Abstract
Romantic love could be considered as a collection of activities associated with the acquisition and retention of emotions needed to survive and reproduce. These emotions change the individual's behavioural strategies in a way that will increase the likelihood of achieving these goals. Love may be defined as an emergent property of an ancient cocktail of neuropeptides and neurotransmitters. It appears that lust, attachment and attraction appear to be distinct but intertwined processes in the brain each mediated by its own neurotransmitters and circuits. These circuits feed on and reinforce each other. Sexual craving is mediated by testosterone and oestrogen and has the amygdala as an important centre. Attraction is mediated by hormones of stress and reward including dopamine, norepinephrine cortisol and the serotinergic system and has the nucleus accumbens the ventral tegmental area as key mediators.
Keywords: Love, monogamy, neuroendocrine, oxytocin, prairie vole, vasopressin
He will not know what all but he do know.
And as he errs, doting on Hermia's eyes.
So I, admiring of his qualities.
Things base and vile, holding no quantity.
Love can transpose to form and dignity.
Love looks not with the eyes, but with the mind.
And therefore is winged Cupid painted blind.
Nor hath Love's mind of any judgment taste.
Wings and no eyes figure unheedy haste.
-William Shakespeare
Midsummer Night's dream (1.1.232-243)
INTRODUCTION
From an evolutionary perspective romantic love could be considered as a collection of activities associated with the acquisition and retention of emotions needed to survive and reproduce. These emotions change the individual's behavioral strategies in a way that will increase the likelihood of achieving these goals.[1] The enduring question for science has been that if these evolutionarily determined behaviors have a biologic substrate and correlation with activation of specific brain areas (and hormones)?[2] This review attempts to summarize our current understanding of the neuroendocrinology of romantic love.
A DEFINITION OF LOVE
While poets and philosophers are more adept at defining love, for the purposes of this review, this particular definition seems to be apt: Love is an emergent property of an ancient cocktail of neuropeptides and neurotransmitters.[3]
THE DICHOTOMY BETWEEN COURTSHIP AND SEX
Despite the intimate intertwining of the sexual drive with courtship, for over 4 decades investigators have suggested that these processes may be distinct [4] but operate in tandem [Table 1]. Human romantic love (hereafter just love) is cross-cultural, universal, and associated with distinct physiologic, psychological, and behavioral traits.[5] Many of these traits are also characteristic of mammalian courtship which includes increased energy, focused attention, obsessive following, affiliative gestures, possessive mate guarding, goal-oriented behavior, and motivation to win a preferred partner.[2]
Table 1.
Comparison of three motivations involved in “love”
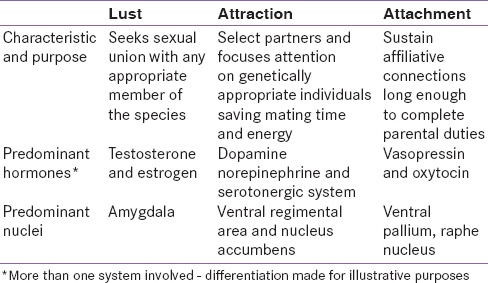
In humans, love often begins when an individual starts to regard another individual as special and unique. This is followed by focused attention, aggrandizement of traits and worth of the object of attention, and minimizing of his or her faults. There is increased ecstasy when things go well, despair when they do not and separation anxiety when apart.[6] Emotional dependence, empathy, sacrifice, and obsessive thinking are common. Sexual desire, intense sexual possessiveness, and mate guarding are present, but the emotional union appears to supersede the craving for sex. Rejection triggers protest and rage, moving into resignation and despair. It has been suggested that love as a preference system is associated with action and conditioning of specific neuroendocrine pathways.
