Abstract
Insulin degludec/insulin aspart (IDegAsp) is a modern coformulation of ultra-long-acting basal insulin degludec, with rapid-acting insulin aspart. IDegAsp provides effective, safe, well-tolerated glycemic control, with a low risk of hypoglycemia while allowing flexibility in meal patterns and timing of administration. This consensus statement describes a pragmatic framework to identify patients who may benefit from IDegAsp therapy. It highlights the utility of IDegAsp in type 2 diabetic patients who are insulin-naive, suboptimally controlled on basal or premixed insulin, or dissatisfied with basal–bolus regimens. It also describes potential IDegAsp usage in type 1 diabetic patients.
Keywords: Basal–bolus, basal insulin, coformulation, flexibility, hypoglycemia, insulin, insulin aspart, insulin degludec, insulin degludec/insulin aspart, premixed insulin
INTRODUCTION
Insulin degludec/insulin aspart (IDegAsp) is a modern coformulation of the ultra-long-acting insulin degludec (IDeg) and the rapid-acting analog insulin aspart (IAsp). IDeg and IAsp, are present in a 70:30 ratio to maintain their distinct pharmacokinetic and pharmacodynamic profile in this coformulation. Thus, IDegAsp is able to provide a safe, well-tolerated fasting as well as prandial blood glucose control.[1]
CLINICAL DEVELOPMENT
IDegAsp is supported by a robust well-designed clinical development program known as BOOST. In phase 3a trials, IDegAsp has been studied in both type 1 and type 2 diabetes.[1] In type 1 diabetes, IDegAsp has been compared, as part of a three-dose regimen (2 doses of IAsp and 1 dose of IDegAsp) with basal–bolus regimens consisting of maximum 4 injections/day. In type 2 diabetes, IDegAsp has been assessed as a once-daily and as a twice-daily dose for both initiation and intensification of therapy.
CLINICAL PHARMACOLOGY
The pharmacokinetic and pharmacodynamic properties of IDegAsp depend on the action of its two constituents. IDeg is an ultra-long-acting basal insulin with a 25.3 h long half-life, a duration of action of 42 h, and a flat, peakless action-time profile, with low levels of variability. The risk of hypoglycemia and nocturnal hypoglycemia, in particular, is markedly reduced with IDeg as compared with comparator basal insulin.[2] IAsp is a rapid-acting insulin analog, with an onset of action of 10–15 min, peak action at 90 min, and duration of action of 4–5 h.[3] This suggests that IDegAsp can be administered with the main meal of the day to provide both prandial and basal control. There may be occasions when once- or twice-daily administration of IAsp is required for adequate prandial control. This can be safely achieved with two doses of IDegAsp, as IDeg does not lead to stacking due to its first-order elimination kinetics. In case a third injection is necessary for prandial glucose control, a separate dose of IAsp can be added with the third meal.[1]
The duration of the action of IDeg (42 h), its flat action profile, and the lack of stacking allow flexibility in its timing of administration.[4] As IDeg has been studied in forced dosage schedules with interdose gap varying from 8 to 40 h, the same flexibility can be used for IDegAsp as well. In practical terms, this implies that IDegAsp can be administered with the major meal(s) of the day, irrespective of which meal it is, provided that a 6-8 h gap is maintained between injections. This gap is required to ensure that the effect of IAsp component of the first dose is over before the next IDegAsp is injected.
In insulin-naive patients, the starting dose recommended as per label is 10 units/day.[5] In persons on other insulins, a unit-to-unit switch is suggested as per the approved label. However, randomized controlled trials (RCTs) report 10–20% lower dose requirements with IDegAsp as compared to other available premix insulin analogs, biphasic IAsp 30.[6,7]
PATIENT PROFILES
Based on the pharmacological properties of IDegAsp, and clinical experience gained with this molecule in three countries (Mexico, India, and Bangladesh), 10 experts from seven countries discussed and created profiles of patients with type 1 and type 2 diabetes, who would benefit from IDegAsp therapy. This work was done at a full day workshop-based advisory board meeting, held on November 29, 2015, at Vancouver, Canada. It was followed by multiple mails and telephonic discussions before the final draft was agreed upon. The shortlisted patient profiles are listed in Table 1.
Table 1.
