Abstract
Antioxidant ingredients present in grape have been extensively investigated for their cancer chemopreventive effects. However, much of the work has been done on individual ingredients, especially focusing on resveratrol and quercetin. Phytochemically, whole grape represents a combination of numerous phytonutrients. Limited research has been done on the possible synergistic/additive/antagonistic interactions among the grape constituents. Among these phytochemical constituents of grapes, resveratrol, quercetin, kaempferol, catechin, epicatechin, and anthocyanins (cyanidin and malvidin) constitute more than 70% of the grape polyphenols. Therefore, these have been relatively well-studied for their chemopreventive effects against a variety of cancers. While a wealth of information is available individually on cancer chemopreventive/anti-proliferative effects of resveratrol and quercetin, limited information is available regarding the other major constituents of grape. Studies have also suggested that multiple grape antioxidants, when used in combination, alone or with other agents/drugs show synergistic or additive anti-proliferative response. Based on strong rationale emanating from published studies, it seems probable that a combination of multiple grape-ingredients alone or together with other agents could impart ‘additive synergism’ against cancer.
Keywords: Combination Chemoprevention, Grape Antioxidants, Resveratrol, Quercetin, Kaempferol, Catechin, Epicatechin, Anthocyanin
Introduction
Despite significant advancement in the understanding of the disease and its management, cancer still remains a global challenge and continues to affect millions of people worldwide. With the steady growth and aging of the world’s population, the global burden of new cancer incidences is estimated to increase from 12.7 million in 2008 to 20.3 million by 2030 [1]. In the United States alone, a total of 1,658,370 new cases of cancer and 589,430 cancer-related deaths were predicted to occur in the year 2015 [2]. The reasons of the predicted rise in the global cancer burden are various and somewhat well established, which include lifestyle risk factors, diet, environment and occupational exposure to carcinogens and socioeconomic status. Carcinogenesis is a multi-step process that takes place over years and often over decades. Arresting one or several of these steps is considered to delay the development of cancer. This provides an opportunity for natural agents to act as a prevention and even intervention mediator in some of the cases. In fact, the cancer chemoprevention by natural dietary agents being a cost-effective and non-toxic approach have received substantial attention as a possible means for controlling the cancer prevalence.
The concept of chemoprevention is based on the use of natural, synthetic, or biological agents to reverse, suppress, or prevent carcinogenic progression to invasive cancer. The importance of chemoprevention could be easily understood with the fact that over 340 human clinical trials have been completed/ongoing on this concept (clinicaltrials.gov: accessed on Oct. 23, 2015). Although several of the results of these clinical trials are still to be revealed, recent studies suggest that combinatorial chemoprevention could be a better approach to deal with the disease having a pleiotropic mode of progression. Cancer tissues are viewed as complex tissues composed of multiple distinct cell types interacting with one another. In 2000, Hanahan and Weinberg proposed the concept of cancer “hallmarks” that comprise six biological capabilities acquired during the multistep development of cancer as they evolve progressively from normal to a neoplastic state [3]. This includes sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. In 2011, Weinberg and Hanahan updated their list by suggesting the addition of two new hallmarks of cancer, i.e. reprogramming of energy metabolism and evading immune destruction [4]. They also suggested that underlying these cancer hallmarks are instability of genome, which makes the genetic diversity that accelerates their acquisition, and inflammation [4]. So far, understanding of the pathogenetic mechanisms of cancer have significantly evolved and it is expected that future discoveries will further elaborate regulatory circuits involved therein. This provides an opportunity to target multiple molecular events that are prone to dysregulation in a given type of cancer, with naturally occurring chemopreventive agents. Thus, a multitude of intracellular sites/processes in tumor development, such as carcinogen metabolism, DNA repair, cell proliferation, apoptosis, differentiation, and angiogenesis and progression, could be simultaneously influenced by natural agents.
We have recently advocated the use of resveratrol-based combinatorial approach for effective cancer management (reviewed in [5]). A combinatorial approach is likely to be more useful because of dysregulation of multiple pathways affecting cancer cell growth and oncogenic signaling. As the grapes contain hundreds of antioxidants [6], the intra-combinations among different grape constituents are expected to provide synergistic cancer chemopreventive responses. Based on recently published studies, in this review we are providing a critical discussion of the possibilities of combinatorial synergism using multiple grape constituents.
Combination chemoprevention: a better strategy for cancer management
Based on recent studies, there is compelling evidence that certain foods may offer advantages over their individual components, suggesting that factors within the whole food may affect the absorption and metabolism, and therefore, exert additive or synergistic effects. For example, a study by Boileau and colleagues has suggested that consumption of tomato powder inhibited N-methyl-N-nitrosourea and testosterone-induced prostate carcinogenesis, but lycopene, one of its principle components failed to do so [7]. This suggests that the other ingredients in tomato may be important in the observed response. It is possible that these other agents synergize with lycopene to impart their effect whereas lycopene alone is not effective in this particular situation. However, on the contrary, other studies suggested that whole product of food may not be as effective as its isolated components. A study by Thiagarajan et.al. demonstrated that the soy flour and full-fat soy flake diets containing 0.049% genistein derivatives (primarily glycosides) were less effective in inhibiting the formation of precancerous colon lesions, foci with aberrant crypts, than the diet containing 0.015% genistein (as the aglycone) [8]. This example suggests that the soy food might have other constituent(s), which can lessen the response by altering the metabolism, absorption, or site of action of the bioactive agent genistein. Therefore, it is very important to increase our efforts to understand the metabolic and mechanistic interactions between bioactive natural agents present within a food. It is possible while some of the ingredients enhance to synergize the beneficial response whereas others may act in a reverse fashion to inhibit the overall response. Thus, if the interactions among individual agents are well-understood, it is conceivable to design ‘personalized approaches’ for specific situations.
