Abstract
Type 2 diabetes mellitus (T2DM) is highly predictive of cardiovascular diseases and can have particularly deleterious health impacts in people with severe mental illness (SMI), i.e. schizophrenia, bipolar disorder or major depressive disorder. This meta‐analysis aimed: a) to describe pooled frequencies of T2DM in people with SMI; b) to analyze the influence of demographic, illness and treatment variables as well as T2DM assessment methods; and c) to describe T2DM prevalence in studies directly comparing persons with each specific SMI diagnosis to general population samples. The trim and fill adjusted pooled T2DM prevalence among 438,245 people with SMI was 11.3% (95% CI: 10.0%‐12.6%). In antipsychotic‐naïve participants, the prevalence of T2DM was 2.9% (95% CI: 1.7%‐4.8%). There were no significant diagnostic subgroup differences. A comparative meta‐analysis established that multi‐episode persons with SMI (N=133,470) were significantly more likely to have T2DM than matched controls (N=5,622,664): relative risk, RR=1.85, 95% CI: 1.45‐2.37, p<0.001. The T2DM prevalence was consistently elevated in each of the three major diagnostic subgroups compared to matched controls. Higher T2DM prevalences were observed in women with SMI compared to men (RR=1.43, 95% CI: 1.20‐1.69, p<0.001). Multi‐episode (versus first‐episode) status was the only significant predictor for T2DM in a multivariable meta‐regression analysis (r2=0.52, p<0.001). The T2DM prevalence was higher in patients prescribed antipsychotics, except for aripriprazole and amisulpride. Routine screening and multidisciplinary management of T2DM is needed. T2DM risks of individual antipsychotic medications should be considered when making treatment choices.
Keywords: Diabetes mellitus, severe mental illness, schizophrenia, bipolar disorder, major depressive disorder, antipsychotics
People with severe mental illness (SMI) – defined as schizophrenia, bipolar disorder or major depressive disorder (MDD) – have a two to three times higher risk for premature death than the general population1, 2. This mortality gap translates to a 10‐20 year shortened life expectancy3, 4 and appears to be widening5. The most important cause for this shortened life expectancy is cardiovascular disease (CVD)6. Major risk factors include antipsychotic medication use and an unhealthy lifestyle7, and these risks are compounded by obstacles in access to medical care8, 9, 10, 11, 12.
Type 2 diabetes mellitus (T2DM) is a major risk factor for CVD. It confers about a two‐fold excess risk for coronary heart disease, major stroke subtypes, and deaths attributed to other vascular causes13, 14. Prevention and treatment of T2DM demand careful consideration in clinical practice, particularly in populations with an increased risk for CVD and associated premature mortality15, 16.
Recent meta‐analyses17, 18, 19, 20 demonstrated that all diagnostic SMI subgroups have a higher risk for developing T2DM than the general population. However, meta‐analytic data comparing T2DM risks across different psychiatric diagnoses are currently lacking. Furthermore, there are no meta‐analytic data that combine all major diagnostic SMI subgroups, and information on the prevalence of T2DM among people with SMI prescribed different antipsychotic medication classes is insufficient.
Large‐scale pooled analyses in the SMI population are relevant, as they enable investigation of risk factors across large numbers of studies and participants, distinguishing risk factors for T2DM associated with specific SMIs from those independent of these illnesses. Pooling data across major diagnostic categories allows for investigation of the effect of demographic variables (gender, age, illness duration, study setting, geographical region) and treatments (particularly mood stabilizers and antipsychotics prescribed for psychotic and non‐psychotic conditions). If risk stratification is observed, this could potentially guide clinicians in monitoring and treatment.
Given the aforementioned gaps within the literature, we conducted a large scale systematic review and meta‐analysis of pooled T2DM prevalences in people with schizophrenia or related psychotic disorders, bipolar disorder or MDD. We aimed to: a) describe pooled T2DM frequencies in people with SMI; b) analyze the influence of demographic, illness and treatment variables as well as T2DM assessment methods; and c) describe T2DM prevalence in studies directly comparing persons with each specific SMI diagnosis to general population samples.
METHODS
Inclusion and exclusion criteria
This systematic review was conducted in accordance with the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) guidelines21 and in line with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) standard22.
We included observational studies (cross‐sectional, retrospective and prospective studies) and randomized controlled trials in adults with a psychiatric diagnosis of schizophrenia or related psychotic disorders, bipolar disorder or MDD according to the DSM‐IV‐TR or the ICD‐10, irrespective of clinical setting (inpatient, outpatient or mixed, community setting), that reported study‐defined T2DM prevalences.
We excluded studies restricted to patients with or without cardiovascular diseases. When required, we contacted the primary or corresponding authors of studies to confirm eligibility, and to obtain the data needed for analysis if they were not available in the published paper.
Search criteria, study selection and critical appraisal
Two independent reviewers (DV, BS) searched Medline, PsycARTICLES, Embase and CINAHL from database inception to August 1, 2015, without language restrictions. Key words used were “diabetes” OR “glucose” AND “severe mental illness” OR “serious mental illness” OR “schizophrenia” OR “psychosis” OR “bipolar disorder” OR “depression” OR “depressive disorder” in the title, abstract or index term fields. Manual searches were also conducted using the reference lists from recovered articles and recent systematic reviews.
