Abstract
Several studies have described a dose-dependent effect of alcohol on human health with light to moderate drinkers having a lower risk of all-cause mortality than abstainers, while heavy drinkers are at the highest risk. In the case of the immune system, moderate alcohol consumption is associated with reduced inflammation and improved responses to vaccination, while chronic heavy drinking is associated with a decreased frequency of lymphocytes and increased risk of both bacterial and viral infections. However, the mechanisms by which alcohol exerts a dose-dependent effect on the immune system remain poorly understood due to a lack of systematic studies that examine the effect of multiple doses and different time courses. This review will summarize our current understanding of the impact of moderate versus excessive alcohol consumption on the innate and adaptive branches of the immune system derived from both in vitro as well as in vivo studies carried out in humans and animal model studies.
Keywords: Alcohol, immunity, infection, vaccination, inflammation, gene expression, HPA axis, glucocorticoid
Overview of the Immune System
The immune system is an intricate network of molecules, cells, tissues, and organs that work together to defend the body against infectious agents and malignant cells. It is broadly divided into innate and adaptive branches, which are both essential for efficient host defense. The innate immune response is immediately available, but is not specific for any one pathogen. Innate immune cells such as natural killer (NK) cells, neutrophils, monocytes/macrophages and dendritic cells (DCs) express pathogen recognition receptors (PRRs) that recognize pathogenassociated molecular patterns (PAMPs), such as the bacterial cell wall component lipopolysaccharide (LPS). The interaction between PRR and PAMPs activates cells of the innate immune system to engulf pathogenic microbes and to secrete cytokines and chemokines, resulting in the induction of an inflammatory response and mobilization of immune cells into the site of infection. In addition, DCs and monocytes process and present peptides derived from foreign antigens bound to specialized molecules called major histocompatibility complex (MHC) molecules on their cell surface to naïve T lymphocytes thereby activating them and initiating the adaptive immune response (Janeway 2008).
The adaptive immune system can be subdivided into cell-mediated immunity, carried out by T cells, and humoral immunity, carried out by B cells. T cells expressing the CD4 T cell co-receptor are known as T helper cells and play a critical role in the activation and maturation of monocytes, cytotoxic T cells and B cells. T cells expressing the CD8 T cell co-receptor are known as cytotoxic T cells and eliminate host cells infected with intracellular pathogens as well as tumor cells. B cells mature into plasma cells that produce antibodies, also known as immunoglobulins (Ig), to eliminate extracellular microorganisms and prevent the spread of infection. The adaptive immune response can be distinguished from innate immunity by the capability of generating immunological memory, or protective immunity against recurring disease caused by the same pathogen (Janeway 2008).
It is known that alcohol consumption alters both innate and adaptive immunity in both humans as well as animal models, however these effects have not been systematically assessed on the basis of the amount of alcohol consumed and duration. Moderate alcohol consumption is defined by the Dietary Guidelines for Americans as up to 1 drink per day for women and up to 2 drinks per day for men. The National Institute on Alcohol Abuse and Alcoholism defines binge drinking as the consumption of 4 drinks for women or 5 drinks for men within 2 hours. The Substance Abuse and Mental Health Services Administration defines heavy drinking as binge drinking on 5 or more days in the past 30 days. Several studies have described a J-shaped curve for the effects of alcohol on human health, with light to moderate consumers having a lower risk of all-cause mortality than abstainers, while heavy drinkers and those with alcohol use disorder (AUD) are at the highest risk (O'Keefe, Bybee et al. 2007). Moreover, moderate alcohol consumption is associated with reduced risk of cardiovascular disease (Klatsky 1999, Holmes, Dale et al. 2014) and seems to exert an immune stimulatory effect, whereas heavy or binge drinking results in an increased risk of cirrhosis of the liver, hypertension, stroke, type 2 diabetes, cancer, and impaired immune function (Ahmed 1995, Szabo 1998). The primary objective of this review is to examine the effects of moderate versus excessive alcohol intake on the immune system in human and animal model studies.
Ethanol Metabolism
Ethanol is primarily metabolized in the stomach and liver by alcohol dehydrogenase (ADH) and cytochrome P450 2E1 (CYP2E1) (Zakhari 2006). ADH is present in the cytosol whereas CYP2E1 is present predominantly in microsomes. Both enzymes convert alcohol to acetaldehyde, which is further metabolized to acetate by acetaldehyde dehydrogenase (ALDH) in the mitochondria. Acetate is then released into the blood where it is oxidized to carbon dioxide in the heart, skeletal muscle, and brain (Zakhari 2006).
Acetaldehyde is the toxic byproduct that contributes to tissue damage, alcohol dependence, and addiction (Zakhari 2006). It can also bind to other proteins to form adducts, such as malondialdehyde (MDA) and MDA-acetaldehyde (MAA), which play a key role in the development of liver injury and stimulate antibody responses that further promote liver inflammation and fibrosis (Tuma and Casey 2003). In addition, oxidation of ethanol by CYP2E1 leads to the formation of reactive oxygen species (ROS). Elevated levels of ROS cause oxidative stress which has been shown to play a role in several harmful processes including cancer development, atherosclerosis, diabetes, and inflammation (Tuma and Casey 2003).
CYP2E1 and catalase can also metabolize alcohol in the brain. Catalase is localized to peroxisomes and requires hydrogen peroxide to oxidize alcohol into water and acetaldehyde. Alcohol metabolism can also take place in the pancreas by acinar and pancreatic stellate cells, which contributes to the development of alcoholic pancreatitis (Vonlaufen, Wilson et al. 2007). Additional studies are required to fully understand the role of ethanol metabolites and adducts in the development of alcoholic liver injury and organ damage.
Modulation of Innate Immunity by Alcohol
Ethanol modulates the function of monocytes, immature innate immune cells that circulate in the blood until recruited into tissues, in a dose and time dependent manner. Monocytes express Toll-like receptor (TLR) 4, which is the PRR responsible for recognizing the endotoxin LPS on the surface of Gram negative bacteria. Upon LPS binding, monocytes become activated, mature into macrophages and migrate into tissues where they respond to infection by secreting various cytokines, recruiting additional leukocytes via production of chemokines and presenting pathogen-derived peptides to T cells to activate them. These events depend on the activation of the nuclear factor kappa B (NFκB) heterodimer p50–p65 and its translocation to the nucleus leading to the expression and production of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-12, and tumor necrosis factor (TNF)-α (Hoffmann, Natoli et al. 2006, Janeway 2008). Often, investigators stimulate with LPS after pre-exposure to ethanol to mimic inflammation observed in trauma patients with high blood alcohol levels and explore the alterations in immunity that lead to frequent subsequent infections among this group.
Several studies have shown a dose-dependent effect of alcohol on the LPS response by monocytes/macrophages. Pre-incubation of human monocytes isolated from healthy men and women in vitro with 25mM alcohol [~0.1g/dL blood alcohol concentration (BAC)] for 24 hours inhibits nuclear translocation of NFκB in response to LPS, and thereby production of pro-inflammatory cytokines (Muralidharan, Ambade et al. 2014). In vitro exposure of the macrophage cell line RAW 264.7 and human peripheral blood monocytes to 25mM ethanol for 24 hours followed by stimulation of LPS also leads to decreased TNF-α production by increasing the expression of IL-1R-associated kinase-monocyte (IRAK-M), a negative regulator of LPS signaling (Mandrekar, Bala et al. 2009). Similarly, exposure of the human monocytic cell line mono Mac 6 cells to 25mM, 50mM, or 75mM ethanol for 24 hours inhibited LPS and phorbol myristate acetate-(PMA) induced TNF-α production in a dose-dependent manner (Zhang, Bagby et al. 2001). This inhibitory effect on NFκB activity is partly due to the increased proteolytic degradation of IκBα kinase (IKK) and consequent decreased phosphorylation of the NFκB p65 subunit (Mandrekar, Jeliazkova et al. 2007). Additional studies also showed that exposure of RAW 264.7 macrophages and human peripheral blood monocytes to 25mM of ethanol for as little as 60 minutes results in the activation of the heat shock transcription factor-1 (HSF-1), which in turn induces heat shock protein hsp70 expression (Mandrekar, Catalano et al. 2008). Hsp70 binds the NFκB subunit p50 and decreases its nuclear translocation while HSF-1 binds to the TNF-α promoter region resulting in negative regulation of TLR4 signaling (Mandrekar, Catalano et al. 2008, Muralidharan, Ambade et al. 2014). Finally, in vitro exposure of human peripheral blood monocytes to 25mM ethanol for 6 hours also inhibited TLR8-induced production of the pro-inflammatory cytokine TNF-α and increased production of the antiinflammatory cytokine IL-10 (Pang, Bala et al. 2011).
These in vitro results have been recapitulated in vivo in rodent models. Measurement of serum cytokine levels 2 hours following a one time administration of ethanol at 6g/kg body weight by oral gavage in female mice (a murine model of binge drinking that yields a peak BAC of approximately 0.4%, which results in loss of consciousness in humans) showed decreased production of inflammatory cytokines IL-6 and IL-12 in response to TLR2/TLR6 (zymosan A Saccharomyces cerevisiae), TLR4 (LPS), TLR5 (bacterial flagellin), TLR7 (R-848) and TLR9 (CpG DNA) ligands administered by intraperitoneal or intravenous injection at the same time as ethanol (Pruett, Zheng et al. 2004). In addition, production of IL-10 in response to TLR2/6 stimulation was increased (Pruett, Zheng et al. 2004). This same treatment also inhibited the in vitro production of IL-6 and IL-12 by peritoneal macrophages harvested 2 hours following injection of LPS (Pruett, Fan et al. 2005). Finally, ethanol administered at 6g/kg but not 3g/kg by oral gavage in mice significantly increased serum concentrations of positive acute phase proteins amyloid A and P that arise early in the inflammatory response and recruit immune cells to the inflammatory site, indicating that ethanol modulates acute phase response in a dose-dependent manner (Pruett and Pruett 2006). This phenomenon was not observed in a TLR4 mutant mouse, indicating that the acute phase response is mediated by TLR4 (Pruett and Pruett 2006).
