Abstract
Introduction
Maintenance of normal weight and higher levels of physical activity are associated with a reduced risk of several types of cancer. As genomic instability is regarded as a hallmark of cancer development, one proposed mechanism is improvement of DNA repair function. We investigated links between dietary weight loss, exercise, and strand break rejoining in an ancillary study to a randomized-controlled trial.
Methods
Overweight/obese postmenopausal women (n=439) were randomized to: a) reduced-calorie weight-loss diet (“diet” n=118); b) moderate-to-vigorous intensity aerobic exercise (“exercise” n=117); c) a combination (“diet+exercise” n=117); or d) control (n=87). The reduced-calorie diet had a 10% weight-loss goal. The exercise intervention consisted of 45 minutes of moderate-to-vigorous aerobic activity 5 days/week for 12 months. DNA repair capacity was measured in a subset of 226 women at baseline and 12 months, from cryopreserved peripheral mononuclear cells using the Comet assay. Anthropometric and body composition measures were performed at baseline and 12 months.
Results
DNA repair capacity did not change significantly with any of the 12 month interventions compared to control; there were also no significant changes when stratified by changes in body composition or aerobic fitness (VO2max). At baseline, DNA repair capacity was positively associated with weight, BMI, and fat mass (r=0.20, p=0.003; r=0.19, p=0.004; r=0.13, p=0.04, respectively) and inversely with lean body mass (r=-0.14, p=0.04).
Conclusion
In conclusion, DNA repair capacity did not change with dietary weight loss or exercise interventions in postmenopausal women within a period of 12 months. Other assays that capture different facets of DNA repair function may be needed.
Keywords: DNA repair, Comet Assay, randomized controlled trial, exercise, caloric restriction, women
Introduction
Overweight, obesity, or a sedentary lifestyle may account for as much as 25% of all cancers, with the risk reduction differing for various cancers (24, 38). Higher levels of regular, moderate-intensity physical activity are associated with a reduced risk of several cancers, including those of the breast, colon, and endometrium (34, 47). Prospective studies suggest that weight loss and maintenance are associated with a reduction in breast cancer risk (22, 25, 34).
DNA damage has been suggested as contributing to aging and chronic diseases, especially cancer. Genomic instability, which may be caused by DNA repair defects, is regarded as a hallmark of cancer (19). DNA repair function is critical in maintaining genome stability. Defective functioning of DNA repair enzymes underlies many of the known cancer syndromes, including Lynch syndrome (also called hereditary non-polyposis colorectal cancer) and xeroderma pigmentosum (32). Regular physical activity can reduce oxidative stress levels, putatively due to adaptation involving an improved capacity to repair oxidative-stress-related damage incurred during exercise (training effect) (37). This is supported by trials showing reduced urinary oxidative stress after regular exercise (8).
There is increasing evidence that weight loss promotes genomic stability by increasing DNA repair capacity, particularly base-excision repair (4, 7). Caloric restriction can reverse the age-related decline in base-excision repair capacity in rats (7). Excess caloric intake and obesity have been shown to increase generation of reactive oxygen species, which can ultimately lead to DNA damage via, for example, the creation of oxidized bases. Human studies have not yet addressed possible effects of caloric restriction on DNA repair capacity. Indirect evidence for such a link comes from studies reporting effects of calorie-restricted diets in humans on oxidative DNA damage levels (13, 23): oxidative DNA damage (5-hydroxymethyl-2′-deoxyuridine) decreased after a calorie-restricted diet for a short period of 2 weeks in women (13) and, likewise, caloric restriction also decreased DNA damage (8-oxo-7,8-dihydroguanine) after 1 year in men and women (23). Rodent studies have shown that caloric restriction increases resistance to oxidative damage (14, 30, 48).
The alkaline Comet Assay is a sensitive technique for the detection of DNA damage and DNA repair, requiring only a small number of cells such as peripheral blood mononuclear cells (PBMCs) and has also been shown to be perform well using cryopreserved blood-cells (10). As noted by Berwick and Vineis, the use of such tests in human populations has yielded consistent positive associations between inferred or actual DNA repair capacity and particularly breast cancer (5, 27). The relevance for other cancer types is currently unknown. A caveat regarding the studies to date is that no prospective investigation of DNA repair capacity (measured by these assays) and future cancer incidence has been undertaken, largely due to the expense of cryopreserving viable lymphocytes in cohort studies.
In an ancillary study to a randomized controlled trial in postmenopausal women, we investigated the independent and combined effects of a moderate-to-vigorous aerobic-exercise intervention and a reduced-calorie weight loss diet on the level of DNA damage and DNA repair in 226 women. We hypothesized that the combination of a reduced-calorie weight-loss diet with moderate-to-vigorous aerobic exercise would result in enhanced DNA repair capacity measured in PBMC by the Comet Assay than either intervention alone, and compared to no lifestyle change (control).
Subjects and Methods
Study design and participants
The Nutrition and Exercise for Women (NEW) study was a 12-month randomized controlled trial, conducted from 2005 to 2009, which tested the effects of exercise and reduced-calorie dietary weight-loss on biomarkers of postmenopausal breast cancer risk (17, 33). Participants were recruited from the greater Seattle area, Washington, US, through mass mailings, media placements, and community outreach.
Inclusion criteria were: 50-75 years of age; BMI ≥25.0 kg/m2 (if Asian-American ≥23.0 kg/m2); <100 minutes/week of leisure time moderate activity; postmenopausal; no postmenopausal hormone therapy for the past 3 months; no history of breast cancer, heart disease, diabetes mellitus, or other serious medical conditions; fasting glucose <126mg/dL and not taking diabetes medication; non-smoking; alcohol intake of <2 drinks/day; ability to attend diet/exercise sessions at the intervention site; and a normal exercise-tolerance test.