After the removal of duplicates, the reviewers screened the titles and abstracts of all potentially eligible articles. They both applied the eligibility criteria, and a list of full text articles was developed through consensus. Next, the two reviewers considered the full texts of these articles and the final list of included articles was reached through consensus. A third reviewer (CC) was available for mediation throughout this process. Methodological appraisal included evaluation of bias (confounding, overlapping data, publication bias).
Statistical analyses
Due to anticipated heterogeneity, a random effects meta‐analysis was employed. Heterogeneity was measured with the Q statistic (which is always presented at the end of the description of the results as a second or final p‐value).
We calculated the relative risk (RR) to investigate the T2DM prevalence within and across SMI subgroups, the latter only in those studies directly comparing diagnostic subgroups. Moreover, we compared the prevalence of T2DM between people with schizophrenia, bipolar disorder and MDD and general population control groups that were matched on age and gender, using data from studies in which they were directly compared. In both analyses, only comparisons of specific SMI groups or a SMI group with a matched general population group were included that had been performed within the same study, in order to minimize variability of T2DM frequencies due to different sampling and assessment procedures.
Furthermore, in the entire dataset, we conducted subgroup analyses to investigate differences between the three main diagnostic subgroups, first‐episode versus multi‐episode illness, males versus females, population based versus non‐population based studies, and differences across medication classes (antipsychotics, antidepressants, mood stabilizers) and geographical regions. In order to reduce heterogeneity, we did not calculate diagnostic and gender differences across studies, but pooled only data of studies that compared these differences on a patient level. Further, we conducted meta‐regression analyses to investigate potential moderators (age, percentage of males, illness duration, smoking prevalence, and T2DM assessment methods) with Comprehensive Meta Analysis (version 3).
Publication bias was tested using the Egger's regression method23 and Begg‐Mazumdar test24, with a p‐value <0.05 suggesting the presence of bias. When we encountered publication bias, we conducted a trim and fill adjusted analysis25 to remove the most extreme small studies from the positive side of the funnel plot, and recalculated the effect size iteratively, until the funnel plot was symmetrical around the (new) effect size.
RESULTS
Search results and included participants
After excluding duplicates and irrevelant hits, our search yielded 323 publications, of which 118 (including 135 T2DM prevalences) met inclusion criteria (Figure 1). A list of the included and excluded studies (with reasons) is available upon request from the first author.
Figure 1.
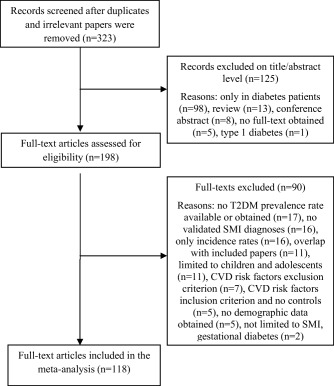
Flow diagram for the search strategy. T2DM — type 2 diabetes mellitus, SMI — severe mental illness, CVD — cardiovascular disease
The final sample comprised 438,245 unique persons with SMI and 5,622,664 matched controls. Sample sizes ranged from 12 to 143,943 participants, with a median sample size of 270. The mean age of participants with SMI was 44.3 years (range 23.1‐77.6 years); 56.8% were male (range 0‐100); 69% were Caucasian (range 0‐100; 37 studies). Mean illness duration was 16.1 years (range 0‐35 years; 29 studies). Thirty‐one studies (N=77,028) reported smoking frequencies, and 44.5% (95% CI: 29.2%‐60.4%) of the included participants smoked.
T2DM prevalence
The estimated weighted mean prevalence of T2DM among 438,245 people with SMI was 10.2% (95% CI: 9.1%‐11.4%; Q=14228.7, p<0.001). The Begg‐Mazumdar (Kendall's tau=0.15, p=0.009) and Egger test (bias=−5.39, 95% CI: −7.33 to −3.45, p<0.001) indicated presence of publication bias. Applying the trim and fill method, adjusting for 13 studies, the prevalence of T2DM was 11.3% (95% CI: 10.0%‐12.6%).
Subgroup analyses and predictors of T2DM
Study setting and design
The pooled prevalences across different treatment settings (inpatients, outpatients, community patients, mixed settings), study designs (cross‐sectional, retrospective and prospective studies, and population versus non‐population based), median year of data collection (before or after the year 2000), methods of T2DM assessment (blood testing, self‐report, charts) are summarized in Table 1. The separate meta‐regressions are presented in Table 2.
Table 1.
