Abstract
Several previous studies indicated that for optimal uptake by the brain, docosahexaenoic acid (DHA) should be present as phospholipid in the plasma. However most of dietary DHA is absorbed as triacylglycerol (TAG) because it is released as free fatty acid during digestion of either TAG-DHA (fish oil) or sn-2-DHA phospholipid (krill oil), and subsequently incorporated into TAG of chylomicrons. We tested the hypothesis that the absorption of DHA as phospholipid can be increased if it is present in the sn-1 position of dietary phospholipid or in lysophosphatidylcholine (LPC), because it would escape the hydrolysis by pancreatic phospholipase A2. We infused micelle containing the DHA either as LPC or as free acid, into the duodenum of lymph cannulated rats, and analyzed the chylomicrons and HDL of the lymph for the DHA-containing lipids. The results show that while the total amount of DHA absorbed was comparable from the two types of micelle, the percentage of DHA recovered in lymph phospholipids was 5 times greater with LPC-DHA, compared to free DHA. Furthermore, the amount of DHA recovered in lymph HDL was increased by 2-fold when LPC-DHA micelle was infused. These results could potentially lead to a novel strategy to increase brain DHA levels through the diet.
Keywords: Fish oil/DHA, chylomicrons/HDL, phospholipids/absorption, micelles, lysophosphatidylcholine, lymph
1. Introduction
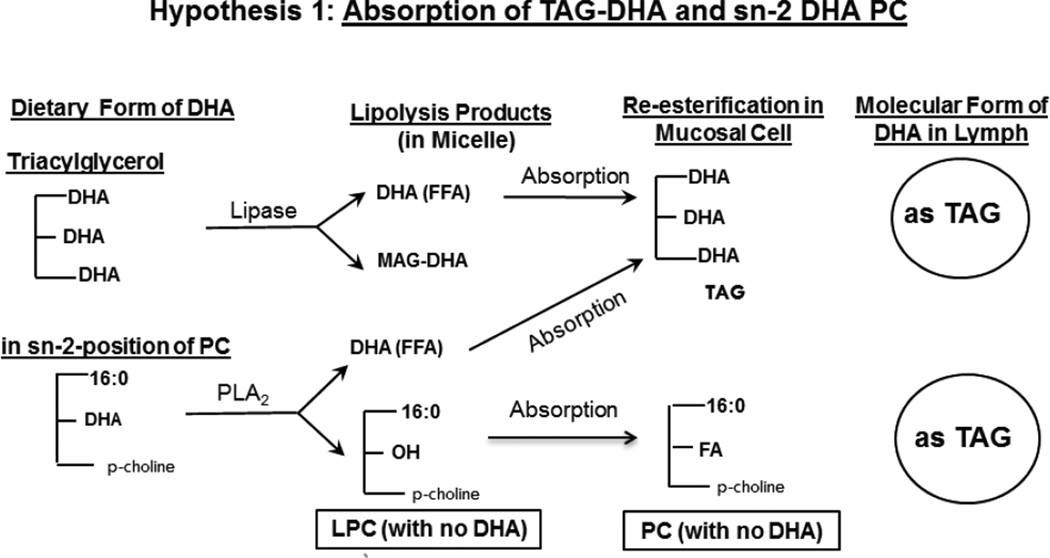
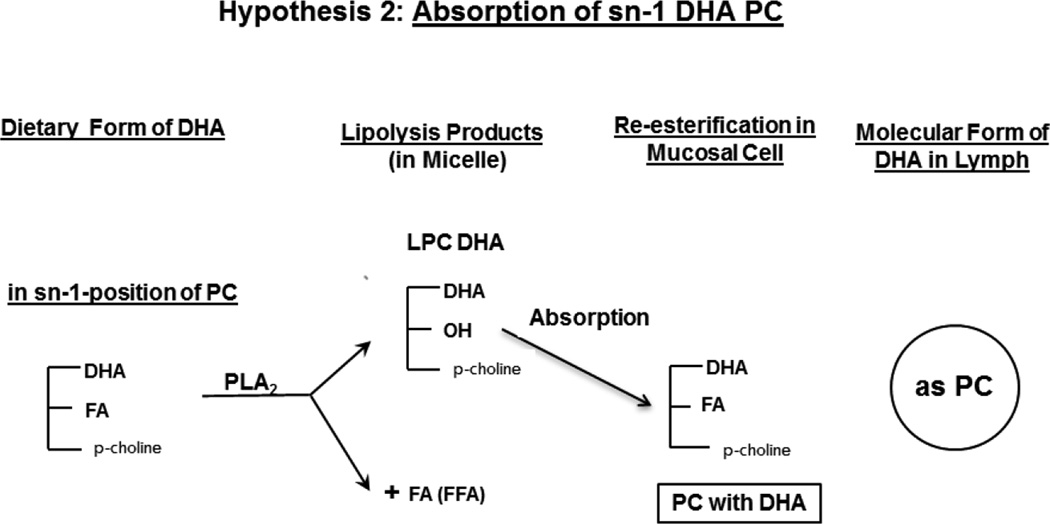
Docosahexaenoic acid (DHA) is an omega 3 fatty acid that is highly concentrated in the brain, and is absolutely essential for the normal development and function of the brain [1,2]. However, it is not synthesized in significant amounts from its precursors in human brain, and has to be imported from plasma through the blood-brain barrier (BBB). Unlike other tissues, the uptake of DHA does not occur through the lipoprotein receptors in the brain [3,4]. There is some controversy regarding the molecular carrier of DHA to the brain. Previous studies in animals by the Lagarde group have reported that DHA in the form of lysophosphatidylcholine (LPC) passes through the BBB about 10 times more efficiently than as free fatty acid [5,6]. On the other hand, the recent kinetic studies of Chen et al [7] suggested that the free DHA in plasma is the major pool supplying the brain, although they also reported that the brain uptake of injected LPC-DHA was higher than that of free DHA. The role of LPC is supported by the recent identification of a specific transporter (Mfsd2a) in the endothelial cells of BBB that selectively transports the LPC form of DHA [8]. Furthermore, the deficiency of this transporter results in defective brain development and impaired brain function in mice [8], as well as in humans [9], showing its physiological relevance. Thus it appears reasonable that the presence of DHA in plasma phospholipids would increase the brain DHA levels more than other molecular forms, and therefore the absorption of dietary DHA in the phospholipid form would be beneficial. There are two major natural sources of dietary DHA, namely fish oil, in which it is present in the form of triacylglycerol (TAG), and krill oil, in which about 35% of DHA is in the phospholipid form (at the sn-2 position of PC) and the rest in TAG form. DHA-rich micro algal oil, which is used in some infant formulae also contains DHA in TAG form [10]. DHA from the sn-2 position of PC is released as free fatty acid (FFA) during digestion because of the specificity of pancreatic phospholipase A2, whereas DHA from TAG is released by the action of gastric and pancreatic lipases, either as MAG or as free fatty acid depending upon the position occupied by DHA in TAG. Consequently, in both cases the DHA is absorbed as FFA (or as MAG) and is then re-esterified to TAG in the intestinal mucosa before being transported in the chylomicrons to various tissues (Hypothesis 1). These dietary forms of DHA are therefore less likely to enrich brain DHA, since the phospholipid form appears to cross the BBB (after conversion to lyso phospholipid) much more rapidly than other forms [5–8]. Previous studies showed that while the dietary DHA enriches most of the tissues, the brain levels are relatively unaffected by the amount of dietary DHA when fed as fish oil [11], ethyl ester concentrate [12], or algal oil [13]. It has also been reported that the liver can directly secrete LPC into the plasma [14], but the amount of LPC-DHA contributed by this pathway is unknown. Therefore absorption of DHA in the phospholipid form would be beneficial for its eventual uptake by the brain. We postulate that if the dietary DHA is present in the sn-1 position of PC, it would survive the hydrolysis by pancreatic PLA2 during digestion, and would be absorbed as LPC and then converted to PC by the intestinal mucosal cells before entering the lymph (Hypothesis 2). The presence of DHA in plasma PC should increase its eventual uptake by the brain. Furthermore the PC generated in the intestine may be incorporated into HDL either directly by assembly in the mucosal cells [15] or due to a transfer of surface components from the chylomicrons during lipolysis by the lipoprotein lipase [16]. The PC from HDL may be hydrolyzed to LPC containing DHA either by the action(s) of endothelial lipase [17,18], hepatic lipase [19], or LCAT [20,21] and thus has a greater potential to cross the BBB. As a first step in testing these hypotheses we investigated the intestinal absorption of two molecular forms of DHA incorporated into micelle by rats with lymph duct cannulation. One type of micelle contained free DHA, representing the DHA released by the pancreatic enzymes from either DHA-TG or sn-2 DHA PC, while the second type of micelle contained LPC-DHA which would be the product of hydrolysis of sn-1 DHA PC by the pancreatic phospholipase A2. The appearance of DHA in TAG and PC of chylomicrons and HDL of lymph was determined following the absorption of the two types of micelle in anesthetized rats. The results show that the incorporation of DHA into lymph PC can be increased about 5–fold, and its incorporation into HDL increased 2-fold by providing the DHA in LPC compared to free DHA.