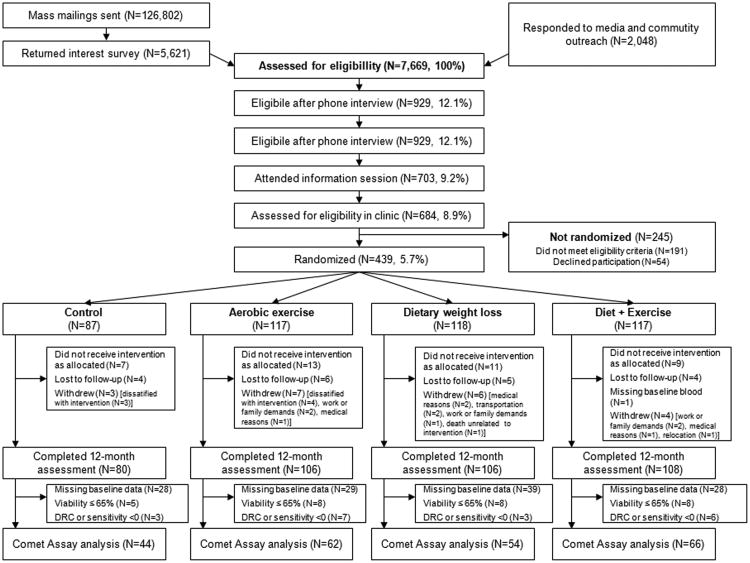
The trial design and recruitment have been previously reported (17). Briefly, a total of 439 eligible women were stratified according to BMI (<30 kg/m2 or ≥30 kg/m2) and race/ethnicity (non-Hispanic White, Black, other), then randomized into one of four groups: 1) reduced-calorie weight-loss diet (“diet”; n=118); 2) moderate-to-vigorous intensity aerobic exercise (“exercise”; n=117); 3) combined reduced-calorie weight-loss diet and moderate-to-vigorous intensity aerobic exercise (“diet+exercise”; n=117); or 4) no diet or exercise change (“control”; n=87). We used permuted-block randomization (ratio 0.75:1:1:1) to assign a proportionally smaller number of women to the control group. In the present ancillary study that began after the parent trial was already underway, we enrolled a subset of 226 women (see Figure 1).
Figure 1.
Flow chart (CONSORT diagram) of the study recruitment and study.
The Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board in Seattle, Washington, approved the study; all participants signed an Informed Consent.
Interventions and control group
The reduced-calorie weight-loss intervention was a modification of the Diabetes Prevention Program (28) and the Look AHEAD (39) lifestyle interventions and has been previously described (17). The diet had a total energy intake goal of 1200-2000 kcal/day based on weight and <30% daily energy intake from fat. The weight-loss goal was 10% by 6 months, with maintenance thereafter. Dietitians with training in behavior modification conducted the sessions. Participants met individually with a study dietitian at least twice and attended weekly dietitian-led group meetings (5-10 women) in months 1-6. In each of months 7-12, participants had one face-to-face individual or group contact, and one contact with a dietitian via phone or email. The diet sessions of the diet+exercise group were held separately from those of the diet group and participants were requested not to discuss diet during facility drop-in exercise training sessions.
The exercise intervention goal was 45 minutes of moderate-to-vigorous intensity exercise 5 days/week for 12 months. Participants attended 3 supervised sessions/week at the facility and exercised 2 days/week at home. They gradually increased exercise training from a 15 minute session at 60–70% maximal heart rate to the target 70-85% of observed maximal heart rate by week 7, and maintained it for the duration of the study. Facility-based exercise consisted of treadmill walking, stationary bicycling, and use of other aerobic machines; while a variety of home exercises were encouraged including walking/hiking, aerobics, and bicycling. Participants recorded exercise mode, duration, peak heart rate, and perceived exertion at each session. Activities of ≥4 METs were counted toward the prescribed target. (17)
Women randomized to the control group were asked not to change their diet or exercise habits. After 12 months, they were offered 4 weight-loss classes and 8 weeks of facility exercise training.
Lifestyle measures
Demographics, lifestyle behaviors, anthropometrics, and body composition were assessed at baseline and 12 months. Type, intensity and duration of physical activity over the previous 3 months were assessed with a modified Minnesota Physical Activity Questionnaire (43). A food frequency questionnaire was employed to assess usual dietary intake (35). Height and weight were measured with a stadiometer and standard scale and BMI was calculated as kg/m2. Body fat was measured by a DXA whole-body scanner (GE Lunar, Madison, WI). Exercise change from baseline was measured by average daily pedometer steps and cardiorespiratory fitness (VO2max. in L/min and ml/kg/min, to allow quantification of fitness change in isolation of weight loss associated with the interventions), measured by a maximal graded treadmill test and a metabolic cart (MedGraphics, St. Paul, MN).
Blood samples, isolation and cryopreservation of PBMCs
At baseline and 12 months, 12-hour fasting blood samples (50mL) were collected. PBMCs were isolated as described (9) using density-gradient centrifugation and stored in aliquots with 3×106 cells at -70°C in freezing medium containing Roswell Park Memorial Institute-1640 (RPMI-1640), fetal calf serum (FCS), and dimethyl sulfoxide (DMSO).
The Comet Assay
The Comet Assay was conducted under alkaline conditions (44), with modifications for cryopreserved PBMCs as described previously (9). For this, cells were gently thawed at 37°C and an aliquot of 4×103 cells was embedded in agarose onto each spot of a CometSlide™. Each measurement consisted of duplicate slides, and each slide contained two gel spots.
To induce damage, two sets of cell-embedded slides were exposed to 1.23 Gy of γ-irradiation at room temperature. Subsequently, one set of slides was immersed immediately in ice-cold lysis buffer, while the other set was incubated at 37°C in 95% air/5% CO2 in complete medium for 30 min to allow repair before immersing in lysis buffer. We observed about 5 to 14-fold increases in DNA damage after γ-irradiation, which was then partially reversed during DNA repair. The mean intra-assay CVs of DRC was 10%.
After lysis, electrophoresis, neutralization, ethanol-washing and air-drying, the slides were stored at room temperature until analysis. Before scoring, the slides were stained with 50μl SYBRGreen I fluorescent dye. Automated analysis of the comet assay was performed by use of a Metacyte CometScan System based on the slide scanning platform Metafer (Metasystems, Altlussheim, FRG), which consists of a motorized microscope (AxioImager M1, Carl Zeiss, Jena, Germany) with fluorescence illumination, a motorized X/Y scanning stage (Maerzhaeuser, Wetzler, Germany) with a CCD camera (CVM4+CL, JAIAS, Glostrup/Copenhagen, Denmark) and a Windows compatible PC running the Metafer software. Percent DNA in tail, tail moment (the product of comet tail length and percent DNA in the tail), and tail moment arm (tail moment divided by the normalized integrated tail intensity to measure the average distance of DNA migration within the tail) were randomly determined from 102 cells (scored for 2 gel spots) with a cell viability by Trypan blue exclusion test of ≥65% as described (9). We also examined the data using both 65% and 80% as a cutoff which did not significantly change the results. DNA repair capacity was calculated as (induced damage level –damage level after 30 min of repair)/(induced damage level) × 100%with higher % values reflecting a greater DNA repair capacity. Assays were performed blinded and samples were excluded with assays being repeated using a second aliquot of the PBMCs if the viability was below 65% or fewer than 100 comets were scanned.
Statistical analyses
We first investigated changes in DNA repair capacity and radiation sensitivity using cell populations with different cell viabilities (≥65% vs. ≥80% viability). There were no differences in results. We thus included all participants with cell viabilities ≥65% in the comparison of the intervention groups compared to control. In addition, we also examined changes stratified by degree of weight change (<5% and ≥5%) and change in VO2max (L/min; <5%, 5-15%, ≥15%) within the intervention groups. We applied the generalized estimating equation (GEE) approach to account for repeated measurements on the same subjects. In addition, associations between baseline DNA repair capacity and age and energy balance (BMI, weight, lean mass, fat mass) were investigated by Pearson correlation. Statistical analyses were performed using SAS (version 9.2, SAS Institute Inc, Cary, NC).
