Abstract
This study aimed to analyze 123 genotypes of Capsicum baccatum L. originating from 22 countries, at two stages of fruit development, for vitamin C content and its relationship with reducing sugars in fruit pericarp. Among the parametric population, vitamin C and reducing sugar concentrations ranged between 2.54 to 50.44 and 41–700 mg g−1 DW of pericarp, respectively. Overall, 14 genotypes accumulated 50–500% of the RDA of vitamin C in each 2 g of fruit pericarp on a dry weight basis. Compared with ripened fruits, matured (unripened) fruits contained higher vitamin C and lower reducing sugars. About 44% variation in the vitamin C content could be ascribed to levels of reducing sugars. For the first time, this study provides comprehensive data on vitamin C in the world collection of C. baccatum genotypes that could serve as a key resource for food research in future.
Keywords: Capsicum baccatum L., Ascorbic acid, Vitamin C, Germplasm, Fruit development, Reducing sugars
1. Introduction
l-ascorbic acid, otherwise known as vitamin C, is commonly in fruits and vegetables. The importance of vitamin C in human health has been reviewed elsewhere (Naidu, 2003; Wahyuni, Ballester, Sudarmonowati, Bino, & Bovy, 2013). Vitamin C is known to have a key role in the maintenance of collagen, a major body structural protein (Levin, 1986), wound repair and healing process (Naidu, 2003; Shukla, 1969), synthesis of muscle carnitine, which is important for fatty acid transport and energy production (Hulse, Ellis, & Henderson, 1978), dietary absorption of iron (Hallberg, 1981), and prevention or relief from the common cold (Pauling, 1970). Although inconclusive, the role of vitamin C in reduced risk of cardiovascular disease and certain cancers cannot be ignored (Naidu, 2003). It has been shown in vitro that the effects of low dose pesticides on cell viability and reactive oxygen species production can be minimized by quantities of vitamin C equivalent to the recommended daily allowance (RDA) (Perla, Perrin, & Greenlee, 2008). The RDA for vitamin C, set by the US Food and Nutrition Board, for adult men is 90 mg/day and for adult women is 75 mg/day. However, the tolerable Upper intake Level (UL) of vitamin C for adults is 2 g/day (US Food and Nutrition Board, 2000).
The amount of vitamin C available in vegetables for human consumption varies widely. In one study, red and green chilli (Capsicum annum var. longum), kale (Brassica oleracea L. var. alboglabra L. H. Bailey), and red cabbage (Brassica oleracea var. capitata L. (f. Rubra)) were recorded as containing very high levels of ascorbic acid among 66 vegetables tested. In fact, the highest amount of vitamin C (>2 mg/g FW) was recorded in red chilli (Isabelle et al., 2010). In another study, 32 accessions belong to four pepper species, viz. Capsicum annuum, Capsicum frutescens, Capsicum chinense and C. baccatum, vitamin C levels ranged from 20.45 (C. baccatum) to 205.94 mg/100 g FW (C. annuum) (Wahyuni, Ballestera, Sudarmonowatib, Binoa, & Bovya, 2011). Similar studies on 63 to 216 accessions of C. chinense, obtained from North, Central, and South America, contained up to 1466 mg vitamin C/100 g FW (Antonious, Lobel, Kochhar, Berke, & Jarret, 2009; Jarret, Berke, Baldwin, & Antonious, 2009). Estimated daily intake of fresh Capsicum fruits by Americans was about 22 g in 2014 (Wells, Bond, & Thornsbury, 2015). Several pepper genotypes are able to supply between 50% to more than 100% of the recommended daily intake (RDI) of vitamin C (Howard, Talcott, Brenes, & Villalon, 2000; Wahyuni et al., 2013). These findings suggest there is a scope for further exploration of Capsicum species for vitamin C supply.
Hancock and Viola (2005) and Gest, Gautier, and Stevens (2013) have reviewed various aspects of vitamin C biosynthesis in plants. Vitamin C synthesis in plants involves myo-inositol, l-gulose, l-galactose and/or d-galacturonic acid pathways. Not all the enzymes in these pathways have been identified in plants. However, l-gulose and l-galactose pathways are routed from GDP-D-mannose. Many biosynthetic pathways for vitamin C in higher plants might have a role in tissue- and organ-specific differences (Hancock & Viola, 2005). The majority of plants and animals synthesize ascorbic acid from d-glucose or d-galactose (Naidu, 2003), both reducing sugars. It is possible that the GDP-mannose pathway is the major vitamin C biosynthesis pathway in Arabidopsis (Dowdle, Ishikawa, Gatzek, Rolinski, & Smirnoff, 2007), but an alternative d-galacturonic acid pathway also exists in strawberry (Agius et al., 2003) and tomato (Badejo et al., 2012). These pathways might regulate vitamin C levels, with other pathways, or exhibit stage-specific response in these fruits (Badejo et al., 2012; Cruz-Rus, Amaya, Sanchez-Sevilla, Botella, & Valpuesta, 2011). Marín, Ferreres, Tomás-Barberán, and Gil (2004) reported that in sweet peppers (C. annuum L.), vitamin C accumulation increased with maturity and reached the highest levels in red ripened fruits. In another study, however, vitamin C levels in tomato and bell pepper (C. annuum) fruits decreased 74 and 51 days, respectively, after the fruit set (Yahiaa, Contreras-Padillaa, & Gonzalez-Aguilarb, 2001). From these reports, it is not clear whether vitamin C accumulation in pepper fruits is specific to genotype or species. Investigation of a large number of genotypes in a species may reveal the true pattern of vitamin C accumulation in this species.
Earlier studies on vitamin C content in Capsicum species were limited to few genotypes, regions or continents. Controversial reports exists on the stage in fruit development at which highest vitamin C is accumulated. Similarly, the relationship between reducing sugars and vitamin C in C. baccatum genotypes, which are widespread throughout the South America, is also not clear. Thus, the objective of this study was to analyze vitamin C in the world collection of 123 C. baccatum genotypes, and understand better the relationships between vitamin C and reducing sugars concentrations and fruit development. In this study, fully matured (un-ripened) and ripened fruit, which are the two major stages in harvest and consumption, were analyzed for vitamin C and reducing sugars, specifically d-glucose and d-galactose. We also attempted to identify the genotypes that might be able to supply at least half the RDA of vitamin C in human diet.
2. Materials and methods
2.1. Collection of fruit samples
Matured unripe and ripened fruits from 123 genotypes of C. baccatum were examined in this study. These genotypes were obtained previously from the Asian Vegetable Research and Development Center (AVRDC), Taiwan. This collection represents 22 countries, viz. Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, France, Germany, Guatemala, Guyana, India, Jamaica, Kenya, Mexico, Netherlands, Paraguay, Peru, UK, USA and Zambia. All these genotypes were grown in a field at Sissonville (WV, USA) during the summer of 2013 using standard cultural practices. Three plants were selected randomly from each genotype for sample collection. For each stage approximately 300 g (not less than five fruits when fruit size was bigger) of fruits were collected from each plant in a plastic zipper bag. Three zipper bags of fruits collected from three independent plants in each genotype were kept in a larger zipper bag and transferred to laboratory on ice.
Fruits from each zipper bag dipped in sufficient liquid nitrogen until frozen and returned to the same zipper bag before being stored at −80 °C for further analysis.
2.2. PBS extraction
From stored samples, approximately 20 g of fruits (not less than five fruits when size was bigger) from each plant were used to isolate pericarp. A small portion of the pericarp from each fruit was pooled to make up 2.5 g. This pooled sample was ground to fine paste with sand in a pestle and mortar, and mixed with 5 ml of ice cold phosphate buffered saline (PBS; without calcium chloride; without magnesium chloride; pH 7.4) (Life Technologies, Grand Island, USA). This mixture was centrifuged at 5000 × g at 5–8 °C for 5 min, and the supernatant collected and stored at −80 °C.
2.3. Estimation of pericarp dry matter
After separating the pericarp for extraction, remainder from all fruit of the same genotype was pooled, weighed, and dried in an oven at 65 ± 2 °C for 72 h. After drying, samples were weighed and the percentage dry matter estimated.
2.4. Estimation of vitamin C
Vitamin C (l-ascorbic acid) in extracted samples was estimated using a protocol adopted from Kapur et al. (2012) with modifications. Briefly, extracts were thawed on equal amounts of ice and water, and kept on ice until use. Samples were vortexed before and after every step in the procedure. 500 µL was mixed with an equal amount of 3% metphosphoric acid (Sigma–Aldrich, St. Louis, USA) and the mixture centrifuged at 10,000×g for 15 min in a table top microcentrifuge (eppendorf, Hauppauge, USA). 280 µL of the supernatant was collected and mixed with 14 µL of bromine concentrate (0.05 mol L−1 commercial solution; Sigma–Aldrich). To this solution, 14 µL of 4% thiourea (Sigma–Aldrich) was added and mixed well. Then, 70 µL of 2% DNPH (2,4-dinitrophenylhydrazine) (Sigma–Aldrich) was added and the mixture incubated at 35 ± 2 °C for 3 h. After incubation, the samples were kept on ice and 350 µL of cold sulfuric acid (85%) (Sigma–Aldrich) added. After 5 min, 80 µL was transferred to a glass 96 well plate (Cayman Chemical, Ann Arbor, USA), and the absorbance read at 520 nm in a microplate reader (Synergy HT™, BioTek Instruments, Winooski, USA).
