Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Prospectively collected data on demographics, complications, and mortality are described for 4899 US men with severe hemophilia.
Analyzing multiple birth cohorts of US men with severe and mild hemophilia demonstrates ongoing morbidity in need of surveillance.
Abstract
The availability of longitudinal data collected prospectively from 1998 to 2011 at federally funded US hemophilia treatment centers provided an opportunity to construct a descriptive analysis of how outcomes of men with severe hemophilia have been altered by the incremental advances and setbacks in hemophilia care in the last 50 years in the United States. This surveillance collaboration with the US Centers for Disease Control and Prevention assembled the largest uniformly examined population with severe hemophilia (n = 4899 men with severe factor VIII and IX deficiency). To address the heterogeneity of this population, 4 successive birth cohorts, differentially affected by eras of hemophilia care, were examined separately in regard to demographics, complications of hemophilia and its treatment, and mortality. Severely affected men in each birth cohort were compared also with the corresponding mild hemophilia birth cohorts (n = 2587 men total) to control for outcomes that might be attributable to aging and environment independent of severely defective hemostasis. The analysis demonstrates improving access to standard of care therapy, correlating the proportion of men on prophylactic factor replacement and reduced bleeding frequency for the youngest men. Frequent bleeding persisted in one third to one half of men across all ages, however, and the disability gap between severe and mild hemophilia did not narrow. The greatest cause of death was liver failure, but attempted anti–hepatitis C virus therapy and cure were low. The study suggests a continued need for national surveillance to monitor and inform hemophilia interventions and outcomes.
Introduction
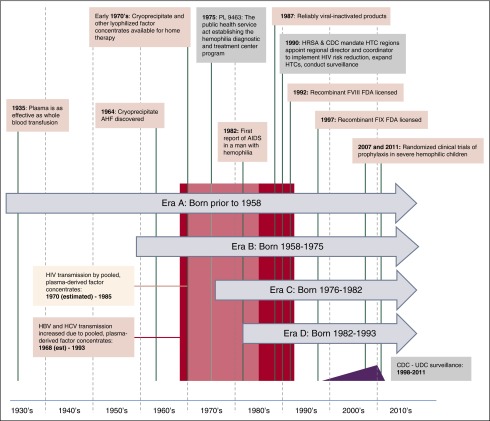
Over approximately the last 5 decades, the experience of men and boys with hemophilia has been characterized by remarkable progress in drug therapies and the delivery of multidisciplinary care,1 interrupted by tragic setbacks from transfusion-transmitted infections (Figure 1).2 The oldest generation of men with hemophilia in the United States experienced childhood with no or little availability of clotting factor replacement. As the first lyophilized therapies became broadly available in the early 1970s, home infusion therapy became possible, allowing rapid on-demand treatment of hemorrhage and, subsequently, prophylactic factor replacement. The devastating effects of HIV, hepatitis C virus (HCV), and hepatitis B virus (HBV) contamination of plasma-derived factor concentrates drove the rapid development and licensure of recombinant factor products in the 1990s.
Figure 1.
Hemophilia eras, landmarks in hemophilia care, and eras. A pictorial description of landmarks in hemophilia and the separate eras representing different birth cohorts of men with hemophilia that are examined in this analysis.
In 1975, the US Congress appropriated funds to create a national network of Hemophilia Diagnostic and Treatment Centers, which grew into the current US hemophilia treatment center network (HTCN).3 The US Centers for Disease Control and Prevention (CDC) partnered with this network in the late 1980s to implement strategies to prevent the spread of HIV infection and subsequently expanded surveillance and prevention programs implemented through the hemophilia treatment centers (HTCs) to address additional complications of bleeding disorders.4 In 1998, this CDC/HTCN collaboration launched a national public health surveillance designated the Universal Data Collection (UDC).5 Modern hemophilia care in the United States is characterized by an adequate supply of pathogen-free clotting factor concentrate infused at home, either prophylactically or on-demand, with the majority of the hemophilia population receiving care through a network of ∼130 regionally organized HTCs practicing a team-based integrated services model.6
Incremental improvements in the standards and access to care, as well as setbacks related to treatment complications, have naturally created eras of care affecting successive birth cohorts of men with severe hemophilia. The availability of longitudinal data collected by the UDC on 4899 men with severe factor VIII and IX deficiency provided an opportunity to construct a descriptive analysis of how outcomes have been altered by the incremental advances and setbacks in hemophilia care in the last 50 years in the United States.
Methods
Data collection
Staff at 130 HTCs (>95% of the US HTCN) used uniform data collection forms to register and annually monitor patients in the UDC database from May 1998 to September 2011. Institutional Review Boards at the CDC and all participating institutions approved the study. All participants gave informed consent. Data for this analysis from the initial registration visit included month and year of birth, self-reported race/ethnicity, age at diagnosis, baseline factor activity, and date of first HTC visit. Deaths (including cause) were reported using a standardized mortality form during the entire study period. All other data came from the most recent HTC visit in which UDC forms were completed. Adults (>18 years of age) with physician-diagnosed mild or severe hemophilia were used for this descriptive analysis.
Hemophilia severity was defined based on the plasma level of baseline factor activity recorded at registration, with severe having levels of <1% of normal and mild having levels of >5% to 50% (note that only 2% of men had levels reported between 40% and 50%, an area of unresolved controversy in the definition of mild hemophilia).7 End points that reflect access to care included age at diagnosis, age at first HTC visit, frequency of HTC utilization, treatment type (episodic vs prophylactic), age at initiation of home infusion, and health insurance type. Data collected to assess potential disease-related complications included self-reported measures of physical function and bleeding complications including target joints, employment status, and body mass index (BMI). Treatment-related complications included the results of serologic tests performed at CDC for HBV, HCV, and HIV infections, reported receipt of antiviral therapy, evidence of hepatitis morbidity, and development of inhibitors. HTCs reported date and a single attributable cause of death. (Please see supplemental Methods, available on the Blood Web site, for detailed variable definitions.)
