Abstract
Hepatitis C virus (HCV) infection is a leading cause of end-stage liver disease that necessitates liver transplantation. The incidence of virus-induced cirrhosis and hepatocellular carcinoma continues to increase, making liver transplantation increasingly common1–3. Infection of the engrafted liver is universal and increases progression to advanced liver disease, with 20–30% displaying cirrhosis within 5 years. While treatments of chronic HCV infection have improved dramatically, albeit with remaining challenges of failure and access, therapeutic options to prevent graft infection during liver transplantation are emerging. Recent developments in directed use of novel direct-acting antiviral (DAA) agents4–6 to eliminate circulating HCV prior or following transplantation bring renewed hope for prevention and treatment of liver graft infection. Identifying the ideal regimen and use of DAAs reveals new paradigms of treatment for this special population6–8. Complementing DAAs, entry inhibitors have been shown to prevent liver graft infection in animal models9–13 and delay graft infection in clinical trials14, providing a perspective to be used concomitant to transplantation. We review the challenges and pathology associated with HCV liver graft infection, highlight current and future strategies of DAA treatment timing, and discuss the potential role of entry inhibitors that might be employed synergistically with DAAs to inhibit graft infection.
Introduction
Hepatitis C virus (HCV) infection is the etiologic agent necessitating more than half of all liver transplantations (LTs) in North America and Europe15–19. The engrafted liver universally becomes infected and undergoes rapid progression to serious liver disease; HCV infection is thereby associated with the poorest post-transplantation survival rates compared to other etiologies leading to LT20. The even more accelerated natural history of allograft HCV in patients undergoing re-transplantation has made re-transplantation an ethically challenging proposition. Recently developed direct-acting antiviral (DAA) therapies have proven effective in treating chronic HCV infection, and appear more effective in the LT setting than conventional interferon (IFN)-based treatments in genotype 1 patients. However, treatment options are still limited for those needing LT consequent to HCV infection, as transplantation requires immunosuppressive reagents to avoid graft rejection with potential drug-drug interactions, the diminished health of this patient population, and the metabolic burden placed on the newly engrafted liver by co-administered pharmaceutical agents.
The most straightforward means of avoiding the pathogenesis of liver graft infection would be to instate preventative measures to avoid graft infection, but the strong efficacy of current DAAs may allow withholding antiviral treatment during operative stage and addressing HCV infection post-operatively. Here, we review the specific hurdles associated with HCV infection in LT, evidence supporting treatment strategies of patients needing transplantation, and the outlooks for prophylactic measures against liver graft infection.
Challenges of HCV liver graft infection
Universal graft infection in HCV RNA positive patients
Due to the current burden of HCV on transplants, the new potent DAAs are hoped to reduce transplantation activity, preemptively reducing the numbers of patients presenting with hepatocellular carcinoma (HCC) and decompensated cirrhosis21. To achieve this goal, however, comprehensive screening is necessary, since the majority of patients with chronic HCV infection only seek medical care following liver-related complications22. A positive outlook is warranted given that a recent analysis indicates that a >90% decline in total infections by 2030 could be achievable, though this will require a 3 to 5-fold increase in diagnosis and treatment23. However, the public health strategy approaching this widespread problem must remain to hope for the best while planning for the worst.
HCV recurrence after LT remains universal in patients with detectable serum HCV RNA pre-transplantation. Even patients who are below detection levels for serum HCV RNA on therapy prior to transplantation have a 30% incidence of relapse, excluding those proven to have sustained virological response (SVR) to therapy for an extended period24. HCV recurrence is a critical medical problem and responsible for an increased risk of death and of graft failure. Positive detection of HCV RNA in recipients prior to transplantation associates with a diminished 5-year patient survival (69.9% vs. 76.6%, P<0.0001) and allograft survival (56.8% vs. 67.7%, P<0.0001)25; reinfection is a serious problem not only for the recipient, but also taxes the precious resource of suitable donated organs.
Rapid fibrosis progression after liver transplantation
The diminished 5-year survival rate is attributed to an accelerated development of pathology due to the immune-suppressive agents administered to prevent graft rejection. While the average time of progression from initial HCV infection to cirrhosis is about 30 years, 20–30% of transplant recipients develop cirrhosis within 5 years26. While only 30% of non-transplant cirrhotic patients have liver decompensation after 10 years of cirrhosis, more than 40% of graft recipients decompensate within the 12 months following LT, of whom less than 50% survive the following year. While the progression to fibrosis in the context of HCV recurrence varies widely depending on individual patient characteristics, the average time of progression to cirrhosis after LT is 10 to 12 years27. Re-transplantation is the only therapeutic option to achieve long-term survival of patients with decompensated cirrhosis after transplantation. Due to both poor patient and graft post-transplant survival rates, and the paucity of suitable organ donations, re-transplantation is not a sustainable option in most countries28.
A critical clinical challenge is to identify scenarios of early and rapid fibrosis development to employ early intervention while minimizing liver damage, highlighting the importance of diagnostic development. The previous consensus opinion was that IFN-based antiviral therapy should be initiated after detecting chronic hepatitis of the liver graft, usually >F1 on the METAVIR scoring system. The diagnosis of HCV recurrence is typically based on liver biopsy detection, since biopsies also can reveal severity of disease progression and exclude other possible diagnoses. It has been recently shown that significant periportal sinusoidal fibrosis in early biopsies (<6 months) is a good predictor of severe HCV recurrence29. The use of serum markers that decisively indicate fibrosis progression and other non-invasive techniques that measure liver stiffness will contribute to future decision-making in post-transplantation HCV treatment. Liver stiffness values of <8.7 kPa have a 90% negative predictive value and can be utilized as a threshold in defining significant fibrosis30. Pressure levels exceeding 6 mmHg of hepatic venous pressure likewise indicate fibrosis31.
Robust recurrence of HCV RNA levels soon after transplantation is associated with poor prognosis, so early monitoring of HCV levels is critical. Robust recurrence occurs in 2 to 8% of patients often resulting in fibrosing cholestatic hepatitis (FCH). FCH is characterized by high levels of cholestatic enzymes and the presence of extensive dense portal fibrosis with immature fibrous bands extending into the sinusoidal spaces, ductular proliferation, cholestasis, and moderate mononuclear inflammation detected in liver graft biopsies32. Without response to antiviral agents, FCH typically proceeds to complete liver failure.
Multiple risk factors contributing to rapid and severe fibrosis progression have been identified. High HCV RNA levels in either serum or liver associates with increased progression to cirrhosis, graft loss, and death33. Recipient and donor characteristics associated with poor outcome include female gender, donor age, and graft steatosis, while human leukocyte antigen (HLA) matching and IL28B genotype negatively associate with poor outcome18,34,35. While some of these factors can be selected for before transplantation, others are unpredictable and only antiviral treatment can improve the prognosis of transplant recipients.
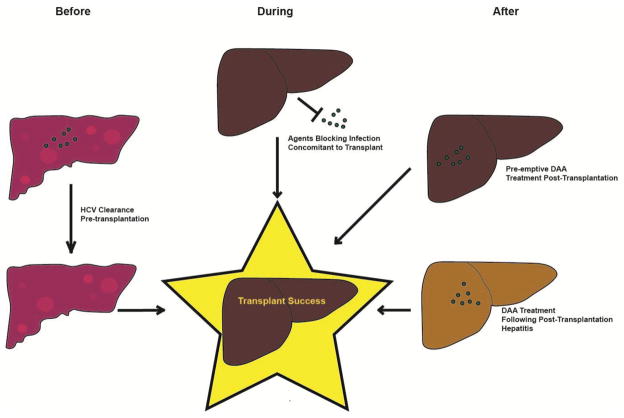
The strategic options of HCV treatment with LT can be divided based on timing of treatment; HCV clearance pre-transplant, inhibition of graft infection concomitant with transplantation, or antiviral treatment after graft infection (Fig. 1).
Figure 1. Timing of Antiviral Strategies for Successful Liver Transplantation in HCV-infected Patients.
