Abstract
PURPOSE OF REVIEW
Recent studies have emerged to reveal the pivotal roles of mTOR signaling not only in the maintenance of physiological functions of renal cells but also in pathogenesis of renal cell dysfunctions and kidney diseases. We introduce current understandings of the mTOR signaling and its crucial roles in glomerular epithelial cell biology as well as the pathophysiology related to kidney diseases.
RECENT FINDINGS
mTOR, a Ser/Thr kinase, forms two distinct functional complexes, mTORC1 and mTORC2. Recent studies revealed physiological levels of mTORC1 and mTORC2 activity play key roles in maintaining podocyte and glomerular functions. However, aberrant activation of mTORC1 and/or loss of mTORC2 activity in podocytes may underlie the pathogenesis of glomerular disorders including diabetic kidney disease.
SUMMARY
An effective treatment for mTORC1-associated podocyte and glomerular dysfunction may require the attenuation of mTORC1 activity in the setting of both an intact mTORC2 pathway and normal basal mTORC1 activity in order to preserve physiological podocyte functions.
Keywords: mTOR, rapamycin, podocytes, glomerular epithelial cells, renal diseases
Introduction
Differentiated podocytes possess elaborated foot processes that form a functional filtration barrier in glomeruli [1]. Because active filtration requires constant maintenance and renewal of the filtration unit, glomerular podocytes may represent a key site of mechanical breakdown in renal failure. One of the major reasons for the vulnerability of podocytes as a filter is that these highly differentiated epithelial cells lack replicative capacity [2]. Various genetic and environmental insults cause podocyte injuries including podocyte foot process effacement and podocyte loss due to cell death or detachment from the glomerular basement membrane [3]. Such podocyte injuries lead to generation of proteinuria and glomerular dysfunction. In this regard, many glomerular disorders that generate proteinuria constitute a spectrum of podocytopathies in which the progression of glomerular dysfunction is often associated with net depletion of podocytes from glomerular tufts [4].
Recent studies suggest that changes in podocyte mass/volume/size in response to stresses may trigger its injury and cause death and/or detachment from the glomerular basement membrane (GBM) [1, 3, 5]. In recent years studies have begun to reveal the molecular mechanisms underlying abnormal cell growth and metabolism that leads to the dysfunction of podocytes [6-12]. Among these, the mechanistic target of rapamycin (mTOR) signaling pathway has been recognized as a key regulatory pathway for normal podocyte function and whose dysregulation under disease conditions such as diabetes leads to their dysfunction and glomerulopathy [11, 12].
mTOR complexes and the effect of rapamycin
Two distinct functional mTOR containing protein complexes have been identified, termed mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) [13-17]. mTORC1 comprises of mTOR, Raptor, PRAS40, mLST8, and Deptor. Raptor is an essential component of mTORC1, functioning as a scaffold for specific mTORC1 substrates such as S6K1 and 4EBP1 [18-20]. Raptor also plays an essential role in tethering mTORC1 to the lysosomal membrane for its activation [21, 22].
In contrast, mTORC2 comprises of mTOR, Rictor, Sin1, mLST8, Deptor, and Protor. Deletion of Rictor, Sin1, or mLST8 abolishes mTORC2 activity as the phosphorylation of mTORC2 substrates such as Akt and SGK1 is largely abolished [14, 17, 23-25].
Rapamycin is a potent and specific inhibitor of mTORC1. After associating with FK-506-binding protein 12 (FKBP12), the rapamycin-FKBP12 complex directly binds to the FKBP12-rapamycin-binding (FRB) domain of mTOR kinase in mTORC1 [26] and allosterically inhibits mTOR kinase activity by blocking the accessibility of substrates to the active site of mTOR kinase [27]. In contrast to its specific effect on mTORC1, rapamycin has little effect on mTOR kinase activity in mTORC2. A recent cryo-EM study of mTORC2 demonstrated that Rictor, an essential mTORC2 component, masks the FRB domain of mTOR kinase of mTORC2 [28]. This suggests that the rapamycin-FKBP12 complex may not be able to access the FRB domain to inhibit mTOR kinase activity once mTORC2 is formed. Interestingly, however, it has been reported that prolonged rapamycin treatment attenuates the formation of mTORC2, thereby preventing mTORC2 from phosphorylating its substrates [29]. These observations suggest that the rapamycin-FKBP12 complex may gain access to newly synthesized mTOR and prevent mTOR from forming functional mTORC2.
