Abstract
This review focuses on current tissue engineering strategies for promoting vascularized bone regeneration. We review the role of angiogenic growth factors in promoting vascularized bone regeneration and discuss the different therapeutic strategies for controlled/sustained growth factor delivery. Next, we address the therapeutic uses of stem cells in vascularized bone regeneration. Specifically, this review addresses the concept of co-culture using osteogenic and vasculogenic stem cells, and how adipose derived stem cells compare to bone marrow derived mesenchymal stem cells in the promotion of angiogenesis. We conclude this review with a discussion of a novel approach to bone regeneration through a cartilage intermediate, and discuss why it has the potential to be more effective than traditional bone grafting methods.
Keywords: Bone regeneration, angiogenesis, osteogenesis, controlled release, stem cells, endochondral ossification
1. Introduction
Bone grafts are crucial for the treatment of a number of conditions that represent a great global burden, including segmental bone defects caused by trauma, tumor excision or chronic osteomyelitis, nonunions, avascular necrosis and spinal fusions1,2. The current gold standard treatment is autografts. However, these are limited in availability, prolong the operation times and are often associated with donor site morbidity3. As an alternative, allografts are widely available. However, they place the patients at risk for infections and rejection by the immune system3. More importantly, allografts demonstrate decreased integration with the host tissue when compared to autografts4, with high failure rates of approximately 25% and reaching up to 60% in patients requiring large grafts5,6. Allografts, as well as synthetic grafts, are associated with complications such as osteonecrosis7–9 as these options lack an endogenous vascular network to facilitate host integration. Recently, vascularized bone grafts have been developed to address these limitations10,11.
The first vascularized bone grafts developed were pedicled grafts, or bone that is transported with its blood supply (or pedicle). Theoretically, such grafts are considered “live”, and include all of the components needed for graft survival and improved bone incorporation, as well as avoid some of the complications associated with allograft, including graft failure and infection. Pedicled grafts, which are commonly used in the treatment of carpal bone pathology10 and femoral head avascular necrosis11, remain connected to the blood supply site of origin, and are therefore limited by their need for proximity to the treatment site. Common harvest sites include the iliac crest, greater trochanter or the fibula for the hip, and the distal radius for the carpal bones. Surgeons must also be able to achieve tension free anastomoses, estimate an adequate pedicle length, and continue monitoring the graft site for blood leakage after the procedure in order to avoid serious complications11.
Free vascularized bone grafts, which keep the vascular connections of vessels to and from a freed segment of bone, were subsequently developed as an alternative to pedicled bone grafts10. However, they are technically difficult and have a relatively high rate of failure, involve intricate handling of the vessels, require specialized equipment, and are associated with morbidity at the harvest site. Therefore, given the difficulties with these vascular grafts, neither is widely used clinically, and there is a need for better solutions to promote vascularized bone regeneration.
The emerging field of tissue engineering holds promise for the development of a much simpler solution for vascularized bone regeneration compared to the complicated pedicled and free vascularized bone grafts while overcoming the problems of structural integration and limited revascularization associated with allografts. In this review, we address the currently available tissue engineering strategies that aim to enhance vascularized bone regeneration. We will start by describing the critical role that angiogenic growth factors play in directing neovascularization during bone regeneration and the importance of scaffolds in generating controlled/sustained release. We will then discuss the therapeutic uses of stem cells as an alternative growth factor delivery method for vascularized bone regeneration, and the potential of cartilage scaffolds in promoting better bone regeneration compared to the currently available bone grafts.
2. Angiogenic growth factors in pre-clinical/clinical trials
Tissue regeneration is largely dependent on cell signaling that is mediated by cellular interactions with growth factors. The therapeutic application of growth factors spans many indications including bone regeneration. Growth factors are capable of promoting osteogenesis by recruiting mesenchymal stem cells (MSCs) and inducing osteoblastic differentiation in addition to stimulating the migration, differentiation and proliferation of stem cells to aid in vascularized bone formation. We will focus on angiogenic growth factors as a means to direct revascularization during bone repair.
2.1. Vascular endothelial growth factor (VEGF)
VEGF has been the target of many investigations for over 3 decades. Scientists identified VEGF target receptors on endothelial cells and found it to be a key player in physiological and pathological angiogenesis12–15. Furthermore, the role of VEGF in vascularization as it relates to endochondral bone formation has long been recognized. Hypertrophic chondrocytes in the epiphyseal growth plate express VEGF16,17, and upon its release, blood vessels invade the cartilage and facilitate bone formation. Experiments with VEGF inactivation showed suppression of blood vessel invasion and significant changes to the growth plate architecture including expansion of the hypertrophic chondrocyte zone17. Furthermore, these effects could be reversed by terminating anti-VEGF treatment. Specifically, vascular invasion and resorption of hypertrophic cartilage resumed and the growth plate architecture returned to normal17.
Subsequently, therapeutic application of VEGF has been proven to enhance both endochondral ossification and intramembranous bone formation. Studies have shown accelerated bone regeneration and improved vascularization with VEGF therapy18,19 and its critical role in healing has been confirmed by evidence of the development of non-unions in experiments with VEGF inhibition20. VEGF is also believed to increase stem cell recruitment to damaged or diseased bone tissue21,22. However, the use of VEGF alone does not yield optimal bone regeneration, which is evident from a study comparing VEGF to platelet-rich plasma in the treatment of critical sized bone defects in New Zealand white rabbits23. Platelet rich plasma is known to contain VEGF in addition to many other growth factors including platelet derived growth factor (PDGF), transforming growth factor beta 1 and 2 (TGF-β1 & TGF-β2), insulin-like growth factor (IGF), epidermal growth factor, and endothelial cell growth factor23. This study concluded that while VEGF was sufficient to improve revascularization, a combination of growth factors led to better bone repair compared to VEGF alone.
The limitation of monotherapy with VEGF therapy has been demonstrated in other studies. A study by Peng et al found that VEGF failed to stimulate bone healing in skull defects in rats despite an increased number of endothelial cells in the non-unions24. Similarly, in a study of fracture healing, it was shown that the extent of vascularization in the non-union group was comparable to the union group in the early stage of repair and even increased after 14 days. Instead, they found that osteogenic proteins, BMP-2 & BMP-4, were decreased. Taken together these studies suggest that there is an essential balance between angiogenic and osteogenic growth factors during bone healing and excessive concentrations of VEGF may favor endothelial cell differentiation over osteogenesis25,26.
Also hindering the translation of VEGF-based therapies to the clinic are practical considerations involved with growth factor delivery. VEGF has a short half-life of 6–8 hours23, which means that controlled delivery is required to ensure sustained activity. Effective VEGF signaling also requires receptor clustering, so designing a delivery system that enables this biological effect could improve outcomes27,28. Conversely, excessive VEGF may put the patients at risk for malignancy, since VEGF is associated with tumor development29.
2.2 Fibroblast growth factor-2 (FGF-2)
FGF-2 is another growth factor that has been extensively studied for its key role in angiogenesis. It has been proven to play a role in both, the induction of angiogenesis and the mitogenesis of mesenchymal progenitors and osteoblasts30,31. A recent review by Hankenson et al highlights the considerable number of preclinical studies conducted in both small and large animal models to demonstrate the potential of FGF-2 to promote angiogenesis and improve fracture healing32. Importantly, the effectiveness of FGF-2 in the induction of angiogenesis and fracture healing is related to its dosage and release kinetics. Studies have shown the minimum required dose to be 100 µg when delivered as a single dose, but could be decreased to 1.4 µg when methods of controlling its release were used33,34. These data emphasize the importance of growth factor delivery methods and controlled release.
2.3 Platelet derived growth factor (PDGF)
A growth factor with demonstrated clinical success is PDGF. It is currently available in the market in the form of a gel for the treatment of chronic foot ulcers in diabetics. It also holds potential in promoting vascularized bone regeneration. In addition to being a chemotactic factor for osteoblasts and stimulating them to undergo proliferation35, PDGF has also been reported to increase the expression of VEGF in endothelial cells36. Systemic administration of recombinant human platelet derived growth factor-BB (rhPDGF-BB) has been shown to improve bone density and biomechanical strength in osteoporotic rat models and improve fracture healing by increasing torsional strength37,38. Similarly, locally administered rhPDGF-bb accelerated bone regeneration in a rabbit osteotomy model through an increase in the size and density of the callus39 and improved bone healing in dogs with periodontal defects40. In human clinical studies, rhPDGF-BB has been used in combination with allograft bone matrix to treat advanced periodontal lesions and promote alveolar bone formation41,42. Furthermore, a triple blinded randomized controlled clinical trial involving 11 clinical centers and 180 patients demonstrated that rhPDGF-BB applied in combination with a beta-tri-calcium phosphate carrier enhanced bone formation43.
2.4 Placental growth factor (PlGF)
PlGF is part of the VEGF family and is a VEGF homolog44. It acts via activation of VEGFR-1, and is thought to potentiate the angiogenic response to VEGF, although there remains controversy surrounding its mechanism of action and net effect on angiogenesis45. Interestingly, when Maes et al examined the role of PlGF in fracture healing in mice, they found it to be responsible for recruiting inflammatory cells to the fracture site, a process important to promoting vascularization early during the healing cascade. Moreover, they discovered that it stimulates MSCs to proliferate and differentiate into osteoblasts to aid in bone formation and stimulates osteoclast progenitor cells to differentiate into osteoclasts to aid in bone remodeling46.
2.5 Insulin-like growth factor (IGF)
IGF has a documented role in regulating endothelial cell migration and tubular formation47,48, but its role in promoting vascularized bone regeneration remains unclear. In vivo studies on segmental defect models in Sprague-Dawley rats49 and Swiss alpine sheep50 showed that IGF has the ability to promote fracture healing. Furthermore, IGF is a critical mediator of the skeletal response to parathyroid hormone which has been shown to promote fracture healing51–53. However, preliminary data indicate that conditional deletion of IGF from osteocytes may result in accelerated boney bridging of a fracture gap indicating that osteocyte-derived IGF may have an inhibitory role during fracture repair54. Further investigation into this model is required to understand the molecular mechanism of IGF during fracture repair, in particular how the timing and source of IGF may influence progression of healing55.
