Abstract
Stroke remains a leading cause of death and disability in the world. Over the past few decades our understanding of the pathophysiology of stroke has increased, but greater insight is required to advance the field of stroke recovery. Clinical treatments have improved in the acute time window, but long-term therapeutics remain limited. Complex neural circuits damaged by ischemia make restoration of function after stroke difficult. New therapeutic approaches, including cell transplantation or stimulation, focus on reestablishing these circuits through multiple mechanisms to improve circuit plasticity and remodeling. Other research targets intact networks to compensate for damaged regions. This review highlights several important mechanisms of stroke injury and describes emerging therapies aimed at improving clinical outcomes.
Pathophysiology of Stroke
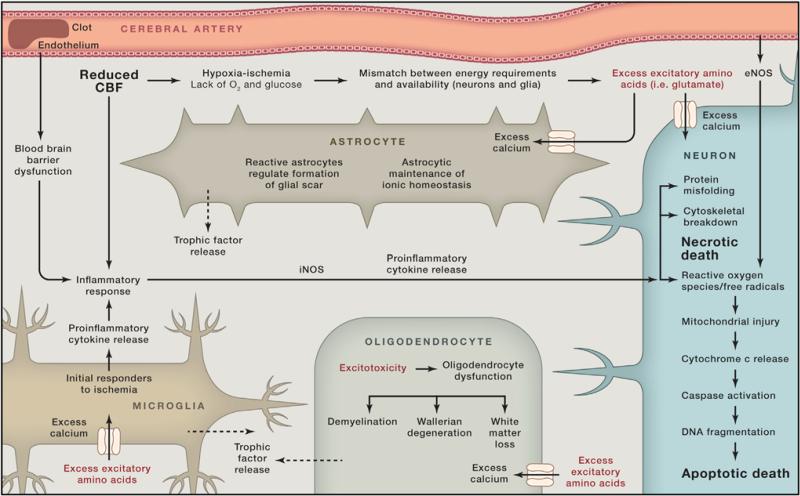
The lack of blood flow during a stroke results in an intricate path-ophysiological response resulting in neural injury, as depicted in Figure 1 (Hossmann, 2006). Multiple mechanisms, including excitotoxicity, mitochondrial response, free radical release, protein misfolding, and inflammatory changes, lead to neural cell loss, but many of these pathways ultimately pave the way for recovery. Injury and death of astrocytes, as well as white matter injury, also contribute to cerebral damage. The delicate balance between detrimental or beneficial effect often relies on the timing and the magnitude of the factors involved. The inflammatory response is a prime example of a system that both propagates ischemic injury and helps promote recovery. Inflammation initially contributes to cellular injury through the release of cytokines and harmful radicals but eventually helps to remove damaged tissue, enabling synaptic remodeling. Glial cells also serve dual roles, helping to regulate the blood-brain barrier, promoting angiogenesis and synaptogenesis, but conversely forming the glial scar that may prevent further plasticity (Gleichman and Carmichael, 2014). The goal for this review is to provide a brief overview of the pathophysiology of stroke followed by a discussion of the current state of stroke recovery research with an emphasis on those approaches that target multiple mechanistic pathways. Many of these therapies are aimed at up-regulating pathways that enhance recovery while reducing the deleterious pathways triggered by the initial ischemic insult. Further understanding and optimizing this delicate balance may facilitate development of effective stroke therapeutics.
Figure 1.
Pathophysiology of Stroke
Excitotoxicity
CNS ischemia results in a deficiency of glucose and oxygen leading to the inability of neuronal cells to maintain normal ionic gradients. Depolarization of these neurons leads to excessive glutamate release resulting in the intracellular influx of calcium, triggering cell death pathways such as apoptosis, autophagocytosis, and necrotic pathways (Lipton, 1999). This process has been termed excitotoxicity and is mediated largely through the glutamatergic pathways involving N-methyl-D-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs), and kainate receptors (Dirnagl et al., 1999; Moskowitz et al., 2010). The role of calcium in excitoxicity also remains complex and has numerous effects in the ischemic environment. The intracellular increase in calcium triggers mitochondrial dysfunction and activation of free radicals, phospholipases, and proteases, which lead to cell death or injury (Szydlowska and Tymianski, 2010). Interestingly, the interplay between the cells is also critical to the spread of injury after ischemic insults. Blockage of the gap junctions between cells in the adult brain reduces neuronal death (Wang et al., 2010), potentially indicating the important interactions that occur between cells during neuronal damage. These processes also promote cerebral edema, which has clinical import in the first few days after a stroke. Numerous therapeutic approaches have centered on interrupting pathways triggered by excitotoxicity to improve stroke recovery, and while often successful in animal models (Yenari et al., 2001; Namura et al., 2013), translation of these findings into the clinic remains challenging.
Mitochondrial Alterations
The mitochondria play a critical role in cell energy homeostasis and are thus prominently involved during ischemia when the energy balance is disrupted and ATP synthesis is altered. The rapid influx of calcium experienced with excitoxicity leads to excess accumulation in the mitochondria, causing dysfunction, which leads to mitochondrial permeability transition pore (mtPTP) opening and cytochrome c release (Liu et al., 1996; Murphy et al., 1999). These events create mitochondrial swelling and membrane collapse, initiating cell death cascades such as apoptosis (Liu et al., 1996). The reactive oxygen species (ROS) created by the mitochondria also play a prominent role in reper-fusion injury and cell death in the ischemic environment (Kalogeris et al., 2014). Maintaining mitochondrial integrity and limiting their induction of apoptotic and oxidative stress pathways in the cell are important avenues to preventing widespread cell toxicity from an ischemic insult.
Free Radicals
Brain ischemia also triggers free radicals, which contribute to the oxidative stresses on neural tissue. The influx of calcium triggers nitric oxide (NO) production by nitric oxide synthase (NOS) that leads to injury through the formation of oxygen free radicals and the production of peroxynitrite (ONOO–) (Iadecola, 1997). The mitochondria undergo dysfunction during ischemia, leading to further oxidative stress (Kalogeris et al., 2014). NADPH oxidase also plays a critical role in ROS production in the setting of excitotoxicty and ischemia (Moskowitz et al., 2010). Furthermore, chimeric bone marrow studies have shown that inflammation contributes with neutrophils releasing inducible NOS (iNOS), which leads to toxic levels of NO (Garcia-Bonilla et al., 2014; Moro et al., 2004). Free radicals trigger the PI3-kinase/Akt pathway as well as upregulate the transcription factor NF-κB. Interestingly, the timing and environment of activation of this pathway likely determine whether stroke recovery is improved or impeded by this signaling cascade (Crack and Taylor, 2005). Other pathways of interest are the transient receptor potential (TRP) channels. TRP channels, TRPM7 specifically, are linked to free radicals in ischemia and likely contribute to increasing the influx of calcium and cellular toxicity experienced during decreased oxygenation (Sun et al., 2009). Not only do free radicals contribute to initial toxicity, they also prevent recovery, which makes them an important post-stroke therapeutic target (Miyamoto et al., 2013). Numerous methods have reduced the oxidative stress from free radicals in ischemic injury and shown neurologic improvement in preclinical models. Combining the regulation of these pathways with other ischemic injury mechanisms may lead to novel therapeutics.
Protein Misfolding
The largest stores of intracellular calcium reside in the endoplasmic reticulum (ER), an organelle that regulates protein synthesis and responds to protein misfolding (Zhang et al., 2014). These processes are largely affected by ER stress induced by ischemic injury (Roussel et al., 2013). As excitotoxic changes occur in neural cells, the sarcoplasmic/ER calcium ATPase (SERCA) pump fails due to energy depletion and adds to the occurrence of cell death (Szydlowska and Tymianski, 2010). The increased accumulation of misfolded proteins also trigger the protein kinase-like ER kinase (PERK) pathway regulating eIF2α kinase activation, which halts new protein synthesis (Althausen et al., 2001). The phosphorylation of eIF2α has been explored as a means to alter damage in cerebral ischemia. Inositol requiring enzyme 1 (IRE1) is another protein involved in the misfolding of proteins that has been shown to induce apoptotic pathways during periods of ER stress (Morimoto et al., 2007). Chaperones (such as oxygen-regulated protein 150 kDa and binding immunoglobulin protein), which normally guide protein synthesis, are also altered in ischemia, and upregulation of these chaperones may reduce apoptosis and limit damage from ischemia (Roussel et al., 2013). The cumulative effect of SERCA pump failure and chaperone misfunctioning make ER stress and its role in protein misfolding important targets for acute stroke therapies.