Downstream of mTORC1 and mTORC2
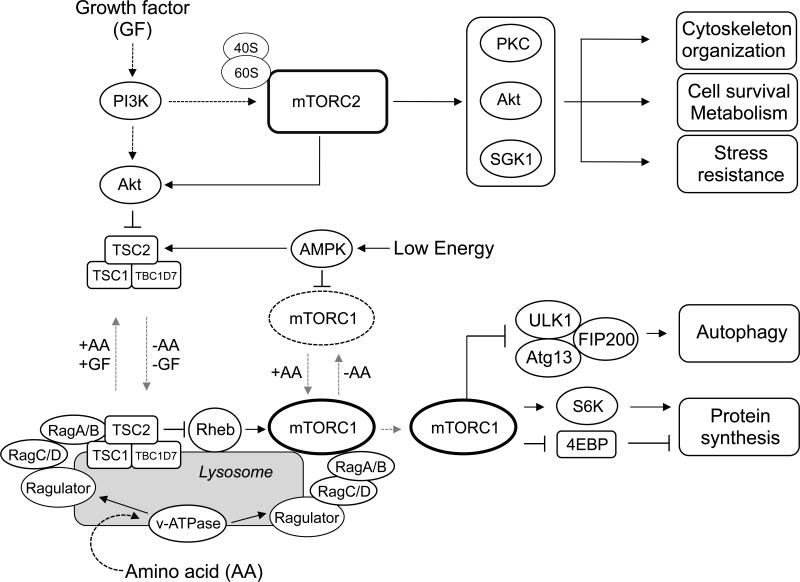
mTORC1 functions as a master regulator of cell growth and proliferation by phosphorylating and regulating multiple downstream effectors, which involve in protein and lipid biosynthesis, glucose metabolism, cellular organelle formation, and protein degradation [30] (Fig.1). Among the group of mTORC1 substrates, S6K1 and 4EBP1 are the best characterized substrates. mTORC1 directly phosphorylates and activates S6K1 [31-33]. Active S6K1 promotes mRNA translation at several different steps including mRNA splicing, translation initiation, and elongation by phosphorylating key translational regulators such as sker, eIF4B, and eEF2K [34]. Interestingly, S6K1 activation negatively feeds back to the PI3K-Akt pathway. By phosphorylating and destabilizing Insulin Receptor Substrates (IRS1/2), mTORC1-mediated S6K1 activation inhibits growth factor-induced PI3K activation [35-37].
Figure 1. The mTOR signaling pathway.
The regulations of mTORC1 and mTORC2, and the major roles of mTORC1 and mTORC2 in mammalian cells.
4EBP1 is also well-characterized mTORC1 substrate. In the absence of mTORC1 activity, hypo-phosphorylated 4EBP1 binds to eIF4E, a 5’ Cap binding protein, to inhibit its interaction with eIF4G1 to form an active initiation complex, thereby suppressing Cap-dependent translation initiation. Phosphorylation of 4EBP1 by mTORC1 leads to its dissociation from eIF4E, which is a critical step of translation initiation [38, 39].
