Abstract
Background
Twenty-five percent of patients with colorectal cancer present with simultaneous liver metastasis. Complete resection is the only potential curative treatment. Due to improvements in surgical and perioperative management, simultaneous liver and colon resections are an accepted procedure at specialized centers for selected patients. Nevertheless, little is known about the long-term oncological results of simultaneous surgical procedures compared to those of staged surgery.
Patients and Methods
Patients with colorectal cancer with simultaneous liver metastases presenting for complete resection at a tertiary cancer center were identified. Patients who received the primary colon resection at an outside institution were excluded from analyses.
Results
Between 1984 and 2008, 429 patients underwent surgical treatment for colorectal cancer with simultaneous liver metastasis. Of these patients, 320 (75%) had simultaneous resection, and 109 had staged resection. There was no difference in the distribution of primary tumor locations between the 2 groups. Mean hepatic metastases size was significantly larger in the staged group (median 4 vs. 2.5cm; p<0.01). Neither disease free nor overall survival rates differed significantly between the two treatment strategies. The extent of the liver procedure (more than 3 segments) was identified as a significant risk factor for decreased disease free and overall survival (both p<0.01).
Conclusion
Simultaneous liver and colorectal resection for metastatic colorectal cancer is associated with similar long-term cancer outcome compared to staged procedures.
Background
Colorectal cancer is the third most common cancer in the Western World.(1) Simultaneous diagnosis of the primary colorectal cancer and liver metastasis occurs in about 25% of cases.(2, 3) R0 resection provides the only chance for cure with 5-year survival rates between 30% and 58%.(4, 5). Technical improvements in liver resection and in detection of liver metastasis, new systemic chemotherapy agents, and the implementation of multidisciplinary tumor boards have resulted in a modification of the surgical treatment strategy for such stage IV colorectal cancer patients.
The historically established standard for the management of synchronous disease involves resecting liver lesions 6 weeks to 6 months after resection of the primary colorectal tumor. (6-9) Previous publications found combined procedures too demanding for these patients due to increased morbidity and mortality rates. Recently, experienced centers published promising perioperative outcome on small series of combined procedures for stage IV colorectal cancer.(6, 7, 10-12) Results regarding mortality and morbidity rates were comparable for simultaneous and staged resections, but with shorter total hospital stays and quicker complete recovery for the simultaneous patients. Nevertheless, combined procedures for patients requiring major liver resection for tumor clearance are still not universally recommended and are an issue of debate.(13)
Nevertheless, limited data is available on differences in the oncological outcome between the two surgical strategies. In addition, the impact and timing of adjuvant chemotherapy on liver metastasis is still an issue of debate.(14, 15) The aim of this study was to examine if a simultaneous resection approach for colorectal stage IV cancers might have an impact on the oncological long-term outcome compared to that of a staged treatment approach.
Patients and methods
From the prospectively documented data base of Memorial Sloan-Kettering Cancer Center (MSKCC) 711 patients were identified who were treated for stage IV colorectal cancer at MSKCC. Synchronous metastasis was defined as patients presenting with colorectal cancer and liver metastasis at the time of diagnosis. Preoperative diagnosis and tumor staging followed the guidelines of the American Joint Committee on Cancer (AJCC).(16)
Patients who underwent surgery for the primary colorectal cancer in an outside hospital were excluded from analysis to assure uniform treatment standards. Furthermore, patients considered for a 2-stage hepatectomy to achieve R0 resection were excluded form analysis. Therefore the final study cohort consisted of 429 patients.
