Abstract
Background:
There are conflicting data regarding the prevalence of colorectal polyp in patients with acromegaly.
Subjects and Methods:
Consecutive forty-seven acromegalic patients (21 men, 26 women), with a mean age of (40 ± 12 years) attending endocrinology outpatient department underwent full colonoscopy. All the patients underwent clinical and biochemical evaluation (glucose suppressed growth hormone (GH), Insulin-like growth factor-1 [IGF-1], fasting insulin, and glucose). The control group (n = 120) for colonoscopy was adult subjects undergoing evaluation for symptoms of irritable bowel syndrome. Clinical and biochemical parameters in acromegalic patients with colonic polyp were compared to those without a polyp.
Results:
Patients with acromegaly had significantly higher prevalence of colonic polyp as compared to control subjects (10.6% vs. 0.8%). None of the patients with polyp had skin tags. There was no significant difference between subjects with and without colonic polyp in duration of illness, basal, and glucose-suppressed GH and most recent IGF-1. Fasting blood sugar was significantly higher (P < 0.05) in adenoma group after adjusting for age, body mass index (BMI), and insulin levels. Patients in adenoma group showed a trend toward male gender and younger age as compared to those without adenoma.
Conclusions:
Subjects with acromegaly as compared to control have a higher prevalence of colonic polyps. There was no association of polyps seen with age, BMI, skin tags, homeostasis model assessment of insulin resistance index, duration of disease, and basal and glucose-suppressed GH and IGF-1 levels. There were no specific predictive factors detected. Screening full colonoscopy is recommended in all cases with acromegaly.
Keywords: Acromegaly, colonoscopy, colon polyp, homeostasis model assessment of insulin resistance index
INTRODUCTION
Acromegaly is characterized by excessive levels of growth hormone (GH) secretion and insulin-like growth factor-1 (IGF-1) levels, usually due to GH-secreting pituitary adenoma. Both GH and IGF-1 play an important role in the regulation of cell proliferation and differentiation.[1,2] Cats et al. reported increased proliferation index of colonic epithelium proportional to their circulating IGF-1 levels.[3] Acromegalic patients are shown to have an increased risk for both benign and malignant lesions.[4] Various studies have reported increased prevalence (9–38%) of colorectal cancer in particular.[5,6,7,8,9,10,11,12,13] These variations in the prevalence of colonic tumors may be due to differences in selection of patients, visualization of the entire colon, autoptic versus colonoscopy series, selection of controls, and sample size. Screening guidelines therefore suggest full colonoscopy should be done at first visit in all acromegalic patients irrespective of age and follow-up should be scheduled in accordance to presence or absence of colonic adenoma and/or elevated IGF-1.[14,15,16] However, a study from North India demonstrated no increased risk of colonic neoplasm in subjects with acromegaly.[17] There is increased incidence of cancer in general population with age and improved survival of acromegaly patients may be one of the reasons of an increased prevalence of cancer. Other factors such as geographical area and ethnicity may play an important role in the development of colorectal neoplasia rather than elevated GH in acromegalic patients.[18] We evaluated North Indian subjects with acromegaly for colonic polyp and its clinical and biochemical predictors.
SUBJECTS AND METHODS
Study design and setting: Cross-sectional, comparative, and tertiary care academic university hospital-based study.
