Abstract
Background
Identifying nutrition- and lifestyle-based risk factors for cognitive impairment and dementia may aid future primary prevention efforts.
Objective
We aimed to examine the association of serum vitamin D levels with incident all-cause dementia, clinically characterized Alzheimer’s disease (AD), MRI markers of brain aging, and neuropsychological function.
Methods
Framingham Heart Study participants had baseline serum 25-hydroxyvitamin D (25(OH)D) concentrations measured between 1986 and 2001. Vitamin D status was considered both as a continuous variable and dichotomized as deficient (<10 ng/mL), or at the cohort-specific 20th and 80th percentiles. Vitamin D was related to the 9-year risk of incident dementia (n= 1663), multiple neuropsychological tests (n= 1291) and MRI markers of brain volume, white matter hyperintensities and silent cerebral infarcts (n = 1139).
Results
In adjusted models, participants with vitamin D deficiency (n = 104, 8% of the cognitive sample) displayed poorer performance on Trail Making B-A (β = −0.03 to −0.05 ±0.02) and the Hooper Visual Organization Test (β = −0.09 to −0.12 ±0.05), indicating poorer executive function, processing speed, and visuo-perceptual skills. These associations remained when vitamin D was examined as a continuous variable or dichotomized at the cohort specific 20th percentile. Vitamin D deficiency was also associated with lower hippocampal volumes (β = −0.01 ±0.01) but not total brain volume, white matter hyperintensities, or silent brain infarcts. No association was found between vitamin D deficiency and incident all-cause dementia or clinically characterized AD.
Conclusions
In this large community-based sample, low 25(OH)D concentrations were associated with smaller hippocampal volume and poorer neuropsychological function.
Keywords: Alzheimer’s disease, brain, dementia, diet, lifestyle, magnetic resonance imaging, neuropsychology, nutritional status, risk factors, vitamin D
INTRODUCTION
As the population continues to age rapidly, it is important to pinpoint modifiable risk factors for dementia, including factors pertaining to lifestyle and diet. Beyond its function in maintaining bone health, vitamin D is increasingly recognized as an important multipurpose steroidal hormone [1]. Vitamin D deficiency has been associated with a number of neurological illnesses including multiple sclerosis [2], Parkinson’s disease [3], cerebrovascular disease [4], and dementia [5, 6].
Meta-analysis of studies comparing patients with and without dementia has indicated that those with Alzheimer’s disease (AD) have lower levels of vitamin D [5, 6]. Similarly, meta-analysis of cross-sectional studies has identified that low levels of vitamin D are associated with poorer global cognitive performance [5] and executive function [7]. However, drawing conclusions from case-control and cross-sectional studies is difficult because individuals with cognitive impairment may have pre-existing risk factors for vitamin D deficiency, including advanced age, comorbid conditions, poor mobilization leading to limiting sun exposure and suboptimal nutritional status [4].
Community-based prospective cohort studies have the ability to relate vitamin D levels to the future risk of incident dementia, yet few studies have been reported. Our objective was to explore the prospective association of serum 25(OH)D concentrations with incident all-cause dementia and clinically characterized AD in the large, community-based, Framingham Heart Study. We also examined the associations of serum 25(OH)D concentrations with neuropsychological function and MRI markers of subclinical brain aging. We hypothesized that low vitamin D status would be associated with evidence of subclinical brain injury on MRI, impairment in executive function, and an increased risk of dementia.
MATERIALS AND METHODS
Study sample
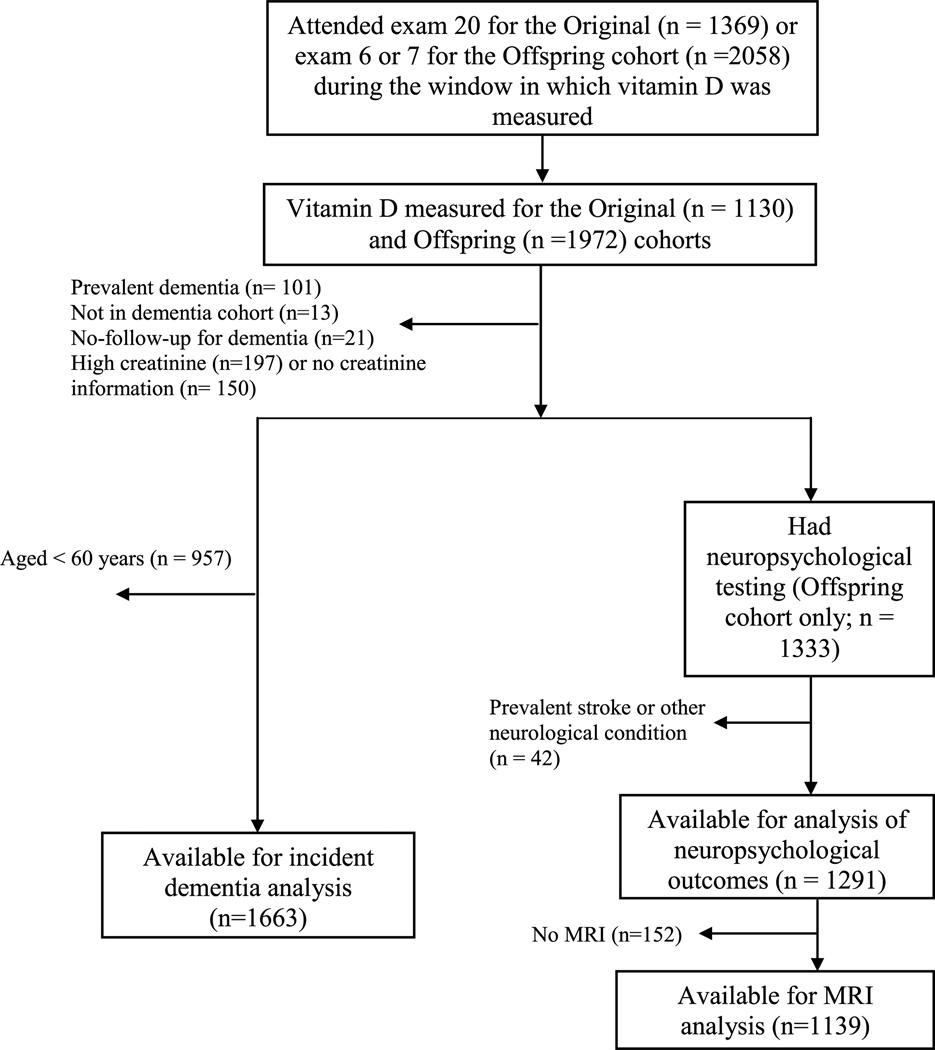
The sample was comprised of participants from the Original and Offspring Framingham Heart Study cohorts. The Original cohort first began in 1948, with participants examined approximately every two years. The Offspring cohort was initiated in 1971, comprised of the offspring of the Original cohort and their spouses. The design and selection criteria of the Framingham Heart Study have been described elsewhere [8, 9]. Vitamin D levels were measured during examination cycle 20 (1986–1990) for the Original cohort and examination cycles 6 (1995–1998) and 7 (1998–2001) for the Offspring cohort. Of the 5209 participants initially enrolled into the Original cohort, 1369 attended the 20th examination cycle. Of the 5124 participants enrolled into the Offspring cohort, 2058 attended examination cycles 6 or 7, during the window in which vitamin D was measured (which straddled the two exams). The participant flow diagram is shown in Fig. 1. The final sample for analysis of incident dementia (n= 1663) comprised participants from both the Original and Offspring study cohorts and was limited to participants aged 60 years or older at the time vitamin D levels were measured. Analysis of neuropsychological (n = 1291) and MRI (n = 1139) outcomes was limited to participants enrolled in the Offspring study cohort, but age restrictions were not imposed. All participants were free from dementia at baseline and almost all participants enrolled in this study were Caucasian. All protocols were approved by the Boston University Medical Center and Tufts Medical Center Institutional Review Boards, and participants provided written informed consent.