Analyses
Critical changes in hemophilia therapy and US HTC health care delivery were identified and used as landmarks to construct four birth cohorts (hereafter referred to as eras; Figure 1). Era A included men born prior to 1958; era B men were born from 1958 to 1975; era C men were born from 1976 to 1982; and era D men were born from 1982 to 1993. US men born within a given era shared broadly similar treatment and life course experiences (Table 1; Figure 1). The eras serve as a framework to evaluate how changes in hemophilia therapies and health care delivery have affected US men with hemophilia within each era over time.
Table 1.
Eras of the experience of hemophilia disease and care, assigned to men with hemophilia for purposes of the descriptive analysis
| Birth years | Description | |
|---|---|---|
| Era A | Prior to 1958 | The assays required to determine specific diagnosis and degree of deficiency were not available in childhood for most men in this cohort, who were diagnosed based on bleeding severity and nonspecific assays. During early childhood and musculoskeletal development, plasma but not clotting factor replacement was available. Men in this cohort had neither access to specialized HTC clinical care nor home-based therapy during childhood. |
| Era B | 1958-1975 | During this transitional era, accurate laboratory diagnosis and treatment with cryoprecipitate or clotting factor VIII concentrate gradually became available during childhood, but only in a limited number of bleeding disorder clinics primarily located in academic, urban hospitals.28 Factor IX concentrates were unavailable during childhood for most in this birth cohort. Congress enacted the law establishing the first HTCs in 1975. |
| Era C | 1976-1982 | Accurate laboratory diagnosis and effective plasma-derived hemostatic agents became more widely available beginning in early childhood. Hemostatic agents for individuals with inhibitors were introduced. The innovation of teaching patients/families to administer factor concentrate in the home29-31 for prompt on demand bleeding treatment was increasingly implemented at specialty hemophilia clinics. The number of HTCs grew, primarily in large urban areas. Nevertheless, treatment with factor concentrates resulted in exposure to the HBV and HCV viruses and HIV during this period. |
| Era D | 1982-1993 | The men in this cohort had access to virally-inactivated clotting factor concentrates (and subsequently recombinant factor) available throughout most of childhood. Simultaneously, HTCs expanded in number and geographic reach through federally mandated regionalization, and national systems for surveillance of blood safety were established. Therapy was prescribed through the growing system of coordinated regional comprehensive HTCs. Home therapy was accepted as standard of care for all ages.32,33 Prophylactic clotting factor replacement was recommended as the standard of care for children in the US, although widespread adoption of primary prophylaxis was slow during the time this group of men were children. |
We chose 2 frameworks to describe the impact of changes in hemophilia care on the outcomes of men with severe hemophilia. The first examines these men within the experiential birth cohorts delineated era A through era D. The second framework attempts to control for the effect of aging on the measured outcomes by comparing outcomes and interventions in men with severe hemophilia in era A to era D to men in the identical aging cohorts having only mild clotting factor deficiency. The second framework acknowledges that differences in demographics, outcomes, and complications for men in each era can be affected by aging even in the absence of a severe hemostatic defect.
Results
Data from the most recent UDC enrollment were available for 7486 men with mild or severe hemophilia (Table 2). Of these, 4899 had severe hemophilia (65.4%) and 2587 had mild hemophilia (34.6%); 6094 men had hemophilia A (81.4%) and 1392 had hemophilia B (18.6%). The younger three eras contained roughly similar numbers of men with severe hemophilia, and within each birth cohort, there were more men with severe hemophilia than mild hemophilia (2.0-2.9 times more severe hemophilia UDC participants in eras B-D). Only in the era of men born before 1958 did men surviving with mild hemophilia outnumber men with severe hemophilia. The mean (median) ages of the men were 59.5 (58), 39.9 (40), 27.2 (28), and 21.9 (21) years in cohorts A to D, respectively.
Table 2.
Demographics and access to care
| Era A | Era B | Era C | Era D | |||||
|---|---|---|---|---|---|---|---|---|
| <1958 | 1958-1975 | 1976-1982 | 1983-1992 | |||||
| Severe | Mild | Severe | Mild | Severe | Mild | Severe | Mild | |
| Total number | 688 | 843 | 1542 | 787 | 1121 | 427 | 1548 | 530 |
| Hemophilia type (%) | ||||||||
| A (FVIII deficiency) | 78.8 | 74.7 | 82.2 | 75.1 | 87.2 | 80.1 | 85.3 | 80.2 |
| B (FIX deficiency) | 21.2 | 25.3 | 17.8 | 24.9 | 12.8 | 19.9 | 14.7 | 19.8 |
| Race/ethnicity (%) | ||||||||
| White (non-Hispanic) | 78.6 | 87.4 | 69.7 | 79.2 | 62.2 | 74 | 62.3 | 73.2 |
| African American (non-Hispanic) | 11.8 | 4.7 | 14.7 | 7.9 | 16.2 | 5.4 | 16.4 | 6.0 |
| Hispanic | 5.2 | 4.5 | 8.8 | 8.9 | 12.4 | 17.6 | 13.8 | 14.3 |
| Asian | 2.5 | 1.1 | 3.4 | 2.0 | 5.0 | 1.6 | 3.2 | 1.5 |
| Other | 1.9 | 2.2 | 3.3 | 2.0 | 4.3 | 1.4 | 4.3 | 4.9 |
| Access to care (%) | ||||||||
| Age at diagnosis: Birth | 2.2 | 0 | 2.3 | 1.4 | 3.2 | 1.9 | 2.6 | 1.1 |
| First HTC visit: ≤2 years old | 7.3 | 1.5 | 23.5 | 11.1 | 53.1 | 23.2 | 69.1 | 32.3 |
| HTC visits: Frequent | 84.2 | 56.2 | 82.8 | 55.4 | 79.2 | 54.1 | 82.9 | 62.4 |
| Treatment regimen: Prophylaxis | 15.8 | 0.2 | 20 | 1.8 | 27.6 | 1.2 | 45.4 | 1.3 |
| Treatment regimen: Home infusion | 70.3 | 25.6 | 76.1 | 32.4 | 74.2 | 34 | 73.9 | 25.8 |
| Start home infusion: <6 years old | 1.6 | 0.4 | 10.0 | 1.2 | 28.5 | 4.4 | 45.7 | 4.7 |
| Health insurance (%) | ||||||||
| Commercial | 36.5 | 52 | 43.2 | 67.3 | 48 | 61.8 | 51.9 | 68.5 |
| Medicaid | 9.2 | 4.6 | 16 | 8.6 | 23.6 | 10.8 | 24.2 | 12.1 |
| Medicare | 47.4 | 33.1 | 29.6 | 7.9 | 10.5 | 4.2 | 4.4 | 0.6 |
| Uninsured | 1.4 | 4.3 | 3.2 | 7.9 | 6.7 | 13.8 | 7.3 | 9.1 |
Demographics and access to care
Race/ethnicity.