LOVE AS A PRIMORDIAL DRIVE
It has been proposed the love is not primarily an emotion but a motivation system (i.e., a system oriented around the planning and pursuit of a specific want or need) designed to enable suitors to build and maintain an intimate relationship with a specific mating partner.[7] Functional magnetic resonance imaging (MRI) studies demonstrate the involvement of areas associated with motivation and goal-oriented behavior in love suggesting that love is a primary motivation system a fundamental human mating drive.[8] Several lines of evidence support this view that love is not an emotion but motivation and are reviewed elsewhere. (8) Love also appears to be stronger than sex drive-those rejected by sexual overtures rarely kill themselves or others. Abandoned lovers sometimes stalk, commit suicide homicide, or fall into clinical depression.[7]
STRESS AS AN INITIATOR/FACILITATOR OF LOVE
The early phase of love represents an extreme neurobiological state somewhat contradictory in a physiologic sense from subsequent phases and states. Stress appears to be the trigger for a quest for pleasure, proximity, and closeness. As a norm, moderate stress encourages social interaction.
Within a homeostatic range, stress-related physiologic processes including the hormones of the hypothalamo-pituitary adrenal axis can help develop and promote social bonding.[9] Indeed, some of the signs commonly associated with love-anxiety palpitations increased peristalsis are manifestations of the stress response (albeit in a pleasurable way). Indeed, subjects in love show higher levels of cortisol when compared to controls.[10] This “love induced hypercortisolemia” may represent a nonspecific stress response to change that characterizes early phases of relationships or a physiologic state of alertness that may help overcome neophobia. Irrespective, this stress response appears to be important in the formation of social contact and attachment.
Central norepinephrine may also be involved. Increased activity of norepinephrine generally produces alertness energy sleeplessness and loss of appetite, increased attention, and increased memory for new stimuli which characterize (the earlier) phases of human love.[11] Norepinephrine is also associated with peripheral sympathetic nervous systems including increased heart rate, sweating trembling which may explain this experience in love.[12]
Positive social interactions and pair bonding (see below) appear to alleviate stress through oxytocin (OT) facilitating security and support. It appears therefore that initial anxiety and stress is an inherent component of early love which reaches its fulfillment through “the chill” rendered by love and deep relationships. These appear to be mediated by a complex interaction between pathways that link stress response to reward mechanisms. Indeed, serotonin-dependent pathways such as the amygdala appear to interact with OT (see below). OT administration appears to decrease anxiety by inhibiting amygdala activity.[13]
GONADAL HORMONES
The role of gonadal hormones in this regard appears to be facilitatory but peripheral in love. Sex hormones may exert developmental effects on neural systems involved in social attachments and may mediate both genetic and environmental influences on the propensity to love and form attachments.[14] Testosterone receptors are distributed in the hypothalamus. Testosterone through these receptors may suppress levels or activity of serotonin which apparently increases aggressiveness. Testosterone further enhances vasopressin levels in the medial amygdala lateral hypothalamus and pre-optical medial area which are involved in aggressive behaviors.[15] Gonadal hormones may further regulate OT and vasopressin through indirect mechanisms. However, social attachment does occur even in the absence of gonadal steroids suggesting that gonadal hormones are only a small piece of an intricately knit puzzle that form the complex phenomenon called love.
Gender differences evident on functional imaging in the partner preference and early phases of love warrant mention. Men show more activity in a region of the right posterior dorsal insula (an area correlating with penile turgidity and viewing of beautiful faces) and in regions associated with the integration of visual stimuli. Women tend to show more activity than men in regions associated with attention, memory, and emotion. Courting men respond more strongly than women to visual signals of youth and beauty. Women appear to be more attracted to men who offer status and resources.[16]
VASOPRESSIN OXYTOCIN AND “PAIR BONDING”
Pair bonding is a very bland scientific term for enduring (romantic) relationships (attachment) and is seen in <5% of the mammalian species.[17] Pair bonding across species is defined as an enduring preferential association formed between two sexually mature adults and is characterized by selective contact, affiliation, and copulation with the partner over a stranger.[18] These are associated with other complex behaviors including mate guarding and biparental care of the young.
