Abstract
Background
Pulmonary vascular (PV) distensibility, defined as the percent increase in pulmonary vessel diameter per mmHg increase in pressure, permits the pulmonary arteries to increase in size to accommodate increased blood flow. We hypothesized that PV distensibility is abnormally low in patients with heart failure (HF) and serves as an important determinant of right ventricular performance and exercise capacity.
Methods and Results
Patients with HF and preserved ejection fraction (HFpEF, n=48), HF and reduced ejection fraction (HFrEF, n=55), pulmonary hypertension without left-heart failure (PAH, n=18), and control subjects (n=30) underwent cardiopulmonary exercise testing with invasive hemodynamic monitoring and first-pass radionuclide ventriculography. PV distensibility was derived from 1257 matched measurements (mean±SD, 8±2 per subject) of PA pressure, PA wedge pressure and cardiac output. PV distensibility was lowest in the PAH group (0.40±0.24% per mmHg) and intermediate in the HFpEF and HFrEF groups (0.92±0.39 and 0.84±0.33% per mmHg, respectively) compared to the control group (1.39±0.32% per mmHg, P<0.0001 for all three). PV distensibility was associated with change in RVEF (ρ=0.39, P<0.0001) with exercise and was an independent predictor of peak VO2. PV distensibility also predicted cardiovascular mortality independent of peak VO2 in HF patients (n=103, Cox HR 0.30, 95% CI 0.10–0.93, P=0.036). In a subset of HFrEF patients (n=26), 12 weeks of treatment with the pulmonary vasodilator sildenafil or placebo led to a 24.6% increase in PV distensibility (P=0.015) in the sildenafil group only.
Conclusions
PV distensibility is reduced in patients with HF and PAH and is closely related to RV systolic function during exercise, maximal exercise capacity, and survival. Furthermore, PV distensibility is modifiable with selective pulmonary vasodilator therapy and may represent an important target for therapy in selected HF patients with pulmonary hypertension.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00309790.
Keywords: pulmonary heart disease, pulmonary hypertension, exercise capacity, heart failure, physiology, pulmonary vascular distensibility
Pulmonary hypertension (PH), as defined by a mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg, is present in the majority of patients with left heart failure with reduced ejection fraction (HFrEF).1–3 PH severity in HFrEF, particularly in conjunction with right ventricular dysfunction, is closely related to worse exercise capacity and prognosis.4–6 Similarly, the prevalence of PH in patients with left heart failure and preserved ejection fraction (HFpEF) is greater than 50%7, 8 and also predicts a poor prognosis.9, 10
mPAP is dependent on the resistance of the pulmonary vessels, pulmonary blood flow, and left-sided filling pressures as represented with the following equation:
| (1) |
where PVR is pulmonary vascular resistance, CO is cardiac output, and PAWP is pulmonary arterial wedge pressure. This equation is limited in that it defines a purely linear relationship between mPAP and CO. During exercise or other states in which CO increases, however, the normal pulmonary vasculature is able to distend and recruit additional closed vessels in order to accommodate increased blood flow, resulting in an attenuated increase in mPAP and a curvilinear relationship with CO.2, 13, 14 Thus, mPAP is also dependent on a fourth variable termed pulmonary vascular (PV) distensibility. The human pulmonary circulation has been shown to lose distensibility under chronic hypoxic conditions, which contributes to PH and increased workload for the right ventricle.14, 15
While PVR only represents the static component of or average right ventricular afterload, other parameters such as PV distensibility, capacitance, and impedance also take into account the dynamic, pulsatile components of afterload and therefore are considered to be potentially better measures of PV function than PVR.16 PV distensibility is a mechanical property of the pulmonary vessels defined as the relative change in pulmonary arterial diameter or area for a given change in pressure, while PV capacitance is the change in volume associated with a change in pressure (calculated as the ratio of stroke volume to pulmonary pulse pressure, SV/PP), and impedance is the ratio of the pulmonary arterial pressure waveform to the flow over the entire cardiac cycle.16, 17 PV distensibility has been estimated with different imaging modalities including magnetic resonance imaging,18–20 echocardiography,21 gated CT,22, 23 and intravascular ultrasound.24 However, these techniques are limited in that they all estimate distensibility based on fractional change in diameter or area of the main PA or large PA segments, and do not account for the distensibility of the entire PV circuit including the medium-sized pulmonary arterioles where much of the abnormal vascular remodeling occurs in PH.25 Furthermore, these techniques only assess PV distensibility or capacitance at rest and not over a range of different flows, which limits the sensitivity of detecting abnormal PV function. To address these limitations, Linehan and colleagues developed a distensible vessel model for the pulmonary circulation that predicts pressure-flow relationships taking into account the PV distensibility and incorporating the entire pulmonary vascular circuit. This model can be utilized to determine average PV distensibility with pressure information (mPAP and PAWP) at different cardiac outputs.13 The model depends on the distensibility (α), in units of percent diameter change per unit mmHg increase in pressure. The equation relating distensibility with mPAP at constant hematocrit is:
| (2) |
where mPAP is in units of mmHg, Pw is the pulmonary arterial wedge pressure (PAWP, mmHg), Ro is total pulmonary resistance at rest calculated as mPAP/Q (in Wood units), and Q is pulmonary blood flow (L/min).13 This distensibility model has been validated in both perfused canine lungs13 and in normal humans.14 A typical PV distensibility value consists of a 1.5–2% increase in pulmonary vessel diameter per mmHg increase in pressure.14, 26
PV distensibility in HF has not been previously defined. Because of the close relationship between PAP and outcomes in HF, it is important to understand relative contributions of the determinants of PAP in HF. The recent recognition that longitudinal PAP monitoring can lead to improved HF outcomes further highlights the importance of characterizing the distensibility component of PAP in HF.27 In this study, we define the clinical correlates and physiologic and prognostic significance of PV distensibility in patients with either HF or with pulmonary arterial hypertension in the absence of left heart disease (PAH) compared to control patients.
METHODS
Patient population
Consecutive patients at the Massachusetts General Hospital who underwent cardiopulmonary exercise testing (CPET) with pulmonary arterial catheter measurements for the evaluation of dyspnea were prospectively enrolled after obtaining informed consent. Patients had chronic NYHA class II–IV symptoms and were classified based on left ventricular ejection fraction (LVEF), resting and exercise pulmonary arterial wedge pressure (PAWP), and resting mean pulmonary arterial pressure (mPAP). Patients were classified by the following criteria: a) HFrEF: chronic LVEF < 0.45 on guideline-based pharmacotherapy; b) HFpEF: LVEF 0.50 and supine PAWP > 15 mmHg at rest; c) PAH: supine mPAP ≥ 25 mmHg at rest (or 21–24 mmHg with a PVR > 3 WU), supine PAWP ≤ 15 mmHg at rest and PAWP < 25 mmHg with exercise. Patients who did not fall into any of the above groups were included as part of the control population. Controls were required to have a normal LVEF, a supine mPAP < 25 mmHg at rest, a supine PAWP ≤ 15 mmHg at rest, exercise PAWP < 25 mmHg and a normal exercise capacity as reflected by a peak VO2 greater than 80% of that predicted on the basis of age, gender, and height.28 While it is possible these patients have subclinical cardiopulmonary disease, this should bias to the null when comparing HF and PAH groups to controls, so that the findings might be even stronger if a truly "normal" population were used. Patients were excluded from study if they had any of the following: 1) known active flow limiting CAD; 2) severe valvular heart disease; 3) intra-cardiac shunting; 4) incomplete pulmonary arterial catheter pressure measurements; 5) submaximal exercise as evidenced by peak respiratory exchange ratio (RER) <1.0; 6) the presence of a pulmonary mechanical limitation to exercise as defined by VE/(forced expiratory volume in 1 second [FEV1] × 35) > 0.7 at the ventilatory threshold.29,30, 31 26 patients in the HFrEF group participated in a previously reported 12-week double-blind, randomized controlled trial of treatment with either placebo (n=12) or sildenafil (n=14).32 This clinical trial was registered (ClinicalTrials.gov #NCT00309790), the study was approved by the institutional review board, and informed consent was obtained.