Fig. 1.
Selection of study participants. MRI, magnetic resonance imaging.
Serum vitamin D status
Serum samples were obtained in the morning after an overnight fast and frozen at −70ΔC for no more than 3 years. At examination 20 for the Original cohort, serum 25(OH)D was determined by a competitive protein-binding assay, as previously described [10]. Intra- and inter- assay coefficients of variation were 7% and 10%, respectively. At Offspring examinations 6 and 7, serum 25(OH)D was determined by radioimmunoassay (DiaSorin, Stillwater, MN), as previously described [11]. The limit of detection for 25(OH)D was 1.5 ng/mL, and no samples had concentrations below the limit of detection. All samples were run in duplicate and the values averaged. Total (intra-assay and inter- assay) coefficients of variation for control values of 14.4 and 54.7 ng/mL were 8.5% and 13.2%, respectively. Vitamin D deficiency was conservatively defined a priori as serum 25(OH)D concentrations below the consensual cut-off value of 10 ng/mL [12].
Incident dementia
We examined the 9-year risk of incident dementia and clinically characterized AD, beginning from the time vitamin D was measured. Dementia was diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV), and AD based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association for definite, probable, or possible AD [13]. Diagnosis was made by the dementia review committee comprising at least one neurologist and one neuropsychologist.
Participants have been monitored for the development of all-cause dementia and AD since 1974 using previously described surveillance techniques [14, 15]. Briefly, participants with suspected dementia or cognitive impairment were either self-referred or referred to us by their primary care provider, family members, or other health care professionals. We also administered the Mini Mental State Examination (MMSE) at each examination cycle in order to detect cognitive impairment. If suspected for cognitive impairment, participants underwent neuropsychological assessment by a neuropsychologist who referred suspected cases of dementia to a neurologist. Participants flagged for potential dementia by a neurologist were reviewed by our dementia review committee. Before making a diagnosis, the committee were required to consider all available evidence including neurologic and neuropsychological assessments, neuroimaging, medical records, and, if available, interviews with family or autopsy data. The review committee determined both type of dementia and date of onset.
Neuropsychological measures
We examined a battery of neuropsychological tests measuring different aspects of cognitive function. Neuropsychological testing was performed an average of 1.9 years after the quantification of vitamin D levels. The individual tests included Trail Making A and B, Logical Memory delayed, Visual Reproductions delayed, Similarities, and the Hooper Visual Organization Test. Trial Making A and B are speeded tasks in which participants have to link a series of scrambled numbers in ascending order (A) and alternate between ascending numbers and letters (B). Outcome scores were calculated as time to complete tasks B – A, with the variable transformed such that higher scores indicated superior task switching (executive control) and processing speed. The test of Similarities from the Wechsler Adult Intelligence Scale involves asking participants to indicate in which way two items are alike. Higher scores reflect better abstraction, reasoning, verbal comprehension, and categorization. Logical Memory delayed from the Wechsler Memory Scale (WMS) is a task of verbal memory with higher scores indicating better access encoding, storage, and retrieval of verbal memory. Visual reproductions delayed from the WMS is primarily a test of visual memory with higher scores reflecting better access encoding, storage and retrieval of visual memory. Finally, the Hooper Visual Organization Test measures visual analysis and synthesis with higher scores indicating better visuo-perceptual skills. Both Trail Making B-A and the Hooper Visual Organization test were natural log transformed to normalize their skewed distributions. All neuropsychological tests have been used previously and have adequate reliability and construct validity [16, 17]. More details of the neuropsychological protocol in the Framingham Heart Study Offspring cohort can be seen elsewhere [17].
