Abstract
In this study we sought to determine if among individuals with urolithiasis, extracorporeal shock wave lithotripsy (SWL) and ureteroscopy are associated with a higher risk of incident arterial hypertension (HTN) and/or chronic kidney disease (CKD). This was measured in a population-based retrospective study of 11,570 participants with incident urolithiasis and 127,464 without urolithiasis in The Health Improvement Network. Patients with pre-existing HTN and CKD were excluded. The study included 1319 and 919 urolithiasis patients with at least one SWL or URS procedure, respectively. Multivariable Cox regression was used to estimate the hazard ratio for incident CKD stage 3–5 and HTN in separate analyses. Over a median of 3.7 and 4.1 years, 1423 and 595 of urolithiasis participants developed HTN and CKD, respectively. Urolithiasis was associated with a significant hazard ratio each for HTN of 1.42 (95% CI: 1.35, 1.51) and for CKD of 1.82 (1.67, 1.98). SWL was associated with a significant increased risk of HTN 1.34 (1.15, 1.57), while ureteroscopy was not. When further stratified as SWL to the kidney or ureter, only SWL to the kidney was significantly and independently associated with HTN 1.40 (1.19, 1.66). Neither SWL nor ureteroscopy was associated with incident CKD. Since urolithiasis itself was associated with a hazard ratio of 1.42 for HTN, an individual who undergoes SWL to the kidney can be expected to have a significantly increased hazard ratio for HTN of 1.96 (1.67, 2.29) compared to an individual without urolithiasis.
Keywords: urolithiasis, shock wave lithotripsy, ureteroscopy, hypertension, chronic kidney disease
INTRODUCTION
Several studies have demonstrated that urolithiasis is associated with increased morbidity and mortality, including a higher risk of cardiovascular events,1 hypertension,2,3 chronic kidney disease (CKD),4 and fractures.5 The mechanisms by which urolithiasis might be either associated with or cause hypertension remain unclear. Altered nephron physiology predisposing to both renal calculi and hypertension, higher sodium intake amongst both stone formers and hypertensive individuals, direct renal injury from urologic interventions, and increased prevalence of metabolic syndrome, gout, or CKD in both populations have been proposed.6 Similarly, the possible mechanisms which might account for the increased risk of CKD associated with urolithiasis include: renal parenchymal crystal deposition;7 prolonged and repeated episodes of obstruction; direct damage from urologic interventions; recurrent episodes of pyelonephritis; or undiagnosed, purportedly rare inherited conditions such as cystinuria, Dent disease, or primary hyperoxaluria.
Historically, ureteric and renal calculi were managed by open surgical techniques. Currently, extracorporeal shock wave lithotripsy (SWL) and ureteroscopy (URS) account for more than 90% of these procedures.8–10 Although SWL was initially thought to be harmless to the kidney,11,12 subsequent animal models have demonstrated that the shock waves cause alterations in renal hemodynamics with resultant ischemic injury to the renal tubules and microvasculature.13 There have also been several clinical reports of acute kidney injury,14 hypertension,15,16 renal morphological changes,17 increased urinary inflammatory cytokines,18 and transient elevation of urinary enzymes, such as N-acetyl-β-glucosaminidase.19
To date there have not been any large epidemiological studies or randomized trials which have assessed the risk of developing CKD or hypertension after SWL or URS treatments. As a result, considerable controversy exists about whether SWL-induced acute changes ultimately result in CKD or long-term hypertension. Most studies suggest that SWL does not result in decreased glomerular filtration rate (GFR).20,21 Studies regarding SWL-associated hypertension have become a matter of debate as there are conflicting data.15,22–25 These studies were generally comprised of small cohorts of fewer than several hundred patients with short follow-up times and relied on surveys or self-reports.12,15,20,22–26 Long-term outcome data on the risk of developing hypertension or CKD after URS is lacking.
The Health Improvement Network (THIN) database has been used to study hypertension,27–31 CKD,32–34 and urolithiasis.5 The objectives of this large population-based cohort study were to determine if among individuals with urolithiasis, SWL is associated with a higher risk of incident hypertension and/or CKD, defined by GFR, if the location of the SWL (kidney versus ureter) impacted these associations, and if URS is associated with a higher risk of incident hypertension and/or CKD.