Hepatic metastases were assessed and staged by means of combinations of computed tomography (CT), magnetic resonance imaging (MRI) and intraoperative ultrasound (IOUS). Preoperative comorbidities were classified as previously described.(10) Chemotherapy before colorectal and/or hepatic resection included any systemic or regional chemotherapy with or without concomitant external beam radiation. The type of liver resection was defined according to the Couinaud classification, (17) and the colorectal resection according to the ASCRS textbook of Colon and Rectal Surgery.(18) For analysis, colorectal resections were classified as follows: right resections including right and extended right colectomies; left resections including left, extended left colectomies, anterior resections and low anterior resections. Resections of 3 or more contiguous liver segments were considered as ‘major liver procedure’.(6) The technique for anesthetic management during surgery has been reported previously.(19)
Statistical analysis
Univariate tests for differences between the simultaneous resection cohort and the staged resection cohort were conducted using Fisher's Exact test for categorical covariates and two-sample t-tests for continuous covariates. ANOVA models were used to estimate correlations between disease treatment variables. Kaplan-Meier estimators are used to model the univariate survival characteristics of categorical risk factors and Cox proportional hazards models are used to model univariate survival of continuous risk factors and for the multivariate survival model. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
At MSKCC 429 patients underwent surgical treatment for colorectal cancer with synchronous liver metastases between 1984 and 2008; 320 (74.6%) of these patients received simultaneous, and 109 staged resection. The mean interval between colorectal and liver resection in staged resected patients was 6.0±6.5 months (median: 3.1 months).
Patient demographics
Mean age was 58.6±13.6 (median: 60 years) in patients resected simultaneously and 59.4±12.3 years (median: 61 years) in patients with staged treatment approach (p=0.60). In the simultaneous group 46.9% (N=150) of patients were female compared to 37.6% (N=41) in the staged group (p=0.10) (Table 1).
Table 1. Tumor characteristics.
| Simultaneous | Staged | p-value | |
|---|---|---|---|
| Patients | 320 | 109 | |
| Age | 58.6±13.6 | 59.4±12.3 | 0.60 |
| Female | 150 (46.9%) | 41 (37.6%) | 0.10 |
| Comorbidities | 194 (60.6%) | 61 (56.0%) | 0.69 |
| Colon primary | |||
| Right | 58 (18.1%) | 14 (12.8%) | p=0.38 |
| Transverse | 23 (7.2%) | 4 (3.7%) | |
| Left | 18 (5.6%) | 9 (8.2%) | |
| Sigmoid | 78 (24.4%) | 29 (26.7%) | |
| Rectum | 143 (44.7%) | 53 (48.6%) | |
| pT 3/4 | 269 (84.1%) | 91 (84.3%) | 1.00 |
| pN 1/2 | 191 (59.7%) | 65 (59.6%) | 1.00 |
| LNN density | 14.1±20.9% | 13.1±18.6% | 0.66 |
| Hepatic metastases | |||
| Size | 3.6±3.2cm | 5.4±3.5cm | <0.01 |
| Number of lesions | 2.1±1.3 | 2.2±1.2 | 0.75 |
| ≥5 lesions | 48 (15.0%) | 10 (9.2%) | 0.15 |
| Fong Score | 2.5±0.9 | 2.8±0.6 | <0.01 |
| Neoadjuvant therapy | |||
| Chemo | 125 (39.1%) | 29 (26.6%) | p=0.13 |
| Chemoradiation | 42 (13.0%) | 14 (12.8%) | |
| Radiation only | 4 (1.3%) | 9 (8.3%) | |
| None | 149 (46.6%) | 57 (52.3%) | |
pT, pathological tumor size; pN, pathological regional lymph node involvement; LNN, lymph node. Hepatic metastases are reported by histopathological assessment.
At least one comorbidity was documented for 194 (60.6%) patients in the simultaneously resected study population. Thirty-nine (12.2%) patients were preoperatively diagnosed with a cardiovascular, 26 (8.1%) with pulmonary comorbidity and 5 (1.6%) patients with combined cardiovascular and pulmonary comorbidity (Table 1).
For staged-treated patients, 61 (56.0%) patients showed preoperatively documented comorbidities. 7 (6.4%) patients were suffering from cardiovascular, 17 (15.6%) patients from pulmonary disease, and 3 (2.7%) from combined disease (p=0.69) (Table 1).
A previous abdominal surgical procedure was documented in around 20% of patients for both study groups (25.6% in simultaneous, 19.3% in staged group, p=0.57) (data not shown).