Structural brain measures
MRI was performed an average of 1.8 years after the quantification of vitamin D levels. MRI techniques used in the Framingham Heart Study Offspring cohort have been described previously [18, 19]. A Siemens Magnetom 1-T field strength MRI machine was used. The images were analyzed by operators blind to serum 25(OH)D concentrations. The following MRI markers of subclinical brain aging were measured: total brain volume (TBV), hippocampal volume (HPV), white matter hyperintensity volume (WMHV), and presence of cover brain infarcts (CBIs). Brain volume was determined by manual outlining of the intracranial vault to determine the total cranial volume and by subsequent mathematical modeling to determine total brain parenchymal volume. We computed TBV as the ratio of total brain parenchymal volume to total cranial volume, thus correcting for differences in head size [18]. HPV was estimated using operator-defined, manually traced boundaries to define the region of interest. WMHV was determined according to previously published methods [19] and participants were categorized as having extensive WMHs if the log-WMHV was more than 1 SD above the age-adjusted mean in the cohort. The presence of CBIs was determined manually in accordance with STRIVE criteria [20]. Such methods have been published previously, demonstrating adequate inter-rater and intra-rater reliabilities [18, 21, 22].
Covariates
A physician-administered medical history and examination were performed at the sixth and seventh Offspring examinations [8]. Vascular risk factors (systolic blood pressure, smoking, diabetes mellitus, and prevalent cardiovascular disease) were defined as in the Framingham Stroke Risk Profile [23]. BMI was defined as weight (in kilograms) divided by the square of height (in meters) [14]. Homocysteine levels were determined as previously described [15], and log transformed. Information regarding vitamin D-containing supplement use was obtained with a detailed food-frequency questionnaire [24]. Educational achievement was studied as a 4-class variable (no high school degree; successful completion of 12 years of education leading to high school degree but no college; some college; college degree).
Statistical analysis
The 25(OH)D measures within each cohort were converted to Z scores, since different assays were used in the two cohorts [10]. Serum vitamin D levels were examined in 4 ways: 1) as a standardized continuous variable; 2) as a dichotomous variable based on the a priori deficiency cut-off value of <10 ng/mL [12]; 3) as a dichotomous variable based on the 20th percentile of the 25(OH)D value of each cohort; and 4) as a dichotomous variable based on the 80th percentile of the 25(OH)D value of each cohort. The 20th and 80th percentile cut-offs of vitamin D levels were <19.0 and ≥40.0 ng/mL for the Original cohort dementia sample; <13.6 and ≥25.5 ng/mL for the Offspring cohort dementia sample; and <13.6 and ≥25.4 ng/mL for the combined neuropsychology and MRI Offspring cohort sample.
The association between baseline serum vitamin D and the risk of developing all cause dementia and AD was investigated using Cox proportional hazards regression. We examined the association between baseline serum vitamin D and the neuropsychological and MRI outcomes using multivariable linear and logistic regression for continuous and dichotomous measures, respectively. The effects of potential confounding variables were dealt with in 3 models. Model 1 adjusted for age and gender (and time from vitamin D measurement to cognitive testing/MRI acquisition for those outcomes, plus attained educational level for cognitive outcomes). Model 2 included additional adjustments for vascular risk factors (smoking, hypertension, diabetes, prevalent cardiovascular disease and homocysteine). Model 3 included further adjustment for BMI and self-reported vitamin supplement use. Analyses were performed with SAS software, version 9.2 (SAS Institute, Cary, NC). A 2-sided probability value of <0.05 was considered statistically significant.
Sensitivity analysis
We performed a sensitivity analysis using the current Institute of Medicine guidelines of vitamin D deficiency (<12 ng/mL), insufficiency (12 to <20 ng/mL), and adequacy (20 to less than 50 ng/mL) [25]. Cox proportional hazards regression were used to separately compare the risk of incident dementia and AD for those with deficient and insufficient vitamin D levels, relative to those with adequate vitamin D levels.
RESULTS
The sample characteristics are summarized in Table 1. The mean age of the dementia cohort was older, given that cohort entry was restricted to participants 60 years of age or older at baseline. Mean 25(OH)D concentrations were 25.1±11.4 ng/mL for the dementia cohort and 19.8 ± 7.4 ng/mL for the cognitive/MRI outcome cohort, although not directly comparable given the aforementioned differences in their determination. Using the predetermined cutoff value of <10 ng/mL, 96 (6%) participants in the dementia cohort, and 104 (8%) in the cognitive outcomes cohort were vitamin D deficient.
Table 1.
