Abstract
Objective
To determine the association between prescription drug monitoring program (PDMP) implementation and emergency department (ED) visits involving opioid analgesics.
Methods
Rates of ED visits involving opioid analgesics per 100,000 residents were estimated from the Drug Abuse Warning Network dataset for 11 geographically diverse metropolitan areas in the United States on a quarterly basis from 2004 to 2011. Generalized estimating equations assessed whether implementation of a prescriber-accessible PDMP was associated with a difference in ED visits involving opioid analgesics. Models were adjusted for calendar quarter, metropolitan area, metropolitan area-specific linear time trends, and unemployment rate.
Results
Rates of ED visits involving opioid analgesics increased in all metropolitan areas. PDMP implementation was not associated with a difference in ED visits involving opioid analgesics (mean difference of 0.8 visits [95% CI: −3.7 to 5.2] per 100,000 residents per quarter).
Conclusions
During 2004–2011, PDMP implementation was not associated with a change in opioid-related morbidity, as measured by emergency department visits involving opioid analgesics. Urgent investigation is needed to determine the optimal PDMP structure and capabilities to improve opioid analgesic safety.
Keywords: Prescription drug monitoring programs, Prescription drug abuse, Opioids, Emergency departments
1. Introduction
In the last two decades, the United States has experienced an epidemic of prescription opioid analgesic use, abuse, addiction, and overdose. From 1999 to 2011, the number of opioid prescriptions in the United States nearly doubled (Volkow, 2014), and overdose deaths involving opioid analgesics quadrupled from 4030 to 16,917 deaths annually (National Center for Health Statistics, 2014a). By 2011, more than 12 million Americans reported using opioid analgesics non-medically (i.e., without a prescription, at higher-than-prescribed doses, or for purposes other than treating pain) and over 488,000 emergency department (ED) visits involved misuse or abuse (henceforth “misuse”) of opioid analgesics (Substance Abuse and Mental Health Services Administration, 2012b, 2013).
Prescription drug monitoring programs (PDMPs) are databases of controlled substance prescriptions that were filled at a pharmacy. In the United States, PDMPs have been developed as a tool to improve safety of prescribed opioid analgesics, identify and decrease diversion of opioid analgesics, and reduce fatal and non-fatal opioid overdose (Centers for Disease Control and Prevention, 2013). From 1989 to 2015, the number of states with an operational PDMP increased from 9 to 49 (Clark et al., 2012; Prescription Drug Monitoring Program Training and Technical Assistance Center, 2015b). In addition to broad expansion, PDMP usage patterns have also changed. Early PDMPs often provided data only to law enforcement officials, but by January 2015 nearly all PDMPs (96%) had adopted regulations to provide data access to prescribers (National Alliance for Model State Drug Laws, 2014b).
The impact of PDMPs on prescribing behavior and opioid safety is unclear. Physician surveys and interviews suggest that PDMP reports may encourage physicians to change their prescribing behaviors, such as reducing the quantity of drug prescribed, forgoing the analgesic prescription or changing it to a non-scheduled (e.g., non-opioid) analgesic, or screening patients for drug abuse before issuing a prescription (Alliance of States with Prescription Monitoring Programs, 2007; Baehren et al., 2010; Feldman et al., 2012; Green et al., 2012; Irvine et al., 2014; Kentucky Cabinet for Health and Family Services and Kentucky Injury Prevention and Research Center, 2010; Perrone et al., 2012; Smith et al., 2015; Weiner et al., 2013). However, on a population level, evidence about the impact of PDMPs is mixed (Haegerich et al., 2014). Studies of PDMPs and state-level opioid consumption have yielded conflicting results; one study found PDMP implementation to be associated with a decrease in opioid consumption (Reisman et al., 2009), while others found no change (Brady et al., 2014) or an increase (Ringwalt et al., 2015). Other studies have associated PDMPs with a decrease in consumption of Schedule II opioid analgesics and a corresponding increase in Schedule III analgesics, suggesting a possible substitution effect (Paulozzi et al., 2011; Simeone and Holland, 2006; Simoni-Wastila and Qian, 2012). PDMPs may be associated with improved opioid safety, as measured by a slower rate of growth of poison center calls involving intentional exposures to opioid analgesics in states with PDMPs (Reifler et al., 2012); however, PDMP implementation was not associated with decreases in state-level opioid overdose mortality (Paulozzi et al., 2011).
While PDMPs have not been associated with lower rates of fatal opioid analgesic overdoses (Paulozzi et al., 2011), nonfatal complications from opioid use are more common (Centers for Disease Control and Prevention, 2015, 2010; Darke et al., 2003; Neale, 2003). These events may necessitate emergency care, and therefore ED visits may be a more sensitive measure of opioid safety than fatal overdoses (Substance Abuse and Mental Health Services Administration, 2012a). To further characterize the association between PDMP implementation and ED visits involving opioids, we conducted a retrospective study using data from 11 metropolitan areas in the United States. We hypothesized that state implementation of a prescriber-accessible PDMP would be associated with lower rates of ED visits involving opioid analgesics.
