Abstract
Background
Central obesity, defined by increased waist circumference (WC) or waist-hip ratio (WHR), is associated with increased cardiovascular (CV) events, including heart failure. However, the pathophysiological link between central obesity and adverse CV outcomes remains poorly understood. We hypothesized that central obesity and larger WHR are independently associated with worse cardiac mechanics (reduced left ventricular [LV] strain and systolic [s’] and early diastolic [e’] tissue velocities).
Methods and Results
We performed speckle-tracking analysis of echocardiograms from participants in the HyperGEN study, a population- and family-based epidemiologic study (N=2181). Multiple indices of systolic and diastolic cardiac mechanics were measured. We evaluated the association between central obesity and cardiac mechanics using multivariable-adjusted linear mixed effects models to account for relatedness among participants. The mean age of the cohort was 51±14 years, 58% were female, and 47% were African-American. Mean body-mass index (BMI) was 30.8±7.1 kg/m2, WC 102±17 cm, WHR 0.91±0.08, and 80% had central obesity based on WC and WHR criteria. After adjusting for multiple potential confounders, including age, sex, race, physical activity, BMI, heart rate, smoking status, systolic blood pressure, fasting glucose, total cholesterol, anti-hypertensive medication use, glomerular filtration rate, LV mass index, wall motion abnormalities, and ejection fraction, central obesity and WHR remained associated with worse global longitudinal strain, early diastolic strain rate, s’ velocity, and e’ velocity (P < 0.05 for all comparisons). There were no significant statistical interactions between WHR and obesity status.
Conclusions
In this cross-sectional study of participants with multiple comorbidities, central obesity was found to be associated with adverse cardiac mechanics.
Keywords: cardiac mechanics, central obesity, strain, echocardiography
Greater than one-third of all adults in the United States are considered obese, and only recently has this trend begun to plateau.1 Obesity is a potent risk factor for the development of cardiovascular disease, including heart failure, yet our understanding of the pathophysiological link is limited.2-4 Further, it is now clear that obesity is not a “one size fits all” disease, and different obesity phenotypes confer various levels of cardiovascular risk. Given the magnitude of the obesity epidemic, targeting high-risk obese individuals will ultimately provide the most cost-efficient and effective care.
While body-mass index (BMI) has been traditionally used in population studies to describe the relationship between adiposity and risk, several studies have shown that body fat distribution (particularly central adiposity) is likely more important than sheer quantity of body fat.5, 6 Waist circumference (WC) and waist-to-hip ratio (WHR), measures of central adiposity, are stronger predictors of cardiovascular risk compared to BMI, yet are infrequently quantified in the clinical setting.7 Central adiposity is linked to adverse metabolic profiles and greater systemic inflammation, both potent risk factors for the development of cardiovascular disease.8 Previous analyses have shown an independent association between measures of central obesity and left ventricular (LV) dysfunction, particularly diastolic dysfunction.2 However, whether central obesity is also associated with subclinical measures of cardiac mechanics, which may precede overt abnormalities in traditional echocardiographic measures, remains unknown.
Evaluating subclinical myocardial dysfunction has recently become feasible through speckle-tracking echocardiography. Speckle-tracking echocardiography quantifies strain (a marker of myocardial deformation) by tracking natural acoustic markers using standard ultrasonographic windows. Strain is a sensitive indicator of cardiomyocyte health that correlates with abnormal calcium transients with cardiomyocytes,9 therefore making it possible to determine the subclinical effects of central obesity on cardiac mechanics.
We sought to study the effect of central obesity on cardiac mechanics, and hypothesized that central obesity, quantified by WHR and WC, is associated with abnormal cardiac mechanics prior to development of symptomatic HF, even in individuals not considered obese by BMI criteria. We therefore performed speckle-tracking analysis for the ascertainment of cardiac mechanics in the Hypertension Genetic Epidemiology Network (HyperGEN) Study, a large population- and family-based study.
METHODS
Study Population
HyperGEN, part of the National Institutes of Health Family Blood Pressure Program (FBPP), is a cross sectional-study consisting of five U.S. sites, with four participating in an ancillary echocardiographic study (Salt Lake City, Utah; Forsyth County, NC; Minneapolis, Minnesota; and Birmingham, Alabama). The goal of HyperGEN was to identify and characterize the genetic basis of familial hypertension.10 Study eligibility required a diagnosis of hypertension prior to the age of 60 and at least one sibling willing to participate in the study. Hypertension was defined by an average systolic blood pressure ≥ 140 mmHg or an average diastolic blood pressure ≥ 90 mmHg (on at least 2 separate clinic visits) or by self-reporting treatment for hypertension. A random sample of normotensive individuals who represented the source cohort from which the HyperGEN affected sibships were identified was also recruited. Individuals with a history of type 1 diabetes mellitus or severe chronic kidney disease were excluded due to the high risk of secondary forms of hypertension. None of the study participants had symptomatic heart failure. All HyperGEN study participants gave written informed consent, and the HyperGEN study was approved by each study site’s local institutional review board.
Clinical Characteristics
Demographic, clinical, and laboratory data were collected during the initial HyperGEN visit. Height, weight, blood pressure, waist/hip circumference, and skinfold thickness were measured by trained personnel, using a standardized protocol. Three consecutive, seated blood pressure measurements were obtained per person and averaged.10 Waist and hip circumferences were measured in the morning while the participants were standing and wearing loose-fitting clothing.11 Skinfold thickness was measured twice and averaged. Physical activity level was quantified as number of blocks walked per day.
