Abstract
Life expectancy in patients with schizophrenia is reduced by 20 years for men and 15 years for women compared to the general population. About 60% of the excess mortality is due to physical illnesses, with cardiovascular disease being dominant. CHANGE was a randomized, parallel‐group, superiority, multi‐centre trial with blinded outcome assessment, testing the efficacy of an intervention aimed to improve cardiovascular risk profile and hereby potentially reduce mortality. A total of 428 patients with schizophrenia spectrum disorders and abdominal obesity were recruited and centrally randomized 1:1:1 to 12 months of lifestyle coaching plus care coordination plus treatment as usual (N=138), or care coordination plus treatment as usual (N=142), or treatment as usual alone (N=148). The primary outcome was 10‐year risk of cardiovascular disease assessed post‐treatment and standardized to age 60. At follow‐up, the mean 10‐year risk of cardiovascular disease was 8.4 ± 6.7% in the group receiving lifestyle coaching, 8.5 ± 7.5% in the care coordination group, and 8.0 ± 6.5% in the treatment as usual group (p=0.41). We found no intervention effects for any secondary or exploratory outcomes, including cardiorespiratory fitness, physical activity, weight, diet and smoking. In conclusion, the CHANGE trial did not support superiority of individual lifestyle coaching or care coordination compared to treatment as usual in reducing cardiovascular risk in patients with schizophrenia spectrum disorders and abdominal obesity.
Keywords: Schizophrenia, abdominal obesity, CHANGE trial, lifestyle coaching, care coordination, cardiovascular risk, cardiorespiratory fitness, physical activity
The gap in life expectancy between patients with schizophrenia and the general population – twenty years shorter for men and fifteen years shorter for women1, 2 – is a major challenge to public health. About 60% of the premature mortality in schizophrenia is due to physical diseases3, with cardiovascular disease explaining the majority4.
Several factors contribute to the early and frequent development of cardiovascular disease in this population, including genetic vulnerability5, metabolic adverse effects of antipsychotics6, 7, insufficient treatment of somatic comorbidity8, and unhealthy lifestyle9. Of these risk factors, medication with antipsychotic drugs can be considered partly modifiable, as reducing doses or switching prescriptions only leads to moderate improvement of metabolic risk factors10, 11. Insufficient treatment of somatic comorbidity and unhealthy lifestyle are potentially fully modifiable and, if they are properly targeted, life expectancy for patients with schizophrenia might improve.
Several clinical trials12, 13, 14 have reported an effect of lifestyle modification in this population, indicating that weight reduction and smoking cessation are possible. However, there are still gaps in the current knowledge. Selecting the optimal outcome for trials aiming to reduce cardiovascular risk remains a challenge: weight reduction or weight gain prevention is the most used outcome, but the correlation between weight loss and mortality remains questionable15. To overcome this, composite surrogate outcomes assessing the risk of cardiovascular disease have been proposed16. Moreover, since the pathogenesis of cardiovascular disease is multifactorial, strategies to reduce multiple, concurrent risk behaviours are needed17. Interventions with long‐term follow‐up are also warranted, since there are no reasons to believe that changes in metabolic risk factors occur faster in patients with severe mental disorders than the general population18. Equally important are follow‐ups after the intervention has ended, as the effect of lifestyle modification tends to vanish, and an intentional weight loss may be followed by an unhealthy weight gain in the majority of participants in behavioural trials19. Finally, it is crucial to evaluate the external validity of trials, which might be compromised by the recruitment of patients with a higher readiness to change and a lower degree of barriers to lifestyle modifications – such as cognitive impairment, anxiety or substance abuse – than the clinical population with severe mental illness as a whole. This can be minimized by pragmatic designs, with few exclusion criteria20.
The CHANGE trial was designed to address the above‐mentioned gaps. We conducted a randomized, pragmatic trial exploring if 12‐month lifestyle coaching plus care coordination plus treatment as usual, compared to care coordination plus treatment as usual and to treatment as usual alone, could reduce the 10‐year risk of cardiovascular disease in patients with schizophrenia spectrum disorders and abdominal obesity.
METHODS
Study design and participants
CHANGE was an investigator‐initiated, independently funded, randomized, parallel‐group, superiority, multi‐centre trial with blinded outcome assessment. Patients were recruited from well‐defined catchment areas in two major Danish cities (Aarhus and Copenhagen). The trial protocol was published in 2015 with no changes made to the original version21.
Patients were eligible if aged 18 or older, receiving a diagnosis of schizophrenia (F20), schizoaffective disorder (F25) or persistent delusional disorder (F22) according to ICD‐10 – as ascertained by the Schedules for Clinical Assessment in Neuropsychiatry (SCAN)22 – and having a waist circumference (measured between the iliac crest and the lowest rib) above 88 cm for women and 102 cm for men23.
Eligible patients were verbally informed by the usual carer and, if accepting, referred to CHANGE research staff by phone or e‐mail. An initial meeting was arranged at the research centre, the outpatient clinic, or patient's home. Verbal and written information on the trial was provided to all patients. Patients reporting current pregnancy or unable to provide informed consent were excluded. If the patient accepted participation in the trial, an informed consent form was signed and an appointment for collection of baseline data was made.
The Danish Ethical Committee (H‐4‐2012‐051) and the Danish Data Protection Agency (referral number 01689 RHP‐2012‐007) approved the trial.
Recruited patients were randomized with a 1:1:1 ratio to lifestyle coaching plus care coordination plus treatment as usual (CHANGE intervention), or care coordination plus treatment as usual, or treatment as usual alone. Randomization was stratified according to site (Copenhagen/Aarhus), gender, and a baseline high/low risk of cardiovascular disease. High risk was defined according to cut‐off points from a Danish population study24, using the Copenhagen risk score16 with age standardized to 60 years.
The randomization was centralized and carried out by the Copenhagen Trial Unit using a computerized sequence with alternating block sizes (9, 12 and 15) unknown to the investigators. After the inclusion of a patient in the trial, one of the lifestyle coaches (see below) contacted the Copenhagen Trial Unit with a unique patient identifier plus stratification variables and in return received the patient allocation. Outcome assessors, statisticians and all investigators involved in the trial were blinded to patient allocation, but patients and the health professionals providing the interventions were not.
Interventions
Lifestyle coaching
Lifestyle coaching was defined as affiliation to a CHANGE team member, offering a tailored, manual‐based intervention targeting physical inactivity, unhealthy dietary habits and smoking, and facilitating contact to the patient's general practitioner to secure medical treatment of somatic comorbidities. The theoretical framework of the lifestyle coaching was based on the theory of stages of change25, motivational interviewing26 and an assertive approach adapted from the assertive community treatment27. Motivational interviewing is a method to help patients elicit their own wishes to change; the assertive approach allows the staff to be respectfully active and persistent in follow‐up, and implement short message services, phone calls, home visits and meetings in the local area. These methods were incorporated into four manuals with detailed descriptions of the interventions addressing four tracks: care coordination, smoking cessation, healthy diet, and physical activity. Manuals are provided in the paper describing the trial protocol21.
