Abstract
Aim:
Chronic iron overload resulting from frequent transfusions, poor compliance to efficient chelation therapy and chronic liver disease is basically responsible for the most severe complications of thalassemia major (TM). Before conventional treatment, TM was entirely childhood disease with a very short survival. Today, survival improved to 40–50 years and becomes a prevalent disease of adulthood and in the near future it will be one of senility. Furthermore, clinical phenotype of TM is changing with age and appearance of severe complications from the heart and endocrine glands that require special health care from well-informed specialists.
Objectives:
The aims of our study were to: (1) Imprint the clinical profile of long-lived TM patients; (2) evaluate retrospectively the cumulative incidence of endocrine diseases; (3) identify potential risk factors; and (4) orient the physicians in the modified clinical phenotype and the relative patients' health needs.
Design:
A retrospective cross-sectional study followed from childhood to adulthood by the same physician in a tertiary thalassemia clinic.
Participants:
Forty-three long-lived TM patients (mean age: 50.3 ± 10.8 years; range: 45.8–59.5 years; 23 females) were studied.
Patients and Methods:
An extensive medical history, with detailed clinical and laboratory data, endocrine complications, and current treatments, was obtained.
Results:
The data indicate that 88.4% of adult TM patients suffered from at least one endocrine complication. The majority of patients developed endocrine complications in the second decade of life when serum ferritin level was very high (12/23 TM female and 8/20 TM male patients, the serum ferritin levels at the diagnosis were above 5.000 ng/ml).
Conclusions:
These data underline that endocrine and bone complications in adult TM patients are highly prevalent and necessitate close monitoring, treatment, and follow-up. Physicians' strategies to optimize chelation therapy include identifying patients who are at risk for developing organ damage, developing chelation plans, promoting compliance, and educating patients. Several clinical aspects remain to be elucidated such as growth and impairment of glucose tolerance in relation to hepatitis C virus infection. Furthermore, affordable worldwide-established long-term treatment protocols for hypogonadism and osteoporosis are needed.
Keywords: Endocrine complications, growth, hepatitis C virus, iron overload, thalassemia
INTRODUCTION
Over the years, advances in the knowledge of physiopathology and in the efficiency of conventional treatment of thalassemia major (TM) resulted in a considerable improvement of patients' survival.[1] Before implementation of transfusions, TM was considered an entire pediatric disease with severe clinical course and short survival, not exceeding the first decade of life. Today in developed countries, survival of patients on conventional treatment increased to 40–50 years; it is also expected in the near future to extent further to the senility. In addition to the improvement of survival, conventional treatment modified the clinical phenotype of the disease, especially in adulthood. Parallel to the significant amelioration of the main clinical features of the disease (severe anemia, bone changes, hepatosplenomegaly, and stunting of growth), adult patients suffer from complications that are mainly related to transfusions and the huge amounts of transfusional iron input that affect the heart, liver, and endocrine glands. These complications, especially those of the endocrine glands are very common among the long-lived patients, requiring special health care and followed by specialists. Other complications related to treatment are transfusion-transmitted viral infections, alloimmunization, iron-chelator-related toxicities, osteoporosis, and psychosocial problems.
The aims of this study on a selected and unique cohort of long-lived patients aged 45–60 years were to: (1) Imprint the clinical phenotype of long-term TM survivors; (2) evaluate retrospectively the cumulative incidence of endocrine diseases; (3) identify potential risk factors; and (4) orient the physicians about patient health needs.
PATIENTS AND METHODS
Setting and research design
Forty-three long-lived TM patients (mean age: 50.3 ± 10.8 years; range: 45.8–59.5 years; 23 females), regularly followed since childhood at the Thalassemia Centre of Ferrara and the Pediatrics and Adolescent Outpatient Clinic for endocrine assessment and followed were studied. These patients underwent periodical evaluations including physical examination as well as laboratory and imaging studies according to National protocols. A detailed cross-retrospective analysis was performed in October 2015.
The inclusion criteria were (1) diagnosis of TM, based on hematological criteria; (2) patients regularly followed for endocrine assessment (every 6–12 months) from childhood to advanced adulthood. The exclusion criteria included: (1) Nontransfusion-dependent thalassemias; (2) bone marrow transplanted patients; (3) patients with HIV positivity or with incomplete clinical and or laboratory data.
Data on demographic variables, diagnosis, treatment and medical history, onset of endocrine problems, and any other late complications during the follow-up (menstrual history, presence of sexual dysfunction or fertility problems, and presence of low bone mineral density [BMD]) were collected.
Approval of the study was obtained in accordance with local institutional requirements and with the Declaration of Helsinki (http://www.wma.net). All procedures were carried out with the adequate understanding and consent of parents (before the age of 18 years) or patients.
Definitions
Short stature was defined as height below the third percentile using the growth charts of Tanner and Whitehouse and of Cacciari et al. after 2002.[2,3] Body mass index (BMI) was calculated (weight in kg/height in m2). A subject was considered overweight when the BMI was between 25 and 30 and obese above 30.[4] Height and weight have been measured according to international recommendations.[5]
In children, growth hormone deficiency (GHD) was diagnosed if the peak GH value after arginine and clonidine test was <10 ng/mL in the two tests and in adults, <3 ng/mL after glucagon stimulation test (GST).[6]
Thyroid dysfunction was classified as: (a) Overt hypothyroidism (low free thyroxine [FT4], increased thyrotropin stimulating hormone [TSH] levels); (b) subclinical hypothyroidism (normal FT4 and TSH >5 mIU/ml); and (c) central hypothyroidism with an inappropriately low serum TSH concentration in the presence of subnormal serum FT4.[7] Antithyroid antibodies, antithyroglobulin, and anti-thyroid peroxidase antibodies were determined by commercially available immunoassays in TM patients with primary hypothyroidism.