RESULTS
Cohort Characteristics
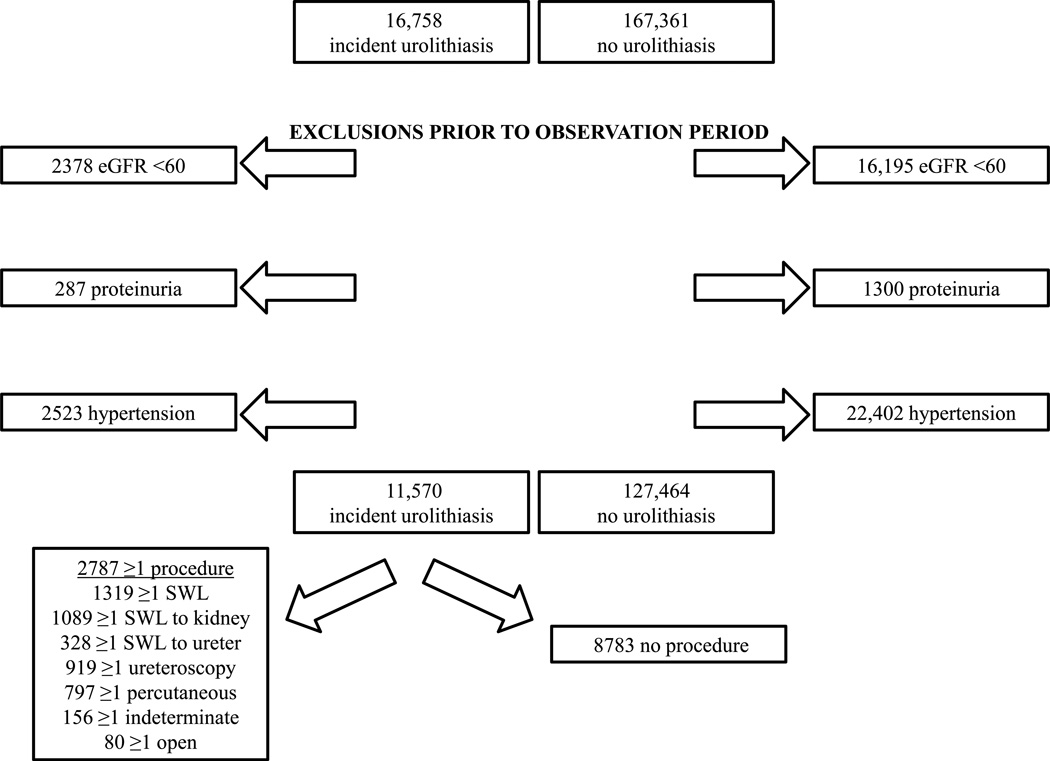
Our cohort comprised 11,570 participants with incident urolithiasis and 127,464 unexposed participants, matched on age, gender, and practice, all of whom at the start of observation had not been diagnosed with hypertension or proteinuria or had a serum creatinine measure consistent with an estimated GFR <60 m/min/1.73m2. 2787 (24%) of the participants with urolithiasis had at least one intervention; SWL and particularly SWL to the kidney were the most common, performed in 47% and 39% of those who had an intervention, respectively (Figure 1). The baseline characteristics of the cohort are shown in Table 1. As expected based on the established epidemiology of urolithiasis,35 there were twice as many men with urolithiasis than women. Also as anticipated and as demonstrated in our prior study,5 diabetes mellitus, gout, and obesity were all more prevalent among the urolithiasis population. 1319 and 919 of the participants with urolithiasis had at least one SWL or URS procedure, respectively. The gender distribution and prevalence of diabetes, gout and obesity in participants who had SWL, URS, both, or neither did not differ, but age distribution did (p <0.001). Among the participants who had SWL, age and prevalence of diabetes, obesity, and gout in those who had SWL to the kidney, ureter, or both did not differ. The proportion of males having SWL to the ureter was greater (p = 0.003). The median calendar year for start of observation was 2006 [inter-quartile range (IQR) 2002–2009].
Figure 1.
Participant flow diagram.
Table 1.
Baseline cohort characteristics.
| N | Age | Male | Diabetes | Gout | BMI ≥30a | |
|---|---|---|---|---|---|---|
| Urolithiasis | 11,570 | 45 (36, 56) | 7648 (66.1) | 584 (5.1) | 226 (2.0) | 1267 (28.8) |
| No Urolithiasis | 127,464 | 46 (36, 57) | 83,802 (65.8) | 3709 (2.9) | 2092 (1.6) | 8908 (22.6) |
| p value | <0.001 | 0.01 | <0.001 | |||
| SWL | 1165 | 46 (38, 57) | 744 (63.9) | 49 (4.2) | 22 (1.9) | 112 (26.9) |
| URS | 765 | 45 (36, 57) | 526 (68.8) | 44 (5.8) | 13 (1.7) | 85 (32.2) |
| SWL and URS | 154 | 49.5 (40, 61) | 108 (70.1) | 10 (6.5) | 4 (2.6) | 19 (35.2) |
| No SWL or URS | 9486 | 45 (35, 56) | 6270 (66.1) | 481 (5.1) | 187 (2.0) | 1051 (28.7) |
| p value | <0.001 | 0.11 | 0.36 | 0.89 | 0.34 | |
| SWL Kidney | 991 | 46 (37, 57) | 615 (62.1) | 48 (4.8) | 17 (1.7) | 99 (28.0) |
| SWL Ureter | 230 | 48 (39, 58) | 164 (71.3) | 10 (4.4) | 6 (2.6) | 18 (22.5) |
| SWL Kidney and Ureter | 98 | 47.5 (38, 57) | 73 (74.5) | 1 (1.0) | 3 (3.1) | 14 (37.8) |
| p value | 0.49 | 0.003 | 0.22 | 0.49 | 0.23 |
Data presented as median (inter-quartile range) or n (%).
N = 4396 for urolithiasis and N = 39,338 for no urolithiasis.
BMI – body mass index, SWL – extracorporeal shock wave lithotripsy, URS – ureteroscopy.
Incidence of Hypertension
Over a median observation period for ascertainment of incident hypertension of 3.7 (IQR 1.6–6.8) and 3.6 (IQR 1.5–6.7) years in participants with and without urolithiasis, respectively, 1423 (12.3%) of those with urolithiasis and 10,934 (8.6%) of unexposed participants developed hypertension. Median age at start of observation was 53 years among participants with urolithiasis who developed hypertension, and 71.8% were male, 6.9% had diabetes, and 2.8% had gout. Median age was 56 years among participants without urolithiasis who developed hypertension, and 69.0% were male, 5.3% had diabetes, and 3.3% had gout. Median time to development of hypertension was 3.1 years (IQR 1.4–5.6), and only 43 individuals (0.03%) had their initial code for hypertension on the same date as their start of observation. Of the 49 diagnosis codes for incident hypertension among these participants, the five most frequently used, accounting for 87%, were: "G20..00: Essential hypertension" (47%), “662..12: Hypertension monitoring” (17%), “G2…00: Hypertensive disease” (14%), “662P.00: Hypertension monitoring” (6%), “G20..11: High blood pressure” (3%). In multivariable cox regression adjusted for age, gender, diabetes, gout, and calendar time, urolithiasis was associated with an increased risk of hypertension (HR 1.42; 95% CI: 1.35, 1.51; p < 0.001).