Studies have also suggested that the use of a combination of natural agents with distinct molecular mechanisms offer an opportunity for maximizing chemopreventive effects while minimizing any adverse effects. Similarly, natural agents have been shown to exert additive/synergistic effects with pharmaceutical drugs, possibly by modifying different molecular targets. Schwartz and colleagues have found that diets encompassing high levels of olive oil show a significant protective effect against colon tumor development that is additive with the inhibitory effect of sulindac, a non-steroidal anti-inflammatory drug [9]. These protective effects were found to be related with the regulation of both expression and activity of key proteins involved in prostaglandin-biosynthesis (COX-2) and apoptosis-induction (Bcl-2 and caspase-3) pathways [9]. Similarly, the soy isoflavone, daidzein, has been shown to improve the efficacy of tamoxifen against mammary tumors [10]. Interestingly, in the same study, a combination of genistein with tamoxifen produced an opposing effect when compared with tamoxifen alone [10]. These evidences suggest that carefully designed combinations of phytochemical with other phytochemical(s) or with pharmaceuticals may be beneficial in cancer management.
Grape: a conglomeration of potentially useful antioxidants
Grape, one of the most popular and widely cultivated and consumed fruits in the world, is very rich in antioxidants. Different forms of grape extracts, such as from the skin, seeds, or grape juice, have been shown to contain a number of phytochemicals including stilbenes, phenolic acids, and flavonoids. Remarkably, grapes are known to possess more than 1600 phytochemicals whose actual composition varies greatly among different varieties [6]. For example, muscadine grapes are known to possess resveratrol, ellagic acid, myricetin, quercetin and kaempferol as major poluphenols. In addition to this, grapes are also known to contain the appropriate amount of vitamin A, vitamin C, thiamine HCl, folic acid, calcium, iron, potassium, phosphorus, sodium, magnesium, manganese, copper, and zinc.
The most precious among the phytonutrients of grape is the “family” of natural agents called polyphenols that have been shown to possess health promoting effects, including prevention of diseases such as cancer. All kinds of grapes, red, green and black, contain a variety of polyphenols that occur in every part of the grape fruit: the flesh, the skin, and the seeds. Studies of grapes and isolated grape constituents such as resveratrol, flavonols, catechins, procyanidins, and anthocyanins suggest that these natural agents may help to protect against certain cancers, nerve and brain disorders, heart disease, arthritis and an array of several health conditions. Several in vitro and in vivo studies have demonstrated that grape phytochemicals may be involved in a broad spectrum of biological activities related to cancer pathophysiology.
Grape antioxidants: efficacy in combination chemoprevention
Based on published studies conducted in animal models, we have earlier proposed that grape antioxidant resveratrol together with other agents and drugs could be useful against cancer (reviewed in [5]). We have also advocated the idea that grape constituents, in its natural combination in whole grape, may have the potential for health promotion and in the management of diseases [11]. In this review, based on recent research with a variety of grape antioxidants, we have discussed a rationale that intra-combinations of certain grape antioxidants may be useful in cancer management. Discussed below, we have limited our discussion to the major antioxidants in grape, namely, resveratrol, quercetin, kaempferol, catechin, epicatechin, and anthocyanins (cyanidin, malvidin, peonidin) [6].
Resveratrol-Quercetin combination
As described above, grapes possess a vast array of constituents, many of which have health-promoting effects. Resveratrol (3,5,4'-trihydroxystilbene), a major polyphenolic component of grapes, has received a great deal of attention of biomedical research community as well as general public and media (reviewed in [12]). Most of the grape types contain resveratrol, albeit at varying levels. Although resveratrol has been under investigation for several of its beneficial effects for a long time, it’s cancer chemopreventive effects were first shown in 1997 by John Pezzuto’s group [13]. Quercetin (3,3′,4′,5,7-pentahydroxyflavone), is another grape antioxidant that has been extensively investigated for its cancer chemopreventive effects [14]. Quercetin possesses high free radical scavenger ability and has been shown to neutralize highly reactive species such as peroxynitrite and the hydroxyl radical [15]. Quercetin is a known inhibitor of PI3K, NF-κB, and several other important targets involved in cell growth and proliferation [14, 16, 17]. A few in vitro and in vivo studies have suggested the usefulness of combining resveratrol and quercetin for cancer management. These studies are discussed below.
In 1999, ElAttar and Virji investigated the combined effect of resveratrol and quercetin in concentrations equivalent to that present in red wines and demonstrated additive inhibition of oral squamous carcinoma cell (SCC-25) growth and proliferation (DNA synthesis) [18]. This group, in a subsequent study, investigated the effects of diluted red wine which contained much lower concentration of resveratrol and quercetin (1.6 μM of each), and found a significant inhibition of cell growth, DNA synthesis and changes in cell morphology than individual agent alone or in combination, which they credited to the synergistic interactions of other wine phenols [19]. In a study by Melzig and Escher, a combination of resveratrol and quercetin has been shown to induce the cellular enzyme activity of neutral endopeptidase and angiotensin-converting enzymes associated with an inhibition of cellular proliferation of the neuroblastoma cell line SK-N-SH [20]. Further, this study demonstrated that the long-term treatment of neuroblastoma cells with quercetin and resveratrol enhanced the differentiation state of the cells [20]. Mertens-Talcott and Percival, have determined the interactions of grape polyphenols ellagic acid and quercetin with resveratrol for the induction of apoptosis and reduction of cell growth in human leukemia cells (MOLT-4) [21]. The authors found a synergistic interaction for the resveratrol and ellagic acid combination (combination index (CI) of 0.64) as well as for resveratrol and quercetin combination (CI of 0.68) [21]. In a previous study from the same group, ellagic acid was shown to significantly potentiate the effects of quercetin in the reduction of cell proliferation and the induction of apoptosis [22]. This group further assessed the antiproliferative activity of resveratrol-quercetin mixture (1:1 ratio) against HT-29 colon cancer cells and found repression of oncogenic microRNA-27a, and induction of zinc finger protein ZBTB10, a specificity protein repressor [23]. In another study, Zamin and colleagues have found that resveratrol (10 μM) or quercetin (25 μM) individually had no effect on apoptosis induction against C6 rat glioma cells. However, when combined together, the combination had a strong effect on caspase 3/7 activation as well as presented a strong synergism in inducing senescence-like growth arrest [24]. Thus, the synergistic interaction among grape antioxidants observed in studies discussed above provides a rationale for further exploring these combinations against cancer in additional in-depth preclinical studies.
