Summary
Nicotinamide Adenine Dinucleotide (NAD) levels decrease during aging, and are involved in age-related metabolic decline. To date, the mechanism responsible for the age-related reduction in NAD has not been elucidated. Here we demonstrate that expression and activity of the NADase CD38 increase with aging and that CD38 is required for the age-related NAD decline and mitochondrial dysfunction via a pathway mediated at least in part by regulation of SIRT3 activity. We also identified CD38 as the main enzyme involved in the degradation of the NAD precursor nicotinamide mononucleotide (NMN) in vivo, indicating that CD38 has a key role in the modulation of NAD-replacement therapy for aging and metabolic diseases.
Keywords: CD38, NAD+, mitochondrial function, glucose intolerance, aging
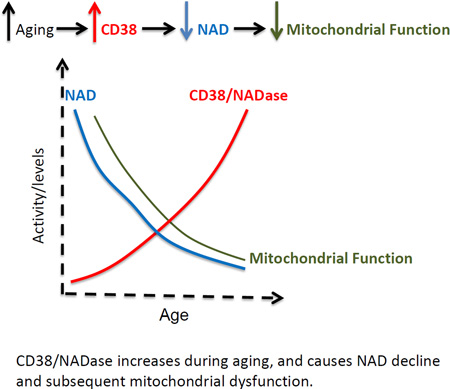
Graphical Abstract
CD38/NADase increases during aging, and causes NAD decline and subsequent mitochondrial dysfunction.
ETOC blurb
Why do NAD levels decrease with age? Camacho et al. now reveal that increased expression of the NADase CD38 is responsible for NAD decline and mitochondrial dysfunction in older mice in a SIRT3-dependent manner. CD38 also metabolizes the NAD precursor NMN and modulates the response to NAD-replacement therapy in vivo.
INTRODUCTION
NAD (nicotinamide adenine dinucleotide) is a cofactor of key enzymes in glycolysis, the tricarboxylic acid cycle, and oxidative phosphorylation (Oxphos), participating in multiple redox reactions in cells. It has been shown that the cellular NAD pool is determined by a balance between the activity of NAD-synthesizing and consuming enzymes (Aksoy et al., 2006, Bai, et al., 2011, Barbosa et al., 2007, Nahimana et al., 2009, Yang et al., 2007, Yoshino et al., 2011). Recently it has been described that NAD levels decline during chronological aging and in progeroid states, leading to mitochondrial dysfunction and metabolic abnormalities (Zhu et al., 2015; Gomes et al., 2013; Braidy et al., 2011; Scheibye-Knudsen et al., 2014, Massudi et al., 2012). Thus, determining the mechanisms that lead to this age-related NAD decline is of great importance for the development of therapies for age-related diseases.
Age-related NAD decline may be caused by an increase in NAD-consuming enzymes (NADases), a decrease in NAD-synthesizing enzymes, or a combination of both. However, to date, the precise contribution of specific metabolic pathways that regulate NAD levels in the age-related NAD-decline has not been determined. In the case of NAD consumption, it has been shown that enzymes such as poly (ADP-ribose) polymerases (PARPs), NAD-dependent deacetylases (SIRTUINS), and NADases such as CD38 can degrade this molecule during their catalytic processes (Bai et al., 2011; Bai and Canto, 2012, Aksoy et al., 2006, Imai and Guarente, 2014). In particular, we have shown that the enzyme CD38 is one of the main NAD-degrading enzymes in mammalian tissues (Aksoy et al., 2006; Barbosa et al., 2007). CD38 was originally identified as a cell surface enzyme that plays a key role in several physiological processes such as immune response, inflammation, cancer, and metabolic disease (Barbosa et al., 2007; Frasca et al., 2006; Guedes et al., 2012; Malavasi et. al., 2008). We have previously shown that CD38 knockout (CD38KO) mice have higher NAD levels and are protected against obesity and metabolic syndrome (Barbosa et al., 2007). In addition, treatment of obese mice with CD38 inhibitors increases intracellular NAD levels and improves several aspects of glucose and lipid homeostasis (Escande et al., 2013). However, the role of CD38 in age-related NAD decline and mitochondrial dysfunction has never been investigated.
Here we show for the first time that CD38 plays an active role in the age-related NAD decline in mammals. NAD levels, mitochondrial respiratory rates, and metabolic functions are preserved during the aging process in CD38KO mice. We further identified that CD38 is the main enzyme metabolizing the NAD precursor NMN in vivo, and demonstrated that ablation of CD38 improves the response to NAD-replacement therapy during aging. We believe that these findings may lead to new strategies for the treatment of diseases related to an imbalance in NAD metabolism and energy homeostasis.
RESULTS
Protein levels and mRNA expression of the NADase CD38 increase with chronological aging
Previous studies have suggested that tissue NAD+ levels decline with aging (Zhu et al., 2015; Gomes et al., 2013; Braidy et al., 2011; Massudi et al., 2012). To confirm the data on NAD-decline in aging, we measured NAD+ and NADH levels in murine tissues by two different methods, the cycling assay and a UPLC-mass spectroscopy assay (see Experimental Procedures section and Supplemental Information)(Figure S1A–C). We also further optimized and validated the cycling assay, and determined that it is very specific for NAD+ and NADH and does not detect any of the other nucleotides or NAD derivatives tested including: NADP, NAAD, NAADP, cADPR, ATP, ADP and others (Figure S1A). The results obtained with both methods confirm that there is indeed a decrease in levels of both NAD+ and NADH in murine tissues during chronological aging (Figure S1B–C). Furthermore, both techniques correlated extremely well for both nucleotides (correlation coefficient of r=0.95 for NAD+ and 0.97 for NADH, Figure S1B–C). In subsequent experiments NAD levels were generally assayed using the cycling method. Because our data agree with previous studies indicating that tissue NAD levels decline with aging, we next investigated the mechanisms leading to this age-related NAD decline in tissues.
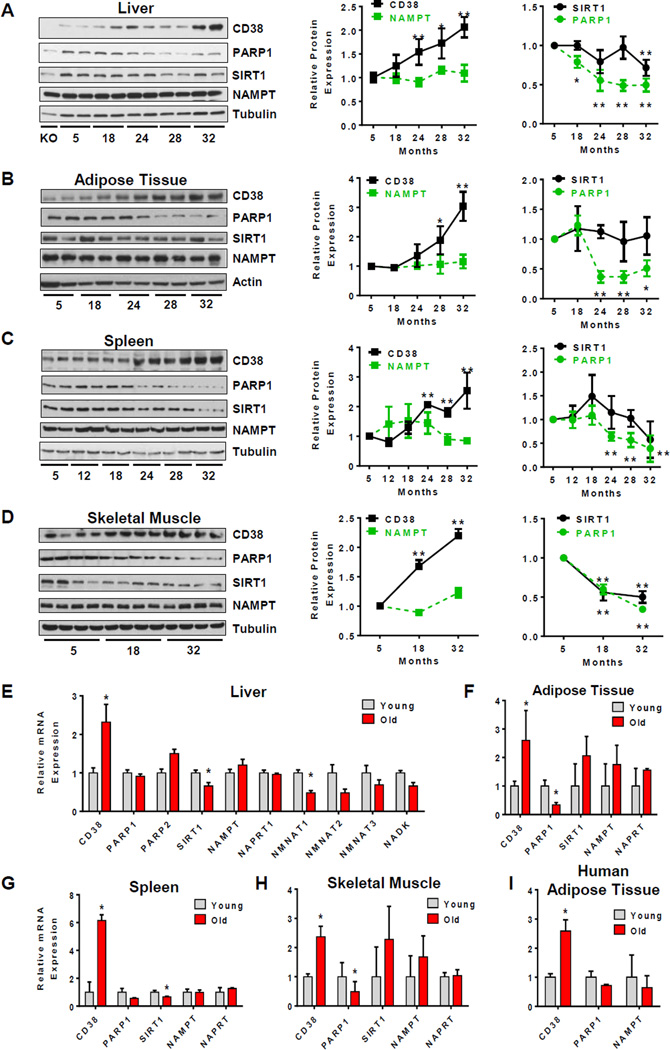
NAD decline could be caused by an increase in NAD-consuming enzymes and/or a decrease in its synthesizing enzymes. Some of the NAD-degrading enzymes in mammalian tissues include CD38, PARPs, and SIRTUINS. We have previously shown that CD38, in particular, is one of the main NAD-degrading enzymes in mammalian tissues (Aksoy et al., 2006; Barbosa et al., 2007). Furthermore, PARP1 has been proposed to be involved in NAD decline in tissues, and SIRT1 is an NAD-dependent deacetylase that plays a key role in age-related metabolic phenotypes (Bai et al., 2011; Bai and Canto, 2012; Imai and Guarente, 2014). Thus, we first investigated the expression of these enzymes in several mouse tissues during aging. As shown in Figure 1, the protein levels of both PARP1 and SIRT1 decreased in all of the tissues tested including liver, white adipose tissue, spleen, and skeletal muscle (Figure 1 A–D, S1D–F). These data indicate that the age-related NAD decline is not mediated by an increase in the expression of either PARP1 or SIRT1. In sharp contrast, levels of the NADase CD38 increased at least 2 to 3 times during chronological aging in all tissues tested, as detected by immunoblotting (Figure 1A–D and S1D–F). Furthermore, in the liver, we observed that the number and intensity of CD38 positive cells increased during aging as determined by flow cytometry (Figure S1G).