We conducted power calculations: with sample sizes for 2 groups being 66 (and 44, respectively), we had 80% power (type-1 error 0.0167) to detect a difference in DNA repair capacity of 7.0 (10.2) if the standard deviation was 11 (16) and the intra-individual correlation between baseline and 12 months was 0.5.
Results
A description of the women participating in the study is given in Table 1: The mean age of participants in all groups was 58.2 years; the majority (83.6%) was non-Hispanic Whites with a higher education (64.6 % had at least a college degree). The average BMI was >30kg/m2; aerobic fitness (22.7-24.0 kg/mL/min), and the caloric intake (about 1900 kcal/d). Baseline characteristics did not differ among the intervention groups.
Table 1. Characteristics of study participants in the parent trial, and ancillary study (and by randomized arm).
| Parent trial | Ancillary study All participants | Ancillary study Control | Ancillary study Diet | Ancillary study Exercise | Ancillary study Diet+Exercise | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | mean ± SD | n | mean ± SD | n | mean ± SD | n | n | mean ± SD | n | mean ± SD | ||
| Age (year) | 439 | 58.0 ± 5.0 | 226 | 58.2 ± 5.3 | 44 | 57.1 ± 4.4 | 54 | 57.9 ± 6.6 | 62 | 59.1 ± 5.1 | 66 | 58.4 ± 4.7 |
| Ethnicity, n (%) | ||||||||||||
| Non-Hispanic White | 373 (85.0%) | 189 (83.6%) | 34 (77.3%) | 47 (87.0%) | 51 (82.3%) | 57 (86.4%) | ||||||
| Others | 66 (15.0%) | 37 (16.4%) | 10 (22.7%) | 7 (13.0%) | 11 (17.7%) | 9 (13.6%) | ||||||
| Education, N (%) | ||||||||||||
| No college degree | 152 (34.6%) | 80 (35.4%) | 15 (34.1%) | 21 (38.9%) | 26 (41.9%) | 18 (27.3%) | ||||||
| College degree | 287 (65.4%) | 146 (64.6%) | 29 (65.9%) | 33 (61.1%) | 36 (58.1%) | 48 (72.7%) | ||||||
| BMI (kg/m2) | 439 | 30.9 ± 4.0 | 226 | 30.6 ± 4.0 | 44 | 30.2 ± 4 | 54 | 30.5 ± 3.9 | 62 | 30.5 ± 3.6 | 66 | 31.2 ± 4.5 |
| Body fat (%) | 439 | 47.2 ± 4.3 | 226 | 47.1 ± 4.3 | 44 | 46.5 ± 4.6 | 54 | 46.8 ± 3.9 | 62 | 47.2 ± 4.3 | 66 | 47.7 ± 4.4 |
| Aerobic fitness (kg/ml/min) | 439 | 22.9 ± 4.0 | 226 | 23.3 ± 4.0 | 44 | 23.8 ± 4.2 | 54 | 22.7 ± 4.6 | 62 | 22.7± 3.5 | 66 | 24.0 ± 3.8 |
| Daily calorie intake (kcal/day) | 427 | 1934 ± 638 | 221 | 1928 ± 643 | 43 | 1948 ± 673 | 53 | 1836 ± 66 | 61 | 2026 ± 629 | 64 | 1898 ± 621 |
| Dietary fiber intake (g/day) | 427 | 20.6 ± 8.5 | 221 | 20.7 ± 8.9 | 43 | 19.3 ± 9 | 53 | 18.7 ± 7.7 | 61 | 23.6 ± 8.7 | 64 | 20.6 ± 9.4 |
| Aerobic fitness (kg/ml/min) | 44 | 23.8 ± 4.2 | 54 | 22.7 ± 4.6 | 62 | 22.7 ± 3.5 | 66 | 24.0 ± 3.8 | ||||
DNA repair capacity was assessed by three analytical variables in parallel (moment arm, percent DNA in tail, tail moment) which all describe the proportion of damaged cells after repair compared to the basal DNA damage. At the end of the 12-month intervention period, none of the interventions: a reduced-calorie diet, a moderate-to-vigorous aerobic-exercise intervention, or a combination of both changed DNA repair capacity compared to control (Table 2a). DNA repair capacity did not differ across groups (∼70%moment arm; ∼62% percent DNA in tail, ∼85% tail moment). The same was true for the radiation sensitivity (initial DNA damage after irradiation) of the PBMCs (∼1% moment arm; ∼7% percent DNA in tail, ∼1% tail moment; data not further shown). Measures of the intervention efficacy, such as weight and change in VO2max from baseline to 12 months have been reported for the parent trial (17); these results are summarized in Table 2a. The diet intervention resulted in a 10 % decrease in body weight (p<0.0001), and the exercise intervention resulted in an 8% improvement of VO2max (P=0.0004). In addition, both variables showed statistically significantly changes in the combined diet and exercise intervention (p<0.0001 and p=0.009, respectively, Table 2a).
Table 2.
Baseline and 12 months DNA repair capacity and intervention efficacy (Table 2a), and baseline and 12 months DNA repair capacity and radiation sensitivity, stratified by intervention and DNA repair capacity stratified by weight loss category (Table 2b).