The coach offered home visits with systematic exploration of possibilities for physical activity in daily life, which were realistic and attractive to the patient. Dietary changes involved concrete examination of the patient's dietary habits, food purchases and cooking practices, and identification of economically realistic, easy and attractive possibilities for change. During home visits, the coach took part in the activities (e.g., physical activity or food purchases), if requested by the patient, to support lifestyle changes. Personal and professional networks were included if possible in individual plans. The smoking cessation program was adapted from that published by the Danish Cancer Society28, and tailored to each patient in order to elicit and enhance motivation and maintain smoking cessation.
The patients were offered affiliation with the team member for one year, with at least one weekly personal meeting of variable duration, often one hour. Further support could be provided by text messages, phone calls and e‐mail messages. The coach to participant ratio was 1:15.
Each participant was encouraged to choose if focus should be on one or more of the four possible tracks, and the lifestyle coach supported the patient in setting individual goals. The staff had access to baseline results regarding cardiorespiratory fitness, forced expiratory volume, anthropometric measures and metabolic variables, and used these in their first consultation with each patient to plan the further course.
The lifestyle coaches performed written registration of all contacts with patients including cancellations. All coaching sessions were classified, according to the focus area of each consultation, into care coordination, smoking cessation, healthy diet or physical activity.
Lifestyle coaches were health professionals (occupational therapists, physiotherapists or dieticians) with clinical experience in psychiatry. They received a 5‐day course in motivational interviewing, a 5‐day course in smoking cessation, a 1‐day course in examination and treatment of lifestyle disorders, and a 2‐day course in healthy dieting, all based on the Danish Health Authority guidelines. During the trial, lifestyle coaches had weekly sessions with supervision to ensure program fidelity. In addition to the intervention described above, the patients in the CHANGE group were offered care coordination (see below) and continued treatment as usual.
Care coordination
Care coordination was incorporated in the CHANGE group and implemented as add‐on to treatment as usual in the care coordination group. The intervention was manual‐based. The care coordinator, a trained psychiatric nurse, facilitated contact to primary care in order to ensure that the patients received optimal treatment of physical health problems. Each care coordinator had 30‐40 participants assigned at a time. Affiliation to the care coordinator was offered for one year.
The care coordinators’ contact with patients comprised personal meetings, phone calls and text messages. The frequency of contact was adjusted according to the individual need. The first meeting with the patient consisted of a general health talk about physical well‐being and an evaluation of test results from the physical examination performed at baseline. Special attention was paid to symptoms of obstructive pulmonary disease, diabetes and cardiovascular disease. The care coordinator used a decision tree to plan the further course. In addition to the care coordination described above, the patients in this group continued treatment as usual.
Treatment as usual
All three groups of patients received treatment as usual for obese patients with schizophrenia. In Denmark all persons have a general practitioner and can consult her/him for free when needed. Patients in secondary mental health services stay affiliated with their general practitioner, who is responsible for treating abnormal results from the mandatory yearly screening of metabolic risk factors. No formalized extra effort was made regarding lifestyle counselling or treatment of physical disorders in the treatment as usual group. Results from the baseline assessment were available if requested by the patient or the usual carer and, if any of the results was a matter of urgent consideration, the CHANGE research team contacted staff at the psychiatric outpatient clinic.
Outcome assessments
The primary outcome was the 10‐year risk of cardiovascular disease, evaluated post‐treatment and standardized to age 60 years. We used the Copenhagen risk score, which is based on data from two large epidemiological studies in the Copenhagen area16 and is recommended by the European Society of Cardiology for screening of cardiovascular risk29. This composite measure incorporates non‐modifiable and modifiable factors. The non‐modifiable factors include: gender, family history of cardiovascular disease (defined as parents suffering from a fatal or non‐fatal cardiovascular event before the age of 55 years for fathers or 60 years for mothers), and prior heart disease (defined as myocardial infarction or verified atherosclerosis of coronary arteries). The modifiable factors include: smoking (defined as daily smoking, yes/no), diabetes mellitus (defined as either haemoglobin A1c >48 mmol/mol or receiving antiglycaemic drugs due to earlier confirmed diagnosis, yes/no), total cholesterol, high density lipoprotein (HDL) cholesterol, systolic blood pressure, and body mass index. Absolute risk was defined as the probability of a clinical event (ischaemic heart disease, myocardial infarction, stroke or death) happening to a person within 10 years. We calculated the risk for each patient, independent of age, as if age was 60, an approach recommended by the European Guidelines on Cardiovascular Disease Prevention in Clinical Practice29 to assess risk in young individuals.
The key secondary outcome was cardiorespiratory fitness (the patient's maximal oxygen uptake was measured using a bicycle cardiopulmonary exercise test). Further secondary outcomes included: forced expiratory volume (measured with Easy‐one® spirometer), waist circumference, systolic blood pressure (average of three values measured on the right upper arm in a sitting position after 10 minutes of rest, and before the bicycle test), resting heart rate, haemoglobin A1c, HDL and non‐HDL cholesterol, and self‐reported moderate and vigorous physical activity (using the Physical Activity Scale30).
The exploratory outcomes included: weight, body mass index, triglycerides, high sensitivity C‐reactive protein, self‐reported time spent sedentary30, daily smoking (using the Fagerström Test for Nicotine Dependence31), diet (using the Dietary Quality Score32), positive and negative symptoms (assessed using the Scale for the Assessment of Positive Symptoms33 and the Scale for the Assessment of Negative Symptoms34), cognition (assessed by the Brief Assessment of Cognition in Schizophrenia35), quality of life (evaluated by the Manchester Short Assessment of Quality of Life36 and the EuroQOL Five Dimensions Questionnaire37), psychosocial functioning (explored by the Global Assessment of Functioning38), perceived health39, and perceived stress40.
Statistical analysis
We expected the experimental interventions to reduce the Copenhagen risk score by 2.5% in the CHANGE group compared with the care coordination group, and by 2.5% in the care coordination group compared with the treatment as usual group. As we planned to compare all three groups, we reduced our alpha level to 0.05/3 = 0.0167. Allowing a power of 90%, we estimated to recruit 150 participants to each intervention group, a total of 450 participants. This calculation was based on a standard deviation of 5.9% of the Copenhagen risk score as found in the Inter99‐trial24.