Figure 1. CD38 expression increases with aging.
(A) Immunoblots for CD38, PARP1, SIRT1, NAMPT and Tubulin in mice liver (left). On the right, graphs show relative protein expression of SIRT1, PARP1, CD38 and NAMPT. Relative expression of each protein was calculated as a ratio to Tubulin levels in each lane, and then calculated relative to 5 month old mice (n=12 for each age, *p<0.05, **p<0.01). In the experiments of Figure 1 A–H animals were obtain from the NIA aging colony.
(B–D) Immunoblots for CD38, PARP1, SIRT1, NAMPT and Tubulin in mice adipose tissue (B), spleen (C), and skeletal muscle (D) (left). On the right, graphs show relative protein expression of SIRT1, PARP1, CD38 and NAMPT in the tissues. Relative expression of each protein was calculated as a ratio to Tubulin in each lane, and then calculated relative to 5 month old mice (n=4–6 for each age, *p<0.05, **p<0.01).
(E) Relative mRNA levels of CD38, PARP1, PARP2, SIRT1, NAMPT, NAPRT1, NMNAT-1, NMNAT-2, NMNAT-3 and NADK in liver of young and old mice (3 and 32 months of age, respectively). (n=4 animals for each age, *p<0.05, versus 3 month old mice).
(F–H) Relative mRNA levels of CD38, PARP1, PARP1, SIRT1 NAMPT, and NAPRT1 in adipose tissue (F), spleen (G), and skeletal muscle (H) (3 and 32 months of age, respectively) (n = 4 animals for each age, *p <0.05, versus 3 month old mice).
(I) Relative mRNA levels of CD38, PARP1, and NAMPT in omental adipose tissue of human subjects with average ages of 34 (young) and 61 (old) (n = 9 for each age, *p <0.05, versus young group).
All values are mean ± SEM.
We further investigated if the age-related NAD decline could be mediated by a decrease in the protein levels of NAD-synthesizing enzymes such as NAMPT and NAPRT1. Neither NAMPT nor NAPRT1 protein levels changed significantly during aging in tissues (Figure 1A–D and S1H). All immunoblots were performed with samples obtained from 6 to14 mice for each age, and several of the original gels are presented in Supplemental Figure S1.
To further confirm and expand our findings, we measured the mRNA levels of these NAD-degrading and synthesizing enzymes in tissues of young (3 months old) and old mice (32 months old). Similar to protein expression, CD38 mRNA levels also increased in old mice when compared to young mice in all tissues tested (Figure 1E–H). The increase in CD38 mRNA in tissues of old mice was about 2.5 times in liver, adipose tissue, and skeletal muscle, and up to 6 times in the spleen (Figure 1E–H). In contrast, PARP-1 and SIRT1 mRNA expression did not change in a way that could explain the age-related NAD decline in any of the tissues tested (Figure 1E–H). In fact, in the case of PARP1, mRNA levels actually declined significantly in several tissues (Figure 1F–H). As for the NAD-synthesizing enzymes, the mRNA levels for neither NAMPT nor NAPRT decreased in any of the tissues during aging (Figure 1 E–H). We also analyzed the mRNA expression of additional NAD-degrading and synthesizing enzymes in the liver of young and old mice (Figure 1E). Expression of the enzymes NMNAT1–3 was lower in older mice, but a significant difference was observed only for NMNAT1 (Figure 1E).
To determine if the increase in CD38 expression observed in mice could also be present in humans, we measured the mRNA expression of some of the key enzymes involved in NAD metabolism in human adipose tissue from young (average age 34) and old (average age 61) subjects. Akin to mouse tissues, the expression of CD38 increased up to 2.5 times in fat tissue obtained from older subjects when compared to younger subjects (Figure 1I). In contrast, levels of neither PARP1 nor NAMPT changed significantly with aging in these samples (Figure 1I).
CD38 plays a key role in the age-related NAD decline
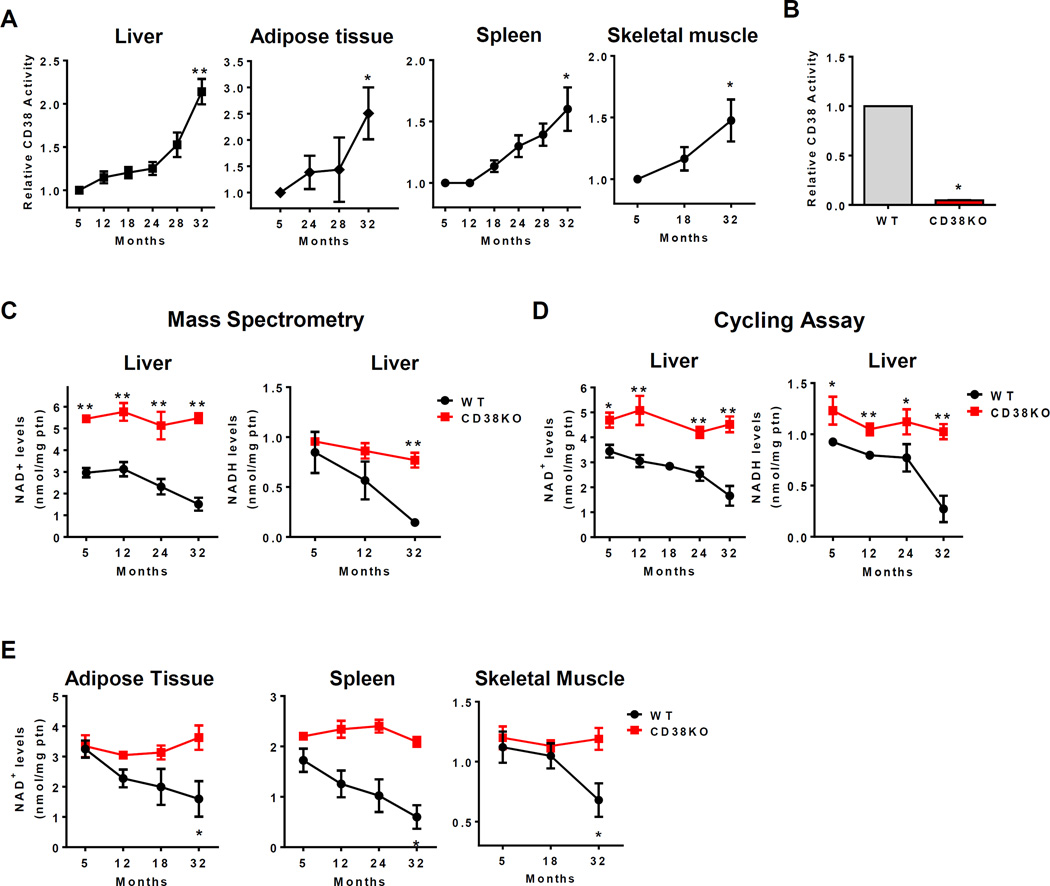
Our data together show that CD38 protein and mRNA expression increase during aging in multiple tissues, and indicate that this enzyme could play a major role in age-related NAD decline. To determine if these changes in expression were associated with an increase in NAD+-degrading activity, we first measured CD38 NADase activity in tissues from mice at different ages. Consistent with CD38 mRNA and protein expression results, CD38 enzymatic activity also increased during aging in liver, white adipose tissue, spleen and skeletal muscle (Figure 2A). We also expanded our observations and determined that the CD38 NADase activity also increased at least 50% in the ilium, jejunum, and kidney during the aging process (Figure S2A). Our control experiments with CD38KO mice confirm the specificity of the NADase assay for CD38 (Figure 2B). In fact, we did not detect significant NADase activity in any of the CD38KO tissues tested.
Figure 2. CD38 regulates the age-related NAD+ decline.