Characteristics of the dementia, cognitive and imaging analysis cohorts
| Variable | Dementia cohort (n = 1663) |
Cognitive and MRI cohort (n= 1291) |
|---|---|---|
| Clinical and demographic | ||
| Age, years | 72.4 ± 6.7 | 59.5± 9.1 |
| Women, n (%) | 973 (59) | 703 (55) |
| Education, n (%) | ||
| No high school degree | 308 (19) | 37 (3) |
| High school degree | 598 (37) | 380 (29) |
| Some college | 369 (23) | 401 (31) |
| College graduate | 345 (21) | 473 (37) |
| 25(OH)D, ng/mL | 25.1± 11.4 | 19.8± 7.4 |
| 25(OH)D <10 ng/mL, n (%) | 96 (6) | 104 (8) |
| Vitamin D supplement use, n (%) | 538 (34) | 571 (48) |
| Body mass index, kg/m2 | 27.3 ± 4.7 | 27.9± 5.3 |
| Smoking, n (%) | 173 (10) | 172 (13) |
| Diabetes, n (%) | 214 (13) | 120 (9) |
| Hypertension, n (%) | 1123 (68) | 477 (37) |
| History of CVD, n (%) | 404 (24) | 98 (8) |
| Homocysteine* | 10 (8,13) | 8 (7,10) |
| Cognitive measures | ||
| Mean time to cognitive assessment | 1.88 (1.31) | |
| Logical Memory Delayed, score | – | 10.55± 3.54 |
| Visual Reproductions Delayed, score | – | 8.19 ±3.37 |
| Trail making, B – A score* | – | 0.67 (0.47,1.00) |
| Similarities, score | – | 16.75± 3.49 |
| Hooper Visual Reproductions, score* | – | 25.5 (23.5,27.0) |
| MRI measures | ||
| Mean time to MRI assessment | 1.85 (1.30) | |
| Total brain volume, % | – | 79.78± 3.29 |
| Hippocampal volume, % | – | 0.34 ±0.06 |
| WMHV, %* | – | 0.04 (0.02,0.08) |
| Extensive WHMV, n (%) | – | 147 (12.9%) |
| Covert brain infarcts, n (%) | – | 133 (11.7%) |
Mean ±SE presented, unless specified otherwise. CVD, cardiovascular disease; WMHV, white matter hyperintensitiy volume.
Median (25th percentile, 75th percentile) reported.
Vitamin D and the incidence of dementia
Over a surveillance period of 9-years, 267 participants (16.1%) developed dementia of any type, out of which 208 (12.5%) fulfilled criteria for AD. 25(OH)D levels were not associated with dementia or clinically characterized AD in any of our adjusted models (Table 2).
Table 2.
Cox adjusted regressions depicting the association between 25(OH)D levels and the risk of all-cause dementia and clinically characterized Alzheimer’s disease
| Model | All-cause dementia |
Alzheimer Disease |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| 25(OH)D as standardized continuous variable | ||||
| 1 | 0.96 (0.85–1.09) | 0.512 | 1.03 (0.90–1.19) | 0.660 |
| 2 | 1.02 (0.89–1.16) | 0.777 | 1.10 (0.95–1.27) | 0.211 |
| 3 | 1.01 (0.88–1.16) | 0.926 | 1.10 (0.94–1.29) | 0.223 |
| 25(OH) D <10 ng/mL | ||||
| 1 | 1.32 (0.76–2.28) | 0.328 | 1.17 (0.61–2.23) | 0.642 |
| 2 | 1.06 (0.57–1.98) | 0.847 | 0.97 (0.47–2.00) | 0.930 |
| 3 | 1.01 (0.51–2.00) | 0.983 | 0.84 (0.37–1.94) | 0.689 |
| 25(OH)D < the cohort-specific 20th percentile | ||||
| 1 | 0.96 (0.71–1.29) | 0.763 | 0.79 (0.56–1.13) | 0.203 |
| 2 | 0.86 (0.63–1.17) | 0.332 | 0.70 (0.49–1.02) | 0.064 |
| 3 | 0.87 (0.63–1.21) | 0.412 | 0.71 (0.48–1.04) | 0.080 |
| 25(OH)D > the cohort-specific 80th percentile | ||||
| 1 | 0.89 (0.64–1.23) | 0.475 | 1.10 (0.77–1.55) | 0.608 |
| 2 | 0.98 (0.70–1.37) | 0.910 | 1.19 (0.83–1.71) | 0.353 |
| 3 | 0.93 (0.66–1.33) | 0.696 | 1.14 (0.78–1.66) | 0.503 |
Hazards ratios for incident all-cause dementia and Alzheimer’s disease are shown per cohort-specific 1-SD increment in 25(OH)D levels, for the presence of a 25(OH)D concentration <10 ng/mL, for<cohort-specific 20th percentile, and for> the cohort-specific 80th percentile. Model 1 includes adjustment for age and gender. Model 2 includes additional adjustment for vascular risk factors (smoking, hypertension, diabetes, prevalent cardiovascular disease, and homocysteine). Model 3 includes additional adjustment for BMI and vitamin D supplement use.
Vitamin D and neuropsychological function
A statistically significant association was found between low serum vitamin D levels (examined as continuous, dichotomized as vitamin D deficient, or at the cohort-specific 20th percentile level) and poorer Trail Making B-A scores (Table 4). Low levels of vitamin D were also associated with poorer performance on the Hooper Visual Reproductions test. The strength of such associations was generally attenuated by the additional adjustment for BMI and vitamin D supplement use. Vitamin D levels were not associated with performance on any of the other neuropsychological tests.
Table 4.