The primary outcome analysis was an intention‐to‐treat one. Multiple imputation was used to handle missing data. The imputations were based on a linear regression model with 100 imputations and 20 iterations. As predictors in the imputation model, we selected variables from a predefined list (age, gender, Global Assessment of Functioning score, duration of illness, daily dose of antipsychotic medication in chlorpromazine equivalents, and research centre) if they were significant predictors of the outcome variable or predictors of dropout (p<0.05 in a univariable model). These variables were, together with the baseline value of the variable and the randomization group, used as predictors for all imputations, if they had less than 5% missing values. Predictor variables with missing values were then simultaneously imputed along with the outcome variables. For the primary outcome, the composite values were imputed.
Analysis of covariance (ANCOVA) was used to calculate any significant differences between the three intervention groups, using the baseline value of each measure and the three stratification variables (gender, research centre and baseline risk of cardiovascular disease) as covariates. All distributions were assessed for normality using visual inspection of histograms and Q‐Q plots. If not normally distributed, variables were log transformed, and if unsuccessful, a non‐parametric test was used. For dichotomous outcomes, we performed multiple logistic regressions with treatment as usual as reference and stratification variables as covariates after having imputed missing values using a logistic regression model.
All tests were two‐tailed. For the primary outcome, the p values were Bonferroni‐adjusted (alpha level=0.05/3=0.0167). We had several secondary and exploratory outcomes, and further Bonferroni correction would have been too conservative, as this approach demands an assumption of independency between outcomes, which was not reasonable in our study. Therefore, p values for secondary and exploratory outcomes are presented unadjusted, and interpreted as follows: no effect of the experimental intervention if p≥0.05; a possible positive effect if p<0.05 but >0.001; a strong indication of a positive effect if p<0.001.
Sensitivity analyses included an analysis of complete cases, removal of outliers (defined as standardized residuals greater than three standard deviations), a per‐protocol analysis defining participants not having a single contact as violating the protocol, and a second per‐protocol analysis including participants with at least 50% of intended personal meetings in the CHANGE group. This second per‐protocol analysis is likely to cause severe selection bias, as the CHANGE group would include the participants with the highest level of motivation. Therefore, it was only considered meaningful to report negative results from this analysis.
RESULTS
Figure 1 illustrates the flow of patients through the trial. Between December 2012 and May 2014, 428 participants were assigned to receive the CHANGE intervention (N=138), or care coordination plus treatment as usual (N=142), or treatment as usual alone (N=148). According to the protocol, we ought to include 450 participants, but had to stop before, due to lack of referrals.
Figure 1.
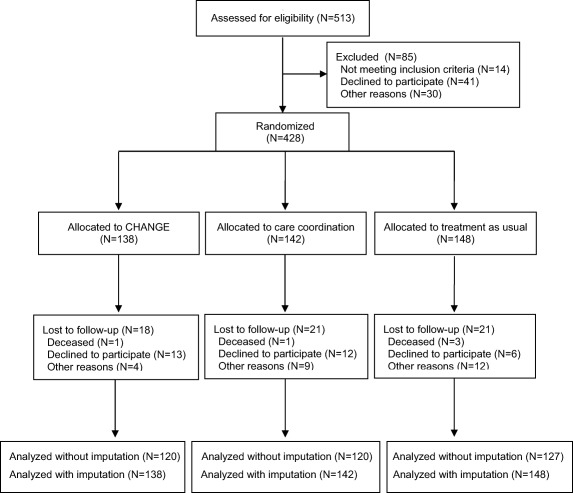
Flow diagram showing the process of recruiting and follow‐up
Retention proportion was 86.0% for the sample as a whole. There was no difference in the dropout rates among the three groups (p=0.68). 365 participants (85.3%) provided information enabling a calculation of the primary outcome at follow‐up. The dropouts did not differ from completers regarding baseline metabolic or psychometric characteristics or pattern of medication, except for a smaller proportion of the former receiving antidepressant treatment (30.0% vs. 46.0%).
Table 1 shows the baseline socio‐demographic and clinical characteristics of the patients. We included slightly more women, and the average age was 38.6 ± 12.4 years. Most patients were diagnosed with schizophrenia (88.0%). The majority were unemployed (92.0%), and a small proportion lived in supported housings (13.8%). There were 52.1% daily smokers and 15.0% had a diagnosis of diabetes. There were no differences between the intervention groups, apart from a higher proportion of participants living in supported housings (16.9% vs. 8.7%) and a smaller proportion having diabetes (9.5% vs. 18.6%) in the treatment as usual group compared with the CHANGE group.
Table 1.
Baseline socio‐demographic and clinical characteristics
| CHANGE (N =138) | CARE (N = 142) | TAU (N = 148) | Total (N=428) | |
|---|---|---|---|---|
| Age (years, mean ±SD) | 37.8 ± 12.6 | 39.5 ± 12.8 | 38.5 ± 11.8 | 38.6 ± 12.4 |
| Gender (female, %) | 55.1 | 57.7 | 54.7 | 56.1 |
| Work status (unemployed, %) | 86.9 | 95.0 | 94.6 | 92.0 |
| Living in supported housing (%) | 8.7 | 15.5 | 16.9 | 13.8 |
| Global Assessment of Functioning (mean±SD) | 44.5 ± 11.3 | 42.9 ± 9.8 | 43.7 ± 9.1 | 43.7 ± 7.5 |
| Risk of cardiovascular disease (high, %) | 5.8 | 7.0 | 5.9 | 6.3 |
| Waist circumference (cm, mean±SD) | 113.7 ± 15.8 | 115.3 ± 14.6 | 114.8 ± 14.2 | 114.6 ± 14.8 |
| Body mass index (mean±SD) | 34.1 ± 6.0 | 34.2 ± 5.9 | 34.2 ± 6.1 | 34.2 ± 6.0 |
| Systolic blood pressure (mm Hg, mean±SD) | 126.5 ± 12.8 | 128.0 ± 13.4 | 128.3 ± 16.0 | 127.6 ± 14.2 |
| HDL cholesterol (mmol/l, mean±SD) | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 |