(A) CD38 activity in liver, adipose tissue, spleen and skeletal muscle of aging mice (n=6 animals for each age, **p<0.01 versus 5 month old mice).
(B) CD38 activity in liver of 1 year old WT and CD38 KO litter mate mice (*p<0.05).
(C) Total liver NAD+ and NADH levels in WT and CD38KO mice during aging measured by mass spectroscopy (n=4 mice for each age, *p<0.05, **p<0.01 versus 5 month old mice).
(D) Total liver NAD+ and NADH levels in WT and CD38KO mice during aging measured by cycling assay (n=14 animals for each age, *p<0.05, **p<0.01 versus 5 month old mice).
(E) NAD+ levels in adipose tissue, spleen, and skeletal muscle of WT and CD38KO mice during aging (n=4 animals for each age, *p<0.05, **p<0.01 versus 5 month old mice).
All values are mean ± SEM.
Interestingly, when CD38 activity or protein expression were plotted against NAD+ decline in aging, an excellent inverse correlation coefficient was observed (r=−0.95 and r=−0.99, respectively, Figure S2B). These results indicate that an increase in CD38 protein expression and activity during aging could be the cause of the age-related NAD+ decrease. The same was not observed for PARP1 (Figure S2C). Consistent with a decrease in PARP1 protein expression, levels of PARylated proteins declined in the liver of aged mice (Figure S2D). This decrease in PARylation correlated positively with a decrease in both PARP1 protein expression and tissue NAD+ levels (Figure S2E). These findings indicate that the decline in PARylation was potentially a consequence of a decrease in either NAD+ or PARP1 levels, and do not support a causal role for PARP1 in the age-related NAD+ decline.
To further demonstrate that CD38 is the main enzyme regulating NAD levels, we examined the role of all three NAD+-degrading enzymes CD38, PARP1, and SIRT1 in maintaining NAD+ levels in cells. First, we measured NAD+ levels in mouse embryonic fibroblasts (MEFs) derived from WT, CD38, PARP1, and SIRT1 KO mice. Only CD38KO MEFs showed a significant increase in cellular NAD+ levels when compared to WT cells (Figure S2F–H). PARP1 KO MEFs showed a trend for an increase in NAD+ but it did not reach statistical significance (Figure S2H). We further tested the role of CD38 and PARP1 in regulating NAD+ levels in vivo, using one year old WT, CD38, and PARP1KO mice (Figure S2I–J). In agreement with the data in cells, CD38KO had higher NAD+ levels than both WT and PARP1KO in all tissues tested. In contrast, although there was a trend for an increase in NAD+ in the liver and adipose tissue of PARP1 KO mice, no statistical significant difference was observed in between WT and these mice (Figure S2J). To directly test the hypothesis that CD38 was responsible for the age-related NAD decline, we measured NAD levels in tissues from WT and CD38KO mice at various ages. Consistent with our hypothesis, we observed that although NAD+ levels declined significantly during chronological aging in all WT tissues (Figure 2C–E). In sharp contrast, in CD38KO mice NAD levels remained constant at all ages (Figure 2C–E). In the liver, in particular, neither NAD+ nor NADH levels significantly decline in CD38KO mice (Figure 2C–D). These results were observed using both the cycling and the UPLC-mass spectroscopy assays (Figure 2 C–D), and demonstrate for the first time that CD38 has a major role in regulating NAD levels during the aging process.
CD38 regulates mitochondrial function in mammalian tissues during aging
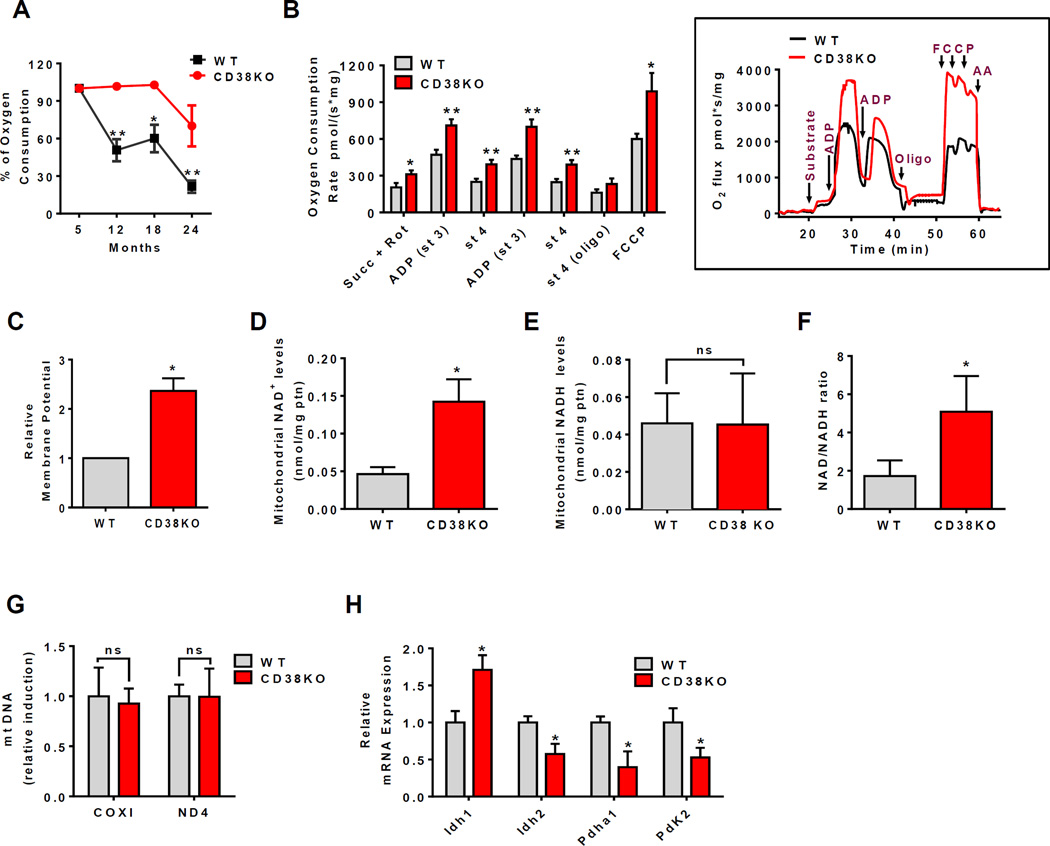
Mitochondrial function decline is a hallmark of aging (Gomes et al., 2013; Lanza and Nair, 2010), and cellular NAD+ decline has been implicated as a potential cause of this age-related mitochondrial dysfunction. Here we investigated the role of CD38 in the development of mitochondrial dysfunction during the aging process in liver tissue. First, we observed that the respiration-driving ATP synthesis decreases almost 70% in WT liver mitochondria (LM) during aging (Figure 3A), and this decrease correlated positively with the age-related decrease in NAD+ levels (r=0.952, Figure S3A). In agreement with the role of CD38 in the age-related NAD+ decrease, the decline in mitochondrial function was forestalled in CD38KO mice (Figure 3A).
Figure 3. CD38 deficiency upregulates mitochondrial function and increases NAD+/NADH ratio in mitochondria.
Liver of WT and CD38KO 12 month old litter mate mice were used for the measurements below in B-H. All values are mean ± SEM.
(A) Percentage of oxygen consumption coupled to ATP synthesis in isolated mitochondria during aging in WT and CD38KO mice (n=4 animals for each age, *p<0.05, **p<0.01 versus 5 month old mice).
(B) Oxygen consumption rates in isolated mitochondria. The following drugs were added for the experimental profile: Succinate 10mM and rotenone 1µM (Succ+Rot), 0.15mM ADP, 1µg/mL oligomycin (Oligo), and 1µM FCCP (inset) (n=4, *p<0.05, **p<0.01 versus WT mice).
(C) Relative membrane potential in mitochondria (*p<0.05 versus WT mice).
(D–F) Total NAD+ levels (D), NADH levels (E), and the NAD+/NADH ratio (F) in isolated mitochondria (n=3, *p<0.05 versus WT mice).
(G) Relative mtDNA quantification of COX I and ND4 as mitochondrial-encoded genes normalized by GAPDH.
(H) Relative mRNA expression of glucose metabolism pathway enzymes (n=6, *p<0.05 versus WT mice).
Oxygen consumption rates in LM were at least 2.5 times higher in all respiratory states in one year old CD38KO mice compared to WT mice, irrespective of the substrate used (Figure 3B, Table S1). Similar results were also observed in mitochondria isolated from spleen of one year old mice (Table S2) or in LM from two-year old mice submitted to normal or high fat diet (Figure S3B–E). These results, together with the higher mitochondrial membrane potential observed in one year old CD38KO mice (Figure 3C), confirm the increased mitochondrial activity in these animals. Higher levels of NAD+, and NAD+/NADH ratio were also detected in the mitochondria of one year old CD38KO mice (Figure 3D–F). Thus, we propose that chronic increases in cellular NAD levels in CD38KO mice also lead to increases in NAD in cellular compartments such as mitochondria.