After a patient presents with a cirrhotic liver (upper left) with HCV infection (green dots) there are multiple strategies that have been proposed for having a successful, non-infected transplantation. Successful treatment eliminating the virus before transplantation (lower left) has proven successful to approximately 70% with DAAs35. Multiple lines of evidence show that immunoprevention and HCV entry antagonists can play a syngergistic role in blocking infection concomitant to transplantation (center, top). Permitting infection and treating later with DAAs (right top and bottom) has proven 70–97% successful depending on the study and drug employed53,54.
DAA-based strategies for prevention and treatment of liver graft infection
HCV cure pre-transplant
The clearly optimal tact is to tackle reinfection early by eliminating HCV infection prior to transplantation. This strategy has been difficult to apply until recent DAA approval, since IFN-based therapies have limited efficacy for those with advanced disease while on a transplantation waitlist with SVR being achieved in only 8–39% of cases. The IFN tolerability is generally poor in these patients and contraindicated in patients with decompensated cirrhosis requiring either dose reduction (70%) or early discontinuation (30%) of treatment. The results of a phase 2 clinical study of administration of sofosbuvir with ribavirin (RBV) to 61 patients on waitlists for LT show that this tact with DAAs is effective6. Of the 43 patients who displayed viral response prior to transplantation, 70% maintained viral clearance 12 weeks post-transplantation. However, the efficacy of this strategy is genotype dependent and managing DAA combinations in the pre-transplant period is challenging. The use of sofosbuvir/RBV in advanced cirrhosis may contribute to lactic acidosis in approximately 14% of patients36. Charlton et al. investigated in the SOLAR-1 trial including the NS5A inhibitor ledipasvir in combination with sofosbuvir and RBV for individuals with cirrhosis and moderate or severe hepatic impairment due to genotype 1 and 4 infections37. SVR-12 was achieved in 86–89% in this difficult-to-treat cohort. The inclusion of ledipasvir or daclatasvir with sofosbuvir and RBV have been also investigated in the SOLAR-2 and ALLY-1 studies, respectively, focusing on patients with advanced liver disease pre-transplantation or with recurrent HCV post-transplantation38–40. In the SOLAR-2 study, patients with the sofosbuvir-sensitive HCV genotypes 1 or 4 were treated for 12 or 24 weeks with sofosbuvir, ledipasvir, and RBV. Preliminary results revealed high SVR rates of 85%–88%, irrespective of treatment duration in genotype 1. Longer treatment duration was superior (SVR rate of 86% vs. 57%) for patients with genotype 4 infection and effective antiviral therapy was associated with improvement in liver histology, MELD and Child-Pugh (CP) scores40. For some decompensated cirrhotic patients, however, MELD or CP scores increased. Searching for prognosis factors of clinical/biological response instead of only viral response is an ongoing and needed area of investigation.
The ALLY-1 phase-III study included 60 patients with advanced cirrhosis treated with daclatasvir, sofosbuvir and RBV39. While overall the SVR12 was 83%, response depended on severity of liver disease; 92%, 94% and 56% for CP A, B and C respectively. These findings suggest that further studies are required to define the best therapy management for CP C patients.
While the inclusion of sofosbuvir has had an impact on the management of genotype 1 infection, the use of this drug has less significantly improved treatment for genotype 3 infection. Foster et al. analyzed addition of NS5A inhibitors to sofosbuvir and RBV in patients with decompensated cirrhosis due to genotypes 1 or 340,41. The response rates varied from 44% to 88%, depending on genotype, NS5A inhibitor, and the use of RBV. Adding ledipasvir to sofosbuvir and RBV was inferior to daclatasvir plus sofosbuvir in patients with genotype 3 infection. Over 40% of patients experienced improvement in liver function with a mean improvement of >2 points of MELD score. Overall, these combinations showed excellent efficacy results and safety profiles, although some patients experienced worsening of their MELD scores. However the severity of cirrhosis remains an impediment to response, even with the new combinations of DAAs. Although the combination of sofosbuvir and NS5A inhibitor velpatasvir for 12 weeks provides SVR in over 95% of patients without cirrhosis42,43, SVR rate was 83% (n=90) in patients with decompensated cirrhosis44. However, the addition of ribavirin to this combination improves the SVR rate of 94%, even in cirrhotic patients (n=87)44.
The critical argument for treatment before transplantation is the prospect of avoiding LT altogether for individuals with liver disease that has not progressed to hepatocellular carcinoma. About 2/3 of patients achieve clinical and biological improvement during treatment in studies enrolling decompensated cirrhotic patients37,39,44,45. However, critical review reveals that 1/3 of patients do not improve or worsen during treatment, regardless of virological response. For those who do improve, the difference is often modest with variations of only 1 or 2 points in MELD score. In a recent meta-analysis involving five studies and including 533 patients, 28% experienced an improvement of MELD score over 346. A number of patients do improve to the point where LT is avoidable. A French cohort study that included 183 patients awaiting transplantation showed that of 53 patients with decompensated cirrhosis, 36% had a complete clinical and biological response, meaning a CP A at the end of treatment45. The best improvement was in those with the least disease progression: those with baseline CP with an area under the curve (AUC) of 0.81, the CP threshold of improvement being 7.5. This raises further questions, since some patients keep improving over longer periods of follow-up. When considering longer times while being on waitlists for LT, comorbidities need to be considered. Optimum conditions and thresholds have not been defined for removing patients from waitlists. Although we could expect significant improvement for 1/3 of patients, this improvement is more likely for patients with less severe disease. For individuals with more severe disease, one needs to practice caution since treating HCV in patients to only get partial biological improvement may be deleterious.
Waitlisted patients with HCC, who normally present with compensated cirrhosis, have several approved regimens available albeit with limited efficacy. In fact, treatment may be futile in about 30% of patients considering the rate of dropout before LT and early HCC recurrence after LT47. The severe disease state of those on the transplantation list can limit treatment options and the 12 weeks needed to confirm SVR status is not always afforded pre-transplantation. Patients with severe end-stage liver disease prior to LT or who require complicated post-operative treatment are frequently ineligible for pre-emptive interferon therapy.
DAA treatment after HCV graft infection
At present, two therapeutic approaches can be considered after transplantation: the pre-emptive strategy involving treatment in the first month following transplantation, or to hold off treatment until chronic hepatitis is observed. Despite the clear benefits of early treatment, the pre-emptive strategy is historically not employed due to safety and efficacy limitations of initiating IFN-based antiviral therapy during the post-operative period48–52. New DAA IFN-free combination therapy revives this strategy although due to the novelty of these therapies, the evidence regarding efficacy is lacking. Factors influencing the future employ of the pre-emptive strategy will depend on safety, cost, and tolerability of next-generation DAAs relative to typical liver graft damage incurred before assessment of HCV recurrence.
On the other hand, treating HCV recurrence has been the standard therapy and until 2011 involved 48 weeks of PEG-IFN and RBV treatment. Three systematic reviews have reported an SVR rate in these conditions of only approximately 30% with limitations of tolerability including bacterial infections, haematological toxicity, and graft rejection53–55. Early virological response (EVR) is a major predictive factor associated with SVR56,57. However, effective antiviral treatment post-transplantation has clear benefits in preventing disease progression58–63. First-generation protease inhibitors, telaprevir or boceprevir, were the initial agents tested in treating recurrent HCV post-transplantation. Their inclusion with PEG-IFN and RBV improved SVR rates by 50–65% in genotype 1 HCV-infected recipients however with a worse safety profile and potent drug-drug interactions64,65. Although feasible, these regimens required close monitoring and great expertise of caregivers leading to their retirement. The new generation of DAAs has further changed the treatment landscape in post-transplantation antiviral treatment, demonstrated in two studies where the initiation of treatment was a year after liver engraftment. Sofosbuvir plus RBV treatment has a 70% efficacy rate in yielding SVR, roughly equivalent to the virological response seen when clearing the virus pre-transplantation 6. Although this response efficacy is not optimal, it demonstrates efficacy and tolerability even in the most severe patients66. However, a more complex cocktail of DAAs can be more efficacious, and several studies describing this have already been communicated and/or published, summarized in Table 1. Data simultaneously comes from both open-label studies and real-life cohorts (HCV-TARGET and CUPILT studies). The SVR 12 rates are usually >90%, better than SVR12 rate treating decompensated cirrhotic prior to LT, and tolerance is excellent. In the SOLAR-1 study assessing post-transplantation treatment, progressive liver disease was associated with lowered response, however all 6 individuals that had FCH achieved SVR 12 weeks after the end of treatment37. Although the treatment of HCV in transplant patients has been significantly improved and simplified, several issues remain to be clarified. This shows the promise of DAAs and combinatorial therapy; multiple targets and mechanisms of action synergize to eliminate the virus.
