Abstract
Context:
The association between depression and thyroid function is well known. Both conditions express many similar symptoms, thus making the diagnosis and treatment difficult.
Aims:
To find the prevalence of anxiety and depressive symptoms among patients with hypothyroid.
Settings and Design:
Cross-sectional study.
Materials and Methodology:
A total of 100 patients diagnosed as hypothyroidism were evaluated using Hamilton depression rating scale (HDRS) and Hamilton scale for anxiety (HAM-A).
Statistical Analysis Used:
The data were analyzed using the SPSS for Windows version 17.0 software. The quantitative data were expressed in number and percentage. The results obtained were compared using the Chi-square test.
Results:
Females constituted 70% of the sample. A total of 60% reported some degree of depression based on HDRS (males – 56.63% and females – 64.29%) whereas about 63% out of the total patients screened showed some degree of anxiety (males –56.66% and females – 65.72%) based on HAM-A. The most common depressive symptom among the males was depressed mood (73.33%) and among females was gastrointestinal somatic symptoms (68.54%). The most common anxiety symptom among the males was depressed mood (70.0%) and among females was anxious mood (92.85%).
Conclusions:
Psychiatric symptoms/disorders are common in patients with thyroid dysfunction.
Keywords: Anxiety, depression, hypothyroid, psychiatry symptoms
INTRODUCTION
Thyroid hormone (TH) has important actions in the adult brain. Varying degree of psychiatric symptoms/disorders is common in patients with thyroid dysfunction both hypo- and hyper-thyroidism.
Thyroid dysfunctions have been recognized to cause significant manifestations in mental health. They may lead to disturbances in emotions and cognition. Both increase and decrease in thyroid function can cause mood abnormalities. Vice versa, depression can also go hand in hand with subtle thyroid dysfunctions.[1]
Before prescribing any antidepressant, almost all doctors must go for the thyroid function tests. Even mild cases of hypothyroid state can cause depression.[2,3]
One of the most common mistakes which happen is that the concerned doctors fail to look for any psychiatric comorbidity in such patients. Many studies have revealed that there are significantly deranged levels of T3, T4, and thyroid-stimulating hormone (TSH) in patients of depression.[4,5] Mood and anxiety disorders have higher prevalence in patients with thyroid dysfunction.[6] Another study showed a prevalence of depression in 20.5% of the patients of hypothyroidism.[7]
Psychiatric manifestations usually appear first in cases of hypothyroidism. They form about 2–12% of the initial symptoms in about all of the reported cases. Initially, it starts with anxiety, memory lapses, progressive mental slowing, and speech deficits.[8,9]
In cases of acute hypothyroidism, anxiety disorders occur in about 30–40% of patients.[10,11] Patients with subclinical hypothyroidism (SCH) can present with anxiety, irritability, poor concentration, slow information processing, and poor learning in comparison to normal subjects.[12,13]
Hypothyroidism impacts certain aspects of cognitive functioning (slowed information processing speed, reduced efficiency in executive functions, and poor learning) and mood. More severe hypothyroidism can mimic melancholic depression and dementia, thus causing reduction in health-related quality of life. Although the results are inconsistent, data do suggest that the neuropsychiatric symptoms tend to improve with treatment for both overt hypothyroidism and SCH and normalization to a euthyroid state.[14]
Assessment of the thyroid function tests can be a good predictor in the treatment of depression and bipolar disorders.[15] THs can be used as augmentation agents and are therapeutically efficient in treatment-resistant depression.[16]
TH has an effect on central nervous system (CNS) through the entire life. It regulates gene expression in myelination, differentiation of neuronal and glial cells, and neuronal viability and function. THs play a role in normal neurological development. It stimulates the development of neuronal processes, axons, and dendrites, increases the rate of neuronal proliferation, end it by acting as a time clock. THs effect on serotonin (5-hydroxytryptamine [5-HT]) is explained by desensitization of 5-HT1A autoreceptor at the site of raphe nuclei which probably results in an increase in the release of serotonin from raphe neurons.[17,18,19]