IGF also plays a documented role in bone growth and development55, which may have analogous implications on fracture healing. Inhibition of the IGF-1 gene by conditional knockout in mice resulted in decreased trabecular and cortical bone mineral density, shortening of hypertrophic chondrocyte zone in the growth plate, and decreased periosteal expansion, suggesting that IGF affects both endochondral ossification and intramembranous bone formation54. In a detailed study of the hypertrophic chondrocyte zone of the growth plate using diffraction phase microscopy, Cooper et al found that IGF may play a critical role in coordinating chondrocyte enlargement56.
2.6 Sonic hedgehog (SHH)
Although classically associated with developmental pathways57, SHH has also been shown to increase neovascularization. In a study using an ischemic hind limb aged mouse model, Pola et al demonstrated that SHH induced significant neovascularization58. SHH may induce angiogenesis indirectly by activating VEGF, the angiopoietins (Ang-1 and Ang-2), PDGF-BB, and TGF-β which are important growth factors for vessel stabilization and maturation58,59. Furthermore, a co-culture study conducted using human primary osteoblasts and outgrowth endothelial cells found that SHH may offer an advantage over VEGF in that its effects have been observed as early as 24 hours following treatment, indicating a potential therapeutic benefit in accelerating vascularization59.
SHH may also facilitate osteogenesis in addition to angiogenesis. A series of co-culture studies conducted by Dohle et al. showed that SHH increased the expression of many osteogenic differentiation markers, improved mineralization, and increased alkaline phosphatase activity60. Similarly, Ho et al found multivalent SHH was sufficient to robustly stimulate mitogenesis and differentiation of bone marrow-derived MSCs into an osteoblastic lineage61.
2.7 Angiopoietins (Ang)
Several recent reviews have discussed angiopoietins and their relationship with the tyrosine kinase receptors (Tie). Tie-1 and Tie-2 receptors are only expressed on vascular endothelial cells and are considered to be crucial for vascular maturation62–64. In a study performed on transgenic mice, Thurston et al found that the combination of VEGF and Ang-1 resulted in the formation of blood vessels that were highly differentiated and covered by periendothelial cells. As a result, they were more resistant to leakage when compared to the vessels that were formed in the VEGF only group65–67. A review by Fagiani et al draws attention to the fact that Ang-1 and VEGF control different aspects of the angiogenesis pathway; Ang-1 stimulates blood vessel remodeling and maturation, whereas VEGF is in charge of vessel development and growth62. The effect of Ang-2, on the other hand, is dependent on the presence or absence of VEGF. It is known to enhance neovascularization in the presence of VEGF and to separate the endothelial cells from the perivascular cells, and result in vascular regression in the absence of VEGF68–70.
The benefits of angiogenic growth factors are numerous. However, despite increasing evidence supporting the role growth factors play in enhancing angiogenesis, the growth factors we have discussed thus far have met with varying degrees of success. Factors limiting their clinical translation include the lack of clinical data describing the optimal choice or combination of growth factors necessary to ensure positive interactions, the right time to deliver each factor and the method of delivery.
3. Delivery of Growth Factors
A possible explanation of what may be hindering growth factors from effectively playing out their role is the method by which we deliver them. As an important example, therapeutic delivery of VEGF has raised concern because continuous infusion of VEGF can result in complications, including, severe vascular leakage leading to hypotension71 and increased risk of developing hemangioma like-vasculature29,72. Utilizing a well-designed delivery system that better recapitulates the native biology of VEGF, could improve its therapeutic effect by stabilizing it against rapid degradation and by modulating its release in an appropriate spatial and temporal manner.
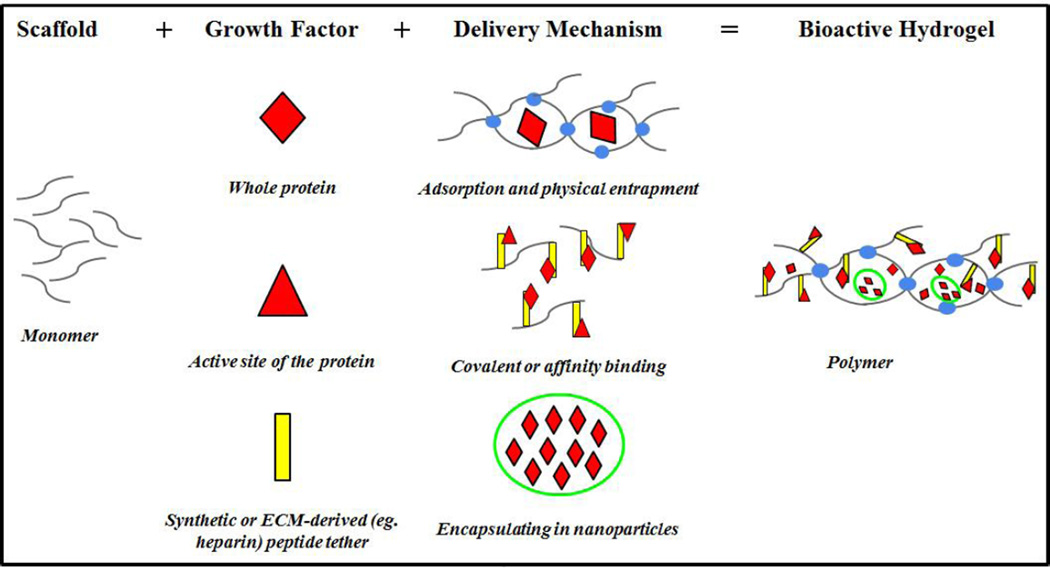
Scaffolds in tissue engineering perform two functions: 1) to provide structural support by acting as an artificial extracellular matrix enabling cell infiltration, adhesion, and proliferation, 2) to serve as a platform for the delivery of growth factors. The later function generates ‘bioactive’ scaffolds that direct cellular behavior by regulating stem cell differentiation and tissue development. ‘Biomimetic’ polymers are synthetic systems that aim to model the native function of the extracellular matrix. One essential role of the native extracellular matrix is to create sustained, and often cell-mediated release, of growth factors. Together bioactive and biomimetic polymers can produce scaffold-based systems that provide engineered growth factor delivery. Important considerations in this design are: scaffold fabrication technique, form factor of the growth factor, and the ability to engineer desired release kinetics. (Figure 1)
Figure 1. Summary of biomaterial based strategies to deliver growth factors.
Techniques to control release growth factors. Thus, when there are several growth factors in the polymer, it is possible to engineer unique temporal release kinetics.
3.1 Physical Entrapment
Physical encapsulation is perhaps the most simplistic method of growth factor delivery and can be accomplished by entrapping growth factors into the scaffold during synthesis, provided the polymerization process does not denature the protein73. A downside of this design is that this method often results in a simple burst release profile of the growth factors, which is often not ideal for stimulating a sustained biological response. Prolonged physical entrapment can also result in the loss of growth factor bioactivity. Release profiles can be moderately altered by tuning the pore size of the scaffold based on the size of the protein or generating a biodegradable scaffold74,75.
3.2 Covalent Binding
Alternatively, growth factors can be covalently bound into polymeric network of scaffolds. This system is advantageous because it ensures localization of the desired tissue response since the growth factor will not diffuse away from the source, and reduces the amount of growth factor that needs to be added since it is not systemically depleted. In order for the cells to access the growth factor, proteins are often presented in a pendant like fashion from the scaffold backbone. This can be accomplished via rapidly clikable Michael Type addition reaction in which cysteine residues within the peptide sequence react with the vinyl sulfone groups engineered onto the synthetic polymer backbone. This strategy was used to covalently link VEGF variants to poly(ethylene glycol) based scaffold resulting in formation of a dense vascular network76,77.
Challenges associated with this delivery system are often centered on ensuring that fusion of the growth factor to the scaffold allows for downstream pathway activation. Specifically, in order to allow for signaling, the site for attaching the growth factor to the scaffold must be away from the receptor-binding domain. This can be complicated when the full-length recombinant form of the protein is used. One technique to engineer appropriate protein presentation is to utilize only the short, bioactive oligopeptide sequence of a protein to induce its functionality. This strategy has been widely used to create biomimetic scaffolds that allow cells to bind directly to the scaffold by incorporating variants of the tripeptide sequence from fibronectin (Arg-Gly-Asp, ‘RGD’) that has been identified as the functional domain mediating cell interaction through integrins. Recently, this concept has been adapted to enhance osteogenic differentiation of MSCs and induce angiogenic response by fusing only the active sequence of the full length SHH protein, located in the N-terminus of the protein, onto synthetic polymer backbones61,76.
Furthermore, it is important that downstream cellular signaling can progress following formation of the receptor-ligand complex by understanding the molecular mechanism of the signaling pathway of interest. For example, downstream activity of a number of proteins in the TGFβ superfamily, such as BMP, are believed to require internalization of the receptor following binding of the ligand77. Alternatively, clustering of some receptors, such as VEGF, allow for signal potentiation78,79. Covalent attachment of the ligand to the scaffold may prevent these things from happening and therefore activation of the pathway would not be substantiated. In these cases you can engineer the attachment of the protein to be temporary, generating the so-called ‘release on demand’ or ‘bioresponsive’ delivery of the growth factor80. For example by incorporating a proteolytically degradable sequence into the linking tether between the scaffold and the peptide, such as those that are MMP-sensitive, one can generate a bioresponsive system in which cellular activity mediates bioavailability of the growth factors81. Alternatively, external cues can be applied to release the peptide at a desired milestone by adding an exogenous enzyme82 to degrade the sequence. More recently an elegant system has been developed in which the growth factor can be released by shining light onto the scaffold by incorporating photolabile peptide sequences into system80.