Patients
Forty-seven consecutive patients with acromegaly were enrolled from endocrinology outpatients department. Criteria for the diagnosis included clinical features, lack of suppression of GH levels below 1 ng/ml after oral 75-g glucose tolerance test, and elevated serum IGF-1 levels for age- and gender-matched healthy subjects. Exclusion criteria were age <18 years and pregnancy. Cure of acromegaly after pituitary surgery was defined as when serum IGF-1 levels were within the normal age- and sex-matched range and serum GH was suppressed <1 ng/ml at 1 h after 75 g glucose as used in international consensus.[19]
Patient characteristics
Detailed clinical history included presenting symptoms and duration, systemic complications of acromegaly and gastrointestinal symptoms as malena, altered bowel habits, abdominal pain were obtained. All subjects underwent complete general physical and systemic examination. Demographic and anthropometric data viz. (height, weight, body mass index [BMI], and waist-hip ratio [WHR]) were recorded. Waist circumference was measured midway between the iliac crest and the lowermost margin of the ribs at the end of normal expiration, and the hip girth was measured at the intertrochanteric level according to the World Health Organization guidelines.[20] WHR was calculated, and a WHR ratio of 0.9 in males and 0.8 in females was taken as a cutoff for abnormal WHR.[21] BMI was calculated (weight [kg]/height [m2]) and the obesity was considered ≥25 kg/m2.[22] Blood sample was obtained after an overnight fast. Plasma blood glucose (fasting and postprandial), serum albumin, adjusted total calcium, phosphorous, alkaline phosphatase, lipid profile, total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, electrolytes, renal function tests, and liver function tests. Endocrine evaluation included serum T4 or FT4, GH suppression (taken at 0 min and after 1 h of 75 g of glucose), IGF-1, follicle stimulating hormone, prolactin (PRL), testosterone (in males), basal and stimulated cortisol (taken1 h after intramuscular injection with 250 µg of synthetic ACTH (1–24), glycosylated hemoglobin (HbA1c), and fasting insulin. Homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated. Stool examination for occult blood was performed. All patients underwent imaging of sella and suprasellar regions using gadolinium-enhanced magnetic resonance, direct ophthalmoscopy for fundus examination, and automated perimetry for visual field defects.
Colonoscopy
Bowel preparation was done using a polyethylene glycol-based electrolyte solution (Peglec-Powder, Tablets India Limited). Full colonoscopy was performed using a video colonoscope (Q180AL, 2008483, and OLYMPUS EVIS EXERA II). All colonoscopies were performed by the same endoscopist. Tumor sites were classified as the right colon (cecum, ascending, and transverse colon), left colon (descending and sigmoid colon), and rectum (distal 18 cm). Location and size of polyps were noted before removal [Figure 1]. All lesions were removed and/or biopsied. Histopathological examination was performed by independent pathologists blinded to clinical findings. Polyps were classified as neoplastic (adenomas and adenocarcinomas) or nonneoplastic (i.e., hyperplastic polyps).[23] No complications occurred during colonoscopy.
Figure 1.
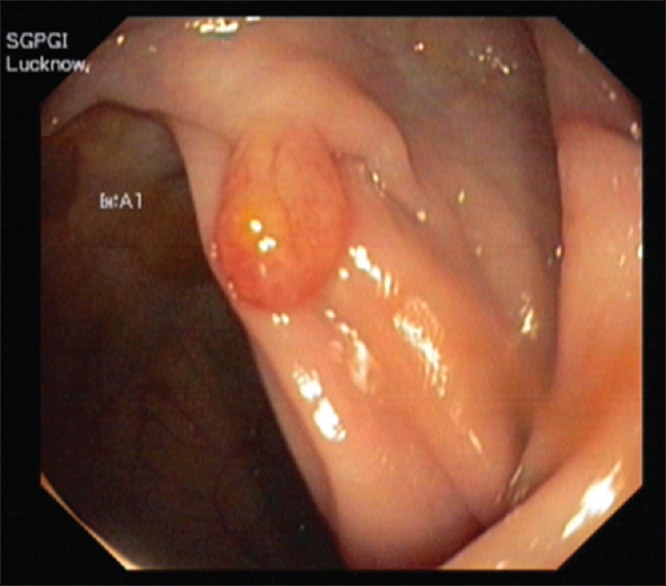
Colonoscopy-pedunculated rectal polyp
Controls
The control group consisted of 120 patients (65 males and 55 women, mean age 39 ± 14) attending gastroenterology outpatient with a diagnosis of irritable bowel syndrome. Hemogram, liver function test, and renal function test were normal. Subsets with diabetes mellitus and hypothyroidism were excluded.