2. Methods
2.1. Study design and data sources
In this retrospective study, we used the Drug Abuse Warning Network (DAWN) public use files to estimate the rate of ED visits involving opioid analgesics in 11 US metropolitan areas from 2004 to 2011. DAWN is a longitudinal public health surveillance program administered by the Substance Abuse and Mental Health Services Administration to identify all ED visits in which illicit or prescription drugs were a cause or contributing factor (Center for Behavioral Health Statistics and Quality, 2013). DAWN data are collected by trained coders through chart review of medical records from a representative sample of non-federal, short stay hospitals. The survey produces nationally representative estimates of ED visits involving drugs, as well as regional estimates for 11 metropolitan areas (Metropolitan Statistical Areas) in which sufficient data are collected to produce statistically reliable results (Center for Behavioral Health Statistics and Quality, 2013). Because metropolitan areas can cross state boundaries, the 11 metropolitan areas include populations in 14 states. The metropolitan areas and their respective states are: Boston (Massachusetts, New Hampshire), Chicago (Illinois, Wisconsin, Indiana), Denver (Colorado), Detroit (Michigan), Houston (Texas), Miami-Dade County (Florida), Minneapolis-St. Paul (Minnesota, Wisconsin), New York City (New York), Phoenix (Arizona), San Francisco (California), and Seattle (Washington).
To determine whether an ED visit was related to drug use, DAWN coders review the medical record for chief complaint, clinician documentation, and final diagnosis. The drug use must be implicated as a direct or indirect cause of the visit for DAWN coders to record the visit. If a drug is incidentally noted to be “on board” (e.g., such as on a urine drug screen) but the ED visit is for an unrelated reason (e.g., appendicitis), the visit is not included in the DAWN results (Center for Behavioral Health Statistics and Quality, 2013). The DAWN classification system further stratifies these ED visits into two subsets. The first subset includes ED visits with an indication of drug misuse, such as patients who use medications prescribed for another person, use a higher-than-prescribed dose, or use opioids for reasons other than treating pain (Center for Behavioral Health Statistics and Quality, 2013). This category also includes drug-related suicide attempts or requests for detoxification services. The second subset includes ED visits with no indication of misuse, such as accidental ingestions, side effects, or allergic reactions.
2.2. Outcome measures
The primary outcome was the rate of ED visits involving opioid analgesics per quarter, per 100,000 metropolitan area residents. This outcome included ED visits classified as involving misuse and those classified as not involving drug misuse. To calculate this outcome, we used DAWN data to estimate the total number of ED visits involving opioid analgesics (Schedules II through V) for each metropolitan area in each calendar quarter (3-month block) during the study period, then divided this visit total by metropolitan area population estimates from the National Center for Health Statistics (National Center for Health Statistics, 2014b,c). Several secondary outcomes were examined: rates of ED visits involving opioid analgesics in three age strata (18–34 years, 35–54 years, and 55 years or greater); rates of ED visits involving misuse of opioid analgesics; the rate of ED visits involving Schedule II opioid analgesics, and the rate of ED visits involving Schedule II opioid analgesic misuse. We examined these age strata because prior research demonstrated that deaths rates from opioid analgesics were highest among individuals aged 35–54 (Centers for Disease Control and Prevention, 2013). We measured Schedule II opioid analgesics separately because they were monitored by all PDMPs throughout the study period, whereas drugs in Schedules III and IV were not monitored by all PDMPs in all study calendar quarters.
2.3. Exposure of interest
The presence of a prescriber-accessible PDMP was our primary exposure of interest. For this analysis, the first date on which a prescriber-accessible PDMP was present in a state is considered the PDMP implementation date. To determine the PDMP implementation date for each state, we first reviewed online data from the National Alliance for State Model Drug Laws (National Alliance for Model State Drug Laws, 2014a) and the University of Kentucky (Blumenschein et al., 2010), and then contacted PDMP administrators in all 14 states for verification. For each state, we classified a PDMP as present for all calendar quarters that included or followed the PDMP implementation date; if a PDMP was present in any part of a quarter, we counted it as present for the entire quarter.
To account for the fact that metropolitan areas can be composed of counties from several states, the presence of a PDMP in each metropolitan area was coded to reflect the proportion of the population residing in a state with a PDMP present. For example, in the first quarter of 2011, Massachusetts had a provider-accessible PDMP, but New Hampshire did not. Because 91% of the population in the Boston metropolitan area resides in Massachusetts, the value of the PDMP variable in this quarter for this metropolitan area was 0.91.
2.4. Statistical analysis
To investigate the association between PDMP implementation and changes in ED visits involving opioids, we first examined the unadjusted ED visit rates in two ways. First, we grouped each of the 11 metropolitan areas by year of PDMP implementation: prior to 2004, 2008–2009, 2010–2011, or after 2011 (no PDMPs were implemented in this cohort of states during 2004–2007). To do this, we used the implementation year of the PDMP in the state that contributed the majority of the population to a given metropolitan area (e.g., Illinois in the case of the Chicago metropolitan area). These results were graphed for visual comparison. Second, we centered metropolitan areas on the date of PDMP implementation and determined the mean ED visit rate in the calendar quarters before and after program implementation. Only metropolitan areas that implemented PDMPs during 2004–2011 (Boston, New York City, Chicago, Minneapolis, Denver, Phoenix, and San Francisco) were included in this analysis. These results were also graphed for visual inspection. If PDMPs had an impact on ED visits, this analysis would potentially reveal a change in the trend after PDMP implementation.