3.3 Affinity Binding
To attain a biologically relevant release profile, growth factors can be electrostatically associated to the scaffold via endogenous binding domains between growth factors and the ECM. Electrostatic binding of growth factors can be advantageous because they are designed to mimic interactions between growth factors and the native extracellular matrix. Natural biopolymers, such as fibrin or collagen, are often used to achieve this style of growth factor delivery, as these protein-based polymers naturally present growth factor binding domains. Fibrin gels have also been used to deliver engineered variants of VEGF protein to stimulate angiogenesis83–85.
To achieve greater and wider engineering design space, synthetic hydrogels have also been developed to contain the functional binding domain of the native ECM. This is frequently accomplished through the chemical addition of negatively charged sulfated polysaccharides. While many negatively charged sulfated polysaccharide can be incorporated into the scaffold to achieve this aim, heparin has become a popular choice due to its linear structure, high affinity binding towards a large variety of growth factors, and ability to preserve the biological activity of the protein. Importantly, heparin also contains an abundance of hydroxyl and carboxylic acid groups allowing it to be easily modified with reactive groups that can be incorporated covalently into hydrogels using chemo-selective chemistry86. Release kinetics of the growth factor is then modulated by the affinity of the growth factor to heparin (i.e. strength of the electrostatic interaction) and the amount of heparin incorporated into the scaffold. Heparin-modified hydrogels have now been used to effectively deliver a variety of growth factors for tissue regeneration, such as, angiogenic factors (VEGF, PDGF, FGF), stem cell derived factor, and TGF-β187–91. An additional benefit of the heparin binding system is that it not only enables engineered release of a desired growth factor to the tissue, but it also can bind and retain growth factors synthesized de novo from encapsulated cells or surrounding tissues.
3.4 Microparticles and Nanoparticles
Particulate systems, such as mico- or nano- particles, can be engineered to deliver growth factors in a controlled manner for regenerative medicine purposes. These are different from the bioactive scaffolds in that they don’t provide any intrinsic structural support, but their small size enables them to penetrate deep into tissues or be embedded into the scaffolds structures92. Growth factor release kinetics from these particles is most often controlled by diffusion. Different sizes of particles will lead to varying delivery times as the size of the carrier particles controls the surface-to-volume ratio93. Similarly, pore size of the particle relative to the size of the growth factor can influence release rates from these systems. For example, PDGF and FGF encapsulated within PLGA–polymer nanoparticles has been shown to enhance angiogenesis94.
Heparin conjugation/or coating techniques have also been applied to these particle systems in order to slow growth factor release. For example heparin (HP)-decorated, hyaluronic acid (HA)-based nanoparticles were synthesized using an inverse emulsion polymerization technique and it was demonstrated that the presence of heparin enabled a higher BMP-2 loading capacity and more sustained release profile92. Similarly, heparin coated methacrylamide microparticles have been effectively used to generate controlled release of BMP-2, VEGF, and FGF295.
An integrative approach combining the use of nanoparticulate systems with bioactive hydrogel scaffolds is becoming increasingly popular for the design of growth factor delivery systems. Using the combinatorial approach enables a differential release of growth factors with distinct delivery kinetics. For example, encapsulating microspheres containing PDGF into a scaffold with physically entrapped VEGF resulted in rapid release of VEGF followed by more sustained delivery of PDGF96. This technique increased local blood vessel density and was the first report of effective sequential growth factor delivery. Engineering the full temporal complexity of the growth factors needed to induce the various phases of tissue repair will become an increasingly active area in materials development.
4. Role of Stem Cells in Vascularized Bone Regeneration
The therapeutic use of stem cells is a promising component in tissue engineering approaches that are designed to promote angiogenesis and osteogenesis for healing bone defects. Angiogenesis is crucial in bone repair97,98 and the formation of new blood vessels depends on the ordered interaction of endothelial cells with different types of cells including, macrophages, pericytes, endothelial progenitor cells (EPCs), and MSCs99–101. It is clear that multiple stem cell types, immunogenic cues, and cytokines participate in, and are necessary, to initiate angiogenesis and osteogenesis. Because vascularized bone regeneration requires interplay between multiple cell types, recent work has focused on co-culturing of different cell types to facilitate improved healing. There has been greater characterization of, and experimentation with, stem cells from different sources in order to most efficiently promote vascularized bone regeneration.
4.1 Mesenchymal Stem Cell (MSCs)
Mesenchymal Stem Cells (MSCs) are the classic adult skeletal progenitor cell due to their established ability to form bone and cartilage both in vivo and in vitro. MSCs can be obtained from a number of adult stem cell niche, including bone marrow, adipose tissue102 and periosteum103. A definitive cell surface marker for the MSC has yet to be determined, but these cells are typically identified by their expression of CD90, CD105, CD73, and CD146, and absence of CD45, CD34, CD14, CD11b, CD79a, CD19, HLA-DR.104,105 Without clear markers for cell sorting, the International Society for Cellular Therapy has proposed a set of basic requirements for a cell to be classified as a MSC. MSCs are defined as a plastic culture adhesive cell with the ability to generate a colony-forming unit and differentiate into bone, cartilage, and adipose tissues106.
The most common, and best characterized, source of MSCs is from human bone marrow (BM-MSCs) and in vitro protocols for their controlled differentiation into bone, and cartilage, and adipose tissues are well-established. More recently, adipose-derived MSCs (ADSCs) have been identified and can also form the bone, cartilage, and adipose lineages. ADSCs offer a potential advantage over BM-MSCs for clinical translation into regenerative therapies due to their relative high abundance and easy access in the adult. Periosteal stem cells are perhaps the most relevant to bone regeneration since they are the primary source of cells that heal the fracture.107 While the native function of these cells is critical to healing, these cells are unlikely to play a large role in therapeutic strategies since periosteal stripping could negatively impact normal bone homeostasis, causes donor site morbidity, and these cells are difficult to access and in low quantity relative to BM-MSCs and ADSCs.
It is the prevailing thought that MSCs must be isolated, enriched, and expanded in vitro prior to clinical use. This may be especially true in older patients since there is a significant age-dependent decrease in the number and function of MSCs in the elderly population108. Newer technologies are looking to create devices that concentrate MSCs in the operating room and some clinical trials have aimed to define the quantity of various MSC populations in different tissues. For example, a recent clinical study, Y Jang et al determined that the BM-MSC population was only 0.42% in the bone marrow and 4.28% in the stromal vascular fraction; the ADSC population was considerably more abundant at 0.16% in the bone marrow and 32% in the stromal vascular fraction.109
4.2 MSC Osteogenesis
The osteogenic potential of MSCs is well known and consequently these cells have a rich history in use for promoting bone regeneration. At a molecular level osteogenic differentiation is transcriptionally regulated by runt related transcription factor-2 (RUNX2)110–112 and nuclear localization of p-catenin through canonical WNT signaling113,114. Together this leads to downstream activation of the canonical bone genes - osterix, osteopontin, and osteocalcin110,115 - and suppression of chondrogenesis116,117. In vitro osteogenesis of MSCs is classically initiated in the plastic adherent cell population by administration of ascorbic acid, β-glycerol phosphate, and dexamethasone or bone morphogenetic protein (BMP). The mechanical microenvironment is also a critical regulator of osteogenesis. Stiffness of the substrate118, or relative in vivo strain environment that the MSCs experience119, can influence osteogenic potential.
While it is assumed that the various MSC populations are similar, recent studies indicate that MSCs have unique characteristics depending on their source, and this may manifest into important functional differences. For example, despite similar cell surface markers, BM-MSCs and ADSCs demonstrate differentially expressed genes by microarray analysis120. Differential expression of genes related to cell proliferation may contribute to the documented increase in proliferative capacity of ADSCs relative to BM-MSCs120,121. In contrast, side-by-side comparative studies suggest that BM-MSCs have an improved differentiation potential as they form better osteogenic and chondrogenic systems in vitro122–124. Definitive comparative studies have not yet been completed and the best source of MSCs for therapeutic application will be an area of continued research as the field develops.
4.3 MSCs as Paracrine Mediators of Tissue Repair
While MSCs are capable of osteogenesis, their ability for de novo bone formation relies on encapsulation or recruitment to a suitable scaffold. In the absence of such a scaffold, the paracrine functionality of MSCs dominates their ability for tissue specific differentiation125–127. MSCs secrete a number of proteins that play a critical role in regulating inflammation and have a trophic function in stimulating tissue regeneration.
The immunomodulatory role of MSCs plays a critical role both in normal healing and for therapeutic use. During fracture healing, circulating MSCs recruited to the site of injury are likely a major factor in influencing macrophage polarization and regulating the immune microenvironment of the fracture callus128. Therapeutically, MSCs are in clinical trials for their ability to modulate immune reactions such as graft versus host rejection129,130. Immunomodulation by the MSCs is accomplished by secretion of immunosuppressive and antiinflammatory cytokines, such as interleukin-10131, nitric oxide132 and prostaglandins133. MSCs can also regulate T-cells in an antigen-independent manner89 through the suppression of the primary and secondary T-cell responses by inhibiting cell proliferation134–136.
MSCs also promote a local healing responses by stimulating proliferation and differentiation of resident stem cell populations, reducing fibrosis, and inhibiting adverse apoptosis137–139. Some research has been done to determine the secretory molecules produced by mSCs, identifying measurable levels of TGF-β, stem cell factor (SCF), insulin-like growth factor (IGF), epidermal growth factor (EGF), and granulocyte and macrophage colony stimulating factors (G/M-CSF)134,140.
Taken together, the immunosuppressive and trophic capabilities of MSCs are powerful and characterizing the secretome of MSCs for therapeutic use remains an active area of research. Of particular interest is how MSCs appear to have a lasting therapeutic effect despite very minimal tissue engraftment. Interestingly, current data also suggests that MSCs are immunoprivileged, or at the least immune evasive, enabling the possibility for allogenic use141. MSCs do not display major histocompatibility complex (MHC) class II cell surface markers, but only MHC class I markers without the co-stimulator molecules, indicating that they will not illicit an immune response142.