| Table 2a | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Study group | Endpoint | Baseline | 12 month | ||||
|
|
|
||||||
| n | mean ± SD | n | mean ± SD | Δ% | P | ||
| Control | DNA repair capacity: | ||||||
| Moment arm (%) | 44 | 69.9 ± 18 | 44 | 71.9 ± 20 | +2.8 | NA | |
| Percent DNA tail (%) | 44 | 64.0 ± 16 | 44 | 65.0 ± 18 | +1.6 | NA | |
| Tail moment (%) | 44 | 84.7 ± 13 | 44 | 85.3 ± 17 | +0.7 | NA | |
| Intervention efficacy1: | |||||||
| Weight (kg) | 44 | 82.9 ± 12 | 44 | 81.6 ± 12 | -1.6 | NA | |
| VO2max (L/min) | 44 | 2.0 ± 0.4 | 41 | 2.0 ± 0.4 | -2.4 | NA | |
|
| |||||||
| Diet | DNA repair capacity: | ||||||
| Moment arm (%) | 54 | 67.4 ± 19 | 54 | 72.8 ± 20 | +8.0 | 0.41 | |
| Percent DNA tail (%) | 54 | 60.2 ± 17 | 54 | 64.9 ± 19 | +7.8 | 0.36 | |
| Tail moment (%) | 54 | 83.7 ± 11 | 54 | 85.2 ± 15 | +1.9 | 0.77 | |
| Intervention efficacy1: | |||||||
| Weight (kg) | 54 | 83.1 ± 13 | 54 | 74.8 ± 13 | -9.9 | <.0001 | |
| VO2max (L/min) | 54 | 1.9 ± 0.3 | 53 | 1.8 ± 0.3 | -2.1 | 0.88 | |
|
| |||||||
| Exercise | DNA repair capacity: | ||||||
| Moment arm (%) | 62 | 67.7 ± 14 | 62 | 70.8 ± 16 | +4.5 | 0.76 | |
| Percent DNA tail (%) | 62 | 61.8 ± 12 | 62 | 63.7 ± 14 | +3.1 | 0.81 | |
| Tail moment (%) | 62 | 84.2 ± 10 | 62 | 86.3 ± 9 | +2.4 | 0.66 | |
| Intervention efficacy1: | |||||||
| Weight (kg) | 62 | 82.7 ± 12 | 61 | 80.3 ± 12 | -2.9 | 0.422 | |
| VO2max (L/min) | 62 | 1.9 ± 0.3 | 57 | 2.0 ± 0.4 | +7.9 | 0.0004 | |
|
| |||||||
| Diet+exercise | DNA repair capacity: | ||||||
| Moment arm (%) | 66 | 72.1 ± 17 | 66 | 73.6 ± 17 | +2.1 | 0.99 | |
| Percent DNA tail (%) | 66 | 63.7 ± 17 | 66 | 64.1 ± 16 | +0.8 | 0.99 | |
| Tail moment (%) | 66 | 86.2 ± 11 | 66 | 85.8 ± 11 | -0.5 | 0.82 | |
| Intervention efficacy1: | |||||||
| Weight (kg) | 66 | 82.9 ± 10.7 | 66 | 73.4 ± 10.7 | -11.4 | <.0001 | |
| VO2max (L/min) | 66 | 2.0 ± 0.3 | 63 | 2.1 ± 0.5 | +6.2 | 0.009 | |
|
| |||||||
| P=intervention vs. control | |||||||
| 1Previously reported for the parent trial (17). | |||||||
| Table 2b | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Weight loss | DNA repair capacity | Radiation sensitivity | |||||||||||
| Baseline | 12 month | Baseline | 12 month | ||||||||||
| n | mean ± SD | n | mean ± SD | Δ% | P | n | mean ± SD | n | mean ± SD | Δ% | P | ||
| Control | |||||||||||||
| Moment arm (%) | <5% | 35 | 71.2 ± 16 | 32 | 74.4 ± 20 | 4.5 | 35 | 1.0 ± 0 | 32 | 0.9 ± 0 | -9.1 | ||
| ≥5% | 9 | 64.9 ± 24 | 8 | 68.9 ± 23 | 6.1 | 9 | 1.1 ± 1 | 8 | 1.0 ± 1 | -4.6 | |||
| Percent DNA tail (%) | <5% | 35 | 65.6 ± 15 | 32 | 66.6 ± 18 | 1.5 | 35 | 7.3 ± 2 | 32 | 6.4 ± 2 | -12 | ||
| ≥5% | 9 | 57.5 ± 17 | 8 | 63.6 ± 21 | 11.0 | 9 | 7.4 ± 3 | 8 | 7.0 ± 3 | -5.6 | |||
| Tail moment (%) | <5% | 35 | 86.0 ± 11 | 32 | 86.2 ± 18 | 0.3 | 35 | 1.1 ± 1 | 32 | 0.9 ± 1 | -17 | ||
| ≥5% | 9 | 79.5 ± 17 | 8 | 85.1 ± 13 | 6.9 | 9 | 1.4 ± 1 | 8 | 1.3 ± 1 | -7.9 | |||
|
| |||||||||||||
| Diet | |||||||||||||
| Moment arm (%) | <5% | 15 | 63.5 ± 21 | 14 | 67.3 ± 24 | 6.0 | 0.77 | 15 | 0.9 ± 1 | 14 | 0.8 ± 1 | -11 | 0.66 |
| ≥5% | 39 | 68.9 ± 18 | 35 | 76.1 ± 19 | 10.0 | 0.79 | 39 | 1.0 ± 0 | 35 | 0.9 ± 0 | -7.4 | 0.90 | |
| Percent DNA tail (%) | <5% | 15 | 54.4 ± 17 | 14 | 57.8 ± 24 | 6.4 | 0.66 | 15 | 6.3 ± 3 | 14 | 5.6 ± 3 | -12 | 0.87 |
| ≥5% | 39 | 62.5 ± 16 | 35 | 68.7 ± 16 | 9.9 | 0.97 | 39 | 7.3 ± 3 | 35 | 6.3 ± 3 | -13 | 0.85 | |
| Tail moment (%) | <5% | 15 | 82.7 ± 12 | 14 | 79.2 ± 21 | -4.3 | 0.52 | 15 | 1.0 ± 1 | 14 | 1.0 ± 2 | 3.7 | 0.20 |
| ≥5% | 39 | 84.0 ± 11 | 35 | 88.0 ± 13 | 4.8 | 0.87 | 39 | 1.1 ± 1 | 35 | 0.9 ± 1 | -16 | 0.92 | |
|
| |||||||||||||
| Exercise | |||||||||||||
| Moment arm (%) | <5% | 45 | 66.3 ± 13 | 40 | 69.3 ± 18 | 4.6 | 0.88 | 45 | 1.0 ± 1 | 40 | 1.0± 0 | -8.5 | 0.98 |
| ≥5% | 16 | 72.8 ± 14 | 16 | 73.3 ± 16 | 0.8 | 0.85 | 16 | 1.1 ± 1 | 16 | 1.0 ± 1 | -9.9 | 0.74 | |
| Percent DNA tail (%) | <5% | 45 | 61.6 ± 12 | 40 | 62.2 ± 16 | 1.0 | 0.99 | 45 | 7.5 ± 3 | 40 | 6.8 ± 2 | -9.3 | 0.68 |
| ≥5% | 16 | 62.8 ± 12 | 16 | 65.5 ± 8.7 | 4.3 | 0.80 | 16 | 7.7 ± 3 | 16 | 7.1 ± 3 | -7.9 | 0.76 | |
| Tail moment (%) | <5% | 45 | 83.9 ± 10 | 40 | 85.0 ± 11 | 1.3 | 0.75 | 45 | 1.2 ± 1 | 40 | 1.0 ± 1 | -16 | 0.81 |
| ≥5% | 16 | 85.6 ± 9 | 16 | 88.4 ± 5.7 | 3.3 | 0.78 | 16 | 0.9 ± 0 | 16 | 1.3 ± 2 | 38 | 0.39 | |
|
| |||||||||||||
| Diet+exercise | |||||||||||||
| Moment arm (%) | <5% | 11 | 73.6 ± 22 | 8 | 80.0 ± 13 | 8.6 | 0.71 | 11 | 0.8 ± 0 | 8 | 2.0 ± 3 | 142 | 0.25 |
| ≥5% | 55 | 71.8 ± 16 | 50 | 73.5 ± 17 | 2.4 | 0.91 | 55 | 1.0 ± 1 | 50 | 0.9 ± 0 | -12 | 0.99 | |
| Percent DNA tail (%) | <5% | 11 | 62.8 ± 24 | 8 | 67.5 ± 13 | 7.5 | 0.72 | 11 | 5.5 ± 2 | 8 | 9.8 ± 12 | 78.1 | 0.17 |
| ≥5% | 55 | 63.8 ± 16 | 50 | 64.8 ± 16 | 1.5 | 0.68 | 55 | 7.1 ± 3 | 50 | 6.3 ± 3 | -12 | 0.60 | |
| Tail moment (%) | <5% | 11 | 84.2 ± 16 | 8 | 90.5 ± 6.1 | 7.4 | 0.37 | 11 | 0.9 ± 1 | 8 | 4.0 ± 10 | 353 | 0.27 |
| ≥5% | 55 | 86.6 ± 9 | 50 | 86.2 ± 11 | -0.5 | 0.50 | 55 | 1.3 ± 1 | 50 | 1.1 ± 1 | -16 | 0.86 | |
P=intervention vs. control
When we stratified by weight loss (<5% and ≥5%); no statistically significant effects of the 12-month interventions compared to control were detectable (Table 2b). In the diet+exercisegroup, a statistically significant increase (p-trend=0.015) in DNA repair capacity was observed among those women with the highest gain in VO2max (≥ 15%) compared to those with the lowest VO2max gain (<5%) (Table 3).