Recently, it was reported that a single episode of binge alcohol consumption in alcohol-experienced human volunteers (men and women) initially (within the first 20 min) increased total number of peripheral blood monocytes and LPS-induced TNF-α production when blood alcohol levels were ~130mg/dL. However, similarly to the in vitro studies described above, at 2 and 5 hours post-binge the numbers of circulating monocytes were reduced and levels of antiinflammatory IL-10 levels were increased (Afshar, Richards et al. 2014).
In contrast to the inhibitory effects of acute alcohol treatment (up to 24 hours), prolonged exposure of human (men and women) peripheral blood monocytes to 25mM ethanol for 7 days increased LPS-induced TNF-α production without affecting IL-10 production (Pang, Bala et al. 2011). Prolonged exposure of Mono Mac 6 cell line to 25mM, 50mM and 75mM ethanol for 7 days also reverses the initial inhibition of LPS or PMA-induced TNF-α production in a dose-dependent manner (Zhang, Bagby et al. 2001). Studies using the RAW 264.7 macrophage cell line and peripheral blood monocytes isolated from healthy men and women demonstrated that this switch to a pro-inflammatory response occurs via decreasing IRAK-M and increasing IRAK-1 and IKK expression resulting in increased phosphorylation of the NFκB p65 subunit, increased NFκB translocation to the nucleus and greater TNF-α production in response to LPS stimulation (Mandrekar, Bala et al. 2009).
Finally, primary alveolar macrophages isolated from female mice cultured in 25–100mM ethanol for 24 hours prior to addition of apoptotic cells showed a dose-dependent decrease in efferocytosis, the process of clearing dying cells that is critical to resolution of the inflammatory process after infection. This defect was rescued when cultures were treated with the Rho kinase inhibitor, Y27632 indicative that ethanol reduced efferocytosis through the induction of Rho kinase activity in a dose-dependent manner (Boe, Richens et al. 2010). In addition, female mice that consumed 20% (w/v) ethanol for 8 weeks showed a reduction in LPS activated efferocytosis (Boe, Richens et al. 2010). In contrast to the effects of high ethanol doses, human monocytes isolated after 30 days of moderate beer consumption (330mL for women and 660mL for men) exhibited increased phagocytic, oxidative burst, and intracellular bactericidal activity when incubated with fluorescence-labeled E. coli compared to basal levels (Romeo, Warnberg et al. 2007).
As described above, in vivo ethanol ingestion of 6.3% (v/v) in the form of a standard Lieber-DeCarli liquid diet (a widely used rodent model of chronic and binge ethanol ad libitum feeding (Bertola, Mathews et al. 2013)) for 4 weeks in male mice up-regulated NFκB activation and increased circulating levels of IL-6 and TNF-α in response to TLR4 ligand LPS stimulation (Maraslioglu, Oppermann et al. 2014). Male rats on a liquid diet with 35% of calories coming from ethanol also showed enhanced mRNA half-life and protein expression of LPS-induced TNF-α by increasing TNF-α in liver monocytes/macrophages (Kishore, McMullen et al. 2001). In humans, peripheral blood monocytes isolated from 16 hospitalized male patients with alcoholic hepatitis (but no detectable blood alcohol levels at the time of blood collection) had significantly increased TNF-α production in response to LPS stimulation when compared to monocytes from healthy volunteers (McClain and Cohen 1989).
The dendritic cell (DC), which plays a critical role in T cell activation and initiation of adaptive immune responses, is another innate immune cell affected by ethanol. DCs uptake antigens in peripheral tissues which leads to their maturation, and then travel to draining lymph nodes where they present them to T cells (Janeway 2008). As described above for monocytes, long-term in vitro treatment of myeloid DCs (mDCs) generated from healthy female and male blood donors with 25mM ethanol for 7 days results in reduced IL-12 production, increased IL-10 production, and a decrease in expression of the co-stimulatory molecules CD80 and CD86 (Mandrekar, Catalano et al. 2004). Similarly, consumption of 10% (w/v) ethanol in tap water ad libitum for 2 days in mice resulted in decreased bone marrow DC generation, decreased expression of CD80 and CD86, impaired induction of T cell proliferation, and a decrease in IL-12 production (Lau, Abe et al. 2006). Also, monocyte-derived mDCs obtained from healthy male and female volunteers shortly after consuming 2mL vodka/kg body weight in a total volume of 300mL orange juice resulting in BACs of 0.095 ± 0.02g/dL, showed reduced ability to induce T cell proliferation in response to allogeneic antigen, super-antigen staphylococcal enterotoxin B, or tetanus toxoid, indicative of impaired antigen presentation (Szabo, Catalano et al. 2004).
Finally, in a nonhuman primate model of ethanol self-administration (Grant et al. 2008), PMA-induced production of the growth factors hepatocyte growth factor (HGF), granulocyte colony-stimulating factor (G-CSF), and vascular-endothelial growth factor (VEGF) by peripheral blood mononuclear cells (PBMCs) isolated from male and female rhesus macaques after 12 months of chronic ethanol exposure were inhibited (Asquith, Pasala et al. 2013). The reduced production of growth factors in this nonhuman primate model of ethanol self-administration was due to increased expression of microRNA miR-181 and miR-221, which led to reduced expression of the transcription factors, signal transducer and activator of transcription 3 (STAT3) and aryl hydrocarbon receptor nuclear translocator (ARNT) (Asquith, Pasala et al. 2013).
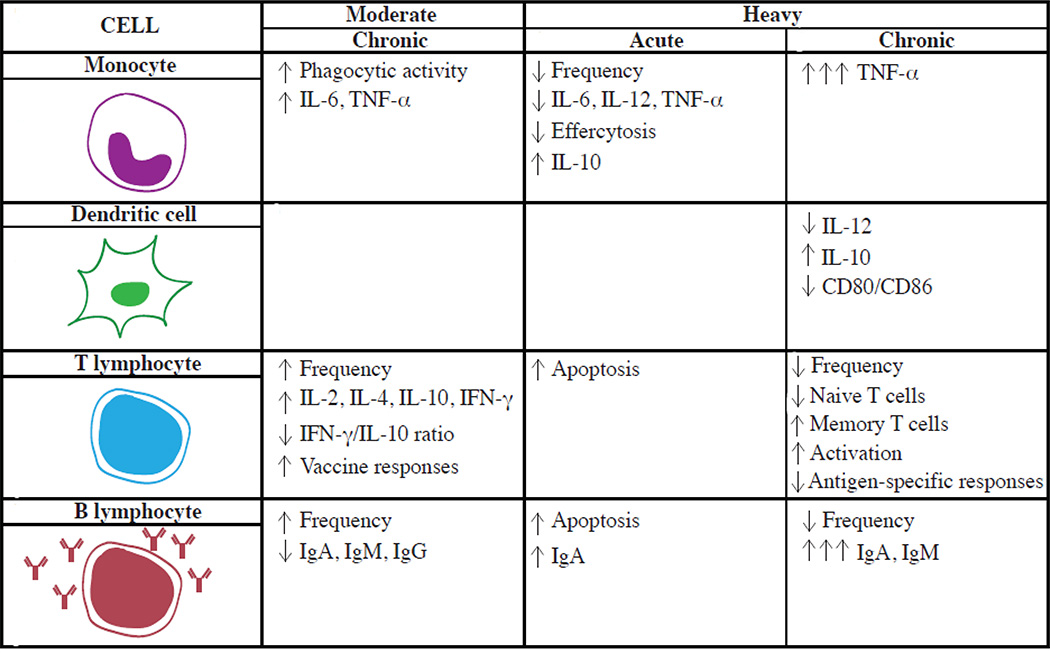
In summary, several in vitro and in vivo studies demonstrate that ethanol modulates the function of innate immune cells (monocytes and DCs) in a dose and time dependent manner (Figure 1). Acute high dose exposures inhibit whereas long-term treatments stimulate proinflammatory cytokine production. In addition, in vivo consumption of moderate amounts enhances phagocytosis and reduces inflammatory cytokine production whereas chronic consumption of large doses inhibits phagocytosis and production of growth factors.
Figure 1.
Opposing Effects of Alcohol Consumption on Immune Cells
Modulation of Adaptive Immunity by Alcohol
Alcohol consumption also impacts cell-mediated and humoral immunity. In a very early study, alcohol abuse was associated with a reduction in CD4 and CD8 T cell numbers (McFarland and Libre 1963). This general finding was confirmed in a cross-sectional study of heavy male drinkers (90–249 drinks/month) who displayed lower B cell numbers than moderate (30–89 drinks/month) or light (<10 drinks/month) drinkers (Mili, Flanders et al. 1992). Additionally, 153 chronic alcoholics (men and women) without liver disease had a decreased ratio of CD4/CD8 T cells in peripheral blood (Gheorghiu, Bara et al. 2004). Thus, in humans it appears that alcohol consumption can lower lymphocyte numbers, and decreases are most apparent in those with AUD.
Ethanol consumption by weanling ICR (outbred) mice (adjusted to 6% in their drinking water) for 8 weeks also resulted in 75% fewer CD3+ T cells (Percival and Sims 2000). Likewise, male rats fed an ethanol-containing liquid diet (8.7% v/v for up to 4 weeks) experienced a progressive loss of both CD4+ and CD8+ T cells (Boyadjieva, Dokur et al. 2002). Increased apoptosis of T and B lymphocytes isolated from the thymus, spleen, and lymph nodes of female mice was observed following 16 hour culture with 0.4%-2% ethanol, concentrations 5 to 25 times the definition of intoxication (Slukvin and Jerrells 1995). In contrast to these observations, moderate consumption of beer (330mL for women and 660mL for men) for 30 days resulted in a significant increase in the number of leukocytes, mature CD3+ T lymphocytes, neutrophils and basophils in women, while only basophils were increased in men (Romeo, Warnberg et al. 2007).