Mechanism of TH activity in the brain is unclear due to the complexity of neurotransmission interaction with thyroid. One hypothesis stated that TH modulates postsynaptic beta-adrenergic receptors in cerebral cortex and cerebellum while others stated that the modulation of 5-HT and its receptors is responsible, which happens due to inhibition caused by the TH at raphe, causing reduction in 5-HT levels.[20,21]
TH affects and governs the CNS both during the development phase and also the entire life. Both the hyper- and hypo-thyroid states have comorbidity with psychiatric conditions. Similarly, patients of mood disorders also show derangement in TSH and thyrotropin releasing hormone whereas T3 and T4 may remain normal.[17,18,22,23]
Multiple data suggest that hypothalamic-pituitary-thyroid (HPT) axis is involved in the pathogenesis of depression. Most of the studies show changes in different hormones at HPT axis, with a few studies showing normal TH range during depressive episode. Data are contradictory regarding the levels of TSH values with some suggesting an increase while others suggesting a decrease.[5,24]
In adult age group, it can cause behavioral disturbances, depressive symptoms, anxiety, learning deficits, memory impairment, and problems in verbal fluency. These can be attributed to neurotransmission impairment in the brain related to learning and memory, such as hippocampus. Thus, decrease in CNS-TH levels can promote alteration in neurotransmission leading to mood disorders such as depression.[25]
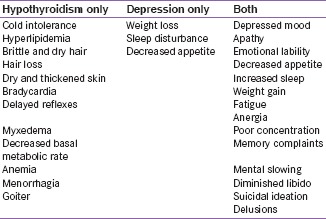
Signs and symptoms common to both depression and hypothyroidism are given below.[26]
Several scales are available to assess depression and anxiety; a few are mentioned here.
-
For depression
- The Hamilton depression rating scale (HDRS/HAM-D): 17 items; 8 items are scored on a 5-point scale ranging from 0 to 4 and 9 items are scored from 0 to 2; clinician-administered
- Beck depression inventory: 21 items; graded ranging 0–4; patient rated
- Clinically useful depression outcome scale: 18 items; rated on a 5-point Likert scale; patient rated
- Patient health questionnaire-9: 9 items; rated on 4-point Likert scale; patient rated
- Quick inventory of depressive symptomatology: 16 items; clinician-rated and self-report versions; total scores on the scale range from 0 to 27.
-
For anxiety
- The Hamilton anxiety rating scale (HAM-A) is a clinician-rated evaluation. The scale consists of 14 items rated on a scale of 0–4
- The Beck anxiety inventory is a 21-question multiple-choice self-report inventory graded from 0 to 3 points
- The Zung self-rating anxiety scale is a 20-item self-report assessment device and is scored on a Likert-type scale of 1–4
- Generalized anxiety disorder 7 is a self-reported questionnaire having seven items and is rated from 0 to 3.
Hence, this study was planned with an aim to find the prevalence of anxiety and depressive symptoms among patients with hypothyroid.
MATERIALS AND METHODOLOGY
A cross-sectional study was conducted at Maharishi Markandeshwar Institute of Medical Sciences and Research, Ambala. Institutional Ethics Committee approval was obtained before the study. The study was conducted for 3 months. After obtaining written informed consent, a total of 100 patients, those fulfilled the inclusion and exclusion criteria, were recruited for the study. All patients were referred from medicine department.
Inclusion criteria
Patients who gave written informed consent
Patients of either sex between the age of 18 and 45 years
Patients with known case of hypothyroidism.
Exclusion criteria
Patients with a history of depressive disorder or anxiety disorder before being diagnosed as hypothyroid
Patients with a history of substance dependence, psychotic disorders, or any other major axis I psychiatric disorders, and epilepsy
Patients with comorbid chronic medical illness except hypothyroidism
Patients with organic disorders such as dementia and delirium
Patients with cognitive impairment
Patients below primary education.