Multivariable adjusted models depicting the association between 25(OH)D levels and neuropsychological function
| Model | Logical Memory delayed |
Visual Reproductions delayed |
Trail Making (B–A) |
Similarities |
Hooper Visual Reproductions |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| β±SE | p | β±SE | p | β ±SE | p | β ±SE | p | β ±SE | p | |
| 25(OH)D as standardized continuous variable | ||||||||||
| 1 | −0.06 ±0.09 | 0.533 | 0.12± 0.09 | 0.176 | 0.02± 0.01 | 0.003 | 0.001± 0.09 | 0.994 | 0.04 ±0.01 | 0.006 |
| 2 | −0.05 ±0.09 | 0.613 | 0.10± 0.09 | 0.240 | 0.02± 0.01 | 0.005 | −0.05± 0.09 | 0.575 | 0.04 ±0.01 | 0.010 |
| 3 | −0.002 ±0.10 | 0.984 | 0.12± 0.09 | 0.182 | 0.01± 0.01 | 0.026 | −0.08± 0.10 | 0.415 | 0.04 ±0.01 | 0.014 |
| 25(OH) D<10 ng/mL | ||||||||||
| 1 | 0.50± 0.34 | 0.135 | −0.02 ±0.31 | 0.951 | −0.05 ± 0.02 | 0.012 | −0.16± 0.31 | 0.612 | −0.12± 0.05 | 0.018 |
| 2 | 0.54± 0.34 | 0.111 | 0.08 ±0.31 | 0.807 | −0.05 ± 0.02 | 0.023 | −0.03± 0.32 | 0.913 | −0.10± 0.05 | 0.036 |
| 3 | 0.58± 0.36 | 0.105 | 0.05 ±0.33 | 0.892 | −0.03 ± 0.02 | 0.129 | 0.07± 0.34 | 0.846 | −0.09± 0.05 | 0.095 |
| 25(OH)D <the cohort-specific 20th percentile | ||||||||||
| 1 | 0.14± 0.23 | 0.539 | 0.05± 0.21 | 0.822 | −0.04± 0.01 | 0.003 | −0.29± 0.22 | 0.175 | −0.08± 0.03 | 0.017 |
| 2 | 0.16± 0.23 | 0.507 | 0.08± 0.22 | 0.702 | −0.04 ± 0.01 | 0.007 | −0.20± 0.22 | 0.359 | −0.07± 0.03 | 0.036 |
| 3 | 0.15± 0.25 | 0.549 | 0.11± 0.23 | 0.649 | −0.03 ± 0.01 | 0.043 | −0.17± 0.24 | 0.467 | −0.06± 0.04 | 0.081 |
| 25(OH)D >the cohort-specific 80th percentile | ||||||||||
| 1 | −0.16 ±0.23 | 0.482 | 0.32± 0.21 | 0.139 | 0.02± 0.01 | 0.211 | 0.06± 0.22 | 0.775 | −0.06± 0.03 | 0.098 |
| 2 | −0.17 ±0.24 | 0.480 | 0.32± 0.22 | 0.142 | 0.01± 0.01 | 0.323 | −0.02± 0.22 | 0.916 | 0.05± 0.03 | 0.140 |
| 3 | −0.03 ±0.24 | 0.888 | 0.40± 0.23 | 0.079 | 0.01± 0.01 | 0.373 | −0.07± 0.23 | 0.759 | 0.06± 0.04 | 0.115 |
Bold highlight denotes p< 0.05; Regression coefficient represents the change in each standardized cognitive variable per cohort-specific 1-SD increment in 25(OH)D levels; or by the presence of a 25(OH)D concentration <10 ng/mL; <cohort-specific 20th percentile; or> the cohort-specific 80th percentile. Model 1 adjusts for age, gender, education and time from vitamin D measurement to neuropsychological testing. Model 2 adjusts for the addition of vascular risk factors (smoking, hypertension, diabetes, history of cardiovascular disease, and homocysteine). Model 3 adjusts for the addition of BMI and vitamin D supplement use.
Vitamin D and markers of subclinical brain aging on MRI
Participants who were vitamin D deficient were found to have smaller HPVs after adjustment for age, gender, and vascular risk factors (Table 3). Vitamin D was not associated with HPV when modeled as a continuous variable or according to cohort specific percentiles. There were no statistically significant associations between vitamin D and the other MRI variables.
Table 3.
Multivariable adjusted models depicting the association between 25(OH)D levels and the MRI variables
| Model | TBV |
HPV |
WMHV |
WMH (extensive) |
SCI |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| β ±SE | p | β±SE | p | β ±SE | p | OR (95% CI) | p | OR (95% CI) | p | |
| 25(OH)D as standardized continuous variable | ||||||||||
| 1 | 0.14 ± 0.08 | 0.075 | 0.003 ±0.002 | 0.103 | −0.03± 0.03 | 0.212 | 1.00 (0.84–1.18) | 0.973 | 0.85 (0.71–1.04) | 0.108 |
| 2 | 0.09 ± 0.08 | 0.268 | 0.003 ±0.002 | 0.114 | −0.03± 0.03 | 0.212 | 1.00 (0.84–1.20) | 0.965 | 0.90 (0.74–1.09) | 0.291 |
| 3 | −0.002± 0.09 | 0.984 | 0.002 ±0.002 | 0.299 | −0.03± 0.03 | 0.295 | 1.02 (0.85–1.23) | 0.815 | 0.91 (0.74–1.12) | 0.374 |
| 25(OH) D <10 ng/mL | ||||||||||
| 1 | −0.34 ±0.29 | 0.249 | −0.01 ±0.01 | 0.033 | −0.004± 0.09 | 0.964 | 1.00 (0.53–1.89) | 0.995 | 1.34 (0.73–2.48) | 0.349 |
| 2 | −0.16 ±0.29 | 0.591 | −0.01 ±0.01 | 0.034 | −0.03± 0.10 | 0.778 | 0.96 (0.50–1.84) | 0.896 | 1.12 (0.60–2.11) | 0.721 |
| 3 | 0.04 ± 0.31 | 0.889 | −0.01 ±0.01 | 0.058 | −0.05± 0.10 | 0.621 | 0.90 (0.45–1.81) | 0.773 | 1.18 (0.61–2.27) | 0.629 |
| 25(OH)D < the cohort-specific 20th percentile | ||||||||||
| 1 | −0.36 ±0.20 | 0.073 | −0.01 ±0.004 | 0.111 | 0.01 ±0.06 | 0.869 | 0.88 (0.56–1.39) | 0.585 | 1.03 (0.65–1.63) | 0.908 |
| 2 | −0.23 ±0.20 | 0.248 | −0.01 ±0.004 | 0.112 | 0.01 ±0.07 | 0.829 | 0.89 (0.56–1.41) | 0.619 | 0.92 (0.57–1.46) | 0.717 |
| 3 | −0.09 ±0.22 | 0.687 | −0.005 ±0.005 | 0.296 | −0.01± 0.07 | 0.890 | 0.88 (0.53–1.43) | 0.595 | 0.89 (0.54–1.47) | 0.652 |
| 25(OH)D > the cohort-specific 80th percentile | ||||||||||
| 1 | 0.28 ± 0.20 | 0.163 | 0.002 ±0.004 | 0.633 | −0.07± 0.06 | 0.277 | 0.91 (0.59–1.41) | 0.676 | 0.87 (0.53–1.41) | 0.557 |