Analytic methods
Serum chemistries were performed on autoanalyzer (Imola™ Randox, UK) within 24 h of sample collection. Serum GH was measured by immunoradiometric assay using commercially available kit (IRMA GH; ©2015 Beckman Coulter, Inc. IMMUNOTECH S.T.O.) with analytical sensitivity of 0.10 mIU/L. Serum IGF-1 was done with (IRMA-IGF-1; Beckman Coulter) after extraction. Analytical sensitivity was 4.5 ng/mL, intra- and inter-assay coefficient of variation (CV) was ≤5.6% and ≤8.3%, respectively. Serum testosterone was done using radioimmunoassay and had analytical sensitivity of 0.02 ng/ml and below 15% and 5.6% was inter- and intra-assay CV. Serum cortisol, PRL, T4, FT4, and insulin were done using chemiluminescent assay (IMMULITE® 1000, SIEMENS) which had an analytical sensitivity of 5.5 nmol/L, 11 mIU/L, 1.67pmol/L, and 2 µIU/ml, respectively. HbA1c was done using (Bio-Rad Variant™ II, hemoglobin A1c Program, USA) which utilizes principles of ion-exchange high-performance liquid chromatography. The range of average values was 3.5–18.6%. Type 2 diabetes mellitus, impaired fasting glucose (IFG), and impaired glucose tolerance (IGT) were defined as per the American Diabetes Association guidelines.[24] IR was determined with the HOMA by HOMA-IR.[25] HOMA-IR = fasting serum insulin (mU/L) × fasting serum glucose (mg/dl)/(22.5 × 18). We used HOMA-IR values higher than 2.5 for indicating IR based on published literature.[26]
Statistical methods
Statistical analyses were performed using SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Komlogorov–Smirnov test or Shapiro-Wilks test was used to assess the normal distribution. Continuous variables were expressed as a mean ± standard deviation. Z-test of proportion was done to compare proportions between two groups (cases vs. control). Chi-square test and Fischer Exact test were used to assess the association or to compare two categorical values. Logistic regression analysis was done to determine various parameters known to affect the presence of a polyp. Those which were significant on univariate analysis were then analyzed using multiple regression. Independent sample t-test or Mann–Whitney test was used to compare variables between two groups. A P < 0.05 was taken as significant.
RESULTS
Forty-seven subjects with mean age of 40 ± 12.3 were enrolled. The colon was visualized fully till caecum in 45 (95%) patients and in two patient it could be done up to hepatic flexure. Of the 47 patients, eight were treatment naive, 24 (65.9%) underwent transsphenoidal surgery (TSS) or transcranial surgery like craniotomy as per neurosurgical indication, while twenty patients had received radiotherapy following TSS. Three subjects took octreotide along with TSS and radiotherapy. One patient was given only octreotide who had ectopic source that was not localized.
There was a median delay of 5 years (range: 1–15) from the onset of symptoms and diagnosis of acromegaly. Anthropometry showed mean BMI of 28 kg/m2 and WHR in male and female was 0.91, respectively. Hypertension was seen in 18 (38%) patients at presentation and eight (17%) had the onset of hypertension before the diagnosis of acromegaly. Type 2 diabetes mellitus was present in 7 and IFG/IGT was present in 12.8% of patients [Tables 1 and 2].
Table 1.