Tumor characteristics
Primary lesions
The distribution of the primary cancer location within the large intestine did not show statistically significant differences (p=0.38). Most of the tumors in both study groups were located in the rectum (44.7 vs. 48.6%). Another quarter of patients presented with sigmoid primaries (24.4 vs. 26.7%) (Table 1).
In both groups more than 80% of patients were histopathologically classified with T3 or 4 lesions in the large intestine (84.1 vs. 84.3%, p=1.00). In almost 50% of the patients loco-regional lymph node involvement was confirmed (59.7% vs. 59.6%, p=1.00). The lymph node ratio was 14.1±20.9% for the simultaneous vs. 13.1±18.6% for the staged group (p=0.66). Further details about the primary tumor location are provided in Table 1.
Hepatic lesions
In the pathological specimens the median number of metastasis was 2 in both groups (p=0.75). In the simultaneous resection group 48 (15.0%) patients with more than 5 metastases were identified, compared to 10 (9.2%) patients in staged resected patients (p=0.15).
The mean size of the largest hepatic lesion was significantly larger (p<0.01) with 5.4±3.5cm (median: 4.0cm) in the staged resected study population compared to 3.6±3.2cm (median: 2.5cm) in the simultaneously resected group. Patients in the simultaneous group had a mean Fong score of 2.5±0.9 compared to 2.8±0.6 in the staged group (p<0.01) (Table 1).(20)
Neoadjuvant treatment
Patients undergoing simultaneous resections received neoadjuvant chemotherapy in 125 (39.1%) cases, chemoradiation in 42 (13.0%) cases and radiation only in 4 (1.3%) cases. The rest of simultaneously resected patients (149; 46.6%) did not receive neoadjuvant treatment.
In the staged group 29 (26.6%) patients received chemotherapy only, 14 (12.8%) patients received chemoradiation and 9 (8.3%) patients received radiation only. The rest (57 patients; 52.3%) of this study population did not receive neoadjuvant treatment (Table 1).
Surgical Details
As there has been a change in practice over time, the distributions of surgical strategy in the course of three time periods are provided in Figure 1. Since 2003, only two patients have been treated with a staged procedure.
Figure 1. Distribution of OR strategy by time.
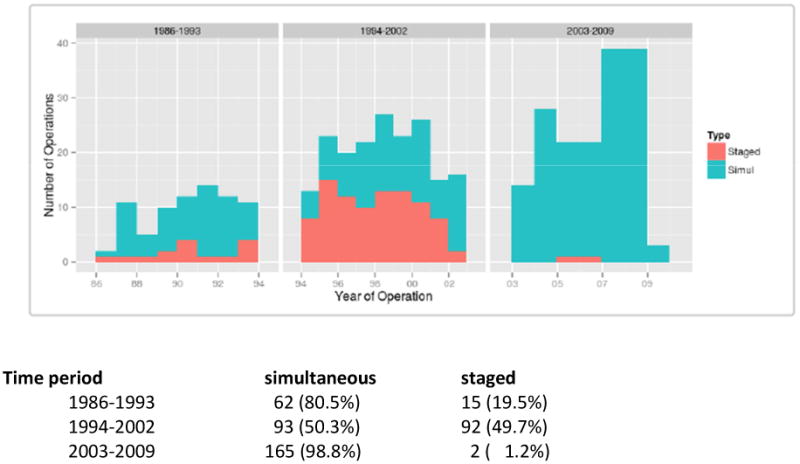
In this series no totally or partially laparoscopic procedures were performed. In about 50% of cases the surgical procedures for the colorectal primary was an anterior (AR) or low anterior resection (LAR) in both groups. Pouch construction was performed in 38 (26.2%) patients of the simultaneous group, and in 4 (7.5%) patients of the staged group (p<0.01). In the simultaneous group 63 (19.7%) patients received a temporary stoma compared to 14 (12.8%) in the staged group (p=0.11) (Table 2).