| 2 | 0.18 ± 0.20 | 0.362 | 0.002 ±0.004 | 0.713 | −0.08± 0.07 | 0.207 | 0.88 (0.56–1.38) | 0.577 | 0.92 (0.56–1.50) | 0.736 |
| 3 | 0.05 ± 0.21 | 0.822 | −0.000 ±0.004 | 0.921 | −0.08± 0.07 | 0.226 | 0.94 (0.59–1.50) | 0.798 | 0.97 (0.59–1.61) | 0.904 |
Bold highlight denotes p< 0.05; HPV, hippocampal volume; SBI, silent cerebral infarcts; TBV, total brain volume; WMHV, white matter hyperintensity volume. Regression coefficients represent the change in mean of each standardized MRI variable per cohort-specific 1-SD increment in 25(OH)D levels; or by the presence of a 25(OH)D concentration <10 ng/mL; < cohort-specific 20th percentile; or > the cohort-specific 80th percentile. Model 1 adjusts for age, gender, and time from vitamin D measurement to MRI. Model 2 adjusts for the addition of vascular risk factors (smoking, hypertension, diabetes, prevalent cardiovascular disease, and homocysteine). Model 3 adjusts for the addition of BMI and vitamin D supplement use.
Sensitivity analysis
Additional adjustment for geographic location and season did not alter the results (data not shown). Vitamin D was not associated with incident dementia or clinically apparent AD when defining thresholds of deficiency and insufficiency as per the Institute of Medicine guidelines (Supplementary Table 1).
DISCUSSION
This large community-based prospective cohort study examined the associations of vitamin D levels with incident dementia, MRI markers of brain aging and neuropsychological function. The primary finding was that low serum 25(OH)D concentrations were associated with domain-specific cognitive performance including executive function, processing speed and visuo-perceptual skills. 25(OH)D deficiency was also associated with lower HPV. There were no associations observed between vitamin D levels and incident dementia or clinically characterized AD.
To our knowledge, the Cardiovascular Health Study is the only other large prospective cohort study to report on the association between serum vitamin D concentrations and the risk of all-cause dementia and AD [26]. Unlike the present study, the Cardiovascular Health Study found that vitamin D deficiency was predictive of incident dementia, including AD. Associations with dementia were evident when comparing those with adequate 25(OH)D levels (≥20 ng/ml) to those who were deficient (<10 ng/ml; the same threshold used in the current study) and to those who were deficient according to a more lenient threshold (≥10 to <20 ng/ml). Similarly, a study of 10,186 Danish individuals reported that reduced vitamin D levels were associated with dementia as determined by review of medical records [27]. A study of 498 women found that higher dietary intake of vitamin D was associated with a lower 7-year risk of AD [29]. However, in line with our study, a reanalysis of 40 women with available serum showed that, unlike vitamin D dietary intakes, serum 25(OH)D concentrations were not associated with the onset of AD after 7 years [28].
Although we did not find associations between vitamin D and incident dementia, we did find that low vitamin D concentrations were associated with poorer executive function, processing speed and visuo-perceptual skills, but not verbal or visual memory. Associations between vitamin D and similar aspects of cognitive function have been observed previously in some cross-sectional [30, 31] and prospective studies [32]. Consistent with other studies, we did not find an association between vitamin D and episodic memory [30, 33–35]. The neuropsychological findings of the present study are also consistent with a recent meta-analysis, which found low vitamin D concentrations to be associated with poorer executive function and processing speed, while associations with episodic memory were reported to be uncertain [7].
Our study found that vitamin D deficiency was associated with smaller HPVs. This association was not found to be linear, suggesting a specific threshold effect. Although hippocampal atrophy is a hallmark of early AD [36, 37], hippocampal atrophy is also associated with vascular pathology [38]. As vitamin D deficiency has been associated with the risk of cardiovascular disease in the Framingham Heart Study Offspring cohort [11], the observed association between vitamin D deficiency and HPV may be mediated through vascular pathways. The associations identified between vitamin D deficiency and executive function, processing speed and visuo-perceptual skills are also consistent with an underlying vascular mechanism. In support of this notion, other studies have identified associations between vitamin D deficiency and the development of atherosclerosis [39, 40], perhaps due to the effects of vitamin D on endothelial dysfunction, vascular smooth muscle cell proliferation and migration, as well as calcification [39].