Subgroup analyses of moderators of type 2 diabetes mellitus (T2DM) in people with severe mental illness
| Meta‐analysis | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|
| Number of studies | Pooled T2DM prevalence (%) | 95% CI | Between‐group difference p‐value | I2 | Q | p‐value | |
| Study design | |||||||
| Cross‐sectional | 70 | 9.2 | 7.9‐10.8 | 0.03 | 97.2 | 2504.2 | <0.001 |
| Retrospective | 43 | 12.3 | 10.3‐4.8 | 99.3 | 6436.5 | <0.001 | |
| Prospective | 21 | 8.5 | 6.2‐11.5 | 98.7 | 1575.0 | <0.001 | |
| Population based or not | |||||||
| Population based | 58 | 10.0 | 8.5‐11.6 | 0.70 | 99.6 | 13491.3 | <0.001 |
| Non‐population based | 76 | 10.4 | 8.9‐12.2 | 84.6 | 486.0 | <0.001 | |
| Study setting | |||||||
| Mixed | 37 | 8.7 | 7.1‐10.5 | 0.26 | 99.5 | 7179.8 | <0.001 |
| Inpatient | 37 | 11.3 | 9.3‐13.8 | 93.5 | 553.7 | <0.001 | |
| Outpatient | 36 | 11.5 | 9.3‐14.1 | 97.1 | 1229.7 | <0.001 | |
| Community | 21 | 9.7 | 7.4‐12.4 | 98.4 | 1221.7 | <0.001 | |
| Diabetes assessment method | |||||||
| Blood testing | 34 | 10.5 | 9.8‐11.2 | <0.001 | 79.3 | 159.3 | <0.001 |
| Self‐report | 26 | 9.3 | 8.8‐9.8 | 97.4 | 980.9 | <0.001 | |
| Charts and files | 53 | 13.0 | 11.0‐15.2 | 99.5 | 11051.8 | <0.001 | |
| Median year data collection | |||||||
| Before 2000 | 18 | 9.5 | 6.9‐12.8 | 0.95 | 97.7 | 728.6 | <0.001 |
| 2000 or later | 116 | 10.2 | 9.0‐11.6 | 99.1 | 13469.1 | <0.001 | |
| Diagnosis | |||||||
| Mixed | 18 | 11.2 | 8.5‐14.6 | 0.003 | 99.6 | 4011.7 | <0.001 |
| Major depressive disorder | 20 | 6.4 | 4.8‐8.4 | 97.8 | 869.4 | <0.001 | |
| Bipolar disorder | 17 | 9.2 | 6.8‐12.4 | 96.6 | 466.8 | <0.001 | |
| Schizophrenia spectrum | 22 | 11.8 | 9.0‐15.2 | 99.0 | 5151.0 | <0.001 | |
| Schizophrenia only | 57 | 11.5 | 9.8‐13.5 | 94.7 | 394.2 | <0.001 | |
| Episode | |||||||
| First‐episode schizophrenia | 14 | 4.0 | 2.5‐6.2 | <0.001 | 62.2 | 34.4 | 0.001 |
| Multi‐episode schizophrenia | 67 | 13.1 | 11.7‐14.8 | 98.9 | 6011.1 | <0.001 | |
| Gender | |||||||
| Male | 31 | 7.9 | 5.9‐10.3 | <0.01 | 97.0 | 1037.5 | <0.001 |
| Female | 31 | 11.3 | 8.6‐14.7 | 97.5 | 1239.8 | <0.001 | |
| Geographical region | |||||||
| North America | 58 | 12.5 | 10.9‐14.3 | 0.007 | 99.0 | 6026.7 | <0.001 |
| Europe | 32 | 7.7 | 6.3‐9.3 | 98.7 | 2486.6 | <0.001 | |
| Asia | 28 | 10.6 | 8.5‐13.1 | 93.1 | 393.9 | <0.001 | |
| Australia | 5 | 9.2 | 5.7‐14.5 | 90.6 | 42.7 | 0.034 | |
| South America | 5 | 8.6 | 4.8‐15.1 | 61.7 | 10.4 | 0.006 | |
| Africa | 2 | 7.0 | 3.1‐15.0 | 86.6 | 7.5 | 0.65 | |
| Middle East | 2 | 10.2 | 4.8‐20.3 | 0 | 0.2 | 0.33 | |
| Antipsychotic medication use | |||||||
| Antipsychotic‐naïve | 10 | 2.9 | 1.7‐4.8 | <0.001 | 78.0 | 41.0 | <0.001 |
| Clozapine | 9 | 15.5 | 11.0‐21.3 | 38.4 | 13.0 | 0.11 | |
| Olanzapine | 9 | 10.6 | 7.0‐15.7 | 2.5 | 8.2 | 0.41 | |
| Risperidone | 9 | 13.2 | 8.8‐19.4 | 54.2 | 17.4 | 0.026 | |
| Quetiapine | 7 | 16.0 | 9.9‐24.7 | 0 | 2.5 | 0.87 | |
| Aripiprazole | 3 | 6.7 | 1.5‐25.0 | 0 | 0.3 | 0.87 | |
| Amisulpride | 2 | 3.9 | 0.5‐25.0 | 0 | 0.6 | 0.44 | |
| Typical antipsychotics | 11 | 10.6 | 7.0‐15.7 | 57.8 | 23.7 | 0.008 | |
Significant between‐group differences are highlighted in bold prints
Table 2.