The diagnosis of diabetes mellitus was based on the American Diabetes Association criteria.[8]
Delayed puberty in girls was defined as the absence of breast development by the age of 13 years and secondary amenorrhea as the absence of menstruation for a period of 6 months at any time after menarche.[4]
In males, an acquired hypogonadotropic hypogonadism was suspected in the presence of decreased libido, erectile dysfunction and strength, reduced volume of ejaculate, and a worsened sense of well-being leading to degraded quality of life.[9]
Hypoparathyroidism was diagnosed when there was low serum calcium concentration, increased serum phosphate, and low serum parathyroid hormone (PTH) or a level inappropriate for the calcium level.[4,10]
Hypoadrenalism was diagnosed when basal cortisol was 3.5 μg/dl (98 nmol/l) or less [4] or in the presence of low cortisol response (250 nmol/l = 9 μg/dl) after GST.[6]
Hyperprolactinemia was defined as a basal level greater than the locally derived normal assay reference range.[11]
The diagnosis of metabolic bone disease was based on the indications of the International Committee for Standards in Bone Measurements (Official Position 2013 available at http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/)). As index of BMD, Z-score was chosen as T-score is not suitable for females before menopause and for males younger than 50 years.[12,13]
Blood sampling and analytical procedures
All blood samples were collected in the morning (08.00–09.00 am) after an overnight fast (1–2 weeks after blood transfusion) to measure the serum concentrations of insulin-like growth factor-1 (IGF-1), glucose, FT4, TSH, urea, creatinine, electrolytes, calcium, and phosphate.
To evaluate liver functions, serum concentrations of alanine aminotransferase (ALT), gamma-glutamyl transferase (γGT), total and direct bilirubin, total proteins, albumin, and International Normalization Ratio (INR) were measured. Screening assays for hepatitis C virus seropositivity (HCV ab) and HCV RNA and virus genotype were performed applying appropriate laboratory methods.
Blood samples for GH secretion after arginine, clonidine, or glucagon were drawn every 30 min from baseline to 180 min for glucose, cortisol, and GH determinations.
GH stimulation test was required if:
Height was below the 3rd percentile or 2 standard deviation (SD) below the mean height for age and sex
Height was within normal percentiles, but growth velocity (GV) was below the <10th percentile over 6–12 months
The patient was excessively short for his/her mid-parental height.
Adolescents with delayed or arrested puberty were evaluated for pituitary–testicular/ovarian axis integrity, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) before and after stimulation with gonadotropin-releasing hormone (Gn-RH stimulation test), prolactin, estradiol in females and for testosterone in males. Blood samples were assayed for FSH and LH before and 20, 40, 60, and 120 min after injection.[4]
Serum GH, FSH, LH, prolactin, estradiol, testosterone, FT4, TSH, PTH, IGF-1, and cortisol in the course of the follow-up were determined using commercially available automated immunoassays. The reported normal IGF-1 values set at the 2.5–97. 5th percentile were: 60.8–297.7 ng/ml for 40–59 years.[14]
Thirty-two TM patients underwent DEXA scan using HOLOGIC (Discovery QDR series) bone densitometer. BMD was evaluated at the lumbar spine or proximal femur.[13]
Assessment of iron overload
Iron overload was assessed by direct and indirect methods and was classified as mild (serum ferritin <1000 ng/ml), moderate (serum ferritin >1000 ng/ml and <2000 ng/ml), and severe (serum ferritin >2000 ng/ml) from three to four times annually.[15]
Serum ferritin was measured by immunoradiometric assay and electrochemiluminescence immunoassays. The manufacturer's normal reference ranges were 30–350 μg/l in males and 15–150 μg/l in females.[16]
Liver iron concentration (LIC) and cardiac T2* were assessed by magnetic resonance imaging (MRI) using a 1.5 T scanner (GE Signa/Excite HD, Milwaukee, WI, USA) in 22 TM patients. Liver T2* values were converted into MRI LIC values using the calibration curve. LIC values were expressed as mg/g dry weight (dw).[17,18,19] LIC (mg Fe/g dw) was classified as mild (LIC >3 and <7), moderate (LIC >7 and <14), and severe overload (LIC >14).[17,18,19]
Compliance to iron chelation therapy, defined as the ratio of actual to recommended chelation treatment, was periodically evaluated on the basis of self-reported questionnaires and patients interviews. It was classified as: Good (>90%), moderate (51–90%), poor (1–50%), or noncompliant (0%).[20]
Statistical analysis
Standard computer program SPSS for Windows, release 13.0 (SPSS Inc., Tulsa, IL, USA) was used for data entry and analysis. All numeric variables were expressed as mean ± SD. Chi-square test was used to compare the frequency of qualitative variables among the different groups. Pearson's and Spearman's correlation tests were used to study correlations between variables with parametric and nonparametric distributions, respectively. P < 0.05 was considered statistically significant.