Table 2. Surgical details classified for simultaneous and staged procedure.
| Simultaneous | Staged | p-value | |
|---|---|---|---|
| OR colorectal | |||
| Right | 80 (25.0%) | 15 (13.8%) | <0.01 |
| Left | 45 (14.1%) | 27 (24.8%) | |
| AR | 37 (11.5%) | 2 (1.8%) | |
| LAR | 134 (41.9%) | 51 (46.8%) | |
| APR | 15 (4.7%) | 13 (11.9%) | |
| Total | 9 (2.8%) | 1 (0.9%) | |
| OR liver | |||
| Wedge | 89 (27.8%) | 7 (6.4%) | |
| Segment | 124 (38.8%) | 23 (21.1%) | |
| Major (≥3) | 107 (33.4%) | 79 (72.5%) | <0.01 |
| Liver first | 215 (67.2%) | ||
| Pump placement | 70 (21.9%) | 19 (17.4%) | 0.76 |
| R positive colorectal | 4 (1.3%) | 5 (4.6%) | 0.97 |
| R status liver | |||
| R1 | 37 (11.6%) | 7 (6.4%) | 0.10 |
| R2 | 22 (6.9%) | 0 (0.0%) | |
OR, surgical procedure; Right, right and extended right colectomy; Left, left and extended left colectomy, AR, anterior resection; LAR, low anterior resection; APR, abdominoperineal resection; Major, resection of 3 or more liver segments.
Details regarding the liver procedures are provided in Table 2. Liver procedures were classified as major (≥3 segments) in 107 (33.4%) patients undergoing simultaneous compared to 79 (72.5%) of patients undergoing staged resection (p<0.01). In 215 (67.2%) simultaneously resected patients, the liver procedure was performed prior to the rectal resection. Pump placement was performed in 70 patients (21.9%) of simultaneously and in 19 (17.4%) of staged resected patients (p=0.76).
The rates for R1 resection in the liver were 11.6% in the simultaneously resected group compared to 6.4% in the staged resected group (p=0.10). A positive resection margin at the colorectal side was detected in 1.3% of patients in the simultaneous group compared to 4.6% in the staged group (p=0.97). None of the patients in either group had positive margins in both the liver and the colon (Table 2).
The mean hospitalization time for patients receiving simultaneous resections was significantly shorter than that of patients treated with a staged approach (11±8 vs. 20±9 days; p<0.01, median 11 vs. 19 days).
Oncologic Outcome
Adjuvant therapy
In the simultaneous group 288 (90.0%) of patients received adjuvant chemotherapy. In the staged study population 97 (89.9%) patients received chemotherapy after staged liver resection (p=1.00) (Table 3).
Table 3. Outcome/Survival.
| Simultaneous | Staged | p-value | |
|---|---|---|---|
| Survival | |||
| Overall median | 47 months | 48 months | |
| Overall 12 months | 90.5% | 92.6% | |
| Overall 60 months | 38.5% | 38.9% | 0.52 |
| DFS 12 months | 51.5% | 72.5% | |
| DFS 60 months | 25.3% | 24.3% | 0.09 |
| Site of initial recurrence | |||
| Patients | 269 | 89 | |
| Liver | 134 (49.8%) | 38 (42.7%) | |
| Lung | 68 (25.3%) | 24 (27.0%) | |
| Lung and liver | 23 (8.6%) | 8 (9.0%) | |
| Peritoneal/intestine | 35 (13.0%) | 15 (16.8%) | |
| Other | 9 (3.3%) | 4 (4.5%) | 0.79 |
DFS, disease-free survival.
Survival/Recurrence
The median follow up time was 36 months in the simultaneous group and 42 months in the staged group (p=0.32). There was no statistically significant difference in the one and five year overall survival (OS) rates between the simultaneously and the staged resected patients (p=0.52). One year survival rates were 90.5% in the simultaneous, and 92.6% in the staged group, 5 years survival rates of 38.5% compared to 38.9%, respectively. Disease-free survival rates were comparable (p=0.09). Details are provided in Table 3. Kaplan Meier curves for OS are shown in Figure 2, for disease-free survival in Figure 3.
Figure 2.