To further explore the role of CD38 in mitochondrial function, we measured the expression of mitochondrial DNA and of the mRNA of mitochondrial enzymes. mt-DNA copy number was not significantly different between one year old WT and CD38KO (Figure 3G), suggesting that the number of mitochondria was not altered in liver of CD38KO mice. This observation was further supported by the relative expression of genes involved in mitochondrial biogenesis such as PPAR-γ, PGC1α, and NRF1 in liver from one year old WT and CD38KO mice (Figure S3F). Interestingly, we found that mRNA levels of Pdha1, and PDK2 that are involved in pyruvate entrance into mitochondria were decreased in CD38KO mice (Figure 3H). We also found a decrease in mRNA expression levels in glycolytic/pentose pathway enzymes and small solute carriers in liver mitochondria of one year old CD38KO mice compared to WT mice (Figure S3G). These changes may be compensatory to an increase in mitochondrial oxidative capacity and mitochondrial NAD levels. Our results in animals indicate that there is an increase in oxygen consumption in CD38KO mice which is related to an increase in mitochondrial NAD, and not mitochondrial biogenesis.
Changes in CD38 expression in cells regulate mitochondrial function
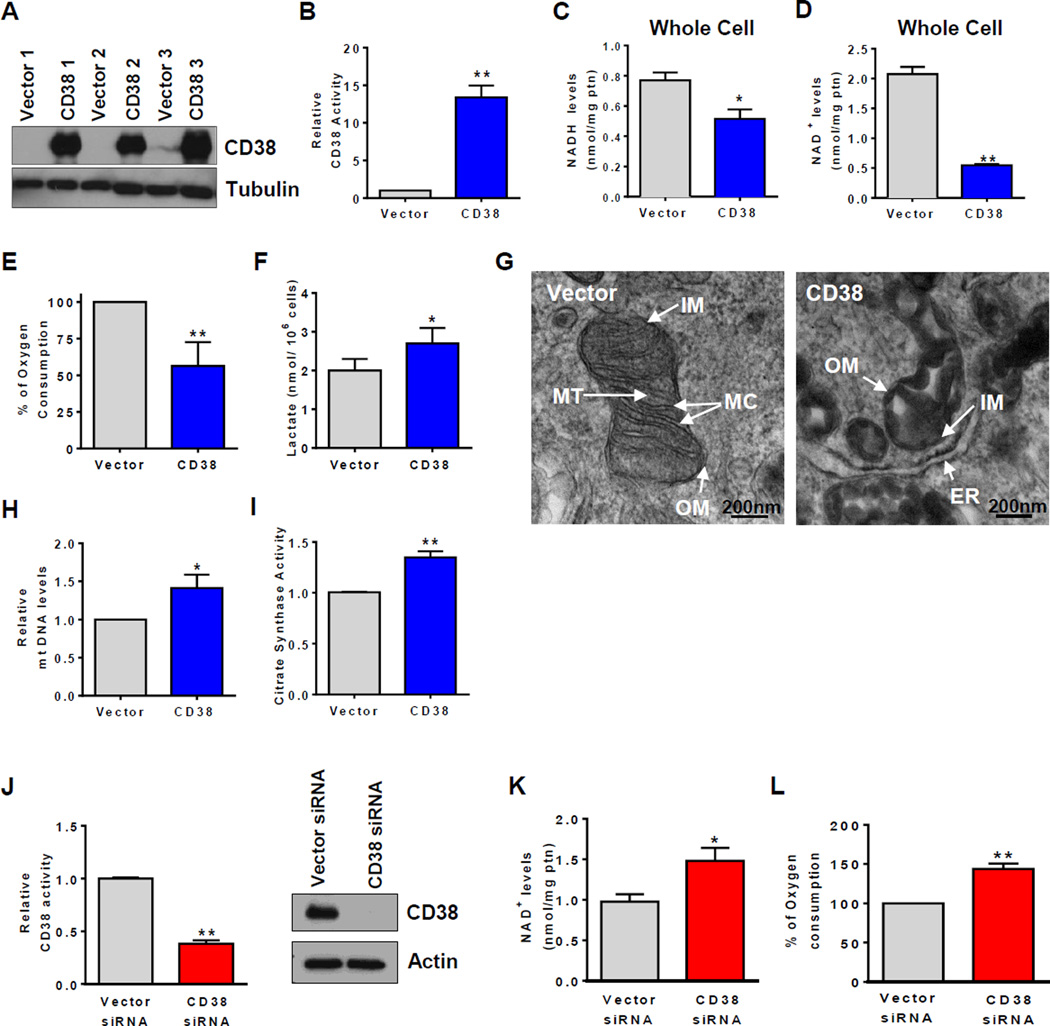
To confirm the role of CD38 in the regulation of mitochondrial function, oxygen consumption experiments were performed in intact cells after transfection with a CD38 plasmid. Expression of CD38 in 293T cells transfected with a control vector was nearly absent (Figure 4A). Cells that were transfected with CD38 plasmid displayed high CD38 expression and NADase activity, lower NAD+ and NADH levels in whole cells (Figure 4B–D). Next we measured the respiratory capacity in intact cells and observed that CD38 transfection induced a sever decrease in total respiratory capacity, and caused a higher lactate release when compared with vector-transfected cells (Figure 4E–F). Our results show that an increase in CD38 expression in cells leads to mitochondrial metabolic dysfunction, and likely to an increase in dependence on glycolysis. We further evaluated the status of the mitochondria in these cells. Electron microscopy shows a very significant abnormal mitochondrial morphology, such as lost and swollen cristae, and other morphological abnormalities in CD38 overexpressing cells (Figure 4G, S4). It has been shown that cellular stress can induce the opening of the mitochondrial transition pore causing swollen of the mitochondria, loss of membrane potential and leakage of mitochondrial matrix components such as NAD and NADH (Di Lisa et. al., 2001). Thus to test the “health” status and integrity of the mitochondria in CD38 over-expressing cells, we isolated mitochondria and measure the content of mitochondrial NAD+ and NADH. We observed a severe loss of NAD+ and NADH in isolated mitochondria from CD38 over-expressing cells (Figure S4I). It is possible that this could be due to degradation via CD38, and/or leakage of these nucleotides from dysfunctional mitochondria either in situ or during the mitochondrial isolation.
Figure 4. Changes in CD38 expression regulate NAD+ levels and mitochondrial function in cells.
293T cells expressing vector or Flag. CD38 were used in experiments from (A–L) and A549 cells were used in (M–O). All values are mean ± SEM. *p<0.05, **p<0.01, compared to control cells.
(A) Immunoblotting for CD38 and Tubulin.
(B) CD38 NADase activity (n=5).
(C) and (D) Total NAD+ and NADH levels in whole cells (n=5).
(E) Mitochondrial oxygen consumption in intact cells. Histogram represents the % oxygen consumption under FCCP-induced maximum respiration (n=6).
(F) Lactate released in media (n=5).
(G) Transmission electron microscopy. Scale bar: 200nm. OM: outer membrane, IM: internal membrane, MT: mitochondrial matrix, MC: mitochondrial cristae, ER: endoplasmic reticulum.
(H) Relative mtDNA quantification (n=4).
(I) Citrate synthase activity (n=9).
(J) CD38 NADase activity (n=5) and immunoblotting for CD38 and Tubulin.
(K) Total NAD+ levels (n=5).
(L) Routine oxygen consumption (n=3).
We further speculated that the severe mitochondrial dysfunction caused by over-expression of CD38 in cells would lead to a compensatory mitochondrial biogenesis. In fact, both the mt-DNA/genomic DNA ratio and citrate synthase activity were higher in cells expressing CD38 than vector-transfected cells (Figure 4H–I). These data suggest that in our over-expression experiments, the decline in cellular NAD levels may cause metabolic dysfunction mediated by intrinsic changes in the mitochondrial machinery, with a “reactive” compensatory mitochondrial biogenesis.
To determine the role of endogenous CD38 in cells, we also performed experiments knocking down CD38 in A549 cells. A549 cells express detectable levels of CD38, and transfection with a CD38 siRNA significantly decreased expression of CD38 and NADase activity, in comparison to control siRNA-transfected cells (Figure 4J). Furthermore, in CD38 siRNA-transfected cells, both NAD+ levels and total respiratory capacity were higher than in control siRNA-transfected cells (Figure 4K–L), confirming that changes in endogenous CD38 expression levels regulate NAD and mitochondrial function.
