of the yeast FAS3 gene, which encodes acetyl-CoA carboxylase (EC 6.4.1.2). The sequence has an open reading frame of 6711 bases coding for a protein of 2237 amino acids with a calculated molecular weight of 250,593. The presence of the unique biotin-binding site, Met-Lys-Met, and the known CNBr peptide and COOH-terminal sequences confirmed the nucleotide-derived amino acid sequence. The yeast, chicken, and rat carboxylases have an overall sequence identity of 34%, suggesting that the eukaryotic carboxylase evolved from a single ancestral gene. The amino acid sequences of yeast fatty acid synthase subunits are least homologous with the animal synthase sequences, whereas carboxylase sequences are highly conserved. The sequences of the ATP, HCO3-, and CoA binding sites of the carboxylases are also well conserved (approximately 50% identical). The sequences surrounding the biotin binding site are poorly conserved, suggesting that this sequence may not be critical as long as the biotin is available for carboxylase reactions. On the basis of this sequence identity, we have defined the putative biotin carboxylase and transcarboxylase domains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai D. H., Moon T. W., López-Casillas F., Andrews P. C., Kim K. H. Analysis of the biotin-binding site on acetyl-CoA carboxylase from rat. Eur J Biochem. 1989 Jun 15;182(2):239–245. doi: 10.1111/j.1432-1033.1989.tb14823.x. [DOI] [PubMed] [Google Scholar]
- Chirala S. S., Kuziora M. A., Spector D. M., Wakil S. J. Complementation of mutations and nucleotide sequence of FAS1 gene encoding beta subunit of yeast fatty acid synthase. J Biol Chem. 1987 Mar 25;262(9):4231–4240. [PubMed] [Google Scholar]
- Davies S. P., Sim A. T., Hardie D. G. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990 Jan 12;187(1):183–190. doi: 10.1111/j.1432-1033.1990.tb15293.x. [DOI] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M., Palmieri R. H., Russell G. A., Kuby S. A. Studies of adenosine triphosphate transphosphorylases. XIV. Equilibrium binding properties of the crystalline rabbit and calf muscle ATP--AMP transphosphorylase (adenylate kinase) and derived peptide fragments. Arch Biochem Biophys. 1979 Jun;195(1):155–177. doi: 10.1016/0003-9861(79)90338-2. [DOI] [PubMed] [Google Scholar]
- Kong I. S., López-Casillas F., Kim K. H. Acetyl-CoA carboxylase mRNA species with or without inhibitory coding sequence for Ser-1200 phosphorylation. J Biol Chem. 1990 Aug 15;265(23):13695–13701. [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Firgaira F., Novotný J., Kalousek F., Williams K. R., Williamson C., Ohura T., Rosenberg L. E. Coding sequence of the precursor of the beta subunit of rat propionyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8049–8053. doi: 10.1073/pnas.83.21.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynen F. New experiments of biotin enzymes. CRC Crit Rev Biochem. 1979 Dec;7(2):103–119. doi: 10.3109/10409237909105428. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Bai D. H., Luo X. C., Kong I. S., Hermodson M. A., Kim K. H. Structure of the coding sequence and primary amino acid sequence of acetyl-coenzyme A carboxylase. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5784–5788. doi: 10.1073/pnas.85.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Kamiryo T., Tanaka A., Fukui S., Numa S. Acetyl-coenzyme-A carboxylase of Candida Lipolytica. 1. Purification and properties of the enzyme. Eur J Biochem. 1976 Dec;71(1):295–300. doi: 10.1111/j.1432-1033.1976.tb11115.x. [DOI] [PubMed] [Google Scholar]
- Mohamed A. H., Chirala S. S., Mody N. H., Huang W. Y., Wakil S. J. Primary structure of the multifunctional alpha subunit protein of yeast fatty acid synthase derived from FAS2 gene sequence. J Biol Chem. 1988 Sep 5;263(25):12315–12325. [PubMed] [Google Scholar]
- Remington S., Wiegand G., Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol. 1982 Jun 15;158(1):111–152. doi: 10.1016/0022-2836(82)90452-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Snyder M., Elledge S., Sweetser D., Young R. A., Davis R. W. Lambda gt 11: gene isolation with antibody probes and other applications. Methods Enzymol. 1987;154:107–128. doi: 10.1016/0076-6879(87)54073-3. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Awad E. S., Arslanian M. J., Gunsberg S., Wakil S. J., Oliver R. M. Studies on the yeast fatty acid synthetase. Subunit composition and structural organization of a large multifunctional enzyme complex. J Biol Chem. 1978 Jun 25;253(12):4464–4475. [PubMed] [Google Scholar]
- Suissa M., Suda K., Schatz G. Isolation of the nuclear yeast genes for citrate synthase and fifteen other mitochondrial proteins by a new screening method. EMBO J. 1984 Aug;3(8):1773–1781. doi: 10.1002/j.1460-2075.1984.tb02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumper M., Riepertinger C. Structural relationship of biotin-containing enzymes. Acetyl-CoA carboxylase and pyruvate carboxylase from yeast. Eur J Biochem. 1972 Sep 18;29(2):237–248. doi: 10.1111/j.1432-1033.1972.tb01980.x. [DOI] [PubMed] [Google Scholar]
- Takai T., Yokoyama C., Wada K., Tanabe T. Primary structure of chicken liver acetyl-CoA carboxylase deduced from cDNA sequence. J Biol Chem. 1988 Feb 25;263(6):2651–2657. [PubMed] [Google Scholar]
- Thampy K. G., Wakil S. J. Activation of acetyl-CoA carboxylase. Purification and properties of a Mn2+-dependent phosphatase. J Biol Chem. 1985 May 25;260(10):6318–6323. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]