Methodology
All patients were briefed regarding the objectives of the study. Written consent was obtained from the patients. The patients were assessed using the following tools:
Sociodemographic information of the patients was obtained using a semi-structured proforma specially designed for the study
HDRS for assessment of depression [27]
The HDRS/HAM-D is the most widely used multiple-item questionnaires to assess the severity of, and change in, depressive symptoms. It is considered the “gold standard” for rating depression in clinical research. It contains 17 items (HDRS-17) pertaining to symptoms of depression experienced over the past week. It is a clinician-administered depression assessment scale; time required for administration is 20–30 min. The questionnaire is designed for adults and is used to rate the severity of their depression by probing mood, feelings of guilt, suicide ideation, insomnia, agitation or retardation, anxiety, weight loss, and somatic symptoms. Each item on the questionnaire is scored on a 3- or 5-point scale depending on the item. This scale should not be used as a diagnostic instrument
-
HAM-A for assessment of anxiety [28]
The HAM-A was one of the first rating scales developed with a main purpose to assess the severity of symptoms of anxiety in adults, adolescents, and children. The scale consists of 14 items, each defined by a series of symptoms, and measures both psychic anxiety (mental agitation and psychological distress) and somatic anxiety (physical complaints related to anxiety). It is a clinician-rated scale; time required for administration is 10–15 min. The scale consists of 14 items designed to assess the severity of a patient's anxiety. Each of the 14 items contains a number of symptoms, and each group of symptoms is rated on a scale of 0–4, with four being the most severe. All of these scores are used to compute an overarching score that indicates a person's anxiety severity.
Both the tools were used in English language.
Statistical analysis
The data was analyzed using the SPSS version 17.0 software. The quantitative data were expressed in number and percentage. The results obtained were compared using the Chi-square test. P < 0.05 was considered statistically significant. For the sociodemographic variables, percentage and P value were calculated. For identifying levels of depression, HDRS was graded as normal (<6), mild (7–17), moderate (18–24), and severe (>24). For identifying levels of anxiety, HAM-A was graded as normal (<6), mild (7–17), moderate (18–24), and severe (>24).
RESULTS
Table 1 shows the sociodemographic data of the sample. A total of 70% females constituted the study sample. Majority of the patients were within the age range of 26–35 years (51%, P = 0.498); 71% were married (P = 0.736); 78% were educated above the level of matric (P = 0.168); 58% stayed in joint family (P = 0.218), and 58% hailed from rural areas (P = 0.250). None of the sociodemographic variable was statistically significant.
Table 1.
Sociodemographic data of the sample
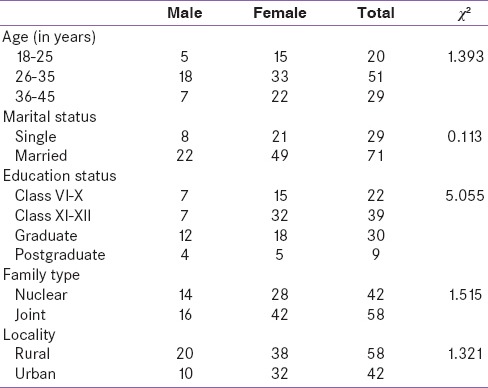
Table 2 shows the grading of HDRS; a total of 60% of patients had some degree of depression. When graded down, 37% had mild depression (male: n = 8; female: n = 29); 14% moderate depression (male: n = 4; female: n = 10); and 9% severe depression (male: n = 3; female: n = 6). The HDRS scores were statistically not significant when compared for both males and females (P = 0.499).
Table 2.
Grading of Hamilton depression rating scale
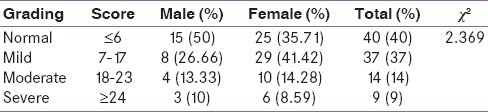
Table 3 shows the grading of HAM-A; a total of 73% patients had some degree of anxiety. When graded down, 29% had mild anxiety (male: n = 7; female: n = 22); 14% moderate anxiety (male: n = 6; female: n = 13); and 9% severe anxiety (male: n = 4; female: n = 11). The HAM-A scores were statistically not significant when compared for both males and females (P = 0.791).