Pair bonding evolved most likely, as an adaptive response to the need of additional parental investment in the rearing of the young and mechanisms through which this relationship was preserved (mate guarding). In other words, romantic relationships and their persistence (through monogamy) was an evolutionary necessity in species in which bi-parental care of the offspring was critical. There are clear benefits to both partners of the relationships as well. In humans, individuals in stable marital relationships live longer than single individuals cross demographic groups. High levels of intimacy correlate negatively with depression and positively with immune function and cardiovascular health.[19]
Most of our knowledge on the neuroendocrinology of intimacy is based on the work on the prairie vole (Microtus ochrogaster) a humble but socially monogamous model from the grasslands of the central United States. In this harsh grassland with scarce resources, the prairie vole evolved into a monogamous animal with breeding pairs living together until one partner dies; the surviving partner does not find another mate. The male prairie vole is highly paternal, helps with nest building guards the nest from conspecific strangers. In general, an animal with low levels of aggression, the male displays enhanced levels of aggression toward strange males. It displays high levels of paternal behavior to litters; this extends to juveniles even after a second litter is born.
Arginine vasopressin (AVP) OT and dopamine (DA) have been reported to be important in regulating social behavior including sexual behavior, aggression, and maternal care. While there are no differences in AVP and OT neurons or their distribution between the monogamous prairie voles and their polygamous cousins, remarkable differences are seen in the receptor distribution (the V1aR and OT receptor [OTR]) and their densities. Interestingly, these densities are stable across the lifespan of the vole. These differences may explain by subtle differences in the promoter region the V1aR and the OTR.[20] The human version of this gene has similar polymorphisms. It is possible that epigenetic modifications of the OTR are also involved.[21]
In the male prairie vole, cohabitation with mating appears to increase AVP synthesis in the bed nucleus of the stria terminalis and AVP release in the limbic system. In the female chemosensory clues altered OTR density in the AOB. Activation of the OT and vasopressin receptors in these centers might result in the development of a conditioned partner preference in prairie voles. Antagonism of the OTR impairs the formation of pair bonds. This effect appears to be larger in the female.
Knockdown of VP production in the paraventricular nucleus of the hypothalamus in the zebra finch increases aggressiveness in the male decreases it the female and reduces gregariousness in both. Knockdown of OT reduces gregariousness, pair bonding, nest cup ownership and side by side perching in females and induces hyperphagia in males.[22] An association between arginine vasopressin receptor 1A polymorphism and human pair bonding behavior analogous to voles has been reported.
AVP activity in the ventral palladium affects partner preference. V1aR activation in this region is necessary for pair bond formation.[23] Similarly, activation of the OT in the nucleus accumbent (NA) also contributes to partner preference and pair bonding.
Functional MRI studies of human partners in long-term relationships show activation the ventral palladium putamen the anterior cingulate cortex and the mid-insular cortex.[24] The ventral putamen/palladium region in particular corresponds to the distribution of V1a receptors in the prairie vole. The areas appear, and connections appear to be distinct but related to those for maternal love. Maternal love activated specific different areas including the lateral orbit frontal cortex but also some same areas as (romantic) love including medial insula, the anterior cingulate gyrus, and caudate nucleus. Both appear to share areas rich in OT and AVP receptors.
LOVE AS A REWARD
From the very beginning of our efforts, in understanding the biologic basis of love it has been clear that it involves reward centers in the brains. In this love and addictions (such as by drugs) are somewhat interconnected the one key difference is that naturally rewarding activities such as love are controlled by feedback mechanisms that activate aversive centers that limit the destructive qualities of addiction seen with drugs.[25] Love activates specific regions in the reward system. The effects include a reduction in emotional judgment and reduced fear and also reduced depression and enhanced mood. It also leads to a reduced need to assess the social validity of that person.[26] It thus appears to deactivate areas mediating negative emotions, avoidance behavior critical social assessment and, on the other hand, triggers mechanisms involved in pleasure reward and appetitive motivation [Figure 1].[27]
Figure 1.