Next, to determine the association between PDMP implementation and our outcomes of interest while adjusting for other covariates, we developed linear regression models. We used a generalized estimating equations framework with a first-order autoregressive (AR1) working covariance matrix to account for repeated measures within metropolitan areas over time. In each regression model, the main independent variable was the presence of a prescriber-accessible PDMP. The coefficient of this variable represented the mean quarterly difference in ED visit rates associated with presence of the PDMP for the full population of a metropolitan area.
In addition to the main independent variable, we adjusted for calendar quarter (to adjust for time trends common to all metropolitan areas) and metropolitan area (to adjust for time-invariant differences between metropolitan areas), as well as an interaction term between quarter (as a continuous variable) and metropolitan area, to allow for differential effects of time in each metropolitan area. Quarterly metropolitan area unemployment rate (Bureau of Labor Statistics, 2015) was also included as a covariate because prior studies have reported higher rates of prescription drug abuse during periods of unemployment (Henkel, 2011; Merline et al., 2004; Spiller et al., 2009). Log transformations of the outcome variables were explored but were not deemed necessary based on graphical assessments of the distributions. To incorporate uncertainty around DAWN estimates of ED visits, we weighted our regression models by the inverse of the variance of the estimated ED visit rate in each metropolitan area in each quarter (French and Heagerty, 2008). All analyses were conducted with SAS 9.4 (SAS Institute, Cary, NC) and STATA 13.1 (College Station, TX) using procedures to account for the complex design of DAWN. The University of Pennsylvania Institutional Review Board reviewed the study protocol and determined it to be exempt.
3. Results
3.1. PDMP implementation
Of the 11 metropolitan areas in our sample, Detroit was the first location in a state with a prescriber-accessible PDMP (2003). Between 2008 and 2009, PDMPs were implemented in states that contained the majority of residents in the Phoenix, San Francisco, Denver, and Chicago metropolitan areas. Following this, PDMPs were implemented in states that contained the majority of residents in the Boston, Miami, Minneapolis, and New York City metropolitan areas between 2010 and 2011. Residents of Seattle and Houston were not covered by a PDMP until after 2011.
3.2. Unadjusted rates of ED visits
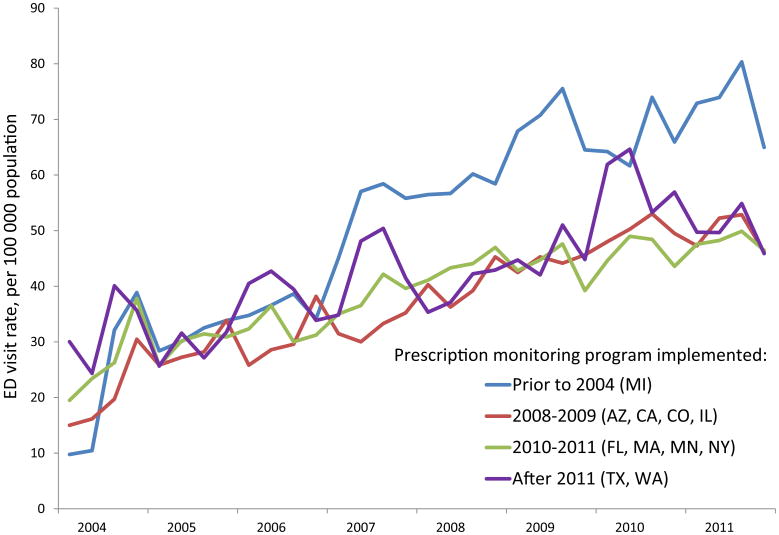
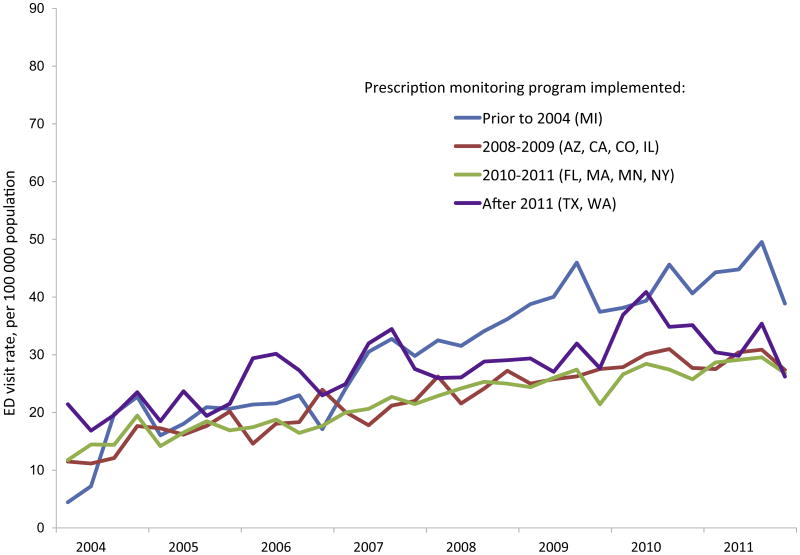
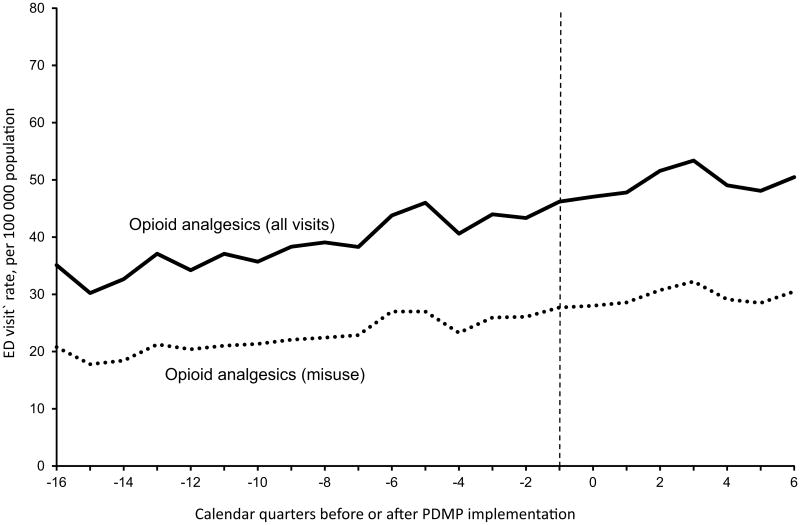
During the study period, unadjusted rates of ED visits involving opioid analgesics generally increased in all metropolitan areas, and the increase was similar when grouped by year of PDMP implementation (Fig. 1). Rates of ED visits involving opioid analgesic misuse grew in all areas (Fig. 2), as did the rates of ED visits involving Schedule II opioids and ED visits involving Schedule II opioid misuse (Supplementary Figs. 1 and 2). When centered on the date of PDMP implementation, unadjusted trends were similar before and after PDMP implementation (Fig. 3 and Supplementary Fig. 3).
