Abstract
Diabetes and related complications are associated with long-term damage and failure of various organ systems. The line of demarcation between the pathogenic mechanisms of microvascular and macrovascular complications of diabetes and differing responses to therapeutic interventions is blurred. Diabetes induces changes in the microvasculature, causing extracellular matrix protein synthesis, and capillary basement membrane thickening which are the pathognomic features of diabetic microangiopathy. These changes in conjunction with advanced glycation end products, oxidative stress, low grade inflammation, and neovascularization of vasa vasorum can lead to macrovascular complications. Hyperglycemia is the principal cause of microvasculopathy but also appears to play an important role in causation of macrovasculopathy. There is thought to be an intersection between micro and macro vascular complications, but the two disorders seem to be strongly interconnected, with micro vascular diseases promoting atherosclerosis through processes such as hypoxia and changes in vasa vasorum. It is thus imperative to understand whether microvascular complications distinctly precede macrovascular complications or do both of them progress simultaneously as a continuum. This will allow re-focusing on the clinical issues with a unifying perspective which can improve type 2 diabetes mellitus outcomes.
Keywords: Complications, diabetes, macrovascular, microvascular
INTRODUCTION
Diabetes mellitus (DM) has routinely been described as a metabolic disorder characterized by hyperglycemia that develops as a consequence of defects in insulin secretion, insulin action, or both. Type 2 diabetes encompasses individuals who have insulin resistance (IR) and usually relative (rather than absolute) insulin deficiency.[1] The pathologic hallmark of DM involves the vasculature leading to both microvascular and macrovascular complications.[2] Chronicity of hyperglycemia is associated with long-term damage and failure of various organ systems mainly affecting the eyes, nerves, kidneys, and the heart.[1]
According to diabetes atlas (7th edition), the global prevalence of diabetes is estimated at 415 million (8.8%), which is predicted to rise to 642 million in next 25 years.[3] In India, there are about 69.2 million people with diabetes and are expected to cross 123.5 million by 2040.[3] Moreover, worldwide approximately 193 million diabetics remain undiagnosed predisposing them to the development of several long-term complications of untreated chronic hyperglycemia.[3] Although intensive glycemic control lowers the incidence and progression of microvascular complications, the morbidity associated with these complications is still increasing.[4] Several landmark studies such as the United Kingdom Prospective Diabetes Study (UKPDS) have demonstrated that strict glycemic control does limit microvascular disease while attempts to improve macrovascular outcomes through glucose-lowering interventions still remain shrouded with controversy. A relative risk (RR) reduction in myocardial infarction (MI) (P = 0.052) has been observed in the 10 years of posttrial follow-up of UKPDS.[5] Similarly, the risk of cardiovascular mortality, nonfatal MI and stroke reduced with pioglitazone in the Prospective Pioglitazone Clinical Trial in Macrovascular Events as compared to placebo group.[6] The action in diabetes and vascular disease: Preterax and Diamicron MR Controlled Evaluation and the Veterans Affairs Diabetes Trial failed to show any significant improvement in cardiovascular risk with the intensification of diabetes therapy.[7,8] To further complicate matters, in Action to Control Cardiovascular Risk in Diabetes trial the use of intensive therapy for 3.5 years increased mortality but did not significantly reduce major cardiovascular events.[9]
In recent years, much attention has been focused on the management of macrovascular complications such as stroke and acute coronary syndromes. It is well-recognized that vascular complications in a given tissue are often accompanied by evidence of pathology in other vascular territories. A linear relationship between microvascular complications and duration of disease was established by the authors where they documented the presence of microvasculopathy across different age groups in their study in 25–40% of diabetic patients aged >25 years with more than 5 years duration of diabetes.[10] Researchers such as Krentz et al. and Al-Wakeel et al. have observed that both microvascular and macrovascular complications develop simultaneously in diabetes.[11,12] On the contrary, Matheus and Gomes described the case report of type 1 DM (T1DM) patient with early and aggressive coronary artery disease (CAD) without evidence of nephropathy, retinopathy, or classical risk factors for CAD.[13] Thus, there is not much clarity over whether microvascular complication precedes macrovascular complications or they progress simultaneously.
The present review attempts an insight into this delicate relationship between microvascular and macrovascular complications of diabetes to understand whether they are discrete entities or in continuum with each other. We propose a unique continuum bridging the microvascular and the macrovascular risk which is based on our evidence-based studies consistently over a decade.