Central adiposity was defined using World Health Organization criteria, including a WC ≥ 88 cm or WHR ≥ 0.85 in women, or a WC ≥ 102 cm or WHR ≥ 0.90 in men.12 Histories of myocardial infarction, transient ischemic attack, and stroke were obtained by self-report. Diabetes mellitus was defined by fasting glucose ≥ 126 mg/dl, use of hypoglycemic medication, or a self-reported history. Dyslipidemia was defined by use of lipid lowering medication, low density lipoprotein cholesterol ≥ 160 mg/dl, triglycerides > 150 mg/dl, or high density lipoprotein cholesterol < 40 mg/dl (for men) or < 50 mg/dl (for women). Obesity was defined by a BMI ≥ 30 kg/m2. Chronic kidney disease was defined by an estimated glomerular filtration rate ≤ 60 ml/min/1.73m2.
Conventional Echocardiography
Echocardiography (including 2D, M-mode, and Doppler imaging) was acquired on all study participants using standardized acquisition protocols and stored in analog format (high grade, medical quality videocassette tapes) at the time of study visit.13, 14 Cardiac structure and function were quantified as recommended by the American Society of Echocardiography (ASE).15, 16 LV ejection fraction (EF) was calculated using the biplane method of discs. LV mass was calculated using the linear method recommended by the ASE and indexed to body surface area. LV hypertrophy was defined by a LV mass index > 95 g/m2 in women or > 115 g/m2 in men. Diastolic function was quantitated using early diastolic (E) and late/atrial diastolic (A) transmitral velocities, E/A ratio, isovolumic relaxation time, and E deceleration time.
Digitization of Echocardiograms and Interpretation of Image Quality
Archived echocardiograms in analog format were converted to digital format using the TIMS 2000 DICOM System (Foresight Imaging, Chelmsford, MA). Cine loops of 2-4 cardiac cycles from the parasternal short axis (papillary muscle level) and apical four chamber views were digitized at a high rate and stored offline in DICOM format. Each study was assessed for image quality by an experienced operator, blinded to all other clinical and echocardiographic data, using a 4-point scale based on the degree of endocardial border visualized (1 = 0-25%; 2 = 25%-50%; 3 = 50%-75%; 4 = 75%-100%), similar to scales used previously.17, 18
Two-Dimensional Speckle-Tracking Analysis
Digitized cine loops were analyzed using 2D wall motion tracking software (2D Cardiac Performance Analysis [CPA], TomTec v4.5, Unterschleisshein, Germany). After isolating the highest quality cardiac cycle by visual estimation, the endocardial and epicardial borders were traced at end-systole in each view. Computerized speckle-tracking analysis was performed and endocardial and epicardial border tracings were manually adjusted to optimize tracking. Indices of LV mechanics included peak global longitudinal strain (GLS), early systolic and diastolic strain rate, and systolic (s’) and early diastolic (e’) tissue velocities. LV filling pressures were estimated using E/e’ ratio. For ease of display, strain values were converted to absolute values. Lower absolute strain values, lower e’ tissue velocities, and higher E/e’ ratio were used to indicate worse cardiac function. A validation of the digitization and speckle-tracking techniques employed here have been published elsewhere.19
Images used for speckle-tracking analysis were generally of high quality. In the parasternal short-axis and apical four chamber views, 85% and 97% of images had an image quality score of ≥ 2, respectively, indicating good image quality for the majority of myocardial segments. Data on interobserver and intraobserver reliability for 96 echocardiograms are presented in Supplemental Table 1, which shows excellent intraclass correlation coefficients for speckle-tracking parameters (>0.75 for all).20
Statistical Analysis
Clinical characteristics, laboratory data, and both conventional echocardiographic parameters and speckle-tracking parameters are displayed for the total cohort and also stratified by the presence or absence of central obesity. Continuous data are presented as mean ± standard deviation. Categorical variables are presented as a count and percentage.
After excluding participants with missing anthropomorphic data (N=25), we compared clinical data between groups using t-tests for normally distributed continuous variables (or non-parametric equivalent when appropriate) and Chi-squared tests for categorical variables (or Fisher’s exact test when appropriate). For our primary analyses, we used multivariable-adjusted regression models to determine whether central obesity and WHR were independently associated with worse indices of cardiac mechanics and increased LV filling pressure (i.e., E/e’ ratio). Central obesity was evaluated as a dichotomous variable, whereas WHR was evaluated as a continuous variable. All regression analyses used linear mixed effects models (with speckle-tracking parameters as the outcome variables), thereby accounting for relatedness among HyperGEN participants. We also created multiplicative interaction terms to determine whether there were sex, race, or obesity interactions with WHR in their association with indices of cardiac mechanics (GLS and e’ velocity). An interaction term P<0.05 in the multivariable regression model was considered significant and explored further.
For the linear mixed effects models, we adjusted for speckle-tracking analyst, image quality, study site (which accounts for differences in sonographers and echocardiography equipment) as fixed effects and familial relatedness as a random effect. Additional covariates were selected using a combination of clinical relevance and association with central obesity either in previous studies or the present one. The additional covariates included in our multivariable models included age, sex, physical activity level, systolic blood pressure, heart rate, BMI, smoking status, anti-hypertensive medication use, LV mass, LV ejection fraction, wall motion score index, estimated glomerular filtration rate, total cholesterol, and fasting glucose.
Several secondary analyses were performed and are presented in the supplementary section. First, on sensitivity analysis, we adjusted for (1) homeostasis model assessment-estimated insulin resistance (HOMA-IR) and (2) triglycerides and HDL cholesterol (as additional elements of the metabolic syndrome) to determine whether the effects of central obesity on cardiac mechanics persisted beyond that predicted by the metabolic syndrome. In addition, in sensitivity analyses, we adjusted for serum aldosterone given its pathophysiologic role in obesity, as well as the number of comorbidities, which has previously been shown to be independently associated with adverse cardiac mechanics.8, 21 Next, we performed multivariable analyses between skinfold thickness measurements (markers of subcutaneous adiposity) and cardiac mechanics. Finally, in order to determine the additive predictive value of central obesity markers versus obesity markers in general, we calculated the residuals from the regression of WHR and BMI, and analyzed the association between these residuals and indices of cardiac mechanics.