| Non‐HDL cholesterol (mmol/l, mean±SD) | 3.8 ± 1.1 | 3.4 ± 1.2 | 3.8 ± 1.1 | 3.8 ± 1.1 |
| Haemoglobin A1c (mmol/mol, mean±SD) | 39.1 ± 8.7 | 38.3 ± 9.1 | 37.7 ± 9.5 | 38.3 ± 9.1 |
| Diabetes (%) | 18.6 | 17.0 | 9.5 | 15.0 |
| Hypercholesterolemia (>5 mmol/l, %) | 46.4 | 52.1 | 47.3 | 48.6 |
| Hypertension (>140 mm Hg, %) | 14.5 | 16.9 | 15.5 | 15.7 |
| Cardiorespiratory fitness (ml O2/kg/min, mean±SD) | 17.3 ± 4.6 | 17.4 ± 5.8 | 17.4 ± 6.1 | 17.4 ± 5.5 |
| Daily smoking (%) | 52.9 | 52.1 | 50.7 | 52.1 |
| Substance dependence (ICD‐10, %) | 5.8 | 2.8 | 3.4 | 4.0 |
| High alcohol consumption (%) | 8.0 | 8.5 | 4.1 | 6.8 |
| Schizophrenia (ICD‐10, %) | 90.6 | 91.5 | 83.1 | 88.0 |
| Duration of illness (years, mean±SD) | 17.2 ± 11.3 | 18.6 ± 11.0 | 16.7 ± 10.4 | 17.5 ± 10.9 |
| Antipsychotic daily dose in chlorpromazine equivalents (mg, mean±SD) | 453.4 ± 398.8 | 502.3 ± 389.5 | 464.7 ± 406.0 | 473.5 ± 397.9 |
| Antidepressant use (%) | 46.4 | 42.2 | 39.2 | 44.2 |
| Mood stabilizers use (%) | 8.7 | 13.4 | 9.5 | 10.5 |
| Positive symptoms (SAPS global score, mean±SD) | 2.2 ± 1.6 | 2.3 ± 1.6 | 2.0 ± 1.7 | 2.2 ± 1.6 |
| Negative symptoms (SANS global score, mean±SD) | 2.5 ± 1.1 | 2.6 ± 1.1 | 2.5 ± 1.3 | 2.6 ± 1.2 |
| Cognition (BACS composite score, mean±SD) | 231.3 ± 51.3 | 221.5 ± 45.5 | 222.7 ± 51.5 | 225.1 ± 49.6 |
CARE – care coordination, TAU – treatment as usual, HDL – high density lipoprotein, HbA1c – haemoglobin A1c, SAPS – Scale for the Assessment of Positive Symptoms, SANS – Scale for the Assessment of Negative Symptoms, BACS – Brief Assessment of Cognition in Schizophrenia
High alcohol consumption was defined as >14 weekly alcohol units for men and >7 for women
In the CHANGE group, the mean number of personal meetings was 24.6 ± 14.5; 60.0% of the participants attended 21 or more of the intended 42 personal meetings; 97.8% had at least one personal meeting with their coach. The 73 daily smokers allocated to the CHANGE group received a mean of 11.2 ± 9.3 sessions focusing on smoking cessation. For the group as a whole, there was a mean of 19.5 ± 13.1 meetings focused on physical activity, 6.3 ± 6.6 on care coordination and 15.8 ± 11.2 on healthy dieting.
Results for primary and secondary outcomes are shown in Table 2. The mean age‐standardized 10‐year risk of cardiovascular disease was 8.4 ± 6.7% in the CHANGE group, 8.5 ± 7.5% in the care coordination group, and 8.0 ± 6.5% in the treatment as usual group (F2,428=1.04, p=0.41).
Table 2.
Results for primary and secondary outcomes
| CHANGE | CARE | TAU | F | p | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| 10‐year risk of cardiovascular disease (%) | |||||
| Mean±SDa | 8.4 ± 6.7 | 8.5 ± 7.5 | 8.0 ± 6.5 | 1.04 | 0.41 |
| Adjusted mean±SEb | 8.3 ± 0.3 | 8.6 ± 0.3 | 8.1 ± 0.3 | ||
| Secondary outcomes | |||||
| Cardiorespiratory fitness (ml O2/min/Kg) | |||||
| Mean±SDa | 18.1 ± 5.5 | 18.0 ± 6.8 | 18.2 ± 6.7 | 0.86 | 0.54 |
| Adjusted mean±SEb | 18.1 ± 0.4 | 17.9 ± 0.4 | 18.3 ± 0.4 | ||
| Forced expiratory volume (l/sec) | |||||
| Mean±SDa | 3.1 ± 0.8 | 3.1 ± 0.8 | 3.0 ± 1.0 | 0.23 | 0.26 |
| Adjusted mean±SEb | 3.0 ± 0.04 | 3.1 ± 0.04 | 3.1 ± 0.04 | ||
| Waist circumference (cm) | |||||
| Mean±SDa | 113.9 ± 16.8 | 115.8 ± 16.3 | 115.0 ± 15.0 | 0.26 | 0.79 |
| Adjusted mean±SEb | 114.8 ± 0.7 | 115.1 ± 0.7 | 114.8 ± 0.6 | ||
| Systolic blood pressure (mm Hg)) | |||||
| Mean±SDa | 128.7 ± 13.9 | 127.6 ± 13.8 | 129.1 ± 14.1 | 1.12 | 0.39 |
| Adjusted mean±SEb | 129.3 ± 1.1 | 127.4 ± 1.0 | 128.7 ± 1.0 | ||
| Resting heart rate (beats/min) | |||||
| Mean±SDa | 86.4 ± 14.9 | 87.5 ± 15.5 | 86.0 ± 14.1 | 0.56 | 0.61 |
| Adjusted mean±SEb | 86.9 ± 1.0 | 86.9 ± 1.0 | 85.9 ± 1.0 | ||
| HbA1c (mmol/mol) | |||||
| Mean±SDa | 38.4 ± 9.7 | 38.7 ± 10.6 | 36.7 ± 6.9 | 3.65 | 0.07 |
| Adjusted mean±SEb | 37.8 ± 0.5 | 38.7 ± 0.5 | 37.2 ± 0.4 | ||
| HDL cholesterol (mmol/l) | |||||
| Mean±SDa | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.24 | 0.34 |
| Adjusted mean±SEb | 1.2 ± 0.02 | 1.2 ± 0.02 | 1.2 ± 0.02 | ||
| Non‐HDL cholesterol (mmol/l) | |||||
| Mean±SDa | 3.8 ± 1.1 | 3.9 ± 1.2 | 3.8 ± 1.1 | 0.29 | 0.77 |
| Adjusted mean±SEb | 3.8 ± 0.1 | 3.8 ± 0.1 | 3.8 ± 0.1 | ||
| Moderate‐vigorous physical activity (hours/week) | |||||
| Mean±SDa | 2.5 ± 4.0 | 3.1 ± 4.4 | 2.5 ± 4.0 | 0.99 | 0.43 |
| Adjusted mean±SEb | 2.6 ± 0.4 | 3.0 ± 0.4 | 2.4 ± 0.3 | ||
CARE – Care coordination, TAU – treatment as usual, HDL – high density lipoprotein, HbA1c – haemoglobin A1c
after multiple imputation
adjusted for gender, research center and baseline risk of cardiovascular disease
The sensitivity analyses of the primary outcome using complete cases, or removing outliers, did not change the results. When analyzing complete cases, we found that the mean age‐standardized 10‐year risk of cardiovascular disease was 8.5 ± 7.0% in the CHANGE group, 8.6 ± 7.8 in the care coordination group and 7.4 ± 5.3% in the treatment as usual group (p=0.46). After removing outliers, we found that it was 7.9 ± 5.2% in the CHANGE group, 7.6 ± 4.9% in the care coordination group and 7.1 ± 4.1% in the treatment as usual group (p=0.18). After removing CHANGE participants who had less than half of the intended 42 sessions, we found that the mean risk was 8.6 ± 7.7% in the CHANGE group, 8.6 ± 7.8% in the care coordination group and 7.4 ± 5.3% in the treatment as usual group (p=0.65). Equally, the per‐protocol analysis removing the three participants with no contact at all to the coach did not change the results.