PATHOPHYSIOLOGICAL BASIS OF MICRO VERSUS MACROVASCULAR COMPLICATIONS
Patients with DM and associated microvascular complications appear particularly at higher risk of accelerated atherosclerosis which ultimately culminates in cerebrovascular and cardiovascular events and premature death.[14] Microvessels are the basic functional unit of the cardiovascular system comprising of arterioles, capillaries, and venules.[2] They differ from macrovessels in both their architecture and cellular components. In contrast to macrovessels supplying blood to organs, microvessels play important roles in maintaining blood pressure and proper nutrient delivery.[2] The microcirculation also has regulatory systems controlling vascular permeability and myogenic responses that can adapt blood flow according to local metabolic needs. Alteration in microvascular function may arise even before overt hyperglycemia and vascular pathologic changes manifest. Diabetes induces pathognomonic changes in the microvasculature, affecting the capillary basement membrane including arterioles in the glomeruli, retina, myocardium, skin, and muscle, by increasing their thickness, leading to the development of diabetic microangiopathy. This thickening eventually leads to abnormality in vessel function, inducing multiple clinical problems such as hypertension, delayed wound healing, and tissue hypoxia. Similarly, neovascularization arising from the vasa vasorum may interconnect macro-and microangiopathy, predict platelet rupture and promote atherosclerosis. The role of microvascular pathology in systemic diabetic complications, including macrovascular atherosclerosis, remains a subject for further debate.[2]
INTERSECTION OF MICROVASCULAR AND MACROVASCULAR COMPLICATIONS OF DIABETES: EVIDENCE-BASED PROOF OF CONCEPT
Diabetic retinopathy
The risk of development of diabetic retinopathy (DR) in patients with (T2DM) has been found to be related to both severities of hyperglycemia and presence of hypertension. Fong et al. attributed approximately 10,000 new cases of blindness to DR in the United States.[15] In India, The Chennai Urban Rural Epidemiology Study (CURES) reported an overall DR prevalence of 17.6% (confidence interval [95% CI]: 15.8–19.5) in the diabetic population.[16] More recently, the Sankara Nethralaya DR Epidemiology and Molecular Genetic Study has estimated an urban prevalence of 18.0% (95% CI: 16.0–20.1) and a rural prevalence of 10.3% (95% CI: 8.53–11.97%) of DR in South India.[17,18] Similar to this, Aravind Comprehensive Eye Study has reported 10.5% prevalence of DR (in self-reported subjects with diabetes) in the rural South Indian population.[19] A DR prevalence of 21.2% has been reported by Chawla et al. in their cohort of North Indian patients. This study also found a significant association between HbA1c, body mass index, duration of diabetes and microalbuminuria in the development of DR (P = 0.001).[20]
Several studies have explored the association between DR and macrovascular complications. As retinal microvasculature shares embryologic and anatomic characteristics with that of cerebral circulation, researchers have studied retinal abnormalities to provide clues to understand the underlying pathophysiology of different cerebrovascular diseases.[21] In the World Health Organization Multinational Study of Vascular Disease in Diabetes, retinopathy was related to the incidence of MI and death from cardiovascular disease (CVD).[22] In the Atherosclerosis Risk in Communities study, increased incidence of clinical stroke was associated with retinal microvascular abnormalities and generalized arteriolar narrowing.[23] Furthermore, retinopathy was significantly associated with combined stroke events (RR 1.7, 95% CI: 1.0–2.8) in persons without diabetes after controlling for age, sex, and systolic blood pressure. This association was stronger in those with two or more retinal microvascular signs (RR 2.7, CI: 1.5–5.2).[24] Targher et al. followed up 2103 T2DM outpatients for 7 years and found a remarkably increased risk of incident CVD in patients with proliferative/laser-treated retinopathy (hazard ratio 2.08 [1.02–3.7] for men and 2.41 [1.05–3.9] for women), after adjustment of hypertension and advanced nephropathy.[25] Contrary to this, Matheus and Gomes has reported cardiovascular events without any presence of retinopathy.[13]
Diabetic nephropathy