Hypothesis 1.
Absorption of TAG-DHA and sn-2 DHA PC
Hypothesis 2.
Absorption of sn-1 DHA PC
2. Materials and Methods
2.1. Materials
All free fatty acids (17:0, 18:1, 22:3, 22:6) as well as monoolein were purchased from Nu-Chek Prep Inc (Elysian, MN). Egg PC, 16:0 LPC, and 16:0–22:6 PC were obtained from Avanti Polar Lipids (Alabaster, AL). Taurocholate, and Mucor lipase (Lipozyme) were purchased from Sigma Chemical (St. Louis, MO). Solid phase extraction cartridges (Supelclean LC-NH2, 1 ml) were obtained from Supelco (Bellefonte, PA).
LPC-DHA was prepared by the hydrolysis of 16:0–22:6 PC by immobilized Mucor lipase (Lipozyme) by a modification of the published procedure [22]. Briefly, 100 mg of Lipozyme was added to 50 mg of 16:0–22:6 PC dissolved in 1ml of 95:5 (v/v) ethanol: water. The reaction mixture was incubated at 37°C for 72 h under nitrogen with shaking. The reaction mixture was dried under nitrogen and extracted with 4 ml of ethanol: water: hexane (2:1:1, by vol) mixture to remove the free fatty acid. The lower layer was concentrated under nitrogen and extracted by Bligh and Dyer procedure [23]. The final sample contained 95% LPC, the rest being unhydrolyzed PC, as determined by lipid phosphorus estimation after TLC separation. Although this enzyme produces sn-2 22:6 LPC, this isomer is unstable, and is non-enzymatically isomerized to sn-1-22:6 LPC [24,25]. The isomer composition of LPC-DHA was not analyzed, but previous studies showed that at equilibrium, the ratio of sn-1 isomer to sn-2 isomer is about 9:1 [24,26].
2.2. Micelle preparation
Two types of micelle, one containing the DHA as free fatty acid, and the other containing the DHA as LPC, were prepared by sonication. The components of the micelle are listed in Table-1. All components except taurocholate were added as chloroform solutions to a glass tube and the solvent was evaporated under nitrogen. Then 10 ml of sodium taurocholate solution (12 mM, in PBS, pH 7.4) was added to the tube and the lipids dispersed by vortexing for 1 min. The mixture was then sonicated in a Sonics Vibro Cell sonicator at 40% setting for four 30 sec pulses at 1 min intervals, while the tube was immersed in ice. The resulting micelle were optically clear, and were used immediately for infusion into rats.
Table -1.
Composition of the infused micelle
| Compound | Micelle 1(Free DHA) | Micelle 2 (LPC-DHA) |
|---|---|---|
| 2-Monoolein (mM) | 2.5 | 2.5 |
| Free Oleic acid (mM) | 5.1 | 7.5 |
| Free DHA (mM) | 2.4 | 0 |
| 16:0 LPC (mM) | 2.4 | 0 |
| 22:6 LPC (mM) | 0 | 2.4 |
| Egg PC (mM) | 0.6 | 0.6 |
| Sod. Taurocholate (mM) | 12 | 12 |
2.3. Lymph duct cannulation and separation of lipoproteins
Male Sprague-Dawley rats (340–480 g) were obtained from Harlan Laboratories (Indianapolis, IN). All protocols were approved by the University of Illinois at Chicago animal care and use committee and were in compliance with the relevant guidelines of the National Research Council for the Care and Use of Laboratory Animals in Research.
Thoracic lymph duct cannulation was carried out as described [27] with slight modifications. The rats were fasted overnight before the procedure. Under analgesics and isoflurane anesthesia, the abdomen was opened, the dorsal parietal peritoneum was incised, and the aorta was dissected free from the surrounding muscle and thoracic duct. The duct was cannulated (3–5 mm) with a heparin (500 units/ml) and saline-rinsed, pre-cut catheter (16 gauge, BD Angiocath). Following insertion, the catheter was connected to ‘U” shaped secured Silastic Laboratory tubing to prevent displacement or dislodgement. Micelle (10 ml) were infused at a rate of 3ml/h into the duodenum (Harvard pump) through a second catheter positioned into the duodenum, followed by the infusion of 10 ml PBS, at 3 ml/h. The abdomen was closed and the lymph was collected for 6 h, in 30 min fractions into tubes containing 1.0 ml of PBS, while the animal was under anesthesia. The body temperature of the animal was maintained at 37 °C with the help of a heating pad.
The volume of each lymph fraction was made up to 2.0 ml with PBS, containing 1 mM EDTA (final). The density of lymph fraction was raised to 1.063 g/ml by the addition of KBr and centrifuged at 100,000 × g for 4 h at 4 °C in the TLA 100.3 rotor of the Beckman TL-100 ultracentrifuge. One ml of the bottom layer was aspirated to a new tube (HDL) and the remaining 1.0 ml was taken as the chylomicrons (CM).
2.4. Extraction and analysis of lipids
The total lipids of HDL and CM were extracted by the Bligh and Dyer method [23] after spiking the samples with 25 µg each of 15:0-15:0-15:0 TAG and 17:0-17:0 PC. The lipid extract was applied to an aminopropyl silica cartridge (100 mg) in 1 ml of chloroform under gravity, and the cartridge was washed with chloroform (2 × 1.0 ml) to elute the TAG and cholesteryl esters. Since we found in initial experiments very little DHA in the cholesteryl esters, we did not separate the TAG and cholesteryl esters before analysis. The PC was then eluted with 2.0 ml of chloroform: methanol (3:2, v/v)). The eluates were evaporated under N2 and methylated by heating under nitrogen at 90 °C for 1 h with BF3-methanol containing 25 µg 22:3 free fatty acid and 250 µg butylated hydroxytoluene. The fatty acid methyl esters (FAME) were extracted twice with 2 ml of hexane after adding 1.0 ml water, and the hexane extracts were evaporated under nitrogen, re-dissolved in 30 µl of hexane, and 1 µl was injected into GC/MS.
The analysis of FAME was carried out by GC/MS using Shimadzu QP2010SE, equipped with a Supelco Omegawax column (30 m × 0.25 mm × 0.25 µ film thickness). The temperature program was as follows: 165 °C for 1 min, raised to 210 °C at the rate of 6.5 ° C/min, followed by raising to 240 °C at the rate of 3.5 ° C/min, and maintaining at 240 °C for 10 min. The total analysis time was 26.5 min. The injection temperature was 250 °C, ion source temperature 230 °C and the interface temperature was 250 °C. Total ion current in the range of 50–400 m/z was used to quantify the FAME, using 17:0 as the internal standard for the FAME derived from PC, and 15:0 for the FAME derived from TAG. The identification of individual FAME was done by comparison of retention times with the standard mixture (PUFA2, Sigma) as well as by the characteristic fragment ions (m/z 74 for saturated, m/z 55 for monounsaturated, m/z 67 for diunsaturated, and m/z 79 for polyunsaturated).