4.4 Endothelial Progenitor Cells (EPCs)
Endothelial progenitor cells (EPCs) may represent another potential adult stem cell population that could be used to promote vascularized bone regeneration with increasing amounts of data providing evidence that EPCs have pro-angiogenic functions in vivo143–145. EPCs are precursor cells of hematologic origin which reside in the bone marrow and peripheral blood. The existence of EPCs was first documented by Asahara et al146 and subsequently these cells have become functionally defined by their ability to generate the endothelial and smooth muscle cells that give rise to a vascular network during in vitro angiogenesis. In vivo, EPCs have been shown to be highly effective in mobilizing to areas of ischemic damage and promoting neovascularization147,148. The pro-angiogenic effect exerted by EPCs appears dependent on their ability to stimulate endothelial cells growth101 and interact with mature endothelial cells to support vascular anastomosis149. Nevertheless, the mechanisms by which the EPCs exert beneficial effects on endothelial cell growth is likely multifactorial and might include the transdifferentiation of subpopulations of EPCs into mature endothelial cells150.
Different experimental approaches for in vitro isolation and functionality have defined two EPC populations: the early and late endothelial progenitor cells. Typically, the endothelial cells used for tissue engineering approaches are human outgrowth endothelial cells (OECs), which are otherwise known as late endothelial progenitor cells, and emerge after 14–21 days of culturing mononuclear cells on type I collagen100,151–153. An alternative source of endothelial cells is early endothelial progenitor cells (eEPCs) that arise following short-term cultures of peripheral and umbilical cord blood-derived mononuclear cells. eEPCs exhibit a hematopoietic cell phenotype that closely resembles monocytes, whereas OECs are differentiated and mature endothelial cells displaying cobblestone morphology and expressing mature endothelial cell-specific markers, such as VE-cadherin and von Willebrand factor100,151–154. OECs behave more like canonical progenitor cells by directly contributing to neovascularization, dividing into new endothelial cells, and forming vascular networks155.
Another potential source of EPCs has been identified in cardiac tissue as a Sca-1+/CD45− progenitor cell. GFP+ Sca-1+/CD45− progenitor cells have been shown to contribute to tissue regeneration in part by undergoing neovascular differentiation characteristic of endothelial cells156. Furthermore, when these GFP+ Sca-1+/CD45− progenitor cells are encapsulated in heparin, bspRGD(15) and TGF β1 containing hyaluronic acid based hydrogel, they demonstrated in vitro vascular-like network formation and in vivo enhanced neovascular response within the hydrogel and significantly anastomosed with the host’s blood vessels88. Unfortunately, like MSCs, no definite cell surface markers have been identified for the EPCs and they display an overlapping phenotype with other endothelial and haematopoitic cells. Current isolation and enrichment strategies for EPCs focus on the surface markers CD34, CD133, and vascular endothelial growth factor receptor 2157–159.
In addition to their direct role in angiogenesis, EPCs may also be capable of differentiating into osteogenic cells. EPCs have been shown to be upregulated in response to orthopaedic trauma and mobilization by signals from bone injury sites may contribute to both neovascularization and bone formation during fracture healing160,161. The therapeutic application of EPCs in orthopedic trauma has been shown to augment fracture healing and local angiogenesis in segmental defect models in rat femurs162 and sheep tibia163,164. Although EPCs hold promise in regenerative therapies, our understanding of EPCs is still in its infancy. Better characterization of EPCs and determining the role of EPCs during angiogenesis and bone formation requires continued research efforts165.
4.5 MSC-ESC Interaction during Neovascularization
MSCs promote neovascularization by releasing pro-angiogenic factors such as angiopoietin-1, FGF-2, and VEGF likely through paracrine communication between EPCs and MSCs.166–168 It has been suggested that MSCs are the major producer of VEGF during vascular bone regeneration.101,167,169 These MSC secreted pro-angiogenic factors stimulate EPC proliferation and differentiation, which in turn causes EPCs to secrete osteogenic growth factors that stimulate MSC differentiation.170,171 While data suggests that BM-MSCs and ADSC secrete comparable levels of angiogenic factors172, one study found that co-culture of these cells together promoted enhanced angiogenesis by increasing VEGF production and improving osteogenesis.173 Furthermore, MSC–EPC co-cultures in vitro found a more pronounced tube formation in co-cultures compared to single cultures.174
It is also suggested that MSCs are the origin of the pericytic cells that wrap around the endothelial cells of capillaries and venules175. Within the blood vessels the pericyte and endothelial cell are in direct cell-to-cell contact creating both a physical and paracrine interaction between these cell types. Pericytes and endothelial cells are functionally interdependent and it is believed the role of the pericyte is to stabilize the vascular network during formation176. Vascular damage during injury may help to mobilize pericytes/MSCs to the site of damage and into circulation. Furthermore, secreted signals from the vascular endothelial cells may help to promote differentiation of the MSCs. Failure of proper communication between the pericyte and endothelial cell has been linked to numerous human pathologies177,178.
4.6 Role of Macrophages in Regulating Vascularized Bone Regeneration
Macrophages play an important role in regulating the process of angiogenesis and bone regeneration. Their role is complex, but it is clear that macrophages have polarizing phenotypes that exhibit either so-called proinflammatory (M1 phenotype) or anti-inflammatory (M2 phenotypes) manner. M1 macrophages dominate the early phase of fracture healing, secreting pro-inflammatory cytokines such as IL-6, IL-8 and TNFα, and contribute to the formation of the hematoma and early angiogenesis.179 The recruitment of inflammatory cells to sites of injury is mediated by four different subfamilies of chemokines (CC, CXC, CX3C and C) based on their biochemical, structural and functional properties180. In a Ccr2 −/− mouse model of decreased macrophage recruitment, it was shown that disruption of Ccr2 signaling impaired vascularization, decreased formation of callus, and delayed maturation of cartilage and bone remodeling up to 21 days after injury181,182. M2 macrophages contribute to the resolution of inflammation and are important to the progression bone healing. These anti-inflammatory M2 macrophages stimulate cell proliferation, survival, and tumor growth in various pathological states183–186. Dysregulation of macrophage polarization, specifically persistence of the M1 phenotype or dampened M2 response, may contribute to age related delays in bone healing.180–182
5. Improved Vascularized Bone Regeneration through Endochondral Ossification
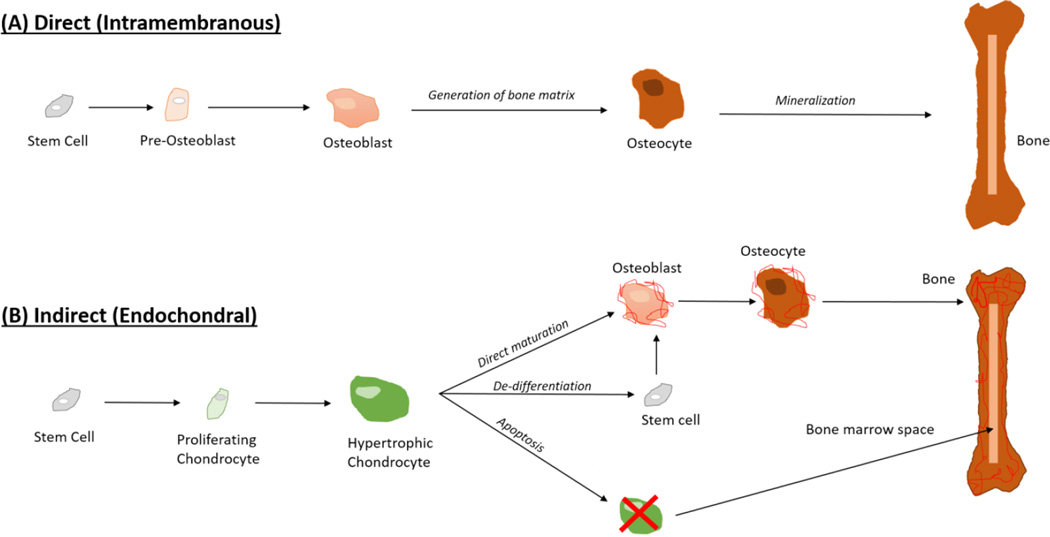
As detailed above, therapeutic strategies to promote vascularized bone regeneration have focused on enhancing bone formation and/or stimulating angiogenesis through the application of growth factors and stem cells. Traditionally, these approaches look to form bone directly through the process of intramembranous ossification. Intramembranous ossification involves direct differentiation of MSCs into osteoblasts. This is the embryonic program by which bones in skull develop, and is the mechanism through which rigidly stabilized fractures heal.
In contrast, a second method for bone formation is endochondral ossification where a temporary cartilage matrix is formed prior to being remodeled into bone. Endochondral ossification is the embryonic developmental pathway for long bone formation, enables post-natal bone elongation, and is the process by which the majority of fractures heal. As an indirect pathway for bone formation, this process involves a complex series of highly regulated steps in which MSCs differentiate into chondrocytes, mature to a hypertrophic state, and then stimulate vascular invasion and mineralization before transforming into bone187,188. Recently, this pathway has been explored as a novel approach to bone regeneration.
5.1 Shortcomings of Bone Grafting Relying on Intramembranous Bone Formation
The gold standard technique for stimulating bone regeneration remains bone autograft, typically through transplantation of morselized iliac crest or structural grafts taken from the rib or fibula. Outcomes from these procedures are typically good since the transplanted tissue contains living osteocytes, osteoclasts, and vascular cells. Together these cells enable bone remodeling and maintain viability and strength of the graft. However, as discussed in the introduction, the limited availability of autograft bone, along with significant donor site morbidity limit overall effectiveness of this procedure. Bone allograft, demineralized bone matrix, synthetic bone grafting substitutes, and tissue engineering approaches to bone regeneration have all emerged as alternatives to autograft. These products attempt to be either osteoinductive, osteoconductive, or osteogenic – with the goal of stimulating direct bone formation and mineralization.