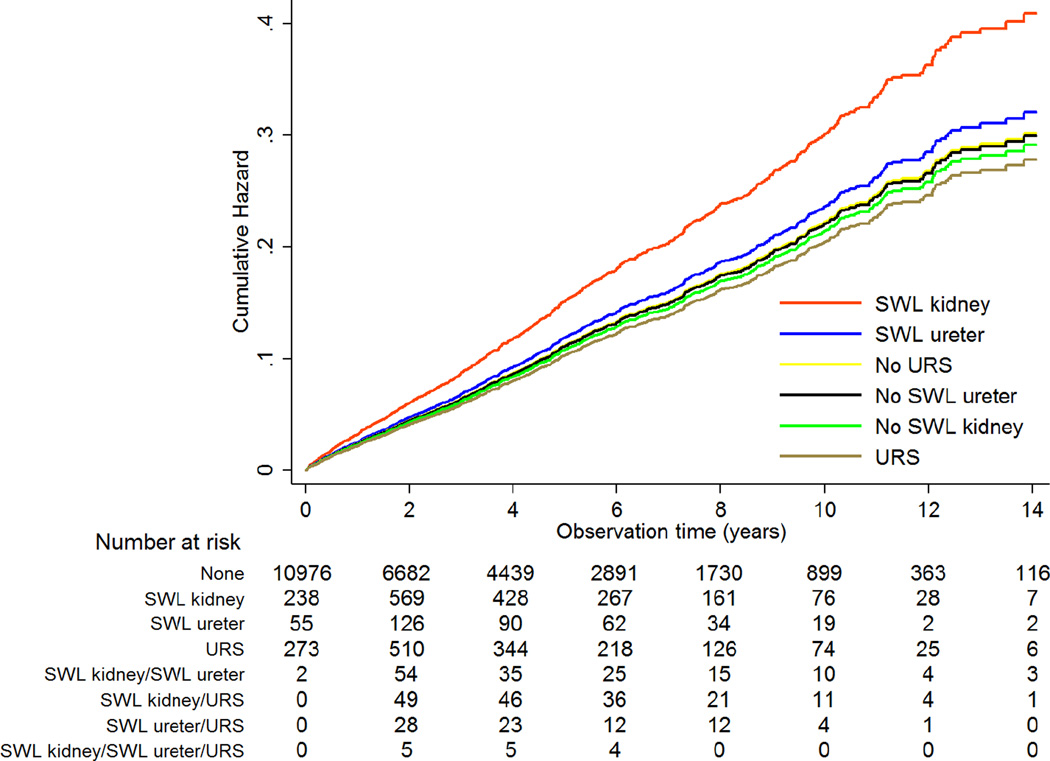
In multivariate analysis among the participants with urolithiasis, adjusted for gender, age, diabetes, gout, and calendar year, exposure to SWL was associated with a significantly increased risk of hypertension (HR 1.34; 95% CI: 1.15, 1.57; p <0.001). As shown in Table 2, when SWL procedures were further stratified by site as SWL to the kidney or ureter, only SWL to the kidney was independently associated with incident hypertension with a HR of 1.40 (95% CI: 1.19, 1.66; p <0.001), as compared to all other urolithiasis participants. The median times from SWL to the kidney, SWL to the ureter, and ureteroscopy to development of incident hypertension were 3.3 years (IQR 1.5–5.2), 3.7 (IQR 1.4–7.0), and 2.8 (1.0–4.7), respectively. SWL to the ureter and URS were not associated not with incident hypertension. Model 4 in Table 2 violated proportional hazards. However, when the model was fit stratified by age at start of observation, the proportional hazards assumption was no longer violated. We then fit model 4 with the addition of a linear time-varying covariate for age, which yielded the same HR for SWL to the kidney of 1.40 (p <0.001), indicating that this HR is robust to violation of proportional hazards. Additional adjustment for percutaneous, open and indeterminate procedures did not attenuate the HR of 1.40 (p <0.001) for SWL kidney, and none of these procedures were associated themselves with hypertension. Specifically for percutaneous procedures which were not infrequent, the HR was 1.12 (95% CI: 0.91–1.36; p = 0.28). Although overall SWL to the ureter was not associated with hypertension, there was a statistically significant interaction with age (p = 0.003); SWL to the ureter was associated with a HR of 2.91 (95% CI: 1.55, 5.48; p = 0.001) in participants <40 years of age, but not in older participants. However, this only represents 11 individuals of the 87 exposed to SWL to the ureter in this age group. An interaction with age was not observed for SWL to the kidney or URS. Figure 2 shows the cumulative hazard for hypertension among the urolithiasis population according to their procedure exposure and illustrates how undergoing SWL to the kidney, relative to other procedures (or no procedure), increases the cumulative hazard for incident hypertension. Given that urolithiasis itself was associated with a HR of 1.42 for hypertension, we performed additional multivariable cox regression analysis within the full cohort to demonstrate that an individual who undergoes SWL to the kidney can be expected to have a HR for incident hypertension of 1.96 (95% CI: 1.67, 2.29; p <0.001) as compared to an individual without urolithiasis (see Supplementary material for details of analysis). We performed additional analyses investigating dose response. We required that there be >14 days between procedure codes to be considered discrete events. Among participants who had SWL to the kidney, 69.5%, 18.9%, and 11.6% had 1, 2, and ≥3 procedures, respectively. Treating SWL to the kidney as a time-varying categorical exposure and adjusted for gender, age, diabetes, gout, calendar year, SWL to the ureter, and URS, the HR for 1, 2, and ≥3 SWL procedures to the kidney was 1.33 (95% CI: 1.09, 1.63), 1.47 (95% CI: 1.03, 2.11), and 1.74 (95% CI: 1.15, 2.64), respectively. Only 14.0% and 5.9% of participants who underwent SWL to the ureter and URS had >1 of these procedures, respectively, but the number of SWL ureter and URS procedures did not influence the risk of hypertension.
Table 2.