For all regression analyses involving WHR and skinfold thickness, beta-coefficients are displayed per 1-standard deviation increase. A two-sided p-value < 0.05 was considered statistically significant. To account for multiple comparisons (n=58 statistical tests in Tables 1 and 2), we used the false discovery rate (FDR) method (Q-value < 0.005 for statistical significance). Statistical analyses were performed using Stata v.12.1 (StataCorp, College Station, TX).
Table 1.
Clinical, Physical, and Laboratory Characteristics of the Study Sample
| Characteristic |
All Participants
(N=2181) |
Non-Centrally Obese
(N=443) |
Centrally Obese
(N=1738) |
P-value* |
|---|---|---|---|---|
| Age, y | 51±14 | 42±14 | 53±13 | <0.001 |
| Female, n (%) | 1272 (58) | 265 (60) | 1007 (58) | 0.47 |
| Race/Ethnicity, n (%) | 0.43 | |||
| White | 1152 (53) | 223 (50) | 929 (53) | |
| African-American | 1021 (46) | 218 (49) | 803 (46) | |
| Other | 8 (1) | 2 (1) | 6 (1) | |
| Blocks walked per day | 6 (2-12) | 8 (2-17) | 6 (1-12) | 0.003 |
| Comorbidities, n (%) | ||||
| Hypertension | 1239 (57) | 124 (37) | 1115 (64) | <0.001 |
| Dyslipidemia | 1319 (60) | 178 (54) | 1141 (66) | <0.001 |
| Diabetes mellitus | 364 (17) | 21 (5) | 343 (20) | <0.001 |
| Chronic kidney disease | 195 (9) | 24 (5) | 171 (9) | 0.004 |
| Myocardial infarction | 128 (6) | 8 (2) | 120 (7) | <0.001 |
| Transient ischemic attack or stroke | 97 (5) | 13 (3) | 84 (5) | 0.08 |
| Medications, n (%) | ||||
| Anti-hypertensive medication | 1080 (50) | 100 (23) | 980 (57) | <0.001 |
| ACE-inhibitor | 438 (20) | 33 (8) | 405 (23) | <0.001 |
| Angiotensin receptor blocker | 55 (3) | 2 (1) | 53 (3) | 0.001 |
| Beta-blocker | 266 (12) | 25 (6) | 241 (14) | <0.001 |
| Calcium channel blocker | 481 (22) | 44 (10) | 437 (25) | <0.001 |
| Loop diuretic | 142 (7) | 7 (2) | 135 (8) | <0.001 |
| Thiazide diuretic | 273 (13) | 30 (7) | 243 (14) | <0.001 |
| Statin | 171 (8) | 11 (2) | 160 (9) | <0.001 |
| Physical examination | ||||
| Systolic blood pressure, mm Hg | 126±21 | 118±18 | 129±20 | <0.001 |
| Diastolic blood pressure, mm Hg | 72±11 | 70±10 | 72±11 | <0.001 |
| Heart rate, beats per minute | 68±12 | 65±11 | 68±12 | <0.001 |
| Body-mass index, kg/m2 | 31±7 | 24±3 | 32±7 | <0.001 |
| Waist circumference, cm | 102±17 | 82±8 | 107±14 | <0.001 |
| Waist-hip ratio | 0.91±0.08 | 0.81±0.05 | 0.94±0.07 | <0.001 |
| Subscapularis skinfold thickness, mm | 25±11 | 17±7 | 27±11 | <0.001 |
| Triceps skinfold thickness, mm | 24±11 | 19±9 | 26±11 | <0.001 |
| Body-mass index classification | ||||
| Body-mass index < 25.0 kg/m2 | 422 (20) | 260 (59) | 162 (9) | <0.001 |
| Body-mass index 25.0-29.9 kg/m2 | 731 (34) | 164 (37) | 567 (33) | 0.08 |
| Body-mass index 30-39.9 kg/m2 | 804 (37) | 18 (4) | 486 (45) | <0.001 |
| Body-mass index ≥ 40.0 kg/m2 | 224 (10) | 1 (1) | 223 (13) | <0.001 |
| Laboratory data | ||||
| Estimated GFR, ml/min/1.73m2 | 85±20 | 89±19 | 84±21 | <0.001 |
| Fasting glucose, mg/dl | 106±43 | 92±39 | 109±43 | <0.001 |
| Total serum cholesterol, mg/dl | 196±39 | 184±38 | 199±38 | <0.001 |
| High density lipoprotein, mg/dl | 51±15 | 57±17 | 49±14 | <0.001 |
| Low density lipoprotein, mg/dl | 119±35 | 108±33 | 121±35 | <0.001 |
| Triglycerides, mg/dl | 139±96 | 99±66 | 149±101 | <0.001 |
| HOMA-IR | 2.14±1.88 | 1.15±0.92 | 2.42±1.98 | <0.001 |
| Aldosterone, pg/ml | 8.6±7.5 | 7.1±6.0 | 9.0±7.8 | <0.001 |
ACE, angiotensin-converting enzyme inhibitor; GFR, glomerular filtration rate; HOMA-IR, homeostatic model assessment - insulin resistance.
P-value threshold for significance based on FDR correction for multiple comparison testing = P<0.005
Table 2.