Astrocytic Changes and White Matter Injury
The glial cells (astrocytes and oligodendrocytes) surrounding neurons and their connections play an integral role in the brain's response to ischemia and recovery. Axons and glial cells are intimately interwoven, forming the connections and signals that compose neural activity and are poised as key therapeutic targets to enhance recovery mechanisms and reduce injurious ones. At baseline, white matter receives less blood supply than gray matter, and this may predispose white matter to ischemic damage with milder variations in blood flow. During ischemic injury, glial cells are damaged by similar injury pathways to neurons including glutamate toxicity (Sánchez-Gómez et al., 2011). Ischemia also triggers P2X7 receptors on oligodendrocytes, which contribute to calcium overload and mitochondrial depolarization (Wang et al., 2009). One of the key differences between the effects of ischemia on white matter compared with gray matter is the reliance on oligodendrocytes for functional deficits as well as the reduced influence of NMDA-type glutamate receptors on white matter injury (Matute et al., 2013).
After the acute response to hypoxic conditions, the glia also help to modulate inflammation and recovery. Although the glial scar has been shown to prevent new growth, it also exhibits positive effects of helping to restore the integrity of the blood-brain barrier. Additionally, reactive astrocytes, associated with formation of the glial scar, also modulate trophic factors, which enhance recovery (Rolls et al., 2009). Thus, glia play a prominent role in modulating the injury cascade and eventual recovery after stroke.
Inflammatory Response and the Role of the Blood-Brain Barrier
The immune system plays a vital role in the CNS's response to ischemia and to eventual recovery of function. An intricate cascade of immune cells and inflammatory factors cause blood-brain barrier breakdown, remodeling of the post-stroke tissue, and also offer a margin of neuroprotection from the harsh excitotoxic post-stroke environment of increased free radicals and enzymes (Iadecola and Anrather, 2011). Initially, microglia respond to the ischemic insult followed by an increase of dendritic cells, macrophages, and lymphocytes, and as astroglia are reduced and blood-brain barrier breakdown occurs, an influx of neutrophilic cells permeates the infarct and peri-infarct region (Gelderblom et al., 2009). Proinflammatory cytokines (i.e., tumor necrosis factor-α and interleukin-1β) are also released as well as free radicals by the immune cells in the post-stroke tissue, which increase the inflammatory response and upregulate cell adhesion molecule expression, further propagating the immune response (Huang et al., 2006). Immune cells also release inducible NO synthetase, which contributes to the detrimental effect of NO in brain ischemia, as noted above (Moro et al., 2004). Additionally, matrix metalloproteins (MMPs) and myeloperoxidase (MPO) production are elevated by the immune response, both of which are major factors leading to blood-brain barrier breakdown (Bao Dang et al., 2013). Inhibiting the acute inflammatory response after stroke has been shown to decrease injury and improve neurologic outcome in rodent stroke models (Arac et al., 2011), but has not yet been translated into the clinic.
Components of the complement cascade play a role in ischemic injury and recovery. The amount of complement proteins increases after ischemia (Pedersen et al., 2004). Evidence suggests that complement proteins tag synapses for removal by microglia to enable synaptic pruning and remodeling (Stephan et al., 2012). Another role of complement proteins (C3a and C5a, in particular) is protecting neurons from the NMDA excitotoxicity that occurs post-stroke (van Beek et al., 2001; Mukherjee et al., 2008). Immune cells such as eosinophils also produce trophic factors such as nerve growth factor (NGF) and neurotrophin-3 that promote neuronal outgrowth and may have a significant impact on post-infarct plasticity (Foster et al., 2011). Microglia also play a prominent role producing glial cell-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF), which promote neural growth and healing (Wang et al., 2013; Yang et al., 2012). Insulin-like growth factor (IGF-1), another molecule modulated by microglia, enhances axonal growth as well as neurogenesis in the subventricular zone (SVZ) to improve stroke recovery (Butovsky et al., 2006; Lalancette-Hébert et al., 2007). Cytokines, such as transforming growth factor-β and interleukin-10, often serve dual roles of driving the inflammatory response but also promoting tissue repair and resolution of inflammation depending on timing and the environment (Iadecola and Anrather, 2011).
The multifaceted immune response has both a beneficial and deleterious effect on the surviving tissue. The timing and levels of inflammatory factors and cells contribute to the balance of post-stroke injury and the restorative process (Peruzzotti-Jametti et al., 2014). The immune response has a positive role on recovery by pruning unwanted synapses and allowing for the formation of new growth and connections. However, there is also a negative effect of the inflammatory response with rodent models showing decreased stroke volume and infarct size in immunodeficient animals (Hurn et al., 2007). While neutrophils release cytokines and radicals that worsen the inflammatory response, inflammatory cells also help remove debris and damaged tissue to facilitate recovery. The balance of the inflammatory response after stroke is critical for recovery, and investigation into the components that lead to improved recovery and plasticity versus those that worsen ischemic damage is an exciting area for further research and translational investigations.
Stroke Therapies
The complex injury pathways described above often disrupt the cortical maps that form the neural representation of our body. Increased spine formation and axonal sprouting weeks after ischemia demonstrate enhanced neural plasticity in the peri-infarct area and contralesional hemisphere as brain regions reorganize, likely to restore function (Brown et al., 2007). Alterations in synaptic function and vasculature have been shown to correlate with behavioral improvement after stroke as the brain remaps to compensate for damaged networks (Winship and Murphy, 2009). Because of the complexity of the restorative processes that occur after the initial ischemic damage, a single mechanistic pathway will likely not be sufficient to greatly improve functional outcomes. Strategies such as cell therapies, stimulation, or mild hypothermia that affect several of these pathways, or a combination of therapeutic approaches, may prove to be the most promising for clinical translation.
Currently, the mainstay of acute stroke therapy is intravenous administration of tissue plasminogen activator (tPA), which has been FDA approved within a narrow time window. Endovascular therapies utilizing intra-arterial mechanical or chemical thrombolysis also improve outcomes. After the acute time period, focused physical rehabilitation of the injured area is the primary current therapy that is proven to be effective (Veerbeek et al., 2014). Re-organization of the cortex has been observed with rehabilitation in pre-clinical models as well as in humans (Liepert et al., 2001). While rehabilitation can be effective, and encouraging results have been demonstrated with constraint induced movement therapy and other techniques (Hoare et al., 2007), the extent of neurologic recovery is still limited and novel approaches to augment or enhance the body's endogenous regenerative abilities are required (Table 1).
Table 1.
Current Approaches for Stroke Therapeutics
| Restoration of Blood Flow (Acute) |
| Intra-arterial and intravenous tPA |
| Mechanical thrombectomy |
| Magnetic resonance-guided focused ultrasound |
| Neuroprotection (Acute) |
| Hypothermia |
| PSD-95 |
| Cell Replacement Therapies (Recovery) |
| Endogenous stem cells |
| Exogenous stem cells |
| Induced stem cells |
| Modulation of Circuits (Recovery) |
| Transcranial direct current stimulation |
| Transcranial magnetic stimulation |
| Optogenetic stimulation |
| MR-guided focus ultrasound |
| Stereotactic radiotherapy |
| Brain-Machine Interface (Recovery) |
| Cortical signals to induce movement |
| Spinal cord signals to induce movement |
Restoring Circulation
Clinical treatments in use currently focus on restoration of blood flow to the penumbral tissue. Dendrites and their spine morphology are adversely affected by ischemia; however, recovery is possible even with severe ischemia if blood flow is restored quickly (Zhang et al., 2005). After many decades of pessimism surrounding stroke therapies, tPA given intravenously showed efficacy in a major clinical trial if given within 3 hr of symptom onset (National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995). More recently, intravenous tPA was shown to be advantageous up to 4.5 hr after stroke in a large European trial (Hacke et al., 2008). Endovascular interventions of mechanical and chemical clot removal are often used clinically and have shown great promise with recent clinical trials demonstrating benefit within the acute timeframe (Berkhemer et al., 2015). The use of noninvasive transcranial Doppler ultrasound-assisted thrombolysis (in combination with intravenous tPA) is also being tested in early phase clinical trials (Barreto et al., 2013). Given the variety of strokes and patient differences in collaterals and vasculature, selecting the correct patients may be critical for the ultimate success of these therapies (Liebeskind et al., 2014). Unfortunately, a vast majority of stroke patients are not able to receive the acute treatments because of the narrow time windows. Further investigations studying the inflammatory and oxidative stresses as blood flow is restored will also help elucidate how the brain heals from ischemic injury. Therapies targeting later time windows are also needed to help recover from tissue that has been damaged before blood flow can be restored.