Metphosphoric acid (3%) was prepared by dissolving 15 g of metphosphoric acid in 40 mL of glacial acetic acid (Sigma–Aldrich) and made up to 500 mL with distilled water. Sulfuric acid (4.5 mol L−1) was used as a solvent for thiourea and DNPH solutions. DNPH solution was filtered using glass microfiber filters (GE Healthcare Bio-Sciences, Pittsburgh, USA). Freshly prepared ascorbic acid in PBS was used as a standard.
2.5. Estimation of reducing sugars
Reducing sugars in the samples were estimated using a protocol adopted from Perla, Holm, and Jayanty (2012). Glucose (Sigma–Aldrich) in PBS was used as a standard.
2.6. Statistical analysis
Unless otherwise stated, all the experiments were conducted with six replicates, and data are presented as mean ± standard deviation. Statistical analysis was performed using SAS® software (Version 9.4, Cary, NC) using BASE SAS, DATA step, PROC IMPORT, PROC CONTENTS, PROC SORT, PROC TRANSPOSE, PROC FORMAT, PROC CORR, PROC REG, PROC UNIVARIATE, PROC SGSCATTER, PROC SGPLOT, PROC TRANSREG, PROC STDIZE, PROC GLM, PROC NPAR1WAY, PROC SQL, PROC MEANS, PROC TABULATE, PROC PRINT and SAS MACRO programs. Pearson correlation analysis, analysis of variance (ANOVA), and Student–Newman–Keuls (SNK) test were performed on parametric datasets at the p ≤ 0.05 significance level. Non-parametric data sets were analyzed using Kruskal–Wallis test.
3. Results and discussion
3.1. Large variation exists in vitamin C levels in the world collection of C. baccatum genotypes
Parametric vitamin C concentrations in the pericarp of matured unripe and ripe fruits from the world collection of C. baccatum genotypes are presented in Table 1. The highest amount of vitamin C was found in the ripened fruits of Bird’s eye hot peppers (strain 3) (50.44 mg g−1 DW) originating from Guyana. The matured unripe fruits of the genotype VI028776 (Argentina) contained the least ascorbic acid (2.54 mg g−1 DW). Combined mean data for the both, matured unripe and ripen fruits suggested the top five genotypes, with highest vitamin C levels, were Bird’s eye hot peppers (strain 3) (49.78 mg g−1 DW; Guyana), VI044347 (39.82 mg g−1 DW; India), PI 543178 01 SD (39.3 mg g−1 DW; Bolivia), VI028794 (29.52 mg g−1 DW; Jamaica), and VI013034 (26.87 mg g−1 DW; UK). Compared with ripe fruit, VI028794 did not accumulate significant amounts of vitamin C at the matured unripe stage. VI028776 (3.31 mg g−1 DW; Argentina), VI029111 (3.49 mg g−1 DW; Brazil), VI029079 (3.66 mg g−1 DW; Ecuador), VI012959 (4.36 mg g−1 DW; Germany), and VI029047 (4.51 mg g−1 DW; India) were the five genotypes with least vitamin C. These findings suggest there is a large variation in parametric vitamin C concentrations among the world collection of C. baccatum genotypes, and this variation could be attributed to accumulation of significant amounts of vitamin C at either one or both stages in fruit development. This kind of large variation in total ascorbic acid values among large number of genotypes was also reported in C. chinense recently (Jarret et al., 2009).
Table 1.
Vitamin C (mg g−1 DW) in the pericarp of matured and ripened fruits of world collection of C. baccatum genotypes.
| S. No. | Genotype | Origin | Matured (M) | Ripened (R) | Combined (C) | SNK^ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Mean | Std | M | R | C | |||
| 1 | 281408 01 SD | Peru | 10.72 | 1.04 | 10.27 | 1.07 | 10.49 | 1.03 | |||
| 2 | Aji amarillo small | Peru | 7.72 | 0.70 | 6.71 | 0.46 | 7.22 | 0.77 | |||
| 3 | Aji benito hot peppers | Bolivia | ND | ND | 6.31 | 0.46 | 6.31 | 0.46 | |||
| 4 | Aji cito hot peppers | Peru | 9.98 | 0.77 | 4.29 | 0.33 | 7.14 | 3.02 | |||
| 5 | Aji colorado hot peppers | Bolivia | ND | ND | 7.06 | 0.66 | 7.06 | 0.66 | |||
| 6 | Aji habanero hot peppers | Mexico | 13.17 | 1.27 | 14.15 | 2.79 | 13.66 | 2.13 | |||
| 7 | Bird’s eye hot peppers (strain 3) | Guyana | 49.12 | 5.85 | 50.44 | 16.87 | 49.78 | 12.06 | a | a | a** |
| 8 | CGN16973 | Bolivia | 25.22 | 2.35 | 16.86 | 10.60 | 21.04 | 8.53 | |||
| 9 | CGN17241 | USA | 6.31 | 0.46 | 6.22 | 0.34 | 6.27 | 0.39 | |||
| 10 | CGN19233 | Peru | 29.40 | 2.84 | 13.76 | 0.97 | 21.58 | 8.42 | |||
| 11 | CGN21479 | Peru | 29.47 | 2.76 | 10.64 | 0.83 | 21.40 | 9.89 | |||
| 12 | CGN21513 | Bolivia | 12.09 | 0.98 | 6.67 | 0.46 | 9.38 | 2.93 | |||
| 13 | CGN22834 | Brazil | 11.50 | 0.75 | 5.82 | 1.16 | 8.66 | 3.11 | |||
| 14 | CGN22858 | Brazil | 6.59 | 0.65 | 7.00 | 0.30 | 6.82 | 0.50 | |||
| 15 | CGN23763 | Brazil | 8.26 | 0.38 | 4.54 | 0.38 | 6.40 | 1.98 | |||
| 16 | GRIF 9219 01 SD | Costa Rica | 8.22 | 0.66 | 8.43 | 0.78 | 8.32 | 0.70 | |||
| 17 | PI 159252 01 SD | USA | 18.44 | 1.77 | 13.13 | 1.09 | 15.79 | 3.10 | |||
| 18 | PI 215699 02 SD | Peru | 16.91 | 1.28 | 8.69 | 0.72 | 12.80 | 4.41 | |||
| 19 | PI 215700 01 SD | Peru | 21.99 | 1.54 | 7.87 | 0.85 | 14.93 | 7.47 | |||
| 20 | PI 238061 01 SD | Bolivia | ND | ND | 7.21 | 0.48 | 7.21 | 0.48 | |||
| 21 | PI 497974 01 SD | Brazil | 12.59 | 0.97 | NP | NP | 14.10 | 1.97 | |||
| (15.60) | (1.48) | ||||||||||
| 22 | PI 543178 01 SD | Bolivia | 41.23 | 4.59 | 37.37 | 4.12 | 39.30 | 4.62 | a | bc | ab* |
| 23 | PI 640885 01 SD | India | 29.08 | 3.40 | 13.76 | 0.94 | 21.42 | 8.35 | |||
| 24 | Uba tuba (Christmas bell) | Brazil | 10.07 | 0.84 | 9.26 | 0.75 | 9.66 | 0.87 | |||
| 25 | VI012279 | Ecuador | 19.17 | 1.33 | 9.72 | 0.63 | 14.44 | 5.04 | |||
| 26 | VI012422 | USA | 9.14 | 0.78 | 10.93 | 0.43 | 10.04 | 1.11 | |||
| 27 | VI012673 | UK | 19.53 | 1.16 | 4.41 | 0.41 | 11.97 | 7.94 | |||
| 28 | VI012898 | France | 12.40 | 1.04 | 16.99 | 1.04 | 14.69 | 2.59 | |||
| 29 | VI012959 | Germany | 4.17 | 0.35 | 4.55 | 0.24 | 4.36 | 0.35 | w | r | |
| 30 | VI012983 | USA | 10.51 | 1.11 | 10.51 | 1.21 | 10.51 | 1.11 | |||
| 31 | VI013034 | UK | 24.88 | 2.31 | 28.86 | 2.67 | 26.87 | 3.16 | b* | ||
| 32 | VI013036 | Germany | 6.28 | 0.39 | 9.34 | 0.92 | 7.81 | 1.74 | |||
| 33 | VI013261 | USA | ND | ND | 30.55 | 2.95 | 30.55 | 2.95 | b* | ||
| 34 | VI013262 | USA | 42.24 | 5.09 | 11.22 | 0.88 | 26.73 | 16.57 | ab | * | |
| 35 | VI013290 | UK | 22.20 | 1.75 | 7.96 | 0.73 | 15.08 | 7.54 | |||
| 36 | VI013395 | Paraguay | 17.22 | 1.22 | 10.04 | 0.86 | 13.63 | 3.88 | |||
| 37 | VI013425 | Argentina | 12.08 | 1.11 | 6.38 | 0.42 | 9.23 | 3.08 | |||
| 38 | VI013462 | UK | 17.35 | 1.37 | 5.09 | 0.50 | 11.22 | 6.48 | |||
| 39 | VI013477 | Netherlands | 8.67 | 0.70 | 7.16 | 1.25 | 7.91 | 1.25 | |||
| 40 | VI013973 | El Salvador | 14.06 | 1.04 | 7.30 | 0.75 | 10.68 | 3.64 | |||