There were no differences between the three groups for any of the secondary outcomes. The means for cardiorespiratory fitness, our key secondary outcome, were 18.1 ± 5.5 ml O2/min/Kg in the CHANGE group, 18.0 ± 6.8 ml O2/min/Kg in the care coordination group, and 18.2 ± 6.7 ml O2/min/Kg in the treatment as usual group (F2,428=0.86, p=0.54).
The analyses revealed no significant differences between the three groups on any exploratory outcomes (Table 3). For weight, the means were 103.1 ± 23.8 Kg in the CHANGE group, 103.7 ± 21.2 Kg in the care coordination group, and 102.9 ± 21.7 Kg in the treatment as usual group (F2,428=1.91, p=0.18). The proportion of daily smokers was 49.0% in the CHANGE group, 49.0% in the care coordination group, and 50.0% in the treatment as usual group (CHANGE group vs. treatment as usual group: p=0.65; care coordination group vs. treatment as usual group: p=0.79).
Table 3.
Results for exploratory outcomes
| CHANGE | CARE | TAU | F | p | |
|---|---|---|---|---|---|
| Weight (Kg) | |||||
| Mean±SDa | 103.1 ± 23.8 | 103.7 ± 21.2 | 102.9 ± 21.7 | 1.91 | 0.18 |
| Adjusted mean±SEb | 102.2 ± 0.7 | 103.8 ± 0.7 | 103.6 ± 0.7 | ||
| Body mass index | |||||
| Mean±SDa | 33.9 ± 5.9 | 34.5 ± 6.3 | 34.4 ± 6.3 | 1.88 | 0.19 |
| Adjusted mean±SEb | 33.9 ± 0.2 | 34.4 ± 0.2 | 34.4 ± 0.2 | ||
| Triglycerides (mmol/l) | |||||
| Mean±SDa | 2.0 ± 1.2 | 2.2 ± 1.5 | 2.2 ± 1.5 | 1.25 | 0.34 |
| Adjusted mean±SEb | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | ||
| Hs‐CRP (mg/l) | |||||
| Mean±SDa | 3.1 ± 2.7 | 3.4 ± 2.8 | 3.1 ± 2.9 | 0.73 | 0.59 |
| Adjusted mean±SEb | 3.2 ± 0.3 | 3.3 ± 0.3 | 3.1 ± 0.3 | ||
| Time spent sedentary (hours/day) | |||||
| Mean±SDa | 9.9 ± 3.6 | 10.5 ± 3.4 | 9.9 ± 3.5 | 1.23 | 0.36 |
| Adjusted mean±SEb | 10.1 ± 0.3 | 10.4 ± 0.3 | 9.9 ± 0.3 | ||
| Daily smoking (yes/no) | 0.65 (CHANGE vs. TAU); 0.79 (CARE vs. TAU) | ||||
| %a | 49.0 | 49.0 | 50.0 | ||
| % (adjusted)b | 49.0 | 49.0 | 50.0 | ||
| Intake of fruit (g/week) | |||||
| Mean±SDa | 393.1 ± 268.5 | 439.8 ± 270.7 | 421.4 ± 258.1 | 1.39 | 0.31 |
| Adjusted mean±SEb | 394.8 ± 20.0 | 428.6 ± 20.3 | 430.5 ± 20.0 | ||
| Intake of vegetables (g/week) | |||||
| Mean±SDa | 507.5 ± 338.8 | 475.7 ± 325.1 | 479.3 ± 307.7 | 1.25 | 0.34 |
| Adjusted mean±SEb | 518.2 ± 28.0 | 477.2 ± 27.3 | 467.9 ± 27.1 | ||
| Intake of fish (g/week) | |||||
| Mean±SDa | 138.1 ± 14.5 | 145.0 ± 13.9 | 140.8 ± 14.4 | 0.35 | 0.73 |
| Adjusted mean±SEb | 136.2 ± 12.3 | 144.9 ± 12.3 | 142.6 ± 12.2 | ||
| Intake of saturated fat (yes/no) | 0.08 (CHANGE vs. TAU); 0.33 (CARE vs. TAU) | ||||
| %a | 52.0 | 62.0 | 66.0 | ||
| % (adjusted)b | 55.0 | 59.0 | 65.0 | ||
| Positive symptoms (SAPS global score) | |||||
| Mean±SDa | 1.7 ± 1.6 | 1.7 ± 1.6 | 1.8 ± 1.6 | 1.44 | 0.29 |
| Adjusted mean±SEb | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.8 ± 0.1 | ||
| Negative symptoms (SANS global score) | |||||
| Mean±SDa | 2.1 ± 1.2 | 2.0 ± 1.2 | 2.0 ± 1.2 | 0.74 | 0.52 |
| Adjusted mean±SEb | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | ||
| Cognition (BACS composite score) | |||||
| Mean±SDa | 244.3 ± 50.1 | 235.8 ± 50.2 | 242.0 ± 49.5 | 2.54 | 0.12 |
| Adjusted mean±SEb | 238.8 ± 2.2 | 239.0 ± 2.2 | 244.1 ± 2.1 | ||
| Quality of life (MANSA score) | |||||
| Mean±SDa | 4.7 ± 0.8 | 4.7 ± 0.8 | 4.7 ± 0.8 | 0.74 | 0.52 |
| Adjusted mean±SEb | 4.7 ± 0.07 | 4.8 ± 0.07 | 4.7 ± 0.07 | ||
| Quality of life (EuroQOL score) | |||||
| Mean±SDa | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.3 ± 0.3 | 1.14 | 0.36 |
| Adjusted mean±SEb | 1.4 ± 0.03 | 1.4 ± 0.03 | 1.3 ± 0.03 | ||
| GAF total score | |||||
| Mean±SDa | 49.4 ± 11.2 | 47.6 ± 9.8 | 47.8 ± 9.4 | 1.19 | 0.35 |
| Adjusted mean±SEb | 49.0 ± 0.8 | 48.1 ± 0.8 | 47.6 ± 0.8 | ||
| Perceived health | |||||
| Mean±SDa | 2.8 ± 1.0 | 2.8 ± 0.9 | 2.7 ± 0.8 | 0.33 | 0.74 |
| Adjusted mean±SEb | 2.7 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 | ||
| Perceived stress | |||||
| Mean±SDa | 26.8 ± 7.8 | 27.0 ± 7.4 | 25.5 ± 7.4 | 1.68 | 0.26 |
| Adjusted mean±SEb | 27.1 ± 0.6 | 26.5 ± 0.6 | 25.7 ± 0.6 |
CARE – care coordination, TAU – treatment as usual, Hs‐CRP – high sensitivity C‐reactive protein, SAPS – Scale for the Assessment of Positive Symptoms, SANS – Scale for the Assessment of Negative Symptoms, BACS – Brief Assessment of Cognition in Schizophrenia, MANSA – Manchester Short Assessment of Quality of Life, GAF – Global Assessment of Functioning
after multiple imputation
adjusted for gender, research center and baseline risk of cardiovascular disease
For dichotomous outcomes, a mean difference in risk ratios was calculated using the risk ratio in the TAU group as reference
Five patients died during the trial. The distribution can be seen in the flow diagram (Figure 1). The causes of death were cancer (N=2), suicide (N=1), and unexplained (N=2). Psychiatric hospitalizations amounted to 18.8% in the CHANGE group, 33.8% in the care coordination group and 24.3% in the treatment as usual group; the difference between the care coordination and the CHANGE group was statistically significant (p=0.004). Somatic hospitalizations amounted to 12.3% in the CHANGE group, 17.6% in the care coordination group and 16.2% in the control group (p=0.40).