Table 1.
Available results of DAA-based regimens to treat HCV recurrence after liver transplantation
| Regimen | Study | N | Genotype | Cirrhosis (%) | SVR12 | Author (ref) |
|---|---|---|---|---|---|---|
| SOFOSBUVIR+RIBAVIRIN 24 weeks |
Prospective Multicenter Open-label |
40 | All (83% G1) | Yes (40%) | 70% | Charlton, M. 65 |
| SOFOSBUVIR+DACLATASVIR+RIBAVIRIN 12 weeks (ALLY-1) |
Prospective Multicenter Open-label |
53 | All (77% G1) | Yes | 94% | Poordad, F. 39 |
| SOFOSBUVIR+DACLATASVIR±RIBAVIRIN 12 or 24 weeks (ANRS CO23 CUPILT) |
Prospective Multicenter Real-life cohort |
130 | All (82% G1) | Yes (31%) | 96% | Coilly, A. 70 |
| PARITAPREVIR+OMBITASVIR/r+DASABUVIR+ RIBAVIRIN 24 weeks |
Prospective Multicenter Open-label |
34 | Only G1 | No | 97% | Kwo, PY. 74 |
| SOFOSBUVIR+LEDIPASVIR+RIBAVIRIN 12 or 24 weeks (SOLAR I and II) |
Prospective randomized phase II study | 444 | G1 (>95%) and G4 | Yes (about 50%) | 92% | Charlton, M. 37 Manns, M. 38 |
| SOFOSBUVIR+SIMEPREVIR±RIBAVIRIN 12 weeks |
Prospective Multicenter Open-label |
109 | Only G1 | F3–F4 (29%) | 90% | Pungpapong, S. 143 |
| SOFOSBUVIR+SIMEPREVIR±RIBAVIRIN 12 weeks (HCV-TARGET) |
Prospective Multicenter Real-life cohort |
143 | All (80% G1) | Yes (56%) | 90% (SVR4) | Sulkowski, M. 144 |
The optimal duration of therapy remains to be defined. While a number of risk factors of treatment failure were identified for IFN-based regimens, no risk factors have been identified for new DAAs except genotype 3. In the non-transplant setting, most studies comparing different treatment durations did not show any benefit of longer treatment and better adherence, fewer side effects, and lower cost associated with a shorter duration. In the transplant setting, robust data is currently lacking and many studies conservatively use 24-weeks of treatment in this special population until more evidence is collected.
The use of RBV in future regimens is not yet established and could be abandoned once next-generation DAAs with higher efficacy are added. There remains a significant benefit of RBV in patients with severe liver disease and recurrent HCV post-transplantation41,67,68.
In LT patients, renal impairment is common and should be properly evaluated before initiating antiviral therapy, especially sofosbuvir-based regimens69. The metabolism of sofosbuvir is renal and its use is not recommended in patients with creatinine clearance below 30 ml/min until an appropriate dosage is determined. A Phase IIb, open-label study of 200 mg or 400 mg sofosbuvir and RBV for 24 weeks in HCV genotype 1 or 3 patients, and ledipasvir/sofosbuvir in individuals with genotype 1 and 4 infection with renal insufficiency is ongoing (Clinicaltrials.gov:NCT01958281). For other available DAAs, the metabolism is hepatic. Although no detrimental effect is expected in RBV-free combination, in a recent communication of the ANRS C023 CUPILT group, a slight but significant reduction in creatinine clearance during treatment was reported (from 72.7±29.0 to 66.3±25.7mL/min. between baseline and end of treatment; p<0.0001) using the combination of sofosbuvir and daclatasvir70. But it should better define which patients who worsen. In a recent multicentre trial of LT recipients with recurrent HCV infection treated with sofosbuvir based regimens, renal improvement was observed in the majority (58%) of patients 71. Those patients with SVR at 12 weeks post-treatment were more likely to have renal improvement, indicating that HCV affects renal health71.
Drug-drug interactions between DAAs and immunosuppressive drugs, mainly calcineurin inhibitors, remain a concern with these regimens. Simeprevir, a second generation protease inhibitor, is a partial cytochrome P450 (CYP) 3A inhibitor. Since the immunosuppressant cyclosporine is likewise a partial CYP3A inhibitor, combination results in accumulation of both drugs in the blood, and coadministration is discouraged72. Ombitasvir, paritaprevir, ritonavir, and dasabuvir require dosing modifications for calcineurin inhibitors tacrolimus and cyclosporine73,74. Conversely, sofosbuvir, ledipasvir and daclatasvir do not seem to interact with calcineurin inhibitors73. However, close monitoring before, during and after DAA therapy remains essential. In the ANRS C023 CUPILT study, 59% of 130 patients treated with sofosbuvir and daclatasvir after LT had to change dosage of one immunosuppressive drug during therapy70.
Finally, the optimal timing for initiation of therapy post-transplantation remains to be determined. Antiviral therapy is usually initiated only when histologically proven recurrent HCV occurs (fibrosis stage ≥2 on the METAVIR score or severe and rapid progression of fibrosis as observed in cholestatic hepatitis). This decision was based on the tolerability of the classic IFN-based regimen, which required post-transplantation recovery time to regain health. Development of IFN-free therapy allows treatment of patients earlier after LT without waiting for disease markers indicating HCV recurrence. This strategy is reasonably based, but without scientific evidence of its efficacy. Nonetheless, earlier treatment appears safe and effective and the potential risk in allowing fibrosis progression on liver graft could raise ethical issues. Treating early after LT could help to overcome the issue of differentiating HCV recurrence and rejection and it could also prevent rejection episodes induced by viral clearance while immunosuppressive levels are still high at early stages after LT. It has been reported that immunosuppressive levels decrease significantly in patients responding to antiviral therapy as the viral clearance improves hepatic microsomal function and elevated regulatory T cell levels may decline75.
Perspective for prevention of graft infection concomitant with transplantation: HCV entry inhibitors
Viral entry has been demonstrated to play an essential role during re-infection of the graft after LT76,77. Thus, concomitant treatment of safe and effective entry inhibitors, including virus-targeting neutralizing antibodies (nAbs), during and immediately after transplantation may prove an effective means of preventing graft infection without allowing allograft damage78. This concept is supported by the experience in prevention of HBV graft infection where hepatitis B immune globulin in combination with nucleos(t)ide analogues can reduce HBV recurrence in LT patients to 4%77,79–81. Entry inhibitors have been shown to effectively inhibit HCV infection, work synergistically with DAAs, and have proven to be safe and effective in humanized mice82. While most of these agents are at a preclinical stage of development, the results of first clinical trials with anti-envelope antibodies14,83,84 and a small molecule host-targeting inhibitor85 suggest that they may be future tools in the antiviral arsenal during transplantation. Strategies for blocking viral entry during liver graft infection can either target the virus or host entry factors:
Anti-envelope antibodies
A high rate of viral diversity, glycosylation of the HCV glycoproteins, and association with apolipoproteins aids the escape of HCV from neutralizing antibodies (nAbs)86–91(reviewed in 89). In the course of HCV infection, nAbs develop that mostly target regions of E2 that interact with the host receptor CD8187,92–94. The crystal structure of the core of glycoprotein E295,96 defined the face of the protein where the majority of nAbs bind. Polyclonal and monoclonal nAbs taken from patients with chronic HCV infection or administered by gene therapeutic approaches are capable of inhibiting infection of human liver chimeric mice9,97–101. In patients, nAbs targeting the HCV envelope glycoprotein (MBL-HCV1) effectively delayed viral rebound, proving the principle that immunotherapy will prove an effective addition to the synergistic antiviral arsenal14. Current studies are underway in combining MBL-HCV1 with DAAs to optimize therapeutic efficacy of this approach with the latest tools. Recent clinical trials of human HCV immune globulin (HCIG) in combination with DAAs show that administration of the immune globulin is safe and more effective than with DAAs alone84. However a potential challenge in utilizing nAbs for prevention of infection is identical to the problem during chronic infection, i.e. genetic adaptation enabling viral escape14. Complementing anti-envelope antibodies, small molecules have been identified to interfere with viral entry102–105.