Patient profiles for insulin degludec/insulin aspart
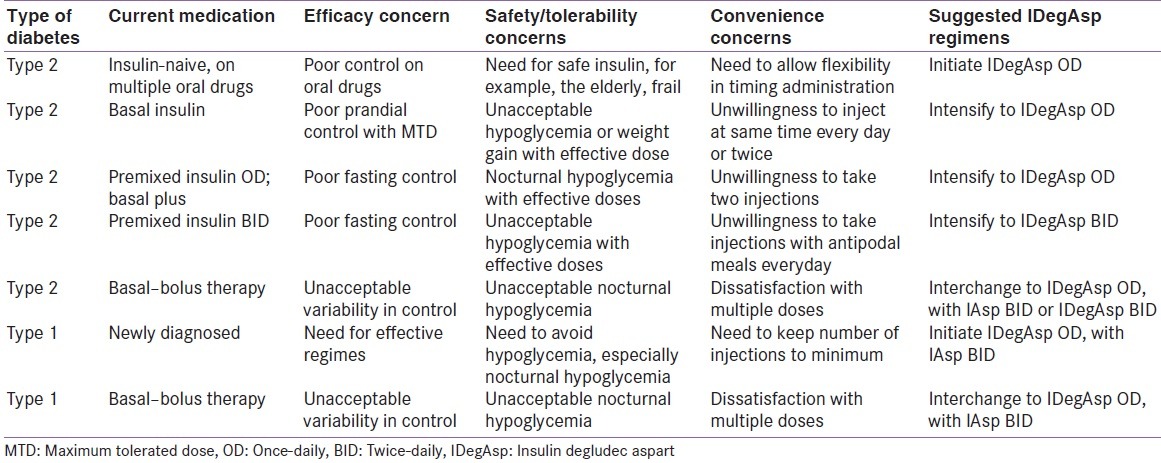
A) TYPE 2 DIABETES
Initiation
The use of dual action insulin (premixed insulin) as first-line therapy is promoted by guidelines from the International Diabetes Federation and various other countries.[8,9] IDegAsp can be used as a drug of choice in insulin-naive type 2 diabetes patients
It provides prandial and basal coverage with one injection and can be administered with the main (largest) meal of the day. Patients retain the flexibility to change the timing of this meal. This convenience is of special importance to persons who have busy or erratic lifestyles and meal patterns, and those who are dependent upon caregivers for injection
The low risk of hypoglycemia is an advantage for the elderly, the frail, and others at high risk of hypoglycemia, for example, those with renal or hepatic impairment. Directly acting oral insulin secretagogues, such as sulfonylureas and meglitinides, should be discontinued when IDegAsp is initiated.
Intensification
IDegAsp can be used to intensify therapy, without increasing the number of injections per day, in persons already on basal insulin or premixed insulin
Such a switch will be necessary only if there are efficacy issues (poor control), safely/tolerability complaints (unacceptable hypoglycemia or weight gain with effective doses), or dissatisfaction with the rigidity of current insulin preparation (e.g., need to inject at specific times or with specific meals)
In such cases, basal insulin being prescribed once-daily or twice-daily can be switched to IDegAsp once-daily with the major meal of the day
While calculating such doses, a unit-to-unit switch should be made for the basal component. Similarly, as per the approved label, patients on conventional or analog premixed insulin should be switched unit-to-unit for the IDegAsp coformulation once-daily or twice-daily, as indicated. If a twice-daily IDegAsp regimen is chosen, a 50:50 distribution of both doses should be begun with, and further titration has to be done as per self-monitoring of blood glucose results. It is not necessary to inject two doses of IDegAsp with antipodal meals
The authors, however, strongly recommend a 10–20% reduction, as compared to the previous premixed dose requirement while calculating starting dose of IDegAsp. This suggestion is supported by data from various BOOST trials, which report a lower dose requirement with IDegAsp than comparator premixed insulin – biphasic IAsp.
Interchange
There are patients with type 2 diabetes, who require basal–bolus or basal plus therapy, but are dissatisfied with their treatment. This dissatisfaction may result from excessive variability in glycemic control, unacceptable nocturnal hypoglycemia with “effective” basal doses, and unwillingness to take multiple injectable doses. In such situations, an interchange to IDegAsp-based therapy provides a rational alternative
Persons on basal plus therapy, taking one basal and one bolus injection, may benefit from the enhanced convenience of a single injection of IDegAsp coformulation
Those on three injections (one basal and two boluses) may interchange to a twice-daily IDegAsp regimen
For those on basal–bolus regimes, requiring 4 or 5 injections/day, two options are available. While some persons may be able to achieve adequate glycemic control with twice-daily IDegAsp, other with severe insulin deficiency and/or resistance may require a three-dose intensive regimen. This will consist of IDegAsp with the main meal and IAsp with the other two meals.