Two-Dimensional, Doppler, and Speckle-Tracking Echocardiographic Parameters of the Study Sample
|
Conventional echocardiographic
parameter |
All participants
(N=2181) |
Non-Centrally
Obese (N=443) |
Centrally
Obese (N=1738) |
P-value*** |
|---|---|---|---|---|
| LV end-diastolic volume, ml | 130±31 | 122±28 | 131±32 | <0.001 |
| LV end-systolic volume, ml | 51±22 | 48±20 | 52±23 | <0.001 |
| LV mass index, g/m2 | 85±22 | 78±21 | 87±22 | <0.001 |
| LV hypertrophy, n (%) | 463 (21) | 65 (15) | 398 (23) | <0.001 |
| Left atrial diameter, cm | 3.5±0.5 | 3.1±0.5 | 3.6±0.5 | <0.001 |
| LV ejection fraction, % | 62±8 | 62±8 | 62±8 | 0.93 |
| Cardiac index, L/min/m2 | 2.6±0.6 | 2.6±0.5 | 2.6±0.6 | 0.53 |
| E velocity, cm/s | 73±20 | 79±17 | 72±20 | <0.001 |
| A velocity, cm/s | 66±19 | 55±16 | 69±18 | <0.001 |
| E/A ratio | 1.21±0.50 | 1.52±0.52 | 1.11±0.45 | <0.001 |
| E deceleration time, ms | 204±58 | 199±51 | 205±60 | 0.07 |
| Isovolumic relaxation time, ms | 80±18 | 77±16 | 81±19 | <0.001 |
|
| ||||
|
Speckle-tracking echocardiographic
parameter |
||||
|
| ||||
| Early diastolic (e’) velocity, cm/s* | 3.5±1.3 | 4.3±1.4 | 3.3±1.2 | <0.001 |
| E/e’ ratio* | 23.5±11.3 | 19.5±7.6 | 24.8±11.9 | <0.001 |
| Early diastolic strain rate, s−1 | 0.8±0.2 | 1.0±0.3 | 0.8±0.2 | <0.001 |
| Systolic (s’) velocity, cm/s* | 3.7±0.9 | 3.9±1.0 | 3.6±0.9 | <0.001 |
| Peak systolic strain rate, s−1 | 0.9±0.2 | 0.9±0.2 | 0.8±0.2 | 0.01 |
| Global radial strain, % | 26.7±11.9 | 28.1±12.0 | 26.4±11.8 | 0.001 |
| Global circumferential strain, % | 20.5±5.3 | 20.8±4.7 | 20.5±5.5 | 0.37 |
| Global longitudinal strain, % | 14.6±3.6 | 15.4±3.5 | 14.4±3.6 | <0.001 |
LV, left ventricular. All strain values are reported as absolute values.
Tissue velocity values derived from speckle-tracking software are lower than values derived from tissue Doppler imaging. Thus, s’ and e’ are lower, and E/e’ ratio is higher, in the present study compared to other studies that use conventional tissue Doppler imaging to measure tissue velocities.
P-value threshold for significance based on FDR correction for multiple comparison testing = P<0.005
RESULTS
Characteristics of Study Participants
Descriptive characteristics of the study sample from HyperGEN are displayed in Table 1, dichotomized by the presence or absence of central obesity. The study cohort consisted of 2181 participants, randomly sampled from all 4 participating sites, representing 1091 unique families. The majority of participants were centrally obese (1738/2181, 80%). The mean age was 51±14 years, 58% were female, and 46% were African American. Comorbidities were common, and. medication use reflected standard therapies used for these comorbidities. Mean blood pressure was 126±21/72±11 mmHg, and obesity was common (mean BMI 31±7 kg/m2, 47% obese [BMI ≥ 30 kg/m2]). Laboratory results revealed largely preserved kidney function (estimated glomerular filtration rate 85±20 ml/min/1.73 m2), mildly elevated fasting glucose (106±43 mg/dl), and mildly elevated total cholesterol (196±39 mg/dl). Individuals with central obesity were older, more often had comorbidities, had higher blood pressure and BMI, and had worse estimated glomerular filtration rate, fasting glucose, and lipids (Table 1).
Table 2 lists the 2D, Doppler, and speckle-tracking echocardiographic parameters of the study participants. Average LV structure fell within normal limits (LV end-systolic volume 51±22 ml; LV end-diastolic volume 130±31 ml; LV mass index 85±22 kg/m2), though roughly one fifth had evidence of LV hypertrophy (21%). Global LV systolic function was preserved (ejection fraction 62±8%) in the majority of participants. Individuals with central obesity notably had larger LV dimensions and worse diastolic function (including lower E/A ratio, longer isovolumic relaxation time, and longer E deceleration time), but no difference was observed in LV ejection fraction.