Host-targeting entry inhibitors
One solution that could feasibly avoid the problem of viral escape from antiviral antibodies would be through targeting host entry factors (Fig. 2)78,106. Indeed, infection of HCV variants that escape host anti-envelope antibodies or exhibit resistance to DAAs are effectively blocked by host-targeting entry inhibitors76,82,107–109, Host-targeting agents have been investigated for multiple steps of viral entry. HCV virions circulate in dynamic complex with lipoproteins and apolipoproteins110,111. The earliest step of HCV attachment is mediated by apolipoprotein E binding to heparan sulfates on the baso-lateral surface of the hepatocyte112. Inhibitors of heparan sulfate attachment such as the green tea polyphenol epigallocatechin-3-gallate (EGCG) are generally safe and can impair infection in cell culture systems113,114, although in HCV mouse models the addition of EGCG adds no observable advantage over anti-envelope antibodies alone115. The next step of the HCV entry process is interaction of the virion with scavenger receptor B1 (SR-B1). Antibodies to SR-B1 markedly inhibit HCV infection in small animal models11,13 and prevent antiviral resistance to DAAs116. Inhibition of the lipid transfer activity of SR-B1 is sufficient to inhibit infection117. A small chemical inhibitor of SR-B1, ITX5061, has been tested in patients undergoing transplantation with HCV infection85. Genotype 1 patients under treatment had sustained reduction of viral load, and the genetic variation of the quasispecies was limited85. After this initial attachment, a sequence of events takes place including the triggering of signaling pathways involving host kinases such as epidermal growth factor receptor (EGFR) to cluster essential entry factor claudin 1 (CLDN1) and CD81118,119. Erlotinib, a small molecule inhibitor of EGFR, has been shown to inhibit HCV infection in both cell culture and animal models118. Antibodies that recognize CD81 have also been shown in small animal models to inhibit HCV infection12,120. Anti-CLDN1-specific antibodies are not only effective in preventing HCV infection, but can prevent and cure HCV infection in humanized mice in monotherapy without resistance and observable side effects10,121,122. The anti-CLDN1 antibody has been shown to be highly synergistic with DAAs82, prevent antiviral resistance by impairing viral spread108. There are a number of other host entry factors such as occludin123,124, Niemann-Pick C1-like 1 (NPC1L1) 125, transferrin receptor 1126, and serum response factor binding protein 1 (SRFBP1)127. A clinically approved small chemical inhibitor of NPC1L1 has likewise shown efficacy in small animal models and to synergize with DAA125,128. Future research will enable the discovery and development of host-targeting entry inhibitors of optimal safety in administration and efficiency in synergizing with DAAs to play a key role in increasing the cure rates in LT.
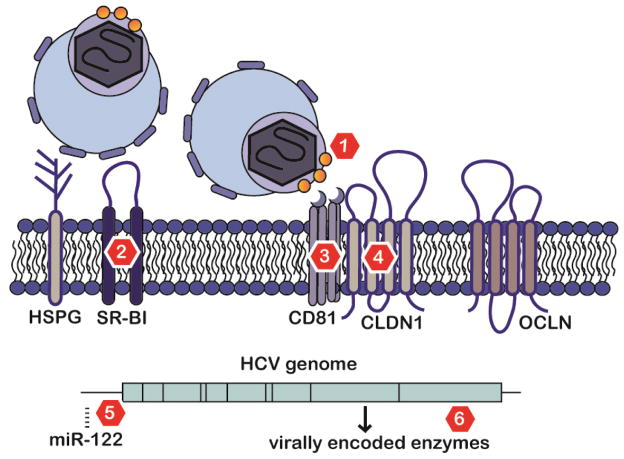
Figure 2. Examples of HCV entry factors as targets to prevent graft infection with completed in vivo proof-of-concept.
Several points of HCV entry are effective targets to prevent initial or ongoing liver graft infection. The HCV glycoprotein E1/E2 is critical for HCV entry (marker 1), and nAbs binding to E1/E2 have proven effective in animal models and clinically9,97,100. Early steps of HCV entry likely involve initial attachment of apoE to HSPG and utilization of SR-B1 (marker 2). SR-B1 inhibitors have been effective in animal models and in the clinic in the context of liver transplantation11,13,85,145. HCV E2 directly binds to host entry factor CD81 (marker 3), and antibodies binding CD81 prevent HCV infection in animal models12,120. Antibodies recognizing CLDN1 (marker 4) have proven effective in curing animal models of HCV infection10,121,122. Furthermore, small molecules erlotinib targeting EGFR118, a kinase promoting CD81-claudin-1 coreceptor formation, and ezetimibe targeting cholesterol transporter NP1CL1 (not shown) have been shown to inhibit HCV infection in humanized mouse models 125. Downstream of entry, microRNA 122 (miRNA) antagonists (antagomirs, marker 5) have been shown to effective and safe in animals and patients139,141. DAAs targeting virally encoded enzymes have revolutionized HCV treatment (marker 6).
Advantages and disadvantages
The success of next-generation DAAs in treatment raises the debate of whether HCV entry inhibitors have a place in future clinical practice128,129. There are a number of advantages and disadvantages to using entry inhibitors78,130. Strengths include their capacity to be used in targeting intervention around transplantation with a short duration of treatment. The barrier for resistance appears to be higher for host-targeting entry inhibitors than for DAA when used in monotherapy10,108. Given their complementary mechanism of action to DAAs106 and efficacy against DAA-resistant viruses108,131 entry inhibitors may offer a perspective for the patient population who fail preemptive therapy while preventing costly post-transplant therapy. The high level of synergy of entry inhibitors with DAAs observed in cell culture and animal models indicates that these agents could shorten treatment time and circumvent the development of antiviral-resistant variants10,82,116. Drawbacks include that a number of entry inhibitors are only now reaching clinical development stages. There are more DAAs in the drug development pipeline that may not be limited by the current safety issues of resistance and complications of renal failure. Targeting host factors will also require careful surveillance of side-effects106 and viral escape has been described14,131–134. Furthermore, the numbers of individuals with serious HCV-related liver disease will decline, as will prices for the DAA regimens.
Other approaches targeting host factors downstream of HCV cell entry106,135 include microRNA (miRNA) antagonists (antagomirs) or cyclophilin inhibitors 136,137. A key miRNA that boosts HCV replication, miR-122, acts by shifting HCV genome activity away from translation and toward replication138. Antisense agents targeting miR-122 have been shown to be safe and efficient in primate models and patients139–141. Cyclophilin, a host factor required for viral replication is efficiently inhibited by alisporivir, a host-targeting agent in clinical development 142. Whether these strategies are feasible in LT remains to be shown.
Summary and conclusion
Treatment of patients in need of LT as a result of HCV-associated advanced disease is a sensitive and complicated tree of decision-making. The successful use of DAAs as prophylactic and therapeutic agents against HCV infection, both before and following transplantation, promises to assist those likely increasing numbers of individuals who find themselves in this historically difficult-to-treat population. Randomized clinical trials are ongoing to define the role of entry inhibitors in prevention of graft infection.