Table 3.
Grading of Hamilton anxiety rating scale
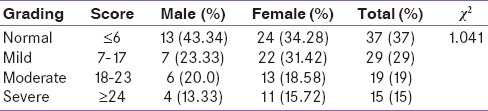
Table 4 shows the symptoms on HDRS and its distribution among the group; the most common symptoms among the males were depressed mood (73.33%), anxiety and genital symptoms (66.67%), insomnia (43.33%), and general somatic symptoms (33.33%) whereas in females, the most common symptoms were gastrointestinal somatic symptoms (68.54%), hypochondriasis (64.28%), depressed mood (60%), anxiety and general somatic symptoms (57.14%), insomnia (47.14%), and suicide (37.14%).
Table 4.
Distribution of Hamilton depression rating scale symptoms and their comparison between males and females
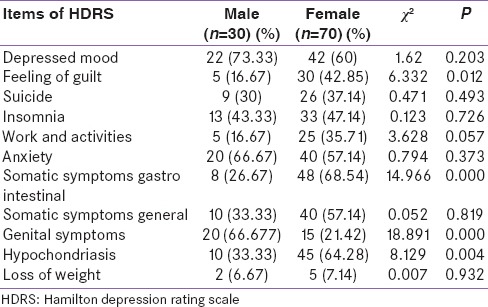
Symptoms such as – feeling of guilt (P = 0.012), gastrointestinal somatic symptoms (P = 0.000), genital symptoms (P = 0.000), and hypochondriasis (P = 0.004) – were statistically significant on HDRS when compared for males and females.
Table 5 shows the symptoms on HAM-A and its distribution among the group; the most common symptoms among the group in males were depressed mood (70.0%), genitourinary symptoms (63.33%), insomnia (43.33%), anxious mood (40%), and tension, fears, gastrointestinal symptoms, and autonomic symptoms (33.33%) whereas in females, the most common symptoms were anxious mood (92.85%), muscular somatic symptoms (78.57%), sensory somatic symptoms and gastrointestinal symptoms (71.43%), tension (64.28%), and depressed mood (62.85%).
Table 5.
Distribution of Hamilton anxiety rating scale symptoms and their comparison between males and females
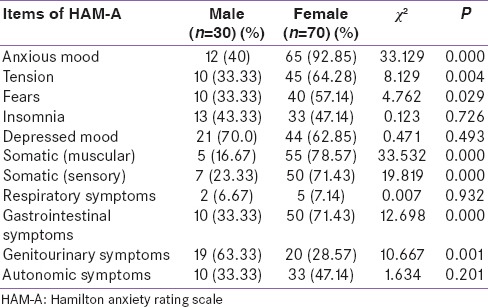
Symptoms such as – anxious mood (P = 0.000), tension (P = 0.004), fears (P = 0.029), muscular somatic (P = 0.000), sensory somatic (P = 0.000), gastrointestinal symptoms (P = 0.000), and genitourinary symptoms (P = 0.001) – were statistically significant on HAM-A when compared for males and females.
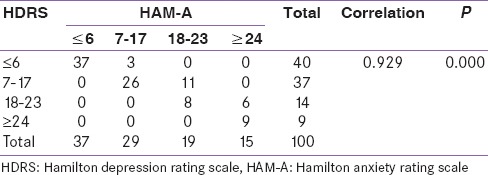
Table 6 shows the correlation of HDRS score and HAM-A score. There was a very strong correlation between the two groups. It is statistically very highly significant (P = 0.000).
Table 6.
Correlation of Hamilton depression rating scale and Hamilton anxiety rating scale score
DISCUSSION
In our study, the major share of patients (n = 51) was of the age group 26–35 years. Of which, 33 were female and 18 were male. This suggests the prevalence of hypothyroidism is more common in females of older age group. These findings are similar to the results of the study conducted by Redmond [29] in 2002 and Chaudhary et al.[30] in 2014. The age group of the sample was chosen to be up to 45 years so as to avoid the inclusion of patients having endogenous depression or psychological manifestation of menopause.