Table 3.
Baseline and 12 month DNA repair capacity in the exercise and diet + exercise group, stratified by change in VO2max (L/min).
| Baseline | 12 month | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| DNA repair capacity | VO2max. change | n | mean ± SD | n | mean ± SD | Δ | Δ% | † P | ‡ P |
| Exercise | |||||||||
|
| |||||||||
| Moment arm (%) | Control | 44 | 69.9 ± 17.9 | 40 | 73.3 ± 19.9 | 3.4 | 4.9 | Ref. | |
| Increase <5% | 17 | 69.1 ± 12.8 | 14 | 71.4 ± 16.2 | 2.3 | 3.4 | 0.94 | Ref. | |
| Increase 5-15% | 27 | 68.4 ± 16.0 | 26 | 72.4 ± 17.7 | 4.0 | 5.8 | 0.86 | 0.85 | |
| Increase ≥15% | 13 | 63.8 ± 10.3 | 13 | 62.2 ± 14.8 | -1.6 | -2.5 | 0.39 | 0.28 | |
| P-trend | *0.67 | ** 0.29 | |||||||
|
| |||||||||
| Percent DNA tail (%) | Control | 44 | 64.0 ± 15.6 | 40 | 66.0 ± 18.3 | 2.1 | 3.2 | Ref. | |
| Increase <5% | 17 | 65.1 ± 9.4 | 14 | 63.9 ± 14.3 | -1.2 | -1.8 | 0.55 | Ref. | |
| Increase 5-15% | 27 | 62.5 ± 12.4 | 26 | 66.2 ± 14.7 | 3.7 | 6.0 | 0.70 | 0.30 | |
| Increase ≥15% | 13 | 55.4 ± 12.3 | 13 | 56.8 ± 14.7 | 1.4 | 2.6 | 0.93 | 0.65 | |
| P-trend | *0.88 | **0.65 | |||||||
|
| |||||||||
| Tail moment (%) | Control | 44 | 84.7 ± 12.6 | 40 | 86.0 ± 16.8 | 1.3 | 1.5 | Ref. | |
| Increase <5% | 17 | 85.4 ± 9.8 | 14 | 85.5 ± 9.4 | 0.1 | 0.2 | 0.80 | Ref. | |
| Increase 5-15% | 27 | 84.1 ± 10.3 | 26 | 87.7 ± 9.9 | 3.6 | 4.3 | 0.53 | 0.25 | |
| Increase ≥15% | 13 | 81.9 ± 8.5 | 13 | 81.7 ± 9.6 | -0.2 | -0.2 | 0.73 | 0.83 | |
| P-trend | *0.90 | **0.87 | |||||||
|
| |||||||||
| Diet+exercise | |||||||||
|
| |||||||||
| Moment arm (%) | Control | 44 | 69.9 ± 17.9 | 40 | 73.3 ± 19.9 | 3.4 | 4.9 | Ref. | |
| Increase <5% | 27 | 72.9 ± 16.8 | 25 | 75.8 ± 16.6 | 2.9 | 4.0 | 0.93 | Ref. | |
| Increase 5-15% | 16 | 80.7 ± 14.5 | 13 | 74.4 ± 13.2 | -6.3 | -7.8 | 0.08 | 0.12 | |
| Increase ≥15% | 20 | 66.5 ± 13.6 | 17 | 74.1 ± 19.8 | 7.6 | 11.5 | 0.49 | 0.45 | |
| P-trend | *0.92 | **0.54 | |||||||
|
| |||||||||
| Percent DNA tail (%) | Control | 44 | 64.0 ± 15.6 | 40 | 66.0 ± 18.3 | 2.1 | 3.2 | Ref. | |
| Increase <5% | 27 | 66.6 ± 15.6 | 25 | 64.0 ± 15.1 | -2.6 | -3.9 | 0.40 | Ref. | |
| Increase 5-15% | 16 | 73.7 ± 13.7 | 13 | 66.2 ± 10.6 | -7.5 | -10.2 | 0.09 | 0.43 | |
| Increase ≥15% | 20 | 54.6 ± 14.1 | 17 | 67.5 ± 18.8 | 12.9 | 23.7 | 0.053 | 0.008 | |
| P-trend | *0.27 | **0.015 | |||||||
|
| |||||||||
| Tail moment (%) | Control | 44 | 84.7 ± 12.6 | 40 | 86.0 ± 16.8 | 1.3 | 1.5 | Ref. | |
| Increase <5% | 27 | 87.4 ± 9.8 | 25 | 87.1 ± 10.2 | -0.3 | -0.3 | 0.72 | Ref. | |
| Increase 5-15% | 16 | 92.0 ± 7.2 | 13 | 87.8 ± 7.2 | -4.1 | -4.5 | 0.17 | 0.27 | |
| Increase ≥15% | 20 | 82.6 ± 8.8 | 17 | 86.2 ± 12.7 | 3.6 | 4.4 | 0.62 | 0.37 | |
| P-trend | *0.95 | **0.43 | |||||||
Testing difference in change from baseline to 12 months in DNA repair capacity compared to controls.
