Abstract
Neurons are especially susceptible to oxidative damage, which is increasingly implicated in neurodegenerative disease. Certain N-acylethanolamines (NAEs) have been shown to protect neurons from oxidative stress. Since glaucoma may be considered a neurodegenerative disorder and the survival of retinal neurons could also be influenced by N-acylethanolamines, our goal was to quantify changes in certain N-acylethanolamine species and their oxylipin derivatives in the retina of a mouse model for glaucoma. We also sought to identify relationships between these and parameters of glaucoma disease development, specifically intraocular pressure, visual acuity, and contrast sensitivity. Five N-acylethanolamine species and three NAE oxylipin derivatives were quantified in retina from young and aged DBA/2Crl mice. N-Acylethanolamines and NAE-oxylipins in retinal extracts were quantified against deuterated standards by isotope dilution gas chromatography–mass spectrometry. Levels (nmol/g dry weight) of N-arachidonoylethanolamine (anandamide; NAE 20:4) were significantly (p = 0.008) decreased in aged (2.875 ± 0.6702) compared to young animals (5.175 ± 0.971). Conversely, the anandamide oxylipin, 15(S)-HETE ethanolamide (15(S)-HETE EA), was significantly (p = 0.042) increased in aged (0.063 ± 0.009) compared to young animals (0.039 ± 0.011). Enzymatic depletion of the anandamide pool by 15-lipoxygenase and consequent accumulation of 15(S)-HETE ethanolamine may contribute to decreased visual function in glaucomatous mice. Since N-acylethanolamines effectively attenuate glaucoma pathogenesis and associated visual impairment, our data provides additional rationale and novel targets for glaucoma therapies.
Keywords: Acylethanolamides, Anandamide, Contrast sensitivity, DBA/2, Intraocular pressure, Lipoxin, Lipoxygenase, Neuroprotection, N-Linoleoylethanolamine, N-Oleylethanolamine, Optokinetic reflex, Optomotor, Visual acuity
Introduction
Neurons are particularly susceptible to oxidative damage due to their high rate of O2 utilization and ATP synthesis, higher lipid concentration and higher levels of lipid peroxidation, and higher levels of oxidation stress resulting from neuro-transmitter signaling [1]. Therefore, it is not surprising that oxidative damage is increasingly implicated in age-related [2], neurodegenerative diseases such as Parkinson disease, Alzheimer disease and amyotrophic lateral sclerosis [3], as well as ocular diseases such as cataract, diabetic retinopathy, and age-related macular degeneration [4]. Glaucoma is a common, complex optic neuropathy associated with degeneration of retinal ganglion cells resulting in structural damage to the optic nerve and the vision loss caused by associated pathological processes [5]. Oxidative stress may contribute to glaucoma pathogenesis not only by increasing IOP through alteration of aqueous outflow via trabecular meshwork degeneration [6], but also by means of apoptosis induction in retinal cells [7] and stimulation of the immune response in the retina [8]. Patients with certain types of glaucoma exhibit decreased antioxidant mechanisms (such as reduced glutathione) as well as increased oxidative DNA damage, protein carbonyl levels, and lipid peroxidation [9].
N-Acylethanolamines (NAEs) are lipid-derived signaling molecules that can be produced in most mammalian cells from the precursor N-acylphosphatidylethanolamine components of the cell membrane via a transacylase-phosphodiesterase pathway. They are synthesized in an on-demand fashion in response to stress or injury. Significant accumulations of NAEs occur under conditions such as necrosis, ischemia, excitotoxicity, and inflammation (reviewed in [10]). N-arachidonoylethanolamine, and other polyunsaturated NAEs interact with cannabinoid receptors 1 and 2 [11] as well as transient receptor potential (TRP) channels (reviewed in [12]). The retina is rich in long-chain polyunsaturated fatty acid (LCPUFA) precursors of NAEs, which are not only highly susceptible to chemical peroxidation, but are also oxygenated by cyclooxygenases, lipoxygenases, and cytochrome P450s. N-Acylethanolamine derivatives of LCPUFAs such as N-linoleoylethanolamine (NAE 18:2) and N-arachidonoylethanolamine (NAE 20:4, anandamide) as well as the saturated NAE, N-palmitoylethanolamine (NAE 16:0), have been shown to protect neurons from glutamate excitotoxicity, in experimental models of retinal ganglion cell layer neuron damage in vivo against glutamate excitotoxicity modeling some aspects of human glaucoma pathology [13, 14], and a rodent in vivo model of stroke [13, 15], and from oxidative stress in an in vitro model of neurodegeneration [16]. Furthermore, changes in N-acylethanolamine levels and metabolites related to N-acylethanolamine signaling have been determined in models of disease and neuronal injury [17], in transgenic models that underwent changes in N-acylethanolamine signaling and metabolism [18], as well as after chronic dosing with N-acylethanolamines [19] or modulators of N-acylethanolamine signaling [20, 21] in pharmacological applications. Since N-acylethanolamine derivatives of LCPUFAs are also susceptible to chemical and enzymatic oxygenation [22, 23], we examined levels of selected N-acylethanolamines and their oxylipin derivatives upon development of visual impairment in the DBA/2 mouse model of glaucoma [24].