Association of Central Obesity with Worse Cardiac Mechanics
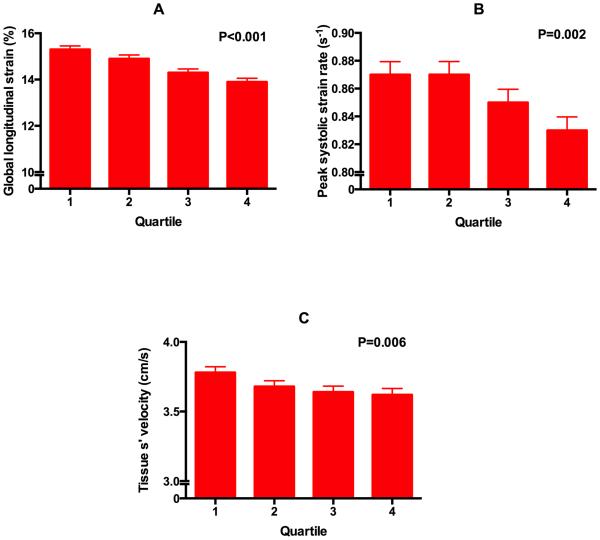
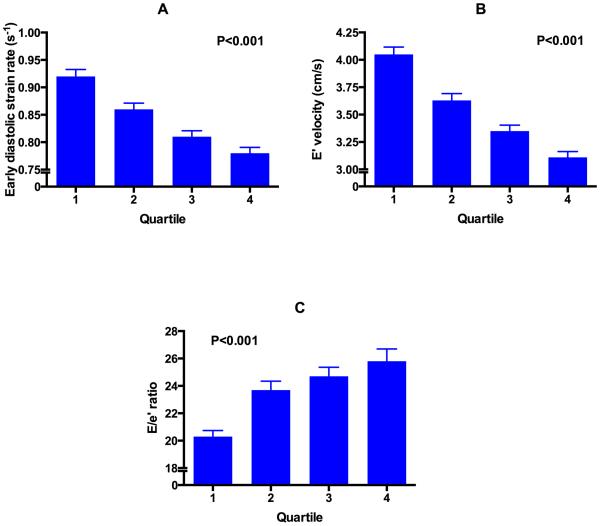
Figure 1 displays the unadjusted relationship between quartiles of WHR with systolic strain parameters and longitudinal systolic tissue velocity (p<0.05 for trend for each parameter). Figure 2 shows a similar relationship of WHR with diastolic parameters (e’ velocity, early diastolic strain rate, and E/e’ ratio [a marker of LV filling pressures]) (P<0.001 for trend for each parameter). On univariate analysis, central obesity and WHR were associated with all indices of cardiac mechanics and elevated LV filling pressure (p<0.05 for all associations). After adjusting for a number of covariates (including speckle-tracking analyst, image quality, center, age, sex, physical activity level, systolic blood pressure, heart rate, BMI, smoking status, anti-hypertensive medication use, LV mass, LV ejection fraction, wall motion score index, estimated glomerular filtration rate, total cholesterol, and fasting glucose), central obesity and WHR were still associated with nearly all indices of cardiac mechanics and elevated LV filling pressure (Tables 3 and 4). In addition, adjusting for each blood pressure medication listed in Table 1 instead of use of anti-hypertensive medications did not alter the significance of our findings (data not shown). We found significant interactions between measures of central obesity and sex for e’ velocity, such that the association of WHR with e’ velocity were stronger in men compared to women (Tables 5 and 6). No interaction was found by race or obesity classification.
Figure 1. Relationship between quartiles of waist-hip-ratio and markers of systolic cardiac mechanics.
(A) global longitudinal strain, (B) peak systolic strain rate, and (C) tissue s’ velocity. Tissue velocities based on speckle-tracking echocardiography are lower than conventional tissue Doppler imaging tissue velocities. P-values are for the linear trend (calculated using linear mixed effects models).
Figure 2. Relationship between quartiles of waist-hip-ratio and diastolic indices.
(A) early diastolic strain rate, (B) tissue e’ velocity, and (C) E/e’ ratio (an estimate of LV filling pressures). Tissue velocities based on speckle-tracking echocardiography are lower than conventional tissue Doppler imaging-based tissue velocities. Thus, e’ velocity is lower, and E/e’ ratio higher, than conventional studies that use tissue Doppler imaging. P-values are for the linear trend (calculated using linear mixed effects models).
Table 3.
Association of Central Obesity with Cardiac Mechanics and Filling Pressures After Multivariable Adjustment *
| Dependent variable |
All participants
(N=2181) |
|
|---|---|---|
|
| ||
| β-Coefficient (95% CI) | P-value | |
| Global longitudinal strain, % | −0.40 (−0.79, −0.01) | 0.044 |
| Peak systolic strain rate, s−1 | −0.02 (−0.05, 0.00) | 0.074 |
| Early diastolic strain rate, s−1 | −0.06 (−0.09, −0.03) | <0.001 |
| Systolic (s’) velocity, cm/s | −0.24 (−0.36, −0.12) | <0.001 |
| Early diastolic (e’) velocity, cm/s | −0.32 (−0.46, −0.18) | <0.001 |
| E/e’ ratio | 2.13 (0.37, 3.90) | 0.018 |
BMI, body-mass index; CI, confidence interval. All strain parameters are reported as absolute values.
Adjusted for age, sex, race, physical activity (average number of blocks walked per day), body-mass index, systolic blood pressure, heart rate, smoking status, serum fasting glucose, total cholesterol, estimated glomerular filtration rate, anti-hypertensive medication use, left ventricular mass, wall motion abnormalities, ejection fraction, center, speckle-tracking analyst, and image quality.
Table 4.
Association of Waist-Hip Ratio with Cardiac Mechanics and Filling Pressures After Multivariable Adjustment*
| Dependent variable |
All participants
(N=2181) |
|
|---|---|---|
|
| ||
| β-Coefficient (95% CI) | P-value | |
| Global longitudinal strain, % | −0.23 (−0.40, −0.06) | 0.009 |
| Peak systolic strain rate, s−1 | −0.01 (−0.02, −0.00) | 0.043 |
| Early diastolic strain rate, s−1 | −0.03 (−0.04, −0.02) | <0.001 |
| s’ velocity, cm/s | −0.09 (−0.14, −0.3) | 0.001 |
| e’ velocity, cm/s | −0.14 (−0.20, −0.07) | <0.001 |
| E/e’ ratio | 1.01 (0.20, 1.81) | 0.014 |
BMI, body-mass index; CI, confidence interval. All strain parameters are reported as absolute values. Beta-coefficients are per 1-SD increase in waist-hip ratio.