A limited number of in vivo studies have also explored the chemopreventive effects of resveratrol in combination with quercetin. Mouria and colleagues have determined the effects of the combination of resveratrol and quercetin against pancreatic cancer in vitro as well as in vivo a nude mouse model [25]. The study demonstrated that the combination exerted a synergistic effect on mitochondrial cytochrome c release and caspase-3 activation. Interestingly, the study found that cyclosporin A (an inhibitor of mitochondrial permeability transition (MTP)) alone inhibited cell death in quercetin treated cancer cells while both cyclosporin A and aristolochic acid (phospholipase A2 inhibitor) were required to inhibit resveratrol-mediated cytochrome c release and apoptosis. The results of this study support the suggestion that resveratrol and quercetin could also act on different pathways in addition to overlapping effects resulting in a synergistic response [25].
In another study, Schlachterman et al. reported the superior combinatorial effect of resveratrol, quercetin, and catechin against metastatic breast cancers in fluorescently tagged breast tumor growth in nude mice model [26]. The authors found the peak tumor inhibition at 5 mg/kg dose (thrice a week starting at 1 day after xenograft implantation until sacrifice at day 118) of each compound in combined dietary polyphenols. In the same study, the in vitro data demonstrated that resveratrol, quercetin, or catechin individually (at 0.5 μM concentrations) were ineffective, however, a combination of the three exerted a synergistic inhibitory effect on ERα (-) ERβ (+) MDA-MB-231 breast cancer cell proliferation and on cell cycle progression [26]. In a subsequent study, these authors explored if resveratrol, quercetin, and catechin in combination inhibit Akt/mTOR signaling and potentiate the effects of gefitinib in breast cancer [27]. It was found that resveratrol, quercetin, and catechin induced apoptosis even in gefitinib-resistant breast cancer cells via regulation of a myriad of pro-apoptotic proteins. Further, using nude mice model, they showed that when combined resveratrol, quercetin, catechin, and gefitinib were more effective than the individual treatments in preventing mammary tumor progression and metastasis in nude mice [27]. Recently, Cote et al. have shown that the combination of resveratrol and quercetin in polymeric micelles, which retained 1.1 mg/mL and 1.42 mg/mL of resveratrol and quercetin, respectively, mitigated doxorubicin-induced cardiotoxicity in vitro and in vivo (in healthy mice) [28]. The in vitro cell viability and combination index data showed a synergistic interaction in human ovarian cancer cells (SKOV-3) and antagonistic in rat cardiomyocytes (H9C2) cells [28]. This study suggested resveratrol and quercetin may be useful in doxorubicin-chemotherapy, to limit the adverse effects of chemotherapy.
In the recent past, a few efforts have also been made to conduct clinical trials with the combination of grape antioxidants, for assessing their efficacy against cancer. For example, as registered at ClinicalTrials.gov, a phase II dietary intervention study (NCT00455416) in patients with Follicular Lymphoma (stage III/IV) was initiated at the Oslo University Hospital (Oslo, Norway) to assess the ability of several dietary factors to inhibit cell proliferation, induce apoptosis, and modulate tumor cell infiltrate in vivo. In this study, among other dietary formulations, resveratrol and quercetin (in the form of grape juice) were included to be studied. This trial was registered in 2007, however, no data is available so far. In another recent trial, steady-state pharmacokinetics and tolerability of trans-resveratrol with other dietary factors was evaluated in healthy human subjects. In this study, eight healthy subjects received resveratrol (2000 mg twice daily) and quercetin (500 mg twice daily) simultaneously, did not show any change in the pharmacokinetics of resveratrol [29]. These trials show an increasing interest of researchers in evaluating chemopreventive combinations of grape-based antioxidants. However, more intense efforts are required in this direction.
Kaempferol in combination with other grape constituents
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), is a flavonol that exists in a variety of plants, including grapes. Several in vitro and in vivo studies have demonstrated the potential anticancer properties of kaempferol against several type of cancer [30]. Kaempferol has been shown to act as a superoxide scavenger and to increases the activity or expression of antioxidant enzymes [31]. The specific combination of resveratrol and kaempferol needs to be carefully evaluated for its potential chemopreventive effects. In this regard, Nöthlings and colleagues estimated intakes of three flavonols, quercetin, kaempferol, and myricetin and examined associations with incidence of pancreatic cancer in a multiethnic cohort study, with 183,518 participants, in Hawaii and California [32]. In this study, quercetin was found to contribute most of total flavonol intake (~70%), followed by kaempferol (~25%). The authors found that all three flavonols in diet, quercetin, kaempferol, and myricetin were associated with a decrease in the incidence of pancreatic cancer among current smokers, but not in never or former smokers, suggesting a potential chemopreventive effect of flavonols (in combination) against pancreatic cancer [32].
Interestingly, resveratrol and kaempferol both are natural aryl hydrocarbon receptor antagonists and estrogen receptor (ER) agonists [33]. The aryl hydrocarbon receptor is a ligand-activated transcription factor intricate in the directive of biological reactions to planar aromatic hydrocarbons, and also known to modulate xenobiotic-metabolizing enzymes like cytochrome P450 [34, 35]. MacPherson and Matthews have demonstrated that inhibition of aryl hydrocarbon receptor-dependent transcription by kaempferol or resveratrol, in human breast cancer cells, was not related with the expression level of ERα [33]. Kowalski and colleagues have demonstrated that kaempferol, resveratrol, and apigenin inhibited TNF-α suggesting their anti-inflammatory activity [36]. However, these studies did not assess resveratrol and kaempferol in combination. It may be useful to study this specific combination against multiple cancers.