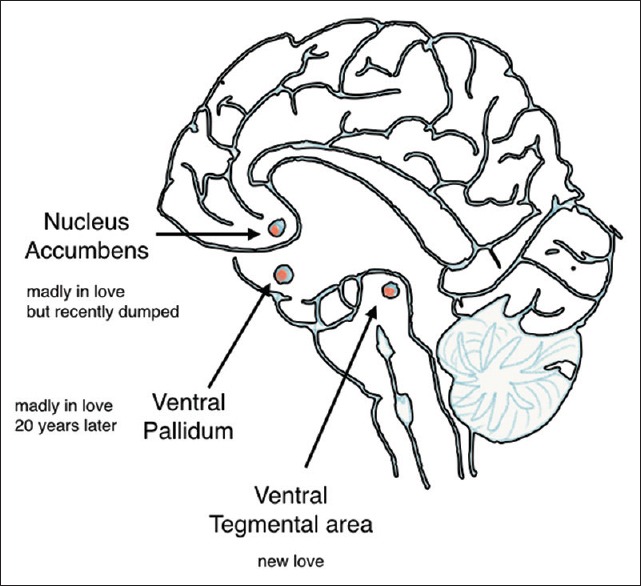
Nuclei associated with motivations collectively involved in “love”
Studies which have examined the OT and AVP receptors strongly suggest that the activation of these receptors in the reward circuitry is important for the development of pair bonding. As a critical part of the reward process, DA appears to be central to the maintenance of love. Differences in DA and its receptor distribution densities have been reported in vole studies (vide above). Dopaminergic pathways appear to be more specific for partner preference that attachment.[28]
While several DA systems exist in the brain, the mesolimbic DA system appears to be the most important in this respect. Both D1 and D2 receptors though partially functional antagonists are both significantly expressed in the NA. Other DA receptors (D3-5) are also linked to the limbic system and are substantially present in the amygdala and the hippocampus. Their functions include reward and motivation and appear to share common morphologic evolutionary and molecular roots. Endogenous opioids may also be involved in this process.
Early studies that involved functional MRI, which used the partner's photograph as a visual stimuli confirm the involvement of the right ventral segmental area (VTA) which is a central region of the brain's reward system [29] associated with pleasure, general arousal, focused attention, and motivation to pursue and acquire rewards.[24] The VTA projects into several regions including the caudate nucleus which plays a role in reward detection, expectation, representation of goals, and integration of sensory inputs to prepare for action. These appear to be true of both early intense (7.4 months) and a little later and not so intense love (28.8 months).[30]
THE ROLE OF SEXUAL ACTIVITY
Clearly, sexual activity is an important component of the reinforcement of the reward system, and this appears to reinforce attachment. Increasing levels of testosterone and oestrogen promote DA release.[31] Similarly, elevated activity of dopaminergic pathways appear to increase the release of testosterone and oestrogen. The relationship between elevated central DA sex steroids, sexual arousal, and performance appears to be conserved in humans (7). The sympathetic nervous system also appears to contribute.
Behavioral data support the complementary but distinct pathways for love and sex drive: (a) While sexual drive is often expressed toward a range of individuals while love is focused on one particular individual (b) the sex drive can be quelled when satiated; love does not decrease with coitus and persists unabated for months or years. Sex drive enables individuals to initiate courtship and mating with a range of partners; love focuses mating energy to specific individuals conserving time and metabolic energy (6).
Pleasure and reward activate behavioral patterns that get memorized for the goal of repetition and faster and better recognition later. There is clear evidence to support a connection between attachment behaviors and pleasure pathways that involve hippocampal mechanisms.
The VP is a major target of the NA. The interaction of OT, AVP, and the DA systems within the reward circuitry appears to be the foundation of monogamy. It is hypothesized that in monogamous species (such as the prairie vole) sex triggers the activity of AVP in the ventral pallidum and OT in the NA and facilitates DA release in these reward regions which in turn motivates the male and female to prefer a current mating partner and initiates attachment or pair bonding. In promiscuous species, the male feels the attraction but does not associate the pleasurable feeling with a specific partner and so does not initiate long-term attachment (23). The relationship with the DA reward systems also appears weaker in these species.[32] Complex interactions between gonadal reward and sympathetic systems demonstrate there are distinct but overlapping neural networks involved in love and sex the latter contributing to the reinforcement of the former. Their interdependence also distinguishes romantic love from more platonic attachments including friendship and maternal love (vide above).