Clinical and biochemical features of patients at baseline (n=47)
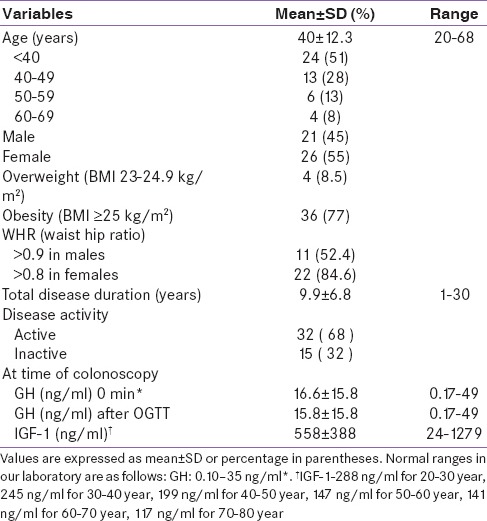
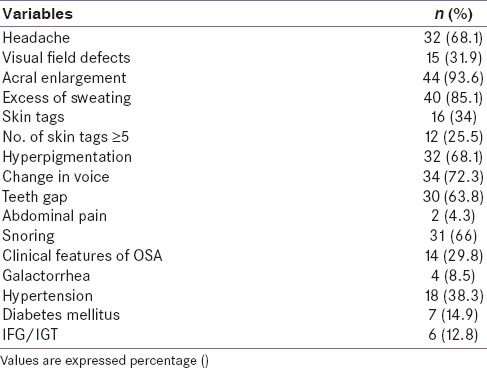
Table 2.
Clinical profile of patients at presentation (n=47)
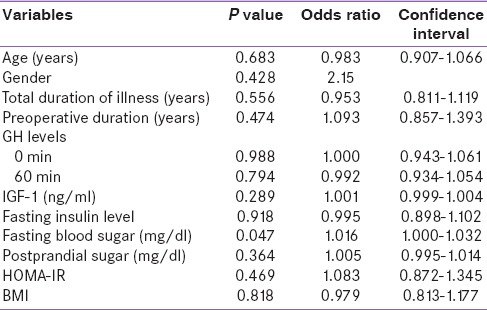
Patients were categorized into two groups (with polyp and those without polyp). Colonic adenoma was documented in five (10.6%) patients. All had single lesion only. In control group (n = 120) only one patient was detected to have polyp (0.8%). Thus, the risk of colonic polyp was significantly higher in patients with acromegaly as compared to control population (P = 0.003). On histopathology, we observed adenomatous polyps with low-grade dysplasia (n = 1) [Figure 2], hyperplastic (n = 1) [Figure 3], and simple polyp with no evidence of dysplasia (n = 2) [Table 3]. All patients with polyp had active disease at the time of colonoscopy. There was no significant difference in the duration of illness, basal GH levels, GH suppression (P = 0.822), and IGF-1 levels between acromegaly subjects with or without polyp [Table 4]. No significant difference was seen in serum fasting insulin levels and calculated HOMA-IR (with polyp vs. without polyp 4.3 ± 4.2 vs. 3.1 ± 3.3, P = 0.501). None in the polyp group was found to have skin tags. One out of five in a polyp group had a history of intermittent abdominal pain and malena with a positive stool for occult blood. No one had a family history of gastrointestinal malignancies [Table 5].
Figure 2.
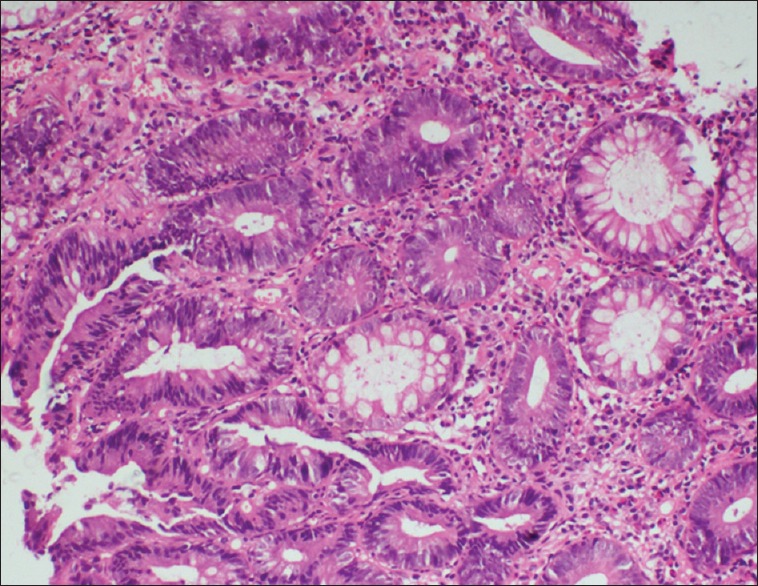
Histopathology-adenomatous colonic polyp
Figure 3.