Protocol for cardiopulmonary exercise testing
Patients were instructed to take their prescribed medications as usual before exercise testing. Subjects underwent placement of a pulmonary arterial catheter via the internal jugular vein and a systemic arterial catheter via the radial artery. Measurements of resting right atrial pressure (RAP), pulmonary arterial pressure (PAP), and PAWP were obtained in the supine and upright positions. Resting first-pass radionuclide biventriculography was performed immediately prior to initiation of exercise (OnePass GVI Medical Devices, Twinsburg, OH) to obtain resting LVEF and RVEF, as previously described.33 Subjects then performed maximum incremental exercise with upright cycle ergometry after an initial 3-minute period of unloaded exercise (MedGraphics, St. Paul, MN). An individualized ramp protocol was used based on the subject's estimated fitness level (5–25 Watts/min), to target at least 5 minutes of incremental exercise. Simultaneous hemodynamic measurements were obtained with exercise (Witt Biomedical Inc, Melbourne, FL), as previously described.31–33 RAP, PAP, PAWP, and systemic arterial pressures were measured in the upright position, at end-expiration, while patients were seated on the cycle, both at rest and at one-minute intervals during exercise. Fick cardiac output (CO)34,35 was determined at one minute intervals throughout exercise by measuring oxygen uptake (VO2) and simultaneous radial arterial and mixed venous O2 saturation to calculate the C(a–v)O2 during a linear ramp protocol. Pulmonary vascular resistance (PVR) was calculated as: [(mean PAP − PAWP)/CO]. Peak VO2 was defined as the highest O2 uptake, averaged over 30 seconds, during the last minute of symptom-limited exercise, as previously described.32 First-pass radionuclide biventriculography was repeated at peak exercise.
Arterial O2 content (CaO2)36 is the amount of O2 carried by blood to the periphery and was calculated as (hemoglobin [Hb, g/dL] × 1.39 × SaO2)+(0.003 × PaO2). Similarly mixed venous O2 content (CvO2) represents the O2 content of blood returning from the peripheral tissues to the right heart which was calculated as (Hb × 1.39 × SvO2) + (0.003 × PvO2).
The Pulmonary Vascular Distensibility Model
As described in the introduction, a previously-developed distensible vessel model (Eqn. 2) was used to calculate the pulmonary vascular pressure-flow relationship.13 In our population, we measured mPAP, PAWP, and Q both at rest and at multiple points during exercise. Utilizing an iterative approach with least-squares methodology, as reported previously,14 we determined the α value that best predicted observed mPAP values.
Subject Follow-up
Vital status of each patient was confirmed by medical record review as of 5/2015. Deaths were considered as cardiovascular deaths if they were primarily attributed to heart failure, arrhythmia or ischemic events.
Statistical methods
STATA 11 (Statacorp, College Station, Texas) was used for statistical analysis. Measurements are presented as mean ± standard deviation (SD), unless otherwise indicated. The Shapiro-Wilk test was used to assess the normality of variables. Group baseline characteristics were compared using either 1-way ANOVA with Bonferroni-adjusted post-hoc pairwise comparisons, Kruskal-Wallis test with Dunn's post-hoc pairwise comparisons, or Fisher’s exact test, as appropriate. Receiver operator characteristic (ROC) analysis was performed to determine the ability of PV distensibility to distinguish HF and PAH populations from the control population. To identify predictors of PV distensibility, we utilized either Pearson's correlation or Spearman's rank correlation, based on whether the data was normally distributed. Multivariable stepwise linear regression using backward elimination was performed to determine independent predictors of peak VO2 in HF patients. Age and gender were required in the multivariable model. In addition to PV distensibility, a comprehensive set of covariates measured at rest were selected for an initial univariable analysis including mPAP, PAWP, Hb, CavO2, cardiac index, LVEF, RVEF, SBP, DBP, systemic pulse pressure, and HR. Some variables were eliminated because they did not serve as a univariable predictor of peak VO2 (P>0.10). These variables included resting SBP, systemic pulse pressure, and HR. The remaining variables were included for multivariable analysis using a backward elimination regression model until all remaining predictor variables had a P≤0.05. Kaplan-Meier survival with Log Rank testing and Cox regression analysis was used to determine if PV distensibility and other variables predict cardiovascular mortality in HF patients. A paired t test was used to determine if placebo or sildenafil treatment altered pulmonary vascular distensibility, and an independent t test was utilized to compare differences between the placebo- and sildenafil-treated arms. A P-value < 0.05 was considered significant unless otherwise noted.
This study was approved by the Partners Healthcare IRB. The authors had full access to the data and take responsibility for its integrity and for the manuscript as written.
RESULTS
Population characteristics
Baseline characteristics for the HFpEF (n=48), HFrEF (n=55), PAH (n=18), and control subjects (n=30) are reported in Table 1. HFrEF patients exhibited a greater male predominance relative to HFpEF patients, while HFpEF patients had a greater BMI relative to the other groups of patients, consistent with the known demographic characteristics of HFpEF and HFrEF populations.37, 38 A greater percentage of patients with HFrEF were treated with diuretics, angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB), beta blockers, and mineralocorticoid receptor antagonists than HFpEF patients. PAH patients consisted of either WHO Group I PH patients (n=10, 7 with idiopathic PAH and 3 with scleroderma) or WHO Group III PH patients (n=8). Only 1 of 18 PAH patients was previously diagnosed with PAH and on PAH-specific pharmacotherapy (sildenafil and bosentan) at the time of CPET. Hemoglobin levels were similar in all four groups.
Table 1.
Baseline Characteristics.
| Characteristics | Controls (30) | HFpEF (48) | HFrEF (55) | PAH (18) |
|---|---|---|---|---|
| Age, yearsa | 58±15 | 63±12 | 59±12 | 61±13 |
| Male Sex, n (%)b | 19 (63) | 19 (40) | 45 (82)* | 9 (50) |
| Caucasian Race, n (%)b | 28 (93) | 46 (96) | 49 (89) | 17 (94) |
| BMI, kg/m2 b | 27.6±4.0 | 33.7±7.9† | 27.9±5.8* | 27.7±4.9 |
| Comorbidities, n (%) | ||||
| Hypertension b | 11 (37) | 29 (60) | 34 (62)‡ | 9 (50) |
| Diabetes Mellitus b | 1 (3) | 12 (25)† | 11 (20) | 2 (11) |
| Hyperlipidemia b | 7 (23) | 25 (52)† | 32 (58)‡ | 9 (50) |
| Pharmacotherapy, n (%) | ||||
| Diuretics b | 2 (7) | 24 (50)† | 48 (87)‡* | 4 (22) |
| ACE Inhibitor or ARB b | 8 (27) | 14 (29) | 45 (82)‡* | 3 (17) |
| β-adrenergic blocker b | 5 (17) | 24 (50)† | 52 (95)‡* | 6 (33) |
| Aldosterone antagonist b | 0 | 4 (8) | 29 (53)‡* | 0 |
| Resting LVEF (%)a | 67±5 | 62±8† | 30±6‡* | 63±7 |
| Resting RVEF (%)a | 53±7 | 50±7 | 38±11‡* | 46±7# |
| Resting Supine mPAP, mmHga | 14±4 | 30±5† | 35±13‡ | 28±5# |
| Resting Supine PAWP, mmHga | 8±3 | 20±3† | 22±9‡ | 10±3 |
| Hemoglobin, g/dla | 13.3±1.3 | 13.2±1.5 | 13.0±2.0 | 14.0±1.8 |
| Upright Rest Hemodynamics | ||||
| mPAP, mmHga | 14.5 ± 1.9 | 20.9±5.5† | 27.2±9‡* | 25.6 ± 5.1# |
| PAWP, mmHga | 5.1 ± 1.4 | 9.4±4.7† | 15.4±7.6‡* | 7.4 ± 2.8# |
| CI, L/min/m2 a | 2.8±0.5 | 2.5±0.7† | 1.8±0.5‡* | 2.6±0.7 |
| SV/PP (mL/mmHg)c | 6.0±2.8 | 3.0±1.2† | 1.8±0.93‡* | 2.7±0.78# |
| Peak Upright Exercise | ||||
| Maximum watts achieveda | 153±52 | 82±33† | 76±36‡ | 83±39# |
| Minutes Exerciseda | 8.5±1.6 | 7.1±2.2† | 5.5±1.7‡* | 7.3±2.1# |
| Peak Exercise RERa | 1.16±0.08 | 1.15±0.11 | 1.16±0.15 | 1.09±0.10 |
| Peak Exercise Lactate, mMa | 7.5±1.5 | 5.3±2.5† | 4.8±2.0‡ | 5.4±2.5# |
| Peak VO2, ml/kg/mina | 25.3±7.5 | 13.9±3.3† | 12.4±3.8‡ | 15.4±4.0# |
| Exercise LVEF (%)a | 71±6 | 64±8† | 33±8‡* | 65±8# |
| Exercise RVEF (%)a | 56±7 | 49±8† | 38±10‡* | 45±7# |
| mPAP, mmHga | 30.3±3.6 | 43.4±9.8† | 49.7±10.2‡* | 50±11.7# |
| PAWP, mmHga | 16.8±4.3 | 28.3±9.0† | 29.8±8.9‡ | 15.3±4.8 |
| CI, L/min/m2 a | 7.5±1.6 | 5.1±1.3† | 3.7±1.2‡* | 5.5±1.2# |
Mean ± SD or n (%).