| 41 | VI014229 | Unknown | 11.39 | 0.89 | 8.03 | 0.71 | 9.71 | 1.91 | |||
| 42 | VI014230 | Peru | 17.09 | 1.32 | 37.38 | 5.03 | 27.23 | 11.16 | bc | * | |
| 43 | VI014270 | Costa Rica | ND | ND | 6.48 | 0.62 | 6.48 | 0.62 | |||
| 44 | VI014892 | Ecuador | 11.39 | 0.77 | 13.55 | 1.28 | 12.47 | 1.52 | |||
| 45 | VI028657 | Costa Rica | 10.94 | 0.71 | 15.89 | 1.83 | 13.42 | 2.90 | |||
| 46 | VI028776 | Argentina | 2.54 | 0.14 | 4.08 | 1.01 | 3.31 | 1.06 | y | w | r |
| 47 | VI028777 | Bolivia | 13.08 | 0.85 | NP | NP | 102.88 | 95.22 | *** | ||
| (192.68) | (24.32) | ||||||||||
| 48 | VI028780 | Bolivia | 17.44 | 1.21 | 11.01 | 0.88 | 14.22 | 3.51 | |||
| 49 | VI028782 | Brazil | 13.67 | 1.35 | 7.55 | 0.60 | 10.61 | 3.35 | |||
| 50 | VI028788 | Chile | 26.43 | 3.51 | 6.15 | 0.47 | 16.29 | 10.85 | |||
| 51 | VI028791 | Costa Rica | 8.91 | 0.70 | 9.87 | 0.96 | 9.39 | 0.95 | |||
| 52 | VI028792 | Ecuador | 9.16 | 0.58 | 3.62 | 0.21 | 6.39 | 2.92 | wx | ||
| 53 | VI028793 | Ecuador | 14.53 | 0.97 | 13.41 | 1.15 | 13.97 | 1.17 | |||
| 54 | VI028794 | Jamaica | 23.93 | 2.58 | 35.11 | 7.74 | 29.52 | 8.02 | cd | b* | |
| 55 | VI028795 | Mexico | 6.26 | 0.50 | 5.44 | 0.47 | 5.85 | 0.63 | |||
| 56 | VI028797 | Mexico | 13.99 | 1.13 | 6.44 | 0.47 | 10.75 | 3.98 | |||
| 57 | VI028798 | Paraguay | 34.45 | 2.92 | 9.01 | 0.89 | 21.73 | 13.44 | |||
| 58 | VI028867 | Brazil | ND | ND | 11.20 | 0.87 | 11.20 | 0.87 | |||
| 59 | VI028870-A | Brazil | 43.45 | 5.74 | 11.75 | 1.12 | 27.60 | 17.02 | ab | * | |
| 60 | VI028870-B | Brazil | 21.03 | 1.87 | 14.95 | 0.48 | 17.99 | 3.43 | |||
| 61 | VI028873 | Brazil | 15.90 | 1.39 | 17.58 | 1.33 | 16.74 | 1.56 | |||
| 62 | VI028878 | Brazil | 11.56 | 1.20 | 16.61 | 1.80 | 14.08 | 3.02 | |||
| 63 | VI028886 | Brazil | 34.45 | 2.78 | 13.94 | 1.01 | 24.20 | 10.90 | * | ||
| 64 | VI028890 | Brazil | 20.57 | 1.67 | 9.47 | 0.81 | 15.02 | 5.93 | |||
| 65 | VI028895 | Brazil | 4.26 | 0.25 | NP | NP | 3.32 | 1.01 | r | ||
| (2.37) | (0.21) | ||||||||||
| 66 | VI028896 | Brazil | 31.44 | 3.47 | 7.83 | 0.81 | 19.63 | 12.56 | |||
| 67 | VI028897 | Brazil | 14.75 | 1.23 | 5.60 | 0.37 | 10.17 | 4.86 | |||
| 68 | VI028901 | Brazil | 13.57 | 0.81 | 6.69 | 0.46 | 10.13 | 3.64 | |||
| 69 | VI028902 | Brazil | 7.93 | 0.59 | 3.65 | 0.27 | 5.79 | 2.28 | wx | ||
| 70 | VI028910 | Brazil | 14.51 | 1.22 | 7.94 | 0.55 | 11.22 | 3.55 | |||
| 71 | VI028911 | Brazil | 16.19 | 1.27 | 9.99 | 0.98 | 13.09 | 3.41 | |||
| 72 | VI028939 | USA | 17.01 | 1.28 | 5.49 | 0.38 | 11.25 | 6.08 | |||
| 73 | VI028942 | USA | 13.26 | 1.27 | 6.67 | 0.56 | 9.97 | 3.57 | |||
| 74 | VI028968 | Chile | 8.26 | 0.72 | 17.71 | 1.43 | 12.98 | 5.05 | |||
| 75 | VI028969 | Colombia | 11.81 | 0.91 | 5.88 | 0.43 | 8.84 | 3.17 | |||
| 76 | VI028971 | Ecuador | 16.22 | 1.25 | 7.09 | 0.46 | 11.66 | 4.86 | |||
| 77 | VI028973 | Ecuador | NP | NP | 10.35 | 0.69 | 5.36 | 5.23 | |||
| (0.37) | (0.02) | ||||||||||
| 78 | VI029021 | Bolivia | 12.14 | 0.65 | 5.63 | 0.39 | 8.89 | 3.40 | |||
| 79 | VI029022 | Bolivia | 8.76 | 0.74 | 5.89 | 0.53 | 7.33 | 1.62 | |||
| 80 | VI029023 | Bolivia | ND | ND | 4.32 | 0.44 | 4.32 | 0.44 | r | ||
| 81 | VI029024 | Bolivia | 7.15 | 0.56 | 11.13 | 0.95 | 9.14 | 2.21 | |||
| 82 | VI029025-A | Bolivia | ND | ND | 9.07 | 1.04 | 9.07 | 1.04 | |||
| 83 | VI029025-B | Bolivia | ND | ND | 3.30 | 0.23 | 3.30 | 0.23 | x | r | |
| 84 | VI029025-C | Bolivia | 8.64 | 0.79 | 5.77 | 0.45 | 7.20 | 1.62 | |||
| 85 | VI029026 | Bolivia | NP | NP | 8.50 | 0.62 | 4.93 | 3.76 | |||
| (1.36) | (0.09) | ||||||||||
| 86 | VI029033-A | Bolivia | ND | ND | 17.46 | 1.63 | 17.46 | 1.63 | |||
| 87 | VI029044 | Brazil | 11.74 | 1.01 | 11.64 | 0.74 | 11.69 | 0.84 | |||
| 88 | VI029047 | India | 4.62 | 0.41 | 4.39 | 0.39 | 4.51 | 0.40 | qr | ||
| 89 | VI029050 | Guatemala | NP | NP | 5.83 | 0.45 | 4.08 | 1.86 | |||
| (2.33) | (0.14) | ||||||||||
| 90 | VI029057 | Brazil | 28.25 | 2.38 | 9.65 | 0.71 | 18.95 | 9.86 | |||
| 91 | VI029060 | Chile | NP | NP | 6.56 | 0.48 | 4.19 | 2.15 | |||
| (2.41) | (0.16) | ||||||||||
| 92 | VI029062 | Ecuador | 20.87 | 1.77 | 12.81 | 0.99 | 16.84 | 4.42 | |||
| 93 | VI029076 | Kenya | 4.17 | 0.30 | 4.91 | 0.35 | 4.54 | 0.49 | w | ||
| 94 | VI029079 | Ecuador | 4.02 | 0.26 | 3.30 | 0.27 | 3.66 | 0.45 | w | x | r |
| 95 | VI029081 | India | 11.61 | 1.30 | 17.83 | 1.99 | 14.72 | 3.62 | |||
| 96 | VI029084 | Brazil | 7.29 | 0.50 | 11.90 | 1.29 | 9.59 | 2.58 | |||
| 97 | VI029085 | Brazil | 6.64 | 0.33 | 7.02 | 0.41 | 6.80 | 0.40 | |||
| 98 | VI029091 | Brazil | 33.06 | 2.50 | NP | NP | 30.46 | 4.09 | ab* | ||
| (27.00) | (3.07) | ||||||||||
| 99 | VI029097 | Brazil | 15.05 | 1.06 | 14.88 | 1.36 | 14.96 | 1.17 | |||
| 100 | VI029098-B | Brazil | 7.90 | 0.62 | 9.25 | 0.71 | 8.67 | 0.95 | |||
| 101 | VI029101 | Brazil | 19.56 | 1.50 | 5.22 | 0.39 | 12.39 | 7.56 | |||
| 102 | VI029104 | Brazil | 8.98 | 0.78 | 6.53 | 0.50 | 7.75 | 1.42 | |||
| 103 | VI029110 | Brazil | 15.27 | 1.26 | 6.23 | 0.42 | 10.75 | 4.81 | |||
| 104 | VI029111 | Brazil | 2.75 | 0.20 | 4.24 | 0.29 | 3.49 | 0.81 | y | r | |
| 105 | VI029112 | Brazil | 19.47 | 1.99 | 7.86 | 0.77 | 13.67 | 6.23 | |||
| 106 | VI029113 | Brazil | 12.54 | 1.13 | 11.52 | 1.06 | 12.03 | 1.17 | |||
| 107 | VI029117 | Brazil | 9.51 | 0.67 | 3.24 | 0.27 | 6.37 | 3.31 | x | ||
| 108 | VI029124-A | Bolivia | 20.10 | 1.43 | 21.17 | 3.89 | 20.64 | 2.85 | |||
| 109 | VI029124-B | Bolivia | 5.30 | 0.16 | 9.59 | 0.64 | 7.45 | 2.29 | |||
| 110 | VI029511 | Colombia | 12.78 | 1.02 | 11.21 | 0.87 | 11.99 | 1.22 | |||
| 111 | VI031222 | Zambia | ND | ND | 6.96 | 0.79 | 6.96 | 0.79 | |||
| 112 | VI037435 | Brazil | 12.67 | 1.03 | 6.92 | 0.51 | 9.80 | 3.10 | |||
| 113 | VI044307 | Unknown | 15.95 | 1.38 | 12.15 | 1.65 | 14.05 | 2.46 | |||
| 114 | VI044310 | Peru | 14.30 | 1.17 | 8.02 | 0.78 | 11.16 | 3.41 | |||
| 115 | VI044347 | India | 48.82 | 4.13 | 30.81 | 2.78 | 39.82 | 9.99 | a | d | ab* |
| 116 | VI044352 | Unknown | 16.94 | 1.22 | 7.68 | 0.64 | 12.31 | 4.92 | |||
| 117 | VI047059-B | Peru | 24.72 | 2.29 | 4.78 | 0.32 | 14.75 | 10.53 | |||
| 118 | VI049287 | USA | 8.23 | 0.83 | 5.74 | 0.43 | 6.99 | 1.45 | |||
| 119 | VI049327 | USA | 10.68 | 0.93 | 18.00 | 1.75 | 14.34 | 4.05 | |||
| 120 | VI057369 | USA | 7.58 | 0.51 | 10.19 | 0.79 | 8.88 | 1.50 | |||
Statistical analysis was performed after logarithmic transformation of data. DW: Dry Weight. Std: Standard deviation. ND: Not Determined. NP: Non-Parametric (Refer Kruskal–Wallis test for NP-data; Fig. 1). Values in parenthesis are NP values. SNK: Student–Newman–Keuls test.