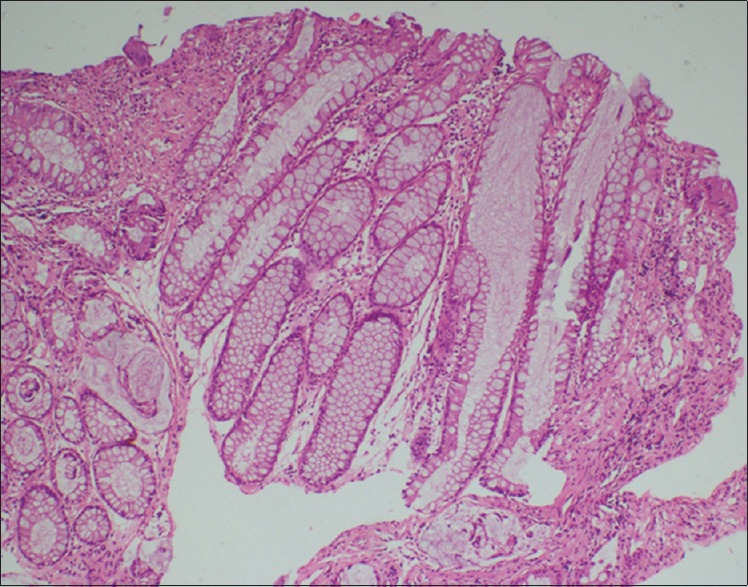
Histopathology-hyperplastic colonic polyp
Table 3.
Localization of colonic adenomas
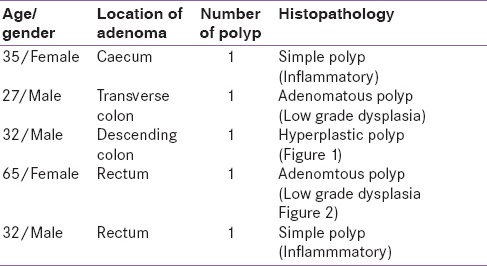
Table 4.
Clinical features of patients between two groups
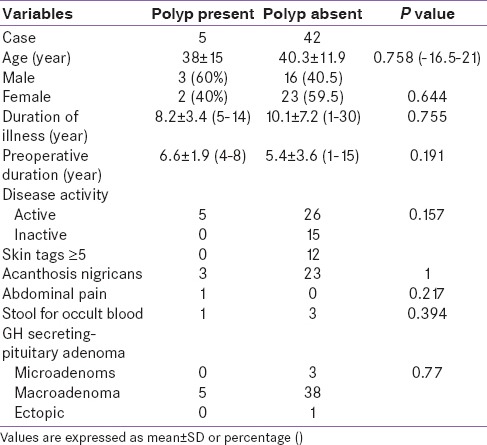
Table 5.
Anthropometric and biochemical features of patients between two groups
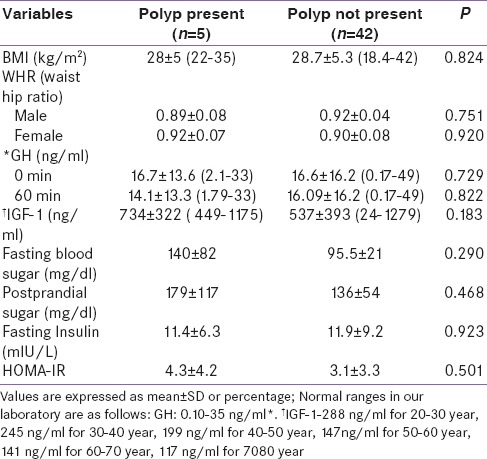
In univariate analysis, fasting blood sugar had a significant association with polyp. In multivariate analysis, it continued to be significant (P < 0.05) after adjusting for age, BMI, gender, and serum insulin [Table 6].
Table 6.