Acknowledgments
The authors would like to thank Dr. Mirjam B. Zeisel and Dr. Che C. Colpitts (both Inserm U1110, University of Strasbourg, France) for critical reading of the manuscript. T.F.B acknowledges grant support by the European Union (ERC-2008-AdG-HEPCENT, ERC-AdG-2014-HEPCIR, FP7 HepaMab, and Interreg IV FEDER-Hepato-Regio-Net 2012), the Agence Nationale de Recherche sur le SIDA (ANRS) and the Direction Générale de l’Offre de Soins (A12027MS). R.T.C. receives grant support from NIH DK078772. This work has been published under the framework of the LABEX ANR-10-LABX-0028_HEPSYS and benefits from a funding from the state managed by the French National Research Agency as part of the Investments for the future program.
Footnotes
Search strategy and selection criteria
References for this review were identified through searches of PubMed for articles published from January, 1995, to January, 2016, by use of the terms “hepatitis c”, “transplantation”, “HCV”, “liver graft”, and “cirrhosis”. Relevant presentations of upcoming publications were identified at the EASL International Liver Congress and the AASLD Liver Meeting. Articles resulting from these searches and relevant references cited in those articles were reviewed. Articles published in English, French, and German were included.
Contributions: DJF, AC, RTC, DS, and TFB wrote and edited the manuscript. DJF and AC contributed equally. DS and TFB contributed equally.
Potential conflicts of interest: DJF-no conflict; AC- Astellas, Novartis, Abbvie, BMS, Gilead and Janssen Cilag; RTC- Gilead, Abbvie, Merck, BMS, Mass Biologics; DS- Astellas, BMS, Gilead, LFB, MSD, Novartis, Roche, Biotest, Abbvie; TFB- Biotest, Indus, Gilead, Vironexx, Co-inventor US patent and patent applications for anti-host cell factor antibodies and small molecules for prevention and treatment of HCV infection.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57(1):249–57. doi: 10.1002/hep.25800. [DOI] [PubMed] [Google Scholar]
- 2.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182–8. e1. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razavi H, Elkhoury AC, Elbasha E, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–70. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curry MP, Smith HL, Chung RT, et al. Peri-transplant treatment with anti-HCV E2 human monoclonal antibody MBL-HCV1 in combintaion with a protease inhibitor results in undetectable HCV RNA in the early post-transplant period. Journal of Hepatology. 2014:S471. [Google Scholar]
- 5.Fontana RJ, Hughes EA, Bifano M, et al. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am J Transplant. 2013;13(6):1601–5. doi: 10.1111/ajt.12209. [DOI] [PubMed] [Google Scholar]
- 6.Curry MP, Forns X, Chung RT, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015;148(1):100–7. e1. doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Coilly A, Roche B, Samuel D. Current management and perspectives for HCV recurrence after liver transplantation. Liver Int. 2013;33(Suppl 1):56–62. doi: 10.1111/liv.12062. [DOI] [PubMed] [Google Scholar]
- 8.Coilly A, Roche B, Dumortier J, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol. 2014;60(1):78–86. doi: 10.1016/j.jhep.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Law M, Maruyama T, Lewis J, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14(1):25–7. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 10.Mailly L, Xiao F, Lupberger J, et al. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol. 2015;33(5):549–54. doi: 10.1038/nbt.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meuleman P, Catanese MT, Verhoye L, et al. A human monoclonal antibody targeting scavenger receptor class B type I precludes hepatitis C virus infection and viral spread in vitro and in vivo. Hepatology. 2012;55(2):364–72. doi: 10.1002/hep.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meuleman P, Hesselgesser J, Paulson M, et al. Anti-CD81 antibodies can prevent a hepatitis C virus infection in vivo. Hepatology. 2008;48(6):1761–8. doi: 10.1002/hep.22547. [DOI] [PubMed] [Google Scholar]
- 13.Vercauteren K, Van Den Eede N, Mesalam AA, et al. Successful anti-scavenger receptor class B type I (SR-BI) monoclonal antibody therapy in humanized mice after challenge with HCV variants with in vitro resistance to SR-BI-targeting agents. Hepatology. 2014;60(5):1508–18. doi: 10.1002/hep.27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung RT, Gordon FD, Curry MP, et al. Human monoclonal antibody MBL-HCV1 delays HCV viral rebound following liver transplantation: a randomized controlled study. Am J Transplant. 2013;13(4):1047–54. doi: 10.1111/ajt.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144–65. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 16.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10(6):1420–7. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlton M, Ruppert K, Belle SH, et al. Long-term results and modeling to predict outcomes in recipients with HCV infection: results of the NIDDK liver transplantation database. Liver Transpl. 2004;10(9):1120–30. doi: 10.1002/lt.20211. [DOI] [PubMed] [Google Scholar]
- 18.Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14(Suppl 2):S36–44. doi: 10.1002/lt.21646. [DOI] [PubMed] [Google Scholar]
- 19.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36(5 Suppl 1):S30–4. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- 20.Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR) J Hepatol. 2012;57(3):675–88. doi: 10.1016/j.jhep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Deuffic-Burban S, Mathurin P, Rosa I, et al. Impact of emerging hepatitis C virus treatments on future needs for liver transplantation in France: a modelling approach. Dig Liver Dis. 2014;46(2):157–63. doi: 10.1016/j.dld.2013.08.137. [DOI] [PubMed] [Google Scholar]
- 22.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55(6):1652–61. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wedemeyer H, Duberg AS, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21(Suppl 1):60–89. doi: 10.1111/jvh.12249. [DOI] [PubMed] [Google Scholar]
- 24.Forns X, Garcia-Retortillo M, Serrano T, et al. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39(3):389–96. doi: 10.1016/s0168-8278(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 25.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122(4):889–96. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 26.Berenguer M, Prieto M, Rayon JM, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32(4 Pt 1):852–8. doi: 10.1053/jhep.2000.17924. [DOI] [PubMed] [Google Scholar]
- 27.Prieto M, Berenguer M, Rayon JM, et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999;29(1):250–6. doi: 10.1002/hep.510290122. [DOI] [PubMed] [Google Scholar]
- 28.Carrion JA, Navasa M, Forns X. Retransplantation in patients with hepatitis C recurrence after liver transplantation. J Hepatol. 2010;53(5):962–70. doi: 10.1016/j.jhep.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Marino Z, Mensa L, Crespo G, et al. Early periportal sinusoidal fibrosis is an accurate marker of accelerated HCV recurrence after liver transplantation. J Hepatol. 2014;61(2):270–7. doi: 10.1016/j.jhep.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Carrion JA, Torres F, Crespo G, et al. Liver stiffness identifies two different patterns of fibrosis progression in patients with hepatitis C virus recurrence after liver transplantation. Hepatology. 2010;51(1):23–34. doi: 10.1002/hep.23240. [DOI] [PubMed] [Google Scholar]
- 31.Blasco A, Forns X, Carrion JA, et al. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology. 2006;43(3):492–9. doi: 10.1002/hep.21090. [DOI] [PubMed] [Google Scholar]
- 32.Berenguer M, McCaughan G. Hepatitis C virus-associated cholestatic hepatitis: we cannot seem to agree on diagnostic criteria. Liver Transpl. 2013;19(2):115–7. doi: 10.1002/lt.23580. [DOI] [PubMed] [Google Scholar]
- 33.Charlton M, Seaberg E, Wiesner R, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998;28(3):823–30. doi: 10.1002/hep.510280333. [DOI] [PubMed] [Google Scholar]
- 34.Fukuhara T, Taketomi A, Motomura T, et al. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010;139(5):1577–85. 85 e1–3. doi: 10.1053/j.gastro.2010.07.058. [DOI] [PubMed] [Google Scholar]