Top as well as bottom 5 genotype-ranks in each stage are denoted separately by lower case letters in descending order; Means with the same letter are not significantly different. More than 5 rankings are displayed when ranked genotypes had ’ND’ or ’NP’ in any stage.
Each 2 g DW sample supplies ≥ 50% RDA of vitamin C.
Each 2 g DW sample supplies ≥ 100% RDA of vitamin C.
Each 2 g DW sample supplies ≥ 200% RDA of vitamin C. RDA of vitamin C for adult men = 90 mg day−1. Number of observations/stage/genotype: 6–8.
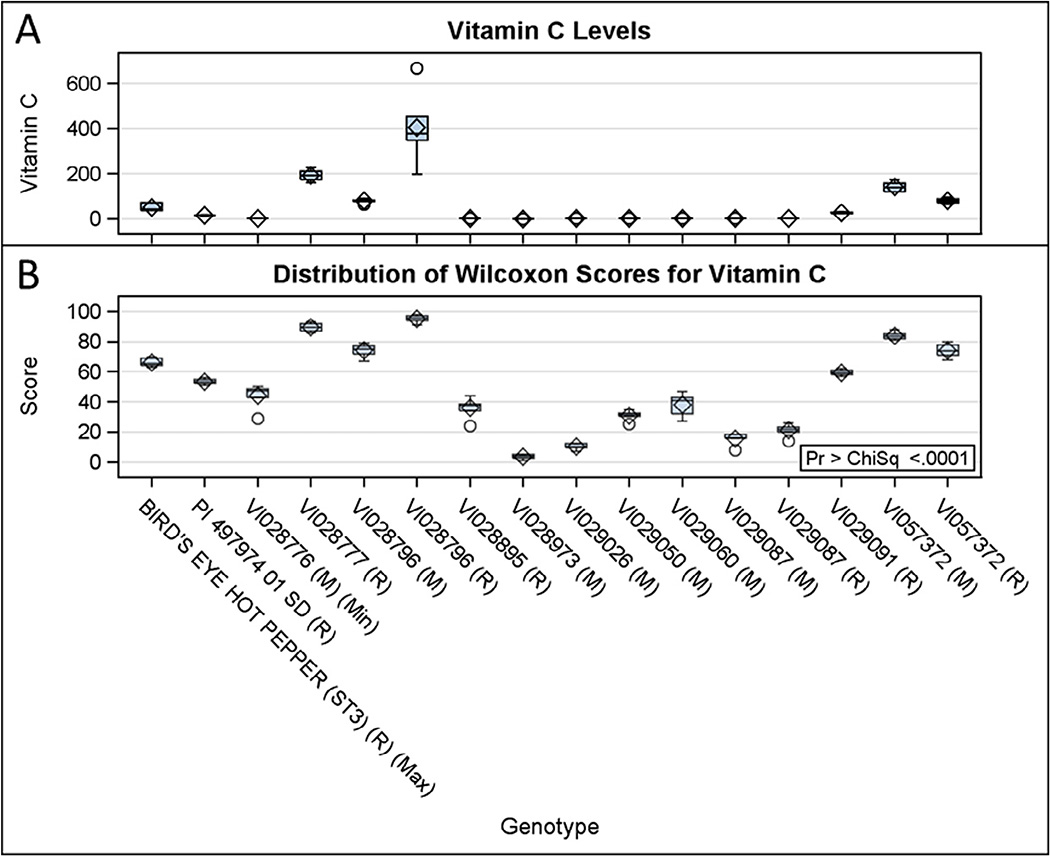
Distribution of Wilcoxon scores for non-parametric vitamin C data is presented in Fig. 1. Among them, only a few genotypes (matured stage of VI028777, Bolivia; and both the stages of VI028796, Mexico; and VI057372, USA) accumulated higher concentrations of vitamin C compared with ripe Bird’s eye hot peppers (strain 3) (Guyana). Similarly, only a few genotypes (matured stage of VI028973, Ecuador; VI029026, Bolivia; and VI029050, Guatemala; and both the stages of VI029087, Brazil) synthesized less vitamin C than the mature unripe stage of VI028776 (Argentina). Vitamin C concentrations in other genotypes (matured stage of VI028895, Brazil; and VI029060, Chile; and ripened stage of PI 49797401 SD, Brazil; and VI029091, Brazil) were within the range identified in the parametric data, as shown in Table 1. Combined mean values of vitamin C for the both, matured unripe and ripen fruits in VI028777 (102.89 mg g−1 DW), VI028796 (242 mg g−1 DW) and VI057372 (110.6 mg g−1 DW) were two- to five-times higher than combined mean value for Bird’s eye hot peppers (strain 3) (49.78 mg g−1 DW) (Table 1; Fig. 1). Combined mean value for vitamin C in VI029091 (30 mg g−1 DW) was much less than combined mean value for Bird’s eye hot peppers (strain 3).
Fig. 1.
Kruskal–Wallis test for non-parametric vitamin C data was performed on either one or two developmental fruit stages of 11 genotypes (n = 6). (A) Vitamin C levels in fruit pericarp (mg g−1 DW); (B) Distribution of Wilcoxon scores for vitamin C levels. Matured and ripened stages of fruits are denoted by ‘M’ and ‘R’, respectively. Genotypes with maximum (‘Max’) and minimum (‘Min’) vitamin C levels from parametric data are included here for reference (refer Table 1). VI028796, VI029087 and VI057372 are originated from Mexico, Brazil and USA, respectively. Refer Table 1 for origin of all other genotypes.
Estimated daily intake of fresh Capsicum fruits by Americans was about 22 g in 2014 (Wells et al., 2015). The moisture content of harvested fruits in the present study was about 90% (data not shown). Thus, 22 g of fresh Capsicum fruits is equal to 2.2 g of dry fruits. Therefore, in the present study, any genotype that accumulated ≥45 mg of vitamin C (combined mean value) in 2 g of fruit pericarp DW would provide more than half the RDA for vitamin C (Supplementary Table S1; Table 1; Fig. 1). We identified one genotype (VI028796, Mexico) that would provide ≥ 500% RDA, two genotypes (VI028777, Bolivia; and VI057372, USA) ≥ 200% RDA, one genotype (BIRD’S EYE HOT PEPPER STRAIN 3, Guyana) ≥ 100% RDA, and ten genotypes (PI 543178 01 SD, Bolivia; VI013034, UK; VI013261, USA; VI013262, USA; VI014230, Peru; VI028794, Jamaica; VI028870-A, Brazil; VI028886, Brazil; VI029091, Brazil; and VI044347, India) with more than half the RDA for vitamin C in the collection. Similar reports, with up to 461% of the RDA for vitamin C, have been published describing different Capsicum species (Howard, Smith, Wagner, Villalon, & Burns, 1994; Howard et al., 2000; Lee, Howard, & Villalon, 1995; Osuna-Garcia, Wall, & Waddell, 1998; Simmone, Simmone, Eitenmiller, Mills, & Green, 1997). Genotypes of C. baccatum from this study might serve as functional foods for vitamin C in the human diet.