The proportions of nearly all minority populations with severe hemophilia consistently increased with each successively younger era: the proportion of nonwhite UDC enrollees increased from 21% in era A to 38% in era D (Table 2).
Access to care.
Comparing eras D vs A, the proportion of men who reported starting home infusion before age 6 was far greater (>45% in era D vs <2% in era A) and those reporting a first HTC visit before age 2 years rose nearly 10-fold (Table 2). Use of a continuous prophylactic factor regimen was nearly threefold greater in the youngest compared with the oldest adults (approaching 50% in era D). The eras differed in regard to insurance, as expected, in line with eligibility criteria for US insurance by age, income, and disability. Participants with severe hemophilia were twice as likely as their mild hemophilia counterparts to have Medicaid and Medicare. With each progressively younger cohort, the likelihood of having commercial insurance grew. Commercial insurance exceeded 50% only for the severe hemophilia cohort in era D, whereas commercial insurance exceeded 50% for all mild hemophilia cohorts. Despite this trend, individuals in the 2 youngest cohorts were also more likely to be uninsured, a pattern that was true regardless of severity of hemophilia. Individuals with mild hemophilia were more likely to be uninsured than individuals with severe hemophilia in each era.
Disease-related complications
Bleeding and target joints.
The proportion of participants reporting frequent bleeds decreased with successively younger era (Table 3). However, frequent bleeding was prevalent even in the era treated with the most modern therapies: >1 in 3 participants with severe hemophilia in era D reported frequent hemorrhages (>5 bleeds in 6 months); 1 in 4 reported a target joint for recurrent hemorrhage.
Table 3.
Disease-related complications
| Era A | Era B | Era C | Era D | |||||
|---|---|---|---|---|---|---|---|---|
| <1958 | 1958-1975 | 1976-1982 | 1983-1992 | |||||
| Severe | Mild | Severe | Mild | Severe | Mild | Severe | Mild | |
| Total N | 688 | 843 | 1542 | 787 | 1121 | 427 | 1548 | 530 |
| Abnormal BMI (%)* | ||||||||
| Obese or overweight (BMI >25) | 48.7 | 73.5 | 52.1 | 69.5 | 49.1 | 56.4 | 44.1 | 50 |
| Underweight (BMI <18.5) | 4.1 | 0.4 | 3.4 | 1.5 | 5.5 | 0.2 | 2.8 | 1.3 |
| Employment and disability status | ||||||||
| Disabled | 45.9 | 12.9 | 37.8 | 14.7 | 15.9 | 3.5 | 5.8 | 1.9 |
| Employed/student | 31.2 | 49.5 | 53.1 | 74.5 | 68.6 | 86.4 | 79.6 | 87.6 |
| Retired | 18.3 | 31.7 | 0.4 | 0.5 | 0 | 0 | 0 | 0 |
| Other | 3.5 | 5.9 | 8.6 | 10.3 | 15.5 | 10.1 | 14.5 | 10.6 |
| Physical function (%) | ||||||||
| Limitation to overall activity level† | 68.8 | 21.1 | 49.4 | 16.3 | 25.9 | 6.6 | 14.9 | 4.3 |
| School/work absenteeism: >10 days missed | 6.9 | 2.6 | 8.5 | 5.2 | 10 | 5.6 | 5.6 | 3 |
| Assistive devices: Intermittent | 39.8 | 16 | 35.1 | 15.4 | 28.8 | 13.6 | 22.9 | 11.3 |
| Assistive devices: Always | 18.5 | 4.6 | 6.6 | 1.5 | 1.7 | 0 | 1.4 | 0.8 |
| Wheelchair: Intermittent | 18.6 | 5.5 | 9.5 | 2.8 | 6.2 | 2.8 | 4.3 | 1.7 |
| Wheelchair: Always | 9 | 0.7 | 4.2 | 0.1 | 1.7 | 0.2 | 0.8 | 0.8 |
| Bleeding complications (%) | ||||||||
| ≤2 joint bleeds in last 6 months | 45.8 | 97.2 | 38.1 | 92.9 | 41.3 | 92.0 | 51.7 | 94.7 |
| ≥5 joint bleeds in last 6 months | 42.6 | 1.7 | 48.8 | 4.3 | 46.6 | 3.8 | 35.5 | 3 |
| Target joint | 32.6 | 3.2 | 36.2 | 7.2 | 35.6 | 5.8 | 24.9 | 2.3 |
BMI was calculated from height and weight measurements taken by clinic staff. Based on this measure, each participant was categorized as underweight if BMI was <18.5, normal if 18.5 to 24.9, overweight if 25 to 29.9, and obese if BMI was ≥30.34
Limitation of activity used UDC definitions as included in the supplemental Methods. The degree of self-reported limitation of activity considered positive for this analysis included limitations of school/work activities or self-care activities or limitation requiring assistance for school/work/self-care and unable to participate in recreational activities.
Physical functioning, activity limitation, and absenteeism.