Catechin and epicatechin in combinatorial strategies
Catechins (flavan-3-ol) are plant-based flavanols, abundantly present in a variety of fruits and plants, including grapes. Epicatechin and catechin are epimers, with L-epicatechin and D-catechin being the most commonly occurring optical isomers in nature. Catechin concentrations are high in red wine, black grapes, blackberries, apples, cherries, pears, raspberries, broad beans, green tea, and chocolate. Typically about 10.5 mg of catechin is present in 100 g of grapes however the content is variable based on variety, location, and time of harvest. Intake of catechins has been connected with a variety of beneficial effects including improved plasma antioxidant activity (the ability to scavenge free radicals), dilation of the brachial artery (blood vessel expansion), oxidation of fat, and resistance of low-density lipoprotein to oxidation [37]. Although extensive cancer chemopreventive studies of catechins isolated from green tea have been done, not much is known about the effects of the monomeric non-gallate catechin, which are abundantly present in grapes [38]. Ebeler et al. observed that catechins from red wine, incorporated in diet, delayed the onset of breast tumors in HTLV-1 tax transgenic mice that develop spontaneous tumors [39]. Similar effects were observed in another study which showed that grape and wine polyphenols downregulated the expression of signal transduction genes and inhibited estrogen receptor-negative MDA-MB231 tumors in nu/nu mouse xenografts [40]. Jara-Palacios et al. evaluated the anti-proliferative effects of white grape pomace extract (PWGPE), and some of its phenolic components on colon cancer cells [41]. PWGPE was found to significantly inhibit the proliferation of cancer cells. The individual ingredients, catechin, epicatechin, quercetin and gallic also inhibited colon cancer cell growth, at specific concentrations. In another study, the antioxidant/antiradical activity and the antiproliferative effect of different polyphenolic mixtures, extracted from grapes, containing catechin, epicatechin, gallic acid, glycosylated flavonols and procyanidin oligomers, were shown to significantly inhibit growth and a cell cycle distribution of Hepa-1c1c7 cells [42]. However, these in vitro studies are very preliminary in nature and needs to be verified in carefully planned detailed investigations, including in vivo animal models of cancer.
Procyanidins, the members of the proanthocyanidin class of flavonoids, are oligomeric compounds derived from catechin and epicatechin molecules. Antioxidant efficacy of five individual polyphenols (catechin, procyanidin B2, procyanidin B5, procyanidin C1 and procyanidin B5-3'-gallate), were assessed in a study, where they were shown to significantly inhibit epidermal lipid peroxidation [43]. Quesada et al. demonstrated that dietary catechins and procyanidins regulated zinc homeostasis in HepG2 human liver hepatocellular carcinoma cells [44]. Although this study used catechins from green tea, grape-seed procyanidin extract was used in the combination regimen. The authors found that the elevation of cytoplasmic labile zinc and extracellular complexation of zinc cations may be important mechanisms underlying the variation of diverse cell signaling and metabolic pathways by catechins and procyanidins [44]. In another study, Hanausek et al. evaluated three of the major antioxidant components found in grapes (resveratrol, catechin, quercetin), and grape seed extract, containing a proanthocyanidin B-2-gallate for their abilities to prevent oxidative stress and to protect the immune system [45]. Tested antioxidants given topically and/or systemically strongly inhibited DMBA-induced epidermal hyperplasia, proliferation, and inflammation. The hydroxylation of 2'-deoxyguanosine was noticeably impeded by dietary as well as topical administration of agents. Concurrent topical and dietary treatment with the antioxidants lessened these biomarkers, showing significant additive and in some cases synergistic effects [45]. In a similar study, resveratrol, quercetin, and catechin in combination has been shown as a potentiation agent for gefitinib against breast cancer, suggesting that combinatorial therapy with grape antioxidant may potentiate the effects of chemotherapeutic agents [27].
Anthocyanins in combination
Anthocyanins are water-soluble pigments responsible for giving blue, purple and red colors to fruits and vegetables. Anthocyanins are abundant in purple grapes. In the European Union, Australia, and New Zealand, anthocyanins are approved for use as a food additive with an ‘E notation’ - E163. The "E" stands for "Europe" and used on food labels throughout the European Union. The existence of multiple anthocyanins together in dietary sources presents an excellent opportunity for their evaluation as cancer chemopreventive or therapeutic agents. The most abundant of the naturally occurring anthocyanins in grapes are the glycosides of cyanidin, malvidin, and peonidin. Although anthocyanins rich extracts or individual agents have been used in several studies, the combination of specific anthocyanins (cyanidin, malvidin, and peonidin) or a combination of specific anthocyanin(s) with other grape antioxidants have not been assessed for potential chemopreventive effects.
The anti-proliferative effect of cyanidin, malvidin, and peonidin have been tested in several studies. For example, Sorrenti and colleagues have shown that cyanidin imparts anti-proliferative effects through activation of caspase-3 and induction of p21 protein in human prostate cancer cells [46]. Pratheeshkumar et al. have found that cyanidin-3-glucoside (C3G) prevented ultraviolet B (UVB)- stimulated oxidative stress and inflammation by modulating MAP kinase and NF-κB signaling pathways in SKH-1 hairless mice skin [47]. Similarly, malvidin has been shown to inhibit TNF-α-induced inflammatory response in endothelial cells, indicating its potential role in preventing chronic inflammation in many diseases including cancer [48]. Liu and colleagues have shown that peonidin-3-glucoside (P3G) and C3G inhibit the phospho-HER2 and phospho-AKT and induce apoptosis in HER2-positive breast cancer cells [49]. Further, in xenograft mouse models, P3G, and C3G, at the dose of 6 mg/kg (oral gavage) for a total of 25 days, were found to reduce the tumor growth. However, this study didn’t notice any response-difference between P3G and C3G treatments, and no combination groups were assessed [49]. Interestingly, C3G has been investigated in combination with trastuzumab (a monoclonal antibody that interferes with the human epidermal growth factor receptor 2; HER2) in HER2-positive breast cancer cell in vitro (in MDA-MB-453, BT474 and HCC1569 cells) and in vivo (in xenograft model), and found to exert synergistic antitumor activity [50].