RESULTS
Patients' characteristics
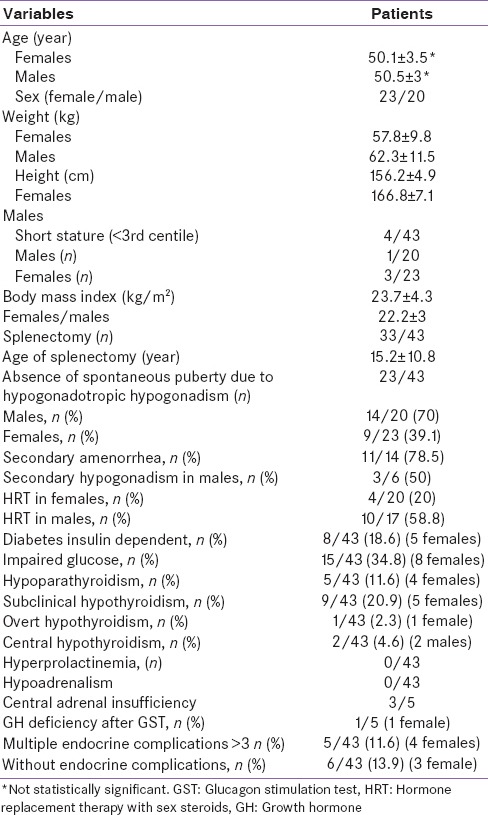
All TM patients were of Italian Ethnic origin. The baseline demographic, anthropometric, and clinical data were summarized in Table 1.
Table 1.
Demographic, clinical, and laboratory data of endocrinopathies in 43 adult thalassemia major patients
Transfusion management
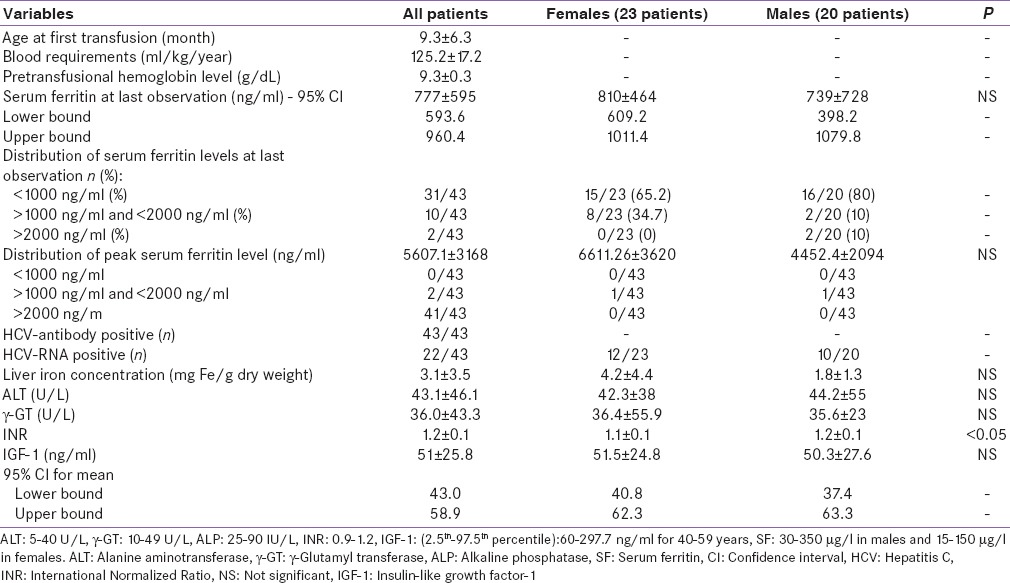
In the past years, the concentration of hemoglobin at which transfusions were given was gradually increased from 5.0–6.0 g/dl in the 1960's to 11.5–12.0 g/dl in the 1980's according to national protocols.[21] In the following years, patients were transfused every 2–3 weeks when the hemoglobin (Hb) level dropped to 9.0–9.5 g/dl. Patients were transfused at intervals of 15–20 days with white cell depleted, genotyped, and washed and packed red blood cells [Table 2]. The mean annual pretransfusional Hb level at the last observation was 9.3 ± 0.3 g/dl.
Table 2.
Hematological, laboratory, and imaging data in 43 adult thalassemia major patients
Splenectomy
Thirty-three patients (13 females) had undergone splenectomy because of increased transfusion requirements of packed red cells (>180–220 ml/kg/year) and/or for the presence of other signs of hypersplenism such as leukopenia, thrombocytopenia, and/or splenomegaly. Splenectomy was done at the mean age of 15.2 ± 10.8 years (range: 2.5–36.9 years).[21]
Chelation therapy
At last evaluation, 26 patients were on treatment with desferrioxamine mesylate (DFO), 10 on oral chelator deferiprone (DFP), 5 on oral chelator deferasirox (DFX), and 2 patients on DFO plus DFP.