Logistic regression of variables that can determine development of polyp
DISCUSSION
Colonic polyps were observed in 10.6% of acromegalic patients, significantly higher as compared to age- and sex-matched control population. This is in concordance with other studies that have shown increased prevalence of colonic polyp in acromegaly patients.[11,27,28,29] A large meta-analysis by Rokkas et al. further highlighted increased risk of developing colorectal adenomatous polyps, as well as colorectal cancer in these patients.[13] In contrast, three studies have shown no association between colonic polyps and acromegaly. In these studies, patients were predominantly younger and colonoscopy was incomplete.[17,18,30]
The mean age of our patients who had polyp was 38 ± 15 years. Of the five patients who had polyp, four (80%) were <40 years of age. Two studies [10,27] reported a higher prevalence of adenoma in younger age group (<55 years) who usually display more aggressive course. None had a family history of colorectal cancer. Males were found to harbor polyp greater than females (60% vs. 40%) although it was statistically not significant. This is consistent with other studies.[7,27]
None of our patients with a polyp had skin tags. The lack of correlation between polyp and skin tags is in agreement with the study done by Terzolo et al.[27] There was no correlation seen between the presence or absence of stool for occult blood and polyps.
Increasing evidence supports IR as the underpinning of the obesity-colorectal neoplasia link. HOMA-IR is a good marker of IR and for central obesity. The presence of adenoma was not influenced with glucose intolerance, fasting insulin levels, HOMA-IR, and WHR. This is in contrast to the study done by Colao et al.,[31] which showed significant association seen with fasting insulin levels, HOMA-IR, and prevalence of polyp. Small sample size could be a plausible explanation. In our study, there was a significant association with fasting blood sugars and it persisted after correction for BMI, age, and insulin levels.
Interestingly, three out of five patients with polyp had <5 years of duration of illness. We failed to demonstrate a significant correlation between the duration of disease, GH, IGF-1 levels, disease activity, and the prevalence of polyps. Similar findings were noted with previous observations.[27] It did not discredit the hypothesis that prolonged and exaggerated secretion of growth-promoting factors, increase cellular mitotic activity of colonic mucosa, thereby predisposing to the development of neoplastic lesions. It has to be considered that hormone measurement in a certain moment of a long-lasting disease such as acromegaly may not give a reliable picture of the degree of hypersecretion during the preceding years. The trend (not significant) toward a greater prevalence of polyps in patients with active disease and pituitary GH-secreting macroadenoma, both conditions associated with more aggressive disease, is consistent with a pathogenic role of GH and/or IGF-1.
Data from India suggest that the prevalence of colonic polyp is 2% which is relatively lower.[32] This has been speculated to be due to high dietary fiber intake and predominently vegetarian habits.[33] This is against the notion that increase in the prevalence is due to increase in life expectancy of patients with acromegaly. Higher prevalence of colonic polyps in patients with acromegaly as compared to control population adds more morbidity and hence, warrants colonic screening in these patients at the time of diagnosis. Further follow-up studies are required to determine how frequently pancolonoscopy be repeated in these patients.
CONCLUSION
The present study demonstrated increased prevalence of colon polyp in acromegaly patients as compared to general population. The prevalence of polyp showed a trend toward males and younger population. This was against the previous notion that with an increase in life expectancy incidence of polyps has increased. There were no specific predictors for the development of polyp. Polyps were detected at different sites without any specific predilection. Hence, all acromegalic patients of any age and duration of illness should undergo screening full colonoscopy at the time of diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors gratefully acknowledge Mr. Satish verma, Mr. Karambir Kumar, Mr. Kshitiz Mishra, and Mrs. Manisha Kakaji for their contributions.