Proteinuria occurs in 15–40% of patients with type 1 diabetes while it ranges from 5 to 20% in patients with T2DM.[26] According to the European Diabetes Prospective Complications Study, the cumulative incidence of microalbuminuria was 12.6% over 7.3 years in patients with T1DM.[26] However, 18 years follow-up study from Denmark reported a prevalence rate of 33% in the T1DM population.[27] Similarly, in the (UKPDS), T2DM patients showed a 2.0% incidence of microalbuminuria per year, which reached up to 25% in 10 years postdiagnosis.[28] The prevalence of diabetic nephropathy was higher in African Americans, Asians, and Native Americans than Caucasians.[26] In India, CURES 45 reported a prevalence of 2.2% for overt diabetic nephropathy and 26.9% for microalbuminuria.[29]
The association of intense control to reduced microvascular complication, as reported by the Diabetes Control and Complications Trial, has emerged as a strong proof of concept for hyperglycemia being an important modifiable risk factor for diabetic nephropathy. A reduction in 10 mm Hg of systolic blood pressure is associated with a 13% decrease in the microvascular complications with minimal risk in patient with a systolic pressure <120 mm Hg.[30] Dyslipidemia with increased low-density lipoprotein (LDL) cholesterol and triglycerides is independently associated with diabetic kidney disease.[31]
The pathogenic mechanisms underlying diabetic nephropathy involve generation of reactive oxygen species (ROS), accumulation of advanced glycation end product (AGE), and activation of intracellular signaling molecules such as protein kinase C (PKC).[32,33] A strong association between diabetic nephropathy and retinopathy was demonstrated by Arora et al. in 2004–2005 in 50 newly diagnosed patients with diabetes.[34] The direct association between the presence of microalbuminuria and macrovascular complications has also been well-established in many studies.[35] In an observational study, Hägg et al(2013). reported the increased incidence of both cerebral infarction and cerebral hemorrhage in patients with severe DR (SDR) and advanced diabetic nephropathy in 4083 patients with T1DM with 36,680 person-years of follow-up. Both nephropathy and SDR were found to be independently increasing the risk for all subtypes of stroke.[36] The increased incidence of stroke in diabetic nephropathy has also been reiterated in the Pittsburgh Epidemiology of Diabetes Complications study, where overt nephropathy increases the risk of ischemic stroke 4.4-fold (but not the risk for hemorrhagic stroke, probably due to the insufficient number of samples).[36] Retinopathy and macroalbuminuria produce higher rates of cardiovascular events among Chinese patients.[37]
Diabetic neuropathy
Diabetic neuropathy, a life-threatening complication involves both peripheral and autonomic nerves, affecting almost half of the diabetic population.[38] The risk of development of diabetic neuropathy is directly proportional to both the duration and magnitude of hyperglycemia. In addition, some individuals may also possess genetic facets that influence their predisposition in developing such complications.[35] The prevalence of diabetic neuropathy varies from country to country. In India, a high prevalence (29.2%) of diabetic peripheral neuropathy was reported among the North Indian population.[39] Further studies in North Indian region (Lucknow) and North-eastern region (Imphal) revealed a prevalence of 29.2% and 29.0%, respectively, among newly diagnosed diabetic patients (duration <6 months).[40,41] Chawla et al. reported the prevalence of 15.3% in their study involving 720 North Indian patients from New Delhi.[20]
Although the precise nature of the injury to the peripheral nerves from hyperglycemia is not yet certain, the mechanisms of hyperglycemia-induced polyol pathway, injury from AGEs, and enhanced oxidative stress have been implicated in its pathogenesis. The damage to peripheral nerves may be mediated by effects on nerve tissue or by endothelial injury or vascular dysfunction.[2] Peripheral neuropathy in diabetes appears in several forms depending on the site, manifesting as sensory, focal/multifocal, and autonomic neuropathies. Diabetic neuropathy has resulted in more than 80% amputations after foot ulceration or injury.[35]
A study conducted by Miguel et al. demonstrated a significant correlation between diabetic neuropathy and the existence of one or more macrovascular complications showing that diabetic patients with peripheral neuropathy presented with significantly higher rates of cardiac events and peripheral vascular disease (PVD) than diabetic patients without neuropathy. Strokes were also numerically higher in the neuropathy group.[42] Chawla et al. in 2011–2012 demonstrated an association between diabetic neuropathy and development of DR and microalbuminuria in 855 patients.[43] Diabetic cardiac autonomic neuropathy have been found to have a strong co-association with DR (22% vs. 14.3%), diabetic neuropathy (14% vs. 6.8%), and poor glycemic control.
