Abstract
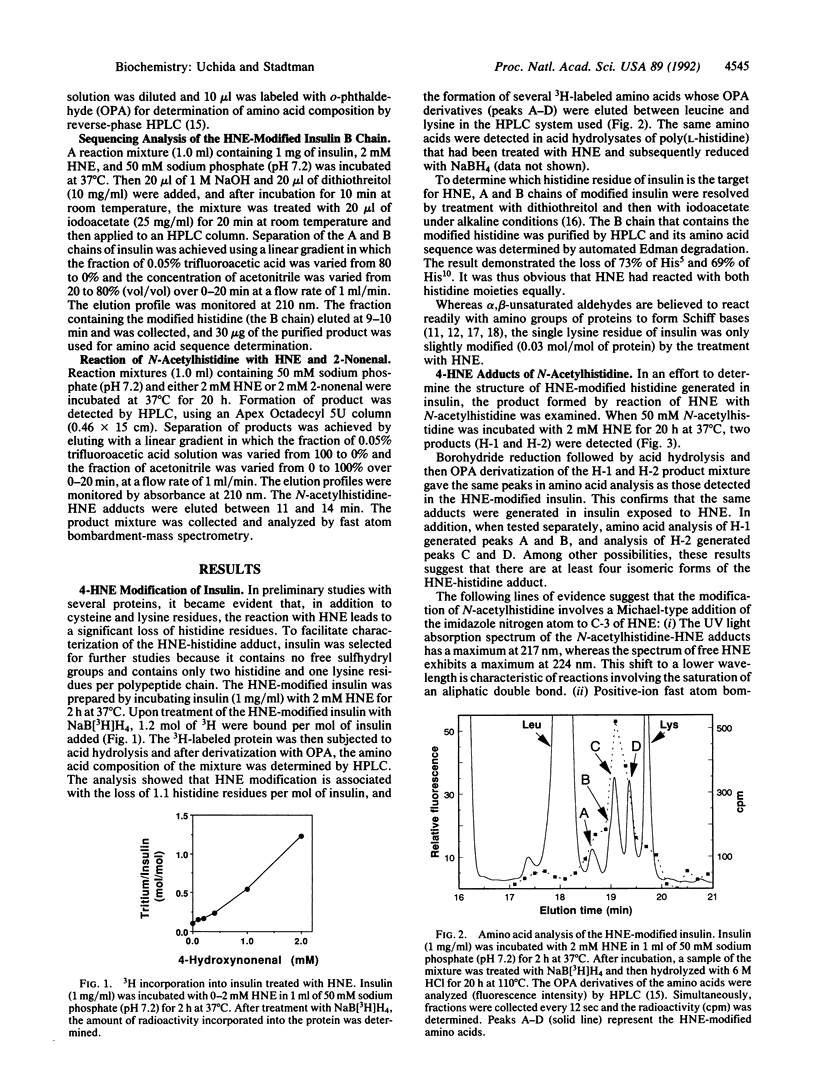
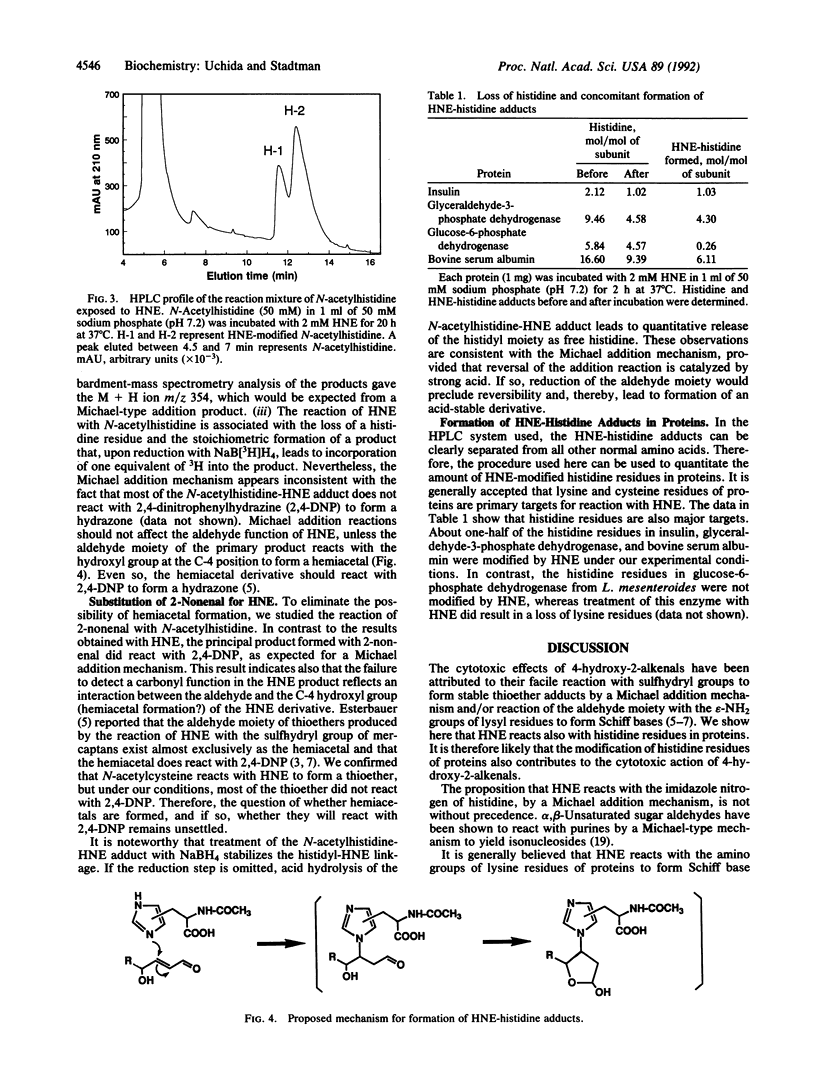
We find that histidine residues in proteins are major targets for reaction with the lipid peroxidation product 4-hydroxynon-2-enal (HNE). Reaction of insulin (which contains no sulfhydryl groups) with HNE leads to the generation of HNE-protein adducts, which are converted to radioactive derivatives upon subsequent treatment with NaB[3H]H4. Amino acid analysis of the modified protein showed that the HNE treatment leads to the selective loss of histidine residues and the stiochiometric formation of 3H-labeled amino acid derivatives. The same labeled products were detected in acid hydrolysates of polyhistidine and N-acetylhistidine after their reactions with HNE and NaB[3H]H4. The reaction of N-acetylhistidine with HNE led to the production of two compounds. Upon acid hydrolysis, both derivatives yielded stoichiometric amounts of histidine. However, after reduction with NaBH4, acid hydrolysis led to a mixture of amino acid derivatives [presumably, isomeric forms of N pi (N tau)-1,4-dihydroxynonanylhistidine] that were indistinguishable from those obtained from insulin and polyhistidine after similar treatment. Although other possibilities are not excluded, it is suggested that the modification of histidine residues in proteins by HNE involves a Michael-type addition of the imidazole nitrogen atom of histidine to the alpha, beta-unsaturated bond of HNE, followed by secondary reaction involving the aldehyde group with the C-4 hydroxyl group of HNE. The reaction of histidine residues with HNE provides the basis for methods by which the contributions of HNE in the modification of proteins can be determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amici A., Levine R. L., Tsai L., Stadtman E. R. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem. 1989 Feb 25;264(6):3341–3346. [PubMed] [Google Scholar]
- Bateman R. C., Jr, Youngblood W. W., Busby W. H., Jr, Kizer J. S. Nonenzymatic peptide alpha-amidation. Implications for a novel enzyme mechanism. J Biol Chem. 1985 Aug 5;260(16):9088–9091. [PubMed] [Google Scholar]