DISCUSSION
We hypothesized that a tailored, multi‐domain intervention, delivered by personal coaching in a community setting, would lead to a meaningfully reduced risk of cardiovascular disease in patients with schizophrenic spectrum disorders and abdominal obesity. However, the findings of this trial suggest that neither the CHANGE intervention nor care coordination were superior to standard treatment in reducing the 10‐year risk of cardiovascular disease.
CHANGE is the first trial, to our knowledge, to evaluate the effect of lifestyle interventions on a composite score estimating the risk of cardiovascular disease in patients with schizophrenic spectrum disorders. One U.S. study had explored the impact of care coordination in patients with severe mental illness, using a composite cardiovascular risk score, finding a significant effect41. Our negative results might be explained by better access to primary care in Denmark. Few of our participants had baseline values of lipids or blood pressure indicating a need for change in medication, according to the current guidelines for cardiovascular prevention42, and only two had haemoglobin A1c values above the cut‐off for diabetes without having being diagnosed and treated beforehand. This might be the result of a successful mandatory examination of blood lipids in the Danish Schizophrenia database, encouraging all clinicians across the three intervention groups to treat risk factors. Thus, the generalizability of results of care coordination might be limited to countries with similar health care systems. Also, we cannot exclude that selecting a subgroup with more severe somatic comorbidities might have changed our results in favour of care coordination or CHANGE intervention.
For our key secondary outcome, cardiorespiratory fitness, few studies have evaluated the effect of lifestyle interventions in patients with schizophrenia, but they reported promising findings43, 44, 45. Trials evaluating the effect of behavioural interventions in reducing metabolic risk factors have shown mixed results17. Weight reduction is the most used outcome46, 47, 48, 49, 50, 51, 52, 53, 54, 55 and the evidence is reported to be favourable17, although long‐term trials are missing18. Trials exploring the effect of behavioural interventions frequently use dyslipidaemia46, 47, 49, 52, haemoglobin A1c46, 56 and blood pressure46, 49, 52, 56, 57 as secondary outcomes, and the evidence is currently low or inadequate17. Thus, our results are not in line with previous trials regarding weight reduction and cardiorespiratory fitness, which might be explained by the clinical characteristics of our sample and the type of intervention.
The clinical characteristics of the sample we recruited reflect our inclusion and exclusion criteria. Our sample might differ from previous trials, as we aimed to optimize the external validity by having as few exclusion criteria as possible, being assertive in the process of recruitment, and offering an intervention without mandatory elements, in order to avoid exclusion of the severely ill (many trials exclude patients with somatic comorbidity, substance abuse or suicidal ideation) and volunteer bias.
The methods used to intervene reflect the chosen outcome variables. As cardiovascular disease is multifactorial, we thought that complex interventions should be the right approach. However, a majority of earlier trials have focused on single risk behaviours, such as diet or smoking or physical inactivity. Our intervention was heterogeneous, as every patient was free to choose the focus area for the intervention in dialogue with the coach. This might have limited our possibility to show an effect on single metabolic outcomes, thus reducing our power.
In spite of a high retention proportion (86.0%), the per‐protocol analysis showed that only 60.0% of patients randomized to the CHANGE group attended at least half of the intended weekly meetings, indicating that offering a higher frequency of sessions or a lower caseload would doubtfully have led to different results.
The CHANGE trial had several strengths. First, the design had central randomization; blinded outcome assessments, data management and data analysis; and independent funding. Second, we planned our sample size to avoid substantial type II errors. Third, we used a manual‐based, well‐described and evidence‐based theoretical framework. Fourth, we implemented a high‐intensity intervention, offering an assertive approach with at least weekly personal contact. Fifth, we had a multifaceted method, allowing the staff to work on all the known risk factors. Sixth, our composite outcome measure integrated the results even though they might be heterogeneous. Seventh, by comparing lifestyle coaching with care coordination, we were able to differentiate between the effect of lifestyle changes and that of sufficient monitoring and treatment of somatic comorbidities. Eighth, all contacts with patients were registered. Ninth, the intervention was developed to be sustainable, using low‐budget possibilities in the neighbourhood.
The ideal outcome measures for trials aiming to reduce mortality from cardiovascular disease are obviously hard ones like death. However, waiting for survival analyses is too time consuming and expensive for most studies, leaving surrogate outcomes as the second best choice. Currently there is no gold standard for surrogate outcomes in trials aiming to improve cardiovascular health, and the outcomes we chose for this trial have strengths and limitations. Strengths are that we used a composite score including several well‐known risk factors. The score consisted of both modifiable and non‐modifiable risk factors. This may be seen as a weakness, since it means that an intervention could affect all the modifiable risk factors, yet not affect the composite outcome measure. This was not an issue in the CHANGE trial, as there were no indications of significant reductions even in the separate modifiable risk factors. Conversely, we view our choice of primary outcome measure as a strength, as constructing a risk score without non‐modifiable risk factors would not yield an accurate estimate of risk. A weakness, though, is the lack of validation of the surrogate measure in a population with schizophrenia. In fact, research published after the initiation of this trial has questioned the generalizability of cardiovascular risk scores to people with severe mental illness58.