There are numerous other neurobiological mechanisms that may explain the links between vitamin D, neuropsychological function, and HPV identified in the current study. Vitamin D receptors, and the enzyme essential for the production of vitamin D in its active form, are ubiquitous in the nervous system in regions of the brain associated with cognition, behavior and neurological disease, including the hippocampus [41–43]. Vitamin D receptors are also expressed in immune cells, with vitamin D able to modify innate and adaptive immune responses, lessen inflammatory cytokine production and boost anti-inflammatory defense [1,44,45]. These mechanisms are of interest given that neuroinflammation and immune system actions may contribute to AD [46]. Vitamin D also has a role in buffering antioxidant defenses [1, 44], stimulating neurotrophin release [47], and downregulating calcium channel expression [48, 49]. Vitamin D may attenuate amyloid-β (A β) accumulation by stimulating the phagocytosis of the Aβ peptide [50] and by enhancing brain-to-blood Aβ efflux transport at the blood-brain barrier [51], with a decreased number of amyloid plaques as a result [52]. Conversely, vitamin D receptor knock-out mice manifest behavioral abnormalities [41], and reduced concentrations of the vitamin D hormone receptor mRNA have been directly linked with AD [53].
Vitamin D deficiency is prevalent in the US and other Western countries, particularly amongst non-Caucasians [54, 55]. Our results suggest that maintaining adequate levels of vitamin D may potentially prevent the accumulation of vascular brain injury, protecting against hippocampal atrophy and executive dysfunction. As vitamin D deficiency can be corrected through supplementation, diet (i.e. oily fish), and increased sun exposure, there is a need to ascertain whether correcting vitamin D deficiency can help mitigate the risk of vascular brain injury and cognitive decline. Should a causal link exist between low vitamin D status and accelerated brain aging, routine screening and treatment for vitamin D deficiency, particularly in high risk populations, may help lower the burden on cognitive impairment.
This study has several strengths including that the sample was relatively large and community-based. Furthermore, the prospective follow-up for dementia allowed us to assess the temporal relationship between vitamin D concentrations and the risk of developing dementia over a 9-year follow-up period. Our study also has limitations. Relatively few participants in our study were found to be deficient in vitamin D. We thus modeled vitamin D deficiency, not only according to a cut-off associated with deficiency, but also according to cohort specific percentiles. As we did not evaluate the concentrations of parathyroid hormone, we cannot determine whether the associations between vitamin D concentrations and neuropsychological function are due to the low vitamin D or to secondary hyperparathyroidism [4], although repletion with vitamin D would be indicated in both cases. The fact that our sample was largely white Caucasian limits the generalizability of our findings to other ethnic groups. Finally, our data is observational and despite several adjustments, one cannot exclude possible residual confounding.
In conclusion, identifying nutritional and lifestyle-based factors that can prevent or ameliorate cognitive decline may have substantial benefits for the individual and noteworthy financial and public health ramifications for society. The current study adds support to a growing body of literature implicating vitamin D deficiency in the development of neuropsychological impairment and subclinical brain injury. Correcting vitamin D deficiency through diet, lifestyle change or supplementation may potentially help prevent brain injury and cognitive decline. However, we did not find an association between levels of vitamin D and the risk of dementia or clinically characterized AD in the Framingham Heart Study. The contribution of vitamin D to dementia thus remains uncertain and requires future investigation. Elucidating why vitamin D appears to be linked to dementia in some cohorts but not others will provide important information for future clinical trials aimed at developing dietary based interventions to protect against cognitive decline and dementia.
Supplementary Material
Acknowledgments
This work was supported by the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195) and by grants from the National Institute of Neurological Disorders and Stroke (R01 NS17950) and from the National Institute on Aging (R01 AG016495, AG008122, AG033193, AG031287). Dr Pase is funded by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1089698).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/manuscript-disclosures/15-0991r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150991.
REFERENCES
- 1.Cherniack EP, Florez H, Roos BA, Troen BR, Levis S. Hypovitaminosis D in the elderly: From bone to brain. J Nutr Health Aging. 2008;12:366–373. doi: 10.1007/BF02982668. [DOI] [PubMed] [Google Scholar]
- 2.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 3.Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Saaksjarvi K, Heliovaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67:808–811. doi: 10.1001/archneurol.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buell JS, Dawson-Hughes B, Scott TM, Weiner DE, Dallal GE, Qui WQ, Bergethon P, Rosenberg IH, Folstein MF, Patz S, Bhadelia RA, Tucker KL. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74:18–26. doi: 10.1212/WNL.0b013e3181beecb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, Llewellyn DJ, Raina P. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology. 2012;79:1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin D concentrations in Alzheimer’s disease: A systematic review and meta-analysis. J Alzheimers Dis. 2013;33:659–674. doi: 10.3233/JAD-2012-121432. [DOI] [PubMed] [Google Scholar]
- 7.Annweiler C, Montero-Odasso M, Llewellyn DJ, Richard-Devantoy S, Duque G, Beauchet O. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. J Alzheimers Dis. 2013;37:147–171. doi: 10.3233/JAD-130452. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 9.Dawber TR, Meadors GF, Moore FE. Epidemiologi-cal approaches to heart disease: The framingham study. Am J Public Health Nations Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rienstra M, Cheng S, Larson MG, McCabe EL, Booth SL, Jacques PF, Lubitz SA, Yin X, Levy D, Magnani JW, Elli-nor PT, Benjamin EJ, Wang TJ. Vitamin D status is not related to development of atrial fibrillation in the community. Am Heart J. 2011;162:538–541. doi: 10.1016/j.ahj.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relationofobesityto cognitive function: Importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 15.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 16.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. USA: Oxford Press; 2006. [Google Scholar]