- 35.Veldt BJ, Duarte-Rojo A, Thompson AJ, et al. Recipient IL28B polymorphism is an important independent predictor of posttransplant diabetes mellitus in liver transplant patients with chronic hepatitis C. Am J Transplant. 2012;12(3):737–44. doi: 10.1111/j.1600-6143.2011.03843.x. [DOI] [PubMed] [Google Scholar]
- 36.Welker MW, Luhne S, Lange CM, et al. Lactic acidosis in patients with hepatitis C virus related cirrhosis and combined ribavirin/sofosbuvir treatment. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 37.Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149(3):649–59. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Manns M, Forns X, Samuel D, et al. Ledipasvir/sofosbuvir with ribavirin is safe and efficacious in decompensated and post liver transplantation patients with HCV infection: preliminary results of the prospective SOLAR 2 trial. J Hepatol. 2015;62(S2):S187. [Google Scholar]
- 39.Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir With Sofosbuvir and Ribavirin for HCV Infection With Advanced Cirrhosis or Post-Liver Transplant Recurrence. Hepatology. 2016 doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gambato M, Villanueva A, Abraldes JG, Altamirano J, Forns X. Clinical Trial Watch: Reports from the EASL International Liver Congress (ILC), Vienna, April 2015. J Hepatol. 2015;63(3):753–62. doi: 10.1016/j.jhep.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Foster GR, McLauchlan J, Irving W, et al. Treatment of decompensated HCV cirrhosis in patients with diverse genotypes: 12 weeks sofosbuvir and NS5A inhibitors with/without ribavirin is effective in HCV genotypes 1 and 3. J Hepatol. 2015;62(S2):S190–S1. [Google Scholar]
- 42.Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373(27):2608–17. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 43.Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373(27):2599–607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 44.Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373(27):2618–28. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 45.Coilly A, Pageaux G, Houssel-Debry P, et al. Improving liver function and delisting of patients awaiting liver transplantation for HCV cirrhosis: do we ask too much to DAA? Hepatology. 2015;62(S1):257A. [Google Scholar]
- 46.Munoz SJ, Reich DJ, Rothstein KD, et al. Curing decompensated wait listed HCV patients with the new DAAs: the potential significant impact on liver transplant wait list and organ allocation. Hepatology. 2015;62(S1):311A. [Google Scholar]
- 47.Toso C, Dupuis-Lozeron E, Majno P, et al. A model for dropout assessment of candidates with or without hepatocellular carcinoma on a common liver transplant waiting list. Hepatology. 2012;56(1):149–56. doi: 10.1002/hep.25603. [DOI] [PubMed] [Google Scholar]
- 48.Bzowej N, Nelson DR, Terrault NA, et al. PHOENIX: A randomized controlled trial of peginterferon alfa-2a plus ribavirin as a prophylactic treatment after liver transplantation for hepatitis C virus. Liver Transpl. 2011;17(5):528–38. doi: 10.1002/lt.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalasani N, Manzarbeitia C, Ferenci P, et al. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005;41(2):289–98. doi: 10.1002/hep.20560. [DOI] [PubMed] [Google Scholar]
- 50.Mazzaferro V, Tagger A, Schiavo M, et al. Prevention of recurrent hepatitis C after liver transplantation with early interferon and ribavirin treatment. Transplant Proc. 2001;33(1–2):1355–7. doi: 10.1016/s0041-1345(00)02508-2. [DOI] [PubMed] [Google Scholar]
- 51.Shergill AK, Khalili M, Straley S, et al. Applicability, tolerability and efficacy of preemptive antiviral therapy in hepatitis C-infected patients undergoing liver transplantation. Am J Transplant. 2005;5(1):118–24. doi: 10.1111/j.1600-6143.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 52.Sugawara Y, Makuuchi M, Matsui Y, et al. Preemptive therapy for hepatitis C virus after living-donor liver transplantation. Transplantation. 2004;78(9):1308–11. doi: 10.1097/01.tp.0000142677.12473.e5. [DOI] [PubMed] [Google Scholar]
- 53.Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49(2):274–87. doi: 10.1016/j.jhep.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006;6(7):1586–99. doi: 10.1111/j.1600-6143.2006.01362.x. [DOI] [PubMed] [Google Scholar]
- 55.Xirouchakis E, Triantos C, Manousou P, et al. Pegylated-interferon and ribavirin in liver transplant candidates and recipients with HCV cirrhosis: systematic review and meta-analysis of prospective controlled studies. J Viral Hepat. 2008;15(10):699–709. doi: 10.1111/j.1365-2893.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 56.Berenguer M, Palau A, Fernandez A, et al. Efficacy, predictors of response, and potential risks associated with antiviral therapy in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2006;12(7):1067–76. doi: 10.1002/lt.20737. [DOI] [PubMed] [Google Scholar]
- 57.Roche B, Sebagh M, Canfora ML, et al. Hepatitis C virus therapy in liver transplant recipients: response predictors, effect on fibrosis progression, and importance of the initial stage of fibrosis. Liver Transpl. 2008;14(12):1766–77. doi: 10.1002/lt.21635. [DOI] [PubMed] [Google Scholar]
- 58.Abdelmalek MF, Firpi RJ, Soldevila-Pico C, et al. Sustained viral response to interferon and ribavirin in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2004;10(2):199–207. doi: 10.1002/lt.20074. [DOI] [PubMed] [Google Scholar]
- 59.Bizollon T, Ahmed SN, Radenne S, et al. Long term histological improvement and clearance of intrahepatic hepatitis C virus RNA following sustained response to interferon-ribavirin combination therapy in liver transplanted patients with hepatitis C virus recurrence. Gut. 2003;52(2):283–7. doi: 10.1136/gut.52.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Firpi RJ, Abdelmalek MF, Soldevila-Pico C, et al. Combination of interferon alfa-2b and ribavirin in liver transplant recipients with histological recurrent hepatitis C. Liver Transpl. 2002;8(11):1000–6. doi: 10.1053/jlts.2002.34968. [DOI] [PubMed] [Google Scholar]
- 61.Picciotto FP, Tritto G, Lanza AG, et al. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007;46(3):459–65. doi: 10.1016/j.jhep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 62.Walter T, Scoazec JY, Guillaud O, et al. Long-term antiviral therapy for recurrent hepatitis C after liver transplantation in nonresponders: biochemical, virological, and histological impact. Liver Transpl. 2009;15(1):54–63. doi: 10.1002/lt.21652. [DOI] [PubMed] [Google Scholar]
- 63.Carrion JA, Navasa M, Garcia-Retortillo M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132(5):1746–56. doi: 10.1053/j.gastro.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 64.Coilly A, Dumortier J, Botta-Fridlund D, et al. Multicenter Experience with Boceprevir or Telaprevir to Treat Hepatitis C Recurrence after Liver Transplantation: When Present Becomes Past, What Lessons for Future? PLoS One. 2015;10(9):e0138091. doi: 10.1371/journal.pone.0138091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Charlton M, Gane E, Manns MP, et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology. 2015;148(1):108–17. doi: 10.1053/j.gastro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Forns X, Charlton M, Denning J, et al. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C after liver transplantation. Hepatology. 2015;61(5):1485–94. doi: 10.1002/hep.27681. [DOI] [PubMed] [Google Scholar]
- 67.Sulkowski MS, Eron JJ, Wyles D, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313(12):1223–31. doi: 10.1001/jama.2015.1328. [DOI] [PubMed] [Google Scholar]
- 68.Reddy KR, Bourliere M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology. 2015;62(1):79–86. doi: 10.1002/hep.27826. [DOI] [PubMed] [Google Scholar]
- 69.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–40. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 70.Coilly A, Fougerou C, De Ledinghen V, et al. The association of sofosbuvir and daclatasvir for treating severe recurrence of HCV infection after liver translplantation; results from a large French prospective multicentric ANRS CO23 CUPILT cohort. J Hepatol. 2015;62(S2):S235. [Google Scholar]
- 71.Faisal N, Renner EL, Bilodeau M, et al. Impact of sofosbuvir-based regimens on renal function in liver transplant recipients: results of a multicenter study. Hepatology. 2015;62(S1):312A. [Google Scholar]