Autophagy is an evolutionarily conserved major catabolic process in which unnecessary, dysfunctional, or toxic cellular materials are captured and delivered by double-membrane vesicles (i.e., autophagosomes) to the lysosome for degradation [40-42]. This process is essential for maintaining cellular energy and nutrient levels under metabolic stress conditions such as starvation. mTORC1 functions as a major suppressor for autophagy by phosphorylating and inhibiting Ulk1, a kinase required for the initiation of autophagy [43-45].
mTORC2 directly phosphorylates and stimulates AGC kinases such as Akt, PKC, and SGK1 [14, 46-49] (Fig.1). mTORC2-dependent Akt phosphorylation and activation play important roles in stimulating glucose uptake, viability, and cytoskeletal organization of cells. For actin remodeling, two small GTPases, RhoA and Rac1, have been reported to play critical roles in cytoskeleton reorganization downstream of mTORC2. Deletion of mTORC2 disrupts formation of the actin cytoskeleton, which is rescued by expression of the constitutively active form of Rac1 or RhoA. Although the precise molecular mechanisms remain elusive, mTORC2 stimulates GTP-loading of Rac1 and RhoA, indicating that mTORC2 functions as an important activator for both Rac1 and RhoA in cytoskeleton formation and organization [14].
Upstream of mTORC1 and mTORC2
Growth factors are activators for both mTORC1 and mTORC2 in a manner dependent on their activation of the PI3K pathway. In the case of mTORC1, PI3K-activated Akt phosphorylates and inactivates TSC2, an essential component of the tuberous sclerosis complex (TSC), which functions as a GTPase activating protein (GAP) for Rheb [50-52]. Rheb is a Ras-related small GTPase, which directly and potently activates mTORC1 [53, 54]. Ablation of TSC2 causes constitutive mTORC1 activation even in the absence of growth factors. Thus, by inactivating the upstream negative regulator, growth factor signaling leads to mTORC1 activation. Interestingly, both the Rheb small GTPase and the TSC complex are mainly expressed on lysosomes [21, 22, 55-57]. These observations suggest that growth factor-dependent mTORC1 activation mainly occurs on the lysosomal membrane. To stimulate mTORC1 activity on the lysosomal membrane, two lysosome-associated protein complexes, Rags and their activator, Ragulator, play important roles in recruiting mTORC1 to the lysosomal membrane in response to amino acids [21, 22, 58]. Rag is an evolutionarily conserved small GTPase and consists of four isoforms (RagA/B/C/D) in mammals. RagA or B forms an obligate heterodimer with Rag C or D [59-61]. Ragulator is a pentameric protein complex, consisting of five subunits, p18 (LAMTOR1), p14, MP1, HBXIP, and C7orf59 [21, 58]. Importantly, Ragulator functions as both a scaffold and a guanine nucleotide exchange factor (GEF) for RagA/B, which directly recruits mTORC1 to the lysosomal membrane [58]. A common picture arising from these recent studies is that amino acids play an essential role in recruiting mTORC1 to the late endosome/lysosome membranes where Rheb directly activates mTORC1. The current model explains why both growth factors and amino acids are required for mTORC1 activity.
The molecular mechanisms underlying growth factor-dependent mTORC2 activation remain obscure. A recently study suggests that ribosomes play important roles in growth factor-mediated mTORC2 activation. mTORC2 physically interacts with some of the ribosome subunits, and this binding is enhanced by insulin-derived PI3K activity. Inhibiting intact ribosome biogenesis by knockdown of a component of the large or small ribosome subunit, but not translational activity, reduces mTORC2 activity [62]. Thus, rather than ongoing mRNA translation, it is the amount of cellular ribosomes that defines a key determinant for mTORC2 activity in cells.