Of the 70 female patients, the highest number of patients (n = 29) were categorized under the HDRS score of mild depression (7–17) followed by 10 patients under the HDRS score of moderate depression (18–23) and finally, 6 patients under the HDRS score of severe depression (more than 24). In males, the highest number of patients (n = 8) were categorized under the HDRS score of mild depression (7–17) followed by 4 patients in moderate (18–23) and finally, 3 patients in severe depression category (more than 24). Similarly, Pies [31] in 1995 and Chaudhary et al.[30] in 2014 had reported similar findings, stating that depression was prevalent in 28–50% and 63% of the sample size, respectively. Saltevo et al.[32] had suggested a much lower prevalence of depression in such population (males – 12.5% and females – 17.5%). Persons with hypothyroidism are at a risk of depression and have also been suggested by several authors.[33,34,35,36] Hence, our study also is in concordance with the previous data regarding the comorbidity of depression and hypothyroidism as 60%.
Of the 70 female patients, the highest number of patients (n = 22) were categorized under the HAM-A score of mild anxiety (7–17) followed by 13 patients under the HAM-A score of moderate anxiety (18–23) and finally 11 patients under the HAM-A score of severe anxiety (more than 24). In males, the highest number of patients (n = 7) were categorized under the HAM-A score of mild anxiety (7–17) followed by 6 patients in moderate (18–23) and finally 4 patients in severe anxiety category (more than 24). However, the earlier findings that have been reported by researchers had stated the prevalence of anxiety symptoms in hypothyroidism to be in the range of 30–40%.[10,11] However, our study shows a prevalence of 63%. Ittermann et al.[34] and Benseñor et al.[37] suggested that persons with hypothyroidism are at a risk of developing anxiety whereas Cosci et al.[35] suggested that anxiety is not a common entity co-occurring with medical disorders.
According to the HDRS symptoms, the most common symptoms among males were depressed mood (73.33%), anxiety and genital symptoms (66.67%), insomnia (43.33%), and general somatic symptoms (33.33%). However, Chaudhary et al.[30] had also the similar occurrence of these symptoms except that insomnia was also a common occurrence in our study, but they had reported gastrointestinal also as one of the commonly occurring symptoms. They had also reported that 50% of the male patients having genital symptoms, whereas we found that 66.67% of male reporting genital symptoms. In females, almost all of the findings are similar to the one reported by Chaudhary et al.,[30] such as gastrointestinal somatic symptoms (68.54%), hypochondriasis (64.28%), depressed mood (60%), anxiety and general somatic symptoms (57.14%), insomnia (47.14%), and suicide (37.14%).
According to the HAM-A symptoms, the most common symptoms among males were genitourinary symptoms (63.33%), anxious mood (40%), and tension, fears, gastrointestinal symptoms and autonomic symptoms (33.33%); these findings are almost similar to as reported by Chaudhary et al.;[30] however, the symptoms such as depressed mood (70.0%), and insomnia (43.33%) were reported by more number of males in our study. In females, the most common symptoms were anxious mood (92.85%), muscular somatic symptoms (78.57%), tension (64.28%), and depressed mood (62.85%); these findings are almost similar to as reported by Chaudhary et al.;[30] however, the symptoms such as sensory somatic symptoms and gastrointestinal symptoms (71.43%) were reported by more number of females in our study. The domain of genitourinary symptoms (includes sexual functions) in our study was more common in males which are contradictory to the results as shown in a study by Krysiak et al.[38] that suggests both thyroid dysfunction and depression together affects female sexual dysfunction.