CD38 regulates metabolic state by a SIRT3-dependent mechanism
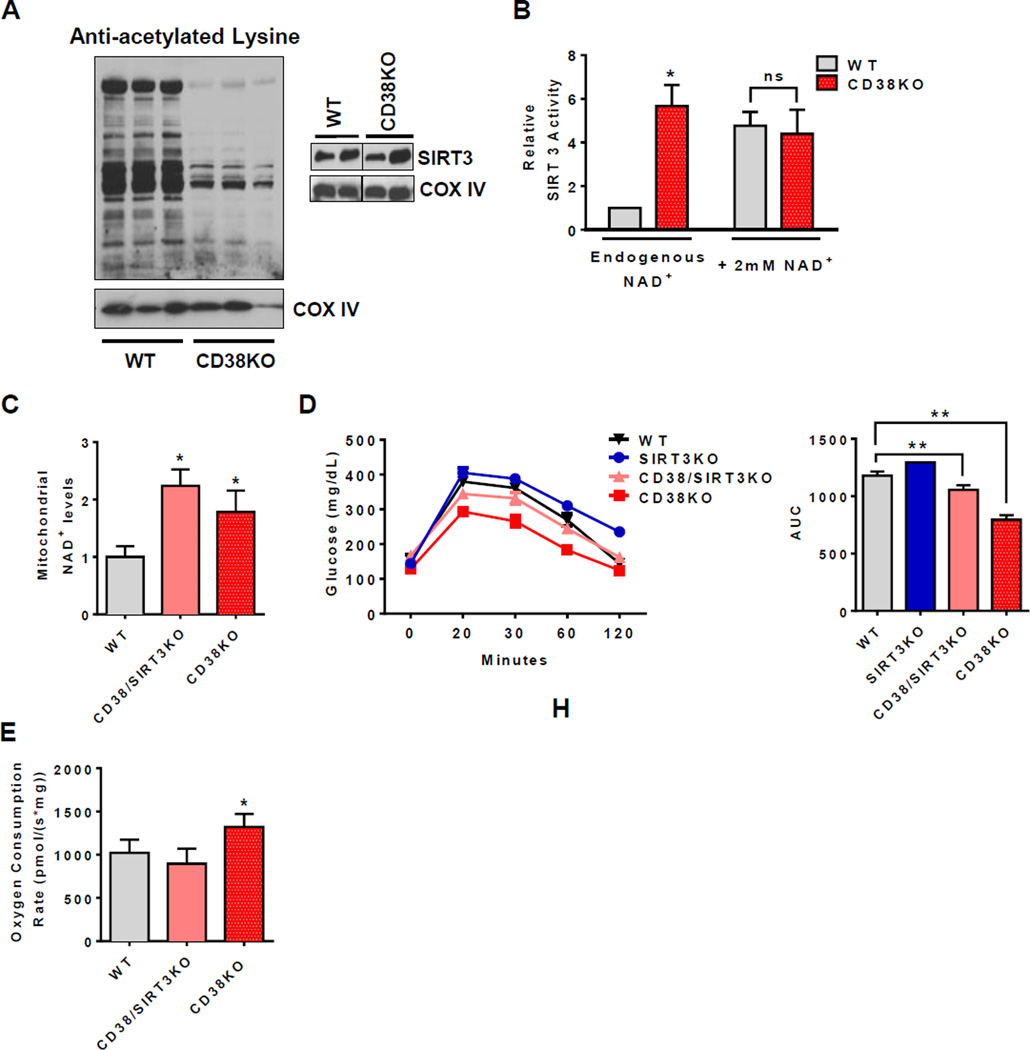
SIRT3 is one the sirtuins localized in mitochondria that regulate key mitochondrial proteins important for metabolism and oxidative homeostasis such as LCAD, IDH2, SDH and MnSOD (Finley et al., 2011; Hirschey et al., 2010; Shimazu et al., 2010; Tao et al., 2010; Yu et al., 2012). To determine if CD38 regulates the NAD+-dependent SIRT3 activity and mitochondrial protein lysine acetylation in vivo, we analyzed the profile of acetylated proteins in mitochondria from one year old WT and CD38KO livers. Although levels of mitochondrial SIRT3 were similar, levels of acetylated proteins were considerably higher in WT than CD38KO LM (Fig 5A, S5A). In addition, SIRT3 activity in the presence of endogenous NAD+ in the liver tissue was 3.5 times higher in CD38KO than WT mice (Figure 5B). In contrast, when saturating levels of exogenous NAD+ were added to the enzymatic assay, similar SIRT3 activities were observed between WT and CD38KO (Figure 5B). These data together indicate that, by changing cellular NAD+, CD38 controls SIRT3 activity without changes in SIRT3 levels.
Figure 5. CD38 controls the metabolic response in aged mice by activating SIRT3-dependent mechanisms.
All values are mean ± SEM. (A) Mitochondrial protein acetylation profile (upper panel) and immunoblotting for SIRT3 in one year old WT and CD38KO litter mate mice (lower panel). Each lane represents one independent mouse.
(B) SIRT3 activity in liver mitochondria isolated from one year old WT and CD38KO litter mate mice with endogenous contaminant NAD+ or addition of a saturating dose of 2mM NAD+ (n=4, *p<0.05, ns= non-significant p>0.05 versus WT control).
(C) NAD+ levels in isolated liver mitochondria from two year old litter mate WT, CD38KO, and CD38/SIRT3KO mice (n=6, *p<0.05 versus WT mice).
(D) Glucose concentration in (F) two year old litter mate WT, SIRT3KO, CD38KO, and CD38/SIRT3KO mice after intraperitoneal injection of glucose (left graphs). Area under the curve for glucose concentrations in different mice (right graphs) (n=8, **p<0.01, *p<0.05 versus WT mice).
(E) Oxygen consumption rates coupled to ATP synthesis in liver mitochondria isolated from two year old litter mate WT, CD38KO, and CD38/SIRT3KO mice (n=6, *p<0.05, versus WT mice).
To address the role of SIRT3 in the mitochondrial metabolic profile observed in CD38KO mice, we generated CD38/SIRT3 double KO mice on a C57BL6/129s background, and used the derived F3 generation of these crosses. As expected, livers of both two years old CD38KO and CD38/SIRT3KO mice have higher mitochondrial NAD+ levels compared to WT mice (Figure 5C). Interestingly, mitochondrial NAD+ was slightly higher in the double KO compared to the CD38KO (Figure 5C). This may indicate a lower utilization of mitochondrial NAD+ in the absence of both CD38 and SIRT3. We further performed a glucose tolerance test and determined the mitochondrial function in these two years old mice. CD38KO mice have an improved glucose tolerance profile compared to WT and SIRT3 KO, which is reversed in the CD38/SIRT3KO mice (Figure 5D).
At 2 years of age CD38 KO mice had an increased oxygen consumption coupled to ATP synthesis over WT that was reverted in the CD38/SIRT3KO mice (Figure 5E). We also determined that ablation of SIRT3 in CD38KO mice abrogated the protection against high fat diet-induced obesity and glucose intolerance that we have previously described in one year old mice (Figure S5B–C, Barbosa et al., 2007). These results suggest that metabolic and mitochondrial function is positively regulated in CD38KO mice by a pathway that requires not only increases in NAD+ levels, but may also require the enzyme SIRT3.