Treatment with intramuscular DFO at a dose of 20 mg/kg body weight (BW) was available for most patients since 1969. Regular subcutaneous (SC) DFO infusion was started in 1978 in patients older than 2 years. Initially, the recommended DFO dose was 20 mg/kg BW administered daily at night, by infusion pump over 8–10 h. Based on transfusional iron input, the dose increased to 40 mg/kg BW in 1982 and up to 60 mg/kg/BW in 1984. Ascorbic acid was added orally at a dose of 2–5 mg/kg (maximum dose 200 mg) in a selected group of patients. Since 1995, oral chelator DFP has been available; it was given at a dose of 75 mg/kg BW to a number of patients over the age of 11 years. In the following years, combined therapy with daily DFP and SC DFO for 3–6 days/week was given to patients with severe iron overload and high iron input. In 2007, the new oral chelating agent DFX was introduced at a dose of 25–30 mg/kg BW for patients in whom treatment with DFO was contraindicated or inadequate.[21]
Iron overload
The mean serum ferritin level at last observation was 777.1 ± 595.3 ng/ml [Table 2]. Iron overload was mild (serum ferritin: <1000 ng/ml) in 31 (72%) patients (15 females: 513.2 ± 178.4 ng/ml and 16 males: 444.5 ± 159.4 ng/ml); moderate (serum ferritin: >1000 ng/ml < 2000 ng/ml) in 10 (24%) (8 females; 1367.5 ± 268 ng/ml and 2 males: 1230 and 1033 ng/ml, respectively); and severe (serum ferritin >2000 ng/ml) in only 2 (4%) patients (1 female: 3070 ng/ml and 1 male: 2335 ng/ml, respectively).
The peak annual mean serum ferritin levels (the highest value registered for each patient) in the 43 TM patients enrolled in this retrospective study were 5.607 ± 3.168 as reported in Table 2. Compliance to chelation therapy was classified as good in 30/43, moderate in 9/43, and poor in 4/43 TM patients.
LIC was assessed in 25/43 TM patients. By SQUID, LIC >7 and <14 was detected in 1 female patient and a value ≥14 mg/g dw in another. Both were on treatment with DFP.
Liver enzymes and hepatitis C virus infection
In our unit, the HCV screening started in 1991. All patients tested after 1990's were HCV seropositive. HCV-RNA positivity was present in 22/43 patients (51.1%). Three different HCV genotypes, 1b (61.1%), 2a (22.2%), and 3a (16.6%), were identified. Eight female and 4 male TM patients had high ALT (>40 U/L); 6 females and 2 male TM patients had a serum γGT concentration above 49 U/L (normal values 10–49 U/L) [Table 2].
Other correlations
A significant indirect correlation was observed between total protein, albumin (range: −0.388; P = 0.010), and INR (range: −0.441; P = 0.004). All other correlations between variables reported in Tables 1 and 2 were negative.
Growth and endocrine complications during childhood, adolescent, and adulthood
Five TM patients (4 males and 1 female) with short stature and GHD aged 10–14 years (mean age: 11.4 ± 1.5 years), with a mean annual serum ferritin at diagnosis 2.400 ± 1.294 ng/ml (range: 1.980–4.100 ng/ml) were treated with conventional doses of GH. After the first 12 months of GH treatment, an increase of GV was observed in 2 patients (4 cm above the previous year), the remaining patients had a partial response (2 cm above the previous year) suggesting some degree of GH resistance. Patients with GHD were not retested after transition because of parents' decision.
Nine patients (5 females) presented with subclinical hypothyroidism (normal FT4 and TSH >8 mUI/ml) at a mean age of 14.3 ± 1.8 years (range: 14.2–16 years) (serum ferritin at diagnosis 5783 ± 3625 ng/ml; range: 2.250–14.130 ng/ml). One female patient and two male patients developed overt hypothyroidism and central hypothyroidism at the age of 13 (serum ferritin at diagnosis 3.700 ng/ml), 21 and 35 years (serum ferritin at diagnosis 2320 and 1.825 ng/ml, respectively).
Eight patients developed insulin-dependent diabetes mellitus at a mean age of 17.3 ± 3.1 years range 13 ± 20.5 years, with serum ferritin at diagnosis 3.648 ± 2.398 ng/ml and range 1.270–8.200 ng/ml. Five out of 8 TM patients with diabetes were HCV-RNA negative.
In 15/43 patients (8 females), a diagnosis of impaired glucose tolerance, after oral glucose tolerance test (OGTT), was made at a mean age of 16.6 ± 1.6 years (mean serum ferritin level at the diagnosis 3.160 ± 1.119 ng/ml; range: 1.447–4.960 ng/ml). Six out of 15 patients were HCV-RNA negative. None presented a significant deterioration or improvement of glucose OGTT during 12 years of observation. Due to due poor patients' acceptance, the OGTT was replaced in the following years by fasting glucose or 2 h postprandial blood sugar. The same approach was followed in the majority of the remaining patients who presented a normal OGTT in the first two decades of life.
Five patients had hypoparathyroidism at a mean age of 20.8 ± 6.5 years (range: 15–32 years) (serum ferritin at diagnosis 6451 ± 5893 ng/ml; range: 1.270–15.200 ng/ml).
Twenty-three adolescents with delayed or arrested puberty (14 males and 9 females) were evaluated for pituitary–gonadal axis integrity. The serum ferritin at diagnosis of hypogonadotropic hypogonadism was 4.569 ± 2.000 and 5.101 ± 2.109 ng/ml, respectively (range in males: 3.000–8.200 ng/ml; in females: 3.150–10.560 ng/ml). In these patients, pubertal development was induced with depot testosterone in males and estrogens/progesterone in females.