ANOVA with Bonferroni-adjusted post-hoc test,a Fisher’s exact testb or Kruskal-Wallis with Dunn's post-hoc pairwise test.c
indicates P<0.05 between HFpEF & controls,
indicates P<0.05 between HFrEF & controls,
indicates P<0.05 between HFpEF & HFrEF,
indicates P<0.05 between PAH & controls
SV, stroke volume; PP, pulmonary pulse pressure
All subjects in the four groups exceeded their ventilatory threshold with peak RER>1.0, indicating a maximal effort during exercise (Table 1).29,30 Peak VO2 was reduced in HFpEF (13.9 ± 3.3 ml/kg/min), HFrEF (12.4 ± 3.8 ml/kg/min), and PAH (15.4 ± 4.0 ml/kg/min) compared to controls (25.3 ± 7.5 ml/kg/min, P<0.001 for all three comparisons). Similarly, maximal watts achieved at peak exercise were reduced in all three groups of patients compared to control subjects.
Pulmonary Vascular Distensibility in Heart Failure and PAH patients
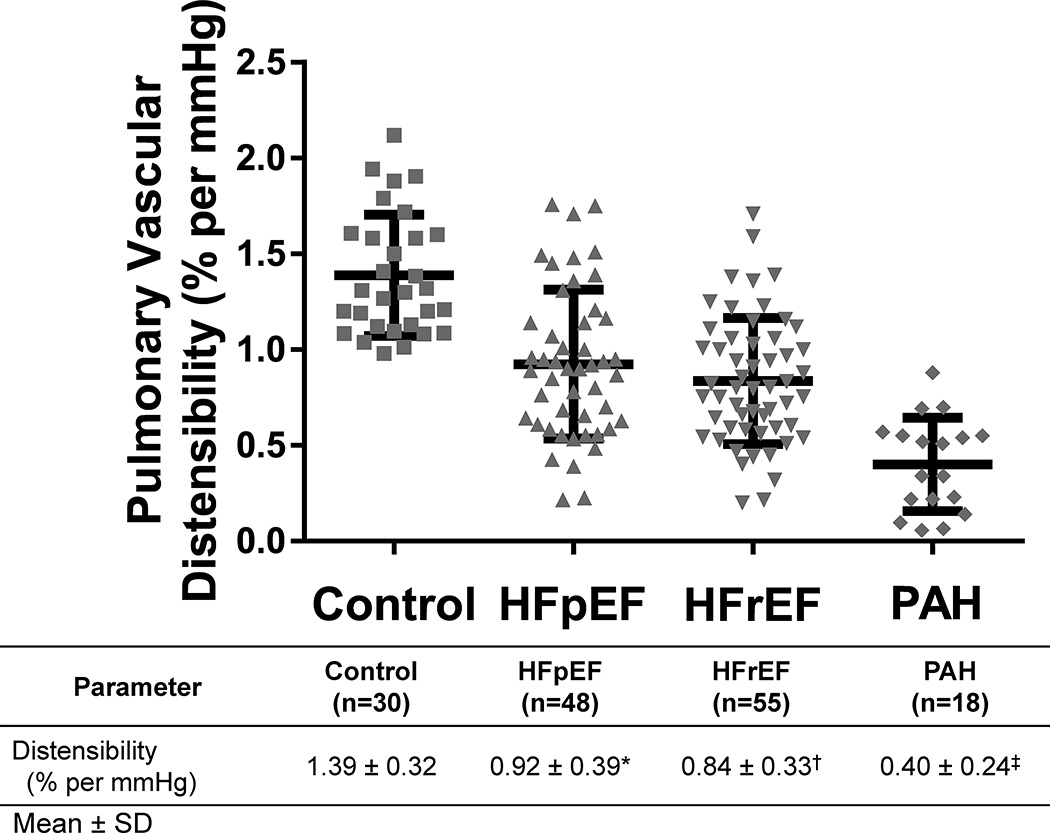
Among the entire cohort studied (n=151), PV distensibility was determined from a total of 1257 matched invasive hemodynamic measurements obtained during exercise (where each measurement consists of mPAP, PAWP, and CO). This represents an average of 8.3 ± 2.8 measurements per patient (with a minimum number of measurements of 4 per subject). Prior studies have established that normal humans have a PV distensibility of 1.5–2% per mmHg.14, 26 Our control population (consisting of patients being evaluated for dyspnea who were older than previously reported normals) exhibited a similar, albeit lower, distensibility of 1.39 ± 0.32% per mmHg (with range of 0.98–2.12). The PV distensibility of patients with HFpEF (0.92 ± 0.39% per mmHg) and HFrEF (0.84 ± 0.33% per mmHg) were significantly lower than control subjects (P<0.001 for both comparisons, Figure 1). Interestingly, patients with HFpEF and HFrEF exhibited similar PV distensibility. Patients with PAH had a PV distensibility of 0.40 ± 0.24% per mmHg, which was reduced when compared to both control subjects and patients with either HFpEF or HFrEF (P<0.001 for all three comparisons). Distensibility in the PAH cohort restricted to patients with resting mPAP ≥ 25mmHg (n=13) was 0.36 ± 0.27 % per mmHg.
Figure 1. Characterization of pulmonary vascular distensibility in heart failure and pulmonary arterial hypertension patients.
Pulmonary vascular distensibility was determined using pulmonary arterial pressure, pulmonary arterial wedge pressure (PAWP), and Fick cardiac output measurements at rest and during exercise, as previously described,13 in control (n=30), HFpEF (n=48), HFrEF (n=55), and PAH (n=18) patients. Mean ± SD is depicted in the graph. HFpEF (*) and HFrEF (†) patients exhibit a reduced pulmonary vascular distensibility compared to control patients (P<0.001 for both comparisons). PAH patients have a reduced distensibility compared to all three other groups (‡ P<0.001 for all three comparisons).
To determine the diagnostic performance of PV distensibility for distinguishing HF patients from controls, we performed ROC area under the curve (AUC ± standard error) analysis. The AUC for distinguishing HF patients (HFpEF and HFrEF, n=103) from control patients (n=30) was 0.86 ± 0.03. The AUC for distinguishing PAH patients (n=18) from control patients (n=30) was 1.0.