mTORC1 in podocytes
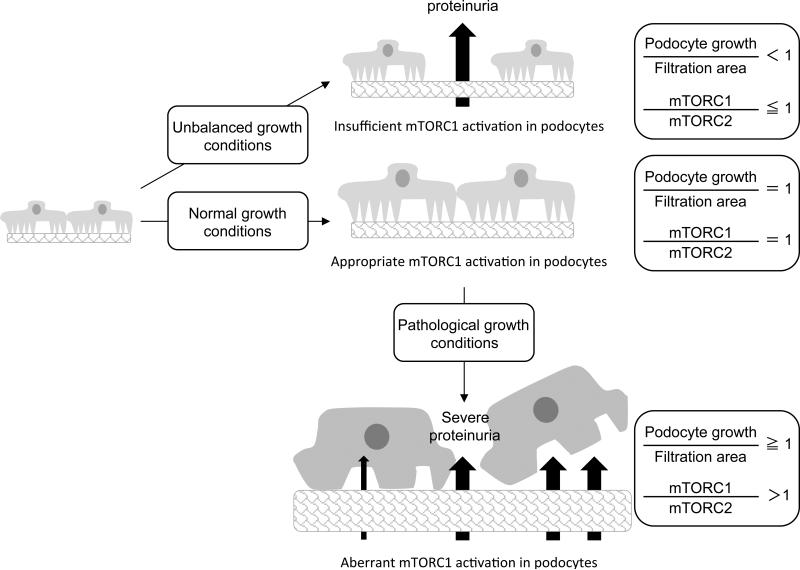
Interestingly, mTORC1 is hyper-activated in podocytes in both type 1 and type 2 diabetic mice and type 2 diabetic patients [11, 12]. Rapamycin treatment, which blocks mTORC1 activity, prevents the development of diabetic nephropathy (DN) in animal models, suggesting that enhanced mTORC1 activity in podocytes may have pathological roles in the onset/development of DN. To further reveal the pathological roles of mTORC1 activation in podocyte dysfuction, podocyte-specific TSC1 knockout (podo-TSC1 KO), where podocytes display aberrant mTORC1 activation, was generated in a non-diabetic mouse background [11]. Importantly, podo-TSC1 KO mice recapitulate many pathological phenotypes seen in DN including podocyte loss, glomerular basement membrane thickening, mesangial expansion, and proteinuria. mTORC1 hyperactivation causes mislocalization of slit diaphragm proteins and induces ER stress and epithelial-mesenchymal transition (EMT)-like phenotypic changes, which may compromise the integrity of podocytes. Furthermore, in both streptozotocin-induced type 1 diabetic mice and obesity-related type 2 diabetic mice (db/db mice), partial reduction of mTORC1 activity specifically in podocytes by the deletion of one allele of the RAPTOR gene effectively attenuates the onset or development of DN [11, 12]. These observations indicate that the aberrant activation of mTORC1 is causative for podocyte injury and the onset/development of DN (Fig.2).
Figure 2. The roles of mTORC1 and mTORC2 in podocytes in physiologic and pathologic conditions.
The roles of mTORC1 and mTORC2 in podocytes for their normal development and under pathological conditions such as diabetic nephropathy.
It is noteworthy that complete ablation of mTORC1 activity in podocytes in podo-Raptor KO mice leads to early onset of proteinuria [11, 12]. These observations imply that while excessive mTORC1 activation is detrimental, basal mTORC1 activity is required for maintaining physiological functions of podocytes. In particular, ablation of Raptor in podocytes during embryonic stages leads to severe proteinuria, while Raptor deletion in podocytes of mature animals shows moderate proteinuria. These findings suggest that basal mTORC1 activity is particularly important during the period of podocyte differentiation, growth, and functional development (Fig. 2).