Adjusted for age, sex, race, physical activity (average number of blocks walked per day), body-mass index, systolic blood pressure, heart rate, smoking status, serum fasting glucose, total cholesterol, estimated glomerular filtration rate, anti-hypertensive medication use, left ventricular mass, wall motion abnormalities, ejection fraction, center, speckle-tracking analyst, and image quality.
Table 5.
Interaction Analysis P-values
| Cardiac mechanics parameter | Interaction term | P-value for the interaction term |
|---|---|---|
| Global longitudinal strain, % | Waist:hip ratio × sex | 0.44 |
| Waist:hip ratio × race* | 0.96 | |
| Waist:hip ratio × obesity | 0.89 | |
| e’ velocity, cm/s | Waist:hip ratio × sex | 0.002 |
| Waisthip ratio × race* | 0.053 | |
| Waisthip ratio × obesity | 0.43 |
The 8 participants who self-identified as “other race” were excluded from these analyses.
In addition to the specified interaction term, the models above were adjusted for age, sex, race, physical activity (average number of blocks walked per day), body-mass index, systolic blood pressure, heart rate, smoking status, serum fasting glucose, total cholesterol, estimated glomerular filtration rate, anti-hypertensive medication use, left ventricular mass, wall motion abnormalities, ejection fraction, center, speckle-tracking analyst, and image quality.
Table 6.
Sex-Stratified Multivariable Regression Analyses
| Cardiac mechanics parameter | Subgroup | Waist:hip ratio | |
|---|---|---|---|
|
| |||
| β-Coefficient (95% CI) | P-value | ||
| Early diastolic (e’) velocity, cm/s | Male (n=909) | −0.29 (−0.42, −0.16) | <0.001 |
| Female (n=1272) | −0.09 (−0.16, −0.01) | 0.02 | |
BMI, body-mass index; CI, confidence interval. Beta-coefficients are per 1-SD increase in waist circumference.
Adjusted for age, sex, race, physical activity (average number of blocks walked per day), body-mass index, systolic blood pressure, heart rate, smoking status, serum fasting glucose, total cholesterol, estimated glomerular filtration rate, anti-hypertensive medication use, left ventricular mass, wall motion abnormalities, ejection fraction, center, speckle-tracking analyst, and image quality.
Additional models, which included additional adjustment for (1) serum aldosterone (with concomitant adjustment for angiotensin-converting enzyme inhibitors and angiotensin receptor blockers); (2) HOMA-IR; (3) HDL and triglycerides instead of total cholesterol (as other key features of the metabolic syndrome, since fasting glucose and blood pressure were already included in the multivariable model); and (4) the number of comorbidities, did not eliminate these associations (p<0.05 for all associations, as shown in Supplemental Table 2).
Association of Subcutaneous Adiposity (Skinfold Thickness Measures) with Cardiac Mechanics
Supplemental Table 3 displays the relationship between skinfold thickness (measured at the subscapular and triceps regions) and cardiac mechanics for the total cohort. On multivariable analysis, only a few relationships were found to be significant. In addition, the magnitude of the association, as measured by the beta-coefficient per standard deviation in these markers of subcutaneous adiposity, was weaker than the associations observed between WHR and cardiac mechanics.
Additive Value of Central Adiposity Markers over General Adiposity on Residuals Analysis
To determine whether central adiposity is better associated with cardiac mechanics over general adiposity markers, we performed a residuals analysis. Supplemental Table 4 shows the significance of association between residuals (from the regression of BMI and WHR) and indices of cardiac mechanics. There was a significant association between the residuals and all indices of cardiac mechanics and E/e’ ratio (p<0.05 for all comparisons).
DISCUSSION
In an analysis of 2181 participants from the HyperGEN study, we found that central obesity (defined by elevated WC and WHR) was associated with multiple measures of cardiac mechanics as determined by speckle-tracking echocardiography. These associations persisted after adjusting for a number of covariates, including BMI, and there was no statistical interaction based on obesity status. In contrast, only few associations were found between skinfold thickness (a marker of subcutaneous or peripheral adiposity) and cardiac mechanics. These data may offer mechanistic insight into why central obesity is associated with worse cardiac outcomes, including heart failure.
Our data underscore the clinical value of measuring waist circumference. BMI, though predictive of cardiovascular events and highly correlated to waist circumference, still fails to identify the highest risk individuals. For example, for a given BMI, those with higher waist circumference have worse cardiometabolic profiles and higher risk of cardiovascular disease.8, 22 We similarly show here that markers of central obesity are associated with an adverse cardiac mechanics profile despite adjusting for BMI. Conversely, we found little evidence to support the measuring of skinfold thickness as a marker of cardiac dysfunction. Skinfold thickness is a regional measure of subcutaneous adiposity and is primarily used as a surrogate marker of percent body fat.23 However, visceral fat deposition denotes a worse metabolic profile and is more highly associated with adverse cardiovascular events.24 Thus, our finding that subcutaneous adiposity is not closely associated with cardiac mechanics compared with central (visceral) adiposity highlights the importance of visceral, metabolically active adipose tissue and adverse cardiac mechanics.
How central obesity may cause intrinsic myocardial dysfunction remains unclear. With excess adipose tissue, particularly visceral deposition, macrophages infiltrate the adipocytes and secrete large amounts of pro-inflammatory molecules.25 Systemic inflammation causes endothelial dysfunction, which may result in abnormal GLS, since GLS reflects the function of longitudinal, subendocardial fibers.26
Previous clinical studies complement our findings. In a four-year longitudinal study of 1402 participants, changes in ventricular stiffness were modestly correlated to changes in body mass.27 A subsequent study of the same cohort showed that central adiposity was linked to increased end-systolic elastance, a measure of systolic ventricular stiffness, in women.28 Smaller studies also found that some diastolic parameters were associated with central obesity as well.29, 30 These important studies buttress the findings of the present study, though notably were performed in studies with smaller sample sizes and did not include speckle-tracking analysis to calculate longitudinal strain.