Meta‐regressions of moderators of type 2 diabetes mellitus (T2DM) in people with severe mental illness
| Number of comparisons | β | 95% CI | p‐value | R2 | ||
|---|---|---|---|---|---|---|
| Design (vs. retrospective) | 0.02 | |||||
| Cross‐sectional | 113 | −0.35 | −0.72 | 0.007 | 0.054 | |
| Prospective | 64 | −0.51 | −1.04 | 0.008 | 0.053 | |
| Population based (yes/no) | 134 | −0.002 | −0.34 | 0.33 | 0.99 | 0.00 |
| Setting (vs. mixed) | 0.02 | |||||
| Inpatients | 74 | 0.32 | −0.12 | 0.77 | 0.15 | |
| Outpatients | 73 | 0.30 | −0.15 | 0.76 | 0.19 | |
| Community patients | 58 | 0.19 | −0.32 | 0.70 | 0.47 | |
| T2DM assessment (vs. self‐report) | 0.09 | |||||
| Blood testing | 60 | −0.02 | −0.53 | 0.49 | 0.92 | |
| Charts | 87 | 0.63 | 0.18 | 1.07 | 0.006 | |
| Publication data (before 2000 or not) | 134 | −0.08 | −0.56 | 0.40 | 0.75 | 0.00 |
| First episode (yes/no) | 81 | 1.31 | 0.80 | 1.81 | <0.001 | 0.19 |
| Mean age (years) | 118 | 0.05 | 0.03 | 0.07 | <0.001 | 0.18 |
| Gender (% male) | 123 | 0.25 | −0.37 | 0.88 | 0.42 | 0.01 |
| Ethnicity (% Caucasian) | 37 | −0.65 | −1.48 | 0.17 | 0.12 | 0.07 |
| Duration of illness (years) | 29 | 0.03 | 0.007 | 0.06 | 0.01 | 0.15 |
| Smoking (% smokers) | 31 | −0.24 | −1.83 | 1.35 | 0.77 | 0.01 |
| Treatment duration (years) | 9 | 0.07 | 0.03 | 0.10 | <0.001 | 0.72 |
| Antidepressants use (%) | 16 | 2.82 | 1.08 | 4.55 | 0.001 | 0.44 |
| Lithium use (%) | 11 | 3.07 | 1.46 | 4.68 | <0.001 | 0.65 |
| Other mood stabilizers use (%) | 13 | −0.47 | −2.09 | 1.14 | 0.57 | 0.06 |
| Geographical region (vs. North America) | 0.06 | |||||
| Europe | 90 | −0.55 | −0.96 | −0.13 | 0.009 | |
| Asia | 86 | −0.23 | −0.67 | 0.22 | 0.31 | |
| Australia | 63 | −0.30 | −1.15 | 0.55 | 0.49 | |
| South America | 63 | −0.48 | −1.44 | 0.48 | 0.32 | |
| Africa | 60 | −0.59 | −1.93 | 0.75 | 0.39 | |
| Middle East | 60 | −0.19 | −1.49 | 1.12 | 0.78 | |
Significant p‐values are highlighted in bold prints
There were no significant differences between the various treatment settings, and data collection before versus after the year 2000. There was also no difference in T2DM prevalence between population based and non‐population based studies. In contrast, a higher T2DM prevalence was observed in studies relying upon clinical data gleaned from file and chart reviews versus self‐report studies. A trend for higher T2DM was found in retrospective studies versus cross‐sectional (p=0.054) and versus prospective (p=0.053) studies.
Diagnostic subgroups
The pooled T2DM prevalences for the different diagnostic subgroups are presented in Table 1. Relative risk meta‐analyses established that there was no significant difference in T2DM in studies directly comparing schizophrenia alone (14.1%, 95% CI: 9.8%‐20.2%; Q=5, p=0.51; N=4,963) versus schizophrenia spectrum disorders (including schizoaffective disorder, schizophreniform disorder and related psychoses) (18.3%, 95% CI: 14.9%‐22.2%; Q=2.1, p=0.34; N=694) (three studies; odds ratio, OR=0.80; 95% CI: 0.52‐1.25, z=−0.97, p=0.33; Q=2.66, p=0.26, I2=24.9).
The same was true for the comparison of schizophrenia (13.7%, 95% CI: 8.2%‐22.1%; Q=131, p<0.01; N=6,005) versus bipolar disorder (13.7%, 95% CI: 9.2%‐20.0%; Q=46, p<0.01; N=3,138) (six studies; OR=1.22, 95% CI: 0.84‐1.77, z=1.08, p=0.28; Q=17.1, p=0.004, I2=70.8); and of schizophrenia (13.7%, 95% CI: 11.6%‐16.1%; Q=0.3, p=0.58; N=893) versus MDD (11.1%, 95% CI: 9.2%‐13.3%; N=911) (two studies; OR=1.27, 95% CI: 0.96‐1.68, z=1.66, p=0.10; Q=6.0, p=0.80, I2=0). There were insufficient studies directly comparing T2DM prevalence in patients with bipolar disorder versus MDD.
Comparing T2DM in first‐ versus multi‐episode patients within the different diagnostic subgroups (see Table 1) demonstrated that first‐episode schizophrenia patients (4.0%, 95% CI: 2.5%‐6.2%) had a significantly lower T2DM prevalence than multi‐episode schizophrenia patients (13.1%, 95% CI: 11.7%‐14.8%, z=−3.89, p<0.001). There were no data in first‐episode bipolar disorder or MDD patients, precluding a comparison with multi‐episode patients.
Demographic variables
A relative risk meta‐analysis across 29 studies (including 32 comparisons) directly comparing T2DM frequencies in men (N=35,400) versus women (N=33,283) with SMI found a higher T2DM prevalence in women (RR=1.43; 95% CI: 1.20‐1.69, p<0.001).
Pooled T2DM prevalences per geographical region are displayed in Table 1. The T2DM prevalence was significantly higher in North America (12.5%, 95% CI: 10.9%‐14.3%; 58 studies) than in Europe (7.7%, 95% CI: 6.3%‐9.3%; 32 studies) (p<0.001). No other significant geographical differences were observed.