Materials and Methods
Animals
Six week old and (approximately) 8.5 month old male DBA/2Crl mice were obtained from Charles Rivers Laboratories (Wilmington, MA). Mice were acclimated to the institutional animal facility for 10 days prior to testing with ad libitum access to food and water and maintained on a 12-h light/dark cycle. All experimental animal procedures were approved by the institutional animal care and use committee and performed in accordance with institutional and federal guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Behavioral Analyses
Intraocular pressure was measured by rebound tonometry without sedation using a Tono-Lab (Tiolat, OY, Helsinki, Finland). Quantification of visual function using measures previously established in this model [25] was performed using an Optomotry™ system (Cerebral Mechanics, Lethbridge, AB). Visual acuity is reported as the maximum spatial frequency that elicits an optomotor response. Acuity testing was performed at 100 % contrast beginning at a low spatial frequency [0.042 cycles/degree (c/d)] with a grating rotating at 12°/s. Contrast sensitivity is reported as the reciprocal of the lowest contrast which elicits an optomotor response. Contrast sensitivity testing was performed by decreasing the contrast while maintaining the spatial frequency at 0.042 c/d. Both tests utilized a random staircase method with the observer blind to the spatial frequency or contrast level.
Extraction and Quantification of NAEs
Tissues were ground in boiling isopropanol (70 °C) using a bead beater. Deuterated NAE standards (100 ng of D4-NAE 16:0 and D4-NAE 20:4; Cayman Chemicals, MI) were added to the tissues before lipid extraction. Total lipids were extracted into chloroform and subsequently purified by solid phase extraction (SPE). Silica SPE cartridges (100 mg; Grace Davison Discovery Sciences) were conditioned with 2 ml methanol and subsequently with 4 ml chloroform. Columns were then loaded with samples suspended in 1 ml chloroform. Columns were washed with 2 ml chloroform and NAEs were eluted in 2 ml of ethyl acetate:acetone (1:1 v/v). The eluate was dried under nitrogen stream prior to derivatization with 50 μl N,O-Bis(trimethylsilyl) trifluoroacetamide (BSTFA; Fisher Scientific, PA) at 55 °C for 30 min [19]. NAEs were quantified as TMS derivatives against deuterated NAE 16:0 and NAE 20:4. Quantification and confirming ions are shown in supplementary Table 1 and coincident chromatographic characteristics of selected confirming and/or quantitative ions for each species are shown in supplementary Fig. 1. The quantification was carried out in SIM (Single Ion Monitoring) mode on an Agilent GC 7890A/MSD 5975C system with a capillary HP-5 MS column (30 × 0.250 mm, 0.25 μm coating thickness; Agilent technologies) as previously described [26]. NAE levels are reported as nanomoles per gram of dry retina.
Extraction and Separation of NAE-oxylipins
NAE-oxylipins were extracted as described previously [27] with some modifications. Tissues were ground in liquid nitrogen and suspended in 4 ml of n-hexane/isopropyl alcohol (3:2 with 0.0025 % w/v 2-butyl-6-hydroxytoluene). Deuterated standards (100 ng of D4-9-HOD and D8-15-HETE) were added prior to extraction. NAE-oxylipins were extracted in hexane-rich phase and combined with 2 ml of 6.6 % potassium sulfate solution. Sodium tetrahydroborate (approximately 5 mg) was added to reduce hydroperoxides to hydroxides. After centrifugation at 4500×g for 10 min, the hexane-rich phase was collected and dried under nitrogen stream. The extract was resuspended in 100 μl of methanol/water (80:20 v/v) for separation by RP-HPLC. NAE-oxylipins were separated as described previously [27] with some modifications. The extracts were separated by RP-HPLC carried out on 150 × 4.6 mm, 5 μm particle size, C18 Nucleosil 120-5 column (Macherey–Nagel, PA) with a binary gradient system (solvent A: methanol/water/acetic acid (80:20:0.1, v/v/v), solvent B: methanol/acetic acid (100:0.1, v/v)). The eluate was collected and dried under nitrogen stream, and derivatized with 50 μl BSTFA (Fisher Scientific, PA) for GC/MS analysis.
Quantification of NAE-oxylipins
NAE-oxylipins were quantified as TMS-derivatives against deuterated standards and are reported in nanomoles per gram dry retina. The quantification was carried out in SIM mode on an Agilent GC 7890A/MSD 5975C system with a capillary HP-5 MS column (30 m × 0.250 mm, 0.25 μm coating thickness; Agilent technologies). Quantification and confirming ions are shown in supplementary Table 1 and coincident chromatographic characteristics of selected confirming and/or quantitative ions for each species are shown in supplementary Fig. 1. Chiral-phase HPLC of selected samples confirmed the enantiomeric nature of NAE oxylipins, as described previously [28].
Statistics
Data are expressed as mean ± standard deviation of the mean. Student’s t test was used to determine statistical significance. p values > 0.05 were not considered statistically significant, 0.05–0.01 were considered significant (*), 0.01–0.001 were considered very significant (**), and < 0.001 were considered extremely significant (***). The Pearson product-moment correlation coefficient (r) was calculated to evaluate relationship strengths. Correlations were identified as strong for r values > 0.6, moderate for r values of 0.4–0.6, weak for r values of 0.2–0.4, and no correlation for r values < 0.2. Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA).