The intramembranous bone formation approach fails to promote adequate vascular invasion as a result of excess mineralization and/or inability of cells to invade the grafts to enable efficient remodeling of the tissue189. The lack of a vascular network hinders the delivery of nutrients and the removal of waste from the center of current bone grafts and tissue engineered constructs190. As a result these bone grafts are met with a number of failure mechanisms, such as, loss of mechanical strength over time, poor osseointegration, and osteonecrosis of the graft.
5.2 Endochondral Ossification Promotes Angiogenesis and Osteogenesis
Poor clinical outcomes using traditional intramembranous approaches to bone regeneration have recently inspired researchers to investigate the therapeutic potential of endochondral ossification as a strategy for promoting well-vascularized bone repair. (Figure 2) In these studies cartilage grafts are used to stimulate bone formation. Murine studies using ectopic transplantation of cartilage have demonstrated that growth plate cartilage and chondrogenically primed hMSCs, but not articular cartilage, have the capacity to form bone191–195. More recently, clinically relevant rodent models for critical sized bone defects have demonstrated the clinical utility of cartilage to promote endochondral bone regeneration196,197. Importantly, these studies demonstrate that the endochondral derived bone is highly vascularized and promotes significantly better osseointegration with the host bone when compared to bone allograft.196,197 Successes with bone regeneration from a cartilage template suggest that a hypertrophic cartilage scaffold has all the inherent signals needed for bone tissue formation and vascular invasion and open the potential for whole bone regeneration in addition to segmental bone repair198,199.
Figure 2. Therapeutic Approaches for Bone Regeneration.
(A) Classic techniques in bone regeneration attempt to stimulate intramembranous ossification, with limited success at producing a vascularized bone regenerate. (B) Transformation of chondrocytes into bone may represent a better therapeutic strategy for promoting vascularized bone regeneration as a result of the strong angiogenic action of the hypertrophic chondrocyte during endochondral ossification.
A potential advantage of using cartilage rather than bone grafts for promoting repair is that cartilage is physiologically adapted to survive in avascular conditions. Consequently, cartilage grafts may be better suited for survival in the low vascular conditions, such as traumatic injury or critical-sized defects, which typically require bone grafting200,201. In a study by Scotti et al., chondrocytes survived hypoxia and then instructed cells to differentiate into osteoblasts194.
Tissue engineering studies for endochondral bone repair have also found that re-vascularization can be optimized by scaffold parameters. Specifically, scaffold chemistry202, porosity197, and ultrastructural architecture203,204 can enhance the inherent bioactivity of the chondrocytes and facilitate vascular invasion. Furthermore, angiogenesis in these systems is an intrinsic function of the hypertrophic chondrocytes in the cartilage template that precedes bone formation. These chondrocytes produce VEGF and PlGF to promote angiogenesis and initiate vascular invasion187,194.
Remodeling of the cartilage template into bone is a complex process that is currently the topic of much debate187,188,205. Degradation of the cartilage matrix occurs in both an intrinsic and extrinsic fashion. Hypertrophic chondrocytes secrete MMP-13 to help degrade the collagen II and aggrecan in the cartilage template. The cartilage matrix is then further degraded as the blood vessels invade due to secretion of MMP-9 from the vascular endothelial cells.190 Dogma held that at this stage the hypertrophic chondrocytes were destined for apoptosis and that new bone forms from osteoblasts invading into the cartilage matrix. However, recent studies using genetic labeling of chondrocytes during bone formation and healing demonstrate that all hypertrophic chondrocytes do not undergo apoptosis, but rather can transdifferentiate into the osteoblasts and osteocytes that directly contribute to new bone formation194,196,206,207. Subsequently, degradation of the cartilage matrix, apoptosis, and osteoclast remodeling facilitate formation of bone marrow cavities. While the molecular mechanisms that regulate cartilage to bone transformation are currently not known, therapeutic manipulation of this pathway represents a novel strategy for promoting vascularized bone regeneration.
6. Summary
Current strategies for bone grafting fail to produce an adequately vascularized bone regenerate. In order to improve clinical outcomes from bone grafting procedures, better technologies to control and direct neovascularization within a mineralized construct need to be developed. Engineering the appropriate growth factors, scaffolds, and cell combinations into a comprehensive system for promoting both angiogenesis and osteogenesis remains a challenge and future goal.
Moving forward, a critical challenge in achieving this goal remains strategic delivery of therapeutic proteins. We have learned from recent experience with BMP that supraphysiological dosages of recombinant proteins can result in off-target effects, and even tumorgenesis208,209. Maintaining growth factors locally and designing controlled release schematics that resemble the native biological demand during healing should be a goal of the next round of smart biomaterials. Furthermore, given the complexity of the normal healing response, it is unlikely that a single protein will generate optimal results. The timing of multiple growth factor signaling will likely be necessary to engineer sophisticated cell differentiation events, as would be needed to encourage endochondral ossification from an MSC population. Consequently, improved systems need to be developed to deliver growth factors with distinct kinetics, especially in situations where delivery of one factor should follow another.
Smart scaffold design will play a critical role in delivering these growth factors, encapsulating target cell populations, enabling vascular invasion and tissue remodeling. Bioactive, biomimetic, and bioresponsive scaffolds aim to recapitulate the native ECM in a dynamic synthetic scaffold. As the building blocks of polymer science continue to grow, we can compliment this toolbox with a better understanding of the molecular and cellular mechanisms that regulate bone repair and neovascularization. The importance of modeling the normal developmental pathway was recently underscored by improved vascularized bone formation upon promoting endochondral, rather than intramembranous, ossification. Coupled with improved nanotechnology, bioprinting, and advances in electrospining we may be able to create ultrastructural microenvironments that are optimized for vascularized bone formation210–212.
Table 1.
Summary of the role of growth factors in angiogenesis and osteogenesis with current status of preclinical and clinical trials
| Growth Factor |
Role in Angiogenesis | Osteogenic Effects | Preclinical/Clinical trials | Refs |
|---|---|---|---|---|
| PlGF |
|
Bone formation and remodeling | Semistabilized bone fractures in WT mice |
46 |
| IGF |
|
|
Knockout mice, segmental defects in Sprague-Dawley rats and Swiss alpine sheep |
49,50, 54 |
| SHH | Proliferation and differentiation of bone marrow-derived MSC’s into an osteoblastic lineage. |
Osteogenic differentiation marker expression, mineralization and alkaline phosphatase |
Adult rats, ischemic hind limb aged mouse model |
58,61 |
| VEGF |
|
Endochondral ossification and intramembranous bone formation |
Rat calvarial defects, critical sized bone defects in New Zealand white rabbits |
18,24 |
| FGF-2 |
|
Increased callus, bone mineral density and mechanical stability |
Many including rat segmental defects, rabbit osteotomies and canine tibial defects |
32 |
| Ang-1 | Blood vessel remodeling and maturation | None | Transgenic mice | 65 |
| PDGF |
|
Improves bone density, biomechanical strength, and torsional strength. Increases callus size and density. |
Osteoporotic rats, rabbit osteotomies, dog periodontal defects, human periodontal defects, diabetic foot ulcers |
37,38, 39,40, 41,42, 43 |
Table 2.
Summary of the advantages & disadvantages of different delivery approaches
| Delivery Method | Advantages | Disadvantages |
|---|---|---|
| Physical Entrapment |
|
|
| Covalent Binding |
|
|
| Electrostatic Binding |
|
The presentation of growth factors may not be uniform and defined in the polymeric network due to the number of variations in the structures of sulfated polysaccharides |
| Micro/Nanoparticles |
|
|
Highlights.
Angiogenic growth factors play a very important role in directing neovascularization.
Delivery methods allowing sustained release can overcome major hurdles that previously prevented the translation of growth factors to the clinic.
Further research is needed to determine the best source of therapeutic stem cells and the benefits of stem cell co-culture.
Cartilage grafts have the potential to promote vascularized bone regeneration through the endochondral ossification pathway.
Acknowledgments
This publication was supported financially by the National Institutes of Health (NIH): National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS) under the following award numbers CSB (#5F32AR062469) and TM (R011057344); the National Institute on Aging under the award number RM (#AG046282); and by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additional research support was provided by the Musculoskeletal Transplant Foundation (CSB: MTF Junior Investigator Award), the AO Foundation Start-Up Grant (CSB, #S-14-114B), the UCSF Core Center for Musculoskeletal Biology and Medicine (P30AR066262), and the UCSF/SFGH Orthopaedic Trauma Institute.