Notably, the association of central obesity with cardiac mechanics persisted despite further adjustment for other markers of the metabolic syndrome (HDL cholesterol, triglycerides, and HOMA-IR), and serum aldosterone. Central obesity, a common comorbidity to the metabolic syndrome, is associated with markers of insulin resistance, dyslipidemia, and hypertension. The National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) has formally recognized this association by including fasting glucose, HDL cholesterol, blood pressure, and triglycerides as part of the criteria for the metabolic syndrome.31 Though adjusting for these cardiometabolic risk factors and HOMA-IR weakened the association between cardiac mechanics and central obesity, it did not eliminate it, implying that the relationship between central obesity and worse cardiac function is not merely confounded by other metabolic disease.
Interestingly, on interaction analyses, we found that the association between central obesity and e’ velocity was stronger in men. Though unclear, it may be that in men compared to women, the abnormal metabolic and inflammatory milieu associated with central adiposity exerts a heightened adverse effect on myocardial relaxation. Conversely, we found no interaction with regards to race or obesity classification.
Strengths of our study include the large number of participants (which allowed for comprehensive multivariable adjustment for several potential confounders), the inclusion of a large number of African Americans, and the novel measurement of myocardial strain with speckle-tracking echocardiography. Certain limitations should be considered when interpreting our results. First, though abdominal obesity is a crude anthropomorphic surrogate marker for visceral adiposity32, it is the latter (as opposed to subcutaneous deposition) which likely plays a key pathophysiological role in the development of metabolic and cardiac dysregulation.24 Quantitative measurements of visceral fat are not available in HyperGEN, and thus we were unable to explore the relationship between visceral adiposity specifically and cardiac mechanics. However, the poor associations observed between skinfold thickness, as a measure of subcutaneous adipose, and cardiac mechanics buttresses the notion that subcutaneous fat is less likely pathologic for intrinsic myocardial disease. In addition, WHR is a much more cost-effective and clinically applicable index compared to quantitative measurement from advanced imaging. Second, due to the cross-sectional nature of our study, we were unable establish causality or evaluate long-term outcomes to determine whether participants with central obesity ultimately developed overt cardiac dysfunction or symptomatic heart failure.
Third, though we used World Health Organization criteria for the diagnosis of central obesity, there is ethnic variability in what may be abnormal.24, 33 However, without validated cutoffs for specific populations, we felt that it was most appropriate to use these widely-used criteria. Fourth, multiple statistical tests were performed which raises the probability of type I error. However, we corrected for this by using FDR analysis. Additional limitation include the lack of dietary data and outcomes data in HyperGEN. Analyzing dietary habits could have provided additional insight into the relationship between central obesity and cardiac mechanics, and association of central obesity and adverse cardiac mechanics with worse outcomes could have enhanced the clinical implications of our findings. Finally, speckle-tracking was performed retrospectively on echocardiograms that were acquired without specific attention to optimizing endocardial border definition. However, the vast majority of images acquired were of at least adequate quality, and image quality was entered into all regression analyses as a covariate.
In summary, in one of the largest speckle-tracking studies to date, we found that central obesity is associated with several measures of adverse cardiac mechanics, independent of BMI and even in non-obese individuals. These data provide support for the adverse cardiovascular effects of central obesity and may explain why individuals with central obesity are at increased risk for heart failure.
Supplementary Material
CLINICAL PERSPECTIVE.
Central obesity is common in the general community and its presence denotes a high-risk phenotype for adverse cardiovascular events, including incident heart failure (HF). However, the pathophysiological link between central obesity and HF remains poorly understood. In this cross-sectional study, we performed speckle-tracking analysis on echocardiograms from greater than 2000 participants enrolled in the Hypertension Genetic Epidemiology (HyperGEN) study—a community and family-based study of hypertension. In multivariable models, we found that the presence of central obesity (increased waist circumference) and higher waist-hip ratio, both of which are indicative of increased visceral adiposity, are associated with worse indices of cardiac mechanics. On the contrary, several markers of subcutaneous fat deposition were, in general, not associated with adverse cardiac mechanics. These findings may have broad implications for the screening, clinical monitoring, or treatment of patients with central obesity or risk factors for its development. Measuring waist and hip circumference can easily be performed in the clinical setting, and may be a useful tool to identify individuals at higher risk for the development of intrinsic myocardial dysfunction and overt HF.