3.2. Large variation exists in reducing sugar levels in the world collection of C. baccatum genotypes
Parametric reducing sugar concentrations in the pericarp of mature unripe and ripen fruits of the world collection of C. baccatum genotypes are presented in Table 2. Among these, ripe VI029085 (Brazil) and mature unripe VI028794 (Jamaica) recorded the highest (700 mg g−1 DW) and lowest (41 mg g−1 DW) amounts of reducing sugars, respectively. Among the combined mean values, the five genotypes with highest amounts of reducing sugars were Uba tuba (christmas bell) (458 mg g−1 DW; Brazil), VI012959 (472 mg g−1 DW; Germany), VI013462 (468 mg g−1 DW; UK), VI028902 (449 mg g−1 DW; Brazil), and VI029101 (483 mg g−1 DW; Brazil). On the other hand, the five genotypes with lowest combined mean values for reducing sugars were VI028794 (109 mg g−1 DW; Jamaica), VI044347 (130 mg g−1 DW; India), VI028896 (155 mg g−1 DW; Brazil), VI013973 (162 mg g−1 DW; El Salvador), and Bird’s eye hot peppers (strain 3) (164 mg g−1 DW; Guyana). As with vitamin C, there was considerable variation in parametric reducing sugar concentrations in the fruits, and this variation could be attributed to the accumulation reducing sugars at different stages in fruit development. Large variations in the concentrations of sugars, such as sucrose, glucose and fructose, were also reported among the genotypes of C. chinense (Jarret et al., 2009). Furthermore, reducing sugars, total soluble solid content, and titratable acidity are known to increase during ripening of peppers (Martínez, Curros, Bermúdez, Carballo, & Franco, 2007; Osuna-Garcia et al., 1998).
Table 2.
Reducing sugars (mg g−1 DW) in the pericarp of matured and ripened fruits of world collection of C. baccatum genotypes.
| S. No. | Genotype | Origin | Matured (M) | Ripened (R) | Combined (C) | SNK^ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Mean | Std | M | R | C | |||
| 1 | 281408 01 SD | Peru | 218.44 | 7.40 | 417.82 | 14.52 | 318.13 | 104.70 | |||
| 2 | Aji amarillo small | Peru | 245.19 | 17.03 | 477.41 | 29.57 | 361.30 | 123.44 | |||
| 3 | Aji benito hot peppers | Bolivia | ND | ND | 669.99 | 15.30 | 669.99 | 15.30 | b | a | |
| 4 | Aji cito hot peppers | Peru | 271.70 | 8.90 | 384.11 | 18.38 | 327.91 | 60.30 | |||
| 5 | Aji colorado hot peppers | Bolivia | ND | ND | 494.74 | 9.80 | 494.74 | 9.80 | b | ||
| 6 | Aji habanero hot peppers | Mexico | 137.71 | 3.14 | 389.63 | 5.30 | 263.67 | 131.63 | |||
| 7 | Bird’s eye hot peppers (strain 3) | Guyana | 67.29 | 1.70 | 260.60 | 22.60 | 163.94 | 102.10 | y | q | |
| 8 | CGN16973 | Bolivia | 87.96 | 5.98 | 343.07 | 2.59 | 215.51 | 133.30 | |||
| 9 | CGN17241 | USA | 149.51 | 7.05 | 327.98 | 19.79 | 238.74 | 94.27 | |||
| 10 | CGN19233 | Peru | 149.95 | 7.21 | 344.28 | 8.16 | 247.12 | 101.75 | |||
| 11 | CGN21479 | Peru | 165.44 | 7.66 | 426.13 | 26.52 | 277.16 | 135.00 | |||
| 12 | CGN21513 | Bolivia | 259.94 | 10.22 | 473.85 | 19.74 | 366.89 | 112.71 | |||
| 13 | CGN22834 | Brazil | 207.69 | 7.10 | 484.76 | 21.14 | 346.23 | 145.48 | |||
| 14 | CGN22858 | Brazil | 152.99 | 2.21 | 311.67 | 37.11 | 243.67 | 85.93 | |||
| 15 | CGN23763 | Brazil | 156.29 | 2.48 | 500.77 | 44.62 | 328.53 | 182.41 | |||
| 16 | GRIF 9219 01 SD | Costa Rica | 147.49 | 9.53 | 323.75 | 38.20 | 235.62 | 95.80 | |||
| 17 | PI 159252 01 SD | USA | 138.32 | 9.62 | 337.79 | 11.80 | 238.05 | 104.68 | |||
| 18 | PI 215699 02 SD | Peru | 170.30 | 10.73 | 607.53 | 75.33 | 388.92 | 234.03 | |||
| 19 | PI 215700 01 SD | Peru | 201.39 | 11.26 | 258.01 | 5.73 | 229.70 | 30.77 | |||
| 20 | PI 238061 01 SD | Bolivia | ND | ND | 351.52 | 8.58 | 351.52 | 8.58 | |||
| 21 | PI 497974 01 SD | Brazil | 252.29 | 5.55 | SP | SP | 456.22 | 213.06 | |||
| (660.15) | (5.55) | ||||||||||
| 22 | PI 543178 01 SD | Bolivia | 167.81 | 12.76 | 376.61 | 33.35 | 272.21 | 111.67 | |||
| 23 | PI 640885 01 SD | India | 136.66 | 4.60 | 418.21 | 8.85 | 277.43 | 147.19 | |||
| 24 | Uba tuba (Christmas bell) | Brazil | 350.46 | 16.69 | 565.76 | 12.35 | 458.11 | 113.31 | r | b | |
| 25 | VI012279 | Ecuador | 195.96 | 11.07 | 498.92 | 29.86 | 347.44 | 159.66 | |||
| 26 | VI012422 | USA | 102.43 | 3.87 | 392.17 | 13.40 | 247.30 | 151.61 | |||
| 27 | VI012673 | UK | 338.22 | 21.72 | 519.32 | 22.89 | 428.77 | 96.94 | r | ||
| 28 | VI012898 | France | 179.83 | 3.60 | 505.91 | 18.62 | 342.87 | 170.77 | |||
| 29 | VI012959 | Germany | 262.98 | 4.91 | 680.48 | 29.15 | 471.73 | 218.94 | ab | b | |
| 30 | VI012983 | USA | 157.27 | 23.94 | 361.51 | 18.82 | 259.39 | 108.62 | |||
| 31 | VI013034 | UK | 72.61 | 3.47 | 272.20 | 4.34 | 172.41 | 104.30 | xy | ||
| 32 | VI013036 | Germany | 215.42 | 13.39 | 465.59 | 6.60 | 340.51 | 131.04 | |||
| 33 | VI013261 | USA | ND | ND | 368.18 | 22.45 | 368.18 | 22.45 | |||
| 34 | VI013262 | USA | 289.85 | 14.92 | 497.40 | 8.26 | 393.63 | 109.00 | yz | ||
| 35 | VI013290 | UK | 210.61 | 13.38 | 532.57 | 15.99 | 371.59 | 168.72 | |||
| 36 | VI013395 | Paraguay | 131.75 | 3.28 | 481.42 | 22.13 | 306.59 | 183.23 | |||
| 37 | VI013425 | Argentina | 175.51 | 13.73 | 448.19 | 5.77 | 311.85 | 142.76 | |||
| 38 | VI013462 | UK | 247.60 | 6.26 | 687.33 | 30.25 | 467.46 | 230.59 | ab | b | |
| 39 | VI013477 | Netherlands | 243.64 | 12.78 | 501.23 | 12.19 | 372.43 | 135.05 | |||
| 40 | VI013973 | El Salvador | 120.44 | 8.57 | 203.55 | 4.25 | 162.00 | 43.88 | mqu | q | |
| 41 | VI014229 | Unknown | 106.79 | 1.07 | 252.29 | 14.87 | 179.54 | 76.65 | |||
| 42 | VI014230 | Peru | 131.67 | 6.09 | 362.76 | 23.21 | 247.22 | 121.76 | |||
| 43 | VI014270 | Costa Rica | ND | ND | 272.55 | 17.77 | 272.55 | 17.77 | |||
| 44 | VI014892 | Ecuador | 184.09 | 6.70 | 480.41 | 3.95 | 332.25 | 154.84 | |||
| 45 | VI028657 | Costa Rica | 156.94 | 5.32 | 404.75 | 9.68 | 280.85 | 129.63 | |||
| 46 | VI028776 | Argentina | 262.53 | 8.85 | 588.01 | 36.75 | 425.27 | 171.88 | |||
| 47 | VI028777 | Bolivia | 162.40 | 8.90 | SP | SP | 256.72 | 99.02 | q | ||
| (351.04) | (11.91) | ||||||||||
| 48 | VI028780 | Bolivia | 152.65 | 10.31 | 496.75 | 2.94 | 324.70 | 179.84 | |||
| 49 | VI028782 | Brazil | 198.53 | 13.09 | 304.67 | 19.47 | 251.60 | 57.64 | |||
| 50 | VI028788 | Chile | 232.10 | 10.56 | 444.52 | 21.81 | 338.31 | 112.13 | |||