Burton and colleagues have shown that anthocyanin containing muscadine grape skin extract inhibited Snail and pSTAT3, and abolished Snail-mediated cathepsin L activity, migration, invasion and osteoclastogenesis in the breast (MCF-7) and prostate (LNCaP, ARCaP-E) cancer cells [51]. Phytochemically, muscadine grapes differ from other varieties of grapes due to the prevalence of anthocyanin 3,5-diglucosides (delphinidin-3,5- diglucoside, cyanidin-3,5-diglucoside and petunidin-3,5-diglucoside), ellagic acid and ellagic acid precursors [52]. As of now, muscadine grape skin extract as a brand name ‘Muscadine Plus’ is in Phase II clinical trial being investigated for the management of prostate cancer (clinical trials.gov NCT01317199). Signorelli et al. have investigated the effect of Liofenol, a lyophilized extract of natural red wine containing miscellaneous polyphenols, flavonoids, and anthocyanins, in HCT116 colon cancer cells [53]. This study demonstrated a reduction in cell proliferation, increase in the levels of p53 and p21, activation of the Nrf2 signaling by Liofenol in HCT116 cells [53]. Jung et al. have determined the effect of purple grape juice rich in polyphenols and anthocyanins in the initiation stage of DMBA-induced rat mammary tumorigenesis in female Sprague-Dawley rats [54]. The authors found that the grape juice inhibited mammary tumor as well as DMBA-DNA adduct formation [54]. In a study by Park and colleagues, anthocyanins extracted from Korea wild berry Meoru (a wild grape) was found to decrease the expression of pGSK3β and β-catenin and increase AMPK activity resulting inhibition of tumorigenic potential of Hep3B hepatocarcinoma cell in a xenografted mouse model [55]. These studies and several others where anthocyanins have been tested in the form of extract or as bioactive agents suggest that it may be worth assessing specific anthocyanin-based combinations in further studies, especially in animal models.
In a recently completed human clinical trial, Czank et al. have determined the absorption, distribution, metabolism, and elimination of a 500 mg isotopically labeled cyanidin-3-glucoside in 8 male humans participants [56]. The study found that anthocyanins were comparatively more bioavailable, with their metabolites present in the circulation for 48 h after ingestion [56]. Thomasset and colleagues have investigated the chemopreventive effect anthocyanins against colorectal cancer in a pilot study, in 25 patients scheduled to undergo resection of primary tumor or liver metastases [57]. The authors found that patients receiving mirtocyan (an anthocyanin-rich standardized bilberry extract containing 0.5–2.0 g anthocyanins) daily for 7 days before surgery, generated enough concentrations of anthocyanins in plasma and tumor tissue, and decrease cell proliferation by 7% compared with pre-intervention values [57]. According to an estimate, total anthocyanin levels in fresh grapes range from 0.50 g/kg to 4.99 g/kg depending on the varieties of the grapes [58]. These findings suggest that anthocyanins may offer chemopreventive and therapeutic potential, and needs to be evaluated in specific combinations.
Challenges and hopes
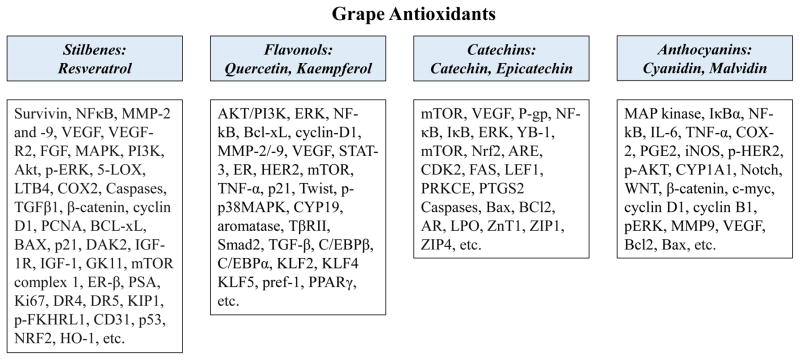
As discussed above and presented in figures 1 and 2, grape antioxidants seem to influence multiple regulatory genes and hallmark characteristic features of cancer, which contribute significantly to cancer development and progression. The ideal combinatorial approaches could be among agents with non-overlapping activities to yield additive or synergistic response, based on actions on different molecular targets. Unfortunately, there is limited literature available in this regard. Indeed, future studies need to be focused to identify such combinations. In addition, other common challenges with combination chemoprevention is the lack of immediate effects (especially for therapy regimens) and fear of unexpected toxic interactions among the agents. However, in the case of intra-combination among grape antioxidants, the toxicity is likely to be minimal due to the natural compatibility of the agents of interest. Moreover, the combination of grape antioxidants within its natural matrix are expected to affect metabolism, and therefore improved bioavailability to be advantageous in cancer management. Resveratrol has been well-appreciated for its beneficial effects. However, the issue of limited bioavailability of resveratrol appears to be hindering its potential use in cancer management. It has been shown that resveratrol is quickly metabolized in vivo to its sulfates and glucuronides, yielding low bioavailability of free agent. In a study, De Santi and colleagues found that several flavonoids (quercetin, apigenin, fisetin, kaempferol, and myricetin) inhibit the hepatic and duodenal sulphation of resveratrol [59]. In continuation, in a separate study, the authors further observed that these flavonoids also inhibit hepatic glucuronidation of resveratrol [60]. Interestingly, all these flavonoids occur naturally in grapes, suggesting that this and other similar combinations need to be carefully analyzed in future well-planned investigations.
Figure 1.
Known molecular targets of selected grape antioxidants
Figure 2.