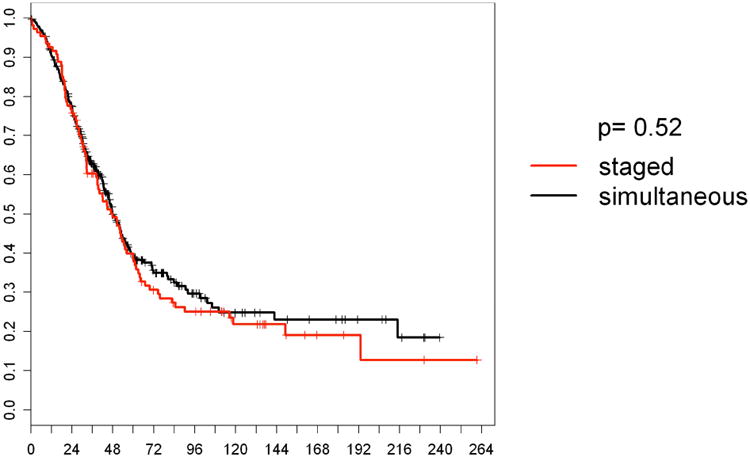
Overall survival according to treatment strategy. Staged: staged resected patients N=109; simultaneous: simultaneously resected patients N=320.
X-axis represents percentages of surviving patients. Y-axis represents survival in months.
Figure 3.
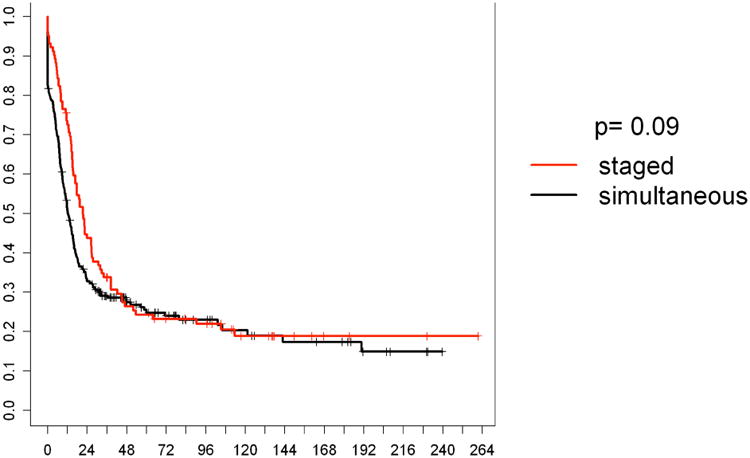
Disease free survival according to treatment strategy. Staged: staged resected patients (n=109); simultaneous: simultaneously resected patients (n=320).
X-axis represents percentages of surviving patients. Y-axis represents survival in months.
Analysis of major liver resections
One hundred-eighty six (43.4%) of all patients underwent major liver resection; 107 (58%) in the simultaneous and 79 (43%) in the staged group (Table 4).
Table 4. Major liver resection.
| Simultaneous | Staged | p-value | |
|---|---|---|---|
| Patients | 107 (33.4%) | 79 (72.5%) | p<0.01 |
| Age | 55±14 | 59±12 | p=0.04 |
| Primary rectum | 28% | 49% | p=0.03 |
| Size liver lesion | 5.8±4.2cm | 6.0±3.6cm | p=0.64 |
| Number liver lesions | 2.5±1.3 | 2.2±1.2 | p=0.43 |
| OR colorectal | |||
| Right | 32 (29.9%) | 10 (12.7%) | p<0.01 |
| Left | 21 (19.6%) | 19 (24.0%) | |
| AR | 8 (7.5%) | 1 (1.3%) | |
| LAR | 44 (41.2%) | 41 (51.9%) | |
| APR | 2 (1.8%) | 8 (10.1%) | |
| R1 resection | 16 (15.0%) | 4 (5.1%) | p=0.03 |
| Survival | |||
| overall | |||
| median | 42% | 47% | p=0.99 |
| 1 year | 88% | 92% | |
| 5 years | 34% | 41% | |
| DFS | |||
| 1 year | 45.0% | 72.0% | p=0.04 |
| 5 years | 22.6% | 22.6% | |
Right, right and extended right colectomy; left, left and extended left colectomy, AR, anterior resection; LAR, low anterior resection; APR, abdominoperineal resection: DFS, disease-free survival. Hepatic metastases are reported by histopathological assessment.