Secondary amenorrhea (serum ferritin at diagnosis 2.715 ± 1.183 ng/ml; range 840–4.025 ng/ml) was registered in 14 patients, from 6 months to 17.5 years (mean 6.2 ± 5.2 years) after menarche. All patients received hormone replacement therapy with sex steroids. Hypocortisolism (basal cortisol 3.5 μg/dl = 98 nmol/L or less) was not reported in our patients.
In adult TM patients, short stature was present in 5 patients (3 females). The maximum height achieved by a male subject being 178 cm and for the females 168 cm.
A BMI between 25 and 30 kg/m2 was documented in 4 patients (3 females), and a value above 30 kg/m2 was registered in 2 patients (1 female).
IGF-1 levels were below 2 SD in 31/43 adult TM patients (6 females) compared to the percentiles of healthy subjects.[14] The IGF-1 level was very low (6.2 ng/ml) in a male patient with liver cirrhosis.
A reassessment of the GH-IGF-1 axis was performed in 3/5 adult patients with childhood diagnosis of GHD. A persisting GHD, after glucagon test, was documented in one female patient (GH peak = 1.3 ng/ml), whereas the others had a normal response. In these patients, cortisol response after glucagon test was below 250 nmol/l (9 μg/dl) in 3 patients indicating central adrenal insufficiency (CAI).[22,23]
Three patients developed acquired hypogonadotropic hypogonadism as adults. The cumulative incidence of endocrine disorders was in total 86.9% in females and 73.9% in males.
Spine and femur BMD Z-scores <−2 occurred in 58% and 42% of participants, respectively. Twenty-one subjects (12 females) married. Eight pregnancies occurred; in 4/8 patients, spermatogenesis or ovulation was induced with gonadotrophins.
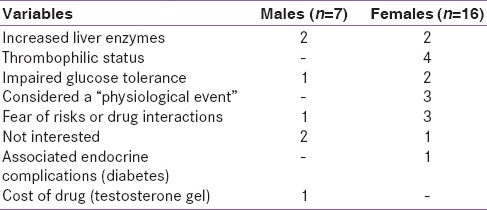
At the last observation, 4/20 hypogonadal female patients (20%) and 10/17 hypogonadal male patients (58.8%) were on treatment with sex steroids or with HCG in males. In the remaining patients, treatment was not recommended or accepted for several reasons [Table 3]. All patients with primary or central hypothyroidism were receiving levothyroxine, those with hypoparathyroidism, calcium, and calcitriol and with diabetes insulin. None of the diabetic patients was on antihypertensive medication or lipid-lowering agents (statins or fibrates). Oral bisphosphonates were given in the course of the years to a limited number of patients, mainly females. At the last observation, only one male patient was taking oral bisphosphonates regularly for the last 12 years. During treatment, a spontaneous rib fracture occurred.
Table 3.
Doctor position and patients opinion for not prescribing or accepting hormone replacement therapy
DISCUSSION
This cross-sectional, analytic study was carried out on a unique cohort of 43 TM patients aged 45–60 years. To the best of our knowledge, this is the first study, reported in the literature, analyzing the prevalence of endocrine complications in a cohort of patients composed entirely of long-lived patients with TM.
The data indicate that 88.4% of adult TM patients of this advanced age group suffered from at least one endocrine complication. The majority of patients developed endocrine complications in the second decade of life when serum ferritin level was very high (12/23 TM female and 8/20 TM male patients the serum ferritin levels at the diagnosis were above 5,000 ng/ml). This finding is alarming, especially for a population that in many countries still does not receive adequate follow-up and efficient treatment. The very high peak level of serum ferritin registered in our patients was probably due to inadequate doses of DFO in the 1st years of life, combined with poor compliance to treatment during the peripubertal or pubertal age and increased amount of transfused blood not balanced by increased chelating treatment. In the 1980s, the transfusion regimen modified, according to the suggestion of Propper et al. proposing transfusion at hemoglobin concentrations of about 11.5–12.0 g/dl,[24] which could worsen the iron overload.
The most common endocrine complication documented was hypogonadotropic hypogonadism. The anterior pituitary is particularly sensitive to iron associated free radical oxidative stress.[5] Even a modest amount of iron deposition in the anterior pituitary by demonstrated by MRI can interfere with its function.[25]
Measurement of peak serum LH following a bolus of Gn-RH may be useful in the evaluation of pituitary reserve.[25] In one study, 72% of patients with absent or very mild pituitary iron loading had a normal increase of LH, whereas only 5% of those with moderate or severe pituitary iron loading had a normal response.[25] Gonadal function generally is less affected, and fertility can be salvaged.[25,26,27,28,29,30]
Several interesting aspects emerged from our survey. First, an higher mean serum ferritin peak in TM girls (6611.26 ± 3620.8 ng/ml) with hypogonadism compared to males (4452.4 ± 2094.6 ng/ml), Although the difference was not statistically significant, 12/23 TM patients had a peak serum ferritin level above 5000 ng/ml versus 7/20 in male patients (P > 0.05). The findings need to be replicated in larger studies and across other ethnic groups.