Results
Retinal N-arachidonoylethanolamine levels significantly decrease and 15(S)-HETE EA levels significantly increase in aged DBA/2Crl mice
Selected N-acylethanolamines and their oxylipin derivatives were extracted and quantified from the pooled retinas of 7-week-old (young) and approximately 8.5-month-old (aged) DBA/2Crl mice (Table 1). For NAE quantification, 4 pools of 2 retinas were analyzed per group. For NAE oxylipins, three pools of three retinas per group were used. A significant (p = 0.0080) decrease in NAE 20:4 was observed in aged retinas. 15(S)-HETE EA levels were significantly (p = 0.0420) increased in aged retinas.
Table 1.
Levels of selected N-acylethanolamines and their oxylipin derivatives in DBA/2Crl retinas
| Lipid | Lipid | NAE level (nmol/g dry weight)a
|
t test | |
|---|---|---|---|---|
| Species | Abbreviation | Young | Aged | p value |
| Palmitoylethanolamine (N-(2-Hydroxyethyl)-hexadecanamide) | NAE 16:0 | 134.9 ± 28.4 | 106.0 ± 23.4 | 0.1673 |
| Stearoylethanolamine (N-(Octadecanoyl)-ethanolamine) | NAE 18:0 | 15.5 ± 4.3 | 11.7 ± 1.9 | 0.1599 |
| N-Oleoylethanolamine (N-(9Z-Octadecenoyl)-ethanolamine) | NAE 18:1 | 6.0 ± 1.4 | 6.3 ± 1.3 | 0.7570 |
| N-Linoleoylethanolamine (N-(2-Hydroxyethyl)-9Z,12Z-octadecadienamide) | NAE 18:2 | 3.1 ± 0.5 | 2.5 ± 0.5 | 0.1578 |
| N-Arachidonoylethanolamine (N-(2-Hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide) | NAE 20:4 | 5.2 ± 1.0 | 2.9 ± 0.7 | 0.0080 |
| (9S,12Z,10E)-9-Hydroxy-10,12 Octadecadienoylethanolamine | 9NAE-HOD | 0.018 ± 0.003 | 0.020 ± 0.003 | 0.4353 |
| (13S,9Z,11E)-13-Hydroxy-9,11-Octadecadienoylethanolamine | 13-NAE-HOD | 0.021 ± 0.006 | 0.021 ± 0.011 | 0.9643 |
| 15(S)-Hydroxy-N-(2-hydroxyethyl)-5Z,8Z,11Z,13E-Eicosatetraenamide | 15(S)-HETE EA | 0.039 ± 0.011 | 0.063 ± 0.009 | 0.0420 |
Data are expressed as mean ± SD
Intraocular Pressure Increases and Visual Function Decreases in Aged DBA/2Crl Mice
To determine if NAE and NAE oxylipin contents in the DBA/2Crl mouse retina were correlated with measures of visual function indicating progression of the glaucoma disease state, intraocular pressure (IOP), visual acuity (VA) and contrast sensitivity (CS) were measured in two experimental cohorts of 7-week-old and ~9 month old mice termed young and aged, respectively.
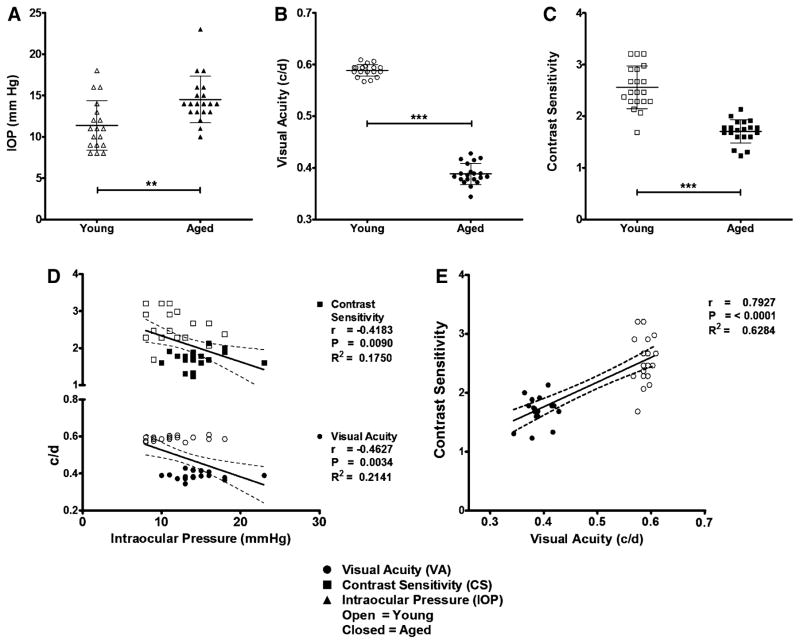
Aged eyes had IOPs of 15 ± 0.63 mmHg (n = 20), which is significantly higher (p = 0.0022) than in young eyes whose IOPs were 11 ± 0.71 mmHg (n = 18) (Fig. 1a). Visual acuity in aged eyes was measured at 0.388 ± 0.005 cycles/degree (c/d; n = 20), which was significantly lower (p < 0.0001) than in young eyes, where VA was 0.589 ± 0.003 c/d (n = 20) (Fig. 1b). Contrast sensitivity of aged eyes was significantly (p < 0.0001) reduced at 1.71 ± 0.05 (n = 20) when compared to young eyes, whose CS was 2.56 ± 0.09 (n = 20) (Fig. 1c). Intraocular pressures showed statistically significant, moderate negative correlations with VA and CS measurements (p = 0.0034, 0.0090 and r = −0.4627, −0.4183, respectively) (Fig. 1d). Contrast sensitivity demonstrated a statistically significant (p ≤ 0.0001), strong, positive correlation (r = 0.7927) with VA (Fig. 1e).