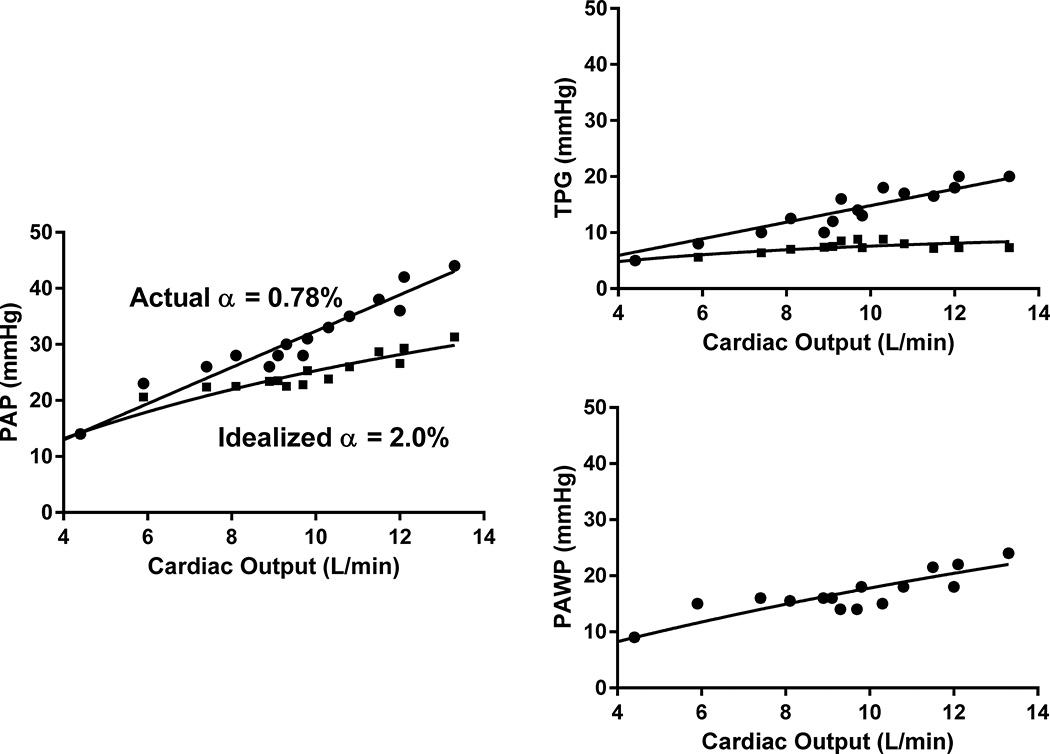
We assessed the effect of PV distensibility on changes in PAP and TPG during exercise. The pulmonary pressure-flow relationship for a representative HFpEF patient is depicted in Figure 2, along with an idealized pressure-flow relationship based on a normal distensibility, while maintaining CO and PAWP at the same values.14 When assessing the entire HF population with distensibility < 1.4% per mmHg (n=94, with a mean resting upright mPAP of 25 mmHg and mean TPG of 12 mmHg), substituting the observed distensibility in controls (α=1.4%) while keeping the cardiac output and PAWP constant, improves the average peak mPAP and TPG from 47 and 19 mmHg to 39 and 11 mmHg, respectively. Therefore, on average, at peak exercise, abnormal distensibility in HF is responsible for an ~20% higher observed mPAP and ~70% higher observed TPG than that expected if distensibility were normal. In PAH patients (n=18, resting upright mPAP 26 mmHg and TPG 18 mmHg), idealizing the distensibility to 1.4% per mmHg improves the average peak mPAP and TPG from 50 and 36 mmHg to 32 and 18 mmHg. Therefore, PV distensibility is a strong determinant of the rise in mPAP and TPG with exercise.
Figure 2. Abnormal pulmonary vascular distensibility is associated with increased pulmonary arterial pressures during exercise.
The pressure-flow relationships for a representative HFpEF patient who underwent cardiopulmonary exercise testing are shown (●). Mean pulmonary arterial pressure (PAP), transpulmonary gradient (TPG), and PAWP are depicted. This patient had a resting cardiac output of 4.4 L/min and resting upright mPAP and PAWP of 14 mmHg and 9 mmHg, respectively. With peak exercise, cardiac output increased to 13.3 L/min and upright mPAP and PAWP peaked at 44 and 24 mmHg, respectively. The transpulmonary gradient (TPG) at peak exercise was 20 mmHg. PV distensibility was calculated to be 0.78% per mmHg. To illustrate the physiologic significance of pulmonary vascular distensibility on pulmonary pressures during exercise, we superimposed an idealized pressure-flow relationship based on a normal distensibility of 2.0% per mmHg, while maintaining PAWP and CO fixed (■).14 At peak exercise, the actual mPAP and TPG are 44 and 20 mmHg, respectively, while the idealized values of mPAP and TPG are 31 and 7 mmHg.
Clinical and Hemodynamic Variables Associated with Pulmonary Vascular Distensibility
PV distensibility decreased with age (r = −0.18, p=0.031), but was not associated with BMI (Table 2) or with gender in each of the four groups of our study. Since PV distensibility reflects a mechanical property of the pulmonary vessels to vasodilate in response to increased blood flow during exercise, we hypothesized that distensibility would exhibit its strongest associations with hemodynamic variables during exercise rather than at rest. Indeed, PV distensibility was associated with VO2, cardiac index, RVEF, and pulse pressure at peak exercise but not with these same parameters at rest (Table 2). The correlation coefficients for PV distensibility with peak VO2, peak cardiac index, peak RVEF, and peak pulse pressure were 0.40, 0.35, 0.36, and 0.42 (P<0.0001 for all). These results suggest that PV distensibility is a potent indicator of cardiovascular functional reserve capacity during exercise.
Table 2.
Predictors of pulmonary vascular distensibility in all patients (n=151).
| Parameter | Correlation | P-value |
|---|---|---|
| Age (years) | −0.18 | 0.031 |
| BMI (kg/m2) | −0.02 | 0.83 |
| Resting Parameters | ||
| VO2 (ml/kg/min)* | −0.10 | 0.24 |
| O2 pulse (ml/beat) | 0.06 | 0.44 |
| CI (L/min/m2) | 0.06 | 0.49 |
| RVEF | 0.10 | 0.24 |
| LVEF | 0.16 | 0.06 |
| Pulse Pressure (mmHg) | −0.01 | 0.87 |
| Parameters at Peak Exercise | ||
| VO2 (ml/kg/min) | 0.40 | <0.0001 |
| O2 pulse (ml/beat) | 0.35 | <0.0001 |
| CI (L/min/m2) | 0.35 | <0.0001 |
| RVEF | 0.36 | <0.0001 |
| LVEF | 0.24 | 0.006 |
| Pulse Pressure (mmHg) | 0.42 | <0.0001 |
| Watts achieved | 0.40 | <0.0001 |
| Lactate (mM) | 0.25 | 0.006 |
Pearson's correlation used except where * indicated for Spearman's correlation.
BMI, body mass index; CI, cardiac index; DPG, diastolic pulmonary gradient
Unadjusted P–values are presented. 17 variables were tested and thus, when adjusting for multiple comparisons the threshold for significance is P<0.003.
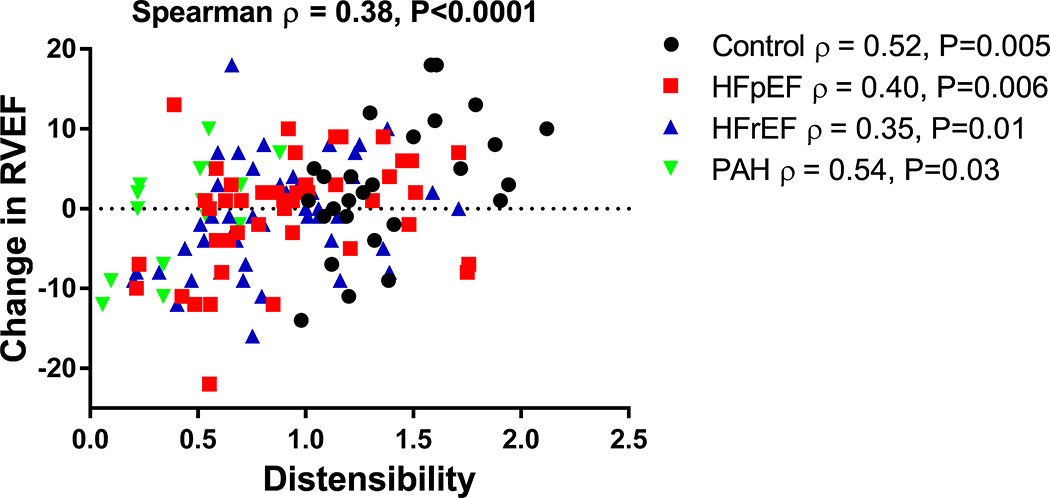
Changes in right ventricular-pulmonary vascular (RV-PV) hemodynamic measurements with exercise are important predictors of outcome in cardiopulmonary diseases.2 We therefore determined the association of PV distensibility with changes in RV-PV functional measurements during exercise. We observed that PV distensibility is associated with both percent change in PVR (Spearman ρ = −0.50, P<0.0001) and absolute change in RVEF (ρ = 0.38, P<0.0001) from rest to peak exercise (Figure 3). These associations were also present when assessing individual groups of our cohort (Figure 3). In patients with HFpEF and HFrEF, PV distensibility was strongly associated with change in RVEF and PVR with exercise (ρ = 0.38 and −0.34, respectively, P<0.001 for both), whereas resting PAWP was not (ρ = 0.04, P=0.72 and ρ = 0.13, P=0.20, respectively). These findings highlight a potentially important role for the distensibility of the pulmonary vasculature, rather than the hemodynamic severity of left-sided heart disease, in determining RV reserve capacity in patients with both HFpEF and HFrEF.