Tumor characteristic/surgical aspects
In the staged group, significantly more patients suffered from a rectal primary (49 vs. 28%; p=0.03). Significantly more APR and LAR procedures were performed in the staged patient group (p<0.01). The size of the largest liver lesion (p=0.64) and the numbers of liver metastasis (p=0.43) did not differ significantly between the groups, R1 resections occurred significantly less in the staged group (5.1 vs. 15.0%; p=0.03) (Table 4).
Discussion
Approximately a quarter of the patients with colorectal cancer present with liver metastases at the time of diagnosis.(2, 21) The only option for potential cure is resection of the colorectal primary and the liver lesions. The optimal strategy for surgical timing is still an issue of debate depending on the location of the primary tumor as well as the extent of the disease in the liver. The originally preferred surgical approach addressed the colorectal tumor first. Then, after adjuvant chemotherapy, the patients underwent staged liver resection. In some centers, only patients without disease progression were selected for hepatic resection. This is based on the high mortality and morbidity of liver resections in the past, and the desire to avoid unnecessary liver resections in patients with unfavorable tumor biology. More recently, liver resections have become quite safe, and initiated impetus for a re-examination of the treatment paradigm.
Since the late 1990's, experienced institutions have reported promising morbidity and mortality results for simultaneous colorectal and hepatic procedures. Comparable perioperative morbidity and mortality rates proved the feasibility and safety of a combined treatment approach. The perceived risk of increased perioperative complication rates and higher mortality rates became refuted.(6, 7, 10, 11) Nevertheless, some authors still recommended simultaneous resections only in patients requiring minor liver resections, as major liver resection was associated with increased morbidity and mortality rates.(6, 22) The most recently presented data have demonstrated the safety even for simultaneous major liver and colorectal resections.(11, 23-26) Further studies will also have to evaluate the impact of laparoscopic procedures on outcome and strategy management in the future.
Despite plenty of perioperative outcome reports, limited and controversial data is available on oncological survival.(27, 28) One of the earliest reports on simultaneous resections for stage IV colorectal cancer patients by Vogt et al. in 1991 showed a trend towards improved oncological outcome following staged resection.(29) More recent data presented comparable overall and disease-free survival rates for the two surgical strategies.(22, 26, 27) In contrast, de Haas et al. reported an increased risk for recurrence with synchronous resections without differences in overall survival rates.(30) Therefore, the focus of this study was set on oncological outcome of synchronous surgical treatment for stage IV colorectal cancer.
Our data show similar OS rates for staged and simultaneous resections after 12 and 60 months. Also disease-free survival rates did not differ significantly. Comparable results were reported by Mayo in a multicenter analysis comparing oncological outcomes between the two treatment strategies of four large international centers.(21)
Several arguments might favor synchronous resection from an oncological point of view: Simultaneous resection avoids a delay in the surgical treatment of metastatic disease. It was even stated that this aggressive approach might result in improved long-term prognosis due to earlier removal of the entire tumor mass and prevention of malignant cell dissemination.(31)
The classical theory to observe the biological behavior of initially resectable liver metastases is no longer universally accepted. Furthermore, the use of chemotherapy prior to staged liver surgery is still an issue of debate, as it only showed a benefit for progression-free but not for overall survival(32, 33). Nevertheless, earlier initiation of adjuvant chemotherapy will be enabled after complete tumor clearance by means of a combined surgical approach.(13)
Another issue in patients treated with a staged approach is the risk of disease progression after resection of the colorectal primary due to a delay of adjuvant chemotherapy caused by perioperative complications. Still, in approximately 30 % of patients at least one complication after colorectal surgery is reported with significant impact on long-term outcome.(34) Hendren et al. could show that complications themselves and the number of complications are associated with omitted chemotherapy.(35) Turrini et al reported undertreated patients in the staged group due to failure of completion of chemotherapy as well as psychological saturation of long course therapy.(36) Furthermore, in staged-resected patients, repeated immunological suppression and thus tumoral growth might have some negative impact on oncological outcome.(37)
Sparing one hospitalization is also associated with increased patient comfort, reduced exposure to pathogens, and reduced costs to health care systems. In our study population we could spare one week of hospitalization with combined procedures (cost analysis for this study population not done). According to Abbott et al. this might result in savings of nearly 20% per case.(26) On the other hand, the combined surgical approach might not be feasible for all institutions due to missing resources and experience.