REFERENCES
- 1.Melmed S. Acromegaly. N Engl J Med. 1990;322:966–77. doi: 10.1056/NEJM199004053221405. [DOI] [PubMed] [Google Scholar]
- 2.Moon HD, Simpson ME, Li CH, Evans HM. Neoplasms in rats treated with pituitary growth hormone. III. Reproductive organs. Cancer Res. 1950;10:549–56. [PubMed] [Google Scholar]
- 3.Cats A, Dullaart RPF, Zwart N, de Vries EGE, Hardonk MJ, Kleibeuker JH. 1993 Increased epithelial cell proliferation in the colon in patients with acromegaly. Neth J Med. 42:A3. [PubMed] [Google Scholar]
- 4.Kurimoto M, Fukuda I, Hizuka N, Takano K. The prevalence of benign and malignant tumors in patients with acromegaly at a single institute. Endocr J. 2008;55:67–71. doi: 10.1507/endocrj.k07e-010. [DOI] [PubMed] [Google Scholar]
- 5.Klein I, Parveen G, Gavaler JS, Vanthiel DH. Colonic polyps in patients with acromegaly. Ann Intern Med. 1982;97:27–30. doi: 10.7326/0003-4819-97-1-27. [DOI] [PubMed] [Google Scholar]
- 6.Brunner JE, Johnson CC, Zafar S, Peterson EL, Brunner JF, Mellinger RC. Colon cancer and polyps in acromegaly: Increased risk associated with family history of colon cancer. Clin Endocrinol (Oxf) 1990;32:65–71. doi: 10.1111/j.1365-2265.1990.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 7.Ezzat S, Strom C, Melmed S. Colon polyps in acromegaly. Ann Intern Med. 1991;114:754–5. doi: 10.7326/0003-4819-114-9-754. [DOI] [PubMed] [Google Scholar]
- 8.Ortego J, Vega B, Sampedro J, Escalada J, Boixeda D, Varela C. Neoplastic colonic polyps in acromegaly. Horm Metab Res. 1994;26:609–10. doi: 10.1055/s-2007-1001769. [DOI] [PubMed] [Google Scholar]
- 9.Vasen HF, van Erpecum KJ, Roelfsema F, Raue F, Koppeschaar H, Griffioen G, et al. Increased prevalence of colonic adenomas in patients with acromegaly. Eur J Endocrinol. 1994;131:235–7. doi: 10.1530/eje.0.1310235. [DOI] [PubMed] [Google Scholar]
- 10.Delhougne B, Deneux C, Abs R, Chanson P, Fierens H, Laurent-Puig P, et al. The prevalence of colonic polyps in acromegaly: A colonoscopic and pathological study in 103 patients. J Clin Endocrinol Metab. 1995;80:3223–6. doi: 10.1210/jcem.80.11.7593429. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins PJ, Fairclough PD, Richards T, Lowe DG, Monson J, Grossman A, et al. Acromegaly, colonic polyps and carcinoma. Clin Endocrinol (Oxf) 1997;47:17–22. doi: 10.1046/j.1365-2265.1997.1911029.x. [DOI] [PubMed] [Google Scholar]
- 12.Koksal AR, Ergun M, Boga S, Alkim H, Bayram M, Altuntas Y, et al. Increased prevalence of colorectal polyp in acromegaly patients: A case-control study. Diagn Ther Endosc. 2014;2014:152049. doi: 10.1155/2014/152049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rokkas T, Pistiolas D, Sechopoulos P, Margantinis G, Koukoulis G. Risk of colorectal neoplasm in patients with acromegaly: A meta-analysis. World J Gastroenterol. 2008;14:3484–9. doi: 10.3748/wjg.14.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P, Lamberts SW, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary. 2013;16:294–302. doi: 10.1007/s11102-012-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–89. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 16.Lois K, Bukowczan J, Perros P, Jones S, Gunn M, James RA. The role of colonoscopic screening in acromegaly revisited: Review of current literature and practice guidelines. Pituitary. 2015;18:568–74. doi: 10.1007/s11102-014-0586-5. [DOI] [PubMed] [Google Scholar]