It is interesting to extrapolate this to humans. Humans engage in sexual activity throughout the cycle which may serve to strengthen the pair bond. Interestingly and in contrast to other species human females have enlarged mammary tissue independent of lactation. Breast and nipple stimulation are integral to human sexual activity.[33] Nipple stimulation during lactation is one of the most potent stimulus for OT release.[34] This part of sexual activity may reinforce pair bonding in humans. OT levels are elevated in women during orgasm and AVP in men increase during sexual arousal adding validity to the notion that sexual activity indeed reinforces the bond. In the postpartum state when sexual activity and desire decreases probably as a trade off in reproductive interests. This appears to be mediated by OT through activation of reward centers in the VTA.[35]
Thus, the neuroendocrine system for sexual attraction and partner attachment appear to work in tandem in a monogamous species motivating individuals to prefer a specific mating partner and also motivating them to form an attachment to this mate. In nonmonogamous species, sexual attraction and partner attachment appear to operate independently. The neuroendocrine networks that mediate these complex relationships appear to themselves be complex, flexible, and interdependent and facilitate individuals of myriad species with the range of motivations, emotions, and behaviors necessary to pursue their species-specific reproductive strategy.[8]
CONCLUSION
Landmark studies in the prairie vole coupled with functional MRI studies have helped us understand the complex interplay of distinct pathways that mediate sexual attraction, romantic love maternal love, and platonic friendships. Further ongoing research is attempting to delineate the biologic basis of complex traits including fidelity, trust, and spirituality. The role of modification of these neurotransmitters particularly OT in the therapy of autism, in trust deficit and in behavioral diseases requires further delineation. For example, OT is generally regarded as a hormone that enhances trust behavior. However, its administration of OT in women who have a forgiving attitude increases the chance that they may punish betrayal.[36] Thus, the response to therapy with OT (and by inference other peptides) appears to be conditional to differences in the individual. These include sex and hormonal status, variations in the OTR, early experiences, epigenetic changes, and neuroplasticity.[37] Large scale trials will further clarify the role of peptide therapy in modulating behavior. And whether successful or not, manipulating love and emotions hoists a slew of ethical red flags. At the time of this writing, however, romantic love retains its mystique continues to tantalize it has revealed some secrets but has manage concealed the vital.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Geary DC, editor. Male, female: The evolution of human sex differences. Washington, DC, US: American Psychological Association; 1998. p. xii. [Google Scholar]
- 2.Fisher H, Aron A, Mashek D, Li H, Strong G, Brown LL. The neural mechanisms of mate choice: A hypothesis. Neuro Endocrinol Lett. 2002;23(Suppl 4):92–7. [PubMed] [Google Scholar]
- 3.Young LJ. Being human: Love: Neuroscience reveals all. Nature. 2009;457:148. doi: 10.1038/457148a. [DOI] [PubMed] [Google Scholar]
- 4.Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–38. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- 5.Jankowiak WR, Fischer EF. A cross-cultural perspective on romantic love. Ethnology. 1992;31:149. [Google Scholar]
- 6.Fisher HE, Aron A, Brown LL. Romantic love: A mammalian brain system for mate choice. Philos Trans R Soc Lond B Biol Sci. 2006;361:2173–86. doi: 10.1098/rstb.2006.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aron A, Paris M, Aron EN. Falling in love: Prospective studies of self-concept change. J Pers Soc Psychol. 1995;69:1102–12. [Google Scholar]
- 8.Fisher H. Why we Love: The Nature and Chemistry of Romantic Love. New York: Henry Holt; 2004. [Google Scholar]