Separate meta‐regression analyses (see Table 2) revealed that higher T2DM frequencies were moderated by older age, longer illness duration, and first‐episode versus multi‐episode status, but not by gender, ethnicity, and smoking status.
When all significant demographic predictors were entered in a multivariable meta‐regression model, multi‐episode versus first‐episode status (β=1.889, 95% CI: 0.1445‐3.6335, z=2.12, p=0.03) remained the only significant moderator of the variance of T2DM. The final multivariable model accounted for just over half of the between‐study heterogeneity in T2DM frequency (r2=0.52, p<0.001).
Medication use
Separate meta‐regression analyses (see Table 2) showed that treatment duration, percentage of antidepressant use and percentage of lithium use, but not percentage of other mood stabilizers use, were significant mediators of T2DM prevalence.
Twenty papers, including 64 analyses, reported on antipsychotics (monotherapy) and T2DM frequencies. The prevalence of T2DM was lowest in antipsychotic‐naïve participants (2.9%, 95% CI: 1.7%‐4.8%). Except for aripiprazole and amisulpride, all individual antipsychotics had significantly (p<0.05) higher T2DM risk compared to antipsychotic‐naïve participants (see Table 1). Except for a higher risk for quetiapine versus olanzapine (p=0.04), we did not find any differences in risk profile between individual medications. The T2DM risk in people treated with clozapine tended (p=0.05) to be higher than the risk in those treated with olanzapine.
Relative risk (RR) of T2DM in diagnostic subgroups compared with general population controls
Thirty‐four studies provided data on T2DM prevalences comparing multi‐episode patients with healthy control subjects, and three studies compared first‐episode schizophrenia patients with controls. In a pooled relative risk meta‐analysis, compared with general population controls (N=5,622,664; 6.2%, 95% CI: 4.8%‐8.0%; Q=18,592, p<0.01), multi‐episode persons with SMI (N=133,470; 12.2%, 95% CI: 9.7‐15.2%; Q=6166, p<0.01) had significantly increased risk of T2DM (RR=1.85, 95% CI: 1.45‐2.37, p<0.001; Q=1302.0, p<0.001; 38 studies). There was no significant difference in T2DM in first‐episode patients (4.4%, 95% CI: 2.5%‐7.6%; Q=2, p=0.4) versus controls (0.9%, 95% CI: 0.03%‐2.4%; Q=3, p=0.3) (RR=4.64, 95% CI: 0.73‐29.3, p=0.10; Q=1302.0, p=0.23; three studies).
Compared to healthy controls, the relative risk of T2DM was 2.04 in patients with schizophrenia or related psychotic disorders (N=115,538; 95% CI: 1.69‐2.49, p<0.001; Q=1302.0, p<0.001, I2=97.8; 29 studies); 1.89 in patients with bipolar disorder (N=4,688; 95% CI: 1.29‐2.77, p<0.001; Q=2.2, p=0.34, I2=7.3; six studies), and 1.43 in patients with MDD (N=10,895; 95% CI: 0.88‐2.25, p=0.029; Q=2.15, p=0.34; three studies).
DISCUSSION
To our knowledge, this is the first meta‐analysis of T2DM including and comparing data from the three main SMIs, namely schizophrenia and related psychotic disorders, bipolar disorder and MDD. Approximately one in 10 individuals with SMI (11.3%; 95% CI: 10.0%‐12.6%) had T2DM, and the relative risk for T2DM in multi‐episode persons with SMI was almost double (RR=1.85, 95% CI: 1.45‐2.37) that found in matched general population comparison samples.
T2DM prevalences were consistently elevated for each of the three diagnostic subgroups compared to the general population, and comparative meta‐analyses found no significant differences across schizophrenia, schizophrenia spectrum disorders, bipolar disorder and MDD. Thus, other diagnostic‐independent factors likely influence T2DM frequency, including hyperglycaemia following psychotropic medication use26 and long‐term exposure to unhealthy lifestyle behaviors27, 28, as well as potential genetic factors linking psychiatric and medical risk29.
We showed for the first time in a large scale meta‐analysis that T2DM risk indeed increased with increasing treatment duration, supported further by a multivariate meta‐regression model in which multi‐episode status remained a unique significant predictor, explaining half of the variance. We also observed a significantly increased prevalence of T2DM in North America versus Europe, in keeping with the overall population prevalences30, which suggests a combined impact of genetic, lifestyle and/or environmental risk factors.
Knowledge of factors associated with a high T2DM risk can help identify individuals at greatest need for intensive monitoring and intervention. In contrast with general population studies31, we found that women with SMI had a higher risk for developing T2DM than men. This finding warrants further investigation, but may be related to a greater propensity to obesity and central obesity in women with SMI compared to men32, since central obesity is a significant risk factor for hyperglycaemia. On the other hand, only a minority of analyzed studies did provide information about the mean age in women and men, and it is possible that women with schizophrenia were older, which could have confounded the results.
Our results also show that T2DM prevalence was higher in individuals with multi‐episode schizophrenia compared with persons in their first episode. The current meta‐analysis adds to the evidence that a first‐episode diagnosis is a unique predictor of lower T2DM prevalence independent of mean age, a finding that was also apparent in a recent analysis of metabolic syndrome prevalences across patients with the same three main SMIs33. Our results point toward the need to adopt a prevention/early intervention approach in order to reduce cardiometabolic risk in people with SMI. Further research is needed to explore the mechanisms underlying this increased T2DM risk with the transition of the illness from an initial episode to a multi‐episode disorder.