Testing difference in change from baseline to 12 months in DNA repair capacity compared to lowest VO2 maximum change group, excluding controls.
Testing for a trend in change from baseline to 12 months in DNA repair capacity measures from controls through gained most VO2 maximum group.
Testing for a trend in change from baseline to 12 months in DNA repair capacity measures from lowest VO2 maximum change group through gained most VO2 maximum group.
At baseline, DNA repair capacity was weakly positively correlated with weight, BMI, and fat mass, and weakly inversely correlated with lean mass (Figure 2a and Table 4). No such associations with 12-month DNA repair capacity or baseline or 12-month radiation sensitivity were observed (data not shown).
Figure 2.
Scatter plot of baseline DNA repair capacity (moment arm) versus participants' baseline body weight.
Table 4. Pearson correlation coefficients: baseline DNA repair capacity with age and body composition.
| Age (year) | Weight (kg) | BMI (kg/m2) | Lean mass (%) | Fat mass (%) | ||
|---|---|---|---|---|---|---|
| DNA repair capacity, moment arm | r | -0.083 | 0.20 | 0.19 | -0.14 | 0.13 |
| p value | 0.2 | 0.003 | 0.004 | 0.04 | 0.04 | |
| DNA repair capacity, percent DNA tail | r | -0.05 | 0.16 | 0.17 | -0.08 | 0.08 |
| p value | 0.42 | 0.016 | 0.011 | 0.21 | 0.22 | |
| DNA repair capacity, tail moment | r | 0.01 | 0.17 | 0.20 | -0.09 | 0.09 |
| p value | 0.90 | 0.009 | 0.003 | 0.17 | 0.20 |
Discussion
This randomized controlled trial showed that an exercise program, a reduced calorie weight loss diet, or a combination of both did not significantly alter DNA repair capacity in PBMCs of postmenopausal women. However, we observed a weak positive correlation between body weight, BMI, and fat mass (as well as a weak negative correlation of lean mass) with baseline DNA repair capacity. To the best of our knowledge, this is the first randomized controlled trial to examine the effects of these interventions on DNA repair capacity in postmenopausal women during a long-term (12-month) intervention period.
A prolonged single bout of exercise, (e.g., 42 km marathon running), results in a significant degree of unrepaired DNA base oxidation in peripheral lymphocytes, despite a concurrent increase in the urinary excretion of the repaired base, 8-hydroxy-2′-deoxyguanine (45). Regular participation in moderate-intensity training (e.g. 30,000 km cycling per year) appears to result in an adaptive response that is beneficial with respect to antioxidant activity and DNA repair function. Mechanisms include the upregulation of DNA-repair enzymes (46). Allgayer et al. have described a short-term (2 weeks), moderate-intensity (0.3-0.4× maximal exercise capacity) exercise program that resulted in decreased levels of urinary 8-oxo-dG (1). In a 12-month randomized, controlled trial of moderate-intensity (at 60-75% of heart rate maximum), 5 days aerobic exercise per week versus usual lifestyle control, decreased levels of the oxidative marker F2-isoprostane were observed only when accompanied by marked gains in aerobic fitness (VO2max) (8). In our study, DNA repair capacity increased in women with the greatest improvement in VO2 max (≥15%) compared to those of the lowest (<5%). However, this finding was limited to women in the diet+exercise group only and may be a chance observation.
Another component of energy balance that can affect oxidative stress is obesity. Major evidence of obesity-induced oxidative stress in humans comes from cross-sectional studies reporting the effects on oxidative biomarkers, such as elevated F2-isoprostanes (6, 26, 42) or elevated thiobarbituric acid reactive substances (TBARS) (18, 29) by BMI categories. Exercise training reduces F2-isoprostane concentrations among women of younger (2, 12, 40) but also older age (8). A benefit of aerobic exercise training was only apparent in young women with high baseline plasma F2-isoprostanes (2). Within longitudinal studies, dietary and exercise interventions have been used to investigate the role of weight loss on markers of oxidative stress. A combined diet and exercise intervention was reported to lower lipid peroxidation after 4% weight loss in obese persons (3). The effects of long-term (12 month) dietary weight loss intervention, in postmenopausal women, by diet and/or exercise on DNA repair capacity and radiation sensitivity had not previously been assessed; here we report no effect.
In contrast to other reports, we did not detect an age-dependent decrease of DNA repair capacity, which may be due to the somewhat limited age range of our study population (aged 50-75 years). Furthermore, we observed a positive correlation between DNA repair capacity and BMI, body weight and body fat. The data from earlier studies are inconsistent [36-38]. We have no explanation for our finding, which is contrary to our hypothesis. However, the correlation coefficients were of medium strength and the results should not be generalized, as our study population was very homogeneous and all participants were overweight or obese (mean BMI >30 kg/m2).
A range of methods are available for the quantification of DNA damage in cells. One of the most commonly employed methods to detect DNA damage in human studies is the Comet Assay (16) which has been previously used in diet-intervention studies (36) and studies on physical activity (21). There are different approaches to analyze comets, including visual inspection and classification, as well as computer-supported image analysis (11). For this study, we used the latter and reported the results by three validated Comet Assay variables (20); moment arm, percent DNA in tail, and tail moment. Generally, percent DNA in tail covers the widest range of damage and is linearly related to break frequencies. Tail moment recommended is expressing both, the tail length and percent DNA in tail but is measured in non-standard units and, therefore, may be difficult to interpret across studies (11). In our study, results by all 3 parameter were comparable. Within longitudinal studies such as ours, cryopreservation of PBMCs is essential in order to avoid batch-effects across samples collected at various time points. The method we applied produces comparable results to those obtained by the use of freshly isolated PBMCs (9). There is still debate whether apoptotic or necrotic cells increase the measured Comet Assay variability and thus impair the results of the Comet Assay (20, 31). We therefore included only results from PBMCs with a cell viability ≥65%; however, the results did not differ when we restricted viability to those ≥80% at both time points. Cryopreserved lymphocytes are not suitable for studying DNA repair capacity after H2O2 treatment (15), the most common genotoxic agent mimicking oxidative stress. Therefore, we used radiation as the genotoxic agent. In contrast to H2O2, which causes single- and double-strand breaks, radiation additionally gives rise to base damage, as well as DNA-strand and DNA-protein cross-links. However, the measurement of H2O2-induced DNA damage is not reliably measureable in cryopreserved lymphocytes, as presented here (15). Future studies might involve other measures of DNA repair pathways, e.g., gene or protein expression associated with repair enzymes in target tissue or a combination of these together with markers of oxidative stress, i.e. F2-isoprostanes.