2.5. LC/MS analysis of molecular species PC and TAG
LC/MS analysis of molecular species of TAG and PC was performed on an ABSciex 6500 QTRAP mass spectrometer coupled with Agilent 2600 UPLC system. Mobile phase A consisted of chloroform/methanol/water, 80/19.5/0.5 (by vol) and mobile phase B consisted of chloroform/methanol/water 60/34.5/5.5 (by vol). Both A and B were supplemented with 0.1% of formic acid and 0.1% ammonium hydroxide. Each sample was dissolved in 100µL of mobile phase A containing 50 ng each of 15:0-15:0-15:0 TAG and 17:0-17:0 PC as internal standards, and 10 µl was injected on to a normal phase silica column (Supelco Ascentis Si 3µM, 100 × 2.1mM). The lipids were separated by a gradient elution of solvent A and solvent B at the flow rate of 0.35ml/min. The gradient program was: 0–5 min with 100% solvent A; 5–30 min with solvent A decreasing linearly from 100% to 0% and 30–35 min 100% B. The column temperature was maintained at 25°C. The spray voltage of the MS was 4.5 kV, and the source temperature was set at 450°C. Mass spectroscopy was performed in positive ionization mode for the quantification of PC species using multiple reaction monitoring (MRM) with transitions from molecular ion to the fragment of choline head group (m/z 184) (Table 1, Supplemental Data). The TAG species containing DHA were quantified by positive MRM with transition from ammonia adducts of the molecular ions to their neutral loss fragments of ammoniated DHA. The identification of the molecular species was confirmed by the fatty acid products under negative ionization mode. However the positional distribution of fatty acids in TAG or PC was not determined. Quantitation of individual molecular species PC and TAG was performed from the relative intensities of the various species and the corresponding internal standards (17:0-17:0 PC and 15:0-15:0-15:0 TAG respectively). The data processing was performed with Analyst 1.6.2 (ABSciex, USA). The DHA content of individual TAG species was corrected for the number of DHA molecules present in each species.
2.6. Statistical analyses
The data are expressed as mean ± SEM. Statistical significance of differences between the results of LPC-DHA and free DHA were determined by Student’s t- test (unpaired) in Microsoft Excel program.
3. Results
3.1. Comparison of secretion of free DHA and LPC-DHA into lymph
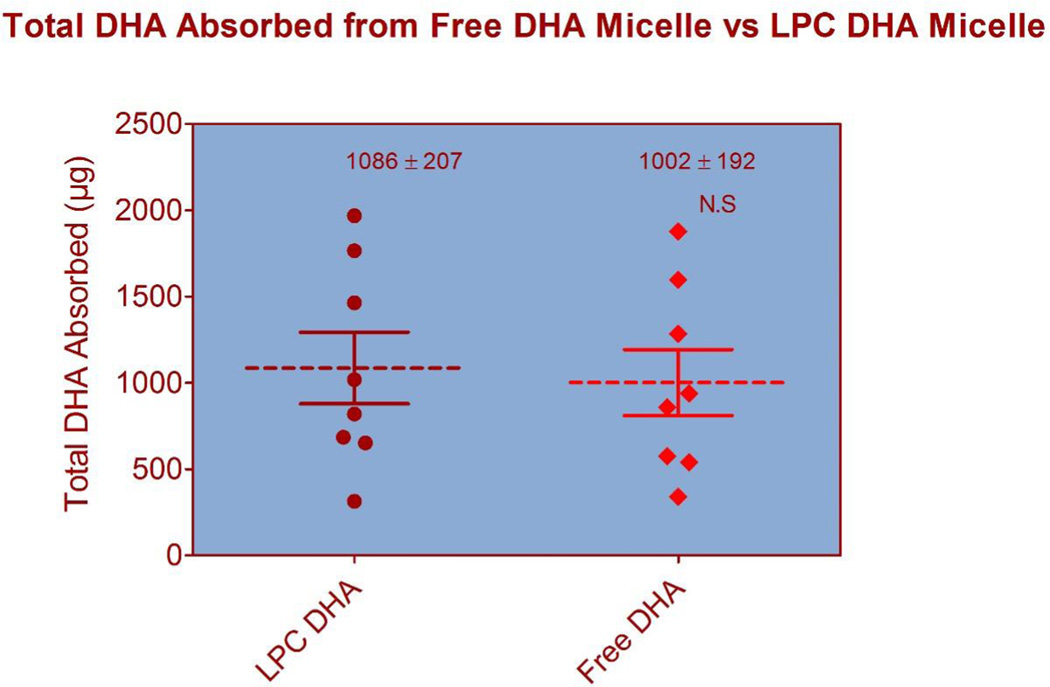
The flow rates of lymph varied from 0.5–1.0 ml/h in individual rats, and the peak of DHA absorption was 3–4 h in both groups (results not shown). Except for the molecular form of DHA, the concentrations of various micellar components (monoacylglycerol, free fatty acid, LPC, PC, bile salt) were exactly the same in the two micellar preparations (Table 1). The free DHA micelle represent the products generated in the intestinal lumen during the digestion of sn-2 DHA PC (or DHA-TAG), whereas the LPC-DHA micelle represent the products generated during the digestion of sn-1 DHA PC, since the pancreatic phospholipase A is specific for the sn-2 ester linkage. The total amount of DHA appearing in the lymph over the 6 h period did not differ significantly between the two types of micelle, although there were wide variations among individual animals (Fig. 1). In some experiments with LPC-DHA, we analyzed the intestinal contents after the 6 h lymph collection, and found only LPC-DHA without substantial amount of free DHA, indicating no hydrolysis of LPC in the lumen.
Fig. 1. Total amount of DHA secreted into lymph.
Total amount of DHA secreted in 6 h in individual experiments is shown. Although there were individual variations among the animals in each group, the mean values were not significantly different between the two groups. The dotted line is the mean value and the solid lines are SEM. N= 8 for both groups. The total amount of DHA infused into the duodenum was 24 µmoles (7.88 mg) in both groups.
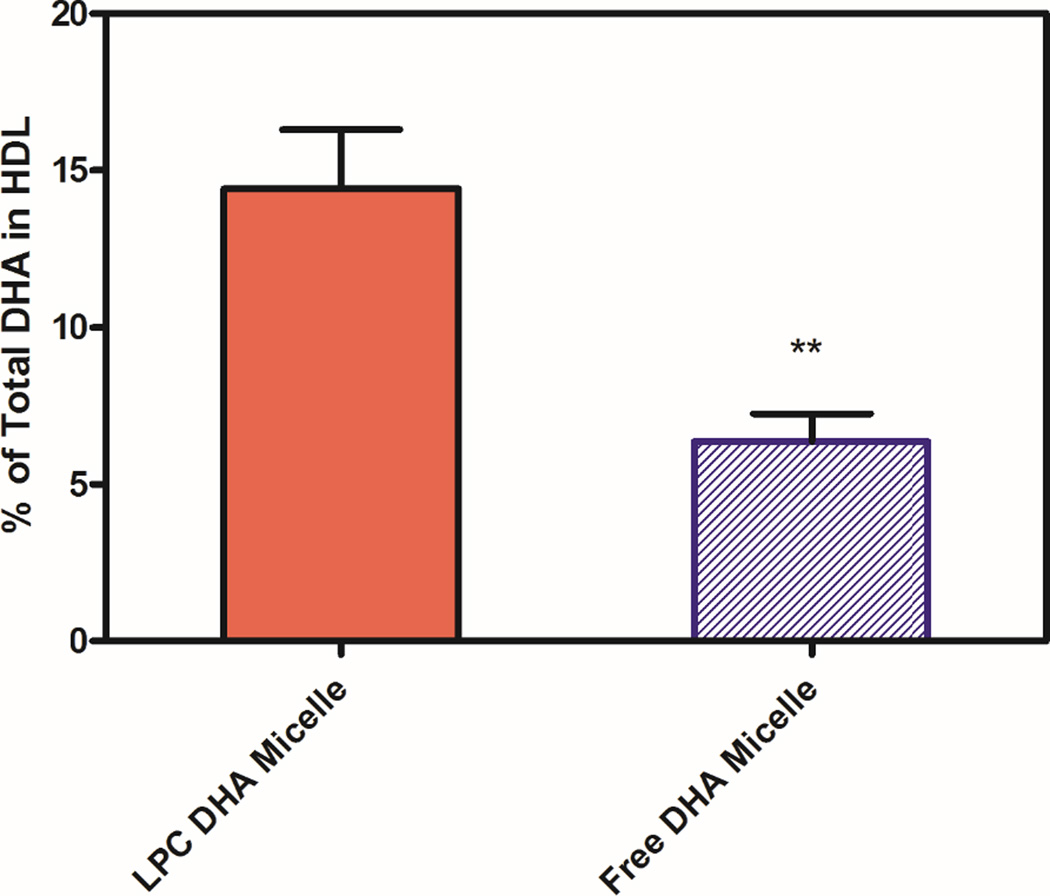
3.2. Incorporation of DHA into HDL and chylomicron (CM) fractions
The individual lymph fractions were fractionated into HDL and CM subfractions by centrifugation at 1.063 g/ml, the total lipids were extracted, and the amount of DHA recovered in the two fractions was determined by GC/MS. As shown in Fig. 2, about 14.4% of lymph DHA was incorporated into HDL (and the rest in CM) when LPC-DHA was infused, whereas only about 6.4% of DHA was present in HDL when free DHA was infused. However, it should be pointed out that the percentage of all the lymph fatty acids recovered in the HDL fraction did not differ significantly between the two groups (9.9 ± 0.6 for free DHA micelle, and 11.7 ± 2.2 for LPC micelle, mean ± SEM), suggesting an enrichment of HDL lipids with DHA after the absorption of LPC-DHA.