As a terminally differentiated cell, podocytes lose their capability for replication once they migrate from the parietal epithelium to the surface of the glomerular tuft, where they cover the filtration area during the capillary loop stage of kidney development. However, during postnatal development, the glomerular tuft volume continues to increase due to increased glomerular capillary number and surface area [63, 64]. In order to cope with the enlarging glomerular tuft, podocytes need to increase their mass (hypertrophy) to cover the enlarged filtration area [65]. In this regard, mTORC1 activity in podocytes may play an important role for their postnatal growth (Fig. 2). Insufficient mTORC1 activity may inhibit podocyte growth that is needed to accommodate the enlarged filtration area and cause the production of proteinuria and the development of focal segmental glomerulosclerosis (FSGS) [66]. In line with this notion, Fukuda et al. have developed podocyte-specific AA-4EBP1 transgenic rats where podocytes have decreased mTORC1-dependent translational activity. AA-4EBP1 is a non-phosphorylatable mutant of the mTORC1 substrate 4EBP1 that interferes mTORC1-dependent translation initiation. In this model, proteinuria and glomerulosclerosis in podocyte-specific AA-4E-BP1 transgenic rats were linearly correlated with increasing body weight and glomerular volume. The histology analysis revealed bare areas of the glomerular basement membrane due to the detachment of podocyte foot processes, followed by adhesion of glomerular tufts to the Bowman's capsule [66]. This study indicated that mTORC1-dependent translation sets the threshold for the adaptive growth capacity of podocytes and provided a plausible explanation for the high incidence of FSGS in fast growing young adults and in the obese population. In addition, these observations also support the idea that ablation of mTORC1 activity in podocytes may restrict their capability for adaptive growth, which is required during the development of the glomerulus or under certain pathological conditions including hypertension, diabetes, and remnant kidney.
mTORC2 in podocytes
In contrast to mTORC1 inhibition in podo-Raptor KO mice, podo-Rictor KO mice in which mTORC2 activity is abolished in podocytes did not display any obvious clinical, histological, or ultrastructural abnormalities in their glomeruli, even in a long term follow up study. However, mTORC2 deletion decreased the ability of podocytes to maintain normal function under stress conditions. Upon BSA overloading stress, levels of proteinuria were significantly increased in podo-Rictor KO mice compared to wild type littermates, suggesting that the function of mTORC2 in podocytes is required for their adaptation under certain stress conditions. Moreover, additional Rictor ablation in podo-Raptor KO mice (podo-Raptor/Rictor double KO mice) exacerbated proteinuria levels and glomerular dysfunction [12]. These observations indicate that in a sensitized mTORC1 mutant background in podocytes, mTORC2 may exert important roles in maintaining the functional structure and viability of podocytes.
Given the important role of mTORC2 in cytoskeleton reorganization [14] and the essential role of actin cytoskeleton in podocyte integrity (Reviewed by [67]), the activity of mTORC2 may be needed to preserve actin reorganization in response to stress. It should be noted that the activity of Akt, which positively regulates cell survival and reorganization of the cytoskeleton, is not completely abolished by the deletion of mTORC2 in many different types of cells including podocytes. This might be one of the reasons why podo-Rictor KO mice appear superficially wild-type under normal conditions. In line with the above observations, Canaud et al. demonstrated that the activity of Akt2 in podocytes is essential for maintaining their physiological functions as podocyte-specific Akt2 KO mice show considerable podocyte injury and glomerular dysfunction [68].
In accordance with the notion that Akt2 activity is essential for podocyte viability, an earlier study suggested that the intact insulin signaling pathway in podocytes is pivotal for maintaining podocyte and glomerular functions during normal kidney development. Welsh et al. demonstrated that podocyte-specific insulin receptor KO mice (podo-IR KO) display proteinuria from 5 weeks of age and develop glomerulosclerosis by week 8 of development. Loss of cytoskeletal architecture in podocyte foot processes was the earliest alteration detected in podo-IR KO mice [69]. Taken together, these recent studies suggest that aberrant activation of mTORC1 in podocytes is likely to be a first step in the pathological signaling that occurs under diabetic conditions, leading to aberrant growth phenotypes including podocyte and glomerular hypertrophy. Simultaneously, hyper-activation of mTORC1 causes a reduction of the IR-PI3K-Akt pathway in podocytes and leads to the impairment of actin cytoskeletal reorganization and the loss of podocyte viability.
Rapamycin analogs such as sirolimus are widely used in renal transplant patients as an immune suppressive agent. Despite of the beneficial effects of rapamycin on experimental DN in animal models, considerable clinical evidence suggests that sirolimus often induces albuminuria in renal transplant recipients with compromised renal function [70-72], which may limit the clinical use of sirolimus in treating any glomerular diseases including DN. Considering that basal mTORC1 activity is required for maintaining normal podocyte functions, complete blockade of mTORC1 activity with long-term sirolimus treatment may evoke adverse outcomes on podocytes. More importantly, prolonged sirolimus treatment also prevents the assembly of newly formed mTORC2 [29], which plays important roles in the activation of Akt2. By reducing both cellular mTORC1 and mTORC2 activity, long-term sirolimus treatment may compromise the integrity and physiological functions of podocytes under stress conditions.