Hence, it is recommended to evaluate autonomic nervous system function at the time of T2DM diagnosis and annually thereafter.[44] Chawla et al. established a positive correlation between autonomic neuropathy and peripheral neuropathy (P = 0.00014); however, the further association between autonomic neuropathy and PVD failed to reach statistical significance.[45] In addition, a direct relationship of lower extremity arterial disease (LEAD) with T2DM was also documented by Chawla et al. They found symptoms of peripheral vascular insufficiency or weak peripheral vessels in 13.8% of patients with foot Doppler confirmed the prevalence of LEAD in 7.4% of patients.[46] The authors validated and advocate the use of neuropathy symptoms score and neuropathy disability score in the clinical and bedside diagnosis of peripheral neuropathy.[47]
COMMON PATHWAYS FOR DEVELOPMENT OF BOTH MICRO AND MACROVASCULAR COMPLICATIONS
Advanced glycation products
AGEs are a heterogeneous group of molecules formed by the nonenzymatic glycation of plasma proteins causing a disruption in their normal functioning by altering their molecular conformation, disrupting enzyme activity, and interfering with receptor functioning. AGEs accumulate in different types of cells and affect their extracellular and intracellular structure and function by cross-linking not only with proteins but also lipids and nucleic acids contributing to a variety of diabetic complications.[48] AGEs crosslink with plasma membrane-localized receptors for AGEs (RAGE) leading to up-regulation of transcription factors such as nuclear factor-κB and its target genes, release of pro-inflammatory molecules and free radicals. Soluble AGEs activate monocytes, and AGEs in the basement membrane inhibit monocyte migration. AGE-bound RAGE increases endothelial permeability to macromolecules. AGEs block nitric oxide activity in the endothelium and cause the production of ROS.[49]
Further, AGEs modify LDL particles and together with vascular damage accelerate atherosclerosis.[50] Kalousová et al. found significant elevation of AGE in patients with T2DM as compared to both healthy (5.11 ± 1.15 × 103 AU/g vs. 4.08 ± 0.71 × 103 AU/g, P < 0.001) as well as T1DM patients (4.14 ± 0.86 × 103 AU/g, P < 0.005).[51] Kilhovd et al. demonstrated a significant increase in the levels of AGEs in patients with T2DM compared with nondiabetic control subjects (7.4 [4.4–10.9] vs. 4.2 [1.6–6.4] U/ml, P < 0.0001). Besides, they also found elevated levels of AGE in patients with coronary heart disease (CHD) than those of without CHD (8.1 [6.4–10.9] vs. 7.1 [3.5–9.8] U/ml, P = 0.03) in patients with T2DM.[52]
Oxidative stress
Oxidative stress, caused by the overproduction of ROS plays an important role in the activation of other pathogenic pathways involved in diabetic complications, including elevated polyol pathway activity, nonenzymatic glycation, and PKC levels which in turn lead to the development of micro-and macrovascular complications.[53] It also inactivates two critical anti-atherosclerotic enzymes, endothelial nitric oxide synthase, and prostacyclin synthase.[54] Hyperglycemia promotes the formation of ROS, which interacts with both deoxyribonucleic acid (DNA) and proteins, causing cellular damage, especially targeting mitochondrial DNA. A study on the human retinal endothelial cell (EC) demonstrated very early mitochondrial DNA damage with hyperglycemia-induced overproduction of ROS.[55] ROS-mediated cellular damage may be a form of pathologic “memory” in the microvasculature that persists even after glucose normalization. Several experimental evidences point to mitochondrial superoxide overproduction as the major culprit in the development of metabolic abnormalities in diabetics.[56] IR induces mitochondrial ROS production from free fatty acids and inhibits anti-atherosclerotic enzymes causing atherosclerosis and cardiomyopathy in T2DM patients. In subjects without diabetes or impaired glucose tolerance, those in the highest quintile of IR had a 2.0-fold increase in CVD risk compared to those in the lowest quintile after adjusting several known cardiovascular risk factors, including LDL, triglycerides, high-density lipoprotein, systolic blood pressure, and smoking.[57]
The other pathways implicated in diabetic complications such as AGE formation, PKC activation, increased polyol flux, and hexosamine formation are also linked to oxidative stress in promoting macrovascular complications through multiple mechanisms. Increased glucose concentrations can activate nuclear factor-κB, a key mediator that regulates multiple pro-inflammatory and pro-atherosclerotic target genes in vascular smooth muscle cells (VSMCs), ECs, and macrophages.[55] Hyperglycemia itself stimulates oxidative stress, which indeed acts as a driving force in accelerated atherosclerosis.