Our study had several strengths: the large study size, with sufficient power to assess long-term changes in DNA repair capacity, a randomized controlled design with three intervention arms, high retention (91%) in the parent trial (the ancillary study was restricted to participants with two repair capacity measurements), excellent adherence to the intervention programs, and gold standard measures of aerobic fitness and body composition (17). We chose a valid and robust method to detect DNA repair capacity, the Comet Assay under alkaline conditions (10), in cryopreserved PBMCs of a randomized controlled trial. Measuring DNA repair in PBMCs is widely used in human biomonitoring studies that provide an easy way to assess DNA repair in a human population (15). There is some evidence that PBMCs are representative of the general organism; however, the level of DNA repair within individual target tissues may vary. Comparing colon cancer tissue and PBMCs of the same individuals revealed that there is a positive correlation between their repair capacities despite tissue-specific differences (41).
We recognize that there are some limitations, including the assay restrictions discussed above. Our finding of no change in DNA repair capacity by any of the interventions could be due to too low radiation-induced DNA damage. With the method applied, we achieved a 5 to 14-fold increase in DNA damage by γ radiation compared to the basal damage. These levels of induced damage are appropriate for cryopreserved lymphocytes and the average DNA repair capacity in cryopreserved lymphocytes is lower than that of fresh samples (9). In order to avoid high assay batch-to-batch variability which is of particular relevance for the Comet Assay, we have chosen to cryopreserve pre- and post-intervention lymphocytes. These were assayed simultaneously in our study. However, compared to frozen lymphocytes, examining DNA repair using fresh lymphocytes would result in higher repair capacities. This could be a reason for a lack of effect in our study. It is possible that a higher level of DNA damage at induction, prior to in vitro DNA repair is more appropriate to cover a broader range of DNA repair capacity. Similarly, repair capacity prior to the interventions was already high (in average 70%), resulting in a possible ceiling effect. One possibility for the lack of effects is that the SD for our DNA repair measures was slightly greater (SD 9.4 – 20, Table 2a) than in our own initial work (9) and as used in the initial power calculation (SD 11 – 16,). We also cannot exclude that stratification by VO2max as a measure of fitness may not fully capture the effects of exercise on DNA repair capacity. In the future, one would include additional measures of DNA repair capacity or markers of cellular stress adaption, such as changes of superoxide dismutase activity or gene expression ultimately directly in the target tissue (e.g. epithelial tissue or muscle). Also, our study population was a relatively homogeneous sample of postmenopausal women, primarily non-Hispanic white, and our results may thus not be generalizable to all populations or the health impact on i.e. specific cancer types. Furthermore, only one exercise program and one dietary weight-loss program were tested; therefore, we are unable to speculate on the effects of other weight-loss methods or other types or intensities of exercise programs on DNA damage and DNA repair capacity changes.
In conclusion, an altered energy balance due to long-term physical activity, dietary restriction, or a combination of both did not affect DNA repair capacity in overweight and obese postmenopausal women. Further studies with other measures of DNA repair function may be needed.
Acknowledgments
Grant Support: This work was supported by the National Cancer Institute at the National Institutes of Health (grants U54-CA116847, R01 CA102504; 5KL2RR025015-03 to K.F.S; R25 CA94880 to A.K.); and Canadian Institutes of Health Research (Fellowships to K.L.C & C.M). None of the funding agencies were involved in the trial design or conduct. During the trial, Dr. Alfano was a faculty member at The Ohio State University, and relocated to NCI following completion of her efforts on the NEW trial. Results of the present study do not constitute endorsement by ACSM
Footnotes
Trial Registration: NCT00470119 http://www.clinicaltrials.gov
Financial Disclosures: None reported.
References
- 1.Allgayer H, Owen RW, Nair J, Spiegelhalder B, Streit J, Reichel C, Bartsch H. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography-electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scand J Gastroenterol. 2008;43(8):971–8. doi: 10.1080/00365520701766111. [DOI] [PubMed] [Google Scholar]
- 2.Arikawa AY, Thomas W, Gross M, Smith A, Phipps WR, Kurzer MS, Schmitz KH. Aerobic training reduces systemic oxidative stress in young women with elevated levels of F2-isoprostanes. Contemporary clinical trials. 2013;34(2):212–7. doi: 10.1016/j.cct.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beard CM, Barnard RJ, Robbins DC, Ordovas JM, Schaefer EJ. Effects of diet and exercise on qualitative and quantitative measures of LDL and its susceptibility to oxidation. Arterioscler Thromb Vasc Biol. 1996;16(2):201–7. doi: 10.1161/01.atv.16.2.201. [DOI] [PubMed] [Google Scholar]
- 4.Benassi-Evans B, Clifton PM, Noakes M, Keogh JB, Fenech M. High protein-high red meat versus high carbohydrate weight loss diets do not differ in effect on genome stability and cell death in lymphocytes of overweight men. Mutagenesis. 2009;24(3):271–7. doi: 10.1093/mutage/gep006. [DOI] [PubMed] [Google Scholar]
- 5.Berwick M, Vineis P. Measuring DNA repair capacity: small steps. J Natl Cancer Inst. 2005;97(2):84–5. doi: 10.1093/jnci/dji038. [DOI] [PubMed] [Google Scholar]
- 6.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. American Journal of Epidemiology. 2002;156(3):274–85. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 7.Cabelof DC, Yanamadala S, Raffoul JJ, Guo Z, Soofi A, Heydari AR. Caloric restriction promotes genomic stability by induction of base excision repair and reversal of its age-related decline. DNA Repair (Amst) 2003;2(3):295–307. doi: 10.1016/s1568-7864(02)00219-7. [DOI] [PubMed] [Google Scholar]
- 8.Campbell PT, Gross MD, Potter JD, Schmitz KH, Duggan C, McTiernan A, Ulrich CM. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Med Sci Sports Exerc. 2010;42(8):1448–53. doi: 10.1249/MSS.0b013e3181cfc908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JL, Chen G, Lampe JW, Ulrich CM. DNA damage and repair measurements from cryopreserved lymphocytes without cell culture--a reproducible assay for intervention studies. Environ Mol Mutagen. 2006;47(7):503–8. doi: 10.1002/em.20219. [DOI] [PubMed] [Google Scholar]