In addition to decreased lymphocyte frequency, alcohol abuse is also associated with shifts in T lymphocyte phenotype. Decreased percentage of CD45RA+ naïve CD4 and CD8 T cells and an increased percentage of CD45RO+ memory subsets was observed in adult males who consumed 30.9 ± 18.7 alcoholic drinks/day (400g/day) for approximately 25.6 ± 11.5 years (Cook, Waldschmidt et al. 1994, Cook, Ballas et al. 1995). Similarly in male and female mice, chronic ethanol consumption of 20% ethanol in water for up to 6 months decreased the percentage of naïve T cells and increased the percentage of memory T cells due to increased homeostatic proliferation (Cho, Rao et al. 2000, Song, Coleman et al. 2002, Zhang and Meadows 2005). Accumulation of memory T cells is associated with increased incidence of chronic inflammatory diseases and age-related pathologies such as osteoporosis, sarcopenia, Alzheimer’s disease, cancer, and cardiovascular disease (Hakim and Gress 2007, Chou and Effros 2013). In addition, loss of naïve T cells would be expected to interfere with the development of efficacious responses to infection and vaccination (Appay and Sauce 2014).
Alcohol abuse also leads to a significant elevation of activated CD8 T cells, measured by increased expression of human leukocyte antigen (HLA)-DR in adult males who consumed an average of 23 drinks/day for approximately 27 years that persisted for up to 10 days of abstinence (Cook, Garvey et al. 1991). Similarly, an increased percentage of CD8 T cells expressing HLA-DR and CD57 was reported in the group of male alcoholics with self reported average alcohol consumption of approximately 400g/day for approximately 26 years (Cook, Ballas et al. 1995). Mice that consumed 20% (w/v) ethanol in water for up to 6 months, also showed an increased percentage of activated T cells as measured by increased expression of CD43, Ly6C, rapid IFN-γ response, and increased sensitivity to low levels of TCR stimulation (Song, Coleman et al. 2002, Zhang and Meadows 2005). Taken together, these studies suggest that chronic alcohol-induced T cell lymphopenia increases T cell activation and homeostatic proliferation resulting in increased proportion of memory T cells relative to naïve T cells. In contrast, moderate alcohol increased frequency of lymphocytes (Figure 1).
Alterations in immunoglobulin (Ig) levels after alcohol consumption have also been observed. An increase in both IgA and IgM levels in heavy drinkers (90–249 drinks/month) compared to light (<9 drinks/month) or moderate drinkers (30–89 drinks/month) was observed in adult males (Mili, Flanders et al. 1992). Similarly, a human study of 460 males and females with 221 alcohol abstainers, 140 light drinkers (1–140g/week), 53 moderate drinkers (141–280g/week), and 46 heavy drinkers (>280g/week) found a dose-dependent increase in serum IgA levels with alcohol consumption (Gonzalez-Quintela, Alende et al. 2008). Additionally, spontaneous IgA synthesis from PBMCs isolated from male and female alcoholic patients with liver disease is higher than that from controls (Wands, Dienstag et al. 1981). Analogously, in Wistar female rats, acute ethanol administration at 4g/kg intraperitoneally for 30 minutes increased the concentration of IgA in the intestinal lamina propria (Budec, Koko et al. 2007). Finally treatment of a mouse hybridoma cell line with 25, 50, 100 and 200mM ethanol for 48 hours resulted in a linear increase in IgM production (Muhlbauer, Karsten et al. 2001). The increased production of immunoglobulins in alcoholics could be due to the adduction of liver proteins by acetaldehyde and lipid peroxidation of membranes by MDA that result in increased immunogenicity of self-proteins and the potential initiation of auto-immune responses (Thiele, Duryee et al. 2010). In contrast to these observations in subjects with alcohol use disorders, IgG, IgM and IgA concentrations, decreased in both men and women following moderate beer consumption (Romeo, Warnberg et al. 2007). Similarly, lower IgG levels were observed in female and male moderate consumers than in abstainers (Gonzalez-Quintela, Alende et al. 2008) (Figure 1).
Infection & Vaccination
Several lines of evidence suggest that alcohol consumption exerts a dose-dependent impact on the host response to infection. Chronic alcohol abuse leads to increased susceptibility to bacterial and viral infections, most notably a 3 to 7-fold increase in susceptibility (Schmidt and De Lint 1972) and severity (Saitz, Ghali et al. 1997) of bacterial pneumonia compared with control subjects. Similarly, the incidence of Mycobacterium tuberculosis infection among alcoholics is increased (Sabot and Vendrame 1969, Hudolin 1975, Kline, Hedemark et al. 1995, Panic and Panic 2001). Alcohol use has also been shown to drive disease progression in chronic viral infections such as human immunodeficiency virus (HIV) (Baum, Rafie et al. 2010) and Hepatitis C (Bhattacharya and Shuhart 2003). In addition, the magnitude of antibody response following vaccination with Hepatitis B is lower in alcoholics compared to controls (Nalpas, Thepot et al. 1993).
This increased susceptibility has been recapitulated in rodent models of chronic alcohol abuse. For instance, increased morbidity and mortality, pulmonary virus titers, and decreased pulmonary influenza-specific CD8 T cell responses were reported in female mice infected with influenza that consumed 20% (w/v) ethanol in their drinking water for 4–8 weeks (Meyerholz, Edsen-Moore et al. 2008). Likewise, higher pathogen burden and decreased CD8 T cell immunity was observed in female mice administered ethanol at 15% (w/v) for 5 days and challenged with Listeria monocytogenes (Gurung, Young et al. 2009). Similar results have been seen in SIV infection of male nonhuman primates (Bagby, Stoltz et al. 2003, Molina, McNurlan et al. 2006, Poonia, Nelson et al. 2006, Marcondes, Watry et al. 2008). Significantly lower protein and mRNA levels of macrophage inflammatory protein-2 (MIP-2) and cytokine-induced neutrophil chemoattractant (CINC), responsible for recruiting immune cells to the lung during early infection as well as delayed neutrophil recruitment were also observed in response to Streptococcus pneumoniae or LPS-induced endotoxemia in male Sprague Dawley rats intraperitoneally injected once with 20% ethanol (5.5g/kg) 30 minutes before infection (Boe, Nelson et al. 2001, Zhang, Bagby et al. 2002).
In contrast to the studies above, moderate alcohol consumption seems to enhance immune response to infection and vaccination (Cohen, Tyrrell et al. 1993, Mendenhall, Theus et al. 1997, Takkouche, Regueira-Méndez et al. 2002, Romeo, Warnberg et al. 2007, Romeo, Warnberg et al. 2007, Ouchi, Niu et al. 2012, Messaoudi, Asquith et al. 2013). A study of 391 males and females intentionally exposed to five different respiratory viruses showed that moderate alcohol consumption (1–2 drinks/day) was associated with decreased incidence of colds (Cohen, Tyrrell et al. 1993). A second study using a cohort of 4272 male and female individuals found the consumption of wine inversely associated with the risk of common cold (Takkouche, Regueira-Méndez et al. 2002). A third retrospective study that followed 899 men reported that non-drinkers were significantly more likely to experience 2 or more episodes of common cold compared with subjects who consumed 11.5–35.8g of alcohol per day (Ouchi, Niu et al. 2012). Moderate beer consumption also enhanced the production of T cell cytokines IL-2, IL-4, IL-10, and IFN-γ and reduced IFN-γ/IL-10 ratio (Romeo, Warnberg et al. 2007).
In male rats that were administered low acute (0.5g/kg, 5 days) or chronic (0.43g/kg/day, 28 days) doses of ethanol in a liquid diet, results revealed significantly higher T cell responses to Mycobacterium bovis and increased clearance of bacterial pathogens compared to controls. In contrast, a high acute (6g/kg for 5 days) or chronic (12g/kg/day for 28 days) dose of ethanol showed an immunosuppressive effect (Mendenhall, Theus et al. 1997). More recently, moderate daily alcohol consumption for over a year (average BAC 40mg/dL) in a rhesus macaque model of ethanol self-administration enhanced CD4+ and CD8+ T cell as well as IgG responses following Modified Vaccinia Ankara (MVA) vaccination, whereas chronic alcohol intoxication in this model (average BAC >80mg/dL) suppressed this response (Messaoudi, Asquith et al. 2013).
Circulating Factors
Alcohol consumption modulates plasma levels of several cytokines, chemokines, growth factors, and hormones in a dose dependent manner. Several large cohort studies (>2000 male and female subjects) have shown a J-shaped association between ethanol consumption and plasma levels of the acute phase protein, C-reactive protein (CRP) (Imhof, Froehlich et al. 2001, Albert, Glynn et al. 2003, Pai, Hankinson et al. 2006). Given the association between CRP levels and cardiovascular disease, the decrease in CRP levels observed with moderate alcohol consumption could explain the reduced incidence of cardiovascular disease in individuals who drink in moderation (Lagrand, Visser et al. 1999). J-shaped associations of acute phase proteins albumin and transferrin (Imhof, Froehlich et al. 2001) as well as IL-6 with alcohol consumption were also reported (Pai, Hankinson et al. 2006). Interestingly, this last study also found a strong inverse linear trend with increasing alcohol consumption in men and women and soluble TNF-α receptors 1 and 2 (sTNF-R1 and sTNF-R2) levels (Pai, Hankinson et al. 2006). TNF-receptors, which mediate TNF-α activity, have been associated with increased risk for adverse cardiovascular outcome, therefore cardiovascular benefits of moderate alcohol consumption may be also mediated by a reduction in TNF-α activity (Albert, Glynn et al. 2003).
A multiplex analysis of plasma collected from 24 healthy male subjects after moderate alcohol consumption (50mL of vodka with 200mL orange juice twice daily or only orange juice daily for 4 weeks during dinner) also showed a significant reduction in acute phase proteins, ferritin and α1-antitrypsin as well as pro-inflammatory cytokines IL-1 receptor antagonist and IL-18 (Joosten, van Erk et al. 2012). In contrast, level of anti-inflammatory protein adiponectin increased (Joosten, van Erk et al. 2012). Similarly, plasma adiponectin concentration was increased after 28 days of daily consumption of 450mL of red wine compared with dealcoholized red wine amongst 34 men, in the absence of changes in subcutaneous and abdominal fat contents as well as body weight (Beulens, van Beers et al. 2006).