Second, although serum ferritin level fell (from 1978 to 2015) from a mean value of 5607.1 ± 3168.4–777.1 ± 595.3 ng/ml in the course of treatment with DFO given SC, secondary amenorrhea was registered in 10 patients (on average 6 years after menarche), two adult patients developed central hypothyroidism, three patients secondary hypogonadotropic hypogonadism, and in 3/5 adult patients presented a CAI after GST. These results indicate that even those patients who comply with long-term iron chelation therapy with DFO may develop hypopituitarism later in life. Such a high prevalence of hypopituitarism may reflect a progressive failure of pituitary function or a low rate of iron accumulation delaying the age of appearance of toxic ferritin levels. This is confirmed by the low ferritin levels of secondary hypogonadism at an advanced age observed in this study compared to primary. Noetzli et al.[31] have reported that patients with transfusional iron overload begin to develop pituitary iron overload in the first decade of life; however, clinically significant volume loss is observed after the second decade of life.
Third, a considerable number of TM patients (51%) were HCV RNA positive and 100% for HVC antibodies. It is likely that the high prevalence of HCV among multitransfused TM patients is due to blood transfusions dating from before the implementation of HCV screening in 1991 as the risk of HCV-associated transfusions has significantly reduced and recently with molecular screening disappeared.
Fourth, 5 out of 8 TM patients with diabetes and 6/15 patients with impaired glucose tolerance were HCV RNA negative. Although iron overload and chronic liver disease and viral infections may play an important role in the development of glucose intolerance, it seems to be not relevant in our patients. Ruhl et al.[32] revisit the association between HCV infection and diabetes in 15,128 adults. The overall prevalence of anti-HCV (+) was 1.7%, of HCV-RNA (+) 1.1%, of diabetes 10.5%, and of prediabetes 32.8%. In multivariate analysis including all relevant variables, the researchers clearly demonstrate that prevalence of prediabetes or diabetes was not associated with HCV infection status, but rather with increased plasma ALT and γGT concentration, regardless of HCV infection status. Although an “amicable divorce” is in the air about the effects of HCV infection and diabetes,[33] Al-Naamani et al. reported that anti-HCV (+) TM patients were more likely to be diabetic than anti-HCV (−) (27% vs. 8%; P < 0.001).[34] Furthermore, Mowla et al.[35] studied 98 TM patients. Forty-six (47%) patients were seropositive for HCV. The prevalence of diabetes in HCV (+) adult TM patients was higher compared to HCV (−) patients (15.2% vs. 1.9%, P = 0.02). Therefore, it is probable that the existence of hemosiderosis makes the effect of HCV infection on glucose metabolism clinically more evident.[34]
In summary, we believe that these findings need to be replicated in larger studies and across other ethnic groups. A better knowledge of the pathogenic mechanisms involved in diabetes associated with HCV infection will enable us not only to further identify those patients at high risk of developing diabetes but also to select the best therapeutic option.[35,36]
Fifth, it has been recommended that early recognition and treatment of endocrine complications is important to prevent late irreversible sequelae. Nevertheless, only a few patients had been treated for osteoporosis, and a large percentage of them did not receive hormone replacement therapy with sex steroids. Some patients perceived medications as not necessary if they were otherwise healthy or were worried about the side effects of treatment or preferred a “natural” treatment or felt that calcium and Vitamin D supplementation were sufficient to preserve bone health [Table 3].
Finally, it is well-known that appropriate iron chelation therapy can improve growth and development. Nevertheless, only 5/43 had a short stature (<3rd centile) although they started late in life iron chelation therapy with DFO and had a high peak of serum ferritin level. These findings emphasize the importance to perform further studies in different groups of patients who started chelation therapy at different ages and with different iron chelating agents.
Strength and limitations
Our retrospective study was conducted in a tertiary care thalassemia clinic involving an adequate number of patients, living in Northeast or Southern of Italy, with a severe form of β-thalassemia followed by the same physician (endocrinologist) for several years. Furthermore, to the best of our knowledge, this is the first time that the validity of the peak annual serum ferritin levels and their relation to the pathogenesis and the diagnosis of endocrine complications in thalassemia are analyzed and reported.
The limitations of the study are the number of patients who had adrenal and GH assessments, and the lack of full information about the protocols used in thalassemia center for the treatment of hepatitis C infection and osteoporosis. However, these limitations are unlikely to have had an important effect on the validity of our findings on endocrine glands disturbances in long-lived patients with TM.