Cellular processes known to be modulated by grape antioxidants
The identification of synergism is generally straightforward when natural agents work in the same direction, such as functioning as antioxidants. However, a challenge is posed when two or more natural agents may negate their response, depending on the cell environment, dose and ratio of combination used. Many antioxidants are shown to have prooxidant activities that could lessen or abrogate the antioxidant properties, depending on specific combinations and conditions. However, the recent studies suggest that the antioxidant or pro-oxidant activity of natural agents closely depends on their dose, often serving as antioxidants to maintain redox homeostasis, at physiological doses [61]. Although antioxidants of grape in combination are likely to have natural compatibility, it is difficult to predict the synergism without well-planned studies. Therefore, the effects of the combination among grape antioxidants should be assessed using isobologram and combination-index methods, derived from the median-effect principle of Cho and Talalay [62]. Once synergism among antioxidants of interest is established, they may be evaluated in smaller human clinical trials.
Conclusion
Although not studied in much detail, it is conceivable that a combination of multiple (more than two) ingredients of grapes alone or together with other chemopreventive- or even chemotherapeutic agents could provide ‘additive synergism’ at multiple levels by more than one of the following: 1) targeting multiple pathways to enhance the response, 2) lowering the effective dose of agents to limit toxicity, 3) modifying the same targets to enhance effectiveness, and 4) enhancing the bio-availability of active agent(s). This may even lead to even broader synergy and, therefore, much-improved outcome. For example, a study by Nadova et al. compared the antioxidant properties of grape flavonoids rutin, myricetin, kaempferol and isorhamnetin with emphasis on the association of these antioxidant properties with their effects on the therapeutic efficacy of cytarabine, a chemotherapy agent against L1210 leukemia cells [63]. The authors found that the anti-proliferative effects were not correlative to their antioxidant properties, and combinational treatment of isorhamnetin, kaempferol, and myricetin with cytarabine led to synergism in their antiproliferative activities [63]. Œlusarz and colleagues investigated the cancer chemoprevention abilities of resveratrol, quercetin, curcumin, apigenin, baicalein, genistein, epigallocatechin 3-gallate (EGCG), both under in vivo conditions in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice as well as in vitro in prostate cancer cell lines [64]. Interestingly, when all seven natural agents were supplemented in combination as pure compounds or as crude plant extracts, well-differentiated carcinoma of the mouse prostate was significantly prevented. The in vitro data showed that all seven compounds inhibit the growth of human and mouse prostate cancer cell lines. The study further found that genistein, curcumin, EGCG, and resveratrol inhibit Hedgehog signaling monitored by Gli1 mRNA concentration or by Gli reporter activity [64].
Similarly, in a recent study, Kausar and colleagues assessed the equimolar combination of five anthocyanidins (sugar-free counterparts of anthocyanins) (cyanidin, malvidin, peonidin, petunidin and delphinidin), and found synergistic cell growth inhibition in aggressive non-small-cell lung cancer cells H1299 and A549, with minimal effects on non-tumorigenic human bronchial epithelial cell (Beas2b) viability [65]. The study suggested that the superior combinatorial effects were possibly due to effect on multiple oncogenic signaling events, including WNT and Notch pathways, and targets like cyclin D1, cyclin B1, β-catenin, c-myc, MMP9, pERK, VEGF, Bcl2, PARP, TNFα, and NF-kB. In vivo in xenografts mouse model, the mixture of anthocyanidins at much lower dose was equally potent, further emphasizing the concept of additive synergism [65]. Keeping these things in consideration, a combination of grape antioxidants alone or in conjunction with other agents or chemotherapeutic modalities likely to provide a much-improved response, possibly even at a late stage of disease progression.
Although the combination of dietary agents derived from the grapes is likely to have limited toxicity, for an optimal combinatorial strategy, it’s critical to pay intense attention to the safety issues associated with novel combinations. Indeed, grapes are considered a superior fruit in a variety of cultures and medicine systems such as in Ayurveda, which is one of the world's oldest medicine systems. The well-known phrase, ‘too much of a good thing can make you sick’, almost always seems to be true. Even the safest food could be toxic in certain situations. For example, a study has reported that 43 dogs who ingested grapes, raisins, or both, developed increased levels of blood urea nitrogen, serum creatinine, or both as well as clinical symptoms like vomiting, lethargy, diarrhea, and anorexia [66]. Thus, the optimal dose regimen for combinatorial strategies needs to be decided based on combination-index methods, derived from the median-effect principle of Cho and Talalay [62]. Accordingly, the synergism should be assessed based on the reduction of dose and toxicity, as well as minimize/delay the induction of drug resistance, if any. Extensive further studies, especially in vivo studies, are needed to design and characterized the best possible disease-specific optimal combinations. This calls for concerted efforts from researchers from multiple disciplines encompassing biomedicine, pharmaceutics, and clinics.
Acknowledgments
This work was supported, in part, by funding from the NIH (R21CA176867) and a grant from the California Table Grape Commission.