Figure 3. Pulmonary vascular distensibility is associated with change in RVEF with exercise.
A scatterplot of change in RVEF versus distensibility of the four patient groups are depicted. Spearman rank correlations were determined. Pulmonary vascular distensibility is a strong determinant of RV function with exercise.
Relationship between Pulmonary Vascular Distensibility, Exercise Capacity and Survival
Peak VO2 is a potent predictor of prognosis in patients with HF.39 We performed multivariable, stepwise linear regression with a backward elimination technique to identify if PV distensibility predicted peak VO2 independently of demographic, functional, and resting hemodynamic parameters (Table 3). PV distensibility was an independent predictor of peak VO2 (normalized β coefficient 0.25, P<0.001) in a model that also included age, gender, hemoglobin, resting cardiac index, resting LVEF, and resting mPAP. These results indicate an important role for PV distensibility in predicting exercise capacity.
Table 3.
Multivariable model for predicting peak VO2 in all HF patients (n=103).
| Parameter | Normalized Beta Coefficient |
P-value |
|---|---|---|
| Age (years) | −0.25 | <0.001 |
| Male Gender | 0.16 | 0.008 |
| Hb (g/dL) | 0.20 | 0.001 |
| Resting Cardiac Index (L/min/m2) | 0.29 | <0.001 |
| Resting PAWP (mmHg) | −0.22 | 0.003 |
| Resting mPAP (mmHg) | −0.19 | 0.018 |
| Distensibility (% per mmHg) | 0.25 | <0.001 |
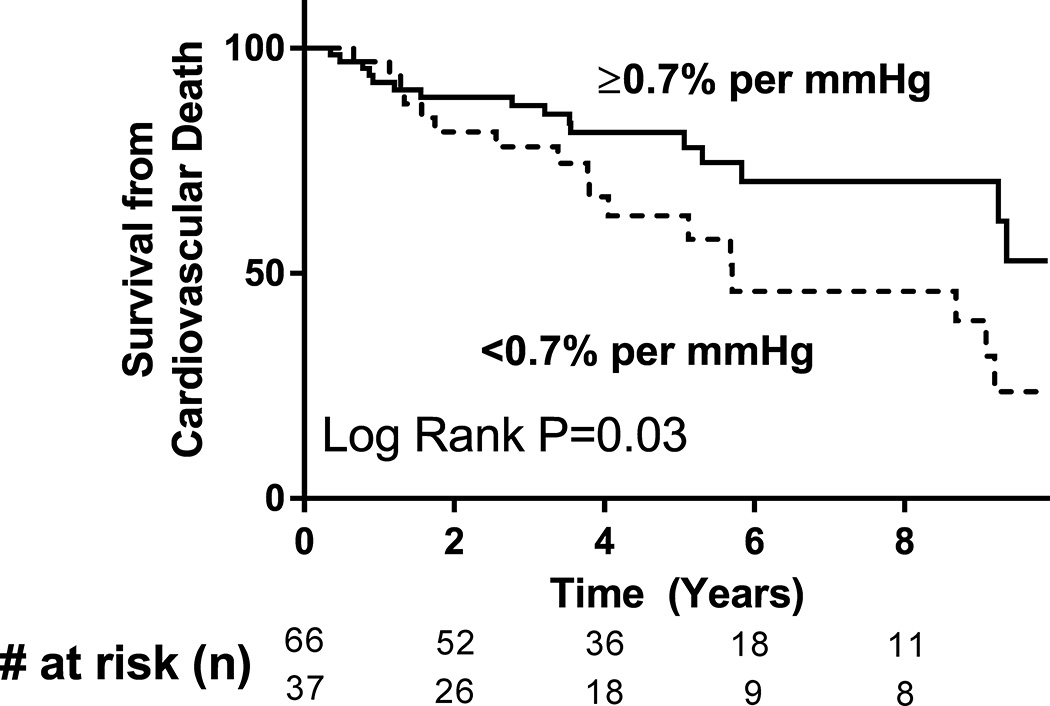
We next sought to determine if PV distensibility predicts cardiovascular mortality in HF patients. Of the 103 patients with HF (HFpEF and HFrEF), median follow-up time was 4.0 years and there was a total of 33 cardiovascular (CV) deaths. 14 patients underwent cardiac transplant or placement of a left ventricular assist device. Distensibility was analyzed both as a continuous variable and when dichotomized (<0.70% and ≥0.70% per mmHg), based on the range of normal distensibility values in our control population and that previously published.40 In both univariable (Cox hazard ratio, HR 0.33 per 1% per mmHg increase, P=0.039) and age- and sex-adjusted multivariable Cox regression analysis (Cox HR 0.33, P=0.034), PV distensibility predicted CV survival. Similarly, PV distensibility predicted transplant-free and LVAD-free CV survival (Cox HR 0.43 per 1% per mmHg increase, P=0.049). Compared to those with a lower distensibility, HF patients with a distensibility ≥0.70% per mmHg had a 55% reduced hazard of CV mortality (Figure 4, Cox HR 0.45, P=0.028). Importantly, PV distensibility was a strong predictor of CV survival independent of peak VO2 (Table 4, Cox HR 0.30 per 1% per mmHg increase, P=0.036). Dichotomized distensibility also predicted survival independent of peak VO2 (Cox HR 0.49 for PV distensibility ≥0.70% per mmHg, P=0.05). Therefore, PV distensibility was an independent predictor of cardiovascular survival for HF patients in our cohort.
Figure 4. Pulmonary vascular distensibility predicts cardiovascular mortality in heart failure patients.
Kaplan-Meier survival curves of HF patients (n=103, both HFpEF and HFrEF) are depicted, dichotomized by pulmonary vascular distensibility value. Compared to those with lower PV distensibility, HF patients with a distensibility ≥0.70% per mmHg exhibit reduced cardiovascular mortality (P=0.03).
Table 4.
Pulmonary vascular distensibility predicts cardiovascular survival in all HF patients (n=103).
| Parameter | Cox Hazard Ratio | 95% Confidence Interval |
P-value |
|---|---|---|---|
| Age (years) | 1.01 | 0.98–1.05 | 0.48 |
| Male Gender | 2.85 | 1.22–6.66 | 0.015 |
| Peak VO2 (ml/kg/min) | 0.79 | 0.70–0.88 | <0.001 |
| Distensibility (% per mmHg) | 0.30 | 0.10–0.93 | 0.036 |
We sought to determine the ability of PV distensibility to predict outcomes after adjusting for PV capacitance (calculated as the ratio of resting stroke volume to pulmonary pulse pressure, SV/PP), determined under resting conditions. SV/PP was lower in the HFpEF, HFrEF, and PAH populations compared to the control group (Table 1). There was a trend towards correlation between SV/PP and α in HF patients (ρ=0.22, P=0.07). We found that SV/PP was not an independent predictor of survival (Cox HR 0.74 per mL/mmHg increase, 95% CI 0.47–1.2, P=0.20) in HF patients. After adjusting for SV/PP, PV distensibility still predicted CV survival (Cox HR 0.22 per 1% per mmHg increase, P=0.03), indicating that the PV distensibility determined over a range of cardiac outputs during exercise performs as a better predictor of outcome in HF than PA capacitance measured under resting conditions.