Fig. 1.
Intraocular pressure increases and visual function decreases in aged DBA/2Crl mice. a Aged (~9 m; n = 18 eyes) DBA/2Crl mice demonstrate significantly (P = 0.0022) elevated intraocular pressure (IOP) compared to young (7 w; n = 20 eyes) controls. b, c Aged mice have a significant (n = 20 eyes; p < 0.0001) deficit in visual acuity (VA) and contrast sensitivity (CS) compared to young controls. d IOP correlates moderately with visual function. e CS and VA correlate strongly with each other. Data are expressed as mean ± SD. IOP measurements are symbolized with triangles, VA with circles, and CS with squares. Open symbols represent young eyes and closed symbols aged eyes. Dashed lines represent the 95 % confidence interval
Decreased 16:0, 18:0, 18:2, and 20:4 N-acylethanolamine Levels in the Retina Correlate with an Increase in Intraocular Pressure and Impaired Visual Function in Aged DBA/2Crl Mice
A Pearson product-moment correlation coefficient calculation was performed to examine relationship strengths between NAE levels in the young and aged DBA/2Crl mouse retina and functional disease parameters of glaucoma.
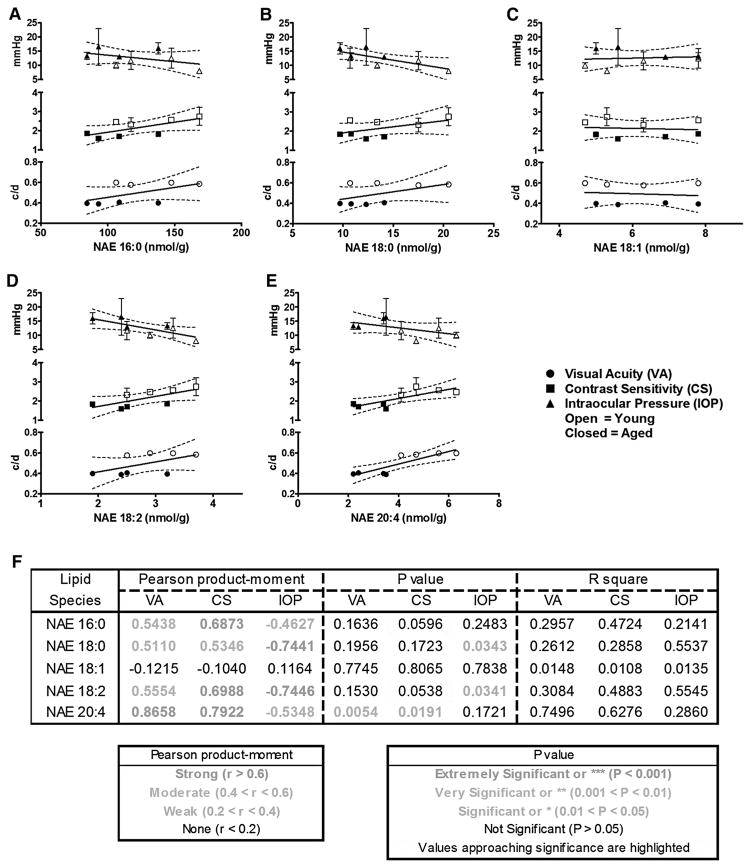
NAE 16:0 levels demonstrated moderate correlations with VA (r = 0.5438) and IOP (r = −0.4627) that were not statistically significant (p = 0.1636 and 0.2483, respectively). However, a strong, positive, correlation (r = 0.6873), approaching statistical significance (p = 0.0596) between NAE 16:0 content and CS, was observed (Fig. 2a).
Fig. 2.
Increased 16:0, 18:0, 18:2, and 20:4 N-acylethanolamine levels in the DBA/2Crl retina correlate with lower intraocular pressure and better visual function. a NAE 16:0 levels correlate moderately with visual acuity (VA) and intraocular pressure (IOP), but not significantly. There is a strong correlation that approaches statistical significance between NAE 16:0 and contrast sensitivity (CS). b NAE 18:0 levels correlate moderately with VA and CS, but not significantly. There is a strong, statistically significant correlation between NAE 18:0 and IOP. c NAE 18:1 levels do not correlate with VA, CS, or IOP. d NAE 18:2 levels correlate moderately with VA, but not significantly. There is a strong correlation that approaches statistical significance between NAE 18:2 and CS. A statistically significant, strong correlation is seen between NAE 18:2 and IOP. e NAE 20:4 levels correlate strongly and significantly with both VA and CS. There is a moderate correlation between NAE 20:4 and IOP that is not statistically significant. f Table of statistical values from correlations a–e. Data are expressed as mean ± SD. IOP measurements are symbolized with triangles, VA with circles, and CS with squares. Open symbols represent young eyes and closed symbols aged eyes. Dashed lines represent the 95 % confidence interval
NAE 18:0 levels showed moderate, positive correlations with VA (r = 0.5110) and CS (r = 0.5346) that were not statistically significant (p = 0.1956 and 0.1723, respectively). A statistically significant (p = 0.0343), strong, negative correlation (r = −0.7441) was shown between NAE 18:0 content and IOP (Fig. 2b).