List of abbreviations
- CI
combination index
- ER
estrogen receptor
- PWGPE
grape pomace extract
- DMBA
7,12-Dimethylbenz[a]anthracene
- C3G
cyanidin-3-glucoside
- P3G
peonidin-3-glucoside
- HER2
human epidermal growth factor receptor 2
- EGCG
epigallocatechin 3-gallate
- UVB
ultraviolet B
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Singh CK, George J, Ahmad N. Resveratrol-based combinatorial strategies for cancer management. Ann N Y Acad Sci. 2013;1290:113–121. doi: 10.1111/nyas.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pezzuto JM. Grapes and human health: a perspective. J Agric Food Chem. 2008;56:6777–6784. doi: 10.1021/jf800898p. [DOI] [PubMed] [Google Scholar]
- 7.Boileau TW, Liao Z, Kim S, Lemeshow S, et al. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 8.Thiagarajan DG, Bennink MR, Bourquin LD, Kavas FA. Prevention of precancerous colonic lesions in rats by soy flakes, soy flour, genistein, and calcium. Am J Clin Nutr. 1998;68:1394S–1399S. doi: 10.1093/ajcn/68.6.1394S. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz B, Birk Y, Raz A, Madar Z. Nutritional-pharmacological combinations--a novel approach to reducing colon cancer incidence. Eur J Nutr. 2004;43:221–229. doi: 10.1007/s00394-004-0462-6. [DOI] [PubMed] [Google Scholar]
- 10.Constantinou AI, White BE, Tonetti D, Yang Y, et al. The soy isoflavone daidzein improves the capacity of tamoxifen to prevent mammary tumours. Eur J Cancer. 2005;41:647–654. doi: 10.1016/j.ejca.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Singh CK, Liu X, Ahmad N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann N Y Acad Sci. 2015;1348:150–160. doi: 10.1111/nyas.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: Challenges for clinical translation. Biochim Biophys Acta. 2015;1852:1178–1185. doi: 10.1016/j.bbadis.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang M, Cai L, Udeani GO, Slowing KV, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 14.Miles SL, McFarland M, Niles RM. Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human disease. Nutr Rev. 2014;72:720–734. doi: 10.1111/nure.12152. [DOI] [PubMed] [Google Scholar]
- 15.Kwon do Y, Kim SJ, Lee JW, Kim YC. Comparison of hydroxyl radical, peroxyl radical, and peroxynitrite scavenging capacity of extracts and active components from selected medicinal plants. Toxicol Res. 2010;26:321–327. doi: 10.5487/TR.2010.26.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun ZJ, Chen G, Hu X, Zhang W, et al. Activation of PI3K/Akt/IKK-alpha/NF-kappaB signaling pathway is required for the apoptosis-evasion in human salivary adenoid cystic carcinoma: its inhibition by quercetin. Apoptosis. 2010;15:850–863. doi: 10.1007/s10495-010-0497-5. [DOI] [PubMed] [Google Scholar]
- 17.Gibellini L, Pinti M, Nasi M, Montagna JP, et al. Quercetin and cancer chemoprevention. Evid Based Complement Alternat Med. 2011;2011:591356. doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ElAttar TM, Virji AS. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anticancer Drugs. 1999;10:187–193. doi: 10.1097/00001813-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Elattar TM, Virji AS. The effect of red wine and its components on growth and proliferation of human oral squamous carcinoma cells. Anticancer Res. 1999;19:5407–5414. [PubMed] [Google Scholar]
- 20.Melzig MF, Escher F. Induction of neutral endopeptidase and angiotensin-converting enzyme activity of SK-N-SH cells in vitro by quercetin and resveratrol. Pharmazie. 2002;57:556–558. [PubMed] [Google Scholar]
- 21.Mertens-Talcott SU, Percival SS. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005;218:141–151. doi: 10.1016/j.canlet.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Mertens-Talcott SU, Talcott ST, Percival SS. Low concentrations of quercetin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in MOLT-4 human leukemia cells. J Nutr. 2003;133:2669–2674. doi: 10.1093/jn/133.8.2669. [DOI] [PubMed] [Google Scholar]
- 23.Del Follo-Martinez A, Banerjee N, Li X, Safe S, Mertens-Talcott S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr Cancer. 2013;65:494–504. doi: 10.1080/01635581.2012.725194. [DOI] [PubMed] [Google Scholar]
- 24.Zamin LL, Filippi-Chiela EC, Dillenburg-Pilla P, Horn F, et al. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009;100:1655–1662. doi: 10.1111/j.1349-7006.2009.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouria M, Gukovskaya AS, Jung Y, Buechler P, et al. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int J Cancer. 2002;98:761–769. doi: 10.1002/ijc.10202. [DOI] [PubMed] [Google Scholar]
- 26.Schlachterman A, Valle F, Wall KM, Azios NG, et al. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl Oncol. 2008;1:19–27. doi: 10.1593/tlo.07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo-Pichardo L, Dharmawardhane SF. Grape polyphenols inhibit Akt/mammalian target of rapamycin signaling and potentiate the effects of gefitinib in breast cancer. Nutr Cancer. 2012;64:1058–1069. doi: 10.1080/01635581.2012.716898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cote B, Carlson LJ, Rao DA, Alani AW. Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J Control Release. 2015;213:128–133. doi: 10.1016/j.jconrel.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 29.la Porte C, Voduc N, Zhang G, Seguin I, et al. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010;49:449–454. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 32.Nothlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;166:924–931. doi: 10.1093/aje/kwm172. [DOI] [PubMed] [Google Scholar]
- 33.Macpherson L, Matthews J. Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor alpha expression in human breast cancer cells. Cancer Lett. 2010;299:119–129. doi: 10.1016/j.canlet.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korashy HM, Abuohashish HM, Maayah ZH. The role of aryl hydrocarbon receptor- regulated cytochrome P450 enzymes in glioma. Curr Pharm Des. 2013;19:7155–7166. doi: 10.2174/13816128113199990583. [DOI] [PubMed] [Google Scholar]
- 36.Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol Rep. 2005;57:390–394. [PubMed] [Google Scholar]
- 37.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 38.Faustino RS, Sobrattee S, Edel AL, Pierce GN. Comparative analysis of the phenolic content of selected Chilean, Canadian and American Merlot red wines. Mol Cell Biochem. 2003;249:11–19. [PubMed] [Google Scholar]