The proportion of participants who reported that pain, loss of motion, or weakness limited their overall activity (school/work activities and/or self-care activities) was highest in era A and decreased with each successively younger era (Table 3). Those with severe hemophilia were consistently ∼3 times more likely to report activity limitations and twice as likely to report intermittent use of assistive devices for mobility/ambulation as their mild hemophilia comparators in all eras. The proportion of participants with severe hemophilia that missed ≥10 days of work or school in the immediately preceding year due to upper or lower extremity joint problems was 2 or 3 times that of men of the same age with mild hemophilia, regardless of era.
Disability and employment.
In era A, nearly half of the participants were disabled and unable to work (Table 3). In successively younger birth cohorts, fewer men who reported that they were neither employed nor in school indicated that their status was “disabled.” Despite this absolute decline in disability, men with severe hemophilia were still ∼3 times as likely to be disabled as their mild hemophilia counterparts, regardless of the era.
Treatment-related complications
HBV, HCV, and HIV.
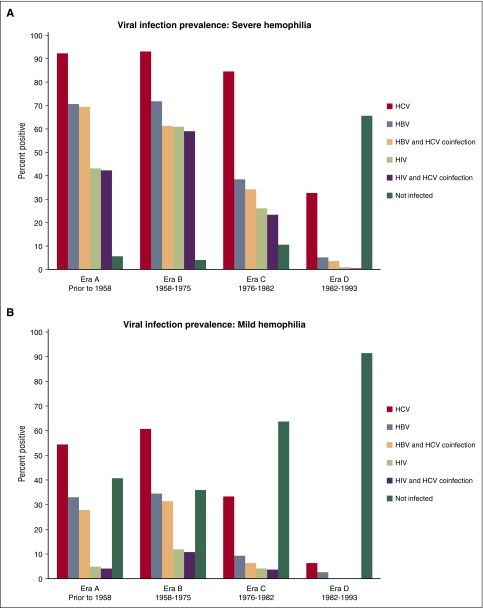
Since 1992, the UDC population experienced no new infections of HBV, HCV, or HIV attributable to plasma-derived or recombinant coagulation factor therapy. Nevertheless, infectious complications remain a critical concern among men with severe hemophilia in all eras: 95% of men with severe hemophilia in the oldest 2 eras, 90% in era C, and 35% in the youngest era D have seroconverted to either HBV, HCV, HIV, or a combination of these agents (Table 4; Figure 2A). Only the youngest cohort of men with severe hemophilia had a prevalence of HCV infection <80%.
Table 4.
Treatment-related complications
| Era A | Era B | Era C | Era D | |||||
|---|---|---|---|---|---|---|---|---|
| <1958 | 1958-1975 | 1976-1982 | 1983-1992 | |||||
| Severe | Mild | Severe | Mild | Severe | Mild | Severe | Mild | |
| Total N | 688 | 843 | 1542 | 787 | 1121 | 427 | 1548 | 530 |
| Viral infections (% positive) | ||||||||
| HBV | 70.5 | 33 | 71.7 | 34.4 | 38.4 | 9.1 | 5.2 | 2.4 |
| HCV | 92.3 | 54.2 | 93 | 60.6 | 84.4 | 33.3 | 32.6 | 6.2 |
| HBV and HCV coinfection | 69.2 | 27.9 | 61.2 | 31.5 | 34.2 | 6.1 | 3.7 | 0.4 |
| HIV | 42.9 | 4.7 | 61 | 11.9 | 26.2 | 4 | 1 | 0.2 |
| HIV and HCV coinfection | 42.2 | 4.2 | 59 | 10.8 | 23.3 | 3.7 | 0.7 | 0 |
| Not infected with HIV, HBV, or HCV | 5.5 | 40.7 | 4.1 | 35.8 | 10.4 | 63.7 | 65.4 | 91.5 |
| Ever received therapy for chronic viral hepatitis | ||||||||
| Any therapy (% of total infected HCV) | 29.0 | 33.0 | 32.7 | 36.9 | 24.2 | 29.6 | 26.7 | 33.3 |
| Sustained virulogic response (% of total infected HCV) | 9.3 | 10.1 | 10.4 | 16.4 | 9.6 | 10.5 | 8.7 | 18.3 |
| Hepatitis morbidity (%) | ||||||||
| Chronically elevated ALT/AST | 29.6 | 16.7 | 29.8 | 16.9 | 17 | 7.7 | 5.6 | 2.4 |
| Signs or symptoms of liver disease | 8 | 3.2 | 6.2 | 3.8 | 2 | 0.2 | 0.2 | 0 |
| Inhibitor development | ||||||||
| Any inhibitor recorded | 17.0 | 3.3 | 13.6 | 3.6 | 11.5 | 2.8 | 15.6 | 2.8 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Figure 2.
Seroprevalence of hepatitis and HIV infections in US men with hemophilia enrolled in the UDC. Of the 7486 participants with severe and mild hemophilia, 2702 have serologically confirmed HBV infection (36.1%), 4629 have serologically confirmed HCV infection (61.8%), and 1696 (22.7%) have serologically confirmed HIV infection. (A) Age distribution-prevalence of viral infection in men with severe hemophilia. (B) Age distribution-prevalence of viral infection in men with mild hemophilia.
The highest observed prevalence of HIV infection was found in era B, with declining prevalence in each successive era to 1% in the youngest era (Table 4; Figure 2). In all eras, regardless of severity, the proportion of men with HIV infection was nearly the same as those coinfected with HIV and HCV. Similarly, for those men infected with HBV, regardless of era and severity, nearly all were coinfected with HCV. The proportion of participants (severe and mild) who reported initiating any viral hepatitis therapy was consistently low (∼1 in 3) across each era; consequently, only 1 in 10 men with severe hemophilia reported having a sustained viral response.
Inhibitor prevalence.
Inhibitors occurred with a similar prevalence across all eras and were consistently more prevalent in men with severe hemophilia (11.5-17.0%) compared with men with mild hemophilia (2.8-3.6%) (Table 4).