CONCLUSIONS
Our data underline that endocrine and bone complications in adult TM patients are highly prevalent and necessitate close monitoring, treatment, and follow-up by specialists. The triad of chronic iron overload, poor compliance to chelation therapy, and chronic liver disease characterizes TM and is mostly responsible for its clinical sequelae which are gradually ameliorated thanks to advances in the implementation of efficient chelation schedules. Physicians' strategies to optimize chelation therapy include identification of patients who are at risk to develop organ damage, formulation of optimum individual chelation plans, promotion of compliance, and education of patients. Therefore, physician–patient interaction including feedback of treatment effects remain the key of treatment for patients with chronic illnesses. Several clinical aspects remain to be elucidated such as growth and impairment of glucose tolerance in relation to HCV infection. Furthermore, affordable worldwide-established long-term treatment protocols for hypogonadism and osteoporosis are still needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.De Sanctis V, Soliman AT. ICET-A: An opportunity for improving thalassemia management. J Blood Disord. 2014;1:1–2. [Google Scholar]
- 2.Cacciari E, Milani S, Balsamo A, Dammacco F, De Luca F, Chiarelli F, et al. Italian cross-sectional growth charts for height, weight and BMI (6-20 y) Eur J Clin Nutr. 2002;56:171–80. doi: 10.1038/sj.ejcn.1601314. [DOI] [PubMed] [Google Scholar]
- 3.Zoppi G, Bressan F, Luciano A. Height and weight reference charts for children aged 2-18 years from Verona, Italy. Eur J Clin Nutr. 1996;50:462–8. [PubMed] [Google Scholar]
- 4.De Sanctis V, Soliman AT, Elsedfy H, Skordis N, Kattamis C, Angastiniotis M, et al. Growth and endocrine disorders in thalassemia: The international network on endocrine complications in thalassemia (I-CET) position statement and guidelines. Indian J Endocrinol Metab. 2013;17:8–18. doi: 10.4103/2230-8210.107808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohmann T, Roche A, Martorell R, editors. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. pp. 1–177. [Google Scholar]
- 6.De Sanctis V, Skordis N, Galati MC, Raiola G, Giovannini M, Candini G, et al. Growth hormone and adrenal response to intramuscular glucagon test and its relationship to IGF-1 production and left ventricular ejection fraction in adult B-thalassemia major patients. Pediatr Endocrinol Rev. 2011;8(Suppl 2):290–4. [PubMed] [Google Scholar]
- 7.De Sanctis V, Soliman A, Candini G, Campisi S, Anastasi S, Iassin M. High prevalence of central hypothyroidism in adult patients with ß-thalassemia major. Georgian Med News. 2013;(222):88–94. [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes – 2015. Diabetes Care. 2015;38(Suppl 1):S8–16. [PubMed] [Google Scholar]
- 9.Salenave S, Trabado S, Maione L, Brailly-Tabard S, Young J. Male acquired hypogonadotropic hypogonadism: Diagnosis and treatment. Ann Endocrinol (Paris) 2012;73:141–6. doi: 10.1016/j.ando.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 10.De Sanctis V, Vullo C, Bagni B, Chiccoli L. Hypoparathyroidism in beta-thalassemia major. Clinical and laboratory observations in 24 patients. Acta Haematol. 1992;88:105–8. doi: 10.1159/000204662. [DOI] [PubMed] [Google Scholar]
- 11.De Sanctis V, Elsedfy H, Soliman AT, Elhakim IZ, Pepe A, Kattamis C, et al. Acquired hypogonadotropic hypogonadism (AHH) in thalassaemia major patients: An underdiagnosed condition? Mediterr J Hematol Infect Dis. 2016;8:e2016001. doi: 10.4084/MJHID.2016.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewiecki EM, Watts NB, McClung MR, Petak SM, Bachrach LK, Shepherd JA, et al. Official positions of the international society for clinical densitometry. J Clin Endocrinol Metab. 2004;89:3651–5. doi: 10.1210/jc.2004-0124. [DOI] [PubMed] [Google Scholar]
- 13.Writing Group for the ISCD Position Development Conference. Indications and reporting for dual-energy x-ray absorptiometry. J Clin Densitom. 2004;7:37–44. doi: 10.1385/jcd:7:1:37. [DOI] [PubMed] [Google Scholar]
- 14.Aimaretti G, Boschetti M, Corneli G, Gasco V, Valle D, Borsotti M, et al. Normal age-dependent values of serum insulin growth factor-I: Results from a healthy Italian population. J Endocrinol Invest. 2008;31:445–9. doi: 10.1007/BF03346389. [DOI] [PubMed] [Google Scholar]
- 15.Casale M, Meloni A, Filosa A, Cuccia L, Caruso V, Palazzi G, et al. Multiparametric cardiac magnetic resonance survey in children with thalassemia major: A multicenter study. Circ Cardiovasc Imaging. 2015;8:e003230. doi: 10.1161/CIRCIMAGING.115.003230. [DOI] [PubMed] [Google Scholar]
- 16.Addison GM, Beamish MR, Hales CN, Hodgkins M, Jacobs A, Llewellin P. An immunoradiometric assay for ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. J Clin Pathol. 1972;25:326–9. doi: 10.1136/jcp.25.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casale M, Meloni A, Filosa A, Cuccia L, Caruso V, Palazzi G, et al. Multiparametric cardiac magnetic resonance survey in children with thalassemia major: A multicenter study. Circ Cardiovasc Imaging. 2015;8:e003230. doi: 10.1161/CIRCIMAGING.115.003230. [DOI] [PubMed] [Google Scholar]