- Benedetti A., Comporti M., Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980 Nov 7;620(2):281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Carney J. M., Starke-Reed P. E., Oliver C. N., Landum R. W., Cheng M. S., Wu J. F., Floyd R. A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church F. C., Porter D. H., Catignani G. L., Swaisgood H. E. An o-phthalaldehyde spectrophotometric assay for proteinases. Anal Biochem. 1985 May 1;146(2):343–348. doi: 10.1016/0003-2697(85)90549-4. [DOI] [PubMed] [Google Scholar]
- Dunn J. A., Ahmed M. U., Murtiashaw M. H., Richardson J. M., Walla M. D., Thorpe S. R., Baynes J. W. Reaction of ascorbate with lysine and protein under autoxidizing conditions: formation of N epsilon-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochemistry. 1990 Dec 11;29(49):10964–10970. doi: 10.1021/bi00501a014. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Schaur R. J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Zollner H., Lang J. Metabolism of the lipid peroxidation product 4-hydroxynonenal by isolated hepatocytes and by liver cytosolic fractions. Biochem J. 1985 Jun 1;228(2):363–373. doi: 10.1042/bj2280363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Zollner H., Scholz N. Reaction of glutathione with conjugated carbonyls. Z Naturforsch C. 1975 Jul-Aug;30(4):466–473. doi: 10.1515/znc-1975-7-808. [DOI] [PubMed] [Google Scholar]
- GARRISON W. M., JAYKO M. E., BENNETT W. Radiation-induced oxidation of protein in aqueous solution. Radiat Res. 1962 Apr;16:483–502. [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M., Edwards P. A. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1712–1716. doi: 10.1073/pnas.79.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff H. F., O'Neil J., Chisolm G. M., 3rd, Cole T. B., Quehenberger O., Esterbauer H., Jürgens G. Modification of low density lipoprotein with 4-hydroxynonenal induces uptake by macrophages. Arteriosclerosis. 1989 Jul-Aug;9(4):538–549. doi: 10.1161/01.atv.9.4.538. [DOI] [PubMed] [Google Scholar]