Fig. 1.
Emergency department visits involving opioid analgesics in 11 metropolitan areas, grouped by year of prescription drug monitoring program implementation.
Fig. 2.
Emergency department visits involving opioid analgesic misuse in 11 metropolitan areas, grouped by year of prescription drug monitoring program implementation.
Fig. 3.
Emergency department visits involving opioid analgesic use and misuse, centered on date of prescription drug monitoring program implementation.a
aFigure includes only metropolitan areas with data pre- and post-implementation: Boston, New York City, Chicago, Minneapolis, Denver, Phoenix, and San Francisco.
3.3. Regression models
In regression models, PDMP implementation was not associated with a difference in the rate of ED visits involving opioid analgesics (mean quarterly difference: 0.8 [95% CI: −3.7 to 5.2] visits per 100,000 population; p = 0.74; Table 1). PDMP implementation was also not associated with a significant difference in ED visits involving opioid analgesics within any of the three age strata, ED visits involving opioid analgesic misuse, ED visits involving Schedule II opioids, or ED visits involving Schedule II opioid misuse.
Table 1.
Association between prescription drug monitoring program implementation and rates of emergency department (ED) visits involving opioids in 11 metropolitan areas, 2004–2011.
| Drug causing ED visit | Mean absolute difference in quarterly ED visit rate per 100,000 population | ||
|---|---|---|---|
|
| |||
| Estimate | 95% CI | p | |
| Opioid analgesic (all visits) | 0.8 | (−3.7 to 5.2) | 0.74 |
| By patient age: | |||
| 18–34 years | 1.3 | (−3.9 to 6.5) | 0.63 |
| 35–54 years | 1.8 | (−7.0 to 10.6) | 0.69 |
| ≥55 years | 0.4 | (−0.6 to 1.3) | 0.49 |
| Opioid analgesic (visits for misuse or abuse) | 0.8 | (−1.9 to 3.4) | 0.57 |
| Schedule II opioid analgesic (all visits) | −1.8 | (−3.9 to 0.3) | 0.09 |
| Schedule II opioid analgesic (visits for misuse or abuse) | −1.1 | (−2.9 to 0.8) | 0.26 |
4. Discussion
This retrospective study is the first analysis to examine the association between PDMP implementation and ED visits involving opioids. We found that PDMP implementation in 11 metropolitan areas in the United States was not associated with a significant difference in the rates of ED visits involving opioid analgesics during 2004–2011.
4.1. PDMPs and prescription opioids
This study expands the limited and conflicting evidence base about the impact of PDMPs on opioid safety. Paulozzi et al. (2011) found that states with PDMPs did not have significantly different total opioid consumption as compared to states without PDMPs, although states with PDMPs appeared to use significantly more Schedule III opioids (e.g., hydrocodone) and nonsignificantly fewer opioids from Schedule II (e.g., oxycodone). Simeone and Holland (2006) found that states with proactive PDMPs (i.e., programs that sent unsolicited reports to prescribers) had lower rates of addiction treatment admissions involving Schedule II opioids during 1997–2003 than did states with non-proactive PDMPs; the analysis did not examine schedule III–V opioids.
Reifler et al. (2012) found that compared to states without PDMPs, states with PDMPs during 2003–2009 had significantly fewer intentional oxycodone exposures reported to poison control centers and nonsignificantly lower rates of individuals seeking care at opioid treatment centers, suggesting a possible improvement in opioid safety. However, no significant association was found between PDMPs and opioid overdose mortality rates during 1999–2005, even among proactive PDMPs and those with high rates of reporting (Paulozzi et al., 2011). Data from Florida suggest that recently implemented state policies may be associated with substantial declines in opioid-associated mortality (Johnson et al., 2014; Rutkow et al., 2015a), but the role of the state PDMP in this decline has not been disentangled from other regulations and enforcement efforts that were launched during the same period. In summary, research to date has not produced consistent evidence that PDMPs have substantially improved opioid safety. Our study builds upon these findings by being first to examine emergency department visits involving opioids. Emergency department visits are a more frequent and thus a potentially more sensitive measure of opioid safety than overdose mortality.