- 72.Ouwerkerk-Mahadevan S, Snoeys J, Peeters M, Beumont-Mauviel M, Simion A. Drug-Drug Interactions with the NS3/4A Protease Inhibitor Simeprevir. Clin Pharmacokinet. 2015 doi: 10.1007/s40262-015-0314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwo PY, Badshah MB. New hepatitis C virus therapies: drug classes and metabolism, drug interactions relevant in the transplant settings, drug options in decompensated cirrhosis, and drug options in end-stage renal disease. Curr Opin Organ Transplant. 2015;20(3):235–41. doi: 10.1097/MOT.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 74.Kwo PY, Mantry PS, Coakley E, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371(25):2375–82. doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 75.Kugelmas M, Osgood MJ, Trotter JF, et al. Hepatitis C virus therapy, hepatocyte drug metabolism, and risk for acute cellular rejection. Liver Transpl. 2003;9(11):1159–65. doi: 10.1053/jlts.2003.50233. [DOI] [PubMed] [Google Scholar]
- 76.Fafi-Kremer S, Fofana I, Soulier E, et al. Viral entry and escape from antibody-mediated neutralization influence hepatitis C virus reinfection in liver transplantation. J Exp Med. 2010;207(9):2019–31. doi: 10.1084/jem.20090766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fofana I, Fafi-Kremer S, Carolla P, et al. Mutations that alter use of hepatitis C virus cell entry factors mediate escape from neutralizing antibodies. Gastroenterology. 2012;143(1):223–33. e9. doi: 10.1053/j.gastro.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fofana I, Jilg N, Chung RT, Baumert TF. Entry inhibitors and future treatment of hepatitis C. Antiviral Res. 2014;104:136–42. doi: 10.1016/j.antiviral.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Gane EJ, Angus PW, Strasser S, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007;132(3):931–7. doi: 10.1053/j.gastro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 80.Crespo G, Marino Z, Navasa M, Forns X. Viral hepatitis in liver transplantation. Gastroenterology. 2012;142(6):1373–83. e1. doi: 10.1053/j.gastro.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 81.Takaki A, Yasunaka T, Yagi T. Molecular Mechanisms to Control Post-Transplantation Hepatitis B Recurrence. Int J Mol Sci. 2015;16(8):17494–513. doi: 10.3390/ijms160817494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao F, Fofana I, Thumann C, et al. Synergy of entry inhibitors with direct-acting antivirals uncovers novel combinations for prevention and treatment of hepatitis C. Gut. 2015;64(3):483–94. doi: 10.1136/gutjnl-2013-306155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davis GL, Nelson DR, Terrault N, et al. A randomized, open-label study to evaluate the safety and pharmacokinetics of human hepatitis C immune globulin (Civacir) in liver transplant recipients. Liver Transpl. 2005;11(8):941–9. doi: 10.1002/lt.20405. [DOI] [PubMed] [Google Scholar]
- 84.Terrault N, Satapathy SK, Therapondos G, et al. Prevention of Hepatitis C Virus (HCV) Recurrence with Peri-Transplant Hepatitis C Immune Globulin Combined with Pre-Transplant (Pre-LT) Antiviral Therapy (AVT) Hepatology. 2014;60(S1):206A. [Google Scholar]
- 85.Rowe IA, Tully DC, Armstrong MJ, et al. Effect of scavenger receptor BI antagonist ITX5061 in patients with hepatitis C virus infection undergoing liver transplantation. Liver Transpl. 2015 doi: 10.1002/lt.24349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Helle F, Vieyres G, Elkrief L, et al. Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J Virol. 2010;84(22):11905–15. doi: 10.1128/JVI.01548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.von Hahn T, Yoon JC, Alter H, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132(2):667–78. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 88.Zhang P, Zhong L, Struble EB, et al. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc Natl Acad Sci U S A. 2009;106(18):7537–41. doi: 10.1073/pnas.0902749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baumert TF, Fauvelle C, Chen DY, Lauer GM. A prophylactic hepatitis C virus vaccine: A distant peak still worth climbing. J Hepatol. 2014;61(1S):S34–S44. doi: 10.1016/j.jhep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Fauvelle C, Felmlee DJ, Crouchet E, et al. Apolipoprotein E Mediates Evasion From Hepatitis C Virus Neutralizing Antibodies. Gastroenterology. 2016;150(1):206–17. e4. doi: 10.1053/j.gastro.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 91.Bankwitz D, Steinmann E, Bitzegeio J, et al. Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol. 2010;84(11):5751–63. doi: 10.1128/JVI.02200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Owsianka AM, Tarr AW, Keck ZY, et al. Broadly neutralizing human monoclonal antibodies to the hepatitis C virus E2 glycoprotein. J Gen Virol. 2008;89(Pt 3):653–9. doi: 10.1099/vir.0.83386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J, Randall G, Higginbottom A, Monk P, Rice CM, McKeating JA. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J Virol. 2004;78(3):1448–55. doi: 10.1128/JVI.78.3.1448-1455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haberstroh A, Schnober EK, Zeisel MB, et al. Neutralizing host responses in hepatitis C virus infection target viral entry at postbinding steps and membrane fusion. Gastroenterology. 2008;135(5):1719–28. e1. doi: 10.1053/j.gastro.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 95.Kong L, Giang E, Nieusma T, et al. Hepatitis C virus E2 envelope glycoprotein core structure. Science. 2013;342(6162):1090–4. doi: 10.1126/science.1243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khan AG, Whidby J, Miller MT, et al. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature. 2014;509(7500):381–4. doi: 10.1038/nature13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vanwolleghem T, Bukh J, Meuleman P, et al. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology. 2008;47(6):1846–55. doi: 10.1002/hep.22244. [DOI] [PubMed] [Google Scholar]
- 98.Akazawa D, Moriyama M, Yokokawa H, et al. Neutralizing antibodies induced by cell culture-derived hepatitis C virus protect against infection in mice. Gastroenterology. 2013;145(2):447–55. e1–4. doi: 10.1053/j.gastro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 99.Meuleman P, Bukh J, Verhoye L, et al. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology. 2011;53(3):755–62. doi: 10.1002/hep.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Jong YP, Dorner M, Mommersteeg MC, et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med. 2014;6(254):254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Desombere I, Fafi-Kremer S, Van Houtte F, et al. Monoclonal anti-envelope antibody AP33 protects humanized mice against a patient-derived hepatitis C virus challenge. Hepatology. 2015 doi: 10.1002/hep.28428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perin PM, Haid S, Brown RJ, et al. Flunarizine prevents hepatitis C virus membrane fusion in a genotype-dependent manner by targeting the potential fusion peptide within E1. Hepatology. 2015 doi: 10.1002/hep.28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.St Vincent MR, Colpitts CC, Ustinov AV, et al. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc Natl Acad Sci U S A. 2010;107(40):17339–44. doi: 10.1073/pnas.1010026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin LT, Chung CY, Hsu WC, et al. Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J Hepatol. 2015;62(3):541–8. doi: 10.1016/j.jhep.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 105.He S, Lin B, Chu V, et al. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci Transl Med. 2015;7(282):282ra49. doi: 10.1126/scitranslmed.3010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeisel MB, Lupberger J, Fofana I, Baumert TF. Host-targeting agents for prevention and treatment of chronic hepatitis C - perspectives and challenges. J Hepatol. 2013;58(2):375–84. doi: 10.1016/j.jhep.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 107.Fofana I, Krieger SE, Grunert F, et al. Monoclonal anti-claudin 1 antibodies prevent hepatitis C virus infection of primary human hepatocytes. Gastroenterology. 2010;139(3):953–64. 64 e1–4. doi: 10.1053/j.gastro.2010.05.073. [DOI] [PubMed] [Google Scholar]