Fig. 2. Percent of total DHA recovered in HDL fraction of the lymph.
HDL and CM from each lymph fraction were separated by centrifugation at a density of 1.063 g/ml, and their fatty acid composition was determined by GC/MS, as described in Section 2.4. The values shown are percentages of total lymph DHA which was recovered in HDL, and are mean ± SEM of 9 experiments for LPC-DHA and 7 experiments for free DHA. The remaining DHA was CM fraction.
** p< 0.01, free DHA vs LPC-DHA (unpaired t test)
3.3. Incorporation of DHA into PC and TAG
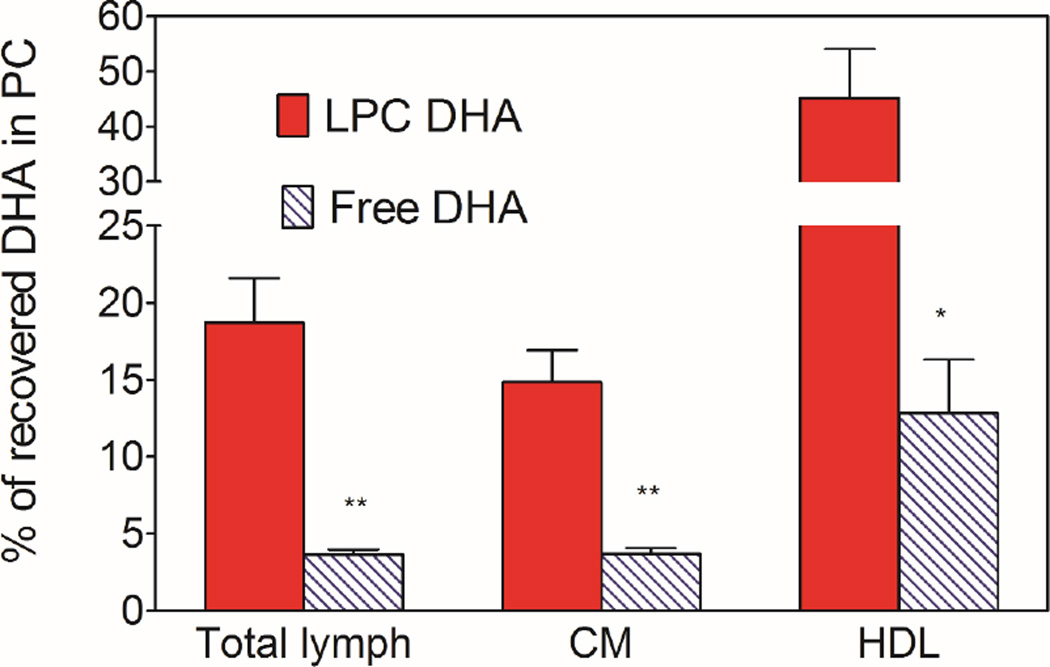
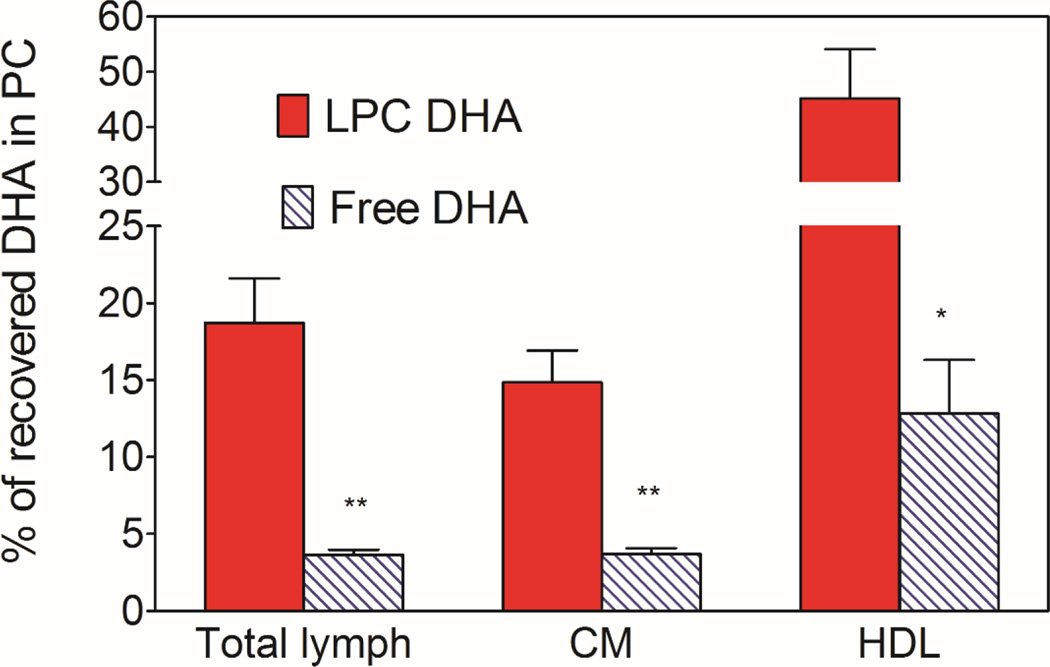
Fig. 3 shows the percent of total DHA recovered in the phospholipids of whole lymph, and the HDL and chylomicron subfractions. The rest of the DHA was present in the neutral lipids (TAG and cholesteryl esters). The LC/MS analyses showed that small amounts of DHA were incorporated into PE with both the micelle preparations (<10% of DHA in PC), but this was included in the PC in the present studies because PC and PE were not well separated under the conditions used. We did not find significant amount of free DHA in the lymph after infusion of either free DHA or LPC-DHA. The percent of whole lymph DHA appearing in PC was 5 times higher when micellar DHA was present as LPC (18.72 ± 2.88, mean ± SEM), compared to its presence as free fatty acid (3.66 ± 0.32, mean ± SEM). The difference between the two micellar forms is more pronounced in HDL compared to chylomicrons. Thus in HDL, about 45% of DHA was present in PC after the absorption of LPC-DHA micelle, compared to only about 13% with free DHA micelle. In the chylomicrons, the majority of DHA was recovered in TAG with both preparations of DHA, although the percent recovered as PC was still higher with LPC-DHA micelle (14.8%) compared to free DHA micelle (3.7%). These results thus show that providing the dietary DHA in the form of LPC results in its increased incorporation into phospholipids of lymph, especially in the HDL fraction. However, the majority of LPC absorbed appears to be hydrolyzed inside the mucosal cells and the resulting free DHA incorporated predominantly into TAG.