There are several potential explanations for our negative findings. First, utilization of PDMPs is highly variable among different states (Alliance of States with Prescription Monitoring Programs, 2007; Green et al., 2012; Ringwalt et al., 2015), and a PDMP may have little impact if few prescribers use it. For example, from January, 2010 through June, 2015, California registered 27,052 practitioners in its PDMP, representing 26% of active licensed physicians in the state as of 2015 (personal communication with PDMP staff; Henry and Kaiser Family Foundation, 2015); only 5136 of these individuals registered during the two years (2010–2011) included in this study. Similarly, the Minnesota PDMP experienced greater than 300% growth in registrants using the system during 2010–2014, yet by the end of this period only 36% of licensed physicians and 21% of licensed dentists in the state were registered for the PDMP (Minnesota Board of Pharmacy, 2015). A 2014 nationwide survey of primary care physicians found that only 53% of participants reported using their state's PDMP (Rutkow et al., 2015b). During 2004–2011, none of the states in this study mandated provider registration or utilization of the PDMP prior to prescribing (Clark et al., 2012). Without these provisions, prescriber uptake of PDMPs may be slow.
Second, even if utilization was high in some metropolitan areas, participation may not ensure PDMP effectiveness. Poor effectiveness might result if there was a substantial delay in pharmacies' data reporting to the PDMP or if individuals used pharmacies in nearby non-PDMP states. Another possibility is that PDMPs may improve opioid safety for some individuals, such as those seeking prescriptions from multiple providers, but this improvement among a small group may not produce a significant change in ED visits on a population scale. For example, PDMPs may reduce the number of prescribers and pharmacies used by individuals who are identified as “doctor-shoppers” or “pharmacy shoppers” (Gonzalez and Kolbasovsky, 2012; PDMP Center of Excellence, 2010, 2011; Pradel et al., 2009; United States General Accounting Office, 2002; Virginia Department of Health Professions, 2010); however, approximately 60% of prescription opioid overdoses occur among patients with a legitimate opioid analgesic prescription from a single provider (Centers for Disease Control and Prevention, 2012), and PDMPs would not necessarily improve prescribing safety for these individuals. Efforts to improve opioid safety should focus not only on “doctor shopping” behavior, but also on other high-risk behaviors such as using high daily doses of opioid analgesics (Bohnert et al., 2011; Dunn et al., 2010; Gomes et al., 2011; Paulozzi et al., 2012; Zedler et al., 2014), use of long-acting or extended-release opioid analgesics (Dhalla et al., 2009; Webster et al., 2011; Zedler et al., 2014), continuous long-term use of opioid analgesics (Paulozzi et al., 2014), or concurrent use of benzodiazepines (Centers for Disease Control and Prevention, 2013; Dunn et al., 2010; Jann et al., 2014; Webster et al., 2011; Zedler et al., 2014).
4.2. Limitations
This study has several limitations. This is an ecological study and therefore we cannot determine the association between PDMP implementation and outcomes for individual state residents. We examined a small number of metropolitan areas over a relatively short period of time, and the results may not be generalizable to other metropolitan areas, to PDMPs that vary substantially in form or content from the ones that we studied, or to effects of PDMPs since 2011. Furthermore, given the relatively small number of PDMPs studied, we were unable to examine the impact of specific features of PDMPs (e.g., longer versus shorter data collection intervals, mandatory registration or utilization, and use of unsolicited reporting).
Another limitation is that we were unable to include measures of PDMP utilization in our models, as utilization data for 2004–2011 were not available for all PDMPs we examined. The PDMPs in our sample provide access to physician assistants and nurse practitioners as of 2015, but we were unable to determine whether all programs provided access to these or other non-physician prescribers (e.g., dentists) during the study period (Prescription Drug Monitoring Program Training and Technical Assistance Center, 2015a). Several states (California, Illinois, Indiana, Massachusetts, New York, and Texas) had PDMPs that provided data to law enforcement officials or licensing boards before prescribers were permitted to access them, which may have diminished the marginal effect of allowing providers access to the PDMP. However, many of these law enforcement-only PDMPs were infrequently used during this period (National Alliance for Model State Drug Laws, 2006a,b,c). Furthermore, DAWN data rely on the accuracy of the medical record. Finally, although we adjusted for several factors in our regression models, there may be residual confounding.
4.3. Conclusions
In summary, implementation of a prescriber-accessible PDMP was not associated with a significant difference in rates of ED visits involving opioids during 2004–2011 in this retrospective analysis of data from 11 metropolitan areas in the United States. As low utilization of PDMPs or incomplete prescription data could explain these findings, states should examine ways to improve PDMP quality and utilization by providers, such as by integrating PDMP functions with clinical information systems, using unsolicited reports to inform providers of worrisome prescribing patterns among their patients, or mandating that prescribers access PDMPs prior to writing certain types of prescriptions. Urgent investigation is needed to examine the impact of different PDMP structures and capabilities on opioid safety and prescriber behavior.