Modulation of Pulmonary Vascular Distensibility by Chronic Phosphodiesterase Type 5 (PDE5) Inhibitor Therapy
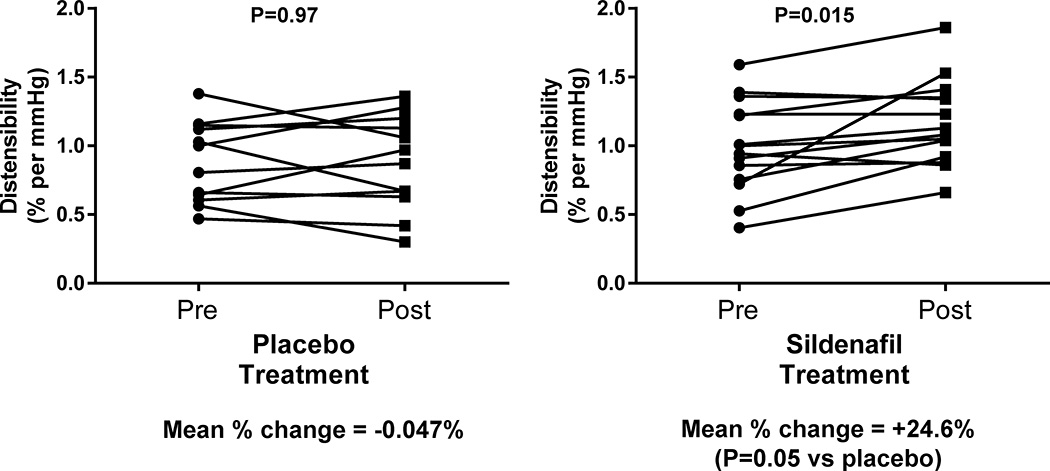
We assessed whether abnormal PV distensibility in HF could be improved with 12 weeks of treatment with the pulmonary vasodilator sildenafil.33 Of 26 patients with HFrEF, 14 patients were treated with sildenafil (25 to 75 mg orally three times per day) and 12 patients were treated with placebo in a double-blinded fashion. These patients underwent comprehensive CPET before and after treatment. Both the sildenafil-treated group (0.99 ± 0.34% per mmHg) and the placebo-treated group (0.88 ± 0.29% per mmHg) had similar baseline PV distensibilities (P=0.38). The average post-treatment distensibility in the sildenafil-treated group was 1.17 ± 0.31% per mmHg and that in the placebo-treated group was 0.88 ± 0.34% per mmHg. The sildenafil-treated group demonstrated a 24.6% increase in distensibility (Figure 5, P=0.015), whereas the placebo-treated arm had no significant change (P=0.05 compared to the sildenafil group). In the sildenafil-treated group, the change in distensibility with treatment was associated with an improved augmentation in RVEF with exercise (Spearman's ρ=0.80, P=0.01). Taken together, these findings indicate that the selective pulmonary vasodilator sildenafil is able to improve PV distensibility in HFrEF and that improvements in PV distensibility are associated with improvements in RV function.
Figure 5. Twelve weeks of treatment with sildenafil improves pulmonary vascular distensibility in patients with heart failure and reduced ejection fraction.
Sildenafil treatment was associated with a 24.6% increase in distensibility (n=14, P=0.015) while no appreciable change in distensibility was observed in the placebo-treated group (n=12). Treatment with a selective pulmonary vasodilator can therefore improve pulmonary vascular distensibility.
DISCUSSION
In this study, we utilized the distensible vessel model13 to characterize pulmonary vascular distensibility for the first time in heart failure patients. Average PV distensibility was significantly lower in HFpEF and HFrEF (34% and 40% lower, respectively) compared to control subjects of similar age. On average, abnormal distensibility in HF patients, compared to controls, led to a more than 70% increased level of peak exercise transpulmonary gradients. PV distensibility was closely associated with change in RVEF in response to exercise as well as peak VO2 and other indices of cardiac function during exercise. PV distensibility was also an independent predictor of CV survival in HF patients. Moreover, treatment of HFrEF patients with a selective pulmonary vasodilator increased PV distensibility by approximately 25% and this improvement was associated with improved RV function with exercise.
The presence of PH in HFpEF and HFrEF predicts functional capacity and long-term outcomes.4, 7 The strategy of regularly measuring PAP and adjusting therapies accordingly in the CHAMPION trial reduced HF-related hospitalizations by 39% and led to recent FDA approval of an implantable device to continuously monitor PAP in HF.27 Moreover, PAP responses to volume challenge and exercise have recently been recognized to provide additive diagnostic and prognostic value in evaluating patients with HF.2, 41, 42 Therefore, understanding the determinants of PAP during exercise or other states of increased blood flow in patients with HF is critically important. While the standard ohmic-Starling model of the pulmonary circulation describes PAP as dependent on three variables (PVR, CO, and PAWP), this model does not account for the curvilinear relationship observed between PAP and flow at higher levels of CO due to the distensible nature and increased recruitment of the pulmonary vessels. Here, we describe the impact that this fourth variable, PV distensibility, has on PAP responses during exercise. In our study, abnormal PV distensibility in HF patients accounted for over 30% of the rise in PAP with exercise. PV distensibility was also a sensitive and specific measure to distinguish either HF (AUC 0.86) or PAH (AUC 1.0) from controls based on ROC analysis. Future studies will need to explore the ability of non-invasive exercise measures of PV distensibility, such as with exercise echocardiography,26 to act as surrogates of PV distensibility derived from invasive measurements, in an effort to broaden its clinical use.
The average PV distensibility in control subjects was 1.39 ± 0.32% per mmHg. This value is lower than that previously observed in a normal healthy population (2.0 ± 0.2% per mmHg);14 however, our control subjects were older than that of the previous study and were undergoing evaluation for dyspnea on exertion. We did not observe a difference in distensibility between genders, in contrast to previous studies that found young females have greater distensibility than males, potentially owing to the older age and post-menopausal status of females in our studies.43 PV distensibility is known to decrease with age (Table 2), as shown in our study and others.14, 43 It is also possible that the lower PV distensibility observed in our control population with dyspnea compared to the normal population in the prior study may reflect signs of early pulmonary vascular disease.14, 40 A recent study by Lau and colleagues found PV distensibility to be abnormally low in patients with pulmonary vascular disease (e.g., confirmed by the presence of thromboembolic disease of the pulmonary circulation or an abnormal lung biopsy) but with mPAP < 25 mmHg at rest, indicating that PV distensibility may be a useful index for early disease detection.40 The study by Lau et al. and our study highlight the ability of exercise-based hemodynamic measurements to add incremental information to resting hemodynamic measurements to carefully define pulmonary vascular function in patients with suspected PH. The striking differences in distensibility between patients with “mild” PAH in our study versus controls indicates that pathological reduction in distensibility may represent a relatively early finding in PAH in light of the fact that the mean PAP was only 28 mmHg in our PAH population.
While PV distensibility did not correlate with resting cardiac indices, it was a strong predictor of cardiac indices at peak exercise (Table 2). Peak VO2 serves as the gold standard measure of exercise capacity and also a significant prognostic indicator in HF patients.39 Our finding that PV distensibility predicts peak VO2 independent of other demographic and CPET variables indicates the important contribution PV distensibility has as a marker of maximal exercise capacity in HF and PAH patients. Similar to our findings, in a study of 24 healthy subjects, those with the greatest PV distensibility exhibited the highest peak VO2, further supporting its possible role in determining exercise capacity.26
Increased stiffness of the pulmonary artery measured by combining right heart catheterization and cardiac magnetic resonance measurements is associated with worse RV performance, dilation, and hypertrophy with chronic PH.44 We observed a strong correlation between impaired PV distensibility, measured with exercise hemodynamic parameters, and abnormal RVEF augmentation during exercise, measured using the independent technique of ventriculography (Figure 3). The association between low PV distensibility and reduced RVEF augmentation with exercise was observed not only in HF and PAH patients, but also in control patients. These results suggest that PV distensibility is an important determinant of RV function that complements imaging-based assessments of RV function. We found that PV distensibility predicted cardiovascular survival independently of peak VO2 in HF patients. RVEF at rest and with exercise are important predictors of outcomes and exercise capacity in HF patients.6, 45 PV distensibility is likely a critical property of the pulmonary vessels that helps to limit the afterload that the right ventricle confronts. Therefore, therapeutically targeting RV function and PV distensibility may improve outcomes in HF.