CD38 degrades NAD+ precursors: implications for NAD+ replacement therapy
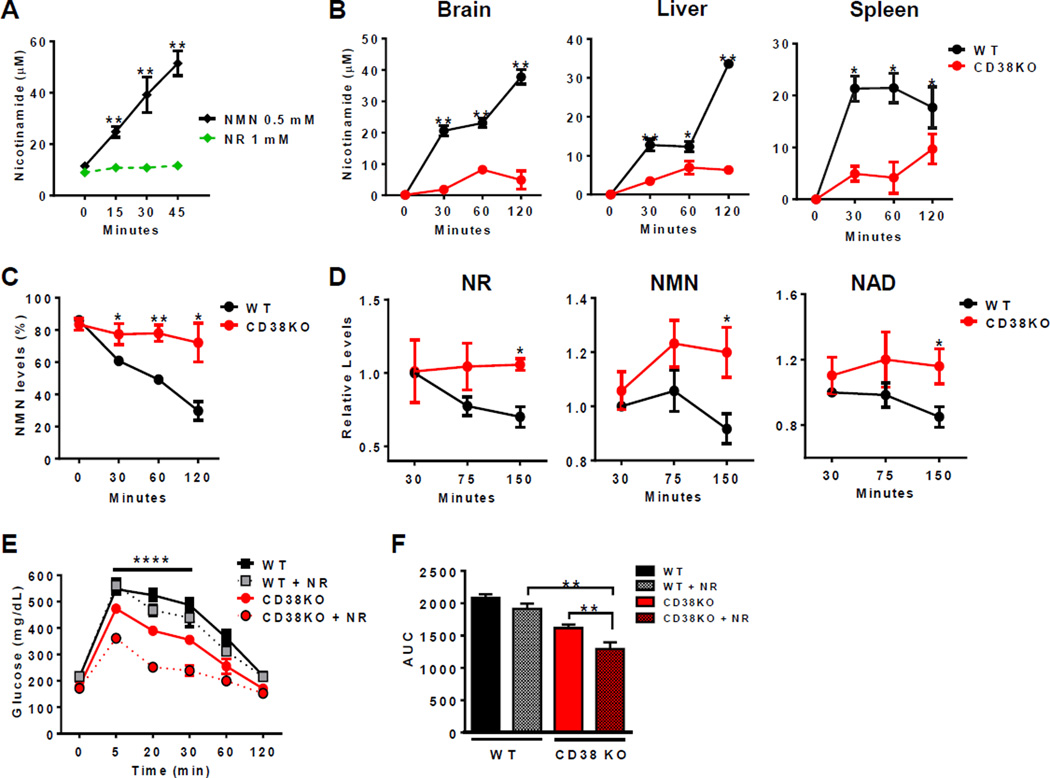
NAD+ precursors such as NMN and NR (nicotinamide riboside) have been proposed as NAD+-replacement therapy for age and diet-induced metabolic dysfunction (Cantó et al., 2012; Gomes et al., 2013; Imai and Guarente, 2014; Prolla and Denu, 2014; Yoshino et al., 2011). Recombinant CD38 has been shown to hydrolyze NMN in vitro (Grozio et al., 2013), as confirmed here by us (Figure 6A). However, it has not been demonstrated whether CD38 has a role in the degradation of these compounds in vivo. We now report, for the first time, that CD38 is one of the main enzymes degrading NMN in mouse tissues (Figure 6B), implying that CD38 has a key role in the pharmacokinetics of NMN. This was confirmed by the rapid disappearance of NMN in blood of WT, but not CD38KO mice (Figure 6C). Due to the rapid degradation of NMN, we performed further in vivo experiments with NR as an NAD+ precursor. NR is resistant to CD38 enzymatic activity in vitro (Figure 6A). However, since NR is converted to NMN (Bieganowski and Brenner, 2004; Grozio et al., 2013), it is still possible that CD38 may have a role in NR-mediated pharmacokinetics. To test this hypothesis, we administered NR to WT and CD38KO mice and followed plasma levels of NR, NMN, and NAD+. We show that, following an i.p. injection of NR (500 mg/kg), the levels of NR, NMN, and NAD+ in the blood of CD38KO mice are more stable than in WT animals (Figure 6D). In fact, levels of NR rapidly decline in WT mice, with a decrease of about 25% in the first 75 minutes, while NR levels are maintained in CD38KO mice. 150 minutes after NR injection, there were still higher levels of NMN and NAD+ in plasma of CD38KO than in WT mice (Figure 6D). These results indicate that CD38 is involved in the metabolism of NAD precursors in vivo. To further explore the role of CD38 in NAD-replacement therapy in vivo, we selected two-year-old C57BL/6 WT and CD38KO mice that had similar normal glycemia, and induced glucose intolerance with high fat diet. After 16 weeks of high fat diet, WT mice become severely glucose intolerant, and although CD38KO were somewhat resistant to the effects of the diet, they still developed a significant degree of glucose intolerance (Figure S6A–B). At the end of 16 weeks we gave an i.p. injection of NR and determined its effect in glucose tolerance tests. Consistent with a role of CD38 in NR-mediated NAD+ replacement therapy, a single dose of NR was able to ameliorate glucose intolerance only in CD38KO mice (Figure 6E–F). These results confirm that CD38 has the ability to influence NAD+-replacement therapy in vivo. We propose that NAD replacement-based therapies with NMN and NR for metabolic dysfunction could be significantly improved by combination with CD38 inhibitors.
Figure 6. CD38 regulates the NR-induced metabolic improvement in high fat diet.
All values are mean ± SEM.
(A) Nicotinamide concentration in vitro after incubation of 1mM NR or 0.5mM NMN with CD38 recombinant protein. The metabolites were measured by HPLC (n=3, **p<0.01 versus NMN incubation).
(B) Nicotinamide concentration in different tissue homogenates from WT and CD38KO mice after incubation with 1mM NMN. The metabolites were measured by a coupled assay (n=3,*p<0.05, **p<0.01 versus WT mice).
(C) Nicotinamide concentration in blood from WT and CD38KO mice after incubation with 1mM NMN. The metabolites were measured by HPLC (n=3, *p<0.05, **p<0.01 versus WT mice).
(D) Levels of NR, NMN, and NAD in blood of WT and CD38KO mice after injection of 500mg/kg NR intraperitoneally (i.p.). The metabolites were measured by HPLC (n=3, *p<0.05 versus WT mice). Due to the technique use it is possible that the NR peak in the figure could contain other metabolites.
(E and F) Glucose concentration in WT and CD38KO mice after 16 weeks of high fat diet, treated with 500mg/Kg of Nicotinamide Riboside (NR) (dotted line) i.p. The controls received PBS injection (solid line) (E). Two-way ANOVA and Bonferroni's post-test with repeated measures show significant interaction between the glucose curve for CD38KO and CD38KO + NR (****p<0.0001). (F) Quantification of glucose AUC (**p<0.01, n=10).
DISCUSSION
The mechanisms that lead to age-related metabolic dysfunction have not been elucidated. Recently, a growing body of evidence indicates that cellular NAD+ levels decrease during the aging process in both rodents and humans (Zhu et al., 2015; Gomes et al., 2013; Braidy et al., 2011; Scheibye-Knudsen et al., 2014, Massudi et al., 2012). Our first goal here was to confirm these findings. We used two different methods to measure NAD levels in mouse tissues, and clearly demonstrate that aging mice experience a sharp decrease in tissue NAD levels that positively correlates with a decrease in mitochondrial function. Thus, our next goal was to understand the mechanisms that lead to this age-related NAD decline and metabolic dysfunction.
One of the current hypotheses to explain the age-related NAD decline is that this phenomenon could be mediated by accumulation of DNA damage and activation of PARP1 during aging (Bai and Cantó, 2012; Imai and Guarente, 2014). However, there is no consensus about the role of PARPs in the aging process, and the concept of a deleterious increase in PARP activity during aging is controversial. In fact, it has been previously observed that levels of PARPs may either decrease or increase with aging (Bakondi et al., 2011; Beneke et al., 2010; Noren Hooten et al., 2012; Zhang et al., 2014). Furthermore, based on the disposable soma evolutionary aging hypothesis, it is expected that the aging organism would present limited repair mechanisms and not necessarily an increase in damage inputs or repair machinery (Kirkwood and Rose, 1991). In fact, in the disposable soma theory, it has been proposed that, to optimize energy use, biological systems may invest most of their energy in growth and development, and little in damage control and repair. In support of this idea, our data indicate that PARP1 levels actually decrease in all mouse tissues tested during the aging process. Thus, we postulated that other NAD-degrading mechanisms besides PARP1 activation are responsible for the age-related NAD+ decline.
We explored the role of the NADase CD38 in NAD decline in several tissues of aging mice. We clearly demonstrate that CD38 levels increase in mouse tissues during aging, and that CD38 is directly involved in the process that mediates the age-related NAD+ decline. Our experiments were comprehensive and included multiple tissues and techniques, and we demonstrated that CD38 protein, mRNA, and NADase activity increased during aging. Although further experiments are necessary to translate our findings to humans, we have also observed an increase in CD38 mRNA expression in white adipose tissue derived from older humans compared to younger subjects. Interestingly, in support of our findings, CD38/NADase activity has been proposed to increase in the blood of aging human subjects (Polzonetti et. al., 2012). In addition, we have recently shown that stable over-expression of CD38 in cancer cells leads to the development of features of cellular senescence (Chini et. al.; 2014).
Our current studies further provide evidence that an increase in CD38 in aging mice correlates with the development of mitochondrial dysfunction. The effects of CD38 on mitochondrial function may be mediated, at least, in part by modulation of the availability of NAD+ as a substrate to mitochondrial enzymes including SIRT3. However, since CD38 regulates both global cellular and mitochondrial NAD levels, we cannot exclude the possibility that the effect of CD38 may be mediated by interference in many of the other cellular NAD+-dependent processes including oxy-reduction reactions, signaling, and epigenetics.