As we did not succeed in recruiting the planned number of participants (we recruited 428 patients, while 450 were expected), we cannot exclude a risk of being underpowered, increasing the risk for type II errors. However, we find it unlikely that including 22 further participants would have changed our results substantially, and we still have a power of 87.2% regarding our primary outcome, which seems an acceptable one compared to most trials.
The lack of effect on individual risk behaviours should be interpreted with caution, due to insufficient power. Furthermore, existing tools measuring lifestyle changes have not been validated in a population with schizophrenia, where cognitive impairment and psychotic symptoms might compromise the validity. As self‐reporting might be subject to both recall problems (introducing random errors and thus increasing the risk of type II errors) and social desirability bias (leading to systematic errors), more direct measurements like actigraphs would have been preferable, but they were not considered in this study due to logistic reasons.
In conclusion, the CHANGE trial provides evidence that a manual‐based individual lifestyle coaching intervention does not reduce the 10‐year risk of cardiovascular disease, compared with treatment as usual, in patients with schizophrenia spectrum disorders and abdominal obesity. Offering lifestyle interventions to this group might seem like a moral imperative, but, seen in the light of the lack of beneficial results and moderate compliance with weekly meetings with the coaches, it is just as imperative to ask whether this is the right approach to improve life for patients with schizophrenia. The general population, and even more, a vulnerable population like this one, is facing major barriers to making healthy choices and powerful pressures to select the unhealthy. We suggest that future research should focus on environmental/structural changes rather than individually anchored health interventions, taking into account the special needs of patients with schizophrenia.
ACKNOWLEDGEMENTS
Funding for this trial was provided by Mental Health Services of the Capital Region of Denmark, the Tryg Foundation, the Lundbeck Foundation, the Dæhnfeldts Foundation, and the Danish Ministry of Health. The authors would like to thank K. Sandberg, H. Lublin, T. Madsen, S. Drivsholm and A. Moltke for participating in the planning of the trial, H.J. Larsen for help with the data collection and organization, and P. Hougaard for statistical advice. H. Speyer and H.C.B. Norgaard contributed equally to this work.
REFERENCES
- 1. Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol 2014;10:425‐48. [DOI] [PubMed] [Google Scholar]
- 2. Chesney E, Goodwin GM, Fazel S. Risks of all‐cause and suicide mortality in mental disorders: a meta‐review. World Psychiatry 2014;13:153‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ 2013;346:f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nordentoft M, Wahlbeck K, Hällgren J et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS One 2013;8:e55176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andreassen OA, Djurovic S, Thompson WK et al. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular‐disease risk factors. Am J Hum Genet 2013;92:197‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daumit GL, Goff DC, Meyer JM et al. Antipsychotic effects on estimated 10‐year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res 2008;105:175‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Correll CU, Detraux J, De Lepeleire J et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015;14:119‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laursen TM, Nordentoft M. Heart disease treatment and mortality in schizophrenia and bipolar disorder − changes in the Danish population between 1994 and 2006. J Psychiatr Res 2011;45:29‐35. [DOI] [PubMed] [Google Scholar]
- 9. McCreadie RG. Diet, smoking and cardiovascular risk in people with schizophrenia. Br J Psychiatry 2003;183:534‐9. [DOI] [PubMed] [Google Scholar]
- 10. Bak M, Fransen A, Janssen J et al. Almost all antipsychotics result in weight gain: a meta‐analysis. PLoS One 2014;9:10‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Correll CU, Joffe BI, Rosen LM et al. Cardiovascular and cerebrovascular risk factors and events associated with second‐generation antipsychotic compared to antidepressant use in a non‐elderly adult sample: results from a claims‐based inception cohort study. World Psychiatry 2015;14:56‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daumit GL, Dickerson FB, Wang N‐Y et al. A behavioral weight‐loss intervention in persons with serious mental illness. N Engl J Med 2013;368:1594‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartels SJ, Pratt SI, Aschbrenner KA et al. Clinically significant improved fitness and weight loss among overweight persons with serious mental illness. Psychiatr Serv 2013;64:729‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green CA, Yarborough BJH, Leo MC et al. The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. Am J Psychiatry 2015;172:71‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ross R, Blair S, de Lannoy L et al. Changing the endpoints for determining effective obesity management. Prog Cardiovasc Dis 2015;57:330‐6. [DOI] [PubMed] [Google Scholar]
- 16. Thomsen TF, Davidsen M, Ibsen H et al. A new method for CHD prediction and prevention based on regional risk scores and randomized clinical trials; PRECARD(R) and the Copenhagen Risk Score. Eur J Cardiovasc Prev Rehabil 2001;8:291‐7. [DOI] [PubMed] [Google Scholar]
- 17. McGinty EE, Baller J, Azrin ST et al. Interventions to address medical conditions and health‐risk behaviors among persons with serious mental illness: a comprehensive review. Schizophr Bull 2016;42:96‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruins J, Jörg F, Bruggeman R et al. The effects of lifestyle interventions on (long‐term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: a meta‐analysis. PLoS One 2014;9:e112276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vink RG, Roumans NJT, Arkenbosch LAJ et al. The effect of rate of weight loss on long‐term weight regain in adults with overweight and obesity. Obesity 2016;24:321‐7. [DOI] [PubMed] [Google Scholar]
- 20. Bartels SJ, Pratt SI, Aschbrenner KA et al. Pragmatic replication trial of health promotion coaching for obesity in serious mental illness and maintenance of outcomes. Am J Psychiatry 2015;172:344‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Speyer H, Norgaard HCB, Hjorthoj C et al. Protocol for CHANGE: a randomized clinical trial assessing lifestyle coaching plus care coordination versus care coordination alone versus treatment as usual to reduce risks of cardiovascular disease in adults with schizophrenia and abdominal obesity. BMC Psychiatry 2015;15:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wing JK, Sartorius N, Ustun TB. Diagnosis and clinical measurement in psychiatry: a reference manual for SCAN. Cambridge: Cambridge University Press, 1998. [Google Scholar]
- 23. World Health Organization . Waist circumference and waist‐hip ratio: report of a WHO expert consultation. Geneva: World Health Organization, 2008. [Google Scholar]
- 24. Jørgensen T, Borch‐Johnsen K, Thomsen TF et al. A randomized non‐pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. Eur J Cardiovasc Prev Rehabil 2003;10:377‐86. [DOI] [PubMed] [Google Scholar]
- 25. Prochaska JO, DiClemente CC. Stages of change in the modification of problem behaviors. Prog Behav Modif 1992;28:183‐218. [PubMed] [Google Scholar]
- 26. Miller WR, Rollnick S. The effectiveness and ineffectiveness of complex behavioral interventions: impact of treatment fidelity. Contemp Clin Trials 2014;37:234‐41. [DOI] [PubMed] [Google Scholar]
- 27. Stein LI, Test MA. Alternative to mental hospital treatment. I. Conceptual model, treatment program, and clinical evaluation. Arch Gen Psychiatry 1980;37:392‐7. [DOI] [PubMed] [Google Scholar]
- 28. Danish Cancer Society . Manual til Rygeafvænning Gruppe. www.cancer.dk.