Disruption of Injury Pathways and Neuroprotection
The peri-infarct region appears to contain the highest potential for plasticity after stroke, with factors promoting growth and axonal sprouting expressed in this territory (Carmichael et al., 2005). Minimizing the damage to these areas and maximizing the potential for restoration are the goals of many of the neuro-protective strategies. Mechanisms that degrade and remodel the extracellular matrix (ECM), such as matrix metalloproteinases, are also upregulated in this region (Zhao et al., 2006). Additionally, angiogenesis and neurotrophic factors such as BDNF, which likely promote plasticity after stroke, are modulated in the area surrounding the infarct (Clarkson et al., 2011). Despite their success in animal models, clinical trials for multiple neuroprotective strategies have proven uninspiring. Multiple explanations exist for this (Dirnagl et al., 1999). One reason is the inadequacy of current animal models. The human brain and response to injury is far more complex than in the rodent. The variability in human anatomy is also difficult to represent in mouse models genetically engineered to be identical. Additionally, young rodents are most commonly used in the laboratory setting due to expenses, although differences in response to stroke are seen between young and older animals. Another difference is that outcome measures can be much more precisely designed and evaluated in the preclinical setting compared with clinical scales that may not truly assess the subtleties of stroke phenotypes. Timing of these therapies is another critical component and is much more controlled in the lab environment compared with clinical application, where administration of a drug can be delayed for hours or days depending on the patient's presentation. A recent trial studying the effect of magnesium given acutely after stroke developed a pathway for rapid delivery of medications. In this trial, magnesium was given in ambulances before the patients arrived at the hospital (Saver et al., 2014). Although this trial was negative, the methodology will serve as a guide for future trials whose treatment effect is contingent on rapid drug administration.
While affecting a single pathway in an animal model is sufficient to prevent injury, multiple pathways may need to be disrupted in humans to yield similar results. Mild brain hypothermia (33°C), which has become the gold standard for acute neuroprotection in rodent stroke models (Zhao et al., 2007), improves neurologic outcomes for patients with a particular type of stroke (global cerebral ischemia) secondary to cardiac arrest and neonatal hypoxic-ischemic encephalopathy (Shankaran et al., 2012; Bernard et al., 2002). Therapeutic hypothermia after cardiac arrest has now become a recommended guideline for clinical care (Peberdy et al., 2010). Mild hypothermia is currently being investigated as an acute stroke therapy, with trials to date proving the feasibility of this approach (Piironen et al., 2014). Another promising acute neuroprotective strategy targets the post-synaptic density-95 protein (PSD-95). PSD-95 connects NMDA receptors to signaling pathways necessary for the excitotoxic cascade and inhibiting these circuits reduces stroke volume in primates (Cook et al., 2012). A recently published prospective, randomized, double-blind controlled trial demonstrated safety and improved neurologic outcome and fewer acute infarcts in patients undergoing endovascular intracranial aneurysm repair who received a PSD-95 inhibitor (Hill et al., 2012). As barriers to accurately mimic clinical practice in the laboratory are reduced and the ability to manipulate multiple recovery pathways are improved, more effective neuroprotective therapies can be developed.
Cell-Based Therapies
Stem cell therapy is an exciting area of research that has entered the clinical arena with multiple ongoing trials. Stem cells are pluripotent or multipotent cells that have the ability to transform into multiple cell types and are self-perpetuating. Endogenous therapeutic strategies focus on increasing mobilization, longevity, and production of neural stem cells in the SVZ and dentate gyrus. Exogenous stem cell treatments refer to transplanted cells from another source into a patient. Exogenous stem cells have been delivered to the brain via the blood stream or direct transplantation and have shown great promise in animal models to enhance stroke recovery.
Endogenous Stem Cells
Neural progenitor cells (NPCs), which in health migrate along the rostral migratory system to the olfactory lobe and other brain regions, traverse to injured areas of the brain in the setting of neurological insult (Goings et al., 2004). Brain ischemia results in upregulation of endogenous NPCs and sometimes differentiation into the predominant cell type of the injured region (Arvidsson et al., 2002; Parent et al., 2002). Therapeutic approaches have focused on augmenting the brain's normal endogenous reaction to injury. Multiple pathways induce neurogenesis, including those triggered by numerous neurotrophic and growth factors such as GDNF, BDNF, granulocyte colony-stimulating factor (G-CSF), and insulin growth factor (IGF-1) (Kobayashi et al., 2006;Dempsey et al., 2003). Alternative mechanisms of increasing endogenous NPC proliferation include anti-inflammatory drugs like indomethacin, non-coding RNA, and hormones such as erythropoietin (Hoehn et al., 2005; Schouten et al., 2012; Wang et al., 2004). Delivering G-CSF and IGF-1 to alter key survival pathways such as the phosphoinositide 3-kinase-Akt pathway are able to reduce NPC death (Lee et al., 2006). Current clinical trials are investigating the ability of G-CSF to mobilize endogenous bone marrow cells as well as utilizing its neuroprotective effects to determine efficacy in stroke recovery (Dunac et al., 2007; Kawada et al., 2006).
Increasing the numbers of migrating endogenous stem cells can be achieved using various chemokine receptors such as stromal derived factor1 and integrin β1 (Ohab et al., 2006; Yan et al., 2007), and manipulation of ECM components or electrical fields to guide endogenous NPCs has been investigated (Lee et al., 2006; Babona-Pilipos et al., 2011). However, currently this research has been limited to the preclinical arena.
Exogenous Stem Cells
Exogenous stem cells are typically divided into three categories: (1) immortalized cell lines, (2) NPCs or neural stem cells, and (3) bone marrow-derived hematopoietic/endothelial progenitors and stromal cells (Bliss et al., 2010). Immortalized cell lines have been developed from tumor cells or from manipulation with oncogenes (such as myc in the human fetal neural cell line ReN001 of ReNeuron). The NT2N cells, derived from teratocarcinoma, differentiate into post-mitotic neuron-like cells with the addition of retinoic acid and mitotic inhibitors (Andrews et al., 1984; Pleasure and Lee, 1993) and have been shown to improve outcome in several ischemic models (Saporta et al., 1999). ReNeuron's cells have shown dose-dependent recovery in stroke rodent models (Stroemer et al., 2009) and creatively have been engineered to be immortalized only in the presence of tamoxifen to reduce the risk of tumor formation (Stroemer et al., 2008).
Human NPCs are derived from embryonic and fetal tissue and have the ability to produce astrocytes, neurons, and oligodendrocytes (Gage, 2000). In stroke models, NPCs are able to migrate to the injured regions and improve recovery (Zhang et al., 2001; Reubinoff et al., 2001; Kelly et al., 2004). NPCs sometimes integrate into the host tissue and differentiate and can demonstrate neuronal characteristics, including expression of synaptic proteins, synapse formation, and electrophysiological properties (Bühnemann et al., 2006; Daadi et al., 2009a, 2009b).
Progenitor cells derived from bone marrow, umbilical cord blood and adipose tissue have all been shown to improve recovery in stroke models (Shen et al., 2007). Many of these sources are already used for the treatment of other disorders clinically such as malignancy and can be obtained from autologous harvesting. Although numerous cell types are included in each of these sources and it appears that the mononuclear or marrow stromal cell component mediates recovery, it is not clear which subtype is responsible for improving functional outcomes. Multiple trials have been performed or are ongoing using these exogenous stem cells (Table 2).
Table 2.