In a monkey model of voluntary ethanol self-administration, chronic ethanol consumption for 32 months by male cynomolgus monkeys with mean daily intakes that reached 4.0g/kg/day (the equivalent of 16 drinks/day and BAC of 400mg/dL) resulted in decreased circulating levels of factors involved in the recruitment of immune cells to the site of infection including chemokines such as CCL3/4, and metalloproteases such as MMP-9 (Helms, Messaoudi et al. 2012). Decreased IL-2 and CCL5 levels provide insight into possible mechanisms of impaired T cell recruitment and proliferation. Increases in IL-7 and IL-15, which are critical for T cell survival, may be compensatory mechanisms for reduced IL-2 levels. Reduced IgE levels were also observed and may be related to the observed decrease in IgE synthesis regulators, IL-13 and CD40 ligand. Increased levels of CCL11, a potent chemokine for IgE-producing eosinophils, may be compensating the reduced IgE levels (Helms, Messaoudi et al. 2012). These changes were most apparent at the highest ethanol intakes and BAC.
Using these data, a 3-(adiponectin, α-2-macroglobulin, and complement component 3) and a 14-(CD40, chemokine ligand 5, factor VII, IgE, IGF-1, IL-2, IL-7, CCL3, CCL4, MMP2, kallikrein-related peptidase 3, glutamic-oxaloacetic transaminase 1, thrombopoietin and VEGFA) plasma protein biomarker panels that showed 100% sensitivity and 88% accuracy of ethanol consumption were developed (Freeman, Salzberg et al. 2010). The same self-administration macaque model also showed that male animals that drank in moderation (1.975g/kg/day) had slightly elevated plasma levels of IL-2, IL-15, IL-12, TNF-α, Regulated on Activation Normal T cell Expressed and Secreted (RANTES), and monokine induced by gamma interferon (MIG) compared to heavy drinkers and controls (Messaoudi, Asquith et al. 2013). These observations could explain why animals drinking moderately generated a more robust response to MVA vaccination compared to controls and animals that drank to intoxication since these factors are critical for lymphocyte proliferation, T cell activation and effector function, and immune cell recruitment.
Molecular Mechanisms of Dose Dependent Modulation of Immunity
Mechanisms that underlie these changes in circulating factors are starting to emerge. Alcohol-induced alterations in gene expression involved in cytokine signaling pathways were examined in male and female subjects with alcohol dependence (AD; more than 25 drinks/week prior to study; n=10), heavy drinkers (HD; defined as regular alcohol use over the past year of at least 8 drinks/week for women and at least 15 drinks/week for me; n=13), and moderate drinkers (MD; defined as up to 7 drinks/week for women and 14 drinks/week for men; n=17). After normalization, 436 differentially expressed genes in blood samples were identified: 291 genes differed between AD and MD subjects, 240 between AD and HD subjects, but only 6 differed between HD and MD subjects (Beech, Qu et al. 2012). Many of the differentially expressed genes were involved in the regulation of the immune response by cytokine signaling and the Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway. Expression of interferon receptor 2 (IFNAR2) was down-regulated in HD subjects compared to MD subjects which could contribute to deficits in both innate and adaptive immunity (Beech, Qu et al. 2012). IL-15, a cytokine critical for promoting survival, proliferation, and activation of NK and CD8 T cells, was found to be up-regulated in AD subjects but there was no difference between HD and MD subjects (Beech, Qu et al. 2012). Expression of the receptor for IL-21, which has been reported in autoimmune diseases, was found to be up-regulated in AD subjects (Beech, Qu et al. 2012).
A second study by Joosten et al. also analyzed gene expression profiles in PBMCs isolated from 24 healthy male subjects who consumed 50mL of vodka with 200mL orange juice or only orange twice daily for 4 weeks during dinner (considered to be moderate). Pathways involving antigen presentation, B and T cell receptor signaling, and IL-15 signaling were altered with moderate vodka consumption (Joosten, van Erk et al. 2012). The most significant change was in glucocorticoid receptor (GR) signaling, which is known to down-regulate immune activity and inflammation by down-regulating NFκB (Pelaia, Vatrella et al. 2003). Indeed, NFκB was down-regulated in the alcohol group compared with the control group (Joosten, van Erk et al. 2012). The observed decrease in expression of NFκB is in line with earlier studies examining decreased pro-inflammatory cytokine production with moderate alcohol consumption.
Interplay Between Alcohol-Induced Stress & Immunity
As discussed above in the gene expression studies, the mechanisms by which ethanol exerts dose-dependent effects on the immune system could also include modulation of the hypothalamic-pituitary-adrenal (HPA) axis, which tightly regulates the stress response, in turn affecting immunity. Response to different stressors is mediated by several neural circuits that converge on the paraventricular nucleus (PVN) of the hypothalamus (Myers, McKlveen et al. 2014). The PVN regulates pituitary hormone production, including adrenocorticotropic hormone (ACTH), which binds to melanocortin type 2 receptors in the adrenal cortex to induce steroidogenesis in distinct layers (Dringenberg, Schwitalla et al. 2013). Primates have a threelayer adrenal cortex with cortisol being the primary glucocorticoid produced in the zona fasciculata (Nguyen and Conley 2008), which is released in response to stress (O'Connor, O'Halloran et al. 2000). Corticosterone is the main glucocorticoid involved in the regulation of stress responses in rodents (Smith and Vale 2006).
The current model of HPA axis-immune system interactions involves glucocorticoid potentiation of the innate immune system and repression of the adaptive immune system (Busillo and Cidlowski 2013). All immune cells express GR at some state of activation, which allows glucocorticoids to have selective, dose-dependent effects on various aspects of immunity such as proliferation of memory T cells (Gutsol, Sokhonevich et al. 2013), macrophage numbers (Zheng, Zhong et al. 2013), and expression of TLRs (Jin, Qin et al. 2009). Impact of glucocorticoids is mediated through changes in gene expression where glucocorticoid/GR complexes bind to specific DNA sequences, called glucocorticoid response elements (GREs), tethered by transcription factors NFκB, AP1, STAT3, and STAT5 (Chinenov and Rogatsky 2007). For instance, glucocorticoids modulate TLR signaling by affecting: 1) downstream protein kinases, 2) suppressors of cytokine signaling (SOCS), 3) GR-inducible leucine zipper protein, and 4) GR-mediated increased expression of TLRs (Chinenov and Rogatsky 2007). In addition to TLR-mediated regulation, GR activity up-regulates the activity of NLRP3, a NOD-like receptor (NLR) involved in the production of IL-1β (Busillo, Azzam et al. 2011). Humans under various stressful environments (where high cortisol levels prevail) show greater TNF-α and IL-6 but lower IL-1β response by PBMCs after LPS stimulation, slower wound healing, increased CRP levels, decreased T lymphocyte counts, and increased CD57+ memory T cells (Kiecolt-Glaser, Marucha et al. 1995, Jaremka, Glaser et al. 2013, Copeland, Wolke et al. 2014, Copertaro, Bracci et al. 2014, Yi, Rykova et al. 2014).
Alcohol is a physiological stressor, which in turn, influences alcohol consumption. The mechanisms underlying this complex interaction, however, remain unknown. Acute alcohol consumption can activate the HPA axis and release glucocorticoids in a dose-dependent manner (Boyd, Kumar et al. 2010). Acute alcohol intake in social drinkers increases cortisol levels, though with the transition from social drinking to alcohol dependence, the HPA axis response is attenuated and the cortisol response is decreased (King, Munisamy et al. 2006). Similarly, acute ethanol voluntary self-administration in rats stimulated release of corticosterone and ACTH, but chronic exposure sufficient to produce dependence decreased the neuroendocrine response (Richardson, Lee et al. 2008). The impact of alcohol on HPA activation/function depends on several factors such as: 1) the timing of alcohol administration relative to stimulus, 2) duration of stressful stimulus (chronic vs. acute), and 3) whether individuals usually experience stimulant-like or sedative-like effects of alcohol under normal conditions. In healthy males given the Trier Social Stress Test, production of salivary cortisol was inhibited when ethanol was administered intravenously after a stressor to achieve a breath alcohol concentration of 40mg/dL (Childs, O'Connor et al. 2011). Similarly, acute ethanol consumption in healthy men blocked activation of the HPA axis by naloxone (at the level of the hypothalamus or above) (Cami, de la Torre et al. 1988), blunted the ACTH and cortisol response to corticotropin-releasing hormone (CRH; at the level of the pituitary) but not ACTH (level of the adrenal) (Waltman, Blevins et al. 1993). Therefore, although acute alcohol consumption can activate the HPA axis in non-dependent subjects, studies indicate that it interferes with the HPA responses to other stressors potentially through a negative feedback loop. Studies show altered expression of steroidogenic enzymes necessary for metabolism of steroid hormones from their precursors with both acute (Kim, Ha et al. 2003) as well as chronic intermittent ethanol consumption (Cagetti, Pinna et al. 2004), but systematic, dose-dependent studies are lacking. One mechanism by which alcohol could alter HPA activity is via modulation of glutamatergic and GABAergic input on parvocellular neurons of the PVN of the hypothalamus. These cells produce CRH and arginine vasopressin (AVP), peptides essential for the stress response, and ACTH release from the anterior pituitary (Rivier and Vale 1983). Additional studies are needed to better understand alcohol-induced alterations in the HPA response and the hyporesponsiveness to stress observed in those with AUD.