NAE 18:1 levels were not correlated with functional visual parameters—VA, CS, or IOP (r = −0.1215, −0.1040, and 0.1164, respectively) (Fig. 2c).
NAE 18:2 levels demonstrated a moderate, positive correlation with VA (r = 0.5554) that was not statistically significant (p = 0.1530). A strong, positive correlation (r = 0.6988), approaching statistical significance (p = 0.0538) with CS was also seen. Finally, a statistically significant (p = 0.0341), strong, negative correlation (r = −0.7446) was demonstrated between NAE 18:2 and IOP (Fig. 2d).
NAE 20:4 levels showed strong, positive, statistically significant correlations with both VA (r = 0.8658, p = 0.0054), and CS (r = 0.7922, p = 0.0191). A moderate, negative correlation (r = −0.5348) with IOP was not statistically significant (p = 0.1721) (Fig. 2e).
To summarize (Fig. 2f), NAE 16:0, 18:0, and 18:2 had moderate, positive correlations with VA that were not statistically significant, while NAE 20:4 showed a strong, positive, significant correlation. NAE 18:0 had a moderate, positive correlation with contrast sensitivity (CS) that was not statistically significant, while NAE 16:0, NAE 18:2, and NAE 20:4 demonstrated strong, positive correlations that surpassed or approached statistical significance. NAE 16:0 and NAE 20:4 had moderate, negative correlations with intraocular pressure (IOP) that were not statistically significant, while NAE 18:0 and NAE 18:2 showed strong, negative, statistically significant correlations with IOP. Levels of NAE 18:1 showed no correlation with VA, CS, or IOP, indicating that the relationships of functional parameters of ocular disease and amounts of tissue NAEs were selective for only certain specific NAE types.
Increased Levels of the NAE Oxylipins, 9-NAE-HOD and 15-(S)-HETE EA Correlate with Higher IOP and Declining Visual Function, and thus with Increased Disease Severity
To examine relationship strengths between NAE oxylipin levels and functional disease parameters of glaucoma in the young and aged DBA/2Crl mouse retina, a Pearson product-moment correlation coefficient calculation was performed.
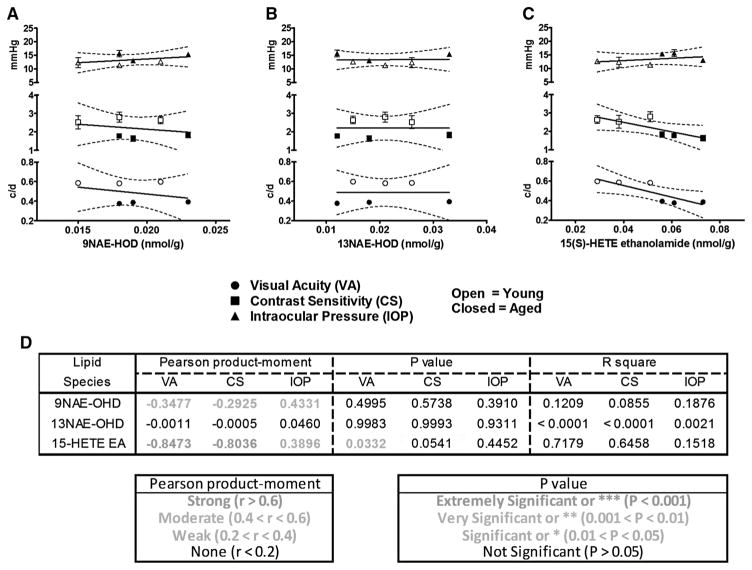
9-NAE-HOD levels demonstrated weak, negative correlations with VA (r = −0.3477) and CS (r = −0.2925) that were not statistically significant (p = 0.4995 and 0.5738, respectively). A moderate, positive, correlation (r = 0.4331) that was not statistically significant (P = 0.3910) between 9-NAE-HOD and IOP was also observed (Fig. 3a).
Fig. 3.
Increased levels of the N-acylethanolamine oxylipins 9NAE-HOD and 15(S)-HETE EA correlate with higher intraocular pressure and declining visual function. a 9NAE-HOD levels correlate moderately with intraocular pressure (IOP) and weakly with visual acuity (VA) and contrast sensitivity (CS), but not significantly. b 13NAE-HOD levels do not correlate with VA, CS, or IOP. c 15(S)-HETE EA levels correlate weakly with IOP, but not significantly. There is a strong correlation that approaches statistical significance between 15(S)-HETE EA and CS. A statistically significant, strong correlation is seen between 15(S)-HETE EA and VA. d Table of statistical values from correlations a–c. Data are expressed as mean ± SD. IOP measurements are symbolized with triangles, VA with circles, and CS with squares. Open symbols represent young eyes and closed symbols aged eyes. Dashed lines represent the 95 % confidence interval
13-NAE-HOD levels were not correlated with any of the functional visual parameters tested—VA, CS, or IOP (r = −0.0011, −0.0005, and 0.0460, respectively) (Fig. 3b).