Multivariable cox regression analysis of incident hypertension risk among participants with urolithiasis.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p |
| Female | 0.82 | (0.73, 0.92) | 0.001 | 0.82 | (0.73, 0.92) | 0.001 | 0.82 | (0.73, 0.92) | 0.001 | 0.82 | (0.73, 0.92) | 0.001 |
| Age (yr) | 1.04 | (1.04, 1.04) | <0.001 | 1.04 | (1.04, 1.04) | <0.001 | 1.04 | (1.04, 1.04) | <0.001 | 1.04 | (1.04, 1.04) | <0.001 |
| Diabetes | 1.37 | (1.10, 1.69) | 0.004 | 1.36 | (1.10, 1.69) | 0.004 | 1.36 | (1.10, 1.69) | 0.004 | 1.37 | (1.11, 1.70) | 0.004 |
| Gout | 1.01 | (0.73, 1.39) | 0.97 | 1.00 | (0.72, 1.38) | 0.99 | 1.00 | (0.72, 1.38) | 0.98 | 1.01 | (0.73, 1.40) | 0.96 |
| Calendar yr | 0.98 | (0.96, 0.99) | 0.003 | 0.98 | (0.96, 0.99) | 0.004 | 0.98 | (0.96, 0.99) | 0.004 | 0.98 | (0.96, 0.99) | 0.004 |
| SWL kidney | 1.41 | (1.19, 1.66) | <0.001 | 1.40 | (1.19, 1.66) | <0.001 | ||||||
| SWL ureter | 1.14 | (0.84, 1.55) | 0.42 | 1.07 | (0.79, 1.46) | 0.67 | ||||||
| URS | 0.93 | (0.76, 1.15) | 0.52 | 0.92 | (0.75, 1.14) | 0.44 | ||||||
SWL – extracorporeal shock wave lithotripsy, URS – ureteroscopy
Figure 2.
Cumulative hazard for incident hypertension according to time-varying procedure exposure.
In a sensitivity analysis among participants with urolithiasis who had BMI data (n = 4387), additional adjustment for obesity in addition to gender, age, diabetes, gout, and calendar time only strengthened model findings. Obesity was associated with a two-fold increased risk of hypertension (HR 2.07; p <0.001), and SWL to the kidney remained independently associated with hypertension (HR of 1.55, 95% CI: 1.17, 2.05; p = 0.002), while SWL to the ureter and URS were not.
Incidence of CKD
Over a median observation period for ascertainment of incident CKD of 4.1 (IQR 1.8–7.3) and 3.9 (IQR 1.7–7.1) years in participants with and without urolithiasis, respectively, 595 (5.1%) of those with urolithiasis and 3814 (3.0%) of those without urolithiasis developed CKD. Median age at start of observation was 62 years among participants with urolithiasis who developed CKD, and 58.7% were male, 11.6% had diabetes, and 5.0% had gout. Median age was 66 years among participants without urolithiasis who developed CKD, and 56.5% were male, 7.4% had diabetes, and 3.7% had gout. In multivariable cox regression adjusted for age, gender, diabetes, gout, and calendar time, urolithiasis was associated with a HR of 1.82 (95% CI: 1.67, 1.98; p <0.001). In multivariate analysis among the participants with urolithiasis, adjusted for gender, age, diabetes, gout, and calendar time, SWL was not associated with incident CKD (HR 1.01, 95% CI: 0.79, 1.29; p = 0.95). There was no association of SWL with CKD when stratified by SWL site (Table 3). Adjusted for gender, age, diabetes, gout, calendar time, and SWL, URS was associated with a borderline significant lower risk of CKD (HR 0.70, 95% CI: 0.49, 1.00; p = 0.05); however, given the multiple different regression models, this finding may be spurious. The median times from SWL to the kidney, SWL to the ureter, and ureteroscopy to development of incident CKD were 4.1 (IQR 2.2–6.9), 4.4 (IQR 2.2–6.6), and 3.9 (IQR 2.2–6.6), respectively. There were no interactions of procedures with age. There was no association of SWL with CKD after additional adjustment for obesity in the subset of the cohort with BMI data.
Table 3.
Multivariable cox regression analysis of incident chronic kidney disease risk among participants with urolithiasis.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p |
| Female | 1.65 | (1.40, 1.95) | <0.001 | 1.65 | (1.40, 1.95) | <0.001 | 1.65 | (1.40, 1.95) | <0.001 | 1.65 | (1.40, 1.95) | <0.001 |
| Age (yr) | 1.09 | (1.08, 1.10) | <0.001 | 1.09 | (1.08, 1.10) | <0.001 | 1.09 | (1.08, 1.10) | <0.001 | 1.09 | (1.08, 1.10) | <0.001 |
| Diabetes | 2.28 | (1.77, 2.94) | <0.001 | 2.29 | (1.77, 2.95) | <0.001 | 2.31 | (1.79, 2.98) | <0.001 | 2.32 | (1.80, 2.99) | <0.001 |
| Gout | 1.94 | (1.34, 2.83) | <0.001 | 1.95 | (1.34, 2.83) | <0.001 | 1.96 | (1.35, 2.84) | <0.001 | 1.96 | (1.35, 2.85) | <0.001 |
| Calendar yr | 0.92 | (0.90, 0.95) | <0.001 | 0.92 | (0.90, 0.95) | <0.001 | 0.92 | (0.90, 0.95) | <0.001 | 0.92 | (0.90, 0.95) | <0.001 |
| SWL kidney | 0.99 | (0.76, 1.30) | 0.95 | 0.99 | (0.76, 1.30) | 0.97 | ||||||
| SWL ureter | 1.15 | (0.72, 1.85) | 0.55 | 1.19 | (0.74, 1.91) | 0.47 | ||||||
| URS | 0.70 | (0.49, 1.00) | 0.05 | 0.70 | (0.49, 1.00) | 0.05 | ||||||
SWL – extracorporeal shock wave lithotripsy, URS – ureteroscopy
DISCUSSION
The increased awareness that urolithiasis is associated with both a greater risk of developing CKD and hypertension requires a thoughtful inquiry into our current treatment modalities. Our study is the first large epidemiological study to examine the association of SWL and URS with the risk of incident CKD and hypertension as compared to an untreated urolithiasis population. Our initial findings revealed that SWL was associated with a 34% higher risk of incident hypertension. Closer inspection revealed that this association was driven by those who received SWL to the kidney (40% higher risk). Importantly, given the 42% higher risk of hypertension associated with having urolithiasis, an individual who undergoes SWL to the kidney can be expected to have a two-fold greater risk of incident hypertension (HR 1.96) as compared to an individual without urolithiasis. Given that the proportion of individuals who developed hypertension after exposure to SWL to the kidney was 15% as compared to 12% of those with urolithiasis who did not undergo SWL to the kidney, the number needed to harm was 33. Overall, SWL to the ureter and URS were not associated with this increased risk of hypertension, though we did observe a three-fold greater risk associated with SWL to the ureter in participants <40 years of age. Furthermore, neither SWL procedure (kidney or ureter) nor URS was associated with an increased risk of developing CKD at an average of 4 years of follow-up.