Fig. 3. Percent of DHA recovered in PC.
The PC and TAG from the lipids of total lymph, HDL, and CM were separated on aminopropyl columns, and the fatty acid composition was determined by GC/MS. The percent of DHA recovered in PC was calculated from this data. The PC fraction contained a small amount of DHA as PE (<10%), since these two lipids were not separated in this procedure. All the remaining DHA was in TAG. The results shown are mean ± SEM. (n= 10 total lymph for LPC-DHA, n=8 for total lymph for free DHA, n=5 each HDL and CM for both micelle).
* P<0.05; ** p<0.005 free DHA vs LPC-DHA (unpaired t test)
3.4. Molecular species of TAG formed from the two types of micellar DHA
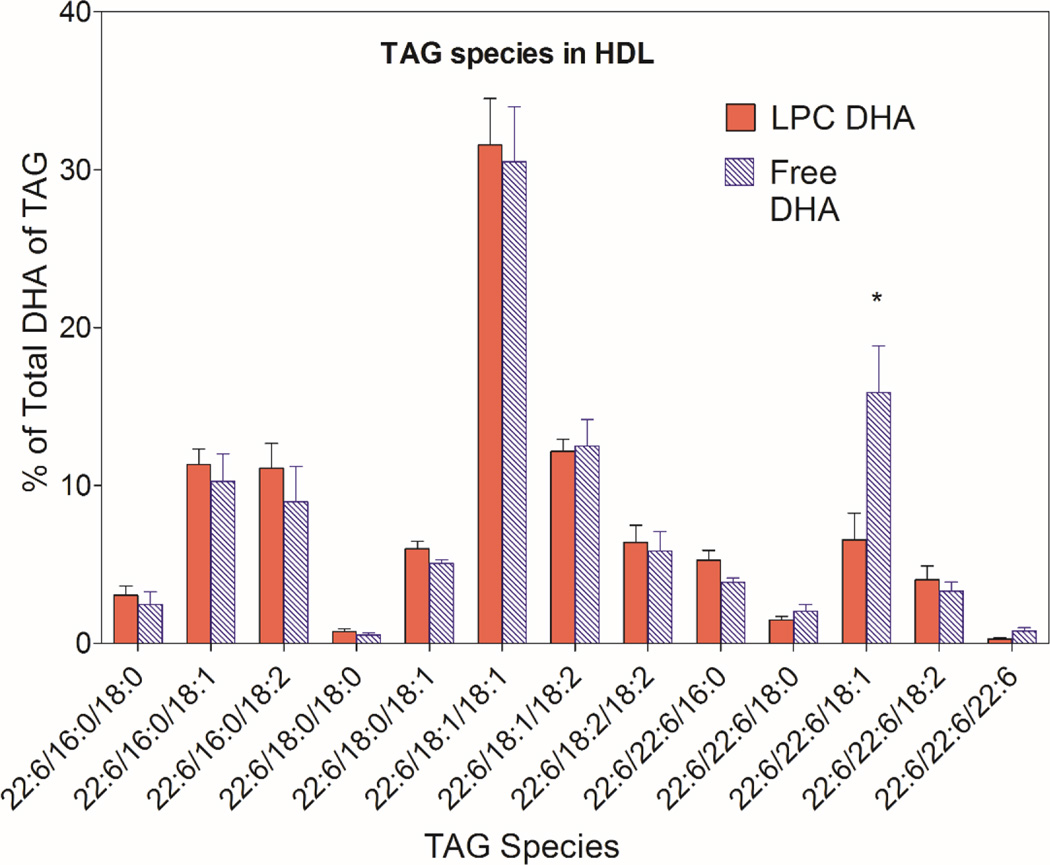
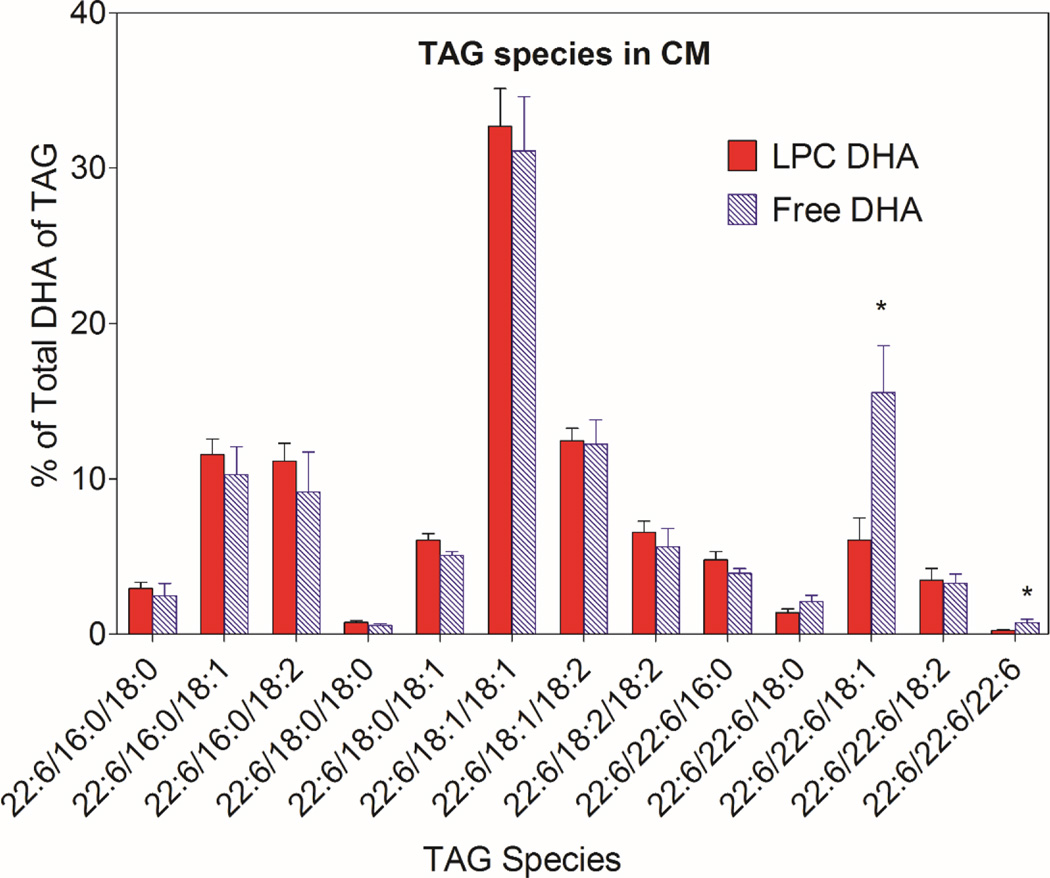
The molecular species of TAG containing DHA were analyzed by LC/MS/MS to determine whether the DHA derived from the two micellar forms of DHA is metabolized differently in the mucosal cells. As shown in Figs. 4 and 5, 13 molecular species of TAG that contained DHA were identified by LC/MS in the HDL and chylomicron subfractions of the lymph. The results shown are the percentages of TAG-DHA in individual TAG species (taking into account the number of DHA molecules per molecule of TAG). Since 2-monoolein was present as the backbone in both types of micelle, it is not surprising that the predominant DHA-containing TAGs in both HDL and CM also contained 18:1. The most abundant DHA-TAG species was 22:6/18:1/18:1 in HDL as well as in CM with both types of the micelle. Statistically, the percentage of 22:6/22:6/18:1 species was significantly higher after the absorption of free DHA, compared to LPC-DHA in both HDL and CM (Figs.4, 5). This suggests the esterification of monoolein with two molecules of free DHA when excess free DHA is available inside the mucosal cell. In addition, the TAG species containing DHA at all 3 positions was also significantly higher after free DHA absorption, compared to LPC-DHA absorption, reflecting the presence of higher concentration of free DHA inside the cells.