- 9.DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci U S A. 1996;93:11980–4. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marazziti D, Canale D. Hormonal changes when falling in love. Psychoneuroendocrinology. 2004;29:931–6. doi: 10.1016/j.psyneuen.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Griffin MG, Taylor GT. Norepinephrine modulation of social memory: Evidence for a time-dependent functional recovery of behavior. Behav Neurosci. 1995;109:466–73. doi: 10.1037//0735-7044.109.3.466. [DOI] [PubMed] [Google Scholar]
- 12.Fisher HE. Lust, attraction, and attachment in mammalian reproduction. Hum Nat. 1998;9:23–52. doi: 10.1007/s12110-998-1010-5. [DOI] [PubMed] [Google Scholar]
- 13.Mottolese R, Redouté J, Costes N, Le Bars D, Sirigu A. Switching brain serotonin with oxytocin. Proc Natl Acad Sci U S A. 2014;111:8637–42. doi: 10.1073/pnas.1319810111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 15.Giammanco M, Tabacchi G, Giammanco S, Di Majo D, La Guardia M. Testosterone and aggressiveness. Med Sci Monit. 2005;11:RA136–45. [PubMed] [Google Scholar]
- 16.Buss DM, Abbott M, Angleitner A, Asherian A, Biaggio A, Blanco-Villasenor A, et al. International preferences in selecting mates: A study of 37 societies. J Cross Cult Psychol. 1990;21:5–47. [Google Scholar]
- 17.Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- 18.Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–5. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 20.Young LJ, Waymire KG, Nilsen R, Macgregor GR, Wang Z, Insel TR. The 5' flanking region of the monogamous prairie vole oxytocin receptor gene directs tissue-specific expression in transgenic mice. Ann N Y Acad Sci. 1997;807:514–7. doi: 10.1111/j.1749-6632.1997.tb51955.x. [DOI] [PubMed] [Google Scholar]
- 21.Puglia MH, Lillard TS, Morris JP, Connelly JJ. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc Natl Acad Sci U S A. 2015;112:3308–13. doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly AM, Goodson JL. Hypothalamic oxytocin and vasopressin neurons exert sex-specific effects on pair bonding, gregariousness, and aggression in finches. Proc Natl Acad Sci U S A. 2014;111:6069–74. doi: 10.1073/pnas.1322554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–34. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- 25.Bozarth MA. Pleasure systems in the brain. In: Wartburton DM, editor. Pleasure: The Politics and the Reality. New York: Wiley & Sons; 1994. pp. 5–14. [Google Scholar]
- 26.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Esch T, Stefano GB. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol Lett. 2004;25:235–51. [PubMed] [Google Scholar]
- 28.Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004;468:555–70. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- 29.Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–37. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- 30.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 31.Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–41. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendrick KM. Oxytocin, motherhood and bonding. Exp Physiol. 2000;85:111S–24S. doi: 10.1111/j.1469-445x.2000.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 33.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 34.Christensson K, Nilsson BA, Stock S, Matthiesen AS, Uvnäs-Moberg K. Effect of nipple stimulation on uterine activity and on plasma levels of oxytocin in full term, healthy, pregnant women. Acta Obstet Gynecol Scand. 1989;68:205–10. doi: 10.3109/00016348909020990. [DOI] [PubMed] [Google Scholar]
- 35.Gregory R, Cheng H, Rupp HA, Sengelaub DR, Heiman JR. Oxytocin increases VTA activation to infant and sexual stimuli in nulliparous and postpartum women. Horm Behav. 2015;69:82–8. doi: 10.1016/j.yhbeh.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao S, Zhao W, Cheng R, Geng Y, Luo L, Kendrick KM. Oxytocin makes females, but not males, less forgiving following betrayal of trust. Int J Neuropsychopharmacol. 2014;17:1785–92. doi: 10.1017/S146114571400090X. [DOI] [PubMed] [Google Scholar]
- 37.Macdonald KS. Sex, receptors, and attachment: A review of individual factors influencing response to oxytocin. Front Neurosci. 2013;6:194. doi: 10.3389/fnins.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]