The immune system, in turn, can modulate the activity of the HPA axis. For instance, IL-1 induces HPA axis activation and glucocorticoid release that suppresses the immune system (Sapolsky, Rivier et al. 1987). Cultures of neonatal rat PVN show norepinephrine-induced CRH release is regulated by IL-1 signaling (Hsieh, Li et al. 2010) and in male rat PVN slices, IL-1β depolarized magnocellular and parvocellular neurons (Ferri and Ferguson 2003, Ferri, Yuill et al. 2005), suggesting that cytokines could impact the stress response at the level of CRH neurons in the hypothalamus. Cytokines are also proposed to cross the blood-brain barrier and produce sickness behavior (Watkins, Maier et al. 1995), which is comorbid with AUD (Dantzer, Bluthe et al. 1998). Ethanol administration (4g/kg) in male rats increased IL-6 but decreased TNF-α expression in PVN, an effect that was blunted or reversed after long-term ethanol self-administration (Doremus-Fitzwater, Buck et al. 2014). Cytokines can also modulate important behavioral functions including learning and memory (Hao, Jing et al. 2014) possibly due to their role in neuroplasticity (Sheridan, Wdowicz et al. 2014). Many gaps remain in our understanding of the stress response, its physiological basis in the HPA, axis and its role in modulating the effects of ethanol on host immunity.
Impact of ethanol on CNS resident immune cells
Alcohol also impacts the function of immune cells of the central nervous system (CNS), particularly astrocytes and microglia. Astrocytes are major glial cells that regulate neuronal function and CNS homeostasis. Their ability to serve as antigen presenting cells and produce cytokines in vivo has been controversial (Dong and Benveniste 2001). In vitro studies have shown that acetaldehyde modulates cytokine production by astrocytes in a dose-dependent manner (Sarc, Wraber et al. 2011). Specifically, 24 hours of exposure to both low (1mM) and high (5mM) concentrations of acetaldehyde stimulate IL-6 secretion, however, 7 days of exposure to the high concentration of acetaldehyde, significantly decrease IL-6 secretion (Sarc, Wraber et al. 2011). In contrast, both acute (24 hours) and prolonged (7 days) exposure to low and high concentrations of acetaldehyde reduce TNF-α secretion by primary rat astrocyte (Sarc, Wraber et al. 2011).
Alcohol-induced effects on microglia are less well understood. Microglia express PRRs, produce cytokines, and modulate neuroinflammatory reactions in brain injury and neurodegenerative diseases (Block, Zecca et al. 2007). Activated microglia respond to neuronal damage by removing damaged cells via phagocytosis, and chronic activation of microglia contributes to the pathology in a number of neurodegenerative diseases including Parkinson’s disease, Alzheimer’s disease, prion diseases, multiple sclerosis, and HIV-dementia (Dheen, Kaur et al. 2007). In Sprague Dawley rats exposed to 25% (w/v) ethanol via intragastric gavage every 8 hours for 4 days, increased activation and proliferation of microglia as evidenced by morphological changes and BrdU incorporation were observed in the hippocampus (McClain, Morris et al. 2011). Changes persisted at least 30 days after alcohol exposure suggestive of longlasting consequences of ethanol on microglia function (McClain, Morris et al. 2011). There is also evidence that ethanol-induced microglia activation is mediated by signaling through TLR4 (Fernandez-Lizarbe, Pascual et al. 2009).
Modulation of Immunity by Nutritional Change in AUD
Alcoholic beverages are energy dense and often become the primary energy source in those with AUD, leading to malnutrition. Individuals with AUD are often deficient in one or more essential nutrients including vitamin A, vitamin C, vitamin D, vitamin E, folate, and thiamine (Hoyumpa 1986). Amongst a group of 30 male and female patients who consumed at least 100g of ethanol daily (on average 51% of total caloric intake) for at least 5 years, all subjects were deficient in vitamin E and folate, 83% were deficient in vitamin C, 80% were deficient in vitamin A, and 73% were deficient in thiamine (Manari, Preedy et al. 2003). These micronutrients have been shown to play an important role in immune system homeostasis and response to infection (Mora, Iwata et al. 2008).
Dendritic cells can convert vitamin A to retinoic acid. T and B cell activation in the presence of retinoic acid results in the up-regulation of gut-homing molecules and generation of IgA-secreting B cells (Mora, Iwata et al. 2008). Consequently, deficiency in vitamin A results in the impairment of mucosal responses (Mora, Iwata et al. 2008). Vitamin D has long been known to have a critical role in calcium and phosphorous homeostasis. In addition, antigen presenting cells convert vitamin D to 1,25(OH)2VD3, a physiologically active form of vitamin D that is highly concentrated in lymphoid tissues (Mora, Iwata et al. 2008) where it can modulate function of T and B cells which express vitamin D receptors. Vitamin D deficiency results in reduced differentiation, phagocytosis and oxidative burst, by monocytes as well as defective bactericidal activity by keratinocytes (Fabri, Stenger et al. 2011, Djukic, Onken et al. 2014).
Vitamin E is one of the most effective antioxidants and its deficiency exacerbates freeradical damage impairing the ability of T cells to respond to pathogenic challenge (Mocchegiani, Costarelli et al. 2014). Similarly, vitamin C, also an antioxidant, is important for phagocytic activity of neutrophils and monocytes, and enhances T cell responses (Strohle and Hahn 2009). Thiamine, also known as vitamin B1, contributes to the activation of T cells, suppresses oxidative stress-induced NFκB activation in macrophages, and serves as an anti-inflammatory factor (Manzetti, Zhang et al. 2014). Antigen-specific responses are decreased in folate-deficient humans and animals (Dhur, Galan et al. 1991).
These observations suggest that immune defects seen in individuals with AUD could also be mediated by nutritional deficiencies in addition to barrier defects and functional changes in immune cells. However, the contributions of each of these changes to increased susceptibility to infection in individuals with AUD remain to be determined.
Conclusion
Molecular mechanisms of the dose-dependent effects of alcohol on the immune system and HPA regulation remain poorly understood due to a lack of systematic studies that examine the effect of multiple doses and different time courses. There may be important differences in the effects of ethanol on the immune system depending on whether the study is conducted in vitro or in vivo, as the latter allows for a complex psychogenic component in which stress-related hormones and immune-signaling molecules interact. In addition, most studies have been done in vitro using primary cells or cell lines in the presence of rather high, constant doses of ethanol. Similarly, most rodent studies to date have focused on acute/short-term binge models utilizing high concentration of ethanol (20% ethanol) as the sole source of fluid, a possible stressor in itself. Therefore, there is a pressing need for in depth studies that examine dose-dependent effects of chronic ethanol consumption on immunity in vivo to allow for the complex interactions between ethanol, its metabolites, HPA signaling, nutritional deficiencies, and the immune system.
Such studies can be challenging to conduct in humans because of difficulties in obtaining accurate medical histories, maintaining adherence, confounding factors such as diet, sleep-wake cycles, and ethical considerations when studying large doses of ethanol. Rodent studies offer several advantages such as availability of transgenic models that can facilitate mechanistic studies. There are however some limitations to rodent studies. Rodents have a much shorter life span and often require forced (i.e., not initiated by the animal) exposure to alcohol, which is stressful. Moreover, a recent systematic comparison examining gene expression changes found that temporal gene response patterns to trauma, burns, and endotoxemia in mouse models correlated poorly with the human conditions (Seok, Warren et al. 2013). Nonhuman primates, on the other hand, voluntarily consume different amounts of alcohol and allow us to conduct studies in an outbred species that shares significant physiological and genetic homology with humans while maintaining rigorous control over diet and other environmental cues. Moreover, immune systems of several nonhuman primate species are similar to those of humans and these animals are susceptible to several clinically important pathogens making them a valuable model to study the impact of ethanol on immunity (Hein and Griebel 2003). Nonetheless, nonhuman primate models come with their disadvantages as well. Costly requirements such as dedicated facilities to house the animals, experienced personnel to perform specialized procedures, and compliance with high standards of care must be considered.
It is also critical to take into consideration that the effects of ethanol on immune function in vivo could involve the actions of its primary metabolite, acetaldehyde. Therefore, more studies looking at the effects of ethanol metabolites in vivo are needed. Acetaldehyde has also been shown to affect NFκB-induced cytokine production in various liver cells. In the presence of acetaldehyde, Kupffer cells, the specialized macrophages in the liver, treated with LPS show decreased NFκB activation (Jokelainen, Thomas et al. 1998), while hepatic stellate cells, the major producers of collagen that accumulate during hepatic fibrosis, show enhanced NFκB activation (Novitskiy, Ravi et al. 2005). Finally, acetaldehyde disrupts intestinal epithelial barrier function and increases paracellular permeability which plays a crucial role in the pathogenesis of alcoholic liver disease by a tyrosine kinase-dependent mechanism (Sheth, Seth et al. 2004).
Future studies aimed at uncovering the mechanisms underlying dose-dependent modulation of immune function should also investigate changes in gene expression patterns, as well as factors that regulate gene expression including microRNAs and epigenetic changes within specific immune cell populations. Additionally, the role of alcohol-induced changes in the microbiome on immunity should be studied. Recent studies have shown that the microbiome modulates immunity in the gut, and in turn, immunity modulates the microbiome in the gut (Belkaid and Hand 2014). Only two studies have examined alcohol-induced changes in colonic (Mutlu, Gillevet et al. 2012) and fecal microbiomes (Chen, Yang et al. 2011), and both studies focused on individuals with AUD. Finally, an emerging informatics approach that can piece together these extensive data sets and build a network between the immune response elements, the HPA axis, and the time-course/dose response of ethanol while emphasizing in vivo studies from rodent, non human primate, and humans is urgently required.
Acknowledgments
This work was supported by NIAAA grants AA021947 (Messaoudi), AA013510 (Grant), and AA109431 (Grant). Tasha Barr was supported by an NRSA T32 training grant (T32 ES018827).