- 29. De Backer G, Ambrosioni E, Borch‐Johnsen K et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003;24:1601‐10. [DOI] [PubMed] [Google Scholar]
- 30. Andersen LG, Groenvold M, Jørgensen T et al. Construct validity of a revised Physical Activity Scale and testing by cognitive interviewing. Scand J Public Health 2010;38:707‐14. [DOI] [PubMed] [Google Scholar]
- 31. Heatherton TF, Kozlowski LT, Frecker RC et al. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Addiction 1991;86:1119‐27. [DOI] [PubMed] [Google Scholar]
- 32. Toft U, Kristoffersen LH, Lau C et al. The Dietary Quality Score: validation and association with cardiovascular risk factors: the Inter99 study. Eur J Clin Nutr 2007;61:270‐8. [DOI] [PubMed] [Google Scholar]
- 33. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa, 1984. [Google Scholar]
- 34. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa, 1984. [Google Scholar]
- 35. Keefe RSE, Goldberg TE, Harvey PD et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004;68:283‐97. [DOI] [PubMed] [Google Scholar]
- 36. Björkman T, Svensson B. Quality of life in people with severe mental illness. Reliability and validity of the Manchester Short Assessment of Quality of Life (MANSA). Nord J Psychiatry 2005;59:302‐6. [DOI] [PubMed] [Google Scholar]
- 37. Luo N, Johnson JA, Shaw JW et al. Self‐reported health status of the general adult U.S. population as assessed by the EQ‐5D and Health Utilities Index. Med Care 2005;43:1078‐86. [DOI] [PubMed] [Google Scholar]
- 38. Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the Global Assessment of Functioning ‐ Split version. Compr Psychiatry 2007;48:88‐94. [DOI] [PubMed] [Google Scholar]
- 39. Mossey JM, Shapiro E. Self‐rated health: a predictor of mortality among the elderly. Am J Publ Health 1982;72:800‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385‐96. [PubMed] [Google Scholar]
- 41. Druss BG, Zhao L, von Esenwein SA et al. The Health and Recovery Peer (HARP) Program: a peer‐led intervention to improve medical self‐management for persons with serious mental illness. Schizophr Res 2010;118:264‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saidj M, Jørgensen T, Prescott E et al. Poor predictive ability of the risk chart SCORE in a Danish population. Dan Med J 2013;60:A4609. [PubMed] [Google Scholar]
- 43. Scheewe TW, Backx FJG, Takken T et al. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatr Scand 2013;127:464‐73. [DOI] [PubMed] [Google Scholar]
- 44. Kimhy D, Vakhrusheva J, Bartels MN et al. The impact of aerobic exercise on brain‐derived neurotrophic factor and neurocognition in individuals with schizophrenia: a single‐blind, randomized clinical trial. Schizophr Bull 2015;41:859‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pajonk F, Wobrock T. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry 2010;67:133‐43. [DOI] [PubMed] [Google Scholar]
- 46. McKibbin CL, Patterson TL, Norman G et al. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial. Schizophr Res 2006;86:36‐44. [DOI] [PubMed] [Google Scholar]
- 47. Wu M‐K, Wang C‐K, Bai Y‐M et al. Outcomes of obese, clozapine‐treated inpatients with schizophrenia placed on a six‐month diet and physical activity program. Psychiatr Serv 2007;58:544‐50. [DOI] [PubMed] [Google Scholar]
- 48. Alvarez‐Jimenez M, Martinez‐Garcia O, Perez‐Iglesias R et al. Prevention of antipsychotic‐induced weight gain with early behavioural intervention in first‐episode psychosis: 2‐year results of a randomized controlled trial. Schizophr Res 2010;116:16‐9. [DOI] [PubMed] [Google Scholar]
- 49. Cordes J, Thunker J, Regenbrecht G et al. Can an early weight management program (WMP) prevent olanzapine (OLZ)‐induced disturbances in body weight, blood glucose and lipid metabolism? Twenty‐four‐ and 48‐week results from a 6‐month randomized trial. World J Biol Psychiatry 2014;15:229‐41. [DOI] [PubMed] [Google Scholar]
- 50. Methapatara W, Srisurapanont M. Pedometer walking plus motivational interviewing program for Thai schizophrenic patients with obesity or overweight: a 12‐week, randomized, controlled trial. Psychiatry Clin Neurosci 2011;65:374‐80. [DOI] [PubMed] [Google Scholar]
- 51. Lovell K, Wearden A, Bradshaw T et al. An exploratory randomized controlled study of a healthy living intervention in early intervention services for psychosis: the INTERvention to encourage ACTivity, improve diet, and reduce weight gain (INTERACT) study. J Clin Psychiatry 2014;75:498‐505. [DOI] [PubMed] [Google Scholar]
- 52. Attux C, Martini LC, Elkis H et al. A 6‐month randomized controlled trial to test the efficacy of a lifestyle intervention for weight gain management in schizophrenia. BMC Psychiatry 2013;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brar JS, Ganguli R, Pandina G et al. Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2005;66:205‐12. [DOI] [PubMed] [Google Scholar]
- 54. Littrell KH, Hilligoss NM, Kirshner CD et al. The effects of an educational intervention on antipsychotic‐induced weight gain. J Nurs Scholarsh 2003;35:237‐41. [DOI] [PubMed] [Google Scholar]
- 55. Wu MH, Lee CP, Hsu SC et al. Effectiveness of high‐intensity interval training on the mental and physical health of people with chronic schizophrenia. Neuropsychiatr Dis Treat 2015;11:1255‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Forsberg KA, Björkman T, Sandman PO et al. Physical health – a cluster randomized controlled lifestyle intervention among persons with a psychiatric disability and their staff. Nord J Psychiatry 2008;62:486‐95. [DOI] [PubMed] [Google Scholar]
- 57. Scheewe TW, Backx FJG, Takken T et al. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatr Scand 2013;127:464‐73. [DOI] [PubMed] [Google Scholar]
- 58. Osbron DPJ, Hardoon S, Omar RZ et al. Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry 2015;72:143‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]