| 51 | VI028791 | Costa Rica | 135.67 | 1.73 | 386.25 | 27.34 | 260.96 | 132.16 | |||
| 52 | VI028792 | Ecuador | 211.51 | 10.19 | 350.13 | 7.32 | 280.82 | 72.88 | |||
| 53 | VI028793 | Ecuador | 186.63 | 2.71 | 421.52 | 7.31 | 304.07 | 122.78 | |||
| 54 | VI028794 | Jamaica | 40.99 | 1.30 | 177.33 | 7.64 | 109.16 | 71.39 | z | u | q |
| 55 | VI028795 | Mexico | 167.27 | 12.28 | 426.36 | 6.54 | 296.82 | 135.63 | |||
| 56 | VI028797 | Mexico | 110.39 | 13.13 | 514.47 | 15.34 | 283.57 | 207.96 | |||
| 57 | VI028798 | Paraguay | 166.49 | 4.13 | 473.76 | 13.01 | 320.12 | 160.73 | |||
| 58 | VI028867 | Brazil | ND | ND | 486.68 | 17.75 | 486.68 | 17.75 | b | ||
| 59 | VI028870-A | Brazil | 170.89 | 2.84 | 467.43 | 9.71 | 319.16 | 155.01 | |||
| 60 | VI028870-B | Brazil | 251.93 | 1.80 | 549.25 | 23.18 | 400.59 | 156.06 | |||
| 61 | VI028873 | Brazil | 124.31 | 1.99 | 377.29 | 11.26 | 250.80 | 132.34 | |||
| 62 | VI028878 | Brazil | 93.11 | 1.63 | 332.34 | 10.49 | 212.72 | 125.14 | |||
| 63 | VI028886 | Brazil | 148.29 | 7.29 | 461.99 | 14.40 | 305.14 | 164.18 | |||
| 64 | VI028890 | Brazil | 138.12 | 2.15 | 395.81 | 10.83 | 266.97 | 134.78 | |||
| 65 | VI028895 | Brazil | 296.17 | 20.08 | SP | SP | 438.47 | 149.34 | xyz | ||
| (580.78) | (7.73) | ||||||||||
| 66 | VI028896 | Brazil | 82.71 | 3.54 | 226.57 | 4.31 | 154.64 | 75.22 | xy | jmq | q |
| 67 | VI028897 | Brazil | 162.11 | 10.12 | 433.29 | 5.06 | 297.70 | 141.82 | |||
| 68 | VI028901 | Brazil | 227.02 | 4.78 | 462.75 | 20.32 | 344.88 | 123.91 | |||
| 69 | VI028902 | Brazil | 273.33 | 12.57 | 623.60 | 11.10 | 448.47 | 183.27 | z | c | b |
| 70 | VI028910 | Brazil | 234.19 | 10.96 | 546.63 | 10.68 | 390.41 | 163.49 | |||
| 71 | VI028911 | Brazil | 209.74 | 7.36 | 536.90 | 1.81 | 373.32 | 170.93 | |||
| 72 | VI028939 | USA | 128.10 | 6.45 | 296.26 | 8.71 | 212.18 | 88.12 | |||
| 73 | VI028942 | USA | 154.40 | 4.77 | 264.96 | 6.84 | 209.68 | 58.01 | |||
| 74 | VI028968 | Chile | 184.24 | 6.70 | 471.68 | 13.19 | 327.96 | 150.44 | |||
| 75 | VI028969 | Colombia | 179.92 | 11.73 | 493.64 | 12.97 | 336.78 | 164.26 | |||
| 76 | VI028971 | Ecuador | 222.23 | 6.09 | 535.45 | 15.40 | 378.84 | 163.95 | |||
| 77 | VI028973 | Ecuador | SP | SP | 335.55 | 20.07 | 255.82 | 84.39 | |||
| (176.10) | (3.37) | ||||||||||
| 78 | VI029021 | Bolivia | 159.76 | 8.53 | 364.28 | 78.89 | 262.02 | 118.71 | |||
| 79 | VI029022 | Bolivia | 165.69 | 6.62 | 375.84 | 13.68 | 270.76 | 110.22 | |||
| 80 | VI029023 | Bolivia | ND | ND | 578.38 | 49.61 | 578.38 | 49.61 | ab | ||
| 81 | VI029024 | Bolivia | 182.76 | 7.53 | 428.71 | 11.93 | 305.74 | 128.80 | |||
| 82 | VI029025-A | Bolivia | ND | ND | 402.51 | 11.34 | 402.51 | 11.34 | |||
| 83 | VI029025-B | Bolivia | ND | ND | 277.87 | 15.04 | 277.87 | 15.04 | |||
| 84 | VI029025-C | Bolivia | 171.76 | 5.60 | 352.03 | 36.32 | 261.90 | 97.35 | |||
| 85 | VI029026 | Bolivia | SP | SP | 481.53 | 15.88 | 313.16 | 176.20 | b | ||
| (144.79) | (4.03) | ||||||||||
| 86 | VI029033-A | Bolivia | ND | ND | 444.98 | 7.22 | 444.98 | 7.22 | |||
| 87 | VI029044 | Brazil | 186.34 | 8.98 | 384.69 | 21.52 | 285.52 | 104.77 | |||
| 88 | VI029047 | India | 222.53 | 4.80 | 363.19 | 13.39 | 292.86 | 74.08 | |||
| 89 | VI029050 | Guatemala | SP | SP | 451.97 | 21.82 | 315.53 | 143.93 | b | ||
| (179.10) | (20.66) | ||||||||||
| 90 | VI029057 | Brazil | 143.78 | 4.21 | 387.44 | 16.10 | 265.61 | 127.74 | |||
| 91 | VI029060 | Chile | SP | SP | 558.14 | 39.10 | 351.94 | 186.98 | ab | ||
| (197.28) | (7.6) | ||||||||||
| 92 | VI029062 | Ecuador | 190.25 | 17.07 | 377.70 | 11.04 | 283.98 | 98.85 | |||
| 93 | VI029076 | Kenya | 191.44 | 1.12 | 470.63 | 12.00 | 331.04 | 146.03 | |||
| 94 | VI029079 | Ecuador | 254.22 | 10.26 | 539.73 | 10.01 | 396.97 | 149.41 | |||
| 95 | VI029081 | India | 138.34 | 6.76 | 371.44 | 10.43 | 254.89 | 122.02 | |||
| 96 | VI029084 | Brazil | 190.08 | 10.39 | 547.47 | 24.58 | 368.78 | 187.50 | |||
| 97 | VI029085 | Brazil | 244.48 | 18.38 | 700.15 | 36.45 | 439.77 | 235.48 | a | ||
| 98 | VI029091 | Brazil | 152.58 | 8.61 | SP | SP | 349.16 | 235.95 | q | ||
| (611.28) | (19.20) | ||||||||||
| 99 | VI029097 | Brazil | 158.62 | 8.51 | 570.91 | 22.29 | 364.77 | 215.91 | |||
| 100 | VI029098-B | Brazil | 223.52 | 12.59 | 426.63 | 40.61 | 339.58 | 108.76 | |||
| 101 | VI029101 | Brazil | 301.25 | 6.97 | 665.48 | 15.90 | 483.36 | 190.58 | xyz | b | b |
| 102 | VI029104 | Brazil | 268.22 | 16.37 | 570.49 | 14.76 | 419.36 | 158.55 | |||
| 103 | VI029110 | Brazil | 162.27 | 7.55 | 366.63 | 16.91 | 264.45 | 107.45 | |||
| 104 | VI029111 | Brazil | 270.50 | 18.34 | 454.23 | 44.75 | 362.37 | 101.34 | |||
| 105 | VI029112 | Brazil | 212.89 | 10.29 | 608.18 | 23.81 | 410.54 | 207.17 | |||
| 106 | VI029113 | Brazil | 206.53 | 7.71 | 426.99 | 12.33 | 316.76 | 115.55 | |||
| 107 | VI029117 | Brazil | 179.58 | 6.53 | 452.55 | 16.24 | 316.06 | 143.04 | |||
| 108 | VI029124-A | Bolivia | 96.13 | 5.32 | 234.80 | 23.85 | 165.46 | 74.27 | jm | ||
| 109 | VI029124-B | Bolivia | 153.00 | 5.05 | 406.81 | 4.76 | 279.90 | 132.63 | |||
| 110 | VI029511 | Colombia | 209.18 | 11.56 | 529.16 | 14.35 | 369.17 | 167.57 | |||
| 111 | VI031222 | Zambia | ND | ND | 469.73 | 6.43 | 469.73 | 6.43 | b | ||
| 112 | VI037435 | Brazil | 236.03 | 9.26 | 511.79 | 17.26 | 373.91 | 144.61 | |||
| 113 | VI044307 | Unknown | 142.70 | 5.62 | 460.24 | 40.00 | 301.47 | 168.05 | |||
| 114 | VI044310 | Peru | 203.46 | 17.51 | 447.41 | 12.96 | 325.43 | 128.24 | |||
| 115 | VI044347 | India | 70.43 | 2.42 | 190.03 | 4.07 | 130.23 | 62.54 | xy | qu | q |
| 116 | VI044352 | Unknown | 182.35 | 7.40 | 497.20 | 38.77 | 339.78 | 166.57 | |||
| 117 | VI047059-B | Peru | 176.18 | 6.45 | 451.31 | 57.08 | 313.75 | 148.81 | |||
| 118 | VI049287 | USA | 191.31 | 11.27 | 573.79 | 31.36 | 382.55 | 201.00 | |||
| 119 | VI049327 | USA | 155.36 | 6.28 | 403.12 | 23.30 | 279.24 | 130.41 | |||
| 120 | VI057369 | USA | 111.75 | 3.73 | 440.67 | 15.26 | 276.21 | 172.10 | |||
DW: Dry Weight. Std: Standard deviation. ND: Not Determined. SP: Separated along with the corresponding non-parametric ascorbic acid data (Refer Kruskal–Wallis test for this data; Fig. 2). Values in parenthesis are SP values. SNK: Student–Newman–Keuls test.