Due to its nonrandomized nature, this study shows clinical bias in selection of patient for simultaneous resections. First, readers will criticize the long period of observation of more than 20 years. Treatment strategies, technical developments and chemotherapy agents have changed over time. Patients requiring major liver resections and with high clinical risk scores for cancer recurrence were more likely to have staged-resections. However, early initial outcomes have encouraged a simultaneous resection practice. Furthermore, the study population was inhomogeneous regarding the location of the colorectal primary. Significantly more patients with rectal primaries were treated at our institution, since patients with stage IV rectal disease are more likely to be treated at a specialized tertiary center. Studies comparing staged versus simultaneous surgery always over-report patients with favorable tumor biology in the staged population, as those with progression of disease are excluded from hepatic resection.
In conclusion, our data show similar outcomes for overall and disease free survival in stage IV colorectal cancer patients treated with synchronous or staged surgery. Shorter hospitalization and the consequent reduction of costs for the health care system are additional advantages of the synchronous over the classic staged surgical treatment concept at specialized institutions with adequate resources and experience.
Footnotes
Conflict of interest: All authors declare that they have no conflict of interests regarding this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93(4):465. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 3.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhir M, Lyden ER, Wang A, Smith LM, Ullrich F, Are C. Influence of margins on overall survival after hepatic resection for colorectal metastasis: a meta-analysis. Ann Surg. 2011;254(2):234. doi: 10.1097/SLA.0b013e318223c609. [DOI] [PubMed] [Google Scholar]
- 5.Shimada H, Tanaka K, Endou I, Ichikawa Y. Treatment for colorectal liver metastases: a review. Langenbecks Arch Surg. 2009;394(6):973. doi: 10.1007/s00423-009-0530-8. [DOI] [PubMed] [Google Scholar]
- 6.Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14(12):3481. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 7.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77(7):1254. [PubMed] [Google Scholar]
- 8.Jenkins LT, Millikan KW, Bines SD, Staren ED, Doolas A. Hepatic resection for metastatic colorectal cancer. Am Surg. 1997;63(7):605. [PubMed] [Google Scholar]
- 9.Verhoef C, van der Pool AE, Nuyttens JJ, Planting AS, Eggermont AM, de Wilt JH. The “liver-first approach” for patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. 2009;52(1):23. doi: 10.1007/DCR.0b013e318197939a. [DOI] [PubMed] [Google Scholar]
- 10.Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197(2):233. doi: 10.1016/S1072-7515(03)00390-9. [DOI] [PubMed] [Google Scholar]
- 11.Martin RC, 2nd, Augenstein V, Reuter NP, Scoggins CR, McMasters KM. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg. 2009;208(5):842. doi: 10.1016/j.jamcollsurg.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, Wang L, Chen C, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastases. J Gastrointest Surg. 2010;14(12):1974. doi: 10.1007/s11605-010-1284-x. [DOI] [PubMed] [Google Scholar]
- 13.Abbott AM, Parsons HM, Tuttle TM, Jensen EH. Short-term outcomes after combined colon and liver resection for synchronous colon cancer liver metastases: a population study. Ann Surg Oncol. 2013;20(1):139. doi: 10.1245/s10434-012-2515-z. [DOI] [PubMed] [Google Scholar]
- 14.Pinto Marques H, Barroso E, de Jong MC, et al. Peri-operative chemotherapy for resectable colorectal liver metastasis: does timing of systemic therapy matter? J Surg Oncol. 2012;105(6):511. doi: 10.1002/jso.22133. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher DJ, Zheng J, Capanu M, et al. Response to neoadjuvant chemotherapy does not predict overall survival for patients with synchronous colorectal hepatic metastases. Ann Surg Oncol. 2009;16(7):1844. doi: 10.1245/s10434-009-0348-1. [DOI] [PubMed] [Google Scholar]