List of 35 Completed or Ongoing Trials Using Exogenous Stem or Progenitor Cells
| Clinical Trial Identifier | Study Type | Cell Type | Planned Enrollment | Timing of Delivery | Delivery Route | Status/Results |
|---|---|---|---|---|---|---|
| NCT00473057 | Ph1-NR-OL | BMMNC | 15 | 3–90 days | IA or i.v. | Complete, no reported results |
| NCT02065778 | Ph1-NR-OL | BMMNC | 30 | chronic | IT | Complete, no reported results |
| NCT01501773 | Ph2-R-OL | BMMNC | 11 | 7–30 days | i.v. | Safe, feasible |
| NCT01849887 | Ph1/2-R-DB | BMMNC | 40 | 1–3 days | i.v. | Not currently recruiting |
| NCT00859014 | Ph1-NR-OL | BMMNC | 10 | 1–3 days | i.v. | Safe, feasible |
| NCT02425670 | Ph2-R-SB | BMMNC | 120 | 7–30 days | i.v. | Safe, feasible, no efficacy benefit |
| NCT01832428 | Ph1/2-NR-OL | BMMNC | 50 | chronic | IT | Recruiting |
| NCT02245698 | Ph1-NR-OL | BMMNC | 200 | subacute/chronic | IT | Recruiting |
| NCT02290483 | Ph2-R-OL | BMMNC | 76 | 1–7 days | IA | Recruiting |
| India, 2011 | Ph1/2-NR-OL | BMMNC | 11 | 3–12 months | i.v. | Safe, feasible, improved neurologic outcomes |
| NCT01436487 | Ph2-R-DB | multistem | 126 | 1–2 days | i.v. | Safe, feasible, no efficacy benefit |
| NCT02117635 | Ph2-NR-OL | CTX0E03, NSC | 41 | 2–3 months | IC | Safe, improved neurologic outcomes |
| NCT01151124 | Ph1-NR-OL | CTX0E03, NSC | 12 | 6–60 months | IC | Not currently recruiting |
| NCT01453829 | Ph1/2- NR-OL | ASC | 10 | subacute | IA | Not currently recruiting |
| NCT01091701 | Ph1/2-R-DB | MSC | 78 | <10 days | i.v. | Not currently recruiting |
| South Korea, 2010 | Ph1/2-R-OL-SB | MSC | 85 | 5–7 weeks | i.v. | Safe, feasible, improved neurologic outcomes |
| NCT00875654 | Ph2-R-OL | MSC | 30 | <6 weeks | i.v. | Not currently recruiting |
| NCT01297413 | Ph1/2-NR-OL | MSC | 35 | >6 months | i.v. | Recruiting |
| NCT01678534 | Ph1/2-R-DB | MSC | 40 | <14 days | i.v. | Not currently recruiting |
| Japan, 2011 | Ph1-NR-OL | 12 | 1–4 months | Safe, feasible, decreased infarct volume | ||
| NCT01714176 | Ph1-NR-OL | MSC | 30 | 3–60 months | IC | Recruiting |
| NCT01716481 | Ph3-R-OL | MSC | 60 | <90 days | i.v. | recruiting |
| NCT0146172 | Ph2-NR-OL | MSC | 50 | 1week to 2months | i.v. | Not currently recruiting |
| NCT01922908 | Ph1/2-R-DB | MSC | 48 | 3–10 days | i.v. | Not currently recruiting |
| NCT01468064 | Ph1/2-R-DB | MSC, EPC | 90 | 5 weeks | i.v. | Recruiting |
| NCT00761982 | Ph1/2-NR-SB | CD34+ | 20 | 5–9 days | IA | Safe, feasible, increased β-NGF |
| NCT00950521 | Ph2-R-OL | CD34+ | 30 | 6–60 months | IC | Complete, no reported results |
| NCT00535197 | Ph1/2-NR-OL | CD34+ | 5 | 7 days | IA | Safe, feasible, reduced infarct volume |
| NCT01518231 | Ph1-R-OL | CD34+ | 40 | <12 months | IA | Recruiting |
| NCT01438593 | Ph1-NR-OL | CD34+ | 6 | 6–60 months | IC | Not currently recruiting |
| NCT01310114 | Ph2-R-DB | PDC | 44 | acute | i.v. | Stopped by sponsor |
| NCT01327768 | Ph1-R-SB | OEC | 6 | 6–60 months | IC | Recruiting |
| NCT01287936 | Ph1/2-NR-OL | SB623 | 18 | 6–36 months | IC | Safe, improved neurologic outcomes |
| BB-IND 7082 | Ph2-R-OL-SB | NT2 | 18 | 1–5 years | IC | Safe, feasible, improved neurologic outcomes in secondary endpoints |
| BB-IND 7082 | Ph1-NR-OL | NT2 | 12 | 6–72 months | IC | Safe, improved neurologic outcomes |
ASC, adipose-derived stromal cells; EPC, endothelial progenitor cells; NSC, neural stem cells; OEC, olfactory ensheathing cells; PDC, placenta-derived stem cells; SB623, human mesenchymal stromal cells; NT2, tetracarcinoma cell-derived neurons; P1, Phase 1 trial; P2, Phase 2 trial; OL, open label; R, randomized; NR, nonrandomized; DB, double blind; SB, single blind; i.v., intravenous; IA, intra-arterial; IC, intracranial.
Induced Stem Cells
The discovery of induced pluripotent stem (iPS) cells created a paradigm shift in cell therapy. The ability to transform host somatic cells such as fibroblasts into pluripotent stem cells bypassed many of the concerns of traditional stem cell therapy, such as ethical discussions, supply limitations, and the possible requirement of immunosuppression (Meissner et al., 2007; Takahashi and Yamanaka, 2006; Wernig et al., 2007). Further development has led to vector- and transgene-free techniques to derive iPS cells that improve functional outcome after brain ischemia (Mohamad et al., 2013). Recently, it has been possible to generate neural cells directly from mouse or human fibroblasts using transcription factors, without passing through a pluripotent phase, which may ultimately have clinical relevance (Pang et al., 2011).
Stem Cell Mechanism
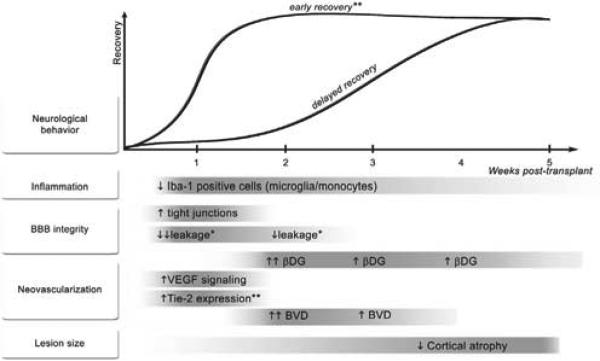
The precise mechanism of action of stem cell therapeutics remains elusive. Until mechanisms are better understood, more intelligent design of trials and applications will be limited. The ability to fabricate and secrete trophic factors is common to all stem cell types and may create the optimum environment for stroke recovery (Bliss et al., 2010). Enhanced recovery most strongly was associated with a reduction in apoptosis in a recent meta-analysis evaluating preclinical stem cell studies (Janowski et al., 2010). Stem cells’ role as local or systemic immunoregulators also may contribute to their ability to improve stroke recovery by decreasing inflammatory effects (Horie et al., 2011). NPCs and bone marrow-derived stem cells augment post-stroke plasticity through upregulation of synapse formation, dendritic branching, and axonal connections (Liu et al., 2008; Andres et al., 2011). Stem cells also enhance angiogenesis and blood-brain barrier repair, which have also shown to improve recovery (Chen et al., 2003; Horie et al., 2011). Although small numbers of transplanted cells may integrate into tissue, the extent of behavioral improvement does not appear to correlate with the number of cells. Additionally, the timing of synapse formation does not always correlate with functional improvement (Song et al., 2002; Englund et al., 2002). Given the complex pathophysiology of stroke, the importance of timing on the effects of factors, and the balance of signals in the pathways of recovery as described above (Figure 2), it is essential to better understand the mechanisms of improvement following stem cell therapy in order to translate these discoveries to clinical applications.
Figure 2. Temporal Profile of Changes Induced by Neural Stem Cell Enhanced Stroke Recovery.
This figure was courtesy of Horie et al., 2011. Neurological behavior indicates motor recovery. The asterisk indicates that Avastin affected all parameters except for the ones marked; two asterisks indicate the inconclusive effects of Avastin. BBB, blood-brain barrier; βDG, β-dystroglycan; BVD, blood vessel density; Iba-1, ionized calcium binding adaptor molecule 1; Tie-2, a receptor tyrosine kinase; VEGF, vascular endothelial growth factor.
Delivery
While intravenous and intra-arterial techniques likely rely on inflammatory modulation or paracrine effects of the cells on the post-ischemic brain, invasive transplantation of stem cells provides a more direct route for cell-to-cell interactions as well as for the stem cells’ trophic effects. More advanced delivery methods are also being developed, including bioengineered polymers to enhance stem cell survival and efficacy. Inert polymer matrices, such as hydrogels and particles, were first described for stem cell delivery (Teng et al., 2002; Zhong et al., 2010). The next step will be developing interactive polymers that are capable of communicating with stem cells in their transplanted environment to enhance recovery.