Top as well as bottom 5 genotype-ranks in each stage are denoted separately by lower case letters in descending order. Means with the same letter are not significantly different. More than 5 rankings are displayed when ranked genotypes had ’ND’ or ’NP’ in any stage. Number of observations/stage/genotype: 6–8.
A second set of parametric reducing sugars data separated with the non-parametric vitamin C data are presented in Fig. 2. Unlike vitamin C (Fig. 1B), Wilcoxon scores for reducing sugars in PI49797401 SD (ripe), VI028777 (ripe), VI028796 (mature unripe and ripe), VI028895 (ripe), VI028973 (mature unripe), VI029026 (mature unripe), VI029050 (mature unripe), VI029060 (mature unripe), VI029087 (mature unripe and ripe), VI029091 (ripe), and VI057372 (mature unripe and ripe) (Fig. 2B) were distributed in the range between the highest (ripe VI029085, Brazil) and lowest (matured stage of VI028794, Jamaica) parametric data (Table 2). These findings also suggest the relationship between vitamin C and reducing sugars in this second group of genotypes is different from the relationship observed in the genotypes presented previously (Tables 1 and 2).
Fig. 2.
Kruskal–Wallis test for parametric data on reducing sugars was performed on either one or two developmental fruit stages of 11 genotypes (n = 6). Although parametric, this reducing sugars data was separated along with the corresponding non-parametric vitamin C data for comparison. (A) Reducing sugar levels in the fruit pericarp (mg g−1 DW); (B) Distribution of Wilcoxon scores for reducing sugar levels. Matured and ripened stages of fruits are denoted by ‘M’ and ‘R’, respectively. Genotypes with maximum (‘Max’) and minimum (‘Min’) reducing sugar levels from parametric data are included here for reference (refer Table 2). VI028796, VI029087 and VI057372 are originated from Mexico, Brazil and USA, respectively. Refer Table 1 for origin of all other genotypes.
3.3. Fruit developmental stage influence the levels of vitamin C and reducing sugars
Accumulation of vitamin C and reducing sugars at mature unripe and ripe stages of fruit development, the two major stages in harvest and consumption, are presented in Fig. 3. Overall, vitamin C levels in ripe fruit were significantly lower than at the mature unripe stage. A significant if weak negative correlation was observed between stage and vitamin C concentration (Pearson correlation coefficient: −0.33; p < 0.0001). Contrary to vitamin C, reducing sugar levels in ripe fruit were significantly higher than at the mature unripe stage. A strong positive correlation was identified between stage and reducing sugars (Pearson correlation coefficient: 0.81; p < 0.0001). Alós, Rodrigo, and Zacarías (2013) argued that the genotypes belonging to C. annuum reliably accumulated more ascorbic acid in ripe fruits. In the present study, a small fraction of C. baccatum genotypes exhibited a similar trend (Table 1; Fig. 1). In contrast, however, the majority of C. baccatum genotypes accumulated most vitamin C at the mature unripe stage, which appears to be a characteristic feature of C. baccatum genotypes (Fig. 3A). Similar genotype-dependent variability in total vitamin C content during ripening has been reported in tomato and watermelon (Ilahy, Hdider, Lenucci, Tlili, & Dalessandro, 2011; Tlili, Hdider, Lenucci, Ilahy, & Jebari, 2011). It has been postulated that these differences might exist due to either genetic variation or comparison of non-uniform or asynchronous stages of ripening (Alós et al., 2013). Similarly, other factors, such as environment, cultural practices and spontaneous mutations, also contribute to variability in vitamin C content at different stages of ripening (Ilahy et al., 2011; Tlili et al., 2011).
Fig. 3.
Effect of fruit developmental stage on accumulation of vitamin C and reducing sugars in the fruit pericarp of the world collection of C. baccatum genotypes. ANOVA was performed on parametric data after logarithmic transformation of vitamin C values (mg g−1 DW) (Refer Table 1 for data). Refer Table 2 for parametric data on reducing sugars (mg g−1 DW). SNK test was performed and means that are significantly different from each other in each analysis are represented by different letter in descending order (n = 688–766).
In certain C. annuum species, ascorbic acid concentrations are inversely correlated with expression of biosynthetic genes, and this feedback regulation of ascorbic acid homeostasis is further controlled by ascorbate oxidase. In tomato and bell pepper (C. annuum), vitamin C decreased from 74 and 51 days, respectively, after the fruit has set. These changes have also been associated with increased levels of ascorbate oxidase (Yahiaa et al., 2001). Although, ascorbate oxidase appeared to have an important role in the regulation of ascorbic acid levels during fruit development and ripening, mRNA levels could not explain differences in ascorbic acid concentration among the varieties examined (Alós et al., 2013). At the end of maturation, between 40 to 60 days after anthesis, there is a global decrease in gene expression in chili pepper fruits (Martínez-López, Ochoa-Alejo, & Martínez, 2014). Fruit ripening is an oxidative process and several antioxidants, including ascorbic acid, might be involved in scavenging reactive oxygen species during ripening (Gest et al., 2013). In one study, compared with green fruits, galactose in ripe red fruits approximately 80% lower. Hydrolysis of galactose by beta-galactosidase is thought to be the first event in bell pepper fruit ripening (Ogasawara, Abe, & Nakajima, 2007). On the other hand, increased levels of reducing sugars during ripening (Martínez et al., 2007; Osuna-Garcia et al., 1998) are an indication of decreased conversion of precursor sugars into vitamin C in the pepper fruits during ripening. Some portion of ascorbic acid might have been converted to organic acids (Ishakawa, Dowdle, & Smirnoff, 2006), which is evident from increased acidity in pepper fruits during ripening (Martínez et al., 2007). Together, it can be postulated that genotypes of C. baccatum accumulate more vitamin C in mature unripe fruits and these levels decrease in ripe fruits, probably due to decreased expression of ascorbic acid biosynthetic genes, hydrolysis of galactose, increased oxidation of ascorbic acid, and or its utilization during antioxidant mechanism or conversion to organic acids.
3.4. Negative relationship exists between vitamin C and reducing sugars
Reducing sugars with a potential aldehyde or keto group include glucose, galactose, lactose, fructose, arabinose and maltose. In plants, ascorbic acid is synthesized via l-gulose, myo-inositol, l-galactose and/or d-galacturonic acid pathways. d-glucose serves as a precursor for l-gulose and l-galactose pathways. l-galactose is an immediate precursor for ascorbic acid synthesis in l-galactose pathway (Hancock, & Viola, 2005). In majority of the plants, ascorbic acid is synthesized from d-glucose or d-galactose (Naidu, 2003). The relationship between reducing sugars and vitamin C at different stages of fruit maturation is presented in Fig. 4. There was a significant moderate negative relationship between the vitamin C and reducing sugars levels in the fruit pericarp (Fig. 4A) in mature unripe fruit. Pearson correlation coefficient of −0.44 (p < 0.0001) between vitamin C and reducing sugars clearly suggests 44% of the variation observed in pericarp vitamin C could be explained by reducing sugar levels in the pericarp of the genotypes shown in Tables 1 and 2. When fruit development stages were separated, this correlation value was reduced to −0.35 (p < 0.0001) in mature unripe and −0.33 (p < 0.0001) in ripe stages (Fig. 4 B and C). Together, these findings suggest that some portion of glucose and/or galactose in the reducing sugar pool might be converted to vitamin C in C. baccatum genotypes. On the other hand, these findings also suggest that non-parametric levels of vitamin C in 11 genotypes cannot be explained by reducing sugar levels alone (Figs. 1 and 2). This might be due to genetic variation associated with vitamin C synthesis in these genotypes or simply macro- or micro-environmental conditions during maturation and ripening of the fruit in the field.
Fig. 4.
Relationship between vitamin C and reducing sugars in the pericarp of fruits of world collection of C. baccatum genotypes. (A) Both stages; (B) matured stage; and (C) ripened stage. Statistical analysis was performed after logarithmic transformation of vitamin C and standardization of reducing sugars data. Total number of genotypes tested = 120; total number of observations = 1454; number of observations/stage/genotype = 6–8.
4. Conclusions
Fruits of 123 genotypes of C. baccatum originating from 22 countries, Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, France, Germany, Guatemala, Guyana, India, Jamaica, Kenya, Mexico, Netherlands, Paraguay, Peru, UK, USA and Zambia, were analyzed for vitamin C and reducing sugars content in mature unripe and ripe fruit collected from field grown plants at Sissonville (WV, USA) during the summer 2013. Among the normal distributed (parametric) population, vitamin C and reducing sugar levels ranged between 2.54 to 50.44 and 41 to 700 mg g−1 DW of pericarp, respectively. Given the latest rate of fresh Capsicum fruit consumption in USA, fruits from 10 genotypes could supply more than half the RDA of vitamin C from 2 g of fruit pericarp on a dry weight basis. Four genotypes accumulated 100–500% the RDA of vitamin C. During fruit development, the highest amounts of vitamin C and reducing sugars accumulated in mature unripe and ripe fruit, respectively. An inverse relationship exists between reducing sugars and vitamin C (R2 = −0.44). Reducing sugars contributed about 44% of variation in the vitamin C content. Some genotypes accumulated higher or lower amounts of vitamin C than the normal population. This variation cannot be explained by reducing sugar levels in the fruits. Genotypes with more than half the RDA of vitamin C could serve as functional foods for vitamin C in human diet.