Fig. 4. Molecular species of TAG containing DHA in HDL.
The molecular species composition of DHA-containing TAG species was determined by MRM in LC/MS as described in Section 2.5 (and supplementary data, Table 1). The nomenclature of the species is based on the fatty acid composition, but the position of the fatty acids on the glycerol backbone has not been determined. The values shown are mean ± SEM of 9 experiments for LPC-DHA and 7 experiments for free DHA.
* p <05, free DHA vs LPC-DHA (unpaired t test)
Fig. 5. Molecular species of TAG containing DHA in lymph CM.
The molecular species composition was determined by MRM in LC/MS as described in Section 2.5 (and supplementary data, Table 1). The values shown are mean ± SEM of 9 experiments for LPC-DHA and 7 experiments for free DHA.
* p <0.05, free DHA vs LPC-DHA (unpaired t test)
3.5. Molecular species of PC formed from the two types of micellar DHA
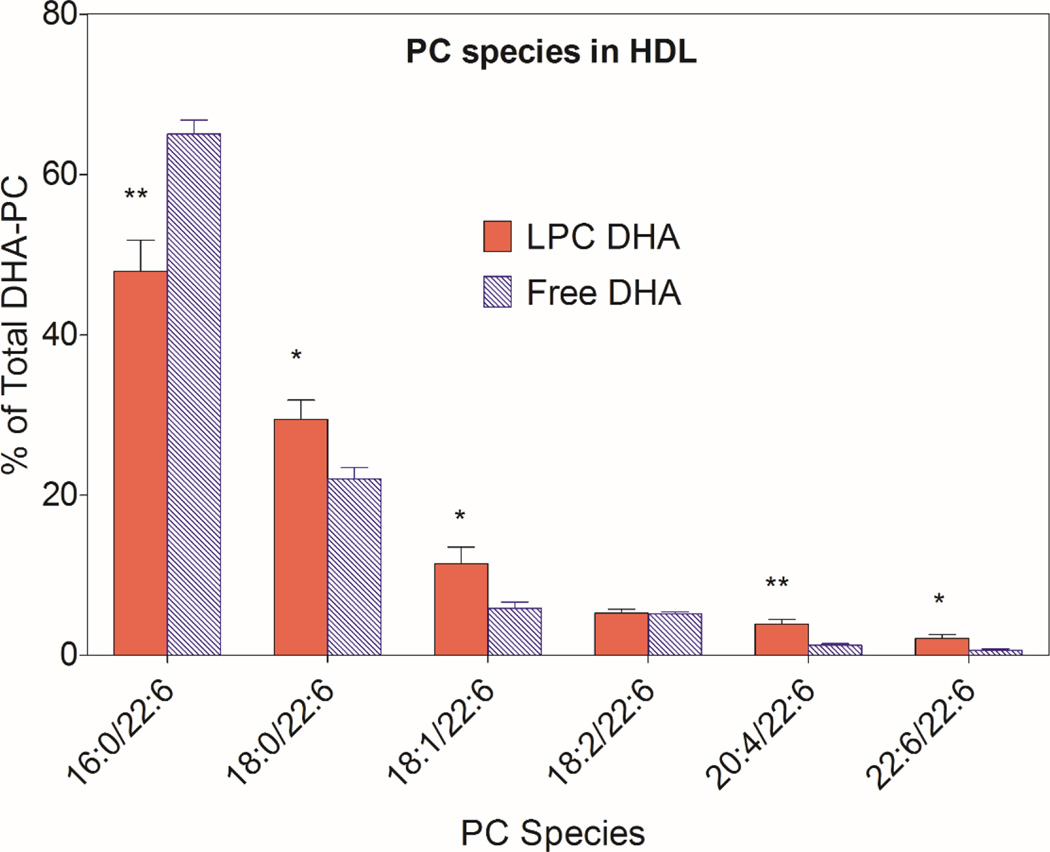
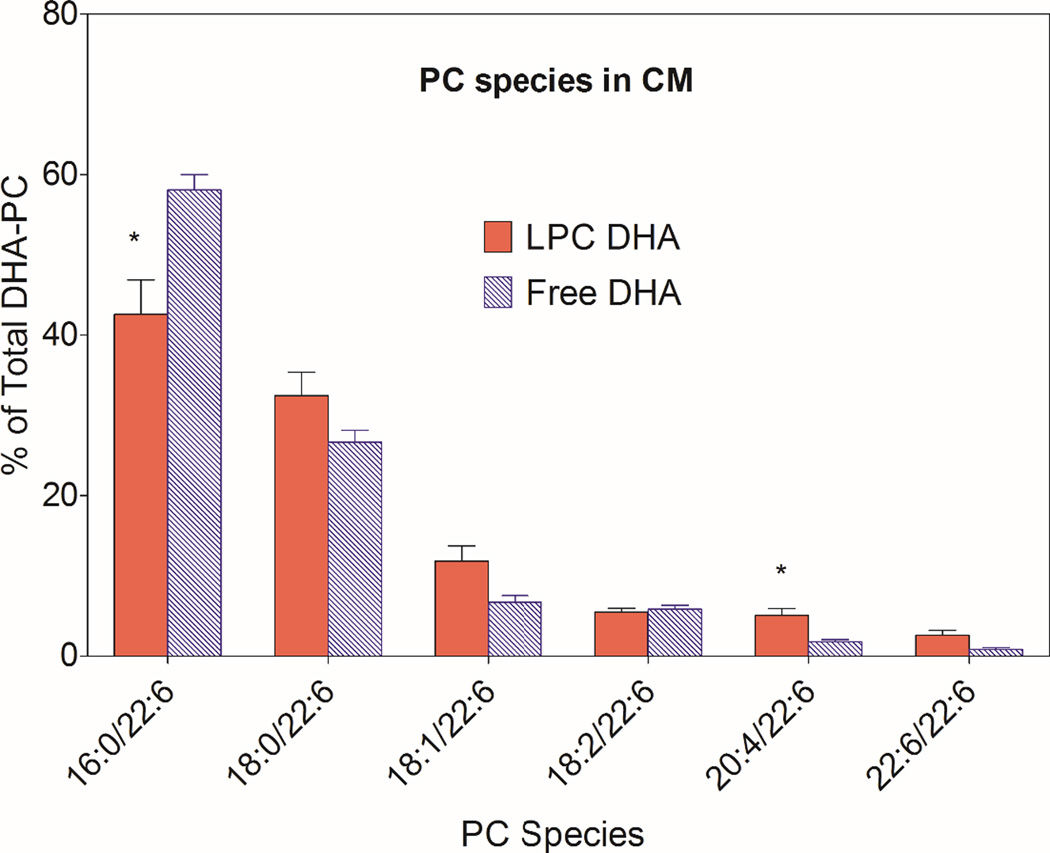
The most abundant DHA-PC was 16:0/22:6, followed by 18:0/22:6 and 18:1/22:6 in both HDL and chylomicron fractions of the lymph after absorption of either type of micelle (Figs. 6 and 7). The percentage of 16:0/22:6 PC was significantly lower following the absorption of LPC-DHA, compared to free DHA absorption, in both HDL and CM. All other species containing 22:6 were increased significantly at the expense of 16:0/22:6, especially in HDL (Fig 6). In CM, only the increase in 20:4/22:6 PC was statistically significant, although most DHA-PC species showed an increase following LPC-DHA absorption compared to free DHA absorption.
Fig. 6. Molecular species of DHA-containing PC in lymph HDL.
The molecular species composition of PCs that contained DHA was determined by MRM, as described in Section 2.5 (and supplementary data, Table 1). The position occupied by DHA was not determined. The values shown are mean ± SEM of 9 experiments for LPC-DHA, and 7 experiments for free DHA
* p < 0.05, ** p < 0.005, Free DHA vs LPC-DHA (unpaired t test)
Fig. 7. Molecular species of DHA-containing PC in CM.