Clinical Trials
Apart from efficacy, safety is an important consideration to move forward with cell transplant therapy. Careful classification and understanding of the biology will be critical for reducing any predilection for tumor formation and adverse effects (Jandial and Snyder, 2009). The immortalized cell lines (NT2N) were the first human cells to be used in a clinical Phase I stroke trial and were implanted into the infarcted region of 12 patients, 6 months to 6 years after a basal ganglia stroke (Kondziolka et al., 2000). No significant adverse events occurred and functional improvement was seen in this small group of patients (p = 0.046). A subsequent Phase II trial with NT2N cells implanted into the peri-infarct or peri-hemorrhagic cavity showed no increase in adverse events (Kondziolka et al., 2005). An open-label, single-blinded randomized trial using mesenchymal stem cells (MSCs) showed significant improvement in functional outcome based on the modified Rankin scale (a functional outcome scale with 0–3 being able to walk with varying degrees of disability) in the treatment group without a difference in adverse events, and multiple other trials have shown safety and feasibility (Lee et al., 2010; Bhasin et al., 2011, 2013). In addition, trials using bone marrow mononuclear cells (BMMNCs) have shown safety and feasibility in the acute and chronic phases of recovery (Friedrich et al., 2012; Savitz et al., 2011; Moniche et al., 2012). A phase 1/2A study that transplanted human modified bone marrow-derived stromal cells showed safety and feasibility of direct intracerebral transplantation 6 months to 5 years post-stroke, with improvement in neurological outcomes (Steinberg et al., 2014). The first neural stem cell trial for ischemic stroke (PISCES) has been completed with results showing promise. In this open-label, dose-escalation study, no adverse events have been observed among the preliminary results in 11 patients with follow up between 9 to 24 months, and functional outcomes were improved after transplantation (D. Kalladka et al., 2014, European Stroke Conference).
Multiple questions have been raised about translating cell therapy to clinical applications. Thus far, tumorgenecity of cell therapies has not been shown to be problematic. Demonstration of efficacy in randomized, double-blinded trials is needed, but numerous clinical trials are underway (Table 2) to determine whether cell-based therapy will become the next modality of restorative stroke therapeutics.
Modulating Circuits to Increase Stroke Recovery
A shift in the excitatory-inhibitory balance in neural networks across the brain occurs after ischemia. In the setting of a long-term depression of inhibitory signals mediated by gamma-aminobutyric acid (GABA) receptors in bilateral hemispheres, cortical hyperexcitability peaks several weeks after stroke and can persist for months (Buchkremer-Ratzmann et al., 1996; Schiene et al., 1996). Sustained increase in glutamate transmission for 4-weeks post-stroke also contributes to greater excitatory signals (Centonze et al., 2007). Modulation of the tonic inhibition regulated by GABA(A) receptors improves functional recovery in animal models (Clarkson et al., 2010). The unaffected hemisphere also can influence the excitatory state of the damaged hemisphere altering recovery (Murase et al., 2004). Further regulation and understanding of the excitatory-inhibitory balance may prove critical when designing therapeutic approaches for stroke recovery.
Stimulation Techniques
Cortical stimulation is an exciting area of research aimed at restoring this excitatory-inhibitory balance of the damaged brain and reorganizing neural circuitry to improve stroke recovery. It is another method targeting multiple signaling pathways as electrical fields are applied across large areas of neural tissue. Noninvasive methods (i.e., repetitive transcranial magnetic stimulation [rTMS] and transcranial direct current stimulation [tDCS]) and invasive methods (i.e., implantable epidural electrodes) exist. Initially, animal models showed functional improvement after stimulation of motor areas and later stimulation of connected but separate pathways have shown efficacy (Kleim et al., 2003; Machado et al., 2009). Utilizing the fact that high-frequency rTMS increases cortical excitability and low-frequency stimulation decreases excitability, ipsilateral or contralateral stimulation has shown to increase functional improvement of the affected extremity for intermediate periods (Khedr et al., 2005; Kirton et al., 2008; Conforto et al., 2012). The role of the contralesional hemisphere after stroke is an area of continued interest and may have beneficial as well as detrimental effects on stroke recovery. The contralesional hemisphere likely can be recruited to improve recovery, but it also imposes increased inhibition on the affected hemisphere especially in the primary motor strip (Murase et al., 2004). This suppressive effect may be beneficial at reducing complications such as seizures, but may prevent plasticity and functional recovery. The majority of therapies aim to restore the excitatory balance between the two hemispheres in order to improve recovery. Stimulation with tDCS has found similar outcomes with improvement after stroke during therapies and for short durations after stimulation (Hummel et al., 2005). Recent Cochrane reviews of rTMS and tDCS both conclude that further studies are required to determine these techniques’ role in stroke recovery (Hao et al., 2013; Elsner et al., 2013). Longer term efficacy is currently being studied with randomized, double-blinded trials to evaluate the utility of these treatment paradigms (Plow et al., 2013).
Invasive cortical stimulation offers the advantage of stimulus patterns of greater duration and at a more stable position. Given that multiple sessions of non-invasive stimulation produce longer sustained improvement, implantable electrodes provide a unique method of delivering more continuous or frequent stimulation (Khedr et al., 2005). Upper-extremity recovery is a significant limitation following stroke with only one-fifth of patients obtaining full recovery at 6 months (Kwakkel et al., 2003). Animal and pilot human studies have demonstrated improved recovery and safety with invasive stimulation techniques (Kleim et al., 2003; Levy et al., 2008). Based on these preliminary studies, the Everest trial was initiated using cortical stimulation combined with rehabilitation to improve upper-extremity recovery after ischemia (Harvey et al., 2009). Unfortunately, this study was discontinued prematurely by the company (Northstar Neuroscience). As we await the results of ongoing clinical trials, further pre-clinical research is underway to help determine the most efficacious locations and patterns for stimulation to improve stroke recovery (Cheng et al., 2014). A better understanding of the proper stimulation sites and paradigms should enable translation of this technique to the clinical arena.
Optogenetics
With the growth in the field of optogenetics, circuits in the brain can be more easily adjusted to tease apart mechanisms of recovery. Because stimulation can be targeted more precisely, the underlying circuits can be more carefully evaluated. Using optogenetic technology, it was discovered that even small ischemic injuries and depression in excitability could lead to relatively large effects on motor circuits (Anenberg et al., 2014). In addition to contributing to our understanding of the circuitry of the brain in the post-stroke environment, optogenetics also has potential as a therapeutic modality. Optogenetic techniques have been used to mitigate seizures and similar strategies could be utilized to alter neural excitability post-stroke (Paz et al., 2013). With the ability to stimulate specific circuits, optogenetics serves as another useful tool for stimulating defined neural pathways in particular brain regions to improve recovery. A recent study demonstrated that utilizing optogenetics to selectively stimulate ipsilesional primary motor cortex neurons after stroke improved functional outcomes in a rodent model, as well as modulated neurotrophic factors in the contralesional cortex (Cheng et al., 2014). A greater understanding of the exact regions that are critical for recovery will allow stimulation to be more effectively tailored to augment recovery. The requirement of gene alteration for optogenetics currently limits it clinical applications, but as clinical gene therapy advances, the use of optogenetics to modulate recovery pathways will be more easily translated to help patients.
Altering Connections
With scientists focusing on the connectome and the complicated neural circuits that integrate in the brain to enable function, technologies that can alter these connections may eventually offer therapeutic applications. One such technology that is being investigated for multiple neurological diseases, including essential tremor, is magnetic resonance-guided focused ultrasound (MRgFUS) (Lipsman et al., 2014). MRgFUS combines advanced MRI and ultrasound technology to accurately focus ultrasonic energy to specific locations in the brain. Several advances make this technology possible, including (1) high-resolution brain and temperature mapping with MRI, (2) merging of software and a phased array, ultrasound transducer helmet to compensate for skull distortion, and (3) technology enabling precise focusing of the ultrasonic energy. MRgFUS allows for controlled thermal ablation of specific brain regions (Clement and Hynynen, 2002). As our understanding of the remodeling of circuits after ischemic injury (some beneficial and some maladaptive) improves, we will be able to apply technology such as MRgFUS to enhance functional recovery. Additionally, MRgFUS has been show to disturb the blood-brain barrier, which allows for therapeutic agents to more easily reach an infarcted area (Hynynen et al., 2001). It may also be possible to modulate circuits after stroke using stereotactic radiosurgery techniques, including CyberKnife, Gamma Knife, linear accelerator (LINAC) and cyclotron, which use focused radiation to place tiny lesions in brain structures. These therapies have already improved outcomes in select patients with Parkinson's disease, essential tremor, chronic pain, epilepsy, and mood disorders (Romanelli et al., 2013; Kim and Lee, 2008; Lad et al., 2007; Ohye et al., 2012). An innovative, non-invasive stimulation method utilizing vibrotactile skin stimulation is currently being planned for patients with epilepsy and may be applicable for stroke patients as well (D'Alonzo and Cipriani, 2012). While these technologies remain in their early stages with regards to clinical applications, their use can provide a method to determine how different brain connections affect recovery and ultimately begin to study their applications as stroke treatments.