Abbreviations
- NK
Natural killer
- DC
dendritic cell
- PRR
pathogen recognition receptor
- PAMP
pathogen-associated molecular pattern
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- Ig
immunoglobulin
- AUD
alcohol use disorder
- ADH
alcohol dehydrogenase
- CYP2E1
cytochrome P450 2E1
- MDA
malondialdehyde
- MAA
MDA-acetaldehyde
- ROS
reactive oxygen species
- TLR
Toll-like receptor
- NFκB
nuclear factor kappa B
- IL
interleukin
- TNF
tumor necrosis factor
- IRAK-M
IL-1R-associated kinase-monocyte
- BAC
blood alcohol concentration
- PBMC
peripheral blood mononuclear cell
- CRP
C-reactive protein
- HPA
hypothalamic-pituitary-adrenal
- PVN
paraventricular nucleus
- ACTH
adrenocorticotropic hormone
- CRH
corticotropin-releasing hormone
- GRE
glucocorticoid response element
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
The authors declare that they have no competing financial interests.
References
- Afshar M, Richards S, Mann D, Cross A, Smith GB, Netzer G, Kovacs E, Hasday J. Acute immunomodulatory effects of binge alcohol ingestion. Alcohol. 2014 doi: 10.1016/j.alcohol.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed FE. Toxicological effects of ethanol on human health. Crit Rev Toxicol. 1995;25(4):347–367. doi: 10.3109/10408449509021614. [DOI] [PubMed] [Google Scholar]
- Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107(3):443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- Appay V, Sauce D. Naive T cells: the crux of cellular immune aging? Exp Gerontol. 2014;54:90–93. doi: 10.1016/j.exger.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, Grant KA, Messaoudi I. Chronic Ethanol Consumption Modulates Growth Factor Release, Mucosal Cytokine Production, and MicroRNA Expression in Nonhuman Primates. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G, Stoltz D, Zhang P, Kolls J, Brown J, Bohm R, Rockar R, Purcell J, Murphey-Corb M, Nelson S. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcoholism, clinical and experimental research. 2003;27(3):495–997. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses. 2010;26(5):511–518. doi: 10.1089/aid.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech RD, Qu J, Leffert JJ, Lin A, Hong KA, Hansen J, Umlauf S, Mane S, Zhao H, Sinha R. Altered expression of cytokine signaling pathway genes in peripheral blood cells of alcohol dependent subjects: preliminary findings. Alcohol Clin Exp Res. 2012;36(9):1487–1496. doi: 10.1111/j.1530-0277.2012.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nature Protocols. 2013;8(3):627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beulens JW, van Beers RM, Stolk RP, Schaafsma G, Hendriks HF. The effect of moderate alcohol consumption on fat distribution and adipocytokines. Obesity (Silver Spring) 2006;14(1):60–66. doi: 10.1038/oby.2006.8. [DOI] [PubMed] [Google Scholar]
- Bhattacharya R, Shuhart MC. Hepatitis C and alcohol: interactions, outcomes, and implications. J Clin Gastroenterol. 2003;36(3):242–252. doi: 10.1097/00004836-200303000-00012. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis. 2001;184(9):1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- Boe DM, Richens TR, Horstmann SA, Burnham EL, Janssen WJ, Henson PM, Moss M, Vandivier RW. Acute and chronic alcohol exposure impair the phagocytosis of apoptotic cells and enhance the pulmonary inflammatory response. Alcohol Clin Exp Res. 2010;34(10):1723–1732. doi: 10.1111/j.1530-0277.2010.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjieva NI, Dokur M, Advis JP, Meadows GG, Sarkar DK. Beta-endorphin modulation of lymphocyte proliferation: effects of ethanol. Alcohol Clin Exp Res. 2002;26(11):1719–1727. doi: 10.1097/01.ALC.0000036925.42090.9D. [DOI] [PubMed] [Google Scholar]
- Boyd KN, Kumar S, O'Buckley TK, Porcu P, Morrow AL. Ethanol induction of steroidogenesis in rat adrenal and brain is dependent upon pituitary ACTH release and de novo adrenal StAR synthesis. J Neurochem. 2010;112(3):784–796. doi: 10.1111/j.1471-4159.2009.06509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budec M, Koko V, Todorovic V, Markovic D, Postic M, Drndarevic N, Spasic A, Mitrovic O. Possible mechanism of acute effect of ethanol on intestinal IgA expression in rat. Int Immunopharmacol. 2007;7(6):858–863. doi: 10.1016/j.intimp.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286(44):38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24(3):109–119. doi: 10.1016/j.tem.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46(4):570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Cami J, de la Torre R, Garcia-Sevilla L, Ugena B, Knobel H, Segura J. Alcohol antagonism of hypercortisolism induced by naloxone. Clin Pharmacol Ther. 1988;43(6):599–604. doi: 10.1038/clpt.1988.82. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- Childs E, O'Connor S, de Wit H. Bidirectional interactions between acute psychosocial stress and acute intravenous alcohol in healthy men. Alcohol Clin Exp Res. 2011;35(10):1794–1803. doi: 10.1111/j.1530-0277.2011.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenov Y, Rogatsky I. Glucocorticoids and the innate immune system: crosstalk with the toll-like receptor signaling network. Mol Cell Endocrinol. 2007;275(1–2):30–42. doi: 10.1016/j.mce.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192(4):549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19(9):1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP. Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health. 1993;83(9):1277–1283. doi: 10.2105/ajph.83.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R, Garvey M, Booth B, Goeken J, Stewart B, Noel M. Activated CD-8 cells and HLA DR expression in alcoholics without overt liver disease. Journal of clinical immunology. 1991;11(5):246–253. doi: 10.1007/BF00918182. [DOI] [PubMed] [Google Scholar]
- Cook RT, Ballas ZK, Waldschmidt TJ, Vandersteen D, LaBrecque DR, Cook BL. Modulation of T-cell adhesion markers, and the CD45R and CD57 antigens in human alcoholics. Alcohol Clin Exp Res. 1995;19(3):555–563. doi: 10.1111/j.1530-0277.1995.tb01548.x. [DOI] [PubMed] [Google Scholar]
- Cook RT, Waldschmidt TJ, Ballas ZK, Cook BL, Booth BM, Stewart BC, Garvey MJ. Fine T-cell subsets in alcoholics as determined by the expression of L-selectin, leukocyte common antigen, and beta-integrin. Alcohol Clin Exp Res. 1994;18(1):71–80. doi: 10.1111/j.1530-0277.1994.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Lereya ST, Shanahan L, Worthman C, Costello EJ. Childhood bullying involvement predicts low-grade systemic inflammation into adulthood. Proc Natl Acad Sci U S A. 2014;111(21):7570–7575. doi: 10.1073/pnas.1323641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copertaro A, Bracci M, Manzella N, Barbaresi M, Copertaro B, Santarelli L. Low perceived social support is associated with CD8+CD57+ lymphocyte expansion and increased TNF-alpha levels. Biomed Res Int. 2014;2014:635784. doi: 10.1155/2014/635784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, Kelley KW. Cytokines and sickness behavior. Ann N Y Acad Sci. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14(11):1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Dhur A, Galan P, Hercberg S. Folate status and the immune system. Prog Food Nutr Sci. 1991;15(1–2):43–60. [PubMed] [Google Scholar]
- Djukic M, Onken ML, Schutze S, Redlich S, Gotz A, Hanisch UK, Bertsch T, Ribes S, Hanenberg A, Schneider S, Bollheimer C, Sieber C, Nau R. Vitamin d deficiency reduces the immune response, phagocytosis rate, and intracellular killing rate of microglial cells. Infect Immun. 2014;82(6):2585–2594. doi: 10.1128/IAI.01814-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36(2):180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, Deak T. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin Exp Res. 2014;38(8):2186–2198. doi: 10.1111/acer.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, Teles R, Montoya D, Iyer SS, Bruns H, Lewinsohn DM, Hollis BW, Hewison M, Adams JS, Steinmeyer A, Zugel U, Cheng G, Jo EK, Bloom BR, Modlin RL. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104):104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183(7):4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Ferri CC, Ferguson AV. Interleukin-1 beta depolarizes paraventricular nucleus parvocellular neurones. J Neuroendocrinol. 2003;15(2):126–133. doi: 10.1046/j.1365-2826.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- Ferri CC, Yuill EA, Ferguson AV. Interleukin-1beta depolarizes magnocellular neurons in the paraventricular nucleus of the hypothalamus through prostaglandin-mediated activation of a non selective cationic conductance. Regul Pept. 2005;129(1–3):63–71. doi: 10.1016/j.regpep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Salzberg AC, Gonzales SW, Grant KA, Vrana KE. Classification of alcohol abuse by plasma protein biomarkers. Biol Psychiatry. 2010;68(3):219–222. doi: 10.1016/j.biopsych.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheorghiu M, Bara C, Pasarica D, Brasoveanu L, Bleotu C, Toparceanu F, Trandafir T, Diaconu CC. Ethanol-induced dysfunction of hepatocytes and leukocytes in patients without liver failure. Roum Arch Microbiol Immunol. 2004;63(1–2):5–33. [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, Vidal C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008;151(1):42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Young BM, Coleman RA, Wiechert S, Turner LE, Ray NB, Waldschmidt TJ, Legge KL, Cook RT. Chronic ethanol induces inhibition of antigen-specific CD8+ but not CD4+ immunodominant T cell responses following Listeria monocytogenes inoculation. J Leukoc Biol. 2009;85(1):34–43. doi: 10.1189/jlb.0208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsol AA, Sokhonevich NA, Seledtsov VI, Litvinova LS. Dexamethasone effects on activation and proliferation of immune memory T cells. Bull Exp Biol Med. 2013;155(4):474–476. doi: 10.1007/s10517-013-2182-5. [DOI] [PubMed] [Google Scholar]
- Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70(3):179–189. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- Hao Y, Jing H, Bi Q, Zhang J, Qin L, Yang P. Intra-amygdala microinfusion of IL-6 impairs the auditory fear conditioning of rats via JAK/STAT activation. Behav Brain Res. 2014;275C:88–95. doi: 10.1016/j.bbr.2014.08.052. [DOI] [PubMed] [Google Scholar]
- Hein WR, Griebel PJ. A road less travelled: large animal models in immunological research. Nat Rev Immunol. 2003;3(1):79–84. doi: 10.1038/nri977. [DOI] [PubMed] [Google Scholar]
- Helms C, Messaoudi I, Jeng S, Freeman W, Vrana K, Grant K. A Longitudinal Analysis of Circulating Stress-Related Proteins and Chronic Ethanol Self-Administration in Cynomolgus Macaques. Alcoholism, clinical and experimental research. 2012;36(6):995–1998. doi: 10.1111/j.1530-0277.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006;25(51):6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- Holmes MV, Dale CE, Zuccolo L, Silverwood RJ, Guo Y, Ye Z, Prieto-Merino D, Dehghan A, Trompet S, Wong A, Cavadino A, Drogan D, Padmanabhan S, Li S, Yesupriya A, Leusink M, Sundstrom J, Hubacek JA, Pikhart H, Swerdlow DI, Panayiotou AG, Borinskaya SA, Finan C, Shah S, Kuchenbaecker KB, Shah T, Engmann J, Folkersen L, Eriksson P, Ricceri F, Melander O, Sacerdote C, Gamble DM, Rayaprolu S, Ross OA, McLachlan S, Vikhireva O, Sluijs I, Scott RA, Adamkova V, Flicker L, Bockxmeer FM, Power C, Marques-Vidal P, Meade T, Marmot MG, Ferro JM, Paulos-Pinheiro S, Humphries SE, Talmud PJ, Mateo Leach I, Verweij N, Linneberg A, Skaaby T, Doevendans PA, Cramer MJ, van der Harst P, Klungel OH, Dowling NF, Dominiczak AF, Kumari M, Nicolaides AN, Weikert C, Boeing H, Ebrahim S, Gaunt TR, Price JF, Lannfelt L, Peasey A, Kubinova R, Pajak A, Malyutina S, Voevoda MI, Tamosiunas A, Maitland-van der Zee AH, Norman PE, Hankey GJ, Bergmann MM, Hofman A, Franco OH, Cooper J, Palmen J, Spiering W, de Jong PA, Kuh D, Hardy R, Uitterlinden AG, Ikram MA, Ford I, Hypponen E, Almeida OP, Wareham NJ, Khaw KT, Hamsten A, Husemoen LL, Tjonneland A, Tolstrup JS, Rimm E, Beulens JW, Verschuren WM, Onland-Moret NC, Hofker MH, Wannamethee SG, Whincup PH, Morris R, Vicente AM, Watkins H, Farrall M, Jukema JW, Meschia J, Cupples LA, Sharp SJ, Fornage M, Kooperberg C, LaCroix AZ, Dai JY, Lanktree MB, Siscovick DS, Jorgenson E, Spring B, Coresh J, Li YR, Buxbaum SG, Schreiner PJ, Ellison RC, Tsai MY, Patel SR, Redline S, Johnson AD, Hoogeveen RC, Hakonarson H, Rotter JI, Boerwinkle E, de Bakker PI, Kivimaki M, Asselbergs FW, Sattar N, Lawlor DA, Whittaker J, Davey Smith G, Mukamal K, Psaty BM, Wilson JG, Lange LA, Hamidovic A, Hingorani AD, Nordestgaard BG, Bobak M, Leon DA, Langenberg C, Palmer TM, Reiner AP, Keating BJ, Dudbridge F, Casas JP C. InterAct. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349:g4164. doi: 10.1136/bmj.g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyumpa AM. Mechanisms of vitamin deficiencies in alcoholism. Alcohol Clin Exp Res. 1986;10(6):573–581. doi: 10.1111/j.1530-0277.1986.tb05147.x. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, Li HY, Chen JC. Nitric oxide and interleukin-1beta mediate noradrenergic induced corticotrophin-releasing hormone release in organotypic cultures of rat paraventricular nucleus. Neuroscience. 2010;165(4):1191–1202. doi: 10.1016/j.neuroscience.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Hudolin V. Tuberculosis and alcoholism. Ann N Y Acad Sci. 1975;252:353–364. doi: 10.1111/j.1749-6632.1975.tb19179.x. [DOI] [PubMed] [Google Scholar]
- Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357(9258):763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- Janeway C. Janeway's Immunobiology. New York, New York: Garland Science, Taylor & Francis Group, LLC; 2008. [Google Scholar]
- Jaremka LM, Glaser R, Loving TJ, Malarkey WB, Stowell JR, Kiecolt-Glaser JK. Attachment anxiety is linked to alterations in cortisol production and cellular immunity. Psychol Sci. 2013;24(3):272–279. doi: 10.1177/0956797612452571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Qin Q, Tu L, Qu J. Glucocorticoids inhibit the innate immune system of human corneal fibroblast through their suppression of toll-like receptors. Mol Vis. 2009;15:2435–2441. [PMC free article] [PubMed] [Google Scholar]
- Jokelainen K, Thomas P, Lindros K, Nanji AA. Acetaldehyde inhibits NF-kappaB activation through IkappaBalpha preservation in rat Kupffer cells. Biochem Biophys Res Commun. 1998;253(3):834–836. doi: 10.1006/bbrc.1998.9863. [DOI] [PubMed] [Google Scholar]
- Joosten MM, van Erk MJ, Pellis L, Witkamp RF, Hendriks HF. Moderate alcohol consumption alters both leucocyte gene expression profiles and circulating proteins related to immune response and lipid metabolism in men. Br J Nutr. 2012;108(4):620–627. doi: 10.1017/S0007114511005988. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346(8984):1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ha M, Park CH, Park SJ, Youn SM, Kang SS, Cho GJ, Choi WS. StAR and steroidogenic enzyme transcriptional regulation in the rat brain: effects of acute alcohol administration. Brain Res Mol Brain Res. 2003;115(1):39–49. doi: 10.1016/s0169-328x(03)00177-3. [DOI] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59(3):203–209. doi: 10.1016/j.ijpsycho.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276(45):41930–41937. doi: 10.1074/jbc.M107181200. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Moderate drinking and reduced risk of heart disease. Alcohol Res Health. 1999;23(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- Kline SE, Hedemark LL, Davies SF. Outbreak of tuberculosis among regular patrons of a neighborhood bar. N Engl J Med. 1995;333(4):222–227. doi: 10.1056/NEJM199507273330404. [DOI] [PubMed] [Google Scholar]
- Lagrand WK, Visser CA, Hermens WT, Niessen HW, Verheugt FW, Wolbink GJ, Hack CE. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100(1):96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- Lau A, Abe M, Thomson A. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. Journal of leukocyte biology. 2006;79(5):941–994. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- Manari AP, Preedy VR, Peters TJ. Nutritional intake of hazardous drinkers and dependent alcoholics in the UK. Addict Biol. 2003;8(2):201–210. doi: 10.1080/1355621031000117437. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183(2):1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173(5):3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Jeliazkova V, Kodys K. Alcohol exposure regulates heat shock transcription factor binding and heat shock proteins 70 and 90 in monocytes and macrophages: implication for TNF-alpha regulation. J Leukoc Biol. 2008;84(5):1335–1345. doi: 10.1189/jlb.0407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Jeliazkova V, Catalano D, Szabo G. Acute alcohol exposure exerts anti-inflammatory effects by inhibiting IkappaB kinase activity and p65 phosphorylation in human monocytes. J Immunol. 2007;178(12):7686–7693. doi: 10.4049/jimmunol.178.12.7686. [DOI] [PubMed] [Google Scholar]
- Manzetti S, Zhang J, van der Spoel D. Thiamin function, metabolism, uptake, and transport. Biochemistry. 2014;53(5):821–835. doi: 10.1021/bi401618y. [DOI] [PubMed] [Google Scholar]
- Maraslioglu M, Oppermann E, Blattner C, Weber R, Henrich D, Jobin C, Schleucher E, Marzi I, Lehnert M. Chronic ethanol feeding modulates inflammatory mediators, activation of nuclear factor-kappaB, and responsiveness to endotoxin in murine Kupffer cells and circulating leukocytes. Mediators Inflamm. 2014;2014:808695. doi: 10.1155/2014/808695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Watry D, Zandonatti M, Flynn C, Taffe MA, Fox H. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2008;32(9):1583–1592. doi: 10.1111/j.1530-0277.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9(3):349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011;25(Suppl 1):S120–S128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland W, Libre EP. Abnormal Leukocyte Response in Alcoholism. Ann Intern Med. 1963;59:865–877. doi: 10.7326/0003-4819-59-6-865. [DOI] [PubMed] [Google Scholar]
- Mendenhall CL, Theus SA, Roselle GA, Grossman CJ, Rouster SD. Biphasic in vivo immune function after low- versus high-dose alcohol consumption. Alcohol. 1997;14(3):255–260. doi: 10.1016/s0741-8329(96)00150-4. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Asquith M, Engelmann F, Park B, Brown M, Rau A, Shaw J, Grant KA. Moderate alcohol consumption enhances vaccine-induced responses in rhesus macaques. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic alcohol consumption increases the severity of murine influenza virus infections. J Immunol. 2008;181(1):641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mili F, Flanders WD, Boring JR, Annest JL, DeStefano F. The associations of alcohol drinking and drinking cessation to measures of the immune system in middle-aged men. Alcohol Clin Exp Res. 1992;16(4):688–694. doi: 10.1111/j.1530-0277.1992.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Costarelli L, Giacconi R, Malavolta M, Basso A, Piacenza F, Ostan R, Cevenini E, Gonos ES, Franceschi C, Monti D. Vitamin E-gene interactions in aging and inflammatory age-related diseases: implications for treatment. A systematic review. Ageing Res Rev. 2014;14:81–101. doi: 10.1016/j.arr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Molina PE, McNurlan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, Bohm RP, Zhang P, Bagby GJ, Nelson S. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res. 2006;30(12):2065–2078. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8(9):685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlbauer E, Karsten U, Rottmann M, Rommelspacher H. Impaired immunoglobulin M production by incubation of hybridoma cells with ethanol. Alcohol. 2001;24(3):179–187. doi: 10.1016/s0741-8329(01)00152-5. [DOI] [PubMed] [Google Scholar]