Multiple mechanisms may contribute to abnormal pulmonary vascular function and distensibility in HF including both functional and structural alterations.1 Studies in both experimental models and patients have suggested a maladaptive imbalance between pulmonary vasodilators and vasoconstrictors in HF. Dysregulated arginine metabolism and nitric oxide (NO) signaling may contribute to the pathophysiology of PH in HF.46 Decreased production of prostacyclin and increased pulmonary expression of endothelin have also been implicated in the pathophysiology of PH in HF.47, 48 Similar to PAH, structural remodeling of the pulmonary vasculature can also occur in HF, including intimal fibrosis and medial vessel hypertrophy.49 In our study, HFrEF patients treated with 12 weeks of PDE5 inhibitor therapy demonstrated a 24.6% increase in PV distensibility (Figure 5). Improvement in PV distensibility with short-term PDE5 inhibitor therapy suggests that a strong contributor to reduced distensibility in HF is via abnormal vasodilator (NO-dependent) signaling. More studies are needed to determine the long-term benefits of treatments directed at the pulmonary vasculature, such as PDE5 therapy, in patients with HF and reduced PV distensibility.
LIMITATIONS
This study was derived from a single-center patient cohort of patients referred to a tertiary care center, which may not be representative of the general HF or PAH populations. Multiple hypotheses were tested regarding the association of PV distensibility with exercise physiology parameters (Table 2), increasing the chance of a type I error. However, exact P-values are included and the Bonferroni-adjusted P value of significance is provided, to limit the type I error. Our control population was limited in size (n=30) because of the low frequency with which subjects without significant cardiopulmonary disease undergo CPET with invasive hemodynamic monitoring. Moreover, our control population cannot be considered as normal individuals given that they were undergoing CPET for the evaluation of dyspnea. The use of clinically referred patients who had normal hemodynamic measurements as controls may underestimate the differences between HF patients and true controls.
The distensible vessel model used in our study makes the assumption of a constant hematocrit.13, 14 However, hemoconcentration does occur to some degree during exercise. Since we perform individualized exercise protocols designed to achieve similar exercise durations, based on our prior experience with cardiopulmonary exercise testing, the degree of hemoconcentration is similar across patients. However, we do not have serial hemoglobin measurements to report in this study. Furthermore, it is important to note that the recruitment of pulmonary vessels during exercise can be differentially affected in HF versus control patients by variability in body position, direct compression of an enlarged heart on pulmonary tissue, and in the setting of elevated filling pressures and PAP in HF, there is less pulmonary vasculature to recruit as flow and pressures increment during exercise.41 The distensible vessel model does not differentiate between the distension that occurs of perfused pulmonary vessels during exercise and the recruitment of previously closed vessels.14
CLINICAL IMPLICATIONS
Despite advances in HF therapy, morbidity and mortality remain high, particularly when PH is present. Chronic elevations in PAP exert negative hemodynamic effects on RV function which range from RV hypertrophy to RV failure. The pulmonary vessels distend to protect the afterload-sensitive RV from excessive increases in pressure. Historically, elevated left-sided hydrostatic pressure has been thought to primarily govern the increased load on the RV at rest and in response to physiologic stressors in HF patients. Our findings that PV distensibility was closely related to change in RVEF during exercise, whereas PAWP was not, suggest that intrinsic properties of the pulmonary circulation play an important role in determining RV reserve capacity in HF. Further study is warranted to determine if PV distensibility can be used to inform candidate selection for heart transplantation or left ventricular assist device therapy, as clinical outcomes after these interventions are dependent on RV-PV function following the procedure.
The RV-PV unit is increasingly recognized as a major determinant of exercise capacity and outcomes in patients with HF. However, the pulmonary circulation has not been effectively targeted for therapeutic interventions. Multicenter clinical trials to date that have evaluated the efficacy of pulmonary vasodilator therapy (i.e. prostacyclins, PDE5 inhibitors, and endothelin antagonists) in patients with heart failure have not shown clinical benefit.1, 50 However, these studies of pulmonary vasodilators in HF have enrolled patients independently of their burden of pre-capillary PH or other indices that specifically reflect PV function. Integrating measurement of PV distensibility into the characterization of PH in HF may help to appropriately identify patients for targeted interventions directed at the pulmonary circulation.
CONCLUSION
Abnormally low PV distensibility in HF is a previously unrecognized important determinant of PAP elevation that is related to impaired cardiac performance during exercise and poor outcomes. Treatment with a PDE5 inhibitor appears to improve PV distensibility. These findings support the pursuit of further studies aimed at understanding and therapeutically targeting right ventricular and pulmonary vascular dysfunction in heart failure.
Clinical Perspective.
Morbidity and mortality in heart failure (HF) remain high, particularly when pulmonary hypertension and/or right ventricular dysfunction are present. Pulmonary vascular (PV) distensibility, defined as the percent increase in pulmonary vessel diameter per mmHg increase in pressure, permits the pulmonary arteries to increase in size to accommodate increased blood flow, thereby reducing the afterload of the right ventricle. This study combined exercise testing and invasive hemodynamic measurements to derive PV distensibility values and to determine that PV distensibility is reduced in HF patients with either reduced ejection fraction or preserved ejection fraction. We show that impaired PV distensibility in HF is associated with worse RV function during exercise and reduced peak VO2, and also predicts poor cardiovascular survival. In a randomized, placebo-controlled study of 26 HF patients with reduced ejection fraction, we demonstrated that treatment with sildenafil can improve PV distensibility. This study highlights an important role for pulmonary vascular responses to exercise in determining prognosis for HF patients and potentially identifies a subset of HF patients who would benefit from therapy selectively targeting impaired pulmonary vascular function.
Acknowledgments
Sources of Funding
Dr. Malhotra was supported by the Fellow-to-Faculty Transition Award 11FTF7290032 from the American Heart Association, the Wild Family Foundation, and the K08HL111210 grant from the National Heart, Lung, and Blood Institute. Dr. Lewis was supported by the American Heart Association Award 15GPSGC24800006, the National Institutes of Health grant R01-HL131029 and the Hassenfeld Clinical Scholar Award. Support for the sildenafil versus placebo trial was provided by Pfizer.
Disclosures
Dr. Malhotra serves as a consultant for Mallinckrodt Pharmaceuticals, formerly Ikaria, Inc.