Our data confirm prior evidence that psychotropic medication use, including that of antidepressants, lithium and antipsychotic medications26, is associated with higher T2DM prevalence. Except for aripiprazole and amisulpride, all antipsychotics were associated with a significantly increased T2DM risk compared to antipsychotic‐naïve patients. Variations in the risk for glucose abnormalities are evident in the literature, with the highest risk being associated with clozapine, olanzapine and quetiapine in carefully designed studies25, 34, 35. In the current meta‐analysis, quetiapine (and a trend for clozapine) was associated with an even higher T2DM risk than olanzapine use. However, this finding should be interpreted with caution, as order effects cannot be excluded, in that patients who acquired marked T2DM risk or developed even frank T2DM on a higher‐risk agent, such as olanzapine, could have been switched to another antipsychotic, including quetiapine, potentially leading to risk misattribution.
Finally, as expected, patient self‐report yielded numerically the lowest T2DM prevalences; the T2DM prevalence was significantly lower compared with chart review data. This finding is likely due to the fact that, in chart review studies, patients were followed back a longer time, extending the detection period. In line with this interpretation, there was a trend for retrospective studies to be associated with higher T2DM prevalences than prospective ones.
Clinical implications
Our meta‐analysis highlighted geographical differences in T2DM, mirroring the different prevalences in the general population, indicating the possible influence of lifestyle and other environmental factors with or without genetic risk differences. Thus, considering the observed increased T2DM risks, screening for and trying to minimize risk factors (including adverse lifestyle factors and specific antipsychotic medication choice) should be a key priority in the multidisciplinary treatment of people with SMI36, 37, 38, 39.
Our data clearly demonstrate that people with SMI should be considered as a “homogeneous and important high‐risk group” that needs proactive screening for T2DM. It is particularly important to establish baseline T2DM risk at initial presentation, so that any subsequent change during treatment can be monitored. The medical history and examination should, at a minimum, include: a) history of previous CVD, T2DM or other related diseases; b) family history of premature CVD, T2DM or other related diseases; c) smoking, dietary and physical activity habits; d) weight and height in order to calculate body mass index, and waist circumference; e) fasting blood glucose and/or hemoglobin A1c (HBA1c); f) blood pressure (measured twice and average taken); and g) past medication history39.
As there are differences in T2DM prevalences across assessment methods, it is recommended that fasting blood glucose measurements (ideally even oral glucose tolerance testing as the gold standard) should be obtained prior to the first prescription of antipsychotic medication. The frequency of glucose metabolism testing will depend on the patient's medical history and the prevalence of baseline risk factors. For patients on antipsychotic medication with normal baseline tests, it is recommended that measurements should be repeated at 12 weeks after initiation of treatment and at least annually thereafter, with more frequent assessments in high‐risk patients, such as those with significant weight gain, post‐partum diabetes or a first‐degree family history of diabetes40. In patients with T2DM (and those with pre‐diabetes), fasting blood glucose and HBA1c should be measured more frequently (approximately every 3‐6 months). An annual examination should include measurement of CVD risk factors, glomerular filtration rate and albumin to creatinine ratio, an eye examination, ideally including fundus photography, and foot examination to diagnose early signs of complications41.
Despite the imperative to screen for T2DM, screening for T2DM and CVD risk factors is still suboptimal, with only slight improvement over the last decade12. The low glucose screening rates (44.3%; 95% CI: 36.3%‐52.4%)12 may reflect both patient and professional barriers. Professional barriers to screening within mental health settings may in their turn reflect lack of clarity about whose clinical responsibility the screening is, lack of understanding about what should be measured and when, uncertainity about how to interpret results, and lack of access to necessary equipment41, as well as incomplete communication between primary and secondary care. Without systematic screening following detailed recommendations and using acceptable and accurate diagnostic tests, the true prevalence of T2DM in patients with SMI will remain unknown and underestimated.
Even after an established diagnosis of T2DM is made, many of those with mental ill health are not offered timely treatment42. Thus, it should be clarified that routine screening is only the first step. Psychiatric centers should cooperate with diabetes centers to establish shared care pathways and ensure an integrated approach for people with mental illness and T2DM. Such an approach would reflect recent calls for the breaking down of the traditional “silo” approach to physical and mental health care, in line with the internationally endorsed Healthy Active Lives Declaration (www.iphys.org).
Those with diagnosed T2DM should also be seen regularly by a multidisciplinary team, including physicians, diabetes nurses, physical therapists or exercise physiologists and dieticians, to advise not just on diabetes but also on other risk factors and medical comorbidities.
When T2DM is detected, people with SMI are likely to require additional pharmacological management, but this is unlikely to be significantly different from the general population. However, clinicians should be aware that any deterioration in mental health may result in compromised management of T2DM, and comprehensive management may require an adjustment to the diabetes care plan.
Limitations
Whilst this is the most comprehensive and thorough meta‐analysis of T2DM in people with SMI conducted to date, we acknowledge some limitations that largely reflect problems in the primary data.