Brain-Computer Interface
Given the elaborate networks connecting motor control regions of the brain to our muscles, several methods of stroke recovery look to bypass damaged areas of the brain and focus on healthy central or peripheral circuits. Because ischemia is usually an isolated event as opposed to an ongoing neurodegenerative process, many of the neural networks not affected by the infarct remain unharmed. Utilizing technology to harness intact circuits in the brain has led to the field of brain-computer interfaces. Through the study of extra-cellular potentials, researchers have deciphered cortical representation of motor movements (Moran and Schwartz, 1999). Often movements are controlled by interpreting cortical activity to induce movements in primates (Churchland et al., 2010), and more recently, cortical signals recorded through high-density microelectrode arrays or electrocorticography grids allowed paralyzed patients the ability to control robotic limbs and computer cursors (Hochberg et al., 2012; Collinger et al., 2013). Alternative approaches stimulated pathways in the spinal cord to induce movements for walking and hand function (Moritz et al., 2007). Activation of spinal circuits has the benefit of triggering multiple muscle groups required to perform a certain task. Closed-loop systems have begun to explore the ability for primates to control limb function by utilizing cortical signals to stimulate spinal circuits to induce upper limb movements (Zimmermann and Jackson, 2014). In patients whose primary cortical areas have been damaged, alternative areas must be trained, or remaining electromyography (EMG) activity has also been used to trigger spinal cord circuits for the performance of certain tasks (Amsuss et al., 2014; Zimmer-mann and Jackson, 2014). Non-invasive methods such as electroencephalography (EEG)-based systems have also been implemented in neurorehabilitation programs, and as this technology is developed further, it may replace implantable arrays (Ang et al., 2014). These methods are still limited by complications in long-term tissue/electronic interfaces and our ability to accurately decipher integrated neural outputs of the cortex. As our ability to interpret cortical signals and robotics continue to advance, brain-computer interfaces offer exciting potential to restore function to patients with hemiplegia or language impairment from stroke.
Conclusions
Ischemic brain injury is a complicated disease affecting a variety of brain regions, resulting in disruption of numerous neural circuits and involving complex injury response. As we look to the horizon for stroke recovery and therapeutics, a more holistic approach may be required. Utilizing treatments that alter multiple cell injury pathways is likely needed to achieve clinically relevant improvements. Currently, cell therapies and stimulation techniques appear closest to clinical application, but many exciting therapeutic approaches are being developed. Advances in genomics, proteomics, and metabolomics will help to better evaluate patients and their individual response to treatments. A focus on modulating circuits that mediate recovery will become increasingly important, and the burgeoning field of brain-computer interface has the potential to have a major impact. As medicine becomes more personalized with a better understanding of genetic implications on therapeutics, stroke treatments will become more tailored to the individual patient and specific stroke types. More sophisticated clinical outcome scales to characterize patient deficits will allow for better evaluation of treatments translated from the lab. As is most often the case, new biomedical discoveries will continue to advance the field and elucidate mechanisms of injury to guide novel therapeutics.
ACKNOWLEDGMENTS
This review was supported in part by funding from NIH National Institute of Neurological Disorders and Stroke grants R01NS058784 and R21NS082894, CIRM grants DR1-01480 and RB5-07363, Bernard and Ronni Lacroute, the William Randolph Hearst Foundation, Russell and Elizabeth Siegelman (to G.K.S.), and the American Brain Foundation (to P.M.G.). Dr. Steinberg serves on the Neuroscience Advisory Board at Medtronics. We thank Cindy H. Samos for assistance with the manuscript.
REFERENCES
- Althausen S, Mengesdorf T, Mies G, Oláh L, Nairn AC, Proud CG, Paschen W. Changes in the phosphorylation of initiation factor eIF-2alpha, elongation factor eEF-2 and p70 S6 kinase after transient focal cerebral ischaemia in mice. J. Neurochem. 2001;78:779–787. doi: 10.1046/j.1471-4159.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- Amsuss S, Goebel PM, Jiang N, Graimann B, Paredes L, Farina D. Self-correcting pattern recognition system of surface EMG signals for upper limb prosthesis control. IEEE Trans. Biomed. Eng. 2014;61:1167–1176. doi: 10.1109/TBME.2013.2296274. [DOI] [PubMed] [Google Scholar]
- Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW, Damjanov I, Simon D, Banting GS, Carlin C, Dracopoli NC, Føgh J. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab. Invest. 1984;50:147–162. [PubMed] [Google Scholar]
- Anenberg E, Arstikaitis P, Niitsu Y, Harrison TC, Boyd JD, Hilton BJ, Tetzlaff W, Murphy TH. Ministrokes in channelrhodopsin-2 transgenic mice reveal widespread deficits in motor output despite maintenance of cortical neuronal excitability. J. Neurosci. 2014;34:1094–1104. doi: 10.1523/JNEUROSCI.1442-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KK, Chua KSG, Phua KS, Wang C, Chin ZY, Kuah CWK, Low W, Guan C. A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clin. EEG Neurosci. 2014 doi: 10.1177/1550059414522229. Published online April 21, 2014. http://dx.doi.org/10.1177/1550059414522229. [DOI] [PubMed]
- Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc. Natl. Acad. Sci. USA. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Babona-Pilipos R, Droujinine IA, Popovic MR, Morshead CM. Adult subependymal neural precursors, but not differentiated cells, undergo rapid cathodal migration in the presence of direct current electric fields. PLoS ONE. 2011;6:e23808. doi: 10.1371/journal.pone.0023808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Dang Q, Lapergue B, Tran-Dinh A, Diallo D, Moreno JA, Mazighi M, Romero IA, Weksler B, Michel JB, Amarenco P, Meilhac O. High-density lipoproteins limit neutrophil-induced damage to the blood-brain barrier in vitro. J. Cereb. Blood Flow Metab. 2013;33:575–582. doi: 10.1038/jcbfm.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto AD, Alexandrov AV, Shen L, Sisson A, Bursaw AW, Sahota P, Peng H, Ardjomand-Hessabi M, Pandurengan R, Rahbar MH, et al. CLOTBUST-Hands Free: pilot safety study of a novel operator-independent ultrasound device in patients with acute ischemic stroke. Stroke. 2013;44:3376–3381. doi: 10.1161/STROKEAHA.113.002713. [DOI] [PubMed] [Google Scholar]
- Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. MR CLEAN Investigators A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, Gaikwad S, Garg A, Airan B. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc. Dis. Extra. 2011;1:93–104. doi: 10.1159/000333381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin A, Srivastava MV, Mohanty S, Bhatia R, Kumaran SS, Bose S. Stem cell therapy: a clinical trial of stroke. Clin. Neurol. Neurosurg. 2013;115:1003–1008. doi: 10.1016/j.clineuro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol. Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J. Neurosci. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW. Electrophysiological transcortical diaschisis after cortical photothrombosis in rat brain. Stroke. 1996;27:1105–1109. doi: 10.1161/01.str.27.6.1105. discussion 1109–1111. [DOI] [PubMed] [Google Scholar]
- Bühnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, Reymann KG, Dihné M. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129:3238–3248. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/ progenitor cells. Mol. Cell. Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp. Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Tortiglione A, Picconi B, Prosperetti C, De Chiara V, Bernardi G, Calabresi P. Synaptic plasticity during recovery from permanent occlusion of the middle cerebral artery. Neurobiol. Dis. 2007;27:44–53. doi: 10.1016/j.nbd.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG, Arac A, Fenno LE, Deisseroth K, Steinberg GK. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc. Natl. Acad. Sci. USA. 2014;111:12913–12918. doi: 10.1073/pnas.1404109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron. 2010;68:387–400. doi: 10.1016/j.neuron.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J. Neurosci. 2011;31:3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys. Med. Biol. 2002;47:1219–1236. doi: 10.1088/0031-9155/47/8/301. [DOI] [PubMed] [Google Scholar]
- Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJC, Velliste M, Boninger ML, Schwartz AB. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381:557–564. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforto AB, Anjos SM, Saposnik G, Mello EA, Nagaya EM, Santos W, Jr., Ferreiro KN, Melo ES, Reis FI, Scaff M, Cohen LG. Transcranial magnetic stimulation in mild to severe hemiparesis early after stroke: a proof of principle and novel approach to improve motor function. J. Neurol. 2012;259:1399–1405. doi: 10.1007/s00415-011-6364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM. Reactive oxygen species and modulation of stroke. Free Radic. Biol. Med. 2005;38:1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- D'Alonzo M, Cipriani C. Vibrotactile sensory substitution elicits feeling of ownership of an alien hand. PLoS ONE. 2012;7:e50756. doi: 10.1371/journal.pone.0050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Lee SH, Arac A, Grueter BA, Bhatnagar R, Maag AL, Schaar B, Malenka RC, Palmer TD, Steinberg GK. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant. 2009a;18:815–826. doi: 10.3727/096368909X470829. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, Wu JC, Steinberg GK. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol. Ther. 2009b;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey RJ, Sailor KA, Bowen KK, Türeyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J. Neurochem. 2003;87:586–597. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dunac A, Frelin C, Popolo-Blondeau M, Chatel M, Mahagne MH, Philip PJ. Neurological and functional recovery in human stroke are associated with peripheral blood CD34+ cell mobilization. J. Neurol. 2007;254:327–332. doi: 10.1007/s00415-006-0362-1. [DOI] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst. Rev. 2013;11:CD009645. doi: 10.1002/14651858.CD009645.pub2. [DOI] [PubMed] [Google Scholar]
- Englund U, Bjorklund A, Wictorin K, Lindvall O, Kokaia M. Grafted neural stem cells develop into functional pyramidal neurons and integrate into host cortical circuitry. Proc. Natl. Acad. Sci. USA. 2002;99:17089–17094. doi: 10.1073/pnas.252589099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, Jacoby DB. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS ONE. 2011;6:e22029. doi: 10.1371/journal.pone.0022029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MA, Martins MP, Araújo MD, Klamt C, Vedolin L, Garicochea B, Raupp EF, Sartori El Ammar J, Machado DC, Costa JC, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012;21(Suppl 1):S13–S21. doi: 10.3727/096368912x612512. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Garcia-Bonilla L, Moore JM, Racchumi G, Zhou P, Butler JM, Iadecola C, Anrather J. Inducible nitric oxide synthase in neutrophils and endothelium contributes to ischemic brain injury in mice. J. Immunol. 2014;193:2531–2537. doi: 10.4049/jimmunol.1400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Gleichman AJ, Carmichael ST. Astrocytic therapies for neuronal repair in stroke. Neurosci. Lett. 2014;565:47–52. doi: 10.1016/j.neulet.2013.10.055. [DOI] [PubMed] [Google Scholar]
- Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. ECASS Investigators Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst. Rev. 2013;5:CD008862. doi: 10.1002/14651858.CD008862.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RL, Winstein CJ, Everest Trial Group Design for the everest randomized trial of cortical stimulation and rehabilitation for arm function following stroke. Neurorehabil. Neural Repair. 2009;23:32–44. doi: 10.1177/1545968308317532. [DOI] [PubMed] [Google Scholar]
- Hill MD, Martin RH, Mikulis D, Wong JH, Silver FL, Terbrugge KG, Milot G, Clark WM, Macdonald RL, Kelly ME, et al. ENACT trial investigators Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11:942–950. doi: 10.1016/S1474-4422(12)70225-9. [DOI] [PubMed] [Google Scholar]
- Hoare BJ, Wasiak J, Imms C, Carey L. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database Syst. Rev. 2007;2:CD004149. doi: 10.1002/14651858.CD004149.pub2. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, et al. Transplanted stem cell-secreted VEGF effects post-stroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell. Mol. Neurobiol. 2006;26:1057–1083. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb. Blood Flow. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandial R, Snyder EY. A safer stem cell: on guard against cancer. Nat. Med. 2009;15:999–1001. doi: 10.1038/nm0909-999. [DOI] [PubMed] [Google Scholar]
- Janowski M, Walczak P, Date I. Intravenous route of cell delivery for treatment of neurological disorders: a meta-analysis of preclinical results. Stem Cells Dev. 2010;19:5–16. doi: 10.1089/scd.2009.0271. [DOI] [PubMed] [Google Scholar]
- Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Kim MC, Lee TK. Stereotactic lesioning for mental illness. Acta Neurochir. Suppl. (Wien) 2008;101:39–43. doi: 10.1007/978-3-211-78205-7_7. [DOI] [PubMed] [Google Scholar]
- Kirton A, Chen R, Friefeld S, Gunraj C, Pontigon AM, Deveber G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet Neurol. 2008;7:507–513. doi: 10.1016/S1474-4422(08)70096-6. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol. Res. 2003;25:789–793. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke. 2006;37:2361–2367. doi: 10.1161/01.STR.0000236025.44089.e1. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, Decesare S, Jovin T, Zafonet R, et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J. Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ, Elder E, Gebel J, Decesare S, Jovin T, Zafonet R, Lebowitz J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- Lad SP, Lipani JD, Gibbs IC, Chang SD, Adler JR, Jr., Henderson JM. Cyberknife targeting the pterygopalatine ganglion for the treatment of chronic cluster headaches. Neurosurgery. 2007;60:E580–E581. E581. doi: 10.1227/01.NEU.0000255348.33582.DE. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J. Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, STARTING collaborators A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- Levy R, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. J. Neurosurg. 2008;108:707–714. doi: 10.3171/JNS/2008/108/4/0707. [DOI] [PubMed] [Google Scholar]
- Liebeskind DS, Jahan R, Nogueira RG, Zaidat OO, Saver JL, SWIFT Investigators Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke. 2014;45:2036–2040. doi: 10.1161/STROKEAHA.114.004781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Uhde I, Gräf S, Leidner O, Weiller C. Motor cortex plasticity during forced-use therapy in stroke patients: a preliminary study. J. Neurol. 2001;248:315–321. doi: 10.1007/s004150170207. [DOI] [PubMed] [Google Scholar]
- Lipsman N, Mainprize TG, Schwartz ML, Hynynen K, Lozano AM. Intracranial applications of magnetic resonance-guided focused ultra-sound. Neurotherapeutics. 2014;11:593–605. doi: 10.1007/s13311-014-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado AG, Baker KB, Schuster D, Butler RS, Rezai A. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res. 2009;1280:107–116. doi: 10.1016/j.brainres.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Domercq M, Pérez-Samartín A, Ransom BR. Protecting white matter from stroke injury. Stroke. 2013;44:1204–1211. doi: 10.1161/STROKEAHA.112.658328. [DOI] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat. Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, Mandeville JB, Kim KW, Lo EH, Arai K. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad O, Drury-Stewart D, Song M, Faulkner B, Chen D, Yu SP, Wei L. Vector-free and transgene-free human iPS cells differentiate into functional neurons and enhance functional recovery after ischemic stroke in mice. PLoS One. 2013;8:e64160. doi: 10.1371/journal.pone.0064160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Piñero P, Espigado I, Garcia-Solis D, Cayuela A, Montaner J, Boada C, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43:2242–2244. doi: 10.1161/STROKEAHA.112.659409. [DOI] [PubMed] [Google Scholar]
- Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J. Neurophysiol. 1999;82:2676–2692. doi: 10.1152/jn.1999.82.5.2676. [DOI] [PubMed] [Google Scholar]
- Morimoto N, Oida Y, Shimazawa M, Miura M, Kudo T, Imaizumi K, Hara H. Involvement of endoplasmic reticulum stress after middle cerebral artery occlusion in mice. Neuroscience. 2007;147:957–967. doi: 10.1016/j.neuroscience.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Lucas TH, Perlmutter SI, Fetz EE. Forelimb movements and muscle responses evoked by microstimulation of cervical spinal cord in sedated monkeys. J. Neurophysiol. 2007;97:110–120. doi: 10.1152/jn.00414.2006. [DOI] [PubMed] [Google Scholar]