- 10.Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 2004;26(3):249–61. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 11.Collins AR, Oscoz AA, Brunborg G, Gaivao I, Giovannelli L, Kruszewski M, Smith CC, Stetina R. The comet assay: topical issues. Mutagenesis. 2008;23(3):143–51. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- 12.Devries MC, Hamadeh MJ, Glover AW, Raha S, Samjoo IA, Tarnopolsky MA. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radic Biol Med. 2008;45(4):503–11. doi: 10.1016/j.freeradbiomed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Djuric Z, Lababidi S, Uhley VE, Heilbrun LK. Levels of 5-hydroxymethyl-2′-deoxyuridine in DNA from women participating in an intervention trial of low-fat and low-energy diets. Biomarkers. 2004;9(1):93–101. doi: 10.1080/13547500310001652151. [DOI] [PubMed] [Google Scholar]
- 14.Djuric Z, Lewis SM, Lu MH, Mayhugh M, Naegeli L, Tang N, Hart RW. Effect of varying caloric restriction levels on female rat growth and 5-hydroxymethyl-2′-deoxyuridine in DNA. Toxicol Sci. 2002;66(1):125–30. doi: 10.1093/toxsci/66.1.125. [DOI] [PubMed] [Google Scholar]
- 15.Duthie SJ, Pirie L, Jenkinson AM, Narayanan S. Cryopreserved versus freshly isolated lymphocytes in human biomonitoring: endogenous and induced DNA damage, antioxidant status and repair capability. Mutagenesis. 2002;17(3):211–4. doi: 10.1093/mutage/17.3.211. [DOI] [PubMed] [Google Scholar]
- 16.Faust F, Kassie F, Knasmuller S, Boedecker RH, Mann M, Mersch-Sundermann V. The use of the alkaline comet assay with lymphocytes in human biomonitoring studies. Mutat Res. 2004;566(3):209–29. doi: 10.1016/j.mrrev.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, Bain CE, Wang CY, Blackburn GL, McTiernan A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012;20(8):1628–38. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, Tice RR. Recommendations for conducting the in vivo alkaline Comet assay. 4th International Comet Assay Workshop. Mutagenesis. 2003;18(1):45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann A, Plappert U, Raddatz K, Grunert-Fuchs M, Speit G. Does physical activity induce DNA damage? Mutagenesis. 1994;9(3):269–72. doi: 10.1093/mutage/9.3.269. [DOI] [PubMed] [Google Scholar]
- 22.Harvie M, Howell A, Vierkant RA, Kumar N, Cerhan JR, Kelemen LE, Folsom AR, Sellers TA. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women's health study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(3):656–61. doi: 10.1158/1055-9965.EPI-04-0001. [DOI] [PubMed] [Google Scholar]
- 23.Hofer T, Fontana L, Anton SD, Weiss EP, Villareal D, Malayappan B, Leeuwenburgh C. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. Rejuvenation Res. 2008;11(4):793–9. doi: 10.1089/rej.2008.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Agency for Research on Cancer and World Health Organization (WHO) IARC Handbooks of Cancer Prevention Weight Control and Physical Activity. Lyon: IARC Press; 2002. [Google Scholar]
- 25.Kawai M, Minami Y, Kuriyama S, Kakizaki M, Kakugawa Y, Nishino Y, Ishida T, Fukao A, Tsuji I, Ohuchi N. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: the Miyagi Cohort Study. Br J Cancer. 2010;103(9):1443–7. doi: 10.1038/sj.bjc.6605885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy DO, Agrawal M, Shen J, Terry MB, Zhang FF, Senie RT, Motykiewicz G, Santella RM. DNA repair capacity of lymphoblastoid cell lines from sisters discordant for breast cancer. J Natl Cancer Inst. 2005;97(2):127–32. doi: 10.1093/jnci/dji013. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konukoglu D, Serin O, Ercan M, Turhan MS. Plasma homocysteine levels in obese and non-obese subjects with or without hypertension; its relationship with oxidative stress and copper. Clin Biochem. 2003;36(5):405–8. doi: 10.1016/s0009-9120(03)00059-6. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Torres M, Gredilla R, Sanz A, Barja G. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Radical Biology & Medicine. 2002;32(9):882–9. doi: 10.1016/s0891-5849(02)00773-6. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzo Y, Costa S, Collins AR, Azqueta A. The comet assay, DNA damage, DNA repair and cytotoxicity: hedgehogs are not always dead. Mutagenesis. 2013;28(4):427–32. doi: 10.1093/mutage/get018. [DOI] [PubMed] [Google Scholar]
- 32.Lynch HT, Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer. 1996;78(6):1149–67. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1149::AID-CNCR1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Mason C, Foster-Schubert KE, Imayama I, Kong A, Xiao L, Bain C, Campbell KL, Wang CY, Duggan CR, Ulrich CM, Alfano CM, Blackburn GL, McTiernan A. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41(4):366–75. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McTiernan A. Mechanisms linking physical activity with cancer. Nature reviews Cancer. 2008;8(3):205–11. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 35.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 36.Pot GK, Habermann N, Majsak-Newman G, Harvey LJ, Geelen A, Przybylska-Philips K, Nagengast FM, Witteman BJ, van de Meeberg PC, Hart AR, Schaafsma G, Hooiveld G, Glei M, Lund EK, Pool-Zobel BL, Kampman E. Increasing fish consumption does not affect genotoxicity markers in the colon in an intervention study. Carcinogenesis. 2010;31(6):1087–91. doi: 10.1093/carcin/bgp255. [DOI] [PubMed] [Google Scholar]
- 37.Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7(1):34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 39.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz KH, Warren M, Rundle AG, Williams NI, Gross MD, Kurzer MS. Exercise effect on oxidative stress is independent of change in estrogen metabolism. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(1):220–3. doi: 10.1158/1055-9965.EPI-07-0058. [DOI] [PubMed] [Google Scholar]
- 41.Slyskova J, Korenkova V, Collins AR, Prochazka P, Vodickova L, Svec J, Lipska L, Levy M, Schneiderova M, Liska V, Holubec L, Kumar R, Soucek P, Naccarati A, Vodicka P. Functional, genetic, and epigenetic aspects of base and nucleotide excision repair in colorectal carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(21):5878–87. doi: 10.1158/1078-0432.CCR-12-1380. [DOI] [PubMed] [Google Scholar]
- 42.Stojiljkovic MP, Lopes HF, Zhang D, Morrow JD, Goodfriend TL, Egan BM. Increasing plasma fatty acids elevates F2-isoprostanes in humans: implications for the cardiovascular risk factor cluster. J Hypertens. 2002;20(6):1215–21. doi: 10.1097/00004872-200206000-00036. [DOI] [PubMed] [Google Scholar]
- 43.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 44.Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35(3):206–21. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Tsai K, Hsu TG, Hsu KM, Cheng H, Liu TY, Hsu CF, Kong CW. Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise. Free Radic Biol Med. 2001;31(11):1465–72. doi: 10.1016/s0891-5849(01)00729-8. [DOI] [PubMed] [Google Scholar]
- 46.Wittwer M, Billeter R, Hoppeler H, Fluck M. Regulatory gene expression in skeletal muscle of highly endurance-trained humans. Acta Physiol Scand. 2004;180(2):217–27. doi: 10.1046/j.0001-6772.2003.01242.x. [DOI] [PubMed] [Google Scholar]
- 47.World Cancer Research Fund (WCRF)/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 48.Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. Faseb J. 2000;14(12):1825–36. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]