- 39.Ebeler SE, Brenneman CA, Kim GS, Jewell WT, et al. Dietary catechin delays tumor onset in a transgenic mouse model. Am J Clin Nutr. 2002;76:865–872. doi: 10.1093/ajcn/76.4.865. [DOI] [PubMed] [Google Scholar]
- 40.Hakimuddin F, Tiwari K, Paliyath G, Meckling K. Grape and wine polyphenols down-regulate the expression of signal transduction genes and inhibit the growth of estrogen receptor-negative MDA-MB231 tumors in nu/nu mouse xenografts. Nutr Res. 2008;28:702–713. doi: 10.1016/j.nutres.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Jara-Palacios MJ, Hernanz D, Cifuentes-Gomez T, Escudero-Gilete ML, et al. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015;183:78–82. doi: 10.1016/j.foodchem.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Matito C, Mastorakou F, Centelles JJ, Torres JL, Cascante M. Antiproliferative effect of antioxidant polyphenols from grape in murine Hepa-1c1c7. Eur J Nutr. 2003;42:43–49. doi: 10.1007/s00394-003-0398-2. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J, Wang J, Chen Y, Agarwal R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3'-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20:1737–1745. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]
- 44.Quesada IM, Bustos M, Blay M, Pujadas G, et al. Dietary catechins and procyanidins modulate zinc homeostasis in human HepG2 cells. J Nutr Biochem. 2011;22:153–163. doi: 10.1016/j.jnutbio.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Hanausek M, Spears E, Walaszek Z, Kowalczyk MC, et al. Inhibition of murine skin carcinogenesis by freeze-dried grape powder and other grape-derived major antioxidants. Nutr Cancer. 2011;63:28–38. doi: 10.1080/01635581.2010.516474. [DOI] [PubMed] [Google Scholar]
- 46.Sorrenti V, Vanella L, Acquaviva R, Cardile V, et al. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int J Oncol. 2015;47:1303–1310. doi: 10.3892/ijo.2015.3130. [DOI] [PubMed] [Google Scholar]
- 47.Pratheeshkumar P, Son YO, Wang X, Divya SP, et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-kappaB signaling pathways in SKH-1 hairless mice skin. Toxicol Appl Pharmacol. 2014;280:127–137. doi: 10.1016/j.taap.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang WY, Wang J, Liu YM, Zheng QS, Li CY. Inhibitory effect of Malvidin on TNF- alpha-induced inflammatory response in endothelial cells. Eur J Pharmacol. 2014;723:67–72. doi: 10.1016/j.ejphar.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 49.Liu W, Xu J, Wu S, Liu Y, et al. Selective anti-proliferation of HER2-positive breast cancer cells by anthocyanins identified by high-throughput screening. PLoS One. 2013;8:e81586. doi: 10.1371/journal.pone.0081586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W, Xu J, Liu Y, Yu X, et al. Anthocyanins potentiate the activity of trastuzumab in human epidermal growth factor receptor 2-positive breast cancer cells in vitro and in vivo. Mol Med Rep. 2014;10:1921–1926. doi: 10.3892/mmr.2014.2414. [DOI] [PubMed] [Google Scholar]
- 51.Burton LJ, Smith BA, Smith BN, Loyd Q, et al. Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion, migration and osteoclastogenesis in prostate and breast cancer cells. Carcinogenesis. 2015;36:1019–1027. doi: 10.1093/carcin/bgv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandhu AK, Gu L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (Muscadine Grapes) As determined by HPLC-DAD-ESI-MS(n) J Agric Food Chem. 2010;58:4681–4692. doi: 10.1021/jf904211q. [DOI] [PubMed] [Google Scholar]
- 53.Signorelli P, Fabiani C, Brizzolari A, Paroni R, et al. Natural grape extracts regulate colon cancer cells malignancy. Nutr Cancer. 2015;67:494–503. doi: 10.1080/01635581.2015.1004591. [DOI] [PubMed] [Google Scholar]
- 54.Jung KJ, Wallig MA, Singletary KW. Purple grape juice inhibits 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 2006;233:279–288. doi: 10.1016/j.canlet.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Park SY, Lee YK, Lee WS, Park OJ, Kim YM. The involvement of AMPK/GSK3-beta signals in the control of metastasis and proliferation in hepato-carcinoma cells treated with anthocyanins extracted from Korea wild berry Meoru. BMC Complement Altern Med. 2014;14:109. doi: 10.1186/1472-6882-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Czank C, Cassidy A, Zhang Q, Morrison DJ, et al. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am J Clin Nutr. 2013;97:995–1003. doi: 10.3945/ajcn.112.049247. [DOI] [PubMed] [Google Scholar]
- 57.Thomasset S, Berry DP, Cai H, West K, et al. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev Res (Phila) 2009;2:625–633. doi: 10.1158/1940-6207.CAPR-08-0201. [DOI] [PubMed] [Google Scholar]
- 58.Balik J, Kumšta M, Rop O. Comparison of anthocyanins present in grapes of Vitis vinifera L. varieties and interspecific hybrids grown in the Czech Republic. Chemical Papers. 2013;67:1285–1292. [Google Scholar]
- 59.De Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of resveratrol, a natural compound present in wine, and its inhibition by natural flavonoids. Xenobiotica. 2000;30:857–866. doi: 10.1080/004982500433282. [DOI] [PubMed] [Google Scholar]
- 60.de Santi C, Pietrabissa A, Mosca F, Pacifici GM. Glucuronidation of resveratrol, a natural product present in grape and wine, in the human liver. Xenobiotica. 2000;30:1047–1054. doi: 10.1080/00498250010002487. [DOI] [PubMed] [Google Scholar]
- 61.Bouayed J, Bohn T. Exogenous antioxidants - Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 63.Nadova S, Miadokova E, Cipak L. Flavonoids potentiate the efficacy of cytarabine through modulation of drug-induced apoptosis. Neoplasma. 2007;54:202–206. [PubMed] [Google Scholar]
- 64.Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, et al. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382–3390. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 65.Kausar H, Jeyabalan J, Aqil F, Chabba D, et al. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012;325:54–62. doi: 10.1016/j.canlet.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 66.Eubig PA, Brady MS, Gwaltney-Brant SM, Khan SA, et al. Acute renal failure in dogs after the ingestion of grapes or raisins: a retrospective evaluation of 43 dogs (1992-2002) J Vet Intern Med. 2005;19:663–674. doi: 10.1111/j.1939-1676.2005.tb02744.x. [DOI] [PubMed] [Google Scholar]