ABSTRACT
Mechanisms of adaptation to acute changes in osmolarity are fundamental for life. When exposed to hyperosmotic stress, cells and organisms utilize conserved strategies to prevent water loss and maintain cellular integrity and viability. The production of glycerol is a common strategy utilized by the nematode Caenorhabditis elegans (C. elegans) and many other organisms to survive hyperosmotic stress. Specifically, the transcriptional upregulation of glycerol-3-phosphate dehydrogenase, a rate-limiting enzyme in the production of glycerol, has been previously implicated in many model organisms. However, what fuels this massive and rapid production of glycerol upon hyperosmotic stress has not been clearly elucidated. We have recently discovered an AMPK-dependent pathway that mediates hyperosmotic stress resistance in C. elegans. Specifically, we demonstrated that the chronic activation of AMPK leads to glycogen accumulation, which under hyperosmotic stress exposure, is rapidly degraded to mediate glycerol production. Importantly, we demonstrate that this strategy is utilized by flcn-1 mutant C. elegans nematodes in an AMPK-dependent manner. FLCN-1 is the worm homolog of the human renal tumor suppressor Folliculin (FLCN) responsible for the Birt-Hogg-Dubé neoplastic syndrome. Here, we comment on the dual role for glycogen in stress resistance: it serves as an energy store and a fuel for osmolyte production. We further discuss the potential utilization of this mechanism by organisms in general and by human cancer cells in order to survive harsh environmental conditions and notably hyperosmotic stress.
KEYWORDS: AMPK, cancer, Folliculin, glycerol, glycogen, hyperosmotic stress
Introduction
When the extracellular osmolarity is higher than the intracellular osmolarity, cells experience hyperosmotic stress, which promotes water flux out of the cell by osmosis, causing cellular shrinkage, severe macromolecular damage, cell cycle arrest, and cell death.1 Most organisms are exposed chronically or accidentally to high salinity environments and the ability to adapt to the availability of water is essential for life. In humans, many organs are exposed to water stress, due to water evaporation such as the skin, or through water osmosis into more concentrated aqueous environments due to physiological processes such as in kidneys, colon, and bladder.1 Cells/tissues/organisms have developed strategies to adapt to threatening hyperosmotic environments. These strategies include cytoskeletal rearrangements to offset the mechanical pressure, the upregulation of antioxidant enzymes to neutralize the sudden increase in reactive oxygen species, the induction of transporters to regulate water transport, and the upregulation of heat shock proteins to ensure protein homeostasis.1-3 In addition to the above-mentioned strategies, the synthesis of compatible organic solutes, also called osmolytes, is a widely-used strategy by all organisms which keeps cellular osmotic pressure equal to that of the external environment.4
The most common organic osmolytes include amino acids and derivatives (glycine, proline, taurine, etc.), carbohydrates, polyols and derivatives (trehalose, glycerol, inositol, myo-inositol, sorbitol, etc.), methylamines such as glycine betaine, and urea.4
In yeast and in the nematode Caenorhabditis elegans (C. elegans), the exposure to hyperosmotic stress causes the rapid accumulation of glycerol via the transcriptional upregulation of glycerol-3-phosphate dehydrogenase, a rate limiting enzyme in glycerol synthesis.5-7 Importantly, several hyperosmotic stress-resistant C. elegans mutants display heightened glycerol levels due to constitutive activation of gpdh-1 and subsequent glycerol accumulation.8-12 Although the use of glycerol in invertebrates to survive hyperosmotic stress is widely accepted, what fuels the rapid glycerol production upon hyperosmotic stress exposure has not been clearly elucidated.
Among pathways that lead to glycerol production, the degradation of glycogen leads to glucose-1-phosphate, which is rapidly converted to glucose-6-phosphate, a major metabolic intermediate that may enter the glycolysis pathway or produce glycerol-3-phosphate, a crucial metabolite for glycerol synthesis (Fig. 1).13 Importantly, our recent work demonstrates that this strategy is utilized to survive hyperosmotic stress by C. elegans wild-type nematodes and is enhanced upon loss of flcn-1, the worm homolog of the renal tumor suppressor protein Folliculin (FLCN), responsible for the Birt-Hogg-Dubé cancer syndrome in humans.14 Specifically, we also highlighted an important role for glycogen reserves in the rapid production of glycerol upon hyperosmotic stress exposure thereby enhancing organismal survival.14
Figure 1.
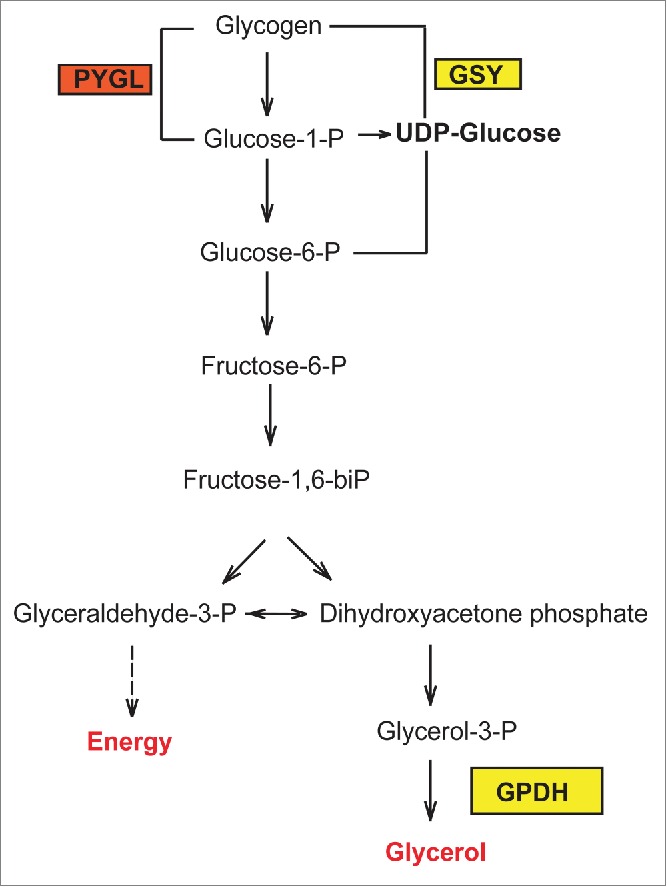
Representative scheme of glycogen metabolism and osmolyte production in C. elegans. PYGL: Glycogen phosphorylase, GSY: Glycogen Synthase.