Our study has several strengths that clarify discrepant findings from prior studies and conclusively confirm the association between SWL to the kidney and development of hypertension. The robust size of the THIN database allowed us to compare nearly 9000 patients with urolithiasis who had never had SWL or URS with more than 2300 patients who had at least one of these procedures. We were able to validate the generalizability of our urolithiasis population by comparing 11,570 individuals with incident urolithiasis, without preexisting hypertension, CKD, or proteinuria, to 127,464 unexposed participants who were matched on age, sex, and practice. Our results are strikingly similar to those of Alexander et al from the Alberta Kidney Disease Network database.4 These authors observed an increased risk of new onset CKD stage 3b-5 (HR 1.74, 95% CI: 1.61–1.88) in patients who had at least one kidney stone, but they did not have data on procedural exposures. We observed a highly similar increased hazard of incident CKD stage 3–5 (HR 1.82, 95% CI: 1.67, 1.98).
Currently, the overwhelmingly preferred surgical treatment options for the treatment of upper urinary tract calculi are SWL and URS. SWL came into widespread use after the introduction of the lithotripter in the 1980s.36 The use of URS has also dramatically increased over the last 30 years.10,36 Until recently, SWL has been the preferred treatment for proximal ureteral calculi and stones confined to the kidney, while URS has been the preferred surgical option for patients with distal and mid-ureteral calculi and for comorbid conditions such as morbid obesity, cardiac pacemaker, pregnancy, or anticoagulation therapy. With recent improvements in ureteroscope size and flexibility, an increasing number of urologists are now using URS instead of SWL for the treatment of both intra-renal and proximal ureteric stones.8,10
Importantly, 24% of individuals with urolithiasis in our study underwent at least one surgical procedure, and 11% of individuals with urolithiasis overall were treated with SWL. Although there is tremendous regional variability, these figures seem plausible given that analysis of Medicare Public Use files in the United States between the years 2001–2010 showed that approximately 38% of all urolithiasis patients underwent surgical treatment, and 15% overall underwent SWL.37 Data from the Hospital Episode Statistics, a database containing details on National Health Service activity, showed that in 2006 approximately 28,619 stone procedures were performed in the United Kingdom (UK).10 Approximately, 65.3% of those were SWL, and 31.9% URS. Our study shows similar findings in that of the 2787 individuals who had procedures 80% had either SWL or URS (47.3% SWL and 33.0% URS). Turney et al10 also noted that between 2000 and 2010, there was a 63% increase in hospitalizations for upper urinary tract stones, with a concomitant 55% increase in the use of SWL. Interestingly, there was an increase of 127% in URS and 83% reduction in open surgeries, confirming the relative increased use of URS over time. Given that the median calendar year in our study was 2006 (range 1994–2012), the slight differences we observed in the number of those who underwent surgical treatments as compared to the 2006 data from the Hospital Episode Statistics likely reflects the shifting practice patterns over time given that some of our participants extend back to 1994.
The association between SWL and incident hypertension remains highly debated. A prospective randomized trial of patients with small asymptomatic calculi and at least one annual follow-up blood pressure measurement found no significant difference in the rate of newly diagnosed hypertension between the 99 patients in the SWL group (11%) and the 93 untreated patients (7%, p = 0.35) at a mean follow up of 2.2 years.22 A registry-based retrospective study of incident urolithiasis patients without pre-existing hypertension comparing 400 patients who underwent SWL with 4382 who did not found no significant association with incident hypertension at a mean follow-up of 6 years.24 The lack of association may have been due to a comparatively small sample size, and it is unknown what proportion of the 400 patients received only SWL to the ureter. In contrast, a questionnaire-based retrospective study of 1892 patients who had previously undergone SWL found a significantly increased risk of developing de novo hypertension as compared to matched NHANES controls (37.8% vs. 32.5%, p = 0.0009) at a mean follow-up of 6 years.15 The major limitations were the reliance on patient report for hypertension diagnosis and the low response rate (27%) to the mailed questionnaire.
To our knowledge, there have been only two studies which have attempted to distinguish the effect of SWL treatment by anatomic location. A retrospective study comparing 772 patients who underwent SWL to the kidney and 505 who underwent SWL for a ureteral stone found no difference in incident hypertension.25 The diagnosis of hypertension in this study was based on patient report, and only 30.2% of patients who underwent SWL during the study period agreed to participate. A prospective study of 925 patients undergoing SWL with at least 3 months of follow-up noted an increase in diastolic blood pressure of more than 10 mmHg in 5% of those who received SWL to the kidney and upper ureter and only 2.8% of those who received SWL to the middle and lower ureter, but this difference was not statistically significant.26 The short follow-up and relatively small numbers of patients make these results difficult to interpret.