In all these scenarios, interchange to IDegAsp or IDegAsp-based regimens allows effective, safe control with lesser number of injections.
B) TYPE 1 DIABETES
IDegAsp can be used as insulin of initiation or as switch (interchange) therapy in type 1 diabetes
In newly diagnosed, insulin-naive type 1 diabetic patients who are stable and do not require admission for intravenous (IV) insulin, a three-dose, intensive IDegAsp-based regimen can be initiated. This will include one dose of IDegAsp with the major meal and two doses of IAsp with other meals
In newly diagnosed type 1 diabetic patients who are being discharged from hospital, the first dose of subcutaneous (SC) IAsp should be injected with the first oral meal. Expert recommendations suggest a 15–30 min overlap between the first SC dose of rapid-acting insulin and continuation of IV insulin infusion. Similarly, continuation of IV infusion for 2 h after SC injection of basal insulin is suggested. A pragmatic suggestion would be to inject IDegAsp, in hospital, with the first meal tray and continue a very low-dose IV infusion for 1–2 h after that
IDegAsp-based intensive therapy can be also used in type 1 diabetic patients already on basal–bolus therapy. Such an interchange or switch will be indicated only if there are efficacy issues (poor fasting control in spite of using intermediate or long-acting insulins such as Neutral Protamine Hagedorn (NPH), glargine, or detemir), safety concerns (unacceptable risk of hypoglycemia with “effective” doses), or inconvenience/dissatisfaction (need for 4–5 doses, with high index of intrusion). In such cases, a three-dose IDegAsp-based intensive regime provides effective, safe, well-tolerated, and a more convenient glycemic control.
SUMMARY
This consensus statement is based on experience and backed by evidence from RCTs and pharmacokinetic/pharmacodynamic data. It provides a pragmatic framework for diabetes care professionals to help identify patients with type 1 and type 2 diabetes who may benefit from the unique properties of IDegAsp. Wider use of this molecule will help understand the utility of this modern coformulation in the management of diabetes in a better and more suitable manner.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kalra S. Insulin degludec aspart: The first co-formulation of insulin analogues. Diabetes Ther. 2014;5:65–72. doi: 10.1007/s13300-014-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalra S, Unnikrishnan AG, Baruah M, Kalra B. Degludec insulin: A novel basal insulin. Indian J Endocrinol Metab. 2011;15(Suppl 1):S12–6. doi: 10.4103/2230-8210.83056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mudaliar SR, Lindberg FA, Joyce M, Beerdsen P, Strange P, Lin A, et al. Insulin aspart (B28 asp-insulin): A fast-acting analog of human insulin: Absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22:1501–6. doi: 10.2337/diacare.22.9.1501. [DOI] [PubMed] [Google Scholar]
- 4.Kalra S, Gupta Y. Clinical use of insulin degludec: Practical experience and pragmatic suggestions. N Am J Med Sci. 2015;7:81–5. doi: 10.4103/1947-2714.153918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tresiba: Package Insert and Label Information. [Last accessed on 2015 Dec 22]. Available from: http://www.druginserts.com/lib/rx/meds/tresiba/
- 6.Niskanen L, Leiter LA, Franek E, Weng J, Damci T, Muñoz-Torres M, et al. Comparison of a soluble co-formulation of insulin degludec/insulin aspart vs biphasic insulin aspart 30 in type 2 diabetes: A randomised trial. Eur J Endocrinol. 2012;167:287–94. doi: 10.1530/EJE-12-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulcher G, Bantwal G, Christiansen JS, Andersen T, Mersebach H, Niskanen LK, et al. Superior FPG Control and Reduced Hypoglycaemia with IDegAsp vs BIAsp 30 in Adults with Type 2 Diabetes Mellitus Inadequately Controlled on Pre/self-mixed Insulin: A Randomised Phase 3 Trial. Poster Presented at EASD 2013 Barcelona. Poster No. 1044. 2013 [Google Scholar]
- 8.Home P, Haddad J, Latif ZA, Soewondo P, Benabbas Y, Litwak L, et al. Comparison of national/regional diabetes guidelines for the management of blood glucose control in non-western countries. Diabetes Ther. 2013;4:91–102. doi: 10.1007/s13300-013-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global Guidelines for Type 2 Diabetes. [Last accessed on 2015 Dec 22]. Available from: http://www.idf.org/guideline-type-2-diabetes .