- 17.Au R, Seshadri S, Wolf PA, Elias M, Elias P, Sullivan L, Beiser A, D’Agostino RB. New norms for a new generation: Cognitive performance in the framingham offspring cohort. Exp Aging Res. 2004;30:333–358. doi: 10.1080/03610730490484380. [DOI] [PubMed] [Google Scholar]
- 18.Seshadri S, Wolf PA, Beiser AS, Selhub J, Au R, Jacques PF, Yoshita M, Rosenberg IH, D’Agostino RB, DeCarli C. Association of plasma total homocysteine levels with subclinical brain injury: Cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol. 2008;65:642–649. doi: 10.1001/archneur.65.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: The Framingham study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 20.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Green-berg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 22.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16:274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semi quantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 25.Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Food and Nutrition Board; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington DC: National Academic Press; 2010. [Google Scholar]
- 26.Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PHM, Fried L, Kestenbaum BR, Kuller LH, Langa KM, Lopez OL, Kos K, Soni M, Llewellyn DJ. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83:920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement. 2014;10:296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- 28.Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Beauchet O. Serum vitamin D deficiency as a predictor of incident non-Alzheimer dementias: A 7-year longitudinal study. Dement Geriatr Cogn Disord. 2011;32:273–278. doi: 10.1159/000334944. [DOI] [PubMed] [Google Scholar]
- 29.Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Herrmann FR, Beauchet O. Higher vitamin D dietary intake is associated with lower risk of alzheimer’s disease: A 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67:1205–1211. doi: 10.1093/gerona/gls107. [DOI] [PubMed] [Google Scholar]
- 30.Buell JS, Scott TM, Dawson-Hughes B, Dallal GE, Rosenberg IH, Folstein MF, Tucker KL. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci. 2009;64:888–895. doi: 10.1093/gerona/glp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menant JC, Close JC, Delbaere K, Sturnieks DL, Trollor J, Sachdev PS, Brodaty H, Lord SR. Relationships between serum vitamin D levels, neuromuscular and neuropsychological function and falls in older men and women. Osteoporos Int. 2012;23:981–989. doi: 10.1007/s00198-011-1637-7. [DOI] [PubMed] [Google Scholar]
- 32.Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, Ferrucci L, Melzer D. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. The Tromso study. J Neurol. 2006;253:464–470. doi: 10.1007/s00415-005-0027-5. [DOI] [PubMed] [Google Scholar]
- 34.McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007;29:49–54. doi: 10.1159/000108918. [DOI] [PubMed] [Google Scholar]
- 35.Tolppanen AM, Williams DM, Lawlor DA. The association of serum ionized calcium and vitamin D with adult cognitive performance. Epidemiology. 2011;22:113–117. doi: 10.1097/EDE.0b013e3181f74683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6:347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laakso MP, Partanen K, Riekkinen P, Jr, Lehtovirta M, Hel-kala EL, Hallikainen M, Hänninen T, Vainio P, Soininen H. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: An MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 39.Kassi E, Adamopoulos C, Basdra EK, Papavassiliou AG. Role of vitamin D in atherosclerosis. Circulation. 2013;128:2517–2531. doi: 10.1161/CIRCULATIONAHA.113.002654. [DOI] [PubMed] [Google Scholar]
- 40.Juonala M, Voipio A, Pahkala K, Viikari JS, Mikkila V, Kahonen M, Hutri-Kahonen N, Jula A, Burgner D, Sabin MA, Marniemi J, Loo BM, Laitinen T, Jokinen E, Tait-tonen L, Magnussen CG, Raitakari OT. Childhood 25-OH vitamin D levels and carotid intima-media thickness in adulthood: The cardiovascular risk in young Finns study. J Clin Endocrinol Metab. 2015;100:1469–1476. doi: 10.1210/jc.2014-3944. [DOI] [PubMed] [Google Scholar]
- 41.Kalueff AV, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr Opin Clin Nutr Metab Care. 2007;10:12–19. doi: 10.1097/MCO.0b013e328010ca18. [DOI] [PubMed] [Google Scholar]
- 42.McCann JC, Ames BN. Isthere convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 43.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: Preventing “D”ecline? Mol Aspects Med. 2008;29:415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heppner FL, Ransohoff RM, Becher B. Immune attack: The role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 47.Kiraly SJ, Kiraly MA, Hawe RD, Makhani N. Vitamin D as a neuroactive substance: Review. Scientific-WorldJournal. 2006;6:125–139. doi: 10.1100/tsw.2006.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuro-protection in parallel with down regulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21:98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer’s disease: Vitamin D receptor suppression by amyloid-beta and preventing the amyloid-beta induced alterations by vitamin D in cortical neurons. J Alzheimers Dis. 2011;23:207–219. doi: 10.3233/JAD-2010-101377. [DOI] [PubMed] [Google Scholar]
- 50.Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, Abel K, Zheng X, Espinosa-Jeffrey A, Mahanian M, Liu PT, Hewison M, Mizwickie M, Cashman J, Fiala M. 1 alpha, 25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2009;17:703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 51.Ito S, Ohtsuki S, Nezu Y, Koitabashi Y, Murata S, Terasaki T. 1alpha,25-Dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-beta peptide(1–40) from mouse brain across the blood-brain barrier. Fluids Barriers CNS. 2011;8:20. doi: 10.1186/2045-8118-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu J, Gattoni-Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni-Celli S, Kindy MS. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AbetaPP transgenic mice. J Alzheimers Dis. 2011;25:295–307. doi: 10.3233/JAD-2011-101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutherland MK, Somerville MJ, Yoong LK, Bergeron C, Haussler MR, McLachlan DR. Reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: Correlation with calbindin-28k mRNA levels. Brain Res Mol Brain Res. 1992;13:239–250. doi: 10.1016/0169-328x(92)90032-7. [DOI] [PubMed] [Google Scholar]
- 54.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Daly RM, Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Sikaris KA, Zimmet PZ, Ebeling PR, Shaw JE. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: A national, population-based study. Clin Endocrinol (Oxf) 2012;77:26–35. doi: 10.1111/j.1365-2265.2011.04320.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.