- 17.Bhansali A, Kochhar R, Chawla YK, Reddy S, Dash RJ. Prevalence of colonic polyps is not increased in patients with acromegaly: Analysis of 60 patients from India. J Gastroenterol Hepatol. 2004;19:266–9. doi: 10.1111/j.1440-1746.2003.03282.x. [DOI] [PubMed] [Google Scholar]
- 18.Ladas SD, Thalassinos NC, Ioannides G, Raptis SA. Does acromegaly really predispose to an increased prevalence of gastrointestinal tumours? Clin Endocrinol (Oxf) 1994;41:597–601. doi: 10.1111/j.1365-2265.1994.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 19.Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, et al. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95:3141–8. doi: 10.1210/jc.2009-2670. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Report of a WHO Expert Committee. Geneva: World Health Organization; 1995. Physical Status: The Use and Interpretation of Anthropometry; pp. 424–38. (WHO Technical Report Series, No. 854). [PubMed] [Google Scholar]
- 21.Lear SA, James PT, Ko GT, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur J Clin Nutr. 2010;64:42–61. doi: 10.1038/ejcn.2009.70. [DOI] [PubMed] [Google Scholar]
- 22.The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Melbourne: Health Communications Australia; 2000. [Last accessed on 2016 Feb 02]. World Health Organization Western Pacific Region/International Assoc for the Study of Obesity/Int Obesity Task Force. Available from: http://www.wpro.who.int/nutrition/documents/docs/Redefiningobesity.pdf . [Google Scholar]
- 23.Sleisenger, Marvin H, Feldman, Mark, Friedman, Lawrence S, (Lawrence Samuel), Brandt, Lawrence J. In: Sleissenger and Fordtran's Gastrointestinal and liver disease: Pathophysiology,diagnosis and management. 8th ed. Mark Feldman MD, Lawrence S, Friedman MD, Lawrence J, Brandt, editors. Elsevier; 2006. pp. 2155–88. [Google Scholar]
- 24.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 27.Terzolo M, Tappero G, Borretta G, Asnaghi G, Pia A, Reimondo G, et al. High prevalence of colonic polyps in patients with acromegaly. Influence of sex and age. Arch Intern Med. 1994;154:1272–6. [PubMed] [Google Scholar]
- 28.Terzolo M, Reimondo G, Gasperi M, Cozzi R, Pivonello R, Vitale G, et al. Colonoscopic screening and follow-up in patients with acromegaly: A multicenter study in Italy. J Clin Endocrinol Metab. 2005;90:84–90. doi: 10.1210/jc.2004-0240. [DOI] [PubMed] [Google Scholar]
- 29.Colao A, Balzano A, Ferone D, Panza N, Grande G, Marzullo P, et al. Increased prevalence of colonic polyps and altered lymphocyte subset pattern in the colonic lamina propria in acromegaly. Clin Endocrinol (Oxf) 1997;47:23–8. doi: 10.1046/j.1365-2265.1997.00253.x. [DOI] [PubMed] [Google Scholar]
- 30.Renehan AG, Bhaskar P, Painter JE, O'Dwyer ST, Haboubi N, Varma J, et al. The prevalence and characteristics of colorectal neoplasia in acromegaly. J Clin Endocrinol Metab. 2000;85:3417–24. doi: 10.1210/jcem.85.9.6775. [DOI] [PubMed] [Google Scholar]
- 31.Colao A, Pivonello R, Auriemma RS, Galdiero M, Ferone D, Minuto F, et al. The association of fasting insulin concentrations and colonic neoplasms in acromegaly: A colonoscopy-based study in 210 patients. J Clin Endocrinol Metab. 2007;92:3854–60. doi: 10.1210/jc.2006-2551. [DOI] [PubMed] [Google Scholar]
- 32.Bhargava DK, Chopra P. Colorectal adenomas in a tropical country. Dis Colon Rectum. 1988;31:692–3. doi: 10.1007/BF02552585. [DOI] [PubMed] [Google Scholar]
- 33.Chiu BC, Ji BT, Dai Q, Gridley G, McLaughlin JK, Gao YT, et al. Dietary factors and risk of colon cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003;12:201–8. [PubMed] [Google Scholar]