Conclusions
Taken together, an effective treatment for mTORC1-associated podocyte dysfunction may require the attenuation of mTORC1 activity in the setting of both an intact mTORC2-Akt pathway and normal basal mTORC1 activity in order to preserve physiological podocyte functions. One plausible but promising approach may be to target the nutrient-sensing mechanism for mTORC1 activation. Amino acids, especially leucine and glutamine, are key nutrients for spatial recruitment and activation of mTORC1 on lysosomal membranes [73, 74]. Unlike the growth factor-PI3K pathway, spatial regulation of mTOR by amino acids is specific to mTORC1 but not mTORC2. Furthermore, a recent study suggested that leucine and glutamine use distinct mechanisms to recruit mTORC1 to the lysosomal membrane [75]. For instance, disruption of the Ragulator-Rag system abolishes leucine-dependent lysosomal localization while glutamine is still able to recruit mTORC1 to lysosomes through an Arf1-dependent manner. Lack of one of these mechanisms significantly reduces lysosomal mTORC1 localization but it does not completely eliminate mTORC1 localization on lysosomes, thus preserving some, albeit lower, mTORC1 activity in these cells. In addition, hypothetically, lower mTORC1 activity in podocytes may sensitize the growth factor-PI3-Akt pathway, which is essential for the integrity of podocytes. Therefore, targeting these amino acid sensing mechanisms may be a promising approach to reduce but not abolish cellular mTORC1 activity without inhibiting the PI3K-mTORC2-Akt pathway in podocytes.
With the notion that the mTOR complexes exist at the nexus of many signaling cascades and have numerous crosstalk with other signaling pathways, such as the Notch and Wnt pathways, it is likely that multiple pathways may coordinately work to maintain a proper balance of mTOR activity, which is essential for normal functions of podocytes. Thus, it is important to identify molecular mechanisms underlying dysregulation of mTOR signaling under different stress and disease conditions including diabetes and obesity. The elucidation of the molecular mechanisms by which mTORC1 or mTORC2 participates in the functions of podocytes and other renal cells will be helpful to develop more precise strategies for the intervention of specific kidney diseases.
Key points.
Mechanistic target of rapamycin complex 1 (mTORC1) is essential for physiological functions of podocytes. However, aberrant activation of mTORC1 causes podocyte injuries, which is one of the important pathomechanisms of glomerulopathies including diabetic nephropathy.
mTORC2 is also important for podocyte functions under stress conditions. The balance of cellular mTORC1 and mTORC2 activity is important for maintaining the functions of differentiated podocytes.
An effective treatment for mTORC1-associated podocyte dysfunction may require the attenuation of mTORC1 activity in the setting of both an intact mTORC2-Akt pathway and normal basal mTORC1 activity in order to preserve physiological podocyte functions.
Acknowledgements
We thank John Kim for comments on this manuscript. We apologize to those authors whose work could not be cited owing to space limitations or our inability to draw connections between elements of the primary literature.
Financial support and sponsorship
This work was supported by the NIH grants (DK083491 and GM110019 to KI).