Supplementary Material
Acknowledgments
This work was supported by a grant from USDA-NIFA (Contract No. 2010-38821-21574); and WV-INBRE Center for Natural Products Research (NIH), sub-award No. P1400846 (NIH Prime award No. 5P20GM103434-13).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.foodchem.2016.01.135.
Contributor Information
Venu Perla, Email: venuperla@yahoo.com.
Padma Nimmakayala, Email: padma@wvstateu.edu.
Marjan Nadimi, Email: marjan.nadimi51@gmail.com.
Suresh Alaparthi, Email: salaparthi@wvstateu.edu.
Gerald R. Hankins, Email: ghankins@wvstateu.edu.
Andreas W. Ebert, Email: andreas.ebert@worldveg.org.
Umesh K. Reddy, Email: ureddy@wvstateu.edu.
References
- Agius F, Gonźalez-Limothe R, Caballero JL, Muñoz-Blanco J, Botella MA, Valpuesta V. Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nature Biotechnology. 2003;21:177–181. doi: 10.1038/nbt777. [DOI] [PubMed] [Google Scholar]
- Alós E, Rodrigo MJ, Zacarías L. Transcriptomic analysis of genes involved in the biosynthesis, recycling and degradation of l-ascorbic acid in pepper fruits (Capsicum annuum L.) Plant Science. 2013;207:2–11. doi: 10.1016/j.plantsci.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Antonious GF, Lobel L, Kochhar T, Berke T, Jarret RL. Antioxidants in Capsicum chinense: Variation among countries of origin. Journal of Environmental Science and Health Part B. 2009;44(6):621–626. doi: 10.1080/03601230903000727. [DOI] [PubMed] [Google Scholar]
- Badejo AA, Wada K, Gao Y, Maruta T, Sawa Y, Shigeoka S, Ishikawa T. Translocation and the alternative d-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the d-mannose/l-galactose pathway. Journal of Experimental Botany. 2012;63:229–239. doi: 10.1093/jxb/err275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Rus E, Amaya I, Sanchez-Sevilla JF, Botella MA, Valpuesta V. Regulation of L-ascorbic acid content in strawberry fruits. Journal of Experimental Botany. 2011;62:4191–4201. doi: 10.1093/jxb/err122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant Journal. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board; Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press; 2000. Vitamin C; pp. 95–185. [PubMed] [Google Scholar]
- Gest N, Gautier H, Stevens R. Ascorbate as seen through plant evolution: The rise of a successful molecule? Journal of Experimental Botany. 2013;64(1):33–53. doi: 10.1093/jxb/ers297. [DOI] [PubMed] [Google Scholar]
- Hallberg L. Bioavailability of dietary iron in man. Annual Review of Nutrition. 1981;1:123–127. doi: 10.1146/annurev.nu.01.070181.001011. [DOI] [PubMed] [Google Scholar]
- Hancock RD, Viola R. Biosynthesis and catabolism of l-ascorbic acid in plants. Critical Reviews in Plant Sciences. 2005;24(3):167–188. [Google Scholar]
- Howard LR, Smith RT, Wagner AB, Villalon B, Burns EE. Provitmain A and ascorbic acid content of fresh pepper cultivars (Capsicum annuum) and processed jalapenos. Journal of Food Science. 1994;59:362–365. [Google Scholar]
- Howard LR, Talcott ST, Brenes CH, Villalon B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum Species) as influenced by maturity. Journal of Agricultural and Food Chemistry. 2000;48(5):1713–1720. doi: 10.1021/jf990916t. [DOI] [PubMed] [Google Scholar]
- Hulse JD, Ellis SR, Henderson LM. Carnitine biosynthesis-beta hydroxylation of trimethyllysine by an α-keto glutarate dependent mitochondrial dioxygenase. The Journal of Biological Chemistry. 1978;253:1654–1659. [PubMed] [Google Scholar]
- Ilahy R, Hdider C, Lenucci MS, Tlili I, Dalessandro G. Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. Journal of Food Composition and Analysis. 2011;24:588–595. [Google Scholar]
- Isabelle M, Lee BL, Lim MT, Koh W-P, Huang D, Ong CN. Antioxidant activity and profiles of common vegetables in Singapore. Food Chemistry. 2010;120:993–1003. [Google Scholar]
- Ishakawa T, Dowdle J, Smirnoff N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiologia Plantarum. 2006;126:343–355. [Google Scholar]
- Jarret RL, Berke T, Baldwin EA, Antonious GF. Variability for free sugars and organic acids in Capsicum chinense. Chemistry & Biodiversity. 2009;6(2):138–145. doi: 10.1002/cbdv.200800046. [DOI] [PubMed] [Google Scholar]
- Kapur A, Hasković A, Čopra-Janićijević A, Klepo L, Topčagić A, Tahirović I, Sofić E. Spectrophotometric analysis of total ascorbic acid content in various fruits and vegetables. Bulletin of the Chemists and Technologists of Bosnia & Herzyovina. 2012;38:39–42. [Google Scholar]
- Lee Y, Howard LR, Villalon B. Flavonoids and antioxidant activity of fresh pepper (Capsicum annuum) cultivars. Journal of Food Science. 1995;60:473–476. [Google Scholar]
- Levin M. New concepts in the biology and biochemistry of ascorbic acid. The New England Journal of Medicine. 1986;31:892–902. doi: 10.1056/NEJM198604033141407. [DOI] [PubMed] [Google Scholar]
- Marín A, Ferreres F, Tomás-Barberán FA, Gil MI. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L.) Journal of Agricultural and Food Chemistry. 2004;52(12):3861–3869. doi: 10.1021/jf0497915. [DOI] [PubMed] [Google Scholar]
- Martínez S, Curros A, Bermúdez J, Carballo J, Franco I. The composition of Arnoia peppers (Capsicum annuum L.) at different stages of maturity. International Journal of Food Sciences and Nutrition. 2007;58(2):150–161. doi: 10.1080/09637480601154095. [DOI] [PubMed] [Google Scholar]
- Martínez-López LA, Ochoa-Alejo N, Martínez O. Dynamics of the chili pepper transcriptome during fruit development. BMC Genomics. 2014;21:15–143. doi: 10.1186/1471-2164-15-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutrition Journal. 2003;2:7. doi: 10.1186/1475-2891-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara S, Abe K, Nakajima T. Pepper beta-galactosidase 1 (PBG1) plays a significant role in fruit ripening in bell pepper (Capsicum annuum) Bioscience, Biotechnology, and Biochemistry. 2007;71(2):309–322. doi: 10.1271/bbb.60179. [DOI] [PubMed] [Google Scholar]
- Osuna-Garcia JA, Wall MM, Waddell CA. Endogenous levels of tocopherols and ascorbic acid during fruit ripening of New Mexican-type chile (Capsicum annuum L.) Journal of Agricultural and Food Chemistry. 1998;46:5093–5096. [Google Scholar]
- Pauling L. Vitamin C and common cold. San Francisco: W.H. Freeman; 1970. p. 122. [Google Scholar]
- Perla V, Holm DG, Jayanty SS. Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT – Food Science and Technology. 2012;45:161–171. [Google Scholar]
- Perla V, Perrin NA, Greenlee AR. Paraquat toxicity in a mouse embryonic stem cell model. Toxicology in Vitro. 2008;22:515–524. doi: 10.1016/j.tiv.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Shukla SP. Level of ascorbic acid and its oxidation in the liver of Scorpion Palamnaeus bengalensis. Experentia. 1969;25:602–604. doi: 10.1007/BF01896537. [DOI] [PubMed] [Google Scholar]
- Simmone AH, Simmone EH, Eitenmiller RR, Mills HA, Green NR. Ascorbic acid and provitamin A contents in some unusually colored bell peppers. Journal of Food Composition and Analysis. 1997;10:299–311. [Google Scholar]
- Tlili I, Hdider C, Lenucci MS, Ilahy R, Jebari H. Bioactive compounds and antioxidant activities during fruit ripening of watermelon cultivars. Journal of Food Composition and Analysis. 2011;24:923–928. [Google Scholar]
- Wahyuni Y, Ballester AR, Sudarmonowati E, Bino RJ, Bovy AG. Secondary metabolites of Capsicum species and their importance in the human diet. Journal of Natural Products. 2013;76(4):83–93. doi: 10.1021/np300898z. [DOI] [PubMed] [Google Scholar]
- Wahyuni Y, Ballestera AR, Sudarmonowatib E, Binoa RJ, Bovya AG. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry. 2011;72(11–12):1358–1370. doi: 10.1016/j.phytochem.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Wells HF, Bond J, Thornsbury S. Vegetables and pulses yearbook data. Economic Research Service, USDA, No. 89011. 2015 [Google Scholar]
- Yahiaa EM, Contreras-Padillaa M, Gonzalez-Aguilarb G. Ascorbic acid content in relation to ascorbic acid oxidase activity and polyamine content in tomato and bell pepper fruits during development, maturation and senescence. LWT -– Food Science and Technology. 2001;34(7):452–457. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.