First, only a limited number of studies assessed T2DM using an oral glucose tolerance test as the gold standard. There are inherent problems with using chart reviews in relation to selection bias and the reliability and validity of the T2DM diagnosis. Second, because our study findings were mainly based on cross‐sectional rather than longitudinal data, directionality of the association between medication use and observed T2DM risk cannot be deduced with certainty; that is, it is possible that those with inherently higher metabolic risk factors may be more likely to receive antipsychotics. Also, given that many of the studies reported cross‐sectional data, it is possible that people with SMI deemed to be at particular risk for glucose abnormalities were preferentially prescribed antipsychotics perceived to be of lower risk, such as aripiprazole and amisulpride.
Third, variables such as clinical subtypes of MDD and bipolar disorder were not reported and controlled for. Fourth, a threat to the validity of any meta‐analysis is publication bias and heterogeneity, which we encountered in most of our analyses. Nevertheless, we adjusted for publication bias using the trim and fill analysis, and were able to explain over half of the between‐study heterogeneity in our multivariable meta‐regression analysis. Fifth, there were inadequate data on lifestyle behaviors, precluding meta‐analytic assessment of these factors as moderating or mediating variables.
Future research
Since antipsychotic medications are increasingly used as first line treatments for bipolar disorder43 and MDD44, additional research on the underlying mechanisms for the development of hyperglycaemia after pharmacotherapy initiation is needed. Moreover, future studies should examine whether different clinical subtypes of depression (i.e., melancholic, psychotic, atypical or undifferentiated) and bipolar disorder (e.g., type 1 or 2), specific mood states (manic, depressive, mixed or euthymic), or different antidepressants or mood stabilizers significantly moderate T2DM risk. For example, previous studies45 found that some antidepressants may, in some circumstances, reduce hyperglycaemia, normalize glucose homeostasis and also increase insulin sensitivity, whereas others, including tricyclic antidepressants, may exacerbate glycaemic dysfunction or have little effect on glucose homeostasis46, 47.
Furthermore, the pathophysiology underlying the association between SMI and T2DM is complex and not well understood, requiring further investigation. Emerging evidence48 suggests that SMI and T2DM share some pathophysiological features, including hypothalamic‐pituitary‐adrenal and mitochondrial dysfunction, neuro‐inflammation, common genetic links and epigenetic interactions.
Future research should also comprehensively assess T2DM risk factors, and evaluate the optimal monitoring regimen and interventions. Finally, long‐term follow‐up is required to accurately document the emergence of more distal outcomes, such as ischemic heart disease, medical costs, and premature mortality49.
REFERENCES
- 1. Chesney E, Goodwin GM, Fazel S. Risks of all‐cause and suicide mortality in mental disorders: a meta‐review. World Psychiatry 2014;13:153‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reininghaus U, Dutta R, Dazzan P et al. Mortality in schizophrenia and other psychoses: a 10‐year follow‐up of the AESOP first‐episode cohort. Schizophr Bull 2015;41:664‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang CK, Hayes RD, Perera G et al. Life expectancy at birth for people with serious mental illness from a secondary mental health care case register in London, UK. PLoS One 2011;6:e19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ 2013;346:f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia. Arch Gen Psychiatry 2007;64:1123‐31. [DOI] [PubMed] [Google Scholar]
- 6. Hoang U, Goldacre MJ, Stewart R. Avoidable mortality in people with schizophrenia or bipolar disorder in England. Acta Psychiatr Scand 2013;127:195‐201. [DOI] [PubMed] [Google Scholar]
- 7. De Hert M, Correll CU, Bobes J et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol 2010;24(Suppl. 4):69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell AJ, Lord O, Malone D. Differences in the prescribing of medication for physical disorders in individuals with v. without mental illness: meta‐analysis. Br J Psychiatry 2012;201:435‐43. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell AJ, Malone D, Doebbeling CC. Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry 2009;194:491‐9. [DOI] [PubMed] [Google Scholar]
- 11. De Hert M, Vancampfort D, Correll CU et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry 2011;199:99‐105. [DOI] [PubMed] [Google Scholar]
- 12. Mitchell AJ, Delaffon V, Vancampfort D et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta‐analysis of screening practices. Psychol Med 2012;42:125‐47. [DOI] [PubMed] [Google Scholar]
- 13. Sarwar N, Gao P, Seshasai SR et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet 2010;375:2215‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murray CJ, Vos T, Lozano R et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197‐223. [DOI] [PubMed] [Google Scholar]
- 15. Grundy SM, Benjamin IJ, Burke GL et al. Diabetes and cardiovascular disease: statement for health professionals from the American Heart Association. Circulation 1999;100:1134‐46. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organization, 1999. [Google Scholar]
- 17. Vancampfort D, Wampers M, Mitchell AJ et al. A meta‐analysis of cardio‐metabolic abnormalities in drug naïve, first‐episode and multi‐episode patients with schizophrenia versus general population controls. World Psychiatry 2013;12:240‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stubbs B, Vancampfort D, De Hert M et al. The prevalence and predictors of type 2 diabetes in people with schizophrenia: a systematic review and comparative meta‐analysis. Acta Psychiatr Scand 2015;132:144‐57. [DOI] [PubMed] [Google Scholar]
- 19. Vancampfort D, Mitchell AJ, De Hert M et al. Prevalence and predictors of type 2 diabetes in people with bipolar disorder: a systematic review and meta‐analysis. J Clin Psychiatry 2015;76:1490‐9. [DOI] [PubMed] [Google Scholar]
- 20. Vancampfort D, Mitchell AJ, De Hert M et al. Type 2 diabetes in patients with major depressive disorder: a meta‐analysis of prevalence estimates and predictors. Depress Anxiety 2015;32:763‐73. [DOI] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008‐12. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J et al. The PRISMA Group . Preferred reporting items for systematic reviews and meta‐Analyses: the PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egger M, Davey SG, Schneider M et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088‐101. [PubMed] [Google Scholar]
- 25. Duval S, Tweedie R. A non‐parametric ‘trim and fill’ method for assessing publication bias in meta‐analysis. J Am Stat Assoc 2000;95:89‐98. [Google Scholar]
- 26. Correll CU, Detraux J, De Lepeleire J et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015;14:119‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vancampfort D, Probst M, Knapen J et al. Associations between sedentary behaviour and metabolic parameters in patients with schizophrenia. Psychiatry Res 2012;200:73‐8. [DOI] [PubMed] [Google Scholar]
- 28. Vancampfort D, De Hert M, Sweers K et al. Diabetes, physical activity participation and exercise capacity in patients with schizophrenia. Psychiatry Clin Neurosci 2013;67:451‐6. [DOI] [PubMed] [Google Scholar]
- 29. Ellingrod VL, Taylor SF, Dalack G et al. Risk factors associated with metabolic syndrome in bipolar and schizophrenia subjects treated with antipsychotics: the role of folate pharmacogenetics. J Clin Psychopharmacol 2012;32:261‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Diabetes Federation. IDF diabetes atlas. Sixth edition update. Brussels: International Diabetes Federation, 2014.