The molecular species composition of PCs containing DHA was determined by MRM, as described in Section 2.5 (and supplementary data, Table 1). The position occupied by DHA was not determined. The values shown are mean ± SEM of 9 experiments for LPC-DHA, and 7 experiments for free DHA
* p< 0.05, Free DHA vs LPC-DHA (unpaired t test)
4. Discussion
Evidence from both biochemical [5,6] and genetic [8,9] studies supports the role of LPC-DHA as a preferred carrier of DHA through the BBB. Although the recent kinetic studies by Chen et al [7] challenged this paradigm, these studies also showed that the uptake of a bolus injected LPC-DHA is greater than that of injected free DHA. Therefore the presence of DHA in plasma phospholipids appears to be beneficial for its brain accrual. The main goal of the present study is to determine whether the amount of dietary DHA absorbed and secreted as phospholipid into the lymph lipoproteins can be increased by feeding sn-1 DHA PC, instead of the naturally occurring sn-2 DHA PC (as present in krill oil) or DHA-TAG (as present in fish oil and krill oil). We used the absorption of LPC-DHA from micelle as substitute for the digestion and absorption of sn-1 DHA PC, and the absorption of free DHA from micelle as substitute for the digestion and absorption of sn-2 DHA PC, based on the expected products of hydrolysis of the two isomers of PC by the pancreatic phospholipase A2. The free DHA also serves as a substitute for the free DHA released from DHA-TAG digestion by the pancreatic lipase. Both the micelle also contained 2-monoolein and free oleic acid to represent the hydrolytic products of dietary TAG. The results presented here show that while the total amount of DHA delivered into lymph is comparable for the two types of DHA micelle, the percentage of DHA incorporated into lymph phospholipids is about 5-fold higher after the absorption of LPC-DHA. Furthermore, the DHA recovered in HDL fraction of the lymph was increased by at least 2-fold. Even though the absorption studies were performed in anesthetized rats in which the rate of absorption may be lower than in the conscious animals, the relative distribution of DHA between TAG and PC in lymph would be expected to be the same as in the conscious animals. While we have not determined the position occupied by DHA in the lymph PC, LPC-DHA could be generated from either sn-1 DHA PC or sn-2 DHA PC in the plasma compartment. Thus, the various PLA1 activities like those of endothelial lipase [17,18], hepatic lipase [19], or LCAT [20,21] could generate LPC-DHA from sn-2 DHA PC, whereas the secretory phospholipases A2 [28,29] could generate LPC-DHA from sn-1 DHA PC. The relative importance of various phospholipase activities of plasma in the generation of LPC-DHA in plasma is not yet known.
Several factors could account for the observation that the majority of DHA is incorporated into CM TAG rather than PC, even when the DHA was provided as LPC-DHA. First and foremost, the amount of phospholipid that can be incorporated into CM is limited, since the CM particle contains only a monolayer of phospholipid which constitutes about 4% of the total lipid, and of this only about 80% is PC [30]. In addition, the dietary PC is diluted with significant amount of biliary PC, which gives rise to saturated LPC during digestion, and thus competes with LPC-DHA for the reacylation and incorporation into CM in the mucosal cell. This further reduces the maximum amount of dietary phospholipid that can be incorporated into CM PC. Scow et al [31] reported that at least 60% of PC in the CM is derived from endogenous sources even after feeding large excess of PC in the diet. Therefore, when LPC-DHA is absorbed, only a fraction of this LPC can be expected to be acylated to PC and incorporated into CM. The remaining LPC-DHA is hydrolyzed by the mucosal lysophospholipase and the resulting free DHA is incorporated into TAG. The extensive degradation of the absorbed LPC has also been reported for 16:0 LPC [31,32], which is naturally formed from the hydrolysis of biliary PC in the intestinal lumen. The percentage of DHA recovered in lymph PC after LPC-DHA infusion in our studies (18%) is lower than the recovery of 16:0 after infusion of 16:0 LPC (25–30%) [32], possibly due to the substrate specificity of the mucosal LPC acyltransferase. Although the specificity of this enzyme towards the polyunsaturated LPCs has not been reported, it is likely that it prefers the more abundant 16:0 LPC which is released from the hydrolysis of biliary PC, based on the results with the human LPCAT3 expressed in yeast [33]. It is also possible that the LPC-DHA employed here contained significant amount of sn-2 DHA isomer, although previous studies showed that at equilibrium, 90% of LPC is sn-1 isomer [24,34]. The sn-2 isomer may be preferentially hydrolyzed in the lumen or mucosal cells, releasing free DHA. Further studies are needed to determine the relative incorporation rates sn-1 and sn-2 isomers of LPC-DHA into lymph phospholipids.
In contrast to CM, the HDL particles secreted by the intestine contain relatively more PC and less TAG per particle. A small but significant amount of absorbed LPC-DHA may be diverted to HDL PC synthesis, thus increasing the amount of DHA secreted via HDL into lymph. In fact previous studies in humans showed that the newly absorbed PC is preferably associated with HDL [35]. We found that there is a >2-fold increase in amount of DHA recovered in lymph HDL after LPC-DHA absorption, compared to free DHA absorption, and about of half of the DHA in lymph HDL was associated with PC. It has been reported that the intestinal HDL is also directly secreted into the plasma, rather than into the lymph [36], in which case the actual amount of DHA incorporated into HDL phospholipids may be much higher than reported here, because we only analyzed the HDL in the lymph. HDL PC is the preferred substrate for all enzymes displaying phospholipase activity in the plasma [18–20,28,29], and therefore the presence of DHA PC in HDL would be beneficial for the generation of LPC-DHA in the plasma compartment and for its potential uptake by the brain through the Mfsd2a [8] pathway. The amount of HDL secreted by the intestine can be increased significantly by LXR agonists which stimulate the transcription of ABCA1 [37]. It would be therefore of interest to study whether the incorporation of DHA into HDL PC can be increased by pretreatment of the animals with an LXR agonist. It is also necessary to perform long term feeding studies with sn-1 DHA PC or DHA LPC to determine whether they will enrich brain DHA more efficiently than either DHA TAG or sn-2 DHA PC commonly employed at present.
5. Conclusions
These studies show that the delivery of DHA in into lymph phospholipids can be increased by 5-fold, and its incorporation into HDL increased by 2-fold, if the DHA is present in the sn-1 position of dietary phospholipids or in LPC, compared to its presence in the sn-2 position of PC or in TAG. The increased concentration of DHA in the plasma phospholipids may be beneficial for its transport into the brain through the Mfsd2a transporter pathway [8].
Acknowledgments
These studies were supported by the National Center for Complementary & Integrative Health of NIH under award number R21 AT008457 (PVS), by a VA Merit Review award IO1 BX001090 (PVS), by American Diabetes Association (1-12-BS-150) (JMO), and by the Office of the Director, NIH, under the award number S10 OD010660 (LC/MS equipment) (PVS). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH, ADA, or the Department of Veterans Affairs.