Although most of the prior literature, consistent with our findings, indicates that SWL does not result in permanently reduced GFR, the data is scant, and long-term follow-up lacking.20,21 A prospective study of 100 patients with a measured GFR at baseline who underwent SWL demonstrated no difference in total GFR or split renal function by repeat nuclear scintigraphy at a mean follow-up of 43 months.20 Another small study followed 35 children who underwent SWL for a mean of 33 months and found no long-term changes in measured GFR or morphological changes as measured by ultrasound.21 Given that most patients undergo unilateral SWL, it is perhaps unsurprising that a majority of patients do not demonstrate any abnormalities in their serum creatinine, as one unaffected kidney would generally be expected to maintain a normal GFR.
Our study has several limitations. The THIN database does not include data on race, and only a subset of the participants had BMI data. Importantly, in the subset of the cohort that did have a BMI recorded, there were no differences in the prevalence of obesity between the SWL and URS groups. Furthermore, although BMI data was limited, the HR for incident hypertension associated with SWL to the kidney was strengthened by adjustment for BMI in this subset, as expected given that obese individuals are less likely to undergo SWL and more likely to develop hypertension. THIN data is documented by the general practitioner and is thus reliant on this individual to accurately enter information from specialists, such as urologists, for our secondary utilization of this data. However, such inaccuracies would be unlikely to create systematic bias. Given that the exposures of SWL to the kidney, SWL to the ureter, and URS were based on Read codes, there was potential for misclassification; however, the face validity of the codes is excellent (see Supplementary Table 1). We were unable to account for urolithiasis burden as it was unclear that each Read code for urolithiasis was an independent event. While it is possible that the individuals who contributed time only as unexposed to any procedure may have had less severe urolithiasis, it should be noted that exposure to SWL to the kidney was associated with incident hypertension, while exposure to SWL to the ureter, URS, open and percutaneous procedures were not. If the severity of urolithiasis was driving the association with hypertension, one would expect that URS, and certainly percutaneous, procedures would also be associated with hypertension. Similarly, the number of SWL or URS procedures could not be reliably determined. Finally, information pertaining to the generation and brand of lithotripter was not available, but given the observation period it is most likely that the vast majority of participants were exposed to third generation lithotripters. Further, we did not have information on the number or intensity of shocks delivered with an SWL procedure.
Given the biological plausibility that SWL to the kidney results in significant parenchymal injury coupled with our findings, we recommend judicious use of SWL if a stone is asymptomatic or URS is a viable option. Although the association between hypertension and urolithiasis is likely multifactorial, SWL to the kidney seems to be an independent risk factor. Given that urolithiasis is itself associated with an increased risk of hypertension, it is imperative that practitioners who care for patients who have previously undergone SWL to the kidney be made aware of this further augmented risk of hypertension (two-fold higher) as compared to the general population.
METHODS
Study Design/Data Source
We conducted a population-based retrospective cohort study using THIN. THIN provides de-identified data from the electronic medical records of 553 general practices in the United Kingdom, including demographics, diagnoses, prescriptions, procedures and select laboratory measures, and comprises data from >10 million people.38 Medical diagnoses and procedures are recorded using Read codes, the standard classification system in the UK.39 The January 2012 version of the database was used. The study adhered to the Declaration of Helsinki, was approved by the THIN Scientific Review Committee, and determined by the Institutional Review Board of the University of Pennsylvania to meet eligibility criteria for IRB exemption authorized by 45 CFR 46.101, category 4.
Study Population
Using THIN, we recently published a study of incident fracture risk associated with urolithiasis across the lifespan in which we compared >50,000 participants with prevalent and incident diagnoses of urolithiasis to >500,000 participants without urolithiasis who were matched 10:1 on age (3-year age groups up to 30 years and 5-year age groups thereafter), sex and practice.5 Participants who only had codes for renal colic, bladder/lower urinary tract calculi (presumed infectious), infectious calculi, hypercalciuria, or nephrocalcinosis were excluded. The analysis described herein was limited to the 16,758 incident urolithiasis participants, and their 167,361 matched unexposed participants, who were 18 years of age or older at the start of the observation period. To be considered incident urolithiasis, a participant had to be registered with his/her practice for 6 months, and the practice using the Vision software as the electronic medical record, prior to or at the time of the initial Read code for urolithiasis. Requiring that the diagnosis occur after 6 months of registration and use of Vision software is standard practice for ascertainment of incident diagnoses in THIN.40 We chose to limit the current population to incident urolithiasis to mitigate against misclassification of historical procedures in prevalent participants and to strengthen the causal inference of any associations. As in our prior study, data on participants 90 years of age or older was excluded. We further excluded participants with an eGFR <60 ml/min/1.73m2, diagnosis of hypertension, or diagnosis of proteinuria (based on Read codes) prior to the start of the observation period, so the study population included 11,570 adult participants with incident urolithiasis and 127,464 participants without urolithiasis (Figure 1). Interventions for urolithiasis (Supplementary Table 1) were categorized as: SWL, URS, percutaneous, open, or indeterminate (for eight codes for which percutaneous versus URS could not be distinguished). SWL was also further categorized as SWL to the kidney or SWL to the ureter.
Outcome Ascertainment
The start of the observation period for participants with urolithiasis was the date of their first entry of a urolithiasis code. The observation period for unexposed participants started on the same date as that of their matched exposed participant. The observation period ended with the earliest of the following: last collection date for the practice, or when applicable, transfer out of the practice, death, or initial outcome event. Given the retrospective observational nature of this medical records database, participants are not recruited for participation. Transfer out of a given practice is a routine occurrence, and the reasons for transferring out of a practice are unknown, but are likely non-differential. Incident hypertension was identified using consistent Read diagnostic codes. In accordance with the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines, adopted in the UK,41,42 and our prior validation work in THIN,33 moderate to severe CKD (stages 3–5) was defined as an eGFR <60 ml/min/1.73m2 on at least two occasions, more than 90 days apart. eGFR was calculated from serum creatinine measures using to the Modification of Diet in Renal Disease (MDRD) formula.43 There were 823,811 measures of serum creatinine available for the individuals in our cohort, a very small proportion of which (0.09%) were excluded, as in our prior validation study,33 for ambiguous or infrequently used units of measure or implausible values (<20 or >1800 umol/L).