Low-grade inflammation
Inflammation has been recognized as one of the potent risk factors in both atherosclerosis and T2DM. Vascular cells encounter many early pathologic changes in response to hyperglycemia, causing a loss of nonadhesive property and adhesion of monocyte to ECs, which is an early step in atherogenesis. Hyperglycemia has been reported to provoke monocyte adhesion to arterial ECs.[58] The association between hyperglycemia and AGEs with oxidative stress is manifested as both can stimulate EC production of superoxide [Figure 1]. Glucose also activates matrix-degrading enzyme metalloproteinase which causes plaque rupture and arterial remodeling as well as VSMC proliferation, migration and altered activity. The role of increased level of tumor necrosis factor alpha in the development of IR is well-documented. IR itself has inflammatory action as described above. The levels of several other inflammatory markers such as C-reactive protein, fibrinogen, plasminogen activator inhibitor I, and interleukine-6 have been shown to increase with the onset of diabetes. Monocyte activation has been documented in the presence of high glucose with induction of inflammatory mediators such as PKC and nuclear factor-κB promoting oxidative stress.[59]
Figure 1.
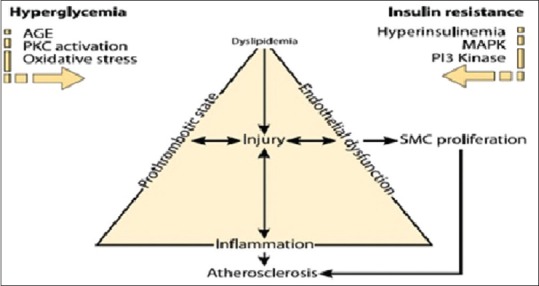
Insulin resistance and hyperglycemia drive the atherosclerotic process
Neovascularization of vasa vasorum
The proliferation of vasa vasorum is associated with increased plaque burden, which subsequently promotes atherosclerosis.[60] Many cellular processes such as inflammation, plaque perfusion and concomitant intra-plaque hemorrhage are critical during the development of atherosclerotic plaques and are linked with vasa vasorum proliferation. Neovascularization develops by the growth from both adventitial layer (outward) and arterial lumen (inward) toward the intima.[61] In T2DM, plaque rupture is associated with increased angiogenesis, and diabetic atherosclerosis is further accelerated by neovasculature microangiopathy.[62] The initial angiogenic response in the adventitial vasa vasorum, an important component of homeostatic mechanisms, appears to be stimulated by hypoxia through identification of increased hypoxia-inducible factor and vascular endothelial growth factor (VEGF) action.[63]
VEGF, a multifunctional cytokine, also contributes to microvascular complications by increasing the vascular permeability to macromolecules, monocyte chemotaxis, and tissue factor production.[64] Up-regulation of VEGF is also reported in experimental diabetic kidney disease models. However, contrary to this, VEGF treatment has been shown to restore microcirculation in the vasa nervorum and limit diabetic neuropathy as demonstrated by rodent VEGF gene transfer experiment.[2] In the eye, a neurotropic factor-pigment epithelium-derived factor (PEDF) may offset VEGF action by its potent angiogenic inhibition.[65] PEDF level is decreased in proliferative DR, whereas VEGF levels are increased. Decreased PEDF levels may also likely contribute to diabetic nephropathy. Other growth factors such as insulin-like growth factor 1, basic fibroblast growth factor, and hepatocyte growth factor may foster proliferative retinopathy.[66]
MICROVASCULAR AND MACROVASCULAR COMPLICATIONS-CONTINUUM OR SEPARATE: THE DEBATE CONTINUES [FIGURE 2]
Figure 2.
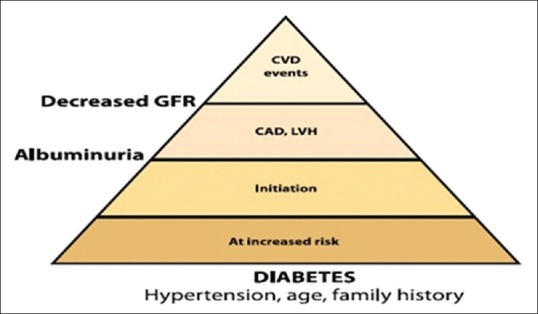
The diabetes continuum
Heart involvement in diabetes may not be only a macrovascular disease where there is Orchestra of contributing factors to the development of diabetic cardiomyopathy concerning fatty acid and glucose complex structural macrovascular derangements such as hypertrophy and loss of function due to glycation but also a microvascular involvement following “common soil” hypothesis of diabetes complications. Atherosclerosis in large arteries as well as cardiomyopathy in diabetes a microvascular component may go hand in hand.[67]
Therefore, changes in small arteries and capillaries are not responsible for only microvascular long-term complications in patients with diabetes (retinopathy, nephropathy, and neuropathy) but also for other manifestations of heart disease in diabetes.