Our studies raise several new important questions that need to be addressed to understand the precise mechanisms leading to age-related NAD+ decline. One question, for example, is what are the signaling mechanisms that lead to CD38 accumulation in tissues during the aging process? We and others have previously shown that lipopolysaccharides (LPS) and inflammatory cytokines such as TNF-α are potent inducers of CD38 expression in cells (Barata et. al., 2004; Lee et. al., 2012). Interestingly, it has been proposed that during the aging process, there is an increase in levels of endotoxins and cytokines (Kim et. al., 2016; Ghosh et. al., 2015.). Therefore, one possibility is that pro-inflammatory agents such as endotoxins may be the main drivers of the age-related increase in CD38 expression.
A second important question is which cells in the tissue express CD38 during aging? Since CD38 is highly expressed in inflammatory cells, it is possible that the low grade inflammation occurring during aging may lead to an increase in the expression of CD38 in inflammatory cells, and accumulation of CD38-positive inflammatory cells in the tissue.
Yet another key topic is if the CD38 ectoenzymatic activity is sufficient to explain its role as a regulator of cellular NAD+ levels in vivo during the aging process. In addition, we do not know if the small fraction of CD38 localized in intracellular compartments can explain its role in the regulation of NAD levels in vivo. Interestingly, CD38 is a type II plasma membrane enzyme with the majority of its catalytic activity facing the outside of the cell. However, CD38/NADase has been shown to be present in intracellular organelles including the nuclei and the mitochondria (Sun et. al., 2002; Yamada, et. al., 1997; Aksoy, et. al., 2006). At this point, it is not clear if the biological effects of CD38 in NAD+ metabolism are exclusively mediated by its extracellular or intracellular NADase activity. The fact that CD38 metabolizes not only NAD, but also NMN, may indicate that CD38 could at least in part decrease the availability of extracellular and intracellular NAD precursors to cells during the aging process.
In conclusion, we present strong evidence that CD38 is a key enzyme involved in the age-related NAD+ decline. The recent development of potent and specific CD38 inhibitors (Escande et al., 2013; Haffner et al., 2015), together with the novel findings highlighting the role of NAD+ replacement therapy and CD38 in age-related diseases such as hearing loss and Alzheimer’s (Prolla and Denu, 2014; Chini 2009; Blacher et al., 2015), indicate that CD38 inhibition combined with NAD precursors may serve as a potential therapy for metabolic dysfunction and age-related diseases.
EXPERIMENTAL PROCEDURES
Animal Studies
CD38KO, SIRT3KO, and PARP1KO mice were bred in our animal facility or purchased from Jackson Laboratories. SIRT3/CD38 double KO mice were generated through heterozygous breeding (see Supplemental Information for details). Studies were performed in male animals at 3 to 32 months of age. Mice received standard chow ad libitum, except for HFD feeding experiments in which an Adjusted Calorie Diet, 42% from fat, was given (TD.88137, Harlan Laboratories, Inc). Fasted glucose levels and glucose tolerance were assessed as described before (Escande et al., 2013). For GTTs, mice were fasted for 15 hours, and 50% dextrose (1.5g/kg body weight) was injected intraperitoneally (i.p.). For NR studies, mice were given a single dose (500 mg/kg body weight) by i.p. injection. Experiments were approved by the Institutional Animal Care and Use Committee (Protocol no. A52112), and adhered to the NIH Guide for the Care and Use of Laboratory Animals. C57BL/6 tissues used in some of the western blot of figure 1 and CD38 activity studies were obtained from the NIA (National Institute on Aging). In all head to head comparisons of wild type, CD38 KO or CD38/SIRT3 KO in house generated litter mates were used.
Western Blot
Mouse tissues and cultured cells were lysed in NETN (20 mM Tris-HCl, pH 8.0; 100 mM NaCl; 1 mM EDTA; and 0.5% NP-40) buffer supplemented with 5 mM NaF, 50 mM 2-glycerophosphate, and a protease inhibitor cocktail (Roche). Western blots were developed using horseradish peroxidase-conjugated secondary antibodies or protein A-HRP and Super Signal West Pico chemiluminescent substrate (Pierce). The anti-human CD38 antibody was from Abcam. Anti-mouse primary antibodies were as follows: CD38 from Santa Cruz Biotechnology; acetylated lysine, PARP1, SIRT1, and SIRT3 from Cell Signaling; COX IV and tubulin from Abcam; PBEF/NAMPT from Bethyl Laboratories, and actin from Sigma. The anti-mouse PAR antibody was a kind gift from Dr. Scott Kaufmann.
Mitochondrial Function
Mitochondria were isolated by differential centrifugation at 4°C followed by percoll (Invitrogen) gradient. Deacetylase inhibitors, trichostatin A (TSA) (5µM) and nicotinamide (5mM), were added to isolation buffer. The final liver mitochondria pellet was re-suspended in MIR05 + fatty acid-free BSA (1mg/mL) at a final protein concentration of 10–15 mg/mL for respiration experiments or frozen for other experiments (see Supplemental Information for more details), The O2 consumption rates were measured polarographically using high-resolution respirometry (Oroboros Oxygraph-O2K) and Data Lab Software (Gnaiger, 2009). Membrane potential was measured using the fluorescence signal of the cationic dye, safranin-O. Tissue mitochondrial content was measured by quantitative real time PCR and expressed as a ratio of mitochondrial DNA to genomic DNA (see Supplemental Information for details).
Determination of NAD+, NADH and NAD+ precursors
Detection of NAD+ and NADH were performed as described before using the cycling assay (Aksoy et al., 2006) and also a UPLC-mass spectroscopy assay (Supplemental methods). The specificity of the cycling assay for NAD+ and NADH was determined as shown in figure S1. Briefly, samples were extracted with 10% TCA at 4°C. TCA was removed with water–saturated ether. NADH was extracted with 500 mM NaOH/ 5 mM EDTA and heated for 30 min at 60°C. The aqueous layer containing the nucleotides was removed and adjusted to pH 8 with 1 M Tris. Fractions were stored at −80°C. In control experiments we have use known concentration of NAD+ and NADH and exposed them to the same extraction protocols use in our studies. We observed that nearly 95% of NAD+ and undetectable levels of NADH remained after the acid extraction with TCA. Furthermore, the reverse was true for NaOH extraction where most of the NAD+ was lost and nearly 95% of the NADH was preserved. We have also measure CD38 NADase activity after the TCA and NaOH treatment and found that both treatments completely destroyed the CD38 NADase activity.
Nicotinamide was measured by a coupled assay containing Nicotinamidase (PNC1) (Smith et al., 2009). NR, NMN, NAM, and NAD+ in blood samples were detected using a HPLC system (Shimadzu Scientific Instruments, Inc) based on (Yoshino and Imai, 2013), details in Supplemental Information.
Enzyme Activity
CD38 activity was measured according to (Aksoy et al., 2006). In vitro CD38 activity was measured using 0.1 unit of recombinant human CD38 (R&D Systems) in 0.25 M sucrose and 40 mM Tris-HCl (pH 7.4). Nicotinamide 1, N6-ethenoadenine di-nucleotide was used to determine NADase activity. Lactate released in media was measured by the lactate dehydrogenase coupled assay (Hamilton and Pardue, 1984). Citrate synthase was measured as described (Kuznetsov et al., 2008). Briefly, cell extract was incubated with a buffer containing 100 mM Tris, pH 8.0, 0.1 mM acetyl-CoA, 0.1 mM 5,5’-dithiobis-(2-nitrobenzoic acid), and 0.1% Triton X-100. The assay started with the addition of 0.2mM oxaloacetate and was monitored at 412 nm for 10 minutes at 25°C. SIRT3 activity was determined by a fluorometric method using SIRT3 Direct Fluorescence Screening Assay Kit (Cayman). Protein concentration was determined using Bio-Rad Protein Reagent.
Statistical Analysis
Data were analyzed by a two-tailed Student’s t test and ANOVA. Statistical analysis was performed using GraphPad Prism 6.
Supplementary Material
Highlights.