Mortality
During the study period, a total of 551 deaths were reported. The era A and B cohorts accounted for 82% of the deaths in the severe hemophilia population and 96% of the deaths in the mild hemophilia population (Table 5). Although the youngest cohorts reported no liver-related deaths, liver failure was the most commonly reported cause of death overall for both severe (33% of deaths) and mild (26% of deaths) hemophilia cohorts. Hemophilia (hemorrhage)-related deaths accounted for 14.6% of deaths in severe hemophilia and 10.7% of deaths in participants with mild hemophilia across all eras.
Table 5.
Mortality
| Era A | Era B | Era C | Era D | |||||
|---|---|---|---|---|---|---|---|---|
| <1958 | 1958-1975 | 1976-1982 | 1983-1992 | |||||
| Severe | Mild | Severe | Mild | Severe | Mild | Severe | Mild | |
| Total N | 688 | 843 | 1542 | 787 | 1121 | 427 | 1548 | 530 |
| Total deaths (N = 551) | 160 | 82 | 202 | 26 | 60 | 3 | 17 | 1 |
| Total deaths (%) | 23.3 | 9.7 | 13.1 | 3.3 | 5.4 | 0.7 | 1.1 | 0.2 |
| Causes (%) | ||||||||
| Hemophilia | 14.4 | 9.8 | 16.3 | 11.5 | 10 | 33.3 | 11.8 | 0 |
| HIV | 10.6 | 1.2 | 26.2 | 7.7 | 25 | 33.3 | 0 | 0 |
| Liver failure | 31.2 | 28 | 38.1 | 23.1 | 25 | 0 | 0 | 0 |
| Suicide | 1.2 | 0 | 0.5 | 11.5 | 5 | 0 | 0 | 0 |
| Other | 32.5 | 47.6 | 15.8 | 34.6 | 26.7 | 33.3 | 82.4 | 100 |
| Unnown | 10 | 14.6 | 5.9 | 11.5 | 8.3 | 0 | 5.9 | 0 |
Discussion
This analysis examines birth cohorts of US men with severe hemophilia to gauge the impact of changes in hemophilia therapeutics and health care delivery over the last half century on clinical outcomes (access to care, physical and social functioning, and complications including mortality). To our knowledge, this is the largest population of men with severe hemophilia to date studied using a single, uniform data collection tool.
Current access to care for severe hemophilia through the national HTC system was relatively uniform across the birth cohorts: 79% to 84% of men across the eras used the HTC at least yearly, with the greatest proportion of frequent users being in the eldest era. The growing composition of minorities in the younger 3 eras aligns with the increasing racial/ethnic diversity in the general US population and provides evidence that minorities have access to the HTC system. In era D, the proportion of men with severe hemophilia of African-American race reached 16.4%, higher than the general 2010 US African-American population of 12.6%. Approximately 14% of era D men were of Hispanic ethnicity, which was comparable to the general US population (16.3%).8
Home infusion of clotting factor was the prevalent approach to care in 70.3% to 76.1% of men regardless of era. Improved access to comprehensive HTC care in more recent decades was evident: eras C and D contained a majority of men who had their initial HTC visit by their second year of life; 28.5% and 45.7%, respectively, had started home infusion before the age of 6 years. The use of continuous prophylactic clotting factor replacement in men with severe hemophilia during 1998 to 2011 was not the standard of care. However, we did observe an increasing proportion reporting the use of prophylaxis therapy with successively younger eras, rising to nearly half in era D.
We observed dramatic differences in the proportion of men with severe hemophilia who reported being disabled or limited in their activity. Fewer men with severe hemophilia used assistive devices and wheelchairs in younger eras. Nearly 4 of 5 men with severe hemophilia in era D were employed or students. Similarly, we observed dramatic declines in HBV infection prevalence starting in era C and declines in the prevalence of HCV infection starting in era D. At the close of the UDC data collection, the prevalence of HIV infection was ∼1% in era D men; nearly 2 of 3 men with severe hemophilia in era D were free of HIV, HBV, and HCV. Mortality was also dramatically lower in eras C/D than in eras A/B, and across all eras, many men with severe hemophilia die with hemophilia rather than from hemophilia-related causes.
The 2 youngest cohorts had the largest proportion of uninsured men. This finding is due to both a larger proportion of minorities compromising the younger eras and an increase in whites who were uninsured. Lack of insurance disproportionately affecting minorities in the United States is widely documented.9,10 The uninsured subset of the hemophilia population is at particularly high risk for long-term morbidity, especially if ready access to clotting factor and to HTC services cannot be maintained. Furthermore, a growing population of men with severe hemophilia who are both uninsured and at greater risk for inhibitor development (due to African-American or Hispanic background)11-14 is particularly concerning given the increased mortality, the high morbidity, and extraordinary financial cost associated with inhibitor development and therapy.15 Focus on this at-risk subpopulation is warranted to optimize musculoskeletal and other outcomes as these younger era C and D birth cohorts age.
Despite similarly frequent HTC utilization across all eras, unacceptably high rates of joint hemorrhages (≥5 hemarthroses per 6 months) were reported by 42% to 49% of men in eras A to C; the proportion with frequent hemorrhages was only slightly lower in era D (35.5%). Additionally, nearly one-fourth of the men in era D reported the presence of a target joint (compared with about one-third of men in eras A-C), despite much more frequent use of prophylaxis by men in era D. Although the prevalence of disability is low in men in era D, the apparent high rates of joint hemorrhages raises concern that the trajectory of joint disease in era D men could follow that of their older comparators unless effective interventions are more consistently implemented.
In an effort to control for the effects of aging on physical function, we compared outcomes of men with severe hemophilia to those of men with mild hemophilia within each era. When observed in this framework, there may be evidence that the effect of frequent bleeds in the younger eras of men with severe hemophilia may already be associated with disability and loss of physical function. As expected, we observed infrequent bleeding in men with mild hemophilia consistently across all eras. Men with mild hemophilia reported low levels of disability or limitations of activity in the youngest era, but these limitations were incrementally more prevalent in each subsequently older birth cohort. Bleeding events were infrequent in each mild hemophilia birth cohort, suggesting the recorded limitations in overall activity represent the effect of aging in combination with some contribution from the infrequent hemorrhage.