This review highlights the need for implementing programs for early detection, screening, and awareness to mitigate the burden of managing the complications.
Good blood glucose control improves microvascular disease and should be implemented early and maintained for the optimum length of time. Appropriate controls of blood pressure as well as dyslipidemia are extremely important in macrovascular disease prevention besides glycemic control. Patients with microvascular complications appear particularly prone to accelerated atherosclerosis and premature death. Neovascularization arising from the vasa vasorum may interconnect macro-and microangiopathy.
A clearer picture on differing responses to therapeutic interventions could lead to better management and improve T2DM outcomes not only regarding microvascular but also macrovascular complications as well. Further systematic research on the above interlinking hypothesis will help us get more clarity whether microvascular complications precede macrovascular complications or they are two ends of the same spectrum of disease existing in continuum.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Standards of medical care in diabetes-2016: Summary of revisions. Diabetes Care. 2016;39(Suppl 1):S4–5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 2.Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53(5 Suppl):S35–42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Atlas. 7th edition. [Last accessed on 2015 Dec 27]. Available from: http://www.diabetesatlas.org .
- 4.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 6.Charbonnel B, Dormandy J, Erdmann E, Massi-Benedetti M, Skene A. PROactive Study Group. The prospective pioglitazone clinical trial in macrovascular events (PROactive): Can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5238 patients. Diabetes Care. 2004;27:1647–53. doi: 10.2337/diacare.27.7.1647. [DOI] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 8.Patel A, Chalmers J, Poulter N. ADVANCE: Action in diabetes and vascular disease. J Hum Hypertens. 2005;19(Suppl 1):S27–32. doi: 10.1038/sj.jhh.1001890. [DOI] [PubMed] [Google Scholar]
- 9.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla A, Chawla R, Bhasin GK, Soota K. Profile of adolescent diabetics in North Indian population. J Clin Diabetol. 2014;1:1–3. [Google Scholar]
- 11.Krentz AJ, Clough G, Byrne CD. Interactions between microvascular and macrovascular disease in diabetes: Pathophysiology and therapeutic implications. Diabetes Obes Metab. 2007;9:781–91. doi: 10.1111/j.1463-1326.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Wakeel JS, Hammad D, Al Suwaida A, Mitwalli AH, Memon NA, Sulimani F. Microvascular and macrovascular complications in diabetic nephropathy patients referred to nephrology clinic. Saudi J Kidney Dis Transpl. 2009;20:77–85. [PubMed] [Google Scholar]
- 13.Matheus AS, Gomes MB. Early aggressive macrovascular disease and type 1 diabetes mellitus without chronic complications: A case report. BMC Res Notes. 2013;6:222. doi: 10.1186/1756-0500-6-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalofoutis C, Piperi C, Kalofoutis A, Harris F, Phoenix D, Singh J. Type II diabetes mellitus and cardiovascular risk factors: Current therapeutic approaches. Exp Clin Cardiol. 2007;12:17–28. [PMC free article] [PubMed] [Google Scholar]
- 15.Fong DS, Aiello LP, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27:2540–53. doi: 10.2337/diacare.27.10.2540. [DOI] [PubMed] [Google Scholar]
- 16.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: The Chennai Urban Rural Epidemiology Study (CURES) eye study, I. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 17.Raman R, Rani PK, Reddi Rachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G, et al. Prevalence of diabetic retinopathy in India: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study report 2. Ophthalmology. 2009;116:311–8. doi: 10.1016/j.ophtha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Raman R, Ganesan S, Pal SS, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care. 2014;2:e000005. doi: 10.1136/bmjdrc-2013-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nirmalan PK, Tielsch JM, Katz J, Thulasiraj RD, Krishnadas R, Ramakrishnan R, et al. Relationship between vision impairment and eye disease to vision-specific quality of life and function in rural India: The Aravind Comprehensive Eye Survey. Invest Ophthalmol Vis Sci. 2005;46:2308–12. doi: 10.1167/iovs.04-0830. [DOI] [PubMed] [Google Scholar]
- 20.Chawla A, Chawla R, Chawla A. Correlation Between Retinopathy Microalbuminuria and Other Modifiable Risk Factors. Presented on American Diabetes Association's 75th Scientific Session; June 5-9; Boston, Massachusetts. 2015. [Google Scholar]