Abbreviations
- MSCs
mesenchymal stem cells
- VEGF
Vascular endothelial growth factor
- PDGF
platelet derived growth factor
- TGF-β1 & TGF-β2
transforming growth factor beta 1 and 2
- IGF
insulin-like growth factor
- FGF-2
Fibroblast growth factor-2
- rhPDGF-BB
recombinant human platelet derived growth factor-BB
- PlGF
Placental growth factor
- Shh
Sonic hedgehog
- Ang
angiopoietins
- Tie
tyrosine kinase receptors
- HA
hyaluronic acid
- EPCs
endothelial progenitor cells
- OECs
outgrowth endothelial cells
- eEPCs
early endothelial progenitor cells
- BM-MSCs
bone marrow derived mesenchymal stem cells
- ADSCs
adipose-derived MSCs
- BMP
Bone morphogenic protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soucacos PN, Kokkalis ZT, Piagkou M, Johnson EO. Vascularized bone grafts for the management of skeletal defects in orthopaedic trauma and reconstructive surgery. Injury. 2013;44(Suppl 1):S70–S75. doi: 10.1016/S0020-1383(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 2.Guerado E, Fuerstenberg CH. What bone graft substitutes should we use in post-traumatic spinal fusion? Injury. 2011;42(Suppl 2):S64–S71. doi: 10.1016/j.injury.2011.06.200. [DOI] [PubMed] [Google Scholar]
- 3.Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9(1) doi: 10.1186/1749-799X-9-18. 18-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrler DM, Vaccaro AR. The use of allograft bone in lumbar spine surgery. Clin Orthop Relat Res. 2000;371(371):38–45. doi: 10.1097/00003086-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324(324):86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435(435):36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen LH, Annabi N, Nikkhah M, et al. Vascularized bone tissue engineering: Approaches for potential improvement. Tissue Eng Part B Rev. 2012;18(5):363–382. doi: 10.1089/ten.teb.2012.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsigkou O, Pomerantseva I, Spencer JA, et al. Engineered vascularized bone grafts. Proc Natl Acad Sci U S A. 2010;107(8):3311–3316. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138(4):745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derby BM, Murray PM, Shin AY, et al. Vascularized bone grafts for the treatment of carpal bone pathology. Hand (N Y) 2013;8(1):27–40. doi: 10.1007/s11552-012-9479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aldridge JM, 3rd, Urbaniak JR. Avascular necrosis of the femoral head: Role of vascularized bone grafts. Orthop Clin North Am. 2007;38(1):13–22. doi: 10.1016/j.ocl.2006.10.012. v. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak HF. VPF/VEGF and the angiogenic response. Semin Perinatol. 2000;24(1):75–78. doi: 10.1016/s0146-0005(00)80061-0. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 16.Harper J, Klagsbrun M. Cartilage to bone--angiogenesis leads the way. Nat Med. 1999;5(6):617–618. doi: 10.1038/9460. [DOI] [PubMed] [Google Scholar]
- 17.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 18.Geiger F, Lorenz H, Xu W, et al. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone. 2007;41(4):516–522. doi: 10.1016/j.bone.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Li R, Stewart DJ, von Schroeder HP, Mackinnon ES, Schemitsch EH. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J Orthop Res. 2009;27(1):8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 20.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29(6):560–564. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 21.Beamer B, Hettrich C, Lane J. Vascular endothelial growth factor: An essential component of angiogenesis and fracture healing. HSS J. 2010;6(1):85–94. doi: 10.1007/s11420-009-9129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury. 2008;39(Suppl 2):S45–S57. doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- 23.Kasten P, Beverungen M, Lorenz H, Wieland J, Fehr M, Geiger F. Comparison of platelet-rich plasma and VEGF-transfected mesenchymal stem cells on vascularization and bone formation in a critical-size bone defect. Cells Tissues Organs. 2012;196(6):523–533. doi: 10.1159/000337490. [DOI] [PubMed] [Google Scholar]
- 24.Peng H, Wright V, Usas A, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110(6):751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia P, Pieruschka A, Klein M, et al. Temporal and spatial vascularization patterns of unions and nonunions: Role of vascular endothelial growth factor and bone morphogenetic proteins. J Bone Joint Surg Am. 2012;94(1):49–58. doi: 10.2106/JBJS.J.00795. [DOI] [PubMed] [Google Scholar]
- 26.Peng H, Usas A, Olshanski A, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20(11):2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Short C, Halasz AM, Edwards JS. The impact of high density receptor clusters on VEGF signaling. Electron Proc Theor Comput Sci. 2013;2013:37–52. doi: 10.4204/EPTCS.??.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leppanen VM, Prota AE, Jeltsch M, et al. Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc Natl Acad Sci U S A. 2010;107(6):2425–2430. doi: 10.1073/pnas.0914318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21(5):735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 30.Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Globus RK, Patterson-Buckendahl P, Gospodarowicz D. Regulation of bovine bone cell proliferation by fibroblast growth factor and transforming growth factor beta. Endocrinology. 1988;123(1):98–105. doi: 10.1210/endo-123-1-98. [DOI] [PubMed] [Google Scholar]
- 32.Hankenson KD, Dishowitz M, Gray C, Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42(6):556–561. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato T, Kawaguchi H, Hanada K, et al. Single local injection of recombinant fibroblast growth factor-2 stimulates healing of segmental bone defects in rabbits. J Orthop Res. 1998;16(6):654–659. doi: 10.1002/jor.1100160605. [DOI] [PubMed] [Google Scholar]
- 34.Inui K, Maeda M, Sano A, et al. Local application of basic fibroblast growth factor minipellet induces the healing of segmental bony defects in rabbits. Calcif Tissue Int. 1998;63(6):490–495. doi: 10.1007/s002239900563. [DOI] [PubMed] [Google Scholar]
- 35.Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87(3):305–312. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- 36.Guo P, Hu B, Gu W, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162(4):1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitlak BH, Finkelman RD, Hill EL, et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res. 1996;11(2):238–247. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- 38.Hollinger JO, Onikepe AO, MacKrell J, et al. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-BB and an injectable beta-tricalcium phosphate/collagen matrix. J Orthop Res. 2008;26(1):83–90. doi: 10.1002/jor.20453. [DOI] [PubMed] [Google Scholar]
- 39.Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15(2):203–208. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- 40.Park JB, Matsuura M, Han KY, et al. Periodontal regeneration in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J Periodontol. 1995;66(6):462–477. doi: 10.1902/jop.1995.66.6.462. [DOI] [PubMed] [Google Scholar]
- 41.Camelo M, Nevins ML, Schenk RK, Lynch SE, Nevins M. Periodontal regeneration in human class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23(3):213–225. [PubMed] [Google Scholar]
- 42.Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74(9):1282–1292. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 43.Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 44.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005;6(2):209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribatti D. The discovery of the placental growth factor and its role in angiogenesis: A historical review. Angiogenesis. 2008;11(3):215–221. doi: 10.1007/s10456-008-9114-4. [DOI] [PubMed] [Google Scholar]
- 46.Maes C, Coenegrachts L, Stockmans I, et al. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J Clin Invest. 2006;116(5):1230–1242. doi: 10.1172/JCI26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bach LA. Endothelial cells and the IGF system. J Mol Endocrinol. 2015;54(1):R1–R13. doi: 10.1530/JME-14-0215. [DOI] [PubMed] [Google Scholar]
- 48.Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Hashizume K. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr J. 1999;46(Suppl):S59–S62. doi: 10.1507/endocrj.46.suppl_s59. [DOI] [PubMed] [Google Scholar]
- 49.Schmidmaier G, Wildemann B, Heeger J, et al. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone. 2002;31(1):165–172. doi: 10.1016/s8756-3282(02)00798-6. [DOI] [PubMed] [Google Scholar]
- 50.Meinel L, Zoidis E, Zapf J, et al. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33(4):660–672. doi: 10.1016/s8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 51.Rhee Y, Allen MR, Condon K, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. Journal of Bone and Mineral Research. 2011;26(5):1035–1046. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakajima A, Shimoji N, Shiomi K, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent Low-Dose human parathyroid hormone (1–34) Journal of Bone and Mineral Research. 2002;17(11):2038–2047. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- 53.Ellegaard M, Kringelbach T, Syberg S, et al. The effect of PTH (1-34) on fracture healing during different loading conditions. Journal of Bone and Mineral Research. 2013;28(10):2145–2155. doi: 10.1002/jbmr.1957. [DOI] [PubMed] [Google Scholar]
- 54.Sheng MH, Zhou XD, Bonewald LF, Baylink DJ, Lau KH. Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone. 2013;52(1):133–144. doi: 10.1016/j.bone.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 55.Sheng MH, Lau KH, Baylink DJ. Role of osteocyte-derived insulin-like growth factor I in developmental growth, modeling, remodeling, and regeneration of the bone. J Bone Metab. 2014;21(1):41–54. doi: 10.11005/jbm.2014.21.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper KL, Oh S, Sung Y, Dasari RR, Kirschner MW, Tabin CJ. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 2013;495(7441):375–378. doi: 10.1038/nature11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teperino R, Aberger F, Esterbauer H, Riobo N, Pospisilik JA. Canonical and non-canonical hedgehog signalling and the control of metabolism. Semin Cell Dev Biol. 2014;33:81–92. doi: 10.1016/j.semcdb.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pola R, Ling LE, Silver M, et al. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7(6):706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 59.Dohle E, Fuchs S, Kolbe M, Hofmann A, Schmidt H, Kirkpatrick CJ. Comparative study assessing effects of sonic hedgehog and VEGF in a human co-culture model for bone vascularisation strategies. Eur Cell Mater. 2011;21:144–156. doi: 10.22203/ecm.v021a12. [DOI] [PubMed] [Google Scholar]
- 60.Dohle E, Fuchs S, Kolbe M, Hofmann A, Schmidt H, Kirkpatrick CJ. Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng Part A. 2010;16(4):1235–1237. doi: 10.1089/ten.tea.2009.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ho JE, Chung EH, Wall S, Schaffer DV, Healy KE. Immobilized sonic hedgehog N-terminal signaling domain enhances differentiation of bone marrow-derived mesenchymal stem cells. J Biomed Mater Res A. 2007;83(4):1200–1208. doi: 10.1002/jbm.a.31355. [DOI] [PubMed] [Google Scholar]
- 62.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328(1):18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 63.Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res. 2013;319(9):1271–1280. doi: 10.1016/j.yexcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12(6):933–941. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- 65.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 66.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 67.Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98(8):1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 69.Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci U S A. 2002;99(12):8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujii T, Kuwano H. Regulation of the expression balance of angiopoietin-1 and angiopoietin-2 by shh and FGF-2. In Vitro Cell Dev Biol Anim. 2010;46(6):487–491. doi: 10.1007/s11626-009-9270-x. [DOI] [PubMed] [Google Scholar]