- Moro MA, Cárdenas A, Hurtado O, Leza JC, Lizasoain I. Role of nitric oxide after brain ischaemia. Cell Calcium. 2004;36:265–275. doi: 10.1016/j.ceca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Thomas S, Pasinetti GM. Complement anaphylatoxin C5a neuroprotects through regulation of glutamate receptor subunit 2 in vitro and in vivo. J. Neuroinflammation. 2008;5:5. doi: 10.1186/1742-2094-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Murphy AN, Fiskum G, Beal MF. Mitochondria in neurodegeneration: bioenergetic function in cell life and death. J. Cereb. Blood Flow Metab. 1999;19:231–245. doi: 10.1097/00004647-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Namura S, Ooboshi H, Liu J, Yenari MA. Neuroprotection after cerebral ischemia. Ann. N Y Acad. Sci. 2013;1278:25–32. doi: 10.1111/nyas.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333:1581–1588. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J. Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohye C, Higuchi Y, Shibazaki T, Hashimoto T, Koyama T, Hirai T, Matsuda S, Serizawa T, Hori T, Hayashi M, et al. Gamma knife thalamotomy for Parkinson disease and essential tremor: a prospective multi-center study. Neurosurgery. 2012;70:526–535. doi: 10.1227/NEU.0b013e3182350893. discussion 535–536. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, et al. 2010 AHA guidelines for cardiopulmonary resuscitation and emergency cardiovascular care science. Circulation. 2010;122:S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- Pedersen ED, Waje-Andreassen U, Vedeler CA, Aamodt G, Mollnes TE. Systemic complement activation following human acute ischaemic stroke. Clin. Exp. Immunol. 2004;137:117–122. doi: 10.1111/j.1365-2249.2004.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzotti-Jametti L, Donega M, Giusto E, Mallucci G, Marchetti B, Pluchino S. The role of the immune system in central nervous system plasticity after acute injury. Neuroscience. 2014;283:210–221. doi: 10.1016/j.neuroscience.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piironen K, Tiainen M, Mustanoja S, Kaukonen KM, Meretoja A, Tatlisumak T, Kaste M. Mild hypothermia after intravenous thrombolysis in patients with acute stroke: a randomized controlled trial. Stroke. 2014;45:486–491. doi: 10.1161/STROKEAHA.113.003180. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Lee VM. NTera 2 cells: a human cell line which displays characteristics expected of a human committed neuronal progenitor cell. J. Neurosci. Res. 1993;35:585–602. doi: 10.1002/jnr.490350603. [DOI] [PubMed] [Google Scholar]
- Plow EB, Cunningham DA, Beall E, Jones S, Wyant A, Bonnett C, Yue GH, Lowe M, Wang XF, Sakaie K, Machado A. Effectiveness and neural mechanisms associated with tDCS delivered to premotor cortex in stroke rehabilitation: study protocol for a randomized controlled trial. Trials. 2013;14:331–340. doi: 10.1186/1745-6215-14-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- Romanelli P, Fardone E, Battaglia G, Bräuer-Krisch E, Prezado Y, Requardt H, Le Duc G, Nemoz C, Anschel DJ, Spiga J, Bravin A. Synchrotron-generated microbeam sensorimotor cortex transections induce seizure control without disruption of neurological functions. PLoS ONE. 2013;8:e53549. doi: 10.1371/journal.pone.0053549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013;12:105–118. doi: 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- Sánchez-Gómez MV, Alberdi E, Pérez-Navarro E, Alberch J, Matute C. Bax and calpain mediate excitotoxic oligodendrocyte death induced by activation of both AMPA and kainate receptors. J. Neurosci. 2011;31:2996–3006. doi: 10.1523/JNEUROSCI.5578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporta S, Borlongan CV, Sanberg PR. Neural transplantation of human neuroteratocarcinoma (hNT) neurons into ischemic rats. A quantitative dose-response analysis of cell survival and behavioral recovery. Neuroscience. 1999;91:519–525. doi: 10.1016/s0306-4522(98)00610-1. [DOI] [PubMed] [Google Scholar]
- Saver JL, Starkman S, Eckstein M, Stratton S, Pratt F, Hamilton S, Conwit R, Liebeskind DS, Sung G, Sanossian N, FAST-MAG Investigators and Coordinators Methodology of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) phase 3 trial: Part 2-prehospital study methods. Int. J. Stroke. 2014;9:220–225. doi: 10.1111/ijs.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Jr., Alderman S, Aisiku I, Kar S, Gee A, Grotta JC. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- Schiene K, Bruehl C, Zilles K, Qü M, Hagemann G, Kraemer M, Witte OW. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J. Cereb. Blood Flow Metab. 1996;16:906–914. doi: 10.1097/00004647-199609000-00014. [DOI] [PubMed] [Google Scholar]
- Schouten M, Buijink MR, Lucassen PJ, Fitzsimons CP. New neurons in aging brains: molecular control by small non-coding RNAs. Front Neurosci. 2012;6:25. doi: 10.3389/fnins.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, et al. Eunice Kennedy Shriver NICHD Neonatal Research Network Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, Lu M, Raginski K, Vanguri P, Smith A, Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J. Cereb. Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat. Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Steinberg GK, Kondziolka D, Schwartz NE, Wechsler L, Lunsford LD, Coburn ML, Billigen JB, Keren-Gill H, McGrogan M, Case C, et al. A novel phase 1/2a study of intraparenchymal transplantation of human modified bone marrow derived cells in patients with stable ischemic stroke. Stroke. 2014;45:A149. [Google Scholar]
- Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Stroemer P, Hope A, Patel S, Pollock K, Sinden J. Development of a human neural stem cell line for use in recovery from disability after stroke. Front. Biosci. 2008;13:2290–2292. doi: 10.2741/2842. [DOI] [PubMed] [Google Scholar]
- Stroemer P, Patel S, Hope A, Oliveira C, Pollock K, Sinden J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil. Neural Repair. 2009;23:895–909. doi: 10.1177/1545968309335978. [DOI] [PubMed] [Google Scholar]
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat. Neurosci. 2009;12:1300–1307. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek J, Nicole O, Ali C, Ischenko A, MacKenzie ET, Buisson A, Fontaine M. Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. Neuroreport. 2001;12:289–293. doi: 10.1097/00001756-200102120-00022. [DOI] [PubMed] [Google Scholar]
- Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, Kwakkel G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE. 2014;9:e87987. doi: 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang LY, Cai WQ, Chen PH, Deng QY, Zhao CM. Down-regulation of P2X7 receptor expression in rat oligodendrocyte precursor cells after hypoxia ischemia. Glia. 2009;57:307–319. doi: 10.1002/glia.20758. [DOI] [PubMed] [Google Scholar]
- Wang Y, Denisova JV, Kang KS, Fontes JD, Zhu BT, Belousov AB. Neuronal gap junctions are required for NMDA receptor-mediated excitotoxicity: implications in ischemic stroke. J. Neurophysiol. 2010;104:3551–3556. doi: 10.1152/jn.00656.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yang Z, Liu C, Zhao Y, Chen Y. Activated microglia provide a neuroprotective role by balancing glial cell-line derived neurotrophic factor and tumor necrosis factor-a secretion after subacute cerebral ischemia. Int. J. Mol. Med. 2013;31:172–178. doi: 10.3892/ijmm.2012.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibro-blasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Winship IR, Murphy TH. Remapping the somatosensory cortex after stroke: insight from imaging the synapse to network. Neuroscientist. 2009;15:507–524. doi: 10.1177/1073858409333076. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuro-blast migration after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Yang H, Feng GD, Liang Z, Vitale A, Jiao XY, Ju G, You SW. In vitro beneficial activation of microglial cells by mechanically-injured astrocytes enhances the synthesis and secretion of BDNF through p38MAPK. Neurochem. Int. 2012;61:175–186. doi: 10.1016/j.neuint.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Minami M, Sun GH, Meier TJ, Kunis DM, McLaughlin JR, Ho DY, Sapolsky RM, Steinberg GK. Calbindin d28k overexpression protects striatal neurons from transient focal cerebral ischemia. Stroke. 2001;32:1028–1035. doi: 10.1161/01.str.32.4.1028. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J. Neurosci. 2005;25:5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Wang ZG, Lu XH, Kong XX, Wu FZ, Lin L, Tan X, Ye LB, Xiao J. Endoplasmic reticulum stress: relevance and therapeutics in central nervous system diseases. Mol. Neurobiol. 2014;51:1343–1352. doi: 10.1007/s12035-014-8813-7. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat. Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J. Cereb. Blood Flow Metab. 2007;27:1879–1894. doi: 10.1038/sj.jcbfm.9600540. [DOI] [PubMed] [Google Scholar]
- Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil. Neural Repair. 2010;24:636–644. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann JB, Jackson A. Closed-loop control of spinal cord stimulation to restore hand function after paralysis. Front Neurosci. 2014;8:87. doi: 10.3389/fnins.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]