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References
- 1.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiological reviews. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 2.Pabst R, Sterzel RB. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983;24:626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- 3.Greka A, Mundel P. Cell biology and pathology of podocytes. Annual review of physiology. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiggins RC. The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 5.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 6.Dai C, Stolz DB, Kiss LP, et al. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. Journal of the American Society of Nephrology : JASN. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flannery PJ, Spurney RF. Transactivation of the epidermal growth factor receptor by angiotensin II in glomerular podocytes. Nephron. Experimental nephrology. 2006;103:e109–118. doi: 10.1159/000092196. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann S, Podlich D, Hahnel B, et al. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. Journal of the American Society of Nephrology : JASN. 2004;15:1475–1487. doi: 10.1097/01.asn.0000127988.42710.a7. [DOI] [PubMed] [Google Scholar]
- 9.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nature medicine. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 10.Tian D, Jacobo SM, Billing D, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Science signaling. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoki K, Mori H, Wang J, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. The Journal of clinical investigation. 2011;121:2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godel M, Hartleben B, Herbach N, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. The Journal of clinical investigation. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 14.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature cell biology. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 15.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 16.Loewith R, Jacinto E, Wullschleger S, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Molecular cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 17.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current biology : CB. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Current biology : CB. 2003;13:797–806. doi: 10.1016/s0960-9822(03)00329-4. [DOI] [PubMed] [Google Scholar]
- 19.Nojima H, Tokunaga C, Eguchi S, et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. The Journal of biological chemistry. 2003;278:15461–15464. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- 20.Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Current biology : CB. 2002;12:632–639. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- 21.Sancak Y, Bar-Peled L, Zoncu R, et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes & development. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Sarbassov DD, Ali SM, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Molecular cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 26.Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273:239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Rudge DG, Koos JD, et al. mTOR kinase structure, mechanism and regulation. Nature. 2013;497:217–223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Gaubitz C, Oliveira TM, Prouteau M, et al. Molecular Basis of the Rapamycin Insensitivity of Target Of Rapamycin Complex 2. Molecular cell. 2015;58:977–988. doi: 10.1016/j.molcel.2015.04.031. [The first demonstration of mTORC2 structure to reveal the mechanism underlying the rapamycin insensitivity of mTORC2.] [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Weng QP, Kozlowski M, Belham C, et al. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. The Journal of biological chemistry. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- 32.Burnett PE, Barrow RK, Cohen NA, et al. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moser BA, Dennis PB, Pullen N, et al. Dual requirement for a newly identified phosphorylation site in p70s6k. Molecular and cellular biology. 1997;17:5648–5655. doi: 10.1128/mcb.17.9.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. The Biochemical journal. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 35.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 36.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Current biology : CB. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 37.Harrington LS, Findlay GM, Gray A, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. The Journal of cell biology. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gingras AC, Gygi SP, Raught B, et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes & development. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes & development. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 40.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung CH, Jun CB, Ro SH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Molecular biology of the cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosokawa N, Hara T, Kaizuka T, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Molecular biology of the cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganley IG, Lam du H, Wang J, et al. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. The Journal of biological chemistry. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikenoue T, Inoki K, Yang Q, et al. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. The EMBO journal. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 48.Yan L, Mieulet V, Lamb RF. mTORC2 is the hydrophobic motif kinase for SGK1. The Biochemical journal. 2008;416:e19–21. doi: 10.1042/BJ20082202. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). The Biochemical journal. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 50.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nature cell biology. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 51.Manning BD, Tee AR, Logsdon MN, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Molecular cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 52.Inoki K, Li Y, Zhu T, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature cell biology. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 53.Sancak Y, Thoreen CC, Peterson TR, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Molecular cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. The Journal of biological chemistry. 2005;280:23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 55*.Menon S, Dibble CC, Talbott G, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [This study demonstraetd the molecular mechanism by which growth factor-induced TSC2 phosphorylation inhibits the function of TSC2 activity towards Rheb. The study first revealed that Akt-dependent TSC2 phosphorylation blocks its lysosomal localization of TSC2 thereby stimulating Rheb-dependent mTORC1 activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Demetriades C, Doumpas N, Teleman AA. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell. 2014;156:786–799. doi: 10.1016/j.cell.2014.01.024. [This study, in contrast, demonstrated the role of Rag small GTPase in regulating lysosome localization of the TSC complex in response to amino acids. The study proposed that amino acid-induced RagA activation induces the dissociation of the TSC complex from lysoosmal membrane.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dibble CC, Elis W, Menon S, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Molecular cell. 2012;47:535–546. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schurmann A, Brauers A, Massmann S, et al. Cloning of a novel family of mammalian GTP-binding proteins (RagA, RagBs, RagB1) with remote similarity to the Ras-related GTPases. The Journal of biological chemistry. 1995;270:28982–28988. doi: 10.1074/jbc.270.48.28982. [DOI] [PubMed] [Google Scholar]
- 60.Hirose E, Nakashima N, Sekiguchi T, Nishimoto T. RagA is a functional homologue of S. cerevisiae Gtr1p involved in the Ran/Gsp1-GTPase pathway. Journal of cell science. 1998;111(Pt 1):11–21. doi: 10.1242/jcs.111.1.11. [DOI] [PubMed] [Google Scholar]
- 61.Sekiguchi T, Hirose E, Nakashima N, et al. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. The Journal of biological chemistry. 2001;276:7246–7257. doi: 10.1074/jbc.M004389200. [DOI] [PubMed] [Google Scholar]
- 62.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 63.Nyengaard JR. Number and dimensions of rat glomerular capillaries in normal development and after nephrectomy. Kidney Int. 1993;43:1049–1057. doi: 10.1038/ki.1993.147. [DOI] [PubMed] [Google Scholar]
- 64.Marcussen N, Nyengaard JR, Christensen S. Compensatory growth of glomeruli is accomplished by an increased number of glomerular capillaries. Laboratory investigation; a journal of technical methods and pathology. 1994;70:868–874. [PubMed] [Google Scholar]
- 65.Wiggins JE, Goyal M, Sanden SK, et al. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. Journal of the American Society of Nephrology : JASN. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 66**.Fukuda A, Chowdhury MA, Venkatareddy MP, et al. Growth-dependent podocyte failure causes glomerulosclerosis. Journal of the American Society of Nephrology : JASN. 2012;23:1351–1363. doi: 10.1681/ASN.2012030271. [This study dempnstated that mTORC1-dependent translation is important for compensately podocyte growth. A lack of this mTORC1-depenednt podocyte growth causes podocyte detachment from the GBM and glomerulosclerosis in response to acute glomerular hypertorophy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faul C, Asanuma K, Yanagida-Asanuma E, et al. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends in cell biology. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 68**.Canaud G, Bienaime F, Viau A, et al. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nature medicine. 2013;19:1288–1296. doi: 10.1038/nm.3313. [This stidy first demonstrated the essential role of Akt2 in podocyte integrity and its physiological functions. The study revealed that reduction of Akt2 activity underlies the pathomecanisms of chronic kidney diseases and glomerular insufficiency in patients with renal transplantation treated with long-term rapamycin.] [DOI] [PubMed] [Google Scholar]
- 69.Welsh GI, Hale LJ, Eremina V, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell metabolism. 2010;12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Izzedine H, Brocheriou I, Frances C. Post-transplantation proteinuria and sirolimus. The New England journal of medicine. 2005;353:2088–2089. doi: 10.1056/NEJM200511103531922. [DOI] [PubMed] [Google Scholar]
- 71.Ruiz JC, Campistol JM, Sanchez-Fructuoso A, et al. Increase of proteinuria after conversion from calcineurin inhibitor to sirolimus-based treatment in kidney transplant patients with chronic allograft dysfunction. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:3252–3257. doi: 10.1093/ndt/gfl447. [DOI] [PubMed] [Google Scholar]
- 72.Butani L. Investigation of pediatric renal transplant recipients with heavy proteinuria after sirolimus rescue. Transplantation. 2004;78:1362–1366. doi: 10.1097/01.tp.0000140868.88149.63. [DOI] [PubMed] [Google Scholar]
- 73.Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duran RV, Oppliger W, Robitaille AM, et al. Glutaminolysis activates Rag-mTORC1 signaling. Molecular cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 75.Jewell JL, Kim YC, Russell RC, et al. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]