- 16.http://www.cancerstaging.org
- 17.Couinaud C. Le foie: Etudes anatomiques et Chirurgicales. Paris: Masson & Cie; 1957. [Google Scholar]
- 18.Wolf Bea. The ASCRS Textbook of Colon and Rectal Surgery [Google Scholar]
- 19.Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187(6):620. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 20.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayo SC, Pulitano C, Marques H, et al. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg. 2013;216(4):707. doi: 10.1016/j.jamcollsurg.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka K, Shimada H, Matsuo K, et al. Outcome after simultaneous colorectal and hepatic resection for colorectal cancer with synchronous metastases. Surgery. 2004;136(3):650. doi: 10.1016/j.surg.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Capussotti L, Ferrero A, Vigano L, Ribero D, Lo Tesoriere R, Polastri R. Major liver resections synchronous with colorectal surgery. Ann Surg Oncol. 2007;14(1):195. doi: 10.1245/s10434-006-9055-3. [DOI] [PubMed] [Google Scholar]
- 24.Muangkaew P, Cho JY, Han HS, et al. Outcomes of Simultaneous Major Liver Resection and Colorectal Surgery for Colorectal Liver Metastases. J Gastrointest Surg. 2015 doi: 10.1007/s11605-015-2979-9. [DOI] [PubMed] [Google Scholar]
- 25.Yoshioka R, Hasegawa K, Mise Y, et al. Evaluation of the safety and efficacy of simultaneous resection of primary colorectal cancer and synchronous colorectal liver metastases. Surgery. 2014;155(3):478. doi: 10.1016/j.surg.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Abbott DE, Cantor SB, Hu CY, et al. Optimizing clinical and economic outcomes of surgical therapy for patients with colorectal cancer and synchronous liver metastases. J Am Coll Surg. 2012;215(2):262. doi: 10.1016/j.jamcollsurg.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210(6):934. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 28.Li ZQ, Liu K, Duan JC, Li Z, Su CQ, Yang JH. Meta-analysis of simultaneous versus staged resection for synchronous colorectal liver metastases. Hepatol Res. 2013;43(1):72. doi: 10.1111/j.1872-034X.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 29.Vogt P, Raab R, Ringe B, Pichlmayr R. Resection of synchronous liver metastases from colorectal cancer. World J Surg. 1991;15(1):62. doi: 10.1007/BF01658964. [DOI] [PubMed] [Google Scholar]
- 30.de Haas RJ, Adam R, Wicherts DA, et al. Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal metastases. Br J Surg. 2010;97(8):1279. doi: 10.1002/bjs.7106. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Li Q, Wang C, Zhu H, Shi Y, Zhao G. Simultaneous vs. staged resection for synchronous colorectal liver metastases: a metaanalysis. Int J Colorectal Dis. 2011;26(2):191. doi: 10.1007/s00384-010-1018-2. [DOI] [PubMed] [Google Scholar]
- 32.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 33.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 34.Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg. 2014;259(5):916. doi: 10.1097/SLA.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 35.Hendren S, Birkmeyer JD, Yin H, Banerjee M, Sonnenday C, Morris AM. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53(12):1587. doi: 10.1007/DCR.0b013e3181f2f202. [DOI] [PubMed] [Google Scholar]
- 36.Turrini O, Viret F, Guiramand J, Lelong B, Bege T, Delpero JR. Strategies for the treatment of synchronous liver metastasis. Eur J Surg Oncol. 2007;33(6):735. doi: 10.1016/j.ejso.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 37.Weese JL, Ottery FD, Emoto SE. Do operations facilitate tumor growth? An experimental model in rats. Surgery. 1986;100(2):273. [PubMed] [Google Scholar]