Supplementary Material
Acknowledgments
Author disclosures: This study was funded by NIH K23DA027719 (Dr. Starrels). Drs. Maughan and Bachhuber received additional support from the Leonard Davis Institute of Health Economics, the United States Department of Veterans Affairs, and the Robert Wood Johnson Foundation Clinical Scholars Program at the University of Pennsylvania. The funding bodies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Author contributions: BCM, MAB, and NM developed the original study concept. MAB and BCM acquired the data, and all authors contributed to analysis and interpretation of the data. BCM and MAB drafted the initial manuscript, and NM and JLS contributed critical revision of the manuscript for important intellectual content. NM offered statistical expertise. JLS supervised the study. All authors reviewed and approved the final manuscript.
Conflicts of interest: None.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2015.09.024.
Contributor Information
Brandon C. Maughan, Email: maughan@gmail.com.
Marcus A. Bachhuber, Email: marcus.bachhuber@gmail.com.
Nandita Mitra, Email: nanditam@mail.med.upenn.edu.
Joanna L. Starrels, Email: jostarre@montefiore.org.
References
- Alliance of States with Prescription Monitoring Programs. An Assessment of State Prescription Monitoring Program Effectiveness and Results. [accessed 07.03.15];2007 Available at http://www.pdmpexcellence.org/pdfs/alliance_pmp_rpt2_1107.pdf.
- Baehren D, Marco C, Droz D, Sinha S, Callan E, Akpunonu P. A statewide prescription monitoring program affects emergency department prescribing behaviors. Ann Emerg Med. 2010;56:e11–e13. 19–23. doi: 10.1016/j.annemergmed.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Blumenschein K, Fink J, Freeman P, James K, Kirsh K, Steinke D, Talbert J. Review of Prescription Drug Monitoring Programs in the United States. [accessed 07.03.15];2010 Available at http://chfs.ky.gov/NR/rdonlyres/85989824-1030-4AA6-91E1-7F9E3EF68827/0/KASPEREvaluationPDMPStatusFinalReport6242010.pdf.
- Bohnert A, Valenstein M, Bair M, Ganoczy D, McCarthy J, Ilgen M, Blow F. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- Brady J, Wunsch H, DiMaggio C, Lang B, Giglio J, Li G. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep. 2014;129:139–147. doi: 10.1177/003335491412900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. Local Area Unemployment Statistics. [accessed 07.03.15];2015 Available at http://www.bls.gov/data/
- Center for Behavioral Health Statistics and Quality. Drug Abuse Warning Network Methodology Report, 2011 Update. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [Google Scholar]
- Centers for Disease Control and Prevention. Emergency department visits involving nonmedical use of selected prescription drugs – United States, 2004–2008. MMWR. 2010;59:705–709. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses – a U.S. epidemic. MMWR. 2012;61:10–13. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers and other drugs among women – United States, 1999–2010. MMWR. 2013;62:537–542. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prescription Drug Overdose in the United States: Fact Sheet. [accessed 07.03.15];2015 Available at http://www.cdc.gov/homeandrecreationalsafety/overdose/facts.html.
- Clark T, Eadie J, Kreiner P, Strickler G. Prescription Drug Monitoring Programs: An Assessment of the Evidence for Best Practices. [accessed 07.03.15];2012 Available at http://www.pdmpexcellence.org/sites/all/pdfs/Brandeis_PDMP_Report_final.pdf.
- Darke S, Mattick R, Degenhardt L. The ratio of non-fatal to fatal heroin overdose. Addiction. 2003;98:1169–1171. doi: 10.1046/j.1360-0443.2003.00474.x. [DOI] [PubMed] [Google Scholar]
- Dhalla I, Mamdani M, Sivilotti M, Kopp A, Qureshi O, Juurlink D. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ. 2009;181:891–896. doi: 10.1503/cmaj.090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K, Saunders K, Rutter C, Banta-Green C, Merrill J, Sullivan M, Weisner C, Silverberg M, Campbell C, Psaty B, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L, Skeel Williams K, Knox M, Coates J. Influencing controlled substance prescribing: attending and resident physician use of a state prescription monitoring program. Pain Med. 2012;13:908–914. doi: 10.1111/j.1526-4637.2012.01412.x. [DOI] [PubMed] [Google Scholar]
- French B, Heagerty P. Analysis of longitudinal data to evaluate a policy change. Stat Med. 2008;27:5005–5025. doi: 10.1002/sim.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T, Mamdani M, Dhalla I, Paterson J, Juurlink D. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Kolbasovsky A. Impact of a managed controlled-opioid prescription monitoring program on care coordination. Am J Manag Care. 2012;18:516–524. [PubMed] [Google Scholar]
- Green T, Mann M, Bowman S, Zaller N, Soto X, Gadea J, Cordy C, Kelly P, Friedmann P. How does use of a prescription monitoring program change medical practice? Pain Med. 2012;13:1314–1323. doi: 10.1111/j.1526-4637.2012.01452.x. [DOI] [PubMed] [Google Scholar]
- Haegerich T, Paulozzi L, Manns B, Jones C. What we know, and don't know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel D. Unemployment and substance use: a review of the literature (1990–2010) Curr Drug Abuse Rev. 2011;4:4–27. doi: 10.2174/1874473711104010004. [DOI] [PubMed] [Google Scholar]
- Henry J. Kaiser Family Foundation. State Health Facts: Total Professionally Active Physicians. [accessed 10.08.15];2015 Available at http://kff.org/other/state-indicator/total-active-physicians/
- Irvine J, Hallvik S, Hildebran C, Marino M, Beran T, Deyo R. Who uses a prescription drug monitoring program and how? Insights from a statewide survey of Oregon clinicians. J Pain. 2014;15:747–755. doi: 10.1016/j.jpain.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann M, Kennedy W, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract. 2014;27:5–16. doi: 10.1177/0897190013515001. [DOI] [PubMed] [Google Scholar]
- Johnson H, Paulozzi L, Porucznik C, Mack K, Herter B. Decline in drug overdose deaths after state policy changes – Florida, 2010–2012. MMWR. 2014;63:569–574. [PMC free article] [PubMed] [Google Scholar]
- Kentucky Cabinet for Health and Family Services, Kentucky Injury Prevention and Research Center. 2010 KASPER Satisfaction Survey Executive Summary. [accessed 07.03.15];2010 Available at http://chfs.ky.gov/NR/rdonlyres/BDC0DFC9-924B-4F11-A10A-5EB17933FDDB/0/2010KASPERSatisfactionSurveyExecutiveSummary.pdf.