By contrast, 15(S)-HETE EA levels showed a strong, negative, statistically significant correlation with VA (r = −0.8473, p = 0.0332). A strong, negative correlation with CS (r = −0.8036) approaching statistical significance (p = 0.0541) was also seen. Finally, a weak, positive correlation (r = 0.3896) was demonstrated between 15(S)-HETE EA and IOP that was not significant (p = 0.4452) (Fig. 3c).
In summary, (Fig. 3d), 9-NAE-HOD and 15(S)-HETE EA had negative correlations with VA and CS (weak and strong, respectively) and positive correlations with IOP (moderate and weak, respectively). The relationship between 15(S)-HETE EA and VA or CS surpassed or approached statistical significance, respectively. Levels of 13-NAE-HOD did not correlate with measures of visual function or IOP, indicating that the relationships of functional parameters of ocular disease and amounts of tissue NAE oxylipins were specific for certain oxidative metabolites.
Discussion
The methodology employed in this study allowed us to quantify selected NAEs and their oxylipin derivatives in mouse retina and associate these concentrations with relevant parameters of visual function in vivo. Although levels of NAE 18:0 and its neuroprotective, polyunsaturated derivative NAE 18:2 did not change significantly upon aging, both correlated strongly and significantly with lower IOP. We identified a very significant decline in NAE 20:4 levels upon aging, which correlated strongly and significantly with the development of visual impairment in the DBA/2 mouse model of glaucoma. Conversely, levels of 15(S)-HETE EA, the oxylipin derivative of NAE 20:4, increased significantly upon aging and correlated strongly and significantly with impairment of visual acuity.
Since NAE 18:2 has previously been found to protect neurons in a rodent model for stroke [16] and retinal ganglion cell layer neurons in vivo against glutamate excitotoxicity, modeling some aspects of human glaucoma pathology [13], it was not surprising that NAE 18:2 and NAE 18:0 levels correlated strongly with lower IOP. Knowing that many cannabinoids, prostamides, and prostaglandin analogues effectively reduce ocular hypertension, it is interesting to consider that NAE 18:2 could enter the cyclooxygenase pathway.
A previous study that examined certain NAE levels in the normal adult rat retina by GC/MS found NAE 20:4 to be undetectable, while NAE 16:0 was measured at 130 ± 35 pmol/g retina, and NAE 18:1 levels were reported as 55 ± 5 pmol/g retina [29]. Although we measured significantly higher levels (nmol/g retina vs. pmol/g retina) of these NAEs in the normotensive DBA/2 mouse retina than were found in adult Sprague–Dawley rats, our results are consistent in ranking order: NAE 16:0 ≫ NAE 18:1 > NAE 20:4. Part of these differences is due to quantification based on dry weight (here in our studies) versus wet weight of tissues. Further, since DBA/2 mice have significantly reduced brain CB1 receptor function [30], perhaps NAE 20:4 production in these animals is upregulated to compensate. Alternatively, these differences may be due to variation between animal species or differences in extraction and detection methods. A more recent study comparing NAE levels in glaucomatous adult human retina with aged-matched controls by LC-APCI-MS was able to detect NAE 20:4 in the range of 10–50 pmol/g retina [31], which is more consistent with our results. We found higher levels of NAE 16:0 in the DBA/2 mouse retina than have been reported in the human retina (135 nmol/g retina vs 100–200 pmol/g retina, respectively), which is consistent with previous results [19].
Previous findings that NAE 20:4 levels are not significantly different in glaucomatous human retina compared to age matched controls [30] seem to conflict with our finding of a very significant decline upon development of visual impairment in the DBA/2 mouse. Since the type and severity of glaucoma, IOP elevation, treatment regimens, gender, or ethnicity was not specified in the human study, it is difficult to speculate on why these results differ from our own. A straightforward explanation would be that the increased sensitivity of our methods allows identification of differences that were not detectable with previous methods. Alternatively, declining levels of neuroprotective anandamide could play a role in the development of neurodegeneration observed in the DBA/2Crl retina.
Visual function, measured here as VA and CS, are not always linearly correlated to IOP as one of the disease phenotypes. In the DBA2 glaucoma model, as in humans, not every animal that demonstrates a functional deficit or optic neuropathy also exhibits the same high level of IOP [32, 33]. It is possible that declining levels of the neuroprotective molecule NAE 20:4 leave retinal neurons susceptible to glaucomatous degeneration. Alternatively or in conjunction with the loss of NAE 20:4, the accumulation of 15(S)-HETE EA may have a negative impact on the survival of retinal neurons as seen in other cell types. Conversely, NAE 18:0 and NAE 18:2 may play a role in lowering IOP, but do not mediate other factors involved in glaucomatous neurodegeneration. Future experiments should examine the effects of exogenously administered compounds.