- Jürgens G., Lang J., Esterbauer H. Modification of human low-density lipoprotein by the lipid peroxidation product 4-hydroxynonenal. Biochim Biophys Acta. 1986 Jan 3;875(1):103–114. doi: 10.1016/0005-2760(86)90016-0. [DOI] [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. I. Inactivation is due to loss of one histidine residue. J Biol Chem. 1983 Oct 10;258(19):11823–11827. [PubMed] [Google Scholar]
- Oliver C. N., Starke-Reed P. E., Stadtman E. R., Liu G. J., Carney J. M., Floyd R. A. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Ylä-Herttuala S., Rosenfeld M. E., Butler S. W., Socher S. A., Parthasarathy S., Curtiss L. K., Witztum J. L. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990 May-Jun;10(3):325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Carney J. M., Starke-Reed P. E., Oliver C. N., Stadtman E. R., Floyd R. A., Markesbery W. R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Covalent modification reactions are marking steps in protein turnover. Biochemistry. 1990 Jul 10;29(27):6323–6331. doi: 10.1021/bi00479a001. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med. 1990;9(4):315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Oliver C. N. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991 Feb 5;266(4):2005–2008. [PubMed] [Google Scholar]
- Starke-Reed P. E., Oliver C. N. Protein oxidation and proteolysis during aging and oxidative stress. Arch Biochem Biophys. 1989 Dec;275(2):559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Winkler P., Lindner W., Esterbauer H., Schauenstein E., Schaur R. J., Khoschsorur G. A. Detection of 4-hydroxynonenal as a product of lipid peroxidation in native Ehrlich ascites tumor cells. Biochim Biophys Acta. 1984 Dec 6;796(3):232–237. doi: 10.1016/0005-2760(84)90122-x. [DOI] [PubMed] [Google Scholar]
- Winkler P., Schaur R. J., Schauenstein E. Selective promotion of ferrous ion-dependent lipid peroxidation in Ehrlich ascites tumor cells by histidine as compared with other amino acids. Biochim Biophys Acta. 1984 Dec 6;796(3):226–231. doi: 10.1016/0005-2760(84)90121-8. [DOI] [PubMed] [Google Scholar]