- 108.Xiao F, Fofana I, Heydmann L, et al. Hepatitis C virus cell-cell transmission and resistance to direct-acting antiviral agents. PLoS Pathog. 2014;10(5):e1004128. doi: 10.1371/journal.ppat.1004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeisel MB, Felmlee DJ, Baumert TF. Hepatitis C virus entry. Curr Top Microbiol Immunol. 2013;369:87–112. doi: 10.1007/978-3-642-27340-7_4. [DOI] [PubMed] [Google Scholar]
- 110.Andre P, Komurian-Pradel F, Deforges S, et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76(14):6919–28. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Felmlee DJ, Sheridan DA, Bridge SH, et al. Intravascular transfer contributes to postprandial increase in numbers of very-low-density hepatitis C virus particles. Gastroenterology. 2010;139(5):1774–83. 83 e1–6. doi: 10.1053/j.gastro.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 112.Jiang J, Cun W, Wu X, Shi Q, Tang H, Luo G. Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate. J Virol. 2012;86(13):7256–67. doi: 10.1128/JVI.07222-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ciesek S, von Hahn T, Colpitts CC, et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology. 2011;54(6):1947–55. doi: 10.1002/hep.24610. [DOI] [PubMed] [Google Scholar]
- 114.Colpitts CC, Schang LM. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J Virol. 2014;88(14):7806–17. doi: 10.1128/JVI.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Shea D, Law J, Egli A, et al. Prevention of HCV infection using a broad cross-neutralizing monoclonal antibody (AR4A) and Epigallocatechin-Gallate. Liver Transpl. 2015 doi: 10.1002/lt.24344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vercauteren K, Brown RJ, Mesalam AA, et al. Targeting a host-cell entry factor barricades antiviral-resistant HCV variants from on-therapy breakthrough in human-liver mice. Gut. 2015 doi: 10.1136/gutjnl-2014-309045. [DOI] [PubMed] [Google Scholar]
- 117.Zahid MN, Turek M, Xiao F, et al. The postbinding activity of scavenger receptor class B type I mediates initiation of hepatitis C virus infection and viral dissemination. Hepatology. 2013;57(2):492–504. doi: 10.1002/hep.26097. [DOI] [PubMed] [Google Scholar]
- 118.Lupberger J, Zeisel MB, Xiao F, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17(5):589–95. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zona L, Lupberger J, Sidahmed-Adrar N, et al. HRas signal transduction promotes hepatitis C virus cell entry by triggering assembly of the host tetraspanin receptor complex. Cell Host Microbe. 2013;13(3):302–13. doi: 10.1016/j.chom.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 120.Ji C, Liu Y, Pamulapati C, et al. Prevention of hepatitis C virus infection and spread in human liver chimeric mice by an anti-CD81 monoclonal antibody. Hepatology. 2014;61(4):1136–44. doi: 10.1002/hep.27603. [DOI] [PubMed] [Google Scholar]
- 121.Yamashita M, Iida M, Tada M, et al. Discovery of Anti-Claudin-1 Antibodies as Candidate Therapeutics against Hepatitis C Virus. J Pharmacol Exp Ther. 2015;353(1):112–8. doi: 10.1124/jpet.114.217653. [DOI] [PubMed] [Google Scholar]
- 122.Fukasawa M, Nagase S, Shirasago Y, et al. Monoclonal antibodies against extracellular domains of claudin-1 block hepatitis C virus infection in a mouse model. J Virol. 2015;89(9):4866–79. doi: 10.1128/JVI.03676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ploss A, Evans MJ, Gaysinskaya VA, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457(7231):882–6. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benedicto I, Molina-Jimenez F, Bartosch B, et al. The tight junction-associated protein occludin is required for a postbinding step in hepatitis C virus entry and infection. J Virol. 2009;83(16):8012–20. doi: 10.1128/JVI.00038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sainz B, Jr, Barretto N, Martin DN, et al. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med. 2012;18(2):281–5. doi: 10.1038/nm.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin DN, Uprichard SL. Identification of transferrin receptor 1 as a hepatitis C virus entry factor. Proc Natl Acad Sci U S A. 2013;110(26):10777–82. doi: 10.1073/pnas.1301764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gerold G, Meissner F, Bruening J, et al. Quantitative Proteomics Identifies Serum Response Factor Binding Protein 1 as a Host Factor for Hepatitis C Virus Entry. Cell Rep. 2015;12(5):864–78. doi: 10.1016/j.celrep.2015.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uprichard SL, Sainz B., Jr Inhibition of hepatitis C entry: too soon to dismiss while many are still being denied treatment. Gut. 2015;64(4):690–1. doi: 10.1136/gutjnl-2014-308396. [DOI] [PubMed] [Google Scholar]
- 129.Pawlotsky JM. Viral entry inhibition: too late for hepatitis C, but promising for other viral infections. Gut. 2015;64(3):362–4. doi: 10.1136/gutjnl-2014-307452. [DOI] [PubMed] [Google Scholar]
- 130.Ploss A, Dubuisson J. New advances in the molecular biology of hepatitis C virus infection: towards the identification of new treatment targets. Gut. 2012;61(Suppl 1):i25–35. doi: 10.1136/gutjnl-2012-302048. [DOI] [PubMed] [Google Scholar]
- 131.Zhu H, Wong-Staal F, Lee H, et al. Evaluation of ITX 5061, a scavenger receptor B1 antagonist: resistance selection and activity in combination with other hepatitis C virus antivirals. J Infect Dis. 2012;205(4):656–62. doi: 10.1093/infdis/jir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haid S, Grethe C, Dill MT, Heim M, Kaderali L, Pietschmann T. Isolate-dependent use of claudins for cell entry by hepatitis C virus. Hepatology. 2014;59(1):24–34. doi: 10.1002/hep.26567. [DOI] [PubMed] [Google Scholar]
- 133.Hopcraft SE, Evans MJ. Selection of a hepatitis C virus with altered entry factor requirements reveals a genetic interaction between the E1 glycoprotein and claudins. Hepatology. 2015;62(4):1059–69. doi: 10.1002/hep.27815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Colpitts CC, Zeisel MB, Baumert TF. When one receptor closes, another opens: Claudins and the hepatitis C virus E1 glycoprotein. Hepatology. 2015;62(4):991–3. doi: 10.1002/hep.27876. [DOI] [PubMed] [Google Scholar]
- 135.Manns MP, von Hahn T. Novel therapies for hepatitis C - one pill fits all? Nat Rev Drug Discov. 2013;12(8):595–610. doi: 10.1038/nrd4050. [DOI] [PubMed] [Google Scholar]
- 136.Bandiera S, Pfeffer S, Baumert TF, Zeisel MB. miR-122--a key factor and therapeutic target in liver disease. J Hepatol. 2015;62(2):448–57. doi: 10.1016/j.jhep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 137.Lin K, Gallay P. Curing a viral infection by targeting the host: the example of cyclophilin inhibitors. Antiviral Res. 2013;99(1):68–77. doi: 10.1016/j.antiviral.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Masaki T, Arend KC, Li Y, et al. miR-122 stimulates hepatitis C virus RNA synthesis by altering the balance of viral RNAs engaged in replication versus translation. Cell Host Microbe. 2015;17(2):217–28. doi: 10.1016/j.chom.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327(5962):198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van Der Ree M, de Vree ML, Stelma F, et al. A single subcutaneous dose of 2 mg/kg or 4 mg/kg of RG-101, a GalNAc-conjugated oligonucleotide with antagonist activity against miR-122, results in significatnt viral load reductions in chronic hepatitis c patients. J Hepatol. 2015;62(S2):S261. [Google Scholar]
- 141.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 142.Guedj J, Yu J, Levi M, et al. Modeling viral kinetics and treatment outcome during alisporivir interferon-free treatment in hepatitis C virus genotype 2 and 3 patients. Hepatology. 2014;59(5):1706–14. doi: 10.1002/hep.26989. [DOI] [PubMed] [Google Scholar]
- 143.Pungpapong S, Werner KT, Aqel B, et al. Multicenter Experience using Sofosbuvir and Simeprevir with/without Rivavirin to Treat HCV Genotype 1 after Liver Transplantation. Hepatology. 2014;60(S1):201A. [Google Scholar]
- 144.Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. Effectiveness of Simeprevir Plus Sofosbuvir, With or Without Ribavirin, in Real-World Patients With HCV Genotype 1 Infection. Gastroenterology. 2016;150(2):419–29. doi: 10.1053/j.gastro.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rowe IAM, Parker R, Guo K, Barton D, McKelvy J, Wong-Staal F, Adams D, McKeating J, Mutimer D. Effect of scavenger receptor B-I antagonist, ITX5061, on early hepatitis C virus kinetics in liver transplantation; results of a phase 1b clinical trial. Lancet. 2014 Feb;:S90. [Google Scholar]