If hemophilia treatment and access to treatment of men with severe hemophilia had improved markedly over the last several decades, then we would expect health outcomes disparities between men with severe hemophilia and men with mild hemophilia to narrow over time. Although joint outcomes related to aging should affect men with mild and severe similarly, joint outcomes related to hemorrhage should affect severe hemophilia disproportionately and would ideally be decreasing with earlier and broader application of modern standards of care. Instead, an approximately threefold greater number of men with severe hemophilia compared with mild hemophilia reported having work disability or limitations of overall ability (due to pain, loss of motion, or weakness) in each of the birth cohorts regardless of whether born before 1958 (era A) or as late as 1992 (era D) (Table 3). Despite improved access to comprehensive care and to pathogen-free clotting factor for those born in recent decades, the gap between severe and mild has not narrowed either for target joints (10 times more common in severe vs mild, whether born in era A or era D) or for the use of assistive devices for mobility (twice as common in severe vs mild). One possible explanation is that there may have been more deaths of individuals with a severe bleeding phenotype in the older cohorts, due to complications of bleeding and/or iatrogenic infection, resulting in survival of a cohort of phenotypically milder severe patients in era A. This possible explanation is supported by the ratio of surviving severe:mild individuals in era A of only 0.8 compared with a ratio of 2.9 (severe:mild) in era D.
The disappointing observations about persistently high bleeding rates and the absence of a narrowing of the disability gap between men with severe and mild hemophilia indicate that, although important improvements in care of men with severe hemophilia have been made, there is evidence of a need for continued improvement in strategies for prevention and treatment of hemophilia-associated hemorrhage.
The CDC UDC hepatitis surveillance demonstrates major declines in the prevalence of HBV infection in men born after 1975 and similar declines in prevalence of HCV in men born after 1983. Much work remains to prevent long-term morbidity and mortality from these chronic infections. We observed a very low proportion of men who attempted therapy for HCV eradication in the US HTC population through 2011 and an even smaller fraction that sustained viral eradication among those who seroconverted. Although the long natural history of HCV infection makes it challenging to estimate the incidence of chronic infection progressing to cirrhosis, for men in the general population, the prevalence of the development of cirrhosis at 20 years is ≥20% to 30% and is potentially accelerated by HIV coinfection.16-18 The rate of development of hepatocellular carcinoma is ∼3% to 5% per year17,19 with a poor prognosis, particularly in patients with cirrhosis and/or poor liver function.20
Our data demonstrate that from 1998 to 2011, most deaths predictably occurred in the era A and era B cohorts, and in these birth cohorts, hepatic failure has eclipsed both HIV and hemorrhage as the leading cause of death. HCV infection was highly prevalent and the mild hemophilia population was not spared: one-third of men with mild hemophilia in eras A and B had HBV, more than half had HCV and more than one-quarter were coinfected. In light of the recent licensure of highly efficacious anti-HCV drugs,21-24 the UDC HCV prevalence, morbidity, and mortality data provide a strong impetus for hemophilia care providers to evaluate and treat HCV infection to address the current greatest contributor to hemophilia mortality. The UDC data presented here support the urgency inherent in the April 2015 recommendation of the Medical and Scientific Advisory Council of the US National Hemophilia Foundation that all individuals with hemophilia who have received blood or plasma-derived products should be evaluated for HCV infection by 31 December 2016 and if active disease is present should be referred for evaluation of the extent of liver disease and consideration of treatment by 31 December 2017.25
Although the mild hemophilia cohorts of eras A to D were examined primarily as a comparator group to men with severe hemophilia, to control for the impact of shared aging and environmental complications (independent of frequent hemorrhage), several unexpected observations suggest that specific surveillance of the population with mild hemophilia is warranted. In addition to the high incidence of HCV in the older cohorts of mild hemophilia patients, the incidence of overweight/obesity was greater in the mild cohorts (era A and B) than in the corresponding severe hemophilia cohorts. Additionally, individuals with mild hemophilia in eras A, B, and C were more than twice as likely to be uninsured as the corresponding severe hemophilia groups. Finally, although most individuals with mild hemophilia reported ≤2 joint hemorrhages per 6 months, half of those who did experience joint bleeding reported ≥5 bleeds per 6 months, suggesting that there is a small subset of individuals with baseline factor levels in the mild range who nonetheless express a more severe bleeding phenotype. Although beyond the scope of this analysis, more specific and/or sensitive surveillance tools are needed to understand the impact of hemophilia on the subset of mildly deficiency patients who nevertheless have relatively poor health care access or relatively poor outcomes.
Although this data set contains the largest number of men with severe hemophilia in any single registry, several limitations apply to this analysis. An inherent limitation to study interpretation is that this is a survival cohort in which patients had to survive to 1998 to be included. As a result of evaluating only survivors, it is probable that the captured outcome measures underestimate the adverse outcomes in severe disease and in the older eras, in particular. Interpretation of some data are limited by lack of historical data (eg, has the patient ever had an inhibitor in the past) and/or detailed treatment data (eg, details of primary vs secondary prophylaxis approaches). The laboratory data that established the severity was reported by the HTC and not determined centrally and patients could be misclassified. The recorded frequency of hemorrhages relied primarily on patient self-report, introducing potential recall bias. It is estimated that 30% of US residents with hemophilia obtain care outside of the network of federally supported HTCs,26 due to preference or insurance restrictions, and the findings of this analysis cannot necessarily be generalized to these individuals.