- 71.Horowitz JR, Rivard A, van der Zee R, et al. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension evidence for a maintenance role in quiescent adult endothelium. Arterioscler Thromb Vasc Biol. 1997;17(11):2793–2800. doi: 10.1161/01.atv.17.11.2793. [DOI] [PubMed] [Google Scholar]
- 72.Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: Potential role for vasculogenesis in adults. Mol Cell. 1998;2(5):549–558. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- 73.Bahney CS, Lujan TJ, Hsu CW, Bottlang M, West JL, Johnstone B. Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur Cell Mater. 2011;22:43–55. doi: 10.22203/ecm.v022a04. discussion 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holloway JL, Ma H, Rai R, Burdick JA. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J Control Release. 2014;191:63–70. doi: 10.1016/j.jconrel.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lutolf MP, Weber FE, Schmoekel HG, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21(5):513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 76.Wall ST, Saha K, Ashton RS, Kam KR, Schaffer DV, Healy KE. Multivalency of sonic hedgehog conjugated to linear polymer chains modulates protein potency. Bioconjug Chem. 2008;19(4):806–812. doi: 10.1021/bc700265k. [DOI] [PubMed] [Google Scholar]
- 77.Penheiter SG, Mitchell H, Garamszegi N, Edens M, Dore JJ, Jr, Leof EB. Internalization-dependent and -independent requirements for transforming growth factor beta receptor signaling via the smad pathway. Mol Cell Biol. 2002;22(13):4750–4759. doi: 10.1128/MCB.22.13.4750-4759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem. 1996;271(2):603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- 79.Almqvist N, Bhatia R, Primbs G, Desai N, Banerjee S, Lal R. Elasticity and adhesion force mapping reveals real-time clustering of growth factor receptors and associated changes in local cellular rheological properties. Biophys J. 2004;86(3):1753–1762. doi: 10.1016/S0006-3495(04)74243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zisch AH, Lutolf MP, Ehrbar M, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17(15):2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 82.Rice MA, Anseth KS. Controlling cartilaginous matrix evolution in hydrogels with degradation triggered by exogenous addition of an enzyme. Tissue Eng. 2007;13(4):683–691. doi: 10.1089/ten.2006.0142. [DOI] [PubMed] [Google Scholar]
- 83.Ehrbar M, Djonov VG, Schnell C, et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res. 2004;94(8):1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 84.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF--fibrin matrices for endothelialization. J Control Release. 2001;72(1–3):101–113. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 85.Ehrbar M, Metters A, Zammaretti P, Hubbell JA, Zisch AH. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J Control Release. 2005;101(1–3):93–109. doi: 10.1016/j.jconrel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 86.Liang Y, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014;10(4):1588–1600. doi: 10.1016/j.actbio.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoon JJ, Chung HJ, Lee HJ, Park TG. Heparin-immobilized biodegradable scaffolds for local and sustained release of angiogenic growth factor. J Biomed Mater Res A. 2006;79(4):934–942. doi: 10.1002/jbm.a.30843. [DOI] [PubMed] [Google Scholar]
- 88.Jha AK, Tharp KM, Ye J, et al. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials. 2015;47:1–12. doi: 10.1016/j.biomaterials.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Purcell BP, Kim IL, Chuo V, Guinen T, Dorsey SM, Burdick JA. Incorporation of sulfated hyaluronic acid macromers into degradable hydrogel scaffolds for sustained molecule delivery. Biomater Sci. 2014;2:693–702. doi: 10.1039/C3BM60227C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110(12):4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jha AK, Mathur A, Svedlund FL, Ye J, Yeghiazarians Y, Healy KE. Molecular weight and concentration of heparin in hyaluronic acid-based matrices modulates growth factor retention kinetics and stem cell fate. J Control Release. 2015;209:308–316. doi: 10.1016/j.jconrel.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu X, Jha AK, Duncan RL, Jia X. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011;7(8):3050–3059. doi: 10.1016/j.actbio.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jha AK, Yang W, Kirn-Safran CB, Farach-Carson MC, Jia X. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30(36):6964–6975. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.d’Angelo I, Garcia-Fuentes M, Parajo Y, et al. Nanoparticles based on PLGA:Poloxamer blends for the delivery of proangiogenic growth factors. Mol Pharm. 2010;7(5):1724–1733. doi: 10.1021/mp1001262. [DOI] [PubMed] [Google Scholar]
- 95.Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE, McDevitt TC. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 2014;35(25):7228–7238. doi: 10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 97.Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8(21):980–989. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 98.Das A, Botchwey E. Evaluation of angiogenesis and osteogenesis. Tissue Eng Part B Rev. 2011;17(6):403–414. doi: 10.1089/ten.TEB.2011.0190. [DOI] [PubMed] [Google Scholar]
- 99.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 100.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi Y, Kramer G, Schroder A, et al. Early endothelial progenitor cells as a source of myeloid cells to improve the pre-vascularisation of bone constructs. Eur Cell Mater. 2014;27:64–79. doi: 10.22203/ecm.v027a06. discussion 79–80. [DOI] [PubMed] [Google Scholar]
- 102.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9 doi: 10.1186/1478-811X-9-12. 12-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Gastel N, Stegen S, Stockmans I, et al. Expansion of murine periosteal progenitor cells with fibroblast growth factor 2 reveals an intrinsic endochondral ossification program mediated by bone morphogenetic protein 2. Stem Cells. 2014;32(9):2407–2418. doi: 10.1002/stem.1783. [DOI] [PubMed] [Google Scholar]
- 104.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the international federation for adipose therapeutics and science (IFATS) and the international society for cellular therapy (ISCT) Cytotherapy. 2013;15(6):641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varma MJ, Breuls RG, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16(1):91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 106.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells the international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 107.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caplan AIBB. Endochondral bone formation: The lineage cascade. In: Hall B, editor. Bone. Vol. 8. Boca Raton, FL: CRC Press; 1994. pp. 1–46. [Google Scholar]
- 109.Jang Y, Koh YG, Choi YJ, et al. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. In Vitro Cell Dev Biol Anim. 2014 doi: 10.1007/s11626-014-9814-6. [DOI] [PubMed] [Google Scholar]
- 110.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 111.Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 112.Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89(5):765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 113.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 114.Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 115.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 116.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Developmental Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 117.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Developmental Cell. 2005;8(5):727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 118.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 119.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. Journal of Orthopaedic Research. 2002;20(5):1091–1098. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 120.Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14(4–6):311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 121.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 122.Im G, Shin Y, Lee K. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis and cartilage. 2005;13(10):845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 123.Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: A patient-matched comparison. Journal of Orthopaedic Research. 2005;23(6):1383–1389. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 124.Toupadakis CA, Wong A, Genetos DC, et al. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am J Vet Res. 2010;71(10):1237–1245. doi: 10.2460/ajvr.71.10.1237. [DOI] [PubMed] [Google Scholar]
- 125.Caplan A. Why are MSCs therapeutic? new data: New insight. J Pathol. 2009;217(2):318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bahney CS, Miclau T. Therapeutic potential of stem cells in orthopedics. Indian J Orthop. 2012;46(1):4–9. doi: 10.4103/0019-5413.91628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bianco P, Robey PG, Saggio I, Riminucci M. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): A critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther. 2010;21(9):1057–1066. doi: 10.1089/hum.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bahney CS, Hu DP, Miclau T, 3rd, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Front Endocrinol (Lausanne) 2015;6:4. doi: 10.3389/fendo.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lalu MM, McIntyre L, Pugliese C, et al. Safe ty of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. 2012 doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. The Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 131.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 132.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 133.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 134.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 135.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 136.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83(1):71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 137.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23(7):845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 138.Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 139.Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 140.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1α. J Cell Physiol. 1996;166(3):585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 141.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 143.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: Regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28(12):519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 144.Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, Kubota Y. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood. 2011;117(19):5264–5272. doi: 10.1182/blood-2011-01-330720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van der Pouw Kraan TC, van der Laan AM, Piek JJ, Horrevoets AJ. Surfing the data tsunami, a bioinformatic dissection of the proangiogenic monocyte. Vascul Pharmacol. 2012;56(5–6):297–305. doi: 10.1016/j.vph.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 146.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 147.Kudo F, Nishibe T, Nishibe M, Yasuda K. Autologous transplantation of peripheral blood endothelial progenitor cells (CD34^ sup ^) for therapeutic angiogenesis in patients with critical limb ischemia. International angiology. 2003;22(4):344. [PubMed] [Google Scholar]