- Merline A, O'Malley P, Schulenberg J, Bachman J, Johnston L. Substance use among adults 35 years of age: prevalence, adulthood predictors, and impact of adolescent substance use. Am J Public Health. 2004;94:96–102. doi: 10.2105/ajph.94.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnesota Board of Pharmacy. Minnesota Prescription Monitoring Program 2014 Annual Report. [accessed 13.08.15];2015 Available at http://pmp.pharmacy.state.mn.us/assets/files/PDFs/Reports/2015/2014_Annual_Report_Updated.pdf.
- National Alliance for Model State Drug Laws. Illinois' Prescription Drug Monitoring Program. [accessed 07.03.15];2006a Available at http://www.namsdl.org/library/2B9E7FA8-1372-636C-DD2E9959BB3C9BFE/
- National Alliance for Model State Drug Laws. Indiana's Prescription Drug Monitoring Program. [accessed 07.03.15];2006b Available at http://www.namsdl.org/library/2BA1B139-1372-636C-DDC1AB6267C4B2F1/
- National Alliance for Model State Drug Laws. Massachusetts' Prescription Drug Monitoring Program. [accessed 07.03.15];2006c Available at http://www.namsdl.org/library/2BBAEA91-65BE-F4BB-A2715595DEFA1C19/
- National Alliance for Model State Drug Laws. PDMP Dates of Operation. [accessed 07.03.15];2014a Available at http://www.namsdl.org/library/1667DC6B-65BE-F4BB-AB7F08135A3A7174/
- National Alliance for Model State Drug Laws. Prescription Drug Monitoring Programs: Types of Authorized Recipients. [accessed 07.03.15];2014b Available at http://www.namsdl.org/library/BD9FB395-CA32-7431-FE92182E4B30E6D5/
- National Center for Health Statistics. Drug-Poisoning Deaths Involving Opioid Analgesics: United States, 1999–2011. [accessed 07.03.15];2014a Available at http://www.cdc.gov/nchs/data/databriefs/db166.htm.
- National Center for Health Statistics. Revised Bridged-Race Intercensal Population Estimates for July 1, 2000–July 1, 2009 by Year, County, Single-Year of Age, Bridged Race, Hispanic Origin, and Sex. [accessed 07.03.15];2014b Available at http://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm#july2009.
- National Center for Health Statistics. Vintage 2013 Bridged-Race Postcensal Population Estimates for April 1, 2010, July 1, 2010–July 1, 2013, by Year, County, Single-Year of Age (0 to 85+years), Bridged-Race, Hispanic Origin, and Sex. [accessed 07.03.15];2014c Available at http://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm#vintage2013.
- Neale J. A response to Darke et al., ‘The ratio of non-fatal to fatal heroin overdose’. Addiction. 2003;98:1171. doi: 10.1046/j.1360-0443.2003.00476.x. [DOI] [PubMed] [Google Scholar]
- Paulozzi L, Kilbourne E, Desai H. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12:747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- Paulozzi L, Kilbourne E, Shah N, Nolte K, Desai H, Landen M, Harvey W, Loring L. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13:87–95. doi: 10.1111/j.1526-4637.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- Paulozzi L, Zhang K, Jones C, Mack K. Risk of adverse health outcomes with increasing duration and regularity of opioid therapy. J Am Board Fam Med. 2014;27:329–338. doi: 10.3122/jabfm.2014.03.130290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDMP Center of Excellence. Trends in Wyoming PMP Prescription History Reporting: Evidence for a Decrease in Doctor Shopping? [accessed 20.03.15];2010 Available at http://www.pdmpexcellence.org/sites/all/pdfs/NFF_wyoming_rev_11_16_10.pdf.
- PDMP Center of Excellence. Nevada's Proactive PMP: The Impact of Unsolicited Reports. [accessed 07.03.15];2011 Available at http://www.pdmpexcellence.org/sites/all/pdfs/nevada_nff_10_26_11.pdf.