Conclusions
To our knowledge, this is the first report of oxylipin-ethanolamine levels in mammalian retina. The significant increase in 15(S)-HETE EA we observed in the aged DBA/2Crl mouse is not surprising, considering that arachidonate 12/15 lipoxygenases are expressed in many types of blood and epithelial cells [34] including the retinal pigment epithelial cell line ARPE-19 [35], that lipoxygenase expression and activity is associated with inflammation, and lipoxygenase action upon NAEs is well described [22, 23]. Our finding of a significant increase in 15(S)-HETE EA levels corresponding to a very significant decrease in anandamide levels upon aging in the DBA/2Crl retina suggests that the 12/15 lipoxygenase pathway may compete with fatty acid amide hydrolase for the hydrolysis of NAE 20:4. Lipoxins, the canonical products of the 12/15 lipoxygenase pathway, generally exert anti-inflammatory, pro-resolving effects and in many cases are considered to be neuroprotective [36]. The strong, significant correlation of 15(S)-HETE EA with visual decline may indicate that this molecule cannot continue down the lipoxin pathway and that its accumulation may have a negative impact on retinal function. Consequently, the lipoxin pathway and signaling molecules identified here may represent novel target for future glaucoma therapies.
Supplementary Material
Acknowledgments
The present study was supported in part by grants from the National Eye Institute (EY022774), the National Institute on Aging (AG010485, AG022550 and AG027956), the National Center for Research Resources and National Institute of General Medical Sciences (RR027093) of the National Institutes of Health (PK). The content of the present study is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Lipid analysis was supported by a grant from the US Department of Energy, Office of Science, Basic Energy Sciences program (DE-FG02-05ER15647; KDC). K.D.C. is grateful to the US National Science Foundation for providing individual research and development leave to assist in the supervision of this research and the preparation of this manuscript by agreement under the Intergovernmental Personnel Act. Additional partial support was provided by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, a Challenge Grant from Research to Prevent Blindness and the Vision Research Foundation of Kansas City (PK) and is gratefully acknowledged. The authors thank Margaret, Richard and Sara Koulen for their generous support and encouragement.
Abbreviations
- 9NAE-HOD
(9S,12Z,10E)-9-Hydroxy-10,12 octadecadienoylethanolamine
- 13NAE-HOD
(13S,9Z,11E)-13-Hydroxy-9,11-octadecadienoylethanolamine
- 15(S)-HETE EA
15(S)-Hydroxy-N-(2-hydroxyethyl)-5Z,8Z,11Z,13E-eicosatetraenamide
- BSTFA
N,O-Bis(trimethylsilyl) trifluoroacetamide
- CS
Contrast sensitivity
- GC/MS
Gas chromatography–mass spectrometry
- IOP
Intraocular pressure
- LC-APCI-MS
Liquid chromatography–atmospheric pressure chemical ionization–mass spectrometry
- LCPUFA
Long chain polyunsaturated fatty acids
- NAE
N-Acylethanolamine
- NAE 16:0
N-Palmitoylethanolamine, N-(2-hydroxyethyl)-hexadecanamide
- NAE 18:0
N-Stearoylethanolamine, N-(octadecanoyl)-ethanolamine
- NAE 18:1
N-Oleoylethanolamine, N-(9Z-octadecenoyl)-ethanolamine
- NAE 18:2
N-Linoleoylethanolamine, N-(2-hydroxyethyl)-9Z,12Z-octadecadienamide
- NAE 20:4
Anandamide, N-arachidonoylethanolamine, N-(2-hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide
- RP-HPLC
Reverse phase-high performance liquid chromatography
- TMS
Trimethylsilyl
- VA
Visual acuity
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11745-016-4161-x) contains supplementary material, which is available to authorized users.
References
- 1.Terland O, Flatmark T, Tangeras A, Gronberg M. Dopamine oxidation generates an oxidative stress mediated by dopamine semiquinone and unrelated to reactive oxygen species. J Mol Cell Cardiol. 1997;29(6):1731–1738. doi: 10.1006/jmcc.1997.0412. [DOI] [PubMed] [Google Scholar]
- 2.Floyd RA, Towner RA, He T, Hensley K, Maples KR. Translational research involving oxidative stress and diseases of aging. Free Radic Biol Med. 2011;51(5):931–941. doi: 10.1016/j.freeradbiomed.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 4.Ohia SE, Opere CA, Leday AM. Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res. 2005;579(1–2):22–36. doi: 10.1016/j.mrfmmm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84(3):389–399. doi: 10.1016/j.exer.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31(2):152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Tezel G. The immune response in glaucoma: a perspective on the roles of oxidative stress. Exp Eye Res. 2011;93(2):178–186. doi: 10.1016/j.exer.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslan M, Dogan S, Kucuksayan E. Oxidative stress and potential applications of free radical scavengers in glaucoma. Redox Rep. 2013;18(2):76–87. doi: 10.1179/1351000212Y.0000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid HH. Pathways and mechanisms of N-acylethanolamine biosynthesis: can anandamide be generated selectively? Chem Phys Lipids. 2000;108(1–2):71–87. doi: 10.1016/s0009-3084(00)00188-2. [DOI] [PubMed] [Google Scholar]
- 11.Di Marzo V. ‘Endocannabinoids’ and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochim Biophys Acta. 1998;1392(2–3):153–175. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 12.Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17(14):1430–1449. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- 13.Sinor AD, Irvin SM, Greenberg DA. Endocannabinoids protect cerebral cortical neurons from in vitro ischemia in rats. Neurosci Lett. 2000;278(3):157–160. doi: 10.1016/s0304-3940(99)00922-2. [DOI] [PubMed] [Google Scholar]