# CD38 levels increase in tissues with age, and correlate with NAD decline.
# NAD and mitochondrial function are preserved in old CD38 knockout mice.
# CD38 metabolizes NMN in vivo and the response to NAD-replacement therapy.
Acknowledgments
This work was supported in part by grants from the American Federation for Aging Research, the Mayo Foundation, the Strickland Career Development Award, National Institutes of Health (NIH) grants from the National Institute of Aging (NIA, grant AG-26094) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, grant DK-084055), Mayo-UOFM Decade of Discovery Grant 63-01, and Minnesota Obesity Council Grant DK-50456-15. J.C.P. is supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico/Brazil(CNPQ) and Fundação de Amparo é Pesquisa do Rio de Janeiro/Brazil (FAPERJ).
Dr. Chini holds patents on CD38 inhibitors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
ENC, JCP, CCSC generated the hypothesis and concept of the manuscript, ENC and JCP was involved in conduction and designs all experiments. JCP, MGT, GMW performed the mitochondrial studies, ENC, JCP and AG analyzed and interpreted the mitochondrial data, JCP, MGT, CCSC performed cell experiments, western-blots, and PCRs. ASP performed flow cytometry, JCP, MGT, CE, VR, RAS, JMR and ENC designed, performed and interpreted nucleotide measurement experiments. JCP, MGT, CE and VN performed animal studies. All authors contributed to writing the manuscript.
Supplemental Information
Supplemental Information includes Supplemental Experimental Procedures, six figures, and two tables and can be found attached to this article.
REFERENCES
- Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun. 2006;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Bai P, Canto C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P, Cantó C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012;16:290–295. doi: 10.1016/j.cmet.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Bakondi E, Catalgol B, Bak I, Jung T, Bozaykut P, Bayramicli M, Ozer NK, Grune T. Age-related loss of stress-induced nuclear proteasome activation is due to low PARP-1 activity. Free Radic. Biol. Med. 2011;50:86–92. doi: 10.1016/j.freeradbiomed.2010.10.700. [DOI] [PubMed] [Google Scholar]
- Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS, Feitoza S, Sieck G, Chini EN. The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells. Endocrinology. 2004;145:881–889. doi: 10.1210/en.2003-0774. [DOI] [PubMed] [Google Scholar]
- Barbosa MTP, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, Chini EN. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- Beneke S, Scherr A-L, Ponath V, Popp O, Bürkle A. Enzyme characteristics of recombinant poly(ADP-ribose) polymerases-1 of rat and human origin mirror the correlation between cellular poly(ADP-ribosyl)ation capacity and species-specific life span. Mech. Ageing Dev. 2010;131:366–369. doi: 10.1016/j.mad.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- Blacher E, Dadali T, Bespalko A, Haupenthal V, Grimm M, Hartmann T, Lund F, Stein R, Levy A. Alzheimer’s disease pathology is attenuated in a CD38 deficient mouse model. Ann. Neurol. 2015;78:88–103. doi: 10.1002/ana.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age-related changes in NAD+ metabolism oxidative stress and SIRT1 activity in wistar rats. PLOS ONE. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux Pa, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini CC, Guerrico AM, Nin V, Camacho-Pereira J, Escande C, Barbosa MT, Chini EN. Targeting of NAD metabolism in pancreatic cancer cells: potential novel therapy for pancreatic tumors. Clin Cancer Res. 2014;20:120–130. doi: 10.1158/1078-0432.CCR-13-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini EN. CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions. Curr. Pharm. Des. 2009;15:57–63. doi: 10.2174/138161209787185788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Menabò R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O’Neil L, White TA, Sinclair DA, Chini EN. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62:1084–1093. doi: 10.2337/db12-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LW, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP, Haigis MC. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One. 2011;6:e23295. doi: 10.1371/journal.pone.0023295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca L, Fedele G, Deaglio S, Capuano C, Palazzo R, Vaisitti T, Malavasi F, Ausiello CM. CD38 orchestrates migration, survival, and Th1 immune response of human mature dendritic cells. Blood. 2006;107:2392–2399. doi: 10.1182/blood-2005-07-2913. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Lertwattanarak R, Garduño Jde J, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S, Musi N. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. Gerontol A Biol Sci Med Sci. 2015;70:232–246. doi: 10.1093/gerona/glu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int. J. Biochem. Cell Biol. 2009;41:1837–1845. doi: 10.1016/j.biocel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, Raffaelli N, De Flora A, Nencioni A, Bruzzone S. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J. Biol. Chem. 2013;288:25938–25949. doi: 10.1074/jbc.M113.470435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes AG, Jude JA, Paulin J, Kita H, Lund FE, Kannan MS. Role of CD38 in TNF- α -induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2008;294:L290–L299. doi: 10.1152/ajplung.00367.2007. [DOI] [PubMed] [Google Scholar]
- Haffner CD, Becherer JD, Boros EE, Cadilla R, Carpenter T, Cowan D, Deaton DN, Guo Y, Harrington W, Henke BR, et al. Discovery, Synthesis, and Biological Evaluation of Thiazoloquin(az)olin(on)es as Potent CD38 Inhibitors. J. Med. Chem. 2015;58:3548–3571. doi: 10.1021/jm502009h. [DOI] [PubMed] [Google Scholar]
- Hamilton SD, Pardue HL. Quantitation of lactate by a kinetic method with an extended range of linearity and low dependence on experimental variables. Clin. Chem. 1984;30:226–229. [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter Ca, Harris C, Biddinger S, Ilkayeva OR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Guarente L. NAD(+) and sirtuins in aging and disease. Trends Cell Biol. 2014;1:1–8. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Jeong JJ, Yoo SY, Kim DH. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. J BMC Microbiol. 2016;16:9. doi: 10.1186/s12866-016-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philosoph Trans: Biol Sci. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Arch. 2010;459:277–289. doi: 10.1007/s00424-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CU, Song EK, Yoo CH, Kwak YK, Han MK. Lipopolysaccharide induces CD38 expression and solubilization in J774 macrophage cells. Mol Cells. 2012;34:573–576. doi: 10.1007/s10059-012-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and Sirt1 activity in wistar rats. PLOS ONE. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahimana A, Attinger A, Aubry D, Greaney P, Ireson C, Thougaard AV, Tiornelund J, Dawson KW, Dupuis M, Duchosal MA. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood. 2009;113:3276–3286. doi: 10.1182/blood-2008-08-173369. [DOI] [PubMed] [Google Scholar]
- Noren Hooten N, Fitzpatrick M, Kompaniez K, Jacob K, Moore B, Nagle J, Barnes J, Lohani A, Evans M. Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) 2012;4:674–685. doi: 10.18632/aging.100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzonetti V, Carpi FM, Micozzi D, Pucciarelli S, Vincenzetti S, Napolioni V. Population variability in CD38 activity: correlation with age and significant effect of TNF-α -308G>A and CD38 184C>G SNPs. Mol Genet Metab. 2012;105:502–507. doi: 10.1016/j.ymgme.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Prolla TA, Denu JM. NAD+ deficiency in age-related mitochondrial dysfunction. Cell Metab. 2014;19:178–180. doi: 10.1016/j.cmet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, Dunn CA, Singh N, Veith S, Hasan-Olive MM, et al. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014;20:840–855. doi: 10.1016/j.cmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654–661. doi: 10.1016/j.cmet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BC, Hallows WC, Denu JM. A continuous microplate assay for sirtuins and nicotinamide-producing enzymes. Anal. Biochem. 2009;394:101–109. doi: 10.1016/j.ab.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Adebanjo OA, Koval A, Anandatheerthavarada HK, Iqbal J, Wu XY, Moonga BS, Wu XB, Biswas G, Bevis PJ, et al. A novel mechanism for coupling cellular intermediary metabolism to cytosolic Ca2+ signaling via CD38/ADP-ribosyl cyclase, a putative intracellular NAD+ sensor. FASEB J. 2002;16:302–314. doi: 10.1096/fj.01-0705com. [DOI] [PubMed] [Google Scholar]
- Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, et al. Sirt3-Mediated Deacetylation of Evolutionarily Conserved Lysine 122 Regulates MnSOD Activity in Response to Stress. Mol. Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Mizuguchi M, Otsuka N, Ikeda K, Takahashi H. Ultrastructural localization of CD38 immunoreactivity in rat brain. Brain Res. 1997;756:52–60. doi: 10.1016/s0006-8993(97)00117-0. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Imai S-I. Accurate measurement of nicotinamide adenine dinucleotide (NAD+) with high-performance liquid chromatography. Methods Mol. Biol. 2013;1077:203–215. doi: 10.1007/978-1-62703-637-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xiong Z-M, Cao K. Mechanisms controlling the smooth muscle cell death in progeria via down-regulation of poly(ADP-ribose) polymerase 1. Proc. Natl. Acad. Sci. 2014;111:E2261–E2270. doi: 10.1073/pnas.1320843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay revels the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.