Clinical implications
For endocrinologists: Any patient on treatment for hypothyroidism not improving or not able to attain premorbid lifestyle must be screened for depression and anxiety symptoms using any of the rating scales and managed accordingly
For psychiatrists: Any patient seeking treatment for either depression or anxiety, not responding to standard dosages of medication or requiring more than usual dosages of the psychotropic drugs, should be screened for the thyroid status. However, if in case of such patients with euthyroid status, still they can also be augmented with thyroxine. If in case of a single isolated elevated TSH level in a depressed patient, he/she should be treated with a rational approach.[39]
The results of the study by Thvilum et al.[40] showed that the patients with hypothyroidism are at increased risk of being diagnosed with psychiatric disorders both before and after the diagnosis of hypothyroidism and being treated with antidepressants, antipsychotics, as well as anxiolytics. Kalra and Balhara [41] had suggested that thyroxine replacement as a monotherapy fails to achieve total remission; whereas the STAR*D trial had suggested that if all fail, add some thyroid as an augmentation to antidepressant;[42] hence, a biopsychosocial-based intervention should be used for the patients treated for hypothyroidism as suggested by Brown et al.[43]
Strengths of the study
This study is first of its kind reported from Haryana that had reported the prevalence of depressive and anxiety symptoms in hypothyroidism patients and also studied the correlation among the males and females.
Limitations of the study
The results of our study cannot be generalized due to certain limitations of this study such as the other psychiatric conditions were not taken into consideration; correlation among the SCH and overt hypothyroidism was not done; small sample size; and symptoms were not correlated with TSH level and duration of hypothyroidism.
CONCLUSIONS
THs play an important role in mood and behavior, and cognition is an established entity. Thus, the correlation between psychiatry disorder and thyroid status is a major area of concern. Furthermore, thyroid dysfunctions can lead to psychiatric comorbidities such as depressive disorder, anxiety disorder, and disturbances in memory and learning also hold true. The patient's presenting with such sign and symptoms should be monitored and treating by both an endocrinologist and a psychiatrist in liaison with each other so as to optimize their management. Moreover, an early recognition of an endocrine condition will help minimize psychiatric morbidity and hence improve health.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge Dr. Shalu Chandna for helping in the preparation of the manuscript.
REFERENCES
- 1.Wolkowitz OM, Rothschild AJ. Psychoneuroendocrinology: The Scientific Basis of Clinical Practice, American Psychiatric. 1st ed. Washington, DC, USA: American Psychiatric Publishing, Inc.; 2003. pp. 419–44. [Google Scholar]
- 2.Davidoff F, Gill J. Myxedema madness: Psychosis as an early manifestation of hypothyroidism. Conn Med. 1977;41:618–21. [PubMed] [Google Scholar]
- 3.Gold MS, Pottash AL, Extein I. Hypothyroidism and depression. Evidence from complete thyroid function evaluation. JAMA. 1981;245:1919–22. doi: 10.1001/jama.245.19.1919. [DOI] [PubMed] [Google Scholar]
- 4.Boral GC, Ghosh AB, Pal SK, Ghosh KK, Nandi DN. Thyroid function in different psychiatric disorders. Indian J Psychiatry. 1980;22:200–2. [PMC free article] [PubMed] [Google Scholar]