Abbreviations
- BBB
Blood brain barrier
- CM
Chylomicrons
- FAME
Fatty acid methyl esters
- LPC
Lysophosphatidylcholine
- MAG
Monoacylglycerol
- MRM
Multiple reaction monitoring
- PC
Phosphatidylcholine
- TAG
Triacylglycerol
References
- 1.Alessandri JM, Guesnet P, Vancassel S FAU - Astorg P, Astorg PF, Denis IF, Langelier BF, Aid S FAU - Poumes-Ballihaut C, Poumes-Ballihaut CF, Champeil-Potokar GF, Lavialle M. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod. Nutr Dev. 2004;44:509–538. doi: 10.1051/rnd:2004063. [DOI] [PubMed] [Google Scholar]
- 2.Lukiw WJ, Bazan NG. Docosahexaenoic acid and the aging brain. J. Nutr. 2008;138:2510–2514. doi: 10.3945/jn.108.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CT, MA DWL, Kim JH, Mount HTJ, Bazinet RP. The low density lipoprotein receptor is not necessary for maintaining mouse brain polyunsaturated fatty acid concentrations. J. Lipid Res. 2008;49:147–152. doi: 10.1194/jlr.M700386-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Rahman T, Taha AY, Jun Song B, Orr SK, Liu Z, Chen CT, Bazinet RP. The very low density lipoprotein receptor is not necessary for maintaining brain polyunsaturated fatty acid concentrations. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2010;82:141–145. doi: 10.1016/j.plefa.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Thies F, Pillon C, Moliere P, Lagarde M, Lecerf J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Amer. J. Physiol-Regul. Integr. C. 1994;36:R1273–R1279. doi: 10.1152/ajpregu.1994.267.5.R1273. [DOI] [PubMed] [Google Scholar]
- 6.Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thies F, Croset M, Lecerf J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001;16:201–204. doi: 10.1385/JMN:16:2-3:201. [DOI] [PubMed] [Google Scholar]
- 7.Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trépanier MO, Lin LE, Ermini L, Post M, Thies F, Bazinet RP. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci Rep. 2015;5:15791. doi: 10.1038/srep15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 9.Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E, Schroth J, Copeland B, Vaux KK, Cazenave-Gassiot A, Quek DQY, Wong BH, Tan BC, Wenk MR, Gunel M, Gabriel S, Chi NC, Silver DL, Gleeson JG. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47:809–813. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arterburn LM, Boswell KD, Lawlor T, Cifone MA, Murli H, Kyle DJ. In vitro genotoxicity testing of ARASCO-® and DHASCO-® oils. Food Chem. Toxicol. 2000;38:971–976. doi: 10.1016/s0278-6915(00)00085-5. [DOI] [PubMed] [Google Scholar]
- 11.Martins SV, Lopes PA, Ramos C, Miguueis S, Alfaia CM, Pinto RMA, Rolo EA, Bispo P, Batista I, Bandarra NM, Prates JAM. Influence of feeding graded levels of canned sardines on the inflammatory markers and tissue fatty acid composition of Wistar rats. British Journal of Nutrition. 2014;112:309–319. doi: 10.1017/S0007114514000853. [DOI] [PubMed] [Google Scholar]
- 12.Saito M, Ueno M, Kubo K, Yamaguchi M. Dose-Response Effect of Dietary Docosahexaenoic Acid on Fatty Acid Profiles of Serum and Tissue Lipids in Rats. J. Agr. Food Chem. 1998;46:184–193. doi: 10.1021/jf970385d. [DOI] [PubMed] [Google Scholar]
- 13.Lin YH, Shah S, Salem N. Altered essential fatty acid metabolism and composition in rat liver, plasma, heart and brain after microalgal DHA addition to the diet. J. Nutr. Biochem. 2011;22:758–765. doi: 10.1016/j.jnutbio.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekas G, Patton GM, Lincoln EC, Robins SJ. Origin of plasma lysophosphatidylcholine: evidence for direct hepatic secretion in the rat. J Lab Clin Med. 1985;105:190–194. [PubMed] [Google Scholar]
- 15.Wang H, Du J, Lu S, Yao Y, Hunter F, Black DD. Regulation of intestinal apolipoprotein A-I synthesis by dietary phosphatidylcholine in newborn swine. Lipids. 2001;36:683–687. doi: 10.1007/s11745-001-0773-x. [DOI] [PubMed] [Google Scholar]
- 16.Tall AR, Green PHR, Glickman RM, Riley JW. Metabolic Fate of Chylomicron Phospholipids and Apoproteins in the Rat. J. Clin. Invest. 1979;64:977–989. doi: 10.1172/JCI109564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Subbaiah PV. Phospholipid and fatty acid specificity of endothelial lipase: Potential role of the enzyme in the delivery of docosahexaenoic acid (DHA) to tissues. Biochim. Biophys. Acta. 2007;1771:1319–1328. doi: 10.1016/j.bbalip.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang P, Belikova NA, Billheimer J, Rader DJ, Hill JS, Subbaiah PV. Inhibition of endothelial lipase activity by sphingomyelin in the lipoproteins. Lipids. 2014;49:987–996. doi: 10.1007/s11745-014-3944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang P, Subbaiah PV. Regulation of hepatic lipase activity by sphingomyelin in plasma lipoproteins. Biochim Biophys Acta. 2015;1851:1327–1336. doi: 10.1016/j.bbalip.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subbaiah PV, Liu M, Paltauf F. Role of sn-2 acyl group of phosphatidyl choline in determining the positional specificity of lecithin-cholesterol acyltransfersae. Biochemistry. 1994;33:13259–13266. doi: 10.1021/bi00249a012. [DOI] [PubMed] [Google Scholar]
- 21.Subbaiah PV, Sowa JM, Davidson MH. Evidence for altered positional specificity of LCAT in vivo: studies with docosahexaenoic acid feeding in humans. J. Lipid Res. 2004;45:2245–2251. doi: 10.1194/jlr.M400197-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Sarney D, Fregapane G, Vulfson E. Lipase-catalyzed synthesis of lysophospholipids in a continuous bioreactor. J Am Oil Chem Soc. 1994;71:93–96. [Google Scholar]
- 23.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Plueckthun A, Dennis EA. Acyl and phosphoryl migration in lysophospholipids: importance in phospholipid synthesis and phospholipase specificity. Biochemistry. 1982;21:1743–1750. doi: 10.1021/bi00537a007. [DOI] [PubMed] [Google Scholar]
- 25.Croset M, Brossard N, Polette A, Lagarde M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 2000;345:61–67. [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, Sammani S, Zhou G, Raj JU, Garcia JG, Berdyshev E, Yuan JX, Natarajan V, Machado RF. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190:1032–1043. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milling SW, Jenkins C, MacPherson G. Collection of lymph-borne dendritic cells in the rat. Nat. Protoc. 2006;1:2263–2270. doi: 10.1038/nprot.2006.315. [DOI] [PubMed] [Google Scholar]
- 28.Singh DK, Subbaiah PV. Modulation of the activity and arachidonic acid selectivity of group X secretory phospholipase A2 by sphingolipids. J. Lipid Res. 2007;48:683–692. doi: 10.1194/jlr.M600421-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Singh DK, Gesquiere LR, Subbaiah PV. Role of sphingomyelin and ceramide in the regulation of the activity and fatty acid specificity of group V secretory phospholipase A2 . Arch. Biochem. Biophys. 2007;459:280–287. doi: 10.1016/j.abb.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zilversmit DB. The surface coat of chylomicrons: lipid chemistry. J. Lipid Res. 1968;9:180–186. [PubMed] [Google Scholar]
- 31.Scow RO, Stein Y, Stein O. Incorporation of Dietary Lecithin and Lysolecithin into Lymph Chylomicrons in the Rat. J. Biol. Chem. 1967;242:4919–4924. [PubMed] [Google Scholar]
- 32.Nilsson A. Intestinal absorption of lecithin and lysolecithin by lymph fistula rats. Biochim Biophys Acta. 1968;152:379–390. doi: 10.1016/0005-2760(68)90047-7. [DOI] [PubMed] [Google Scholar]
- 33.Kazachkov M, Chen Q, Wang L, Zou J. Substrate preferences of a lysophosphatidylcholine acyltransferase highlight its role in phospholipid remodeling. 2008;43:895–902. doi: 10.1007/s11745-008-3233-y. [DOI] [PubMed] [Google Scholar]
- 34.Kielbowicz G, Smuga D, Gladkowski W, Chojnacka A, Wawrzenczyk C. An LC method for the analysis of phosphatidylcholine hydrolysis products and its application to the monitoring of the acyl migration process. Talanta. 2012;94:22–29. doi: 10.1016/j.talanta.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Zierenberg O, Grundy SM. Intestinal absorption of polyenephosphatidylcholine in man. J. Lipid Res. 1982;23:1136–1142. [PubMed] [Google Scholar]
- 36.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato M, Kawata Y, Erami K, Ikeda I, Imaizumi K. LXR Agonist Increases the Lymph HDL Transport in Rats by Promoting Reciprocally Intestinal ABCA1 and apo A-I mRNA Levels, Lipids. 2008;43:125–131. doi: 10.1007/s11745-007-3131-8. [DOI] [PubMed] [Google Scholar]