- 18.Positano V, Pepe A, Santarelli MF, Scattini B, De Marchi D, Ramazzotti A, et al. Standardized T2* map of normal human heart in vivo to correct T2* segmental artefacts. NMR Biomed. 2007;20:578–90. doi: 10.1002/nbm.1121. [DOI] [PubMed] [Google Scholar]
- 19.Maggio A, Capra M, Pepe A, Mancuso L, Cracolici E, Vitabile S, et al. A critical review of non invasive procedures for the evaluation of body iron burden in thalassemia major patients. Pediatr Endocrinol Rev. 2008;6(Suppl 1):193–203. [PubMed] [Google Scholar]
- 20.Lee WS, Toh TH, Chai PF, Soo TL. Self-reported level of and factors influencing the compliance to desferrioxamine therapy in multitransfused thalassaemias. J Paediatr Child Health. 2011;47:535–40. doi: 10.1111/j.1440-1754.2011.02017.x. [DOI] [PubMed] [Google Scholar]
- 21.Cao A, Gabutti W, Masera G, Vullo C. Protocollo per la terapia della β thalassaemia. Prospect Pediatr. 1981;43:261–71. [Google Scholar]
- 22.Berg C, Meinel T, Lahner H, Yuece A, Mann K, Petersenn S. Diagnostic utility of the glucagon stimulation test in comparison to the insulin tolerance test in patients following pituitary surgery. Eur J Endocrinol. 2010;162:477–82. doi: 10.1530/EJE-09-0824. [DOI] [PubMed] [Google Scholar]
- 23.Hamrahian AH, Yuen KC, Gordon MB, Pulaski-Liebert KJ, Bena J, Biller BM. Revised GH and cortisol cut-points for the glucagon stimulation test in the evaluation of GH and hypothalamic-pituitary-adrenal axes in adults: Results from a prospective randomized multicenter study. Pituitary. 2016 doi: 10.1007/s11102-016-0712-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Propper RD, Button LN, Nathan DG. New approaches to the transfusion management of thalassemia. Blood. 1980;55:55–60. [PubMed] [Google Scholar]
- 25.Berkovitch M, Bistritzer T, Milone SD, Perlman K, Kucharczyk W, Olivieri NF. Iron deposition in the anterior pituitary in homozygous beta-thalassemia: MRI evaluation and correlation with gonadal function. J Pediatr Endocrinol Metab. 2000;13:179–84. doi: 10.1515/jpem.2000.13.2.179. [DOI] [PubMed] [Google Scholar]
- 26.Bronspiegel-Weintrob N, Olivieri NF, Tyler B, Andrews DF, Freedman MH, Holland FJ. Effect of age at the start of iron chelation therapy on gonadal function in beta-thalassemia major. N Engl J Med. 1990;323:713–9. doi: 10.1056/NEJM199009133231104. [DOI] [PubMed] [Google Scholar]
- 27.De Sanctis V, Vullo C, Katz M, Wonke B, Nannetti C, Bagni B. Induction of spermatogenesis in thalassaemia. Fertil Steril. 1988;50:969–75. doi: 10.1016/s0015-0282(16)60382-5. [DOI] [PubMed] [Google Scholar]
- 28.De Sanctis V, Vullo C, Katz M, Wonke B, Tanas R, Bagni B. Gonadal function in patients with beta thalassaemia major. J Clin Pathol. 1988;41:133–7. doi: 10.1136/jcp.41.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Sanctis V, Vullo C, Negri P, Codemi L, Mollica G, Bagni B. Induction of ovulation in a thalassaemic patient. Acta Eur Fertil. 1989;20:223–5. [PubMed] [Google Scholar]
- 30.Seracchioli R, Porcu E, Colombi C, Ciotti P, Fabbri R, De Sanctis V, et al. Transfusion-dependent homozygous beta-thalassaemia major: Successful twin pregnancy following in-vitro fertilization and tubal embryo transfer. Hum Reprod. 1994;9:1964–5. doi: 10.1093/oxfordjournals.humrep.a138368. [DOI] [PubMed] [Google Scholar]
- 31.Noetzli LJ, Panigrahy A, Mittelman SD, Hyderi A, Dongelyan A, Coates TD, et al. Pituitary iron and volume predict hypogonadism in transfusional iron overload. Am J Hematol. 2012;87:167–71. doi: 10.1002/ajh.22247. [DOI] [PubMed] [Google Scholar]
- 32.Ruhl CE, Menke A, Cowie CC, Everhart JE. Relationship of hepatitis C virus infection with diabetes in the U.S. population. Hepatology. 2014;60:1139–49. doi: 10.1002/hep.27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cusi K. The relationship between hepatitis C virus infection and diabetes: Time for a divorce? Hepatology. 2014;60:1121–3. doi: 10.1002/hep.27252. [DOI] [PubMed] [Google Scholar]
- 34.Al-Naamani K, Al-Zakwani I, Al-Sinani S, Wasim F, Daar S. Prevalence of hepatitis C among multi-transfused thalassaemic patients in Oman: Single centre experience. Sultan Qaboos Univ Med J. 2015;15:e46–51. [PMC free article] [PubMed] [Google Scholar]
- 35.Mowla A, Karimi M, Afrasiabi A, De Sanctis V. Prevalence of diabetes mellitus and impaired glucose tolerance in beta-thalassemia patients with and without hepatitis C virus infection. Pediatr Endocrinol Rev. 2004;2(Suppl 2):282–4. [PubMed] [Google Scholar]
- 36.De Sanctis V, Soliman AT, Candini G, Elsedfy H. Hepatitis C virus infection in thalassemic patients with and without insulin dependent diabetes. Indian J Endocrinol Metab. 2015;19:303–4. doi: 10.4103/2230-8210.149327. [DOI] [PMC free article] [PubMed] [Google Scholar]