- 31. Hammerman A, Dreiher J, Klang SH et al. Antipsychotics and diabetes: an age‐related association. Ann Pharmacother 2008;42:1316‐22. [DOI] [PubMed] [Google Scholar]
- 32. Gardner‐Sood P, Lally J, Smith S et al. Cardiovascular risk factors and metabolic syndrome in people with established psychotic illnesses: baseline data from the IMPaCT randomized controlled trial. Psychol Med 2015;45:2619‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vancampfort D, Stubbs B, Mitchell AJ et al. Risk of metabolic syndrome and its components in people with schizophrenia, bipolar and major depressive disorders: a large scale meta‐analysis of 198 studies. World Psychiatry 2015;14:339‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic‐naïve schizophrenia patients. Neuropsychopharmacology 2010;35:1997‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kessing LV, Thomsen AF, Mogensen UB et al. Treatment with antipsychotics and the risk of diabetes in clinical practice. Br J Psychiatry 2010;197:266‐71. [DOI] [PubMed] [Google Scholar]
- 36. De Hert M, Dekker JM, Wood D et al. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 2009;24:412‐24. [DOI] [PubMed] [Google Scholar]
- 37. McIntyre RS, Alsuwaidan M, Goldstein BI et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid metabolic disorders. Ann Clin Psychiatry 2012;24:69‐81. [PubMed] [Google Scholar]
- 38. Vancampfort D, De Hert M, Skjerven LH et al. International Organization of Physical Therapy in Mental Health consensus on physical activity within multidisciplinary rehabilitation programmes for minimising cardio‐metabolic risk in patients with schizophrenia. Disabil Rehabil 2012;34:1‐12. [DOI] [PubMed] [Google Scholar]
- 39. Gierisch JM, Nieuwsma JA, Bradford DW et al. Pharmacologic and behavioral interventions to improve cardiovascular risk factors in adults with serious mental illness: a systematic review and meta‐analysis. J Clin Psychiatry 2014;75:424‐40. [DOI] [PubMed] [Google Scholar]
- 40. De Hert M, Detraux J, van Winkel R et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011;8:114‐26. [DOI] [PubMed] [Google Scholar]
- 41. De Hert M, Cohen D, Bobes J et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, and recommendations at the system and individual levels. World Psychiatry 2011;10:138‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holt RI. The prevention of diabetes and cardiovascular disease in people with schizophrenia. Acta Psychiatr Scand 2015;132:86‐96. [DOI] [PubMed] [Google Scholar]
- 43. Pillarella J, Higashi A, Alexander GC et al. Trends in use of second‐generation antipsychotics for treatment of bipolar disorder in the United States, 1998‐2009. Psychiatr Serv 2012;63:83‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry 2010;71(Suppl. 1):e04. [DOI] [PubMed] [Google Scholar]
- 45. Hennings JM, Schaaf L, Fulda S. Glucose metabolism and antidepressant medication. Curr Pharm Des 2012;18:5900‐19. [DOI] [PubMed] [Google Scholar]
- 46. Mojtabai R. Antidepressant use and glycemic control. Psychopharmacologia 2013;227:467‐77. [DOI] [PubMed] [Google Scholar]
- 47. Lamers F, Vogelzangs N, Merikangas KR et al. Evidence for a differential role of HPA‐axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2013;18:692‐9. [DOI] [PubMed] [Google Scholar]
- 48. Manu P, Correll CU, Wampers M et al. Markers of inflammation in schizophrenia: association vs. causation. World Psychiatry 2014;13:189‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Correll CU, Joffe BI, Rosen LM et al. Cardiovascular and cerebrovascular risk factors and events associated with second‐generation antipsychotic compared to antidepressant use in a non‐elderly adult sample: results from a claims‐based inception cohort study. World Psychiatry 2015;14:55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]