Analysis
Descriptive statistics were reported as median and inter-quartile range (IQR) for continuous variables and frequencies for categorical variables. Group differences were assessed using the Kruskal-Wallis and chi-square test for continuous and categorical variables, respectively. Cox proportional hazards regression in the full cohort was used to assess the association between urolithiasis and outcomes of CKD and hypertension in separate analyses. Cox proportional hazards regression among the urolithiasis population was used to assess the association between the interventions of SWL and URS as time-varying exposures and the outcomes of CKD and hypertension in separate analyses. Thus, exposure pertained to time rather than an individual. An individual who underwent either procedure would be considered unexposed prior to their first code for that procedure and exposed thereafter, contributing time as both unexposed and exposed to that procedure. An individual who underwent both procedures would therefore, contribute exposure time to both covariates based on the timing of the respective procedure. For example, an individual who had four years of observation time and had a code for SWL one year after start of observation and a subsequent code for URS two years after start of observation, would contribute one year of time unexposed to any procedure, three years of exposure time to SWL, and two years of exposure time to URS. Multivariable cox regression analysis was used to assess confounding by covariates of age, gender, diabetes mellitus, obesity, and gout. Covariate exposure was defined as having a consistent Read code recorded by the start of observation. Data on body mass index (BMI) within two years prior to start of observation was available in a subset of the cohort, among whom sensitivity analyses were performed adjusting for the covariate of obesity (BMI ≥30 kg/m2). Final models were also adjusted for time-varying covariate exposure to other procedures (percutaneous, open, and indeterminate). Multiplicative interactions of SWL and URS with age at start of observation were assessed. A two-sided p value of <0.05 was considered statistically significant. Analyses were performed using STATA 13.0 (Stata Corporation, College Station, TX). Violation of proportional hazards was tested through the estat phtest command in STATA. A cumulative hazard figure for hypertension was generated with the stcurve command, using the estimates from the final multivariable model.
Supplementary Material
ACKNOWLEDGEMENTS
This project was supported by NIH Grants UL1 RR024134 and K23 DK093556 (MRD). Dr. Denburg was also funded by The Nephcure Foundation-American Society of Nephrology Research Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
The authors receive(d) funding from the following sponsors for research outside the submitted work: Genentech, Inc and Mallinckrodt Pharmaceuticals (MRD), Bristol-Myers Squibb Co (KH), and AstraZeneca Plc (KH). The authors have the following consultancy agreements: Infiniti Medical (MRD).
Footnotes
DISCLOSURES
The remaining authors have no disclosures.
Contributor Information
Michelle R. Denburg, The Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, 34th Street and Civic Center Blvd, Philadelphia, PA 19104.
Thomas Jemielita, Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine at the University of Pennsylvania.
Gregory Tasian, Center for Pediatric Clinical Effectiveness, The Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania.
Kevin Haynes, Center for Clinical Epidemiology and Biostatistics.
Phillip Mucksavage, Perelman School of Medicine at the University of Pennsylvania.
Justine Shults, The Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, Center for Clinical Epidemiology and Biostatistics.
Lawrence Copelovitch, The Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania.
REFERENCES
- 1.Alexander RT, Hemmelgarn BR, Wiebe N, et al. Kidney stones and cardiovascular events: a cohort study. Clin J Am Soc Nephrol. 2014;9:506–512. doi: 10.2215/CJN.04960513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. American journal of hypertension. 1998;11:46–53. doi: 10.1016/s0895-7061(97)00371-3. [DOI] [PubMed] [Google Scholar]
- 3.Madore F, Stampfer MJ, Willett WC, Speizer FE, Curhan GC. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998;32:802–807. doi: 10.1016/s0272-6386(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 4.Alexander RT, Hemmelgarn BR, Wiebe N, et al. Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345:e5287. doi: 10.1136/bmj.e5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denburg MR, Leonard MB, Haynes K, et al. Risk of fracture in urolithiasis: a population-based cohort study using the health improvement network. Clin J Am Soc Nephrol. 2014;9:2133–2140. doi: 10.2215/CJN.04340514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. American journal of hypertension. 2008;21:257–264. doi: 10.1038/ajh.2007.62. [DOI] [PubMed] [Google Scholar]
- 7.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 8.Pearle MS, Calhoun EA, Curhan GC Urologic Diseases of America P. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848–857. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 9.Scales CD, Jr, Krupski TL, Curtis LH, et al. Practice variation in the surgical management of urinary lithiasis. J Urol. 2011;186:146–150. doi: 10.1016/j.juro.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turney BW, Reynard JM, Noble JG, Keoghane SR. Trends in urological stone disease. BJU international. 2012;109:1082–1087. doi: 10.1111/j.1464-410X.2011.10495.x. [DOI] [PubMed] [Google Scholar]
- 11.de Cogain M, Krambeck AE, Rule AD, et al. Shock wave lithotripsy and diabetes mellitus: a population-based cohort study. Urology. 2012;79:298–302. doi: 10.1016/j.urology.2011.07.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jocham D, Liedl B, Lunz C, Schuster C, Chaussy C. Long-term experiences following extracorporeal shockwave lithotripsy in patients with urinary calculi. Der Urologe Ausg A. 1989;28:134–137. [PubMed] [Google Scholar]
- 13.Blomgren PM, Connors BA, Lingeman JE, Willis LR, Evan AP. Quantitation of shock wave lithotripsy-induced lesion in small and large pig kidneys. The Anatomical record. 1997;249:341–348. doi: 10.1002/(SICI)1097-0185(199711)249:3<341::AID-AR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Evan AP, Willis LR, Connors B, Reed G, McAteer JA, Lingeman JE. Shock wave lithotripsy-induced renal injury. Am J Kidney Dis. 1991;17:445–450. doi: 10.1016/s0272-6386(12)80639-1. [DOI] [PubMed] [Google Scholar]