- 21.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, et al. Retinal microvascular abnormalities and incident stroke: The atherosclerosis risk in communities study. Lancet. 2001;358:1134–40. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 22.Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: The WHO mutinational study of vascular disease in diabetes. Diabetologia. 2001;44(Suppl 2):S54–64. doi: 10.1007/pl00002940. [DOI] [PubMed] [Google Scholar]
- 23.Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR. ARIC Study Investigators. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis risk in communities study. Stroke. 2010;41:1349–55. doi: 10.1161/STROKEAHA.110.580837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005;65:1005–9. doi: 10.1212/01.wnl.0000179177.15900.ca. [DOI] [PubMed] [Google Scholar]
- 25.Targher G, Bertolini L, Zenari L, Lippi G, Pichiri I, Zoppini G, et al. Diabetic retinopathy is associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabet Med. 2008;25:45–50. doi: 10.1111/j.1464-5491.2007.02327.x. [DOI] [PubMed] [Google Scholar]
- 26.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–76. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 27.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH. EURODIAB Prospective Complications Study Group. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: The EURODIAB prospective complications study (PCS) Diabetes Care. 2008;31:1360–6. doi: 10.2337/dc08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: Inception cohort study. BMJ. 2004;328:1105. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. UKPDS Group. Development and progression of nephropathy in type 2 diabetes: The United Kingdom prospective diabetes study (UKPDS 64) Kidney Int. 2003;63:225–32. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 30.Unnikrishnan RI, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, et al. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: The Chennai Urban Rural Epidemiology Study (CURES 45) Diabetes Care. 2007;30:2019–24. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 31.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. BMJ. 2000;321:412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trevisan R, Dodesini AR, Lepore G. Lipids and renal disease. J Am Soc Nephrol. 2006;17(4 Suppl 2):S145–7. doi: 10.1681/ASN.2005121320. [DOI] [PubMed] [Google Scholar]
- 33.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–35. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arora GS, Chawla R, Ahuja CP. To Evaluate the Clinical Profile and Determine the Prevalence of Complications in Newly Detected Type-2 Diabetes Patients. Presented on Research Society for the Study of Diabetes in India (RSSDI) rd Annual Conference; September rd-th, Bangalore; [Google Scholar]
- 35.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 36.Hägg S, Thorn LM, Putaala J, Liebkind R, Harjutsalo V, Forsblom CM, et al. Incidence of stroke according to presence of diabetic nephropathy and severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care. 2013;36:4140–6. doi: 10.2337/dc13-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd CE, Klein R, Maser RE, Kuller LH, Becker DJ, Orchard TJ. The progression of retinopathy over 2 years: The Pittsburgh epidemiology of diabetes complications (EDC) study. J Diabetes Complications. 1995;9:140–8. doi: 10.1016/1056-8727(94)00039-q. [DOI] [PubMed] [Google Scholar]
- 38.Tong PC, Kong AP, So WY, Ng MH, Yang X, Ng MC, et al. Hematocrit, independent of chronic kidney disease, predicts adverse cardiovascular outcomes in chinese patients with type 2 diabetes. Diabetes Care. 2006;29:2439–44. doi: 10.2337/dc06-0887. [DOI] [PubMed] [Google Scholar]
- 39.Tapp R, Shaw J. In: Epidemiology of diabetic neuropathy. Tesdaye S, Boulton A, editors. Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- 40.Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of development of peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig. 2014;5:714–21. doi: 10.1111/jdi.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutta A, Naorem S, Singh TP, Wangjam K. Prevalence of peripheral neuropathy in newly diagnosed type 2 diabetics. Int J Diabetes Dev Ctries. 2005;25:30–3. [Google Scholar]
- 42.Miguel GA, Fernández EG, Rodríguez JCR, Pablos DL, Díaz-Guerra GM, Hawkins FG. Association Between Peripheral Neuropathy and Macrovascular Disease in Diabetic Patients. Presented at Endocrine Society's 97th Annual Meeting and Expo, March 5-8; San Diego. 2015 [Google Scholar]