- Muralidharan S, Ambade A, Fulham MA, Deshpande J, Catalano D, Mandrekar P. Moderate Alcohol Induces Stress Proteins HSF1 and hsp70 and Inhibits Proinflammatory Cytokines Resulting in Endotoxin Tolerance. J Immunol. 2014;193(4):1975–1987. doi: 10.4049/jimmunol.1303468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu E, Gillevet P, Rangwala H, Sikaroodi M, Naqvi A, Engen P, Kwasny M, Lau C, Keshavarzian A. Colonic microbiome is altered in alcoholism. American journal of physiology. Gastrointestinal and liver physiology. 2012;302(9):78. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalpas B, Thepot V, Driss F, Pol S, Courouce A, Saliou P, Berthelot P. Secondary immune response to hepatitis B virus vaccine in alcoholics. Alcoholism, clinical and experimental research. 1993;17(2):295–303. doi: 10.1111/j.1530-0277.1993.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Novitskiy G, Ravi R, Potter JJ, Rennie-Tankersley L, Wang L, Mezey E. Effects of acetaldehyde and TNF alpha on the inhibitory kappa B-alpha protein and nuclear factor kappa B activation in hepatic stellate cells. Alcohol Alcohol. 2005;40(2):96–101. doi: 10.1093/alcalc/agh116. [DOI] [PubMed] [Google Scholar]
- O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50(11):1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- Ouchi E, Niu K, Kobayashi Y, Guan L, Momma H, Guo H, Chujo M, Otomo A, Cui Y, Nagatomi R. Frequent alcohol drinking is associated with lower prevalence of self-reported common cold: a retrospective study. BMC Public Health. 2012;12:987. doi: 10.1186/1471-2458-12-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186(1):113–120. doi: 10.1016/j.atherosclerosis.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Pang M, Bala S, Kodys K, Catalano D, Szabo G. Inhibition of TLR8- and TLR4-induced Type I IFN induction by alcohol is different from its effects on inflammatory cytokine production in monocytes. BMC Immunol. 2011;12:55. doi: 10.1186/1471-2172-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panic E, Panic I. Chronic alcoholics' knowledge regarding tuberculosis. Pneumologia. 2001;50(4):232–235. [PubMed] [Google Scholar]
- Pelaia G, Vatrella A, Cuda G, Maselli R, Marsico SA. Molecular mechanisms of corticosteroid actions in chronic inflammatory airway diseases. Life Sci. 2003;72(14):1549–1561. doi: 10.1016/s0024-3205(02)02446-3. [DOI] [PubMed] [Google Scholar]
- Percival SS, Sims CA. Wine modifies the effects of alcohol on immune cells of mice. J Nutr. 2000;130(5):1091–1094. doi: 10.1093/jn/130.5.1091. [DOI] [PubMed] [Google Scholar]
- Poonia B, Nelson S, Bagby G, Veazey R. Intestinal lymphocyte subsets and turnover are affected by chronic alcohol consumption: implications for SIV/HIV infection. Journal of acquired immune deficiency syndromes (1999) 2006;41(5):537–584. doi: 10.1097/01.qai.0000209907.43244.ee. [DOI] [PubMed] [Google Scholar]
- Pruett B, Pruett S. An explanation for the paradoxical induction and suppression of an acute phase response by ethanol. Alcohol (Fayetteville, N.Y.) 2006;39(2):105–110. doi: 10.1016/j.alcohol.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Schwab C. Differences in IL-10 and IL-12 production patterns and differences in the effects of acute ethanol treatment on macrophages in vivo and in vitro. Alcohol. 2005;37(1):1–8. doi: 10.1016/j.alcohol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan RP, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004;33(2):147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28(8):1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305(5932):325–327. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- Romeo J, Warnberg J, Diaz LE, Gonzalez-Gross M, Marcos A. Effects of moderate beer consumption on first-line immunity of healthy adults. J Physiol Biochem. 2007;63(2):153–159. doi: 10.1007/BF03168226. [DOI] [PubMed] [Google Scholar]
- Romeo J, Warnberg J, Nova E, Diaz LE, Gonzalez-Gross M, Marcos A. Changes in the immune system after moderate beer consumption. Ann Nutr Metab. 2007;51(4):359–366. doi: 10.1159/000107679. [DOI] [PubMed] [Google Scholar]
- Sabot, Vendrame GG. Incidence of pulmonary tuberculosis in alcoholics. Study based on investigations made at the Ospedale Psichiatrico Provinciale di Udine in the decade 1958–1967. Minerva Med. 1969;60(101):5190–5194. [PubMed] [Google Scholar]
- Saitz R, Ghali W, Moskowitz M. The impact of alcohol-related diagnoses on pneumonia outcomes. Archives of internal medicine. 1997;157(13):1446–1498. [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Sarc L, Wraber B, Lipnik-Stangelj M. Ethanol and acetaldehyde disturb TNF-alpha and IL-6 production in cultured astrocytes. Hum Exp Toxicol. 2011;30(9):1256–1265. doi: 10.1177/0960327110388533. [DOI] [PubMed] [Google Scholar]
- Schmidt W, De Lint J. Causes of death of alcoholics. Q J Stud Alcohol. 1972;33(1):171–185. [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG Inflammation L.S. C. R. P. Host Response to Injury. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan GK, Wdowicz A, Pickering M, Watters O, Halley P, O'Sullivan NC, Mooney C, O'Connell DJ, O'Connor JJ, Murphy KJ. CX3CL1 is up-regulated in the rat hippocampus during memory-associated synaptic plasticity. Front Cell Neurosci. 2014;8:233. doi: 10.3389/fncel.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth P, Seth A, Thangavel M, Basuroy S, Rao RK. Epidermal growth factor prevents acetaldehyde-induced paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 2004;28(5):797–804. doi: 10.1097/01.alc.0000125358.92335.90. [DOI] [PubMed] [Google Scholar]
- Slukvin II, Jerrells TR. Different pathways of in vitro ethanol-induced apoptosis in thymocytes and splenic T and B lymphocytes. Immunopharmacology. 1995;31(1):43–57. doi: 10.1016/0162-3109(95)00032-4. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Coleman R, Zhu X, Alber C, Ballas Z, Waldschmidt T, Cook R. Chronic ethanol consumption by mice results in activated splenic T cells. Journal of leukocyte biology. 2002;72(6):1109–1125. [PubMed] [Google Scholar]
- Strohle A, Hahn A. Vitamin C and immune function. Med Monatsschr Pharm. 2009;32(2):49–54. quiz 55-46. [PubMed] [Google Scholar]
- Szabo G. Monocytes, alcohol use, and altered immunity. Alcohol Clin Exp Res. 1998;22(5 Suppl):216S–219S. doi: 10.1097/00000374-199805001-00002. [DOI] [PubMed] [Google Scholar]
- Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin Exp Res. 2004;28(5):824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- Takkouche B, Regueira-Méndez C, García-Closas R, Figueiras A, Gestal-Otero JJ, Hernán MA. Intake of Wine, Beer, and Spirits and the Risk of Clinical Common Cold. American Journal of Epidemiology. 2002;155(9):853–858. doi: 10.1093/aje/155.9.853. [DOI] [PubMed] [Google Scholar]
- Thiele GM, Duryee MJ, Willis MS, Tuma DJ, Radio SJ, Hunter CD, Schaffert CS, Klassen LW. Autoimmune hepatitis induced by syngeneic liver cytosolic proteins biotransformed by alcohol metabolites. Alcohol Clin Exp Res. 2010;34(12):2126–2136. doi: 10.1111/j.1530-0277.2010.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma DJ, Casey CA. Dangerous byproducts of alcohol breakdown--focus on adducts. Alcohol Res Health. 2003;27(4):285–290. [PMC free article] [PubMed] [Google Scholar]
- Vonlaufen A, Wilson JS, Pirola RC, Apte MV. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res Health. 2007;30(1):48–54. [PMC free article] [PubMed] [Google Scholar]
- Waltman C, Blevins LS, Jr, Boyd G, Wand GS. The effects of mild ethanol intoxication on the hypothalamic-pituitary-adrenal axis in nonalcoholic men. J Clin Endocrinol Metab. 1993;77(2):518–522. doi: 10.1210/jcem.77.2.8393888. [DOI] [PubMed] [Google Scholar]
- Wands JR, Dienstag JL, Weake JR, Koff RS. In vitro studies of enhanced IgG synthesis in severe alcoholic liver disease. Clin Exp Immunol. 1981;44(2):396–404. [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 1995;57(11):1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- Yi B, Rykova M, Feuerecker M, Jager B, Ladinig C, Basner M, Horl M, Matzel S, Kaufmann I, Strewe C, Nichiporuk I, Vassilieva G, Rinas K, Baatout S, Schelling G, Thiel M, Dinges DF, Morukov B, Chouker A. 520-d Isolation and confinement simulating a flight to Mars reveals heightened immune responses and alterations of leukocyte phenotype. Brain Behav Immun. 2014;40:203–210. doi: 10.1016/j.bbi.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29(4):245–254. [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78(5):1070–1080. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Boe DM, Zhong Q, Schwarzenberger P, Kolls JK, Summer WR, Nelson S. Acute alcohol intoxication suppresses the CXC chemokine response during endotoxemia. Alcohol Clin Exp Res. 2002;26(1):65–73. [PubMed] [Google Scholar]
- Zhang Z, Bagby GJ, Stoltz D, Oliver P, Schwarzenberger PO, Kolls JK. Prolonged ethanol treatment enhances lipopolysaccharide/phorbol myristate acetate-induced tumor necrosis factor-alpha production in human monocytic cells. Alcohol Clin Exp Res. 2001;25(3):444–449. [PubMed] [Google Scholar]
- Zheng G, Zhong S, Geng Y, Munirathinam G, Cha I, Reardon C, Getz GS, van Rooijen N, Kang Y, Wang B, Chen A. Dexamethasone promotes tolerance in vivo by enriching CD11clo CD40lo tolerogenic macrophages. Eur J Immunol. 2013;43(1):219–227. doi: 10.1002/eji.201242468. [DOI] [PMC free article] [PubMed] [Google Scholar]