- 15.Barbosa PV, Makhlouf AA, Thorner D, Ugarte R, Monga M. Shock wave lithotripsy associated with greater prevalence of hypertension. Urology. 2011;78:22–25. doi: 10.1016/j.urology.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Janetschek G, Frauscher F, Knapp R, Hofle G, Peschel R, Bartsch G. New onset hypertension after extracorporeal shock wave lithotripsy: age related incidence and prediction by intrarenal resistive index. J Urol. 1997;158:346–351. doi: 10.1016/s0022-5347(01)64475-6. [DOI] [PubMed] [Google Scholar]
- 17.Kaude JV, Williams CM, Millner MR, Scott KN, Finlayson B. Renal morphology and function immediately after extracorporeal shock-wave lithotripsy. AJR American journal of roentgenology. 1985;145:305–313. doi: 10.2214/ajr.145.2.305. [DOI] [PubMed] [Google Scholar]
- 18.Goktas C, Coskun A, Bicik Z, et al. Evaluating ESWL-induced renal injury based on urinary TNF-alpha, IL-1alpha, and IL-6 levels. Urological research. 2012;40:569–573. doi: 10.1007/s00240-012-0467-1. [DOI] [PubMed] [Google Scholar]
- 19.Assimos DG, Boyce WH, Furr EG, et al. Selective elevation of urinary enzyme levels after extracorporeal shock wave lithotripsy. J Urol. 1989;142:687–690. doi: 10.1016/s0022-5347(17)38853-5. [DOI] [PubMed] [Google Scholar]
- 20.Eassa WA, Sheir KZ, Gad HM, Dawaba ME, El-Kenawy MR, Elkappany HA. Prospective study of the long-term effects of shock wave lithotripsy on renal function and blood pressure. J Urol. 2008;179:964–968. doi: 10.1016/j.juro.2007.10.055. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 21.Sarica K, Kupei S, Sarica N, Gogus O, Kilic S, Saribas S. Long-term follow-up of renal morphology and function in children after lithotripsy. Urologia internationalis. 1995;54:95–98. doi: 10.1159/000282697. [DOI] [PubMed] [Google Scholar]
- 22.Elves AW, Tilling K, Menezes P, Wills M, Rao PN, Feneley RC. Early observations of the effect of extracorporeal shockwave lithotripsy on blood pressure: a prospective randomized control clinical trial. BJU international. 2000;85:611–615. doi: 10.1046/j.1464-410x.2000.00571.x. [DOI] [PubMed] [Google Scholar]
- 23.Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Segura JW. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175:1742–1747. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 24.Krambeck AE, Rule AD, Li X, Bergstralh EJ, Gettman MT, Lieske JC. Shock wave lithotripsy is not predictive of hypertension among community stone formers at long-term followup. J Urol. 2011;185:164–169. doi: 10.1016/j.juro.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato Y, Tanda H, Kato S, et al. Shock wave lithotripsy for renal stones is not associated with hypertension and diabetes mellitus. Urology. 2008;71:586–591. doi: 10.1016/j.urology.2007.10.072. discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 26.Ehreth JT, Drach GW, Arnett ML, et al. Extracorporeal shock wave lithotripsy: multicenter study of kidney and upper ureter versus middle and lower ureter treatments. J Urol. 1994;152:1379–1385. doi: 10.1016/s0022-5347(17)32425-4. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald TM, Morant SV. Prevalence and treatment of isolated and concurrent hypertension and hypercholesterolaemia in the United Kingdom. British journal of clinical pharmacology. 2008;65:775–786. doi: 10.1111/j.1365-2125.2007.03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serumaga B, Ross-Degnan D, Avery AJ, et al. Effect of pay for performance on the management and outcomes of hypertension in the United Kingdom: interrupted time series study. BMJ. 2011;342:d108. doi: 10.1136/bmj.d108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshita J, Wang S, Shin DB, et al. Effect of psoriasis severity on hypertension control: a population-based study in the United kingdom. JAMA dermatology. 2015;151:161–169. doi: 10.1001/jamadermatol.2014.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W, Goldberg SI, Shubina M, Turchin A. Optimal systolic blood pressure target, time to intensification, and time to follow-up in treatment of hypertension: population based retrospective cohort study. BMJ. 2015;350:h158. doi: 10.1136/bmj.h158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins G, Altman D. Predicting the risk of chronic kidney disease in the UK: an evaluation of QKidney(R) scores using a primary care database. The British journal of general practice : the journal of the Royal College of General Practitioners. 2012;62:e243–e250. doi: 10.3399/bjgp12X636065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denburg MR, Haynes K, Shults J, Lewis JD, Leonard MB. Validation of The Health Improvement Network (THIN) database for epidemiologic studies of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2011;20:1138–1149. doi: 10.1002/pds.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan J, Wang S, Haynes K, Denburg MR, Shin DB, Gelfand JM. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi: 10.1136/bmj.f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. European urology. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright AE, Rukin NJ, Somani BK. Ureteroscopy and stones: Current status and future expectations. World journal of nephrology. 2014;3:243–248. doi: 10.5527/wjn.v3.i4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seklehner S, Laudano MA, Jamzadeh A, Del Pizzo JJ, Chughtai B, Lee RK. Trends and inequalities in the surgical management of ureteric calculi in the USA. BJU international. 2014;113:476–483. doi: 10.1111/bju.12372. [DOI] [PubMed] [Google Scholar]
- 38.CSD Medical Research UK. THIN data statistics. [Accessed 10/21/2013]; at http://csdmruk.cegedim.com/our-data/statistics.shtml. [Google Scholar]
- 39.Chisholm J. The Read clinical classification. BMJ. 1990;300:1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14:443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 41.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 42.Archibald G, Bartlett W, Brown A, et al. UK Consensus Conference on Early Chronic Kidney Disease--6 and 7 February 2007. Nephrol Dial Transplant. 2007;22:2455–2457. doi: 10.1093/ndt/gfm268. [DOI] [PubMed] [Google Scholar]
- 43.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.