- Perrone J, DeRoos F, Nelson L. Prescribing practices, knowledge, and use of prescription drug monitoring programs (PDMP) by a national sample of medical toxicologists, 2012. J Med Toxicol. 2012;8:341–352. doi: 10.1007/s13181-012-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel V, Frauger E, Thirion X, Ronfle E, Lapierre V, Masut A, Coudert C, Blin O, Micallef J. Impact of a prescription monitoring program on doctor-shopping for high dosage buprenorphine. Pharmacoepidemiol Drug Saf. 2009;18:36–43. doi: 10.1002/pds.1681. [DOI] [PubMed] [Google Scholar]
- Prescription Drug Monitoring Program Training and Technical Assistance Center. Authorized Requestors of PDMP Data. [accessed 06.08.15];2015a Available at http://www.pdmpassist.org/content/authorized-requestors-pdmp-data.
- Prescription Drug Monitoring Program Training and Technical Assistance Center. Status of Prescription Drug Monitoring Programs (PDMPs) [accessed 07.03.15];2015b Available at http://www.pdmpassist.org/pdf/PDMP_Program_Status_2015.pdf.
- Reifler L, Droz D, Bailey J, Schnoll S, Fant R, Dart R, Bucher Bartelson B. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13:434–442. doi: 10.1111/j.1526-4637.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- Reisman R, Shenoy P, Atherly A, Flowers C. Prescription opioid usage and abuse relationships: an evaluation of state prescription drug monitoring program efficacy. Subst Abuse. 2009;3:41–51. doi: 10.4137/sart.s2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwalt C, Garrettson M, Alexandridis A. The effects of North Carolina's prescription drug monitoring program on the prescribing behaviors of the state's providers. J Prim Prev. 2015;36:131–137. doi: 10.1007/s10935-014-0381-0. [DOI] [PubMed] [Google Scholar]
- Rutkow L, Chang H, Daubresse M, Webster D, Stuart E, Alexander G. Effect of Florida's prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Intern Med. 2015a doi: 10.1001/jamainternmed.2015.3931. http://dx.doi.org/10.1001/jamainternmed.2015.3931 (epub) [DOI] [PubMed]
- Rutkow L, Turner L, Lucas E, Hwang C, Alexander G. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Aff (Millwood) 2015b;34:484–492. doi: 10.1377/hlthaff.2014.1085. [DOI] [PubMed] [Google Scholar]
- Simeone R, Holland L. An Evaluation of Prescription Monitoring Programs. [accessed 07.03.15];2006 Available at http://www.simeoneassociates.com/simeone3.pdf.
- Simoni-Wastila L, Qian J. Influence of prescription monitoring programs on analgesic utilization by an insured retiree population. Pharmacoepidemiol Drug Saf. 2012;21:1261–1268. doi: 10.1002/pds.3342. [DOI] [PubMed] [Google Scholar]
- Smith R, Kilaru A, Perrone J, Paciotti B, Barg F, Gadsden S, Meisel Z. How, why, and for whom do emergency medicine providers use prescription drug monitoring programs? Pain Med. 2015;16:1122–1131. doi: 10.1111/pme.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller H, Lorenz D, Bailey E, Dart R. Epidemiological trends in abuse and misuse of prescription opioids. J Addict Dis. 2009;28:130–136. doi: 10.1080/10550880902772431. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2010: National Estimates of Drug-Related Emergency Department Visits. [accessed 07.03.15];2012a Available at http://www.samhsa.gov/data/2k13/DAWN2k10ED/DAWN2k10ED.htm. [PubMed]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. [accessed 07.03.15];2012b Available at http://media.samhsa.gov/data/NSDUH/2k11Results/NSDUHresults2011.htm.
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. [accessed 07.03.15];2013 Available at http://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. [PubMed]
- United States General Accounting Office. Prescription drugs: state monitoring programs provide useful tool to reduce diversion. [accessed 07.03.15];2002 Available at http://www.gao.gov/products/GAO-02-634.
- Virginia Department of Health Professions. Report on the Collection of Data and Information about Utilization of the Prescription Monitoring Program pursuant to SJR 73 and SJR 75. [accessed 07.03.15];2010 Available at http://www.dhp.virginia.gov/dhp_programs/pmp/docs/SD13PMPReportSJR73-75.pdf.
- Volkow N. America's Addiction to Opioids: Heroin and Prescription Drug Abuse. Testimony to Senate Caucus on International Narcotics Control. [accessed 07.03.15];2014 Available at http://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2014/americas-addiction-to-opioids-heroin-prescription-drug-abuse.
- Webster L, Cochella S, Dasgupta N, Fakata K, Fine P, Fishman S, Grey T, Johnson E, Lee L, Passik S, Peppin J, Porucznik C, Ray A, Schnoll S, Stieg R, Wakeland W. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(Suppl. 2):S26–S35. doi: 10.1111/j.1526-4637.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- Weiner S, Griggs C, Mitchell P, Langlois B, Friedman F, Moore R, Lin S, Nelson K, Feldman J. Clinician impression versus prescription drug monitoring program criteria in the assessment of drug-seeking behavior in the emergency department. Ann Emerg Med. 2013;62:281–289. doi: 10.1016/j.annemergmed.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Zedler B, Xie L, Wang L, Joyce A, Vick C, Kariburyo F, Rajan P, Baser O, Murrelle L. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15:1911–1929. doi: 10.1111/pme.12480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.