- 5.Saxena J, Singh PN, Srivastava U, Siddiqui AQ. A study of thyroid hormones (T3, T4& TSH) in patients of depression. Indian J Psychiatry. 2000;42:243–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Placidi GP, Boldrini M, Patronelli A, Fiore E, Chiovato L, Perugi G, et al. Prevalence of psychiatric disorders in thyroid diseased patients. Neuropsychobiology. 1998;38:222–5. doi: 10.1159/000026545. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Saha PK, Mukhopadhyay A. Prevalence of hypothyroidism and importance of cholesterol estimation in patients suffering from major depressive disorder. J Indian Med Assoc. 2008;106:240–2. [PubMed] [Google Scholar]
- 8.Hall RC, Stickney S, Beresford TP. Endocrine disease and behaviour. Integr Psychiatry. 1986;4:122–35. [Google Scholar]
- 9.Popkin MK, Mackenzie TB. Psychiatric presentations of endocrine dysfunction. In: Hall RC, editor. Psychiatric Presentations of Medical Illness. New York: Spectrum Books; 1980. pp. 142–3. [Google Scholar]
- 10.Haug TT, Mykletun A, Dahl AA. The association between anxiety, depression, and somatic symptoms in a large population: The Hunt-II study. Psychosom Med. 2004;66:845–51. doi: 10.1097/01.psy.0000145823.85658.0c. [DOI] [PubMed] [Google Scholar]
- 11.Hall RC. Psychiatric effects of thyroid hormone disturbance. Psychosomatics. 1983;24:7–22. doi: 10.1016/s0033-3182(83)73255-x. [DOI] [PubMed] [Google Scholar]
- 12.Whybrow PC, Prange AJ, Jr, Treadway CR. Mental changes accompanying thyroid gland dysfunction. A reappraisal using objective psychological measurement. Arch Gen Psychiatry. 1969;20:48–63. doi: 10.1001/archpsyc.1969.01740130050004. [DOI] [PubMed] [Google Scholar]
- 13.Haggerty JJ, Jr, Garbutt JC, Evans DL, Golden RN, Pedersen C, Simon JS, et al. Subclinical hypothyroidism: A review of neuropsychiatric aspects. Int J Psychiatry Med. 1990;20:193–208. doi: 10.2190/ADLY-1UU0-1A8L-HPXY. [DOI] [PubMed] [Google Scholar]
- 14.Davis JD, Tremont G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007;32:49–65. [PubMed] [Google Scholar]
- 15.Gitlin M, Altshuler LL, Frye MA, Suri R, Huynh EL, Fairbanks L, et al. Peripheral thyroid hormones and response to selective serotonin reuptake inhibitors. J Psychiatry Neurosci. 2004;29:383–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho AF, Machado JR, Cavalcante JL. Augmentation strategies for treatment-resistant depression. Curr Opin Psychiatry. 2009;22:7–12. doi: 10.1097/YCO.0b013e32831be9ef. [DOI] [PubMed] [Google Scholar]
- 17.Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: Of synergy and significance in the adult brain. Mol Psychiatry. 2002;7:140–56. doi: 10.1038/sj.mp.4000963. [DOI] [PubMed] [Google Scholar]
- 18.Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: A brief review. Neurosci Biobehav Rev. 2002;26:45–60. doi: 10.1016/s0149-7634(01)00037-9. [DOI] [PubMed] [Google Scholar]
- 19.Bernal J, Nunez J. Thyroid hormones and brain development. Eur J Endocrinol. 1995;133:390–8. doi: 10.1530/eje.0.1330390. [DOI] [PubMed] [Google Scholar]
- 20.Atterwill CK, Bunn SJ, Atkinson DJ, Smith SL, Heal DJ. Effects of thyroid status on presynaptic alpha 2-adrenoceptor function and beta-adrenoceptor binding in the rat brain. J Neural Transm. 1984;59:43–55. doi: 10.1007/BF01249877. [DOI] [PubMed] [Google Scholar]
- 21.Belmaker RH, Agam G. Mechanisms of Disease: Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 22.Linkowski P, Brauman H, Mendlewicz J. Thyrotrophin response to thyrotrophin-releasing hormone in unipolar and bipolar affective illness. J Affect Disord. 1981;3:9–16. doi: 10.1016/0165-0327(81)90014-8. [DOI] [PubMed] [Google Scholar]
- 23.Loosen PT. The TRH-induced TSH response in psychiatric patients: A possible neuroendocrine marker. Psychoneuroendocrinology. 1985;10:237–60. doi: 10.1016/0306-4530(85)90002-2. [DOI] [PubMed] [Google Scholar]
- 24.Forman-Hoffman V, Philibert RA. Lower TSH and higher T4 levels are associated with current depressive syndrome in young adults. Acta Psychiatr Scand. 2006;114:132–9. doi: 10.1111/j.1600-0447.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 25.Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS. Health status, mood, and cognition in experimentally induced subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:2545–51. doi: 10.1210/jc.2007-0011. [DOI] [PubMed] [Google Scholar]