The prospectively collected data from 7500 adults with severe and mild hemophilia, when examined taking into account experiential differences dictated by evolving eras of treatment and health care delivery, indicate that some but not all of the goals for modern therapy are being met. Our descriptive analysis demonstrates that 1) access to an improved standard of hemophilia care has grown over time; 2) hemophilic joint bleeding remains higher than expected given widespread availability of effective therapies accessible for home use27; 3) frequency of good joint bleeding outcomes correlates with that of continuous prophylaxis use in younger adults; nevertheless, a minority of adults used continuous prophylaxis in every era during the UDC observation period; 4) the relative health status disparities between adult men with severe vs mild hemophilia have not really diminished over time, despite the improved standard of care with each subsequently younger era; and 5) eradicating active HCV is an immediate imperative if morbidity and mortality are to be reduced. If the surveillance data had been examined aggregating men of all ages and severities rather than using the eras framework, some potential areas that deserve prospective focus might have been less obvious. Of particular concern is that the youngest era of men with hemophilia continues to be at risk for morbidity due to excessive bleeding, to a high proportion of uninsured men, and to population demographics that include an increasingly diverse racial/ethnic makeup that may be associated with higher risk for inhibitors and their complications. The use of mild hemophilia as a comparison group also revealed a subgroup of individuals with mild hemophilia who experience worse bleeding outcomes and are otherwise not well characterized. Surveillance specifically tailored to examine non-severe hemophilia may be revealing. The UDC experience demonstrates that national surveillance in the US hemophilia population remains vital to inform improvements in HTC access, therapeutics, and outcomes.
Acknowledgments
The authors acknowledge with gratitude the staff of the US HTCN for recruiting patients to the UDC surveillance project and collecting the data. The authors also thank the patients and their families for their participation. This national database analysis was supported by the Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, CDC. The authors also acknowledge Rodney Presley for data analysis. Additionally, P.E.M. and M.A.M. are indebted to Brenda I. Nielsen for her mentorship regarding UDC data collection.
The UDC Project was funded by a cooperative agreement (“Prevention of Bleeding Disorder Complications through Regional Hemophilia Treatment Centers”) between the CDC and the US HTCN, which is comprised of >130 clinical centers located throughout the United States.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Contributor Information
Collaborators: Doreen B. Brettler, Christopher E. Walsh, Regina B. Butler, Trish Dominic, Ruth Brown, Thomas C. Abshire, Christine L. Kempton, Ivan C. Harner, Deborah L. Brown, Brian M. Wicklund, Marilyn J. Manco-Johnson, Diane J. Nugent, Robina E. Ingram-Rich, Ann D. Forsberg, Mariam Voutsis, Amanda Wade, Steven Humes, Karen Droze, Tami Wood-Lively, Mary Anne Schall, John H. Drake, and Becky Dudley
Authorship
Contribution: P.E.M., J.R.B., B.K.R., and J.M.S. conceptualized the study design, analyzed the data, wrote and edited the manuscript, and edited the figures; J.M.S. additionally conducted statistical data analyses and created the tables; and M.A.M. analyzed the data, created the figures, wrote and edited the manuscript.
Conflict-of-interest disclosure: P.E.M. receives research support through the University of North Carolina from Asklepios and Novo Nordisk and has received research support in the past from Baxter Healthcare, Novo Nordisk, Pfizer, and Prolor. He holds patents that have been licensed to Asklepios, for which he receives royalties. He has received payment for consultation, services, and for speaking for Asklepios, Chatham LLC, Baxter Healthcare, and Pfizer and has additionally consulted for Bayer, Novo Nordisk, and Biogen. All other authors declare no competing financial interests.
A complete list of the members of the US Hemophilia Treatment Center Network appears in “Appendix.”
Correspondence: Paul E. Monahan, 141 Graylyn Dr, Chapel Hill, NC 27516-4456; e-mail: pablomonoloco@gmail.com.
Appendix: study group members
The US Hemophilia Treatment Center Network (HTCN) includes ∼130 regionally organized hemophilia treatment centers. The CDC UDC Cooperative Agreement Grantees/Regional Directors of the 12 regions of the US HTCN at the time of final UDC data cleaning and research evaluations include the author P.E.M. and Doreen B. Brettler, New England Hemophilia Center, Worcester, MA; Christopher E. Walsh, Mount Sinai School of Medicine, New York, NY; Regina B. Butler, Children’s Hospital of Philadelphia, Philadelphia, PA; Trish Dominic and Ruth Brown, Hemophilia of Georgia; Thomas C. Abshire and Christine L. Kempton, Children’s Health Care of Atlanta, GA; Ivan C. Harner, Hemophilia Foundation of Michigan; Deborah L. Brown, Gulf States Hemophilia and Thrombophilia Center, Houston, TX; Brian M. Wicklund, Kansas City Regional Hemophilia Center, Kansas City, MO; Marilyn J. Manco-Johnson, University of Colorado Hemophilia and Thrombosis Center, Aurora, CO; Diane J. Nugent, Children’s Hospital of Orange County, Orange, CA; Robina E. Ingram-Rich, Oregon Hemophilia Treatment Center, Portland, OR. In addition to coordinating regional data collection, the Regional Coordinators from the 12 regions of the US HTCN validated specific data elements and categories to verify the precision of this study and include the authors J.R.B. and B.K.R., Ann D. Forsberg, Mariam Voutsis, Amanda Wade, Steven Humes, Karen Droze, Tami Wood-Lively, Mary Anne Schall, John H. Drake, and Becky Dudley.