- 148.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yoon CH, Hur J, Park KW, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 150.Bellik L, Musilli C, Vinci MC, Ledda F, Parenti A. Human mature endothelial cells modulate peripheral blood mononuclear cell differentiation toward an endothelial phenotype. Exp Cell Res. 2008;314(16):2965–2974. doi: 10.1016/j.yexcr.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 151.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28(9):1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 153.Ferreras C, Cole CL, Urban K, Jayson GC, Avizienyte E. Segregation of late outgrowth endothelial cells into functional endothelial CD34- and progenitor-like CD34+ cell populations. Angiogenesis. 2015;18(1):47–68. doi: 10.1007/s10456-014-9446-1. [DOI] [PubMed] [Google Scholar]
- 154.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110(4):624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27(7):1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 156.Ye J, Boyle A, Shih H, et al. Sca-1+ cardiosphere-derived cells are enriched for Isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS One. 2012;7(1):e30329. doi: 10.1371/journal.pone.0030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;58(2):390–398. doi: 10.1016/s0008-6363(02)00785-x. [DOI] [PubMed] [Google Scholar]
- 158.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–5012. [PubMed] [Google Scholar]
- 159.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- 160.Laing AJ, Dillon JP, Condon ET, et al. Mobilization of endothelial precursor cells: Systemic vascular response to musculoskeletal trauma. J Orthop Res. 2007;25(1):44–50. doi: 10.1002/jor.20228. [DOI] [PubMed] [Google Scholar]
- 161.Lee DY, Cho TJ, Kim JA, et al. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone. 2008;42(5):932–941. doi: 10.1016/j.bone.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 162.Atesok K, Li R, Stewart DJ, Schemitsch EH. Endothelial progenitor cells promote fracture healing in a segmental bone defect model. J Orthop Res. 2010;28(8):1007–1014. doi: 10.1002/jor.21083. [DOI] [PubMed] [Google Scholar]
- 163.Rozen N, Bick T, Bajayo A, et al. Transplanted blood-derived endothelial progenitor cells (EPC) enhance bridging of sheep tibia critical size defects. Bone. 2009;45(5):918–924. doi: 10.1016/j.bone.2009.07.085. [DOI] [PubMed] [Google Scholar]
- 164.Li R, Atesok K, Nauth A, et al. Endothelial progenitor cells for fracture healing: A microcomputed tomography and biomechanical analysis. J Orthop Trauma. 2011;25(8):467–471. doi: 10.1097/BOT.0b013e31821ad4ec. [DOI] [PubMed] [Google Scholar]
- 165.Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: Identity defined? J Cell Mol Med. 2009;13(1):87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 167.Kolbe M, Xiang Z, Dohle E, Tonak M, Kirkpatrick CJ, Fuchs S. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng Part A. 2011;17(17–18):2199–2212. doi: 10.1089/ten.TEA.2010.0474. [DOI] [PubMed] [Google Scholar]
- 168.Gershovich JG, Dahlin RL, Kasper FK, Mikos AG. Enhanced osteogenesis in cocultures with human mesenchymal stem cells and endothelial cells on polymeric microfiber scaffolds. Tissue Eng Part A. 2013;19(23–24):2565–2576. doi: 10.1089/ten.tea.2013.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang L, Fan H, Zhang ZY, et al. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized beta-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials. 2010;31(36):9452–9461. doi: 10.1016/j.biomaterials.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 170.Ern C, Krump-Konvalinkova V, Docheva D, et al. Interactions of human endothelial and multipotent mesenchymal stem cells in cocultures. Open Biomed Eng J. 2010;4:190–198. doi: 10.2174/1874120701004010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guerrero J, Catros S, Derkaoui SM, et al. Cell interactions between human progenitor-derived endothelial cells and human mesenchymal stem cells in a three-dimensional macroporous polysaccharide-based scaffold promote osteogenesis. Acta Biomater. 2013;9(9):8200–8213. doi: 10.1016/j.actbio.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 172.Hsiao ST, Asgari A, Lokmic Z, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem cells and development. 2011;21(12):2189–2203. doi: 10.1089/scd.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim KI, Park S, Im GI. Osteogenic differentiation and angiogenesis with cocultured adipose-derived stromal cells and bone marrow stromal cells. Biomaterials. 2014;35(17):4792–4804. doi: 10.1016/j.biomaterials.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 174.Duttenhoefer F, Lara de Freitas R, Meury T, et al. 3D scaffolds co-seeded with human endothelial progenitor and mesenchymal stem cells: Evidence of prevascularisation within 7 days. Eur Cell Mater. 2013;26:49–64. doi: 10.22203/ecm.v026a04. discussion 64-5. [DOI] [PubMed] [Google Scholar]
- 175.Loibl M, Binder A, Herrmann M, et al. Direct cell-cell contact between mesenchymal stem cells and endothelial progenitor cells induces a pericyte-like phenotype in vitro. Biomed Res Int. 2014;2014:395781. doi: 10.1155/2014/395781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goerke SM, Plaha J, Hager S, et al. Human endothelial progenitor cells induce extracellular signal-regulated kinase-dependent differentiation of mesenchymal stem cells into smooth muscle cells upon cocultivation. Tissue Eng Part A. 2012;18(23–24):2395–2405. doi: 10.1089/ten.TEA.2012.0147. [DOI] [PubMed] [Google Scholar]
- 177.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33(3):136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21(2):216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 180.Xing Z, Lu C, Hu D, Miclau T, 3rd, Marcucio RS. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J Orthop Res. 2010;28(8):1000–1006. doi: 10.1002/jor.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xing Z, Lu C, Hu D, et al. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3(7–8):451–458. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Slade Shantz JA, Yu YY, Andres W, Miclau T, 3rd, Marcucio R. Modulation of macrophage activity during fracture repair has differential effects in young adult and elderly mice. J Orthop Trauma. 2014;28(Suppl 1):S10–S14. doi: 10.1097/BOT.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 184.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118(11):3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014;6:13–13. doi: 10.12703/P6-13. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bahney CS, Hu DP, Miclau T, 3rd, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Front Endocrinol (Lausanne) 2015;6:4. doi: 10.3389/fendo.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yeung Tsang K, Wa Tsang S, Chan D, Cheah KS. The chondrocytic journey in endochondral bone growth and skeletal dysplasia. Birth Defects Res C Embryo Today. 2014;102(1):52–73. doi: 10.1002/bdrc.21060. [DOI] [PubMed] [Google Scholar]
- 189.Sheehy EJ, Vinardell T, Buckley CT, Kelly DJ. Engineering osteochondral constructs through spatial regulation of endochondral ossification. Acta Biomater. 2013;9(3):5484–5492. doi: 10.1016/j.actbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 190.Thompson EM, Matsiko A, Farrell E, Kelly DJ, O’Brien FJ. Recapitulating endochondral ossification: A promising route to in vivo bone regeneration. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1918. [DOI] [PubMed] [Google Scholar]
- 191.Oliveira SM, Mijares DQ, Turner G, Amaral IF, Barbosa MA, Teixeira CC. Engineering endochondral bone: In vivo studies. Tissue Eng Part A. 2009;15(3):635–643. doi: 10.1089/ten.tea.2008.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–1166. doi: 10.1242/jcs.113.7.1161. (Pt 7) [DOI] [PubMed] [Google Scholar]
- 193.Vacanti CA, Kim W, Upton J, Mooney D, Vacanti JP. The efficacy of periosteal cells compared to chondrocytes in the tissue engineered repair of bone defects. Tissue Eng. 1995;1(3):301–308. doi: 10.1089/ten.1995.1.301. [DOI] [PubMed] [Google Scholar]
- 194.Scotti C, Tonnarelli B, Papadimitropoulos A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 2010;107(16):7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dickhut A, Pelttari K, Janicki P, et al. Calcification or dedifferentiation: Requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219(1):219–226. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 196.Bahney CS, Hu DP, Taylor AJ, et al. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res. 2014;29(5):1269–1282. doi: 10.1002/jbmr.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Harada N, Watanabe Y, Sato K, et al. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials. 2014;35(27):7800–7810. doi: 10.1016/j.biomaterials.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 198.Scotti C, Piccinini E, Takizawa H, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci U S A. 2013;110(10):3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang JI, Durbhakula MM, Angele P, Johnstone B, Yoo JU. Lunate arthroplasty with autologous mesenchymal stem cells in a rabbit model. J Bone Joint Surg Am. 2006;88(4):744–752. doi: 10.2106/JBJS.E.00669. [DOI] [PubMed] [Google Scholar]
- 200.Gawlitta D, Farrell E, Malda J, Creemers LB, Alblas J, Dhert WJ. Modulating endochondral ossification of multipotent stromal cells for bone regeneration. Tissue Eng Part B Rev. 2010;16(4):385–395. doi: 10.1089/ten.TEB.2009.0712. [DOI] [PubMed] [Google Scholar]
- 201.Dennis SC, Berkland CJ, Bonewald LF, Detamore MS. Endochondral ossification for enhancing bone regeneration: Converging native extracellular matrix biomaterials and developmental engineering in vivo. Tissue Eng Part B Rev. 2014 doi: 10.1089/ten.teb.2014.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A. 2012;18(11–12):1161–1170. doi: 10.1089/ten.tea.2011.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sheehy EJ, Vinardell T, Toner ME, Buckley CT, Kelly DJ. Altering the architecture of tissue engineered hypertrophic cartilaginous grafts facilitates vascularisation and accelerates mineralisation. PLoS One. 2014;9(3):e90716. doi: 10.1371/journal.pone.0090716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sheehy EJ, Buckley CT, Kelly DJ. Chondrocytes and bone marrow-derived mesenchymal stem cells undergoing chondrogenesis in agarose hydrogels of solid and channelled architectures respond differentially to dynamic culture conditions. J Tissue Eng Regen Med. 2011;5(9):747–758. doi: 10.1002/term.385. [DOI] [PubMed] [Google Scholar]
- 205.Tsang KY, Chan D, Cheah KS. Fate of growth plate hypertrophic chondrocytes: Death or lineage extension? Dev Growth Differ. 2015;57(2):179–192. doi: 10.1111/dgd.12203. [DOI] [PubMed] [Google Scholar]
- 206.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111(33):12097–12102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014;10(12):e1004820. doi: 10.1371/journal.pgen.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chrastil J, Low JB, Whang PG, Patel AA. Complications associated with the use of the recombinant human bone morphogenetic proteins for posterior interbody fusions of the lumbar spine. Spine (Phila Pa 1976) 2013;38(16):E1020–E1027. doi: 10.1097/BRS.0b013e3182982f8e. [DOI] [PubMed] [Google Scholar]
- 209.DeVine JG, Dettori JR, France JC, Brodt E, McGuire RA. The use of rhBMP in spine surgery: Is there a cancer risk? Evidence-based spine-care journal. 2012;3(2):35. doi: 10.1055/s-0031-1298616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Song S, Kim EJ, Bahney CS, Miclau T, Marcucio R, Roy S. The synergistic effect of microtopography and biochemical culture environment to promote angiogenesis and osteogenic differentiation of human mesenchymal stem cells. Acta biomaterialia. 2015;18:100–111. doi: 10.1016/j.actbio.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 211.Caliari SR, Mozdzen LC, Armitage O, Oyen ML, Harley BA. Award winner in the young investigator category, 2014 society for biomaterials annual meeting and exposition, denver, colorado, april 16–19, 2014: Periodically perforated core-shell collagen biomaterials balance cell infiltration, bioactivity, and mechanical properties. J Biomed Mater Res A. 2014;102(4):917–927. doi: 10.1002/jbm.a.35058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]