- 26.Tremont G, Stern RA, Westervelt HJ, Bishop CL, Davis JD. Neurobehavioral functioning in thyroid disorders. Med Health R. 2003;86:318–22. [PubMed] [Google Scholar]
- 27.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 29.Redmond GP. Hypothyroidism and women's health. Int J Fertil Womens Med. 2002;47:123–7. [PubMed] [Google Scholar]
- 30.Chaudhary R, Chabra S, Singla M, Mishra BP, Sharma A. Psychiatric morbidity among hypothyroid patients – A hospital based study. Delhi Psychiatry J. 2014;17:35–8. [Google Scholar]
- 31.Pies RW. Women, mood, and the thyroid. Women Psychiatry Health. 1995;4:4–10. [Google Scholar]
- 32.Saltevo J, Kautiainen H, Mäntyselkä P, Jula A, Keinänen-Kiukaanniemi S, Korpi-Hyövälti E, et al. The relationship between thyroid function and depressive symptoms-the FIN-D2D population-based study. Clin Med Insights Endocrinol Diabetes. 2015;8:29–33. doi: 10.4137/CMED.S24111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kvetny J, Ellervik C, Bech P. Is suppressed thyroid-stimulating hormone (TSH) associated with subclinical depression in the Danish General Suburban Population Study? Nord J Psychiatry. 2015;69:282–6. doi: 10.3109/08039488.2014.972454. [DOI] [PubMed] [Google Scholar]
- 34.Ittermann T, Völzke H, Baumeister SE, Appel K, Grabe HJ. Diagnosed thyroid disorders are associated with depression and anxiety. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1417–25. doi: 10.1007/s00127-015-1043-0. [DOI] [PubMed] [Google Scholar]
- 35.Cosci F, Fava GA, Sonino N. Mood and anxiety disorders as early manifestations of medical illness: A systematic review. Psychother Psychosom. 2015;84:22–9. doi: 10.1159/000367913. [DOI] [PubMed] [Google Scholar]
- 36.Blum MR, Wijsman LW, Virgini VS, Bauer DC, den Elzen WP, Jukema JW, et al. Subclinical thyroid dysfunction and depressive symptoms among elderly: A prospective cohort study. Neuroendocrinology. 2015 doi: 10.1159/000437387. DOI: 10.1159/000437387. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Benseñor IM, Nunes MA, Sander Diniz MF, Santos IS, Brunoni AR, Lotufo PA. Subclinical thyroid dysfunction and psychiatric disorders: Cross-sectional results from the Brazilian Study of Adult Health (ELSA-Brasil) Clin Endocrinol (Oxf) 2015;84:250–6. doi: 10.1111/cen.12719. [DOI] [PubMed] [Google Scholar]
- 38.Krysiak R, Drosdzol-Cop A, Skrzypulec-Plinta V, Okopien B. Sexual function and depressive symptoms in young women with thyroid autoimmunity and subclinical hypothyroidism. Clin Endocrinol (Oxf) 2015 doi: 10.1111/cen.12956. DOI: 10.1111/cen.12956. [DOI] [PubMed] [Google Scholar]
- 39.Joffe RT, Sullivan TB. The significance of an isolated elevated TSH level in a depressed patient: A clinical commentary. Int J Psychiatry Med. 2014;48:167–73. doi: 10.2190/PM.48.3.b. [DOI] [PubMed] [Google Scholar]
- 40.Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedüs L. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: A nationwide register study. Thyroid. 2014;24:802–8. doi: 10.1089/thy.2013.0555. [DOI] [PubMed] [Google Scholar]
- 41.Kalra S, Balhara YP. Euthyroid depression: The role of thyroid hormone. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:38–41. doi: 10.2174/1872214807666131229130540. [DOI] [PubMed] [Google Scholar]
- 42.Huynh NN, McIntyre RS. What are the implications of the STAR*D trial for primary care? A review and synthesis. Prim Care Companion J Clin Psychiatry. 2008;10:91–6. doi: 10.4088/pcc.v10n0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown BT, Graham PL, Bonello R, Pollard H. A biopsychosocial approach to primary hypothyroidism: Treatment and harms data from a randomized controlled trial. Chiropr Man Therap. 2015;23:24. doi: 10.1186/s12998-015-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