- 43.Chawla R, Rathore P, editors. To Study the Prevalence of Diabetic Peripheral Neuropathy by Biothesiometric Evaluation & It's Co-association with Other Complications 2004(NNDU Proceedings 2004); 2004 [Google Scholar]
- 44.Chawla R, Poddar A, Chawla R. High Prevalence of CAN in New Onset T-2 Diabetes Patients. Presented on American Diabetes Association's 68th Scientific Session; June 8; San Francisco, CA. 2008 [Google Scholar]
- 45.Chawla R, Gupta S, Punyani H. Correlation Between Autonomic Neuropathy and Distal Peripheral Neuropathy and Its Co-association with Peripheral Vascular Disease in Type-2 Diabetes Mellitus Patients. Presented on American Diabetes Association's 70th Scientific Session; June 25; Orlando, FL, USA. 2010 [Google Scholar]
- 46.Chawla R, Rathore P. Evaluation of Lower Extremity Arterial Disease (LEAD/PVD) in Type-2 Diabetics and Its Co-association with Other Macro Vascular Complications. Presented on Annual Conference of Diabetic Foot Society of India's Scientific Session; September 4-5; Bhopal, India. 2004 [Google Scholar]
- 47.Chawla A, Bhasin GK, Chawla R. Validation of neuropathy symptoms score (NSS) and neuropathy disability score (NDS) in the clinical diagnosis of peripheral neuropathy in middle aged people with diabetes. Internet J Fam Pract. 2013;12:1. [Google Scholar]
- 48.Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL, Stern D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14:1521–8. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- 50.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 51.Kalousová M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51:597–604. [PubMed] [Google Scholar]
- 52.Kilhovd BK, Berg TJ, Birkeland KI, Thorsby P, Hanssen KF. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care. 1999;22:1543–8. doi: 10.2337/diacare.22.9.1543. [DOI] [PubMed] [Google Scholar]
- 53.Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53:131–42. [PubMed] [Google Scholar]
- 54.Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. 2011;7:313–24. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 55.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie L, Zhu X, Hu Y, Li T, Gao Y, Shi Y, et al. Mitochondrial DNA oxidative damage triggering mitochondrial dysfunction and apoptosis in high glucose-induced HRECs. Invest Ophthalmol Vis Sci. 2008;49:4203–9. doi: 10.1167/iovs.07-1364. [DOI] [PubMed] [Google Scholar]
- 57.Duncan JG. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta. 2011;1813:1351–9. doi: 10.1016/j.bbamcr.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Syed Ikmal SI, Zaman Huri H, Vethakkan SR, Wan Ahmad WA. Potential biomarkers of insulin resistance and atherosclerosis in type 2 diabetes mellitus patients with coronary artery disease. Int J Endocrinol. 2013;2013:698567. doi: 10.1155/2013/698567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otsuka A, Azuma K, Iesaki T, Sato F, Hirose T, Shimizu T, et al. Temporary hyperglycaemia provokes monocyte adhesion to endothelial cells in rat thoracic aorta. Diabetologia. 2005;48:2667–74. doi: 10.1007/s00125-005-0005-6. [DOI] [PubMed] [Google Scholar]
- 60.Tian J, Hu S, Sun Y, Yu H, Han X, Cheng W, et al. Vasa vasorum and plaque progression, and responses to atorvastatin in a rabbit model of atherosclerosis: Contrast-enhanced ultrasound imaging and intravascular ultrasound study. Heart. 2013;99:48–54. doi: 10.1136/heartjnl-2012-302775. [DOI] [PubMed] [Google Scholar]
- 61.Hayden MR, Tyagi SC. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: A malignant transformation. Cardiovasc Diabetol. 2004;3:1. doi: 10.1186/1475-2840-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel A. Does the role of angiogenesis play a role in atherosclerosis and plaque instability. Anat Physiol. 2014;4:147. [Google Scholar]
- 63.Xu J, Lu X, Shi GP. Vasa vasorum in atherosclerosis and clinical significance. Int J Mol Sci. 2015;16:11574–608. doi: 10.3390/ijms160511574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonnefond A, Saulnier PJ, Stathopoulou MG, Grarup N, Ndiaye NC, Roussel R, et al. What is the contribution of two genetic variants regulating VEGF levels to type 2 diabetes risk and to microvascular complications? PLoS One. 2013;8:e55921. doi: 10.1371/journal.pone.0055921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schratzberger P, Walter DH, Rittig K, Bahlmann FH, Pola R, Curry C, et al. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J Clin Invest. 2001;107:1083–92. doi: 10.1172/JCI12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–6. doi: 10.1016/s0014-5793(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 67.Laakso M. Heart in diabetes: A microvascular disease. Diabetes Care. 2011;34(Suppl 2):S145–9. doi: 10.2337/dc11-s209. [DOI] [PMC free article] [PubMed] [Google Scholar]