References
- 1.Evatt BL. The natural evolution of haemophilia care: developing and sustaining comprehensive care globally. Haemophilia. 2006;12(Suppl 3):13–21. doi: 10.1111/j.1365-2516.2006.01256.x. [DOI] [PubMed] [Google Scholar]
- 2.Evatt BL. The tragic history of AIDS in the hemophilia population, 1982-1984. J Thromb Haemost. 2006;4(11):2295–2301. doi: 10.1111/j.1538-7836.2006.02213.x. [DOI] [PubMed] [Google Scholar]
- 3. Public Law 9463: The Public Health Service Act Establishing the Hemophilia Diagnostic and Treatment Center Program. N01131 of Public Law 9463. Washington, DC: Government Printing Office; 1975. [Google Scholar]
- 4.Baker JR, Crudder SO, Riske B, Bias V, Forsberg A. A model for a regional system of care to promote the health and well-being of people with rare chronic genetic disorders. Am J Public Health. 2005;95(11):1910–1916. doi: 10.2105/AJPH.2004.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soucie JM, McAlister S, McClellan A, Oakley M, Su Y. The universal data collection surveillance system for rare bleeding disorders. Am J Prev Med. 2010;38(4 Suppl):S475–S481. doi: 10.1016/j.amepre.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Grosse SD, Schechter MS, Kulkarni R, Lloyd-Puryear MA, Strickland B, Trevathan E. Models of comprehensive multidisciplinary care for individuals in the United States with genetic disorders. Pediatrics. 2009;123(1):407–412. doi: 10.1542/peds.2007-2875. [DOI] [PubMed] [Google Scholar]
- 7.Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A Subcommittee on Factor VIII, Factor IX and Rare Coagulation Disorders of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
- 8.Humes K, Jones N, Ramirez R. Overview of race and Hispanic origin: 2010. US Census Bureau. Washington, DC: Government Printing Office; 2011:1-24. [Google Scholar]
- 9.Smedley BD. Moving beyond access: achieving equity in state health care reform. Health Aff (Millwood) 2008;27(2):447–455. doi: 10.1377/hlthaff.27.2.447. [DOI] [PubMed] [Google Scholar]
- 10.DeNavas-Walt C, Proctor BD, Smith JC. Income, poverty and health insurance coverage in the United States: 2009. US Census Bureau. Washington, DC: Government Printing Office; 2010:60-238. [Google Scholar]
- 11.Aledort LM, Dimichele DM. Inhibitors occur more frequently in African-American and Latino haemophiliacs. Haemophilia. 1998;4(1):68. doi: 10.1046/j.1365-2516.1998.0146c.x. [DOI] [PubMed] [Google Scholar]
- 12.Goudemand J, Rothschild C, Demiguel V, et al. FVIII-LFB and Recombinant FVIII study groups. Influence of the type of factor VIII concentrate on the incidence of factor VIII inhibitors in previously untreated patients with severe hemophilia A. Blood. 2006;107(1):46–51. doi: 10.1182/blood-2005-04-1371. [DOI] [PubMed] [Google Scholar]
- 13.Miller CH, Benson J, Ellingsen D, et al. Hemophilia Inhibitor Research Study Investigators. F8 and F9 mutations in US haemophilia patients: correlation with history of inhibitor and race/ethnicity. Haemophilia. 2012;18(3):375–382. doi: 10.1111/j.1365-2516.2011.02700.x. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter SL, Michael Soucie J, Sterner S, Presley R Hemophilia Treatment Center Network (HTCN) Investigators. Increased prevalence of inhibitors in Hispanic patients with severe haemophilia A enrolled in the Universal Data Collection database. Haemophilia. 2012;18(3):e260–e265. doi: 10.1111/j.1365-2516.2011.02739.x. [DOI] [PubMed] [Google Scholar]
- 15.Walsh CE, Soucie JM, Miller CH United States Hemophilia Treatment Center Network. Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 2015;90(5):400–405. doi: 10.1002/ajh.23957. [DOI] [PubMed] [Google Scholar]
- 16.Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014–1018. doi: 10.1053/he.2000.5762. [DOI] [PubMed] [Google Scholar]
- 17.Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 18.Alberti A, Chemello L, Benvegnù L. Natural history of hepatitis C. J Hepatol. 1999;31(Suppl 1):17–24. doi: 10.1016/s0168-8278(99)80369-9. [DOI] [PubMed] [Google Scholar]
- 19.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 20.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20(6):1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 21.Kowdley KV, Lawitz E, Poordad F, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370(3):222–232. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
- 22.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 23.Liang TJ, Ghany MG. Therapy of hepatitis C--back to the future. N Engl J Med. 2014;370(21):2043–2047. doi: 10.1056/NEJMe1403619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 25. Medical and Scientific Advisory Council of the National Hemophilia Foundation: MASAC Recommendations on Treatment of Hepatitis C in Individuals with Hemophilia and Other Bleeding Disorders. New York, NY: Medical and Scientific Advisory Council; 2015. [Google Scholar]
- 26.Baker JR, Riske B, Drake JH, et al. US Hemophilia Treatment Center population trends 1990-2010: patient diagnoses, demographics, health services utilization. Haemophilia. 2013;19(1):21–26. doi: 10.1111/j.1365-2516.2012.02915.x. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Siddiqi AE, Soucie JM, et al. Joint Outcomes Committee of Universal Data Collection and the Hemophilia Treatment Centres Network. The effect of secondary prophylaxis versus episodic treatment on the range of motion of target joints in patients with haemophilia. Br J Haematol. 2013;161(3):424–433. doi: 10.1111/bjh.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petit C, Klein H. Hemophilia, Hemophiliacs and the Health Care Delivery System. Washington, DC: National Heart and Lung Institute, Division of Blood Disease and Resources, Office of Prevention, Control, and Education; 1976:76-871. [Google Scholar]
- 29.Rabiner SF, Telfer MC. Home transfusion for patients with hemophilia A. N Engl J Med. 1970;283(19):1011–1015. doi: 10.1056/NEJM197011052831902. [DOI] [PubMed] [Google Scholar]
- 30.Levine PH. Efficacy of self-therapy in hemophilia. A study of 72 patients with hemophilia A and B. N Engl J Med. 1974;291(26):1381–1384. doi: 10.1056/NEJM197412262912604. [DOI] [PubMed] [Google Scholar]
- 31.Strawczynski H, Stachewitsch A, Morgenstern G, Shaw ME. Delivery of care to hemophilic children: home care versus hospitalization. Pediatrics. 1973;51(6):986–991. [PubMed] [Google Scholar]
- 32.Teitel JM, Barnard D, Israels S, Lillicrap D, Poon MC, Sek J. Home management of haemophilia. Haemophilia. 2004;10(2):118–133. doi: 10.1046/j.1365-2516.2003.00853.x. [DOI] [PubMed] [Google Scholar]
- 33.Baker JR, Riske B, Voutsis M, Cutter S, Presley R. Insurance, home therapy, and prophylaxis in U.S. youth with severe hemophilia. Am J Prev Med. 2011;41(6 Suppl 4):S338–S345. doi: 10.1016/j.amepre.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 34.National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—the evidence report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]