Acknowledgments
SOURCES OF FUNDING
The HyperGEN cardiac mechanics ancillary study was funded by the National Institutes of Health (R01 HL107577 to S.J.S.). The HyperGEN parent study was funded by cooperative agreements (U10) with the National Heart, Lung, and Blood Institute: HL54471, HL54472, HL54473, HL54495, HL54496, HL54497, HL54509, HL54515.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ammar KA, Redfield MM, Mahoney DW, Johnson M, Jacobsen SJ, Rodeheffer RJ. Central obesity: association with left ventricular dysfunction and mortality in the community. Am. Heart J. 2008;156:975–981. doi: 10.1016/j.ahj.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 4.Wong YW, Thomas L, Sun JL, McMurray JJ, Krum H, Hernandez AF, Rutten GE, Leiter LA, Standl E, Haffner SM, Mazzone T, Martinez FA, Tognoni G, Giles T, Califf RM. Predictors of incident heart failure hospitalizations among patients with impaired glucose tolerance: insight from the Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research study. Circulation. Heart failure. 2013;6:203–210. doi: 10.1161/CIRCHEARTFAILURE.112.000086. [DOI] [PubMed] [Google Scholar]
- 5.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol. Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 7.Dunkley AJ, Stone MA, Patel N, Davies MJ, Khunti K. Waist circumference measurement: knowledge, attitudes and barriers in patients and practitioners in a multi-ethnic population. Fam. Pract. 2009;26:365–371. doi: 10.1093/fampra/cmp048. [DOI] [PubMed] [Google Scholar]
- 8.Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 9.Shah SJ, Aistrup GL, Gupta DK, O'Toole MJ, Nahhas AF, Schuster D, Chirayil N, Bassi N, Ramakrishna S, Beussink L, Misener S, Kane B, Wang D, Randolph B, Ito A, Wu M, Akintilo L, Mongkolrattanothai T, Reddy M, Kumar M, Arora R, Ng J, Wasserstrom JA. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol. 2014;306:H88–100. doi: 10.1152/ajpheart.00642.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams RR, Rao DC, Ellison RC, Arnett DK, Heiss G, Oberman A, Eckfeldt JH, Leppert MF, Province MA, Mockrin SC, Hunt SC. NHLBI family blood pressure program: methodology and recruitment in the HyperGEN network. Hypertension genetic epidemiology network. Ann. Epidemiol. 2000;10:389–400. doi: 10.1016/s1047-2797(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 11.de Simone G, Devereux RB, Kizer JR, Chinali M, Bella JN, Oberman A, Kitzman DW, Hopkins PN, Rao DC, Arnett DK. Body composition and fat distribution influence systemic hemodynamics in the absence of obesity: the HyperGEN Study. Am. J. Clin. Nutr. 2005;81:757–761. doi: 10.1093/ajcn/81.4.757. [DOI] [PubMed] [Google Scholar]
- 12.Waist Circumference and Waist-Hip Ratio, Report of a WHO Expert Consultation. 2011 [Google Scholar]
- 13.Palmieri V, Dahlof B, DeQuattro V, Sharpe N, Bella JN, de Simone G, Paranicas M, Fishman D, Devereux RB. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J. Am. Coll. Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 14.Devereux RB, Roman MJ, de Simone G, O'Grady MJ, Paranicas M, Yeh JL, Fabsitz RR, Howard BV. Relations of left ventricular mass to demographic and hemodynamic variables in American Indians: the Strong Heart Study. Circulation. 1997;96:1416–1423. doi: 10.1161/01.cir.96.5.1416. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 17.Galema TW, Geleijnse ML, Yap SC, van Domburg RT, Biagini E, Vletter WB, Ten Cate FJ. Assessment of left ventricular ejection fraction after myocardial infarction using contrast echocardiography. Eur J Echocardiogr. 2008;9:250–254. doi: 10.1016/j.euje.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Peteiro J, Pinon P, Perez R, Monserrat L, Perez D, Castro-Beiras A. Comparison of 2- and 3-dimensional exercise echocardiography for the detection of coronary artery disease. J. Am. Soc. Echocardiogr. 2007;20:959–967. doi: 10.1016/j.echo.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Aguilar FA, Selvaraj S, Martinez EE, Beussink L, Kim K-Y, Ping J, Rasmussen-Torvik L, Sha J, Irvin R, Arnett DK, Shah SJ. Archeological Echocardiography: Digitization and Speckle-Tracking Analysis of Archival Echocardiograms in the HyperGEN Study. J. Am. Soc. Echocardiogr. 2012;25:B10. doi: 10.1111/echo.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz DH, Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim KY, Peng J, Sha J, Irvin MR, Eckfeldt JH, Turner ST, Freedman BI, Arnett DK, Shah SJ. Association of low-grade albuminuria with adverse cardiac mechanics: findings from the hypertension genetic epidemiology network (HyperGEN) study. Circulation. 2014;129:42–50. doi: 10.1161/CIRCULATIONAHA.113.003429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim KY, Peng J, Rasmussen-Torvik L, Sha J, Irvin MR, Gu CC, Lewis CE, Hunt SC, Arnett DK, Shah SJ. Association of comorbidity burden with abnormal cardiac mechanics: findings from the HyperGEN study. Journal of the American Heart Association. 2014;3:e000631. doi: 10.1161/JAHA.113.000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr., Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 23.Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P. Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 24.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 25.Lemieux I, Pascot A, Prud'homme D, Almeras N, Bogaty P, Nadeau A, Bergeron J, Despres JP. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler. Thromb. Vasc. Biol. 2001;21:961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 26.Badimon L, Romero JC, Cubedo J, Borrell-Pages M. Circulating biomarkers. Thromb. Res. 2012;130(Suppl 1):S12–15. doi: 10.1016/j.thromres.2012.08.262. [DOI] [PubMed] [Google Scholar]
- 27.Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, Rodeheffer RJ. Longitudinal changes in left ventricular stiffness: a community-based study. Circulation. Heart failure. 2013;6:944–952. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, Borlaug BA. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC. Heart failure. 2014;2:489–499. doi: 10.1016/j.jchf.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canepa M, Strait JB, Abramov D, Milaneschi Y, AlGhatrif M, Moni M, Ramachandran R, Najjar SS, Brunelli C, Abraham TP, Lakatta EG, Ferrucci L. Contribution of central adiposity to left ventricular diastolic function (from the Baltimore Longitudinal Study of Aging) Am. J. Cardiol. 2012;109:1171–1178. doi: 10.1016/j.amjcard.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canepa M, Strait JB, Milaneschi Y, AlGhatrif M, Ramachandran R, Makrogiannis S, Moni M, David M, Brunelli C, Lakatta EG, Ferrucci L. The relationship between visceral adiposity and left ventricular diastolic function: results from the Baltimore Longitudinal Study of Aging. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2013;23:1263–1270. doi: 10.1016/j.numecd.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, Nadeau A, Despres JP. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–184. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 33.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.