- 14.Duncan RS, Xin H, Goad DL, Chapman KD, Koulen P. Protection of neurons in the retinal ganglion cell layer against excitotoxicity by the N-acylethanolamine, N-linoleoylethanolamine. Clin Ophthalmol. 2011;5:543–548. doi: 10.2147/OPTH.S18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg P, Duncan RS, Kaja S, Zabaneh A, Chapman KD, Koulen P. Lauroylethanolamide and linoleoylethanolamide improve functional outcome in a rodent model for stroke. Neurosci Lett. 2011;492(3):134–138. doi: 10.1016/j.neulet.2011.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan RS, Chapman KD, Koulen P. The neuroprotective properties of palmitoylethanolamine against oxidative stress in a neuronal cell line. Mol Neurodegener. 2009;4:50. doi: 10.1186/1750-1326-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilaru A, Tamura P, Garg P, et al. Changes in N-acylethanolamine pathway related metabolites in a rat model of cerebral ischemia/reperfusion. J Glycom Lipidom. 2011;1(1):101. [PMC free article] [PubMed] [Google Scholar]
- 18.Kilaru A, Isaac G, Tamura P, et al. Lipid profiling reveals tissue-specific differences for ethanolamide lipids in mice lacking fatty acid amide hydrolase. Lipids. 2010;45(9):863–875. doi: 10.1007/s11745-010-3457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grillo SL, Keereetaweep J, Grillo MA, et al. N-Palmitoylethanolamine depot injection increased its tissue levels and those of other acylethanolamide lipids. Drug Des Dev Ther. 2013;7:747–752. doi: 10.2147/DDDT.S48324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faure L, Nagarajan S, Hwang H, et al. Synthesis of phenoxyacyl-ethanolamides and their effects on fatty acid amide hydrolase activity. J Biol Chem. 2014;289(13):9340–9351. doi: 10.1074/jbc.M113.533315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faure L, Cavazos R, Khan BR, et al. Effects of synthetic alkamides on Arabidopsis fatty acid amide hydrolase activity and plant development. Phytochemistry. 2015;110:58–71. doi: 10.1016/j.phytochem.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: crosstalk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev. 2011;111(10):5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urquhart P, Nicolaou A, Woodward DF. Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases. Biochim Biophys Acta. 2015;1851(4):366–376. doi: 10.1016/j.bbalip.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 24.McKinnon SJ, Schlamp CL, Nickells RW. Mouse models of retinal ganglion cell death and glaucoma. Exp Eye Res. 2009;88(4):816–824. doi: 10.1016/j.exer.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burroughs SL, Kaja S, Koulen P. Quantification of deficits in spatial visual function of mouse models for glaucoma. Invest Ophthalmol Vis Sci. 2011;52(6):3654–3659. doi: 10.1167/iovs.10-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venables BJ, Waggoner CA, Chapman KD. N-Acylethanolamines in seeds of selected legumes. Phytochemistry. 2005;66(16):1913–1918. doi: 10.1016/j.phytochem.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Kilaru A, Herrfurth C, Keereetaweep J, et al. Lipoxygenase-mediated oxidation of polyunsaturated N-acylethanolamines in Arabidopsis. J Biol Chem. 2011;286(17):15205–15214. doi: 10.1074/jbc.M110.217588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keereetaweep J, Blancaflor EB, Hornung E, et al. Ethanolamide oxylipins of linolenic acid can negatively regulate Arabidopsis seedling development. Plant Cell. 2013;25(10):3824–3840. doi: 10.1105/tpc.113.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straiker A, Stella N, Piomelli D, et al. Cannabinoid CB1 receptors and ligands in vertebrate retina: localization and function of an endogenous signaling system. Proc Natl Acad Sci USA. 1999;96(25):14565–14570. doi: 10.1073/pnas.96.25.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hungund BL, Basavarajappa BS. Role of endocannabinoids and cannabinoid CB1 receptors in alcohol-related behaviors. Ann N Y Acad Sci. 2004;1025:515–527. doi: 10.1196/annals.1316.064. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Matias I, Dinh T, et al. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochem Biophys Res Commun. 2005;330(4):1062–1067. doi: 10.1016/j.bbrc.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 32.Scholz M, Buder T, Seeber S, et al. Dependency of intraocular pressure elevation and glaucomatous changes in DBA/2J and DBA/2J-Rj mice. Invest Ophthalmol Vis Sci. 2008;49(2):613–621. doi: 10.1167/iovs.07-0745. [DOI] [PubMed] [Google Scholar]
- 33.Harazny J, Scholz M, Buder T, et al. Electrophysiological deficits in the retina of the DBA/2J mouse. Doc Ophthalmol. 2009;119(3):181–197. doi: 10.1007/s10633-009-9194-5. [DOI] [PubMed] [Google Scholar]
- 34.Conrad DJ. The arachidonate 12/15 lipoxygenases. A review of tissue expression and biologic function. Clin Rev Allergy Immunol. 1999;17(1–2):71–89. doi: 10.1007/BF02737598. [DOI] [PubMed] [Google Scholar]
- 35.Calandria JM, Marcheselli VL, Mukherjee PK, et al. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J Biol Chem. 2009;284(26):17877–17882. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martini AC, Forner S, Bento AF, Rae GA. Neuroprotective effects of lipoxin A4 in central nervous system pathologies. Biomed Res Int. 2014;2014:316204. doi: 10.1155/2014/316204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.