REFERENCES
- 1.Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, Gomberg-Maitland M, Murali S, Frantz RP, McGlothlin D, Horn EM, Benza RL. World health organization pulmonary hypertension group 2: Pulmonary hypertension due to left heart disease in the adult--a summary statement from the pulmonary hypertension council of the international society for heart and lung transplantation. J Heart Lung Transplant. 2012;31:913–933. doi: 10.1016/j.healun.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–1479. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 3.Benza RL, Raina A, Abraham WT, Adamson PB, Lindenfeld J, Miller AB, Bourge RC, Bauman J, Yadav J. Pulmonary hypertension related to left heart disease: Insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant. 2015;34:329–337. doi: 10.1016/j.healun.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34:1802–1806. doi: 10.1016/s0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 5.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 6.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 7.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung CC, Moondra V, Catherwood E, Andrus BW. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am J Cardiol. 2010;106:284–286. doi: 10.1016/j.amjcard.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 9.Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Rande JL, Hittinger L, Clark AL, Cleland JG. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (tapse) among out-patients referred for the evaluation of heart failure. J Card Fail. 2012;18:216–225. doi: 10.1016/j.cardfail.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed SF, Hussain I, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: A community-based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naeije R, Chesler N. Pulmonary circulation at exercise. Compr Physiol. 2012;2:711–741. doi: 10.1002/cphy.c100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naeije R, Vanderpool R, Dhakal BP, Saggar R, Vachiery JL, Lewis GD. Exercise-induced pulmonary hypertension: Physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187:576–583. doi: 10.1164/rccm.201211-2090CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linehan JH, Haworth ST, Nelin LD, Krenz GS, Dawson CA. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol (1985) 1992;73:987–994. doi: 10.1152/jappl.1992.73.3.987. [DOI] [PubMed] [Google Scholar]
- 14.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol. 2005;288:L419–L425. doi: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- 15.Frise MC, Robbins PA. The pulmonary vasculature - lessons from tibetans and from rare diseases of oxygen sensing. Exp Physiol. 2014;100:1233–1241. doi: 10.1113/expphysiol.2014.080507. [DOI] [PubMed] [Google Scholar]
- 16.Ghio S, Schirinzi S, Pica S. Pulmonary arterial compliance: How and why should we measure it? Glob Cardiol Sci Pract. 2015;2015:58. doi: 10.5339/gcsp.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanz J, Kariisa M, Dellegrottaglie S, Prat-Gonzalez S, Garcia MJ, Fuster V, Rajagopalan S. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2:286–295. doi: 10.1016/j.jcmg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Kang KW, Chang HJ, Kim YJ, Choi BW, Lee HS, Yang WI, Shim CY, Ha J, Chung N. Cardiac magnetic resonance imaging-derived pulmonary artery distensibility index correlates with pulmonary artery stiffness and predicts functional capacity in patients with pulmonary arterial hypertension. Circ J. 2011;75:2244–2251. doi: 10.1253/circj.cj-10-1310. [DOI] [PubMed] [Google Scholar]
- 19.Swift AJ, Rajaram S, Condliffe R, Capener D, Hurdman J, Elliot C, Kiely DG, Wild JM. Pulmonary artery relative area change detects mild elevations in pulmonary vascular resistance and predicts adverse outcome in pulmonary hypertension. Invest Radiol. 2012;47:571–577. doi: 10.1097/RLI.0b013e31826c4341. [DOI] [PubMed] [Google Scholar]
- 20.Bogren HG, Klipstein RH, Mohiaddin RH, Firmin DN, Underwood SR, Rees RS, Longmore DB. Pulmonary artery distensibility and blood flow patterns: A magnetic resonance study of normal subjects and of patients with pulmonary arterial hypertension. Am Heart J. 1989;118:990–999. doi: 10.1016/0002-8703(89)90235-4. [DOI] [PubMed] [Google Scholar]
- 21.Pasierski TJ, Starling RC, Binkley PF, Pearson AC. Echocardiographic evaluation of pulmonary artery distensibility. Chest. 1993;103:1080–1083. doi: 10.1378/chest.103.4.1080. [DOI] [PubMed] [Google Scholar]
- 22.Revel MP, Faivre JB, Remy-Jardin M, Delannoy-Deken V, Duhamel A, Remy J. Pulmonary hypertension: Ecg-gated 64-section ct angiographic evaluation of new functional parameters as diagnostic criteria. Radiology. 2009;250:558–566. doi: 10.1148/radiol.2502080315. [DOI] [PubMed] [Google Scholar]
- 23.Kasai H, Sugiura T, Tanabe N, Sakurai Y, Yahaba M, Matsuura Y, Shigeta A, Kawata N, Sakao S, Kasahara Y, Tatsumi K. Electrocardiogram-gated 320-slice multidetector computed tomography for the measurement of pulmonary arterial distensibility in chronic thromboembolic pulmonary hypertension. PLoS One. 2014;9:e111563. doi: 10.1371/journal.pone.0111563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau EM, Iyer N, Ilsar R, Bailey BP, Adams MR, Celermajer DS. Abnormal pulmonary artery stiffness in pulmonary arterial hypertension: In vivo study with intravascular ultrasound. PLoS One. 2012;7:e33331. doi: 10.1371/journal.pone.0033331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellofiore A, Henningsen J, Lepak CG, Tian L, Roldan-Alzate A, Kellihan HB, Consigny DW, Francois CJ, Chesler NC. A novel in vivo approach to assess radial and axial distensibility of large and intermediate pulmonary artery branches. J Biomech Eng. 2015;137:044501. doi: 10.1115/1.4029578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalande S, Yerly P, Faoro V, Naeije R. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol. 2012;590:4279–4288. doi: 10.1113/jphysiol.2012.234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, Group CTS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 28.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of exercise testing and interpretation. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 29.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. The American review of respiratory disease. 1984;129:S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 30.Gandevia B, Hugh-Jones P. Terminology for measurements of ventilatory capacity; a report to the thoracic society. Thorax. 1957;12:290–293. doi: 10.1136/thx.12.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: The role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 33.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 34.Stringer WW, Hansen JE, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol. 1997;82:908–912. doi: 10.1152/jappl.1997.82.3.908. [DOI] [PubMed] [Google Scholar]
- 35.Agostoni PG, Wasserman K, Perego GB, Guazzi M, Cattadori G, Palermo P, Lauri G, Marenzi G. Non-invasive measurement of stroke volume during exercise in heart failure patients. Clin Sci (Lond) 2000;98:545–551. [PubMed] [Google Scholar]
- 36.Finch CA, Lenfant C. Oxygen transport in man. The New England journal of medicine. 1972;286:407–415. doi: 10.1056/NEJM197202242860806. [DOI] [PubMed] [Google Scholar]
- 37.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 38.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. European journal of heart failure. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 40.Lau EM, Chemla D, Godinas L, Zhu K, Sitbon O, Savale L, Montani D, Jais X, Celermajer DS, Simonneau G, Humbert M, Herve P. Loss of vascular distensibility during exercise is an early hemodynamic marker of pulmonary vascular disease. Chest. Chest. 2016;149:353–361. doi: 10.1378/chest.15-0125. [DOI] [PubMed] [Google Scholar]
- 41.Lewis GD, Murphy RM, Shah RV, Pappagianopoulos PP, Malhotra R, Bloch KD, Systrom DM, Semigran MJ. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail. 2011;4:276–285. doi: 10.1161/CIRCHEARTFAILURE.110.959437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Argiento P, Vanderpool RR, Mule M, Russo MG, D'Alto M, Bossone E, Chesler NC, Naeije R. Exercise stress echocardiography of the pulmonary circulation: Limits of normal and sex differences. Chest. 2012;142:1158–1165. doi: 10.1378/chest.12-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens GR, Garcia-Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J. Rv dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging. 2012;5:378–387. doi: 10.1016/j.jcmg.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Baker BJ, Wilen MM, Boyd CM, Dinh H, Franciosa JA. Relation of right ventricular ejection fraction to exercise capacity in chronic left ventricular failure. Am J Cardiol. 1984;54:596–599. doi: 10.1016/0002-9149(84)90256-x. [DOI] [PubMed] [Google Scholar]
- 46.Shao Z, Wang Z, Shrestha K, Thakur A, Borowski AG, Sweet W, Thomas JD, Moravec CS, Hazen SL, Tang WH. Pulmonary hypertension associated with advanced systolic heart failure: Dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase-1. J Am Coll Cardiol. 2012;59:1150–1158. doi: 10.1016/j.jacc.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galie N, Manes A, Branzi A. Prostanoids for pulmonary arterial hypertension. Am J Respir Med. 2003;2:123–137. doi: 10.1007/BF03256644. [DOI] [PubMed] [Google Scholar]
- 48.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: The role of the endothelium in pathophysiology and management. Circulation. 2000;102:1718–1723. doi: 10.1161/01.cir.102.14.1718. [DOI] [PubMed] [Google Scholar]
- 49.Dupuis J, Guazzi M. Pathophysiology and clinical relevance of pulmonary remodelling in pulmonary hypertension due to left heart diseases. Can J Cardiol. 2015;31:416–429. doi: 10.1016/j.cjca.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA : the journal of the American Medical Association. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]