FLCN-1/AMPK regulates hyperosmotic stress resistance in C. elegans
We have previously shown that FLCN-1 regulates resistance to energy stresses in C. elegans and mammalian cells including oxidative stress, anoxia, heat, and serum starvation.15-17 We also showed that the increased resistance to energy stresses is evolutionarily conserved and requires the 5'AMP-activated protein kinase (AMPK), a major regulator of cellular energy homeostasis and stress response.15,17 In our recent work, we demonstrate an important role for FLCN-1/AMPK in the regulation of resistance to hyperosmotic stress in C. elegans.14 Specifically, we showed that loss of flcn-1 enhanced the resistance of C. elegans nematodes to high NaCl conditions (400mM and 500mM NaCl) and improved their recovery from acute salinity attacks. Using the flcn-1(ok975); aak-1(tm1944); aak-2 (ok524) triple mutant animals that we generated, we showed that this FLCN-1-dependent hyperosmotic stress resistant phenotype strictly requires both AMPKα catalytic subunits AAK-1 and AAK-2.14
FLCN-1/AMPK regulates glycogen metabolism in C. elegans
Using electron microscopy and iodine staining, we observed a prominent accumulation of glycogen in different tissues of C. elegans nematodes upon loss of FLCN-1, especially in the hypodermis.14 Glycogen is a polymer of glucose molecules widely used as an energy storage in animals. Glycogen is synthesized from UDP-glucose by glycogen synthase and is degraded into glucose-1-phosphate using glycogen phospharylase, and both enzymes are highly evolutionarily conserved (Fig. 1).13 Importantly, we observed that the inhibition of glycogen synthesis and degradation by RNAi against glycogen synthase and glycogen phosphorylase, respectively, abrogated the resistance of wild-type animals to hyperosmotic stress and strongly suppressed the advantageous resistance mediated by loss of flcn-1.14
Since the chronic AMPK activation has been shown to lead to glycogen accumulation in multiple model systems, and because we have previously demonstrated that loss of flcn-1 chronically activates AMPK in C. elegans and in mammalian cells,15 we hypothesized that the increased accumulation of glycogen in flcn-1 animals depends on AMPK. Indeed, we demonstrated using iodine staining, that AMPK is required for glycogen accumulation in both wild-type and flcn-1 animals.14 This result explains why loss of AMPK or inhibition of glycogen metabolism lead to the same phenotypic outcome in regards to hyperosmotic stress resistance in C. elegans.
Glycogen breakdown fuels glycerol production and enhances hyperosmotic stress survival
While glycogen has been also shown to mediate a parental-associated effect of stress resistance in C. elegans embryos,18 two recent reports that were published while our manuscript was under review, have also linked glycogen to hypoosmotic-anoxic stress resistance in C. elegans.19,20 However, how glycogen is leading to hyperosmotic stress resistance specifically has not been clearly elucidated. In our recent work, we showed that following hyperosmotic stress exposure, glycerol is rapidly produced in both wild-type and flcn-1 animals, but more prominently in flcn-1 nematodes, which is consistent with the massive glycogen breakdown in these animals.14 We also showed that the enzymes responsible for glycogen synthesis, glycerol-3-phosphate dehydrogenases (gpdh-1 and gpdh-2) are strongly transcriptionally induced in both wild-type and flcn-1 animals, but more prominently upon loss of flcn-1. Supporting the important role of glycerol in the resistance to hyperosmotic stress, we generated the flcn-1; gpdh-1; gpdh2 triple mutant and determined its resistance to the gpdh-1; gpdh-2 double mutant animals. Indeed, we found that the loss of glycerol-3-phosphate dehydrogenases, strongly suppressed the increased resistance to hyperosmotic stress conferred by loss of flcn-1.14
The glycogen accumulation conferred by loss of FLCN-1 is evolutionarily conserved
In this work, we also highlighted an evolutionary conserved role of FLCN/AMPK in the regulation of glycogen metabolism. Specifically, we showed that glycogen accumulates in the tumors of BHD patients and in renal tissues of kidney-specific Flcn KO mice. This result implies that glycogen could play an important role in BHD tumorigenesis. In accordance, heightened glycogen levels were also reported in the muscle tissues of muscle-specific Flcn KO mice as compared to the controls.21,22
A dual role for glycogen
The role of glycogen as an energy source has been widely demonstrated in multiple organisms. However, its role as a reservoir for the production of osmolytes upon acute exposure to hypertonic stress has not been clearly reported. In Corynebacterium glutamicum, the exposure to hyperosmotic shock was shown to result in glycogen degradation and the synthesis of the osmoprotectant trehalose.23 In C. elegans, recent reports demonstrate an important role for glycogen in mediating survival to hypoosmotic-anoxic stress.19,20 Our data suggest that glycogen degradation leads to different outcomes depending on the type of stress. It is possible that the glycogen degraded by energy stresses generates ATP while the glycogen degraded by hyperosmotic stress produces glycerol. In support to this, we observed that the pretreatment of wild-type and flcn-1 mutant animals with Paraquat (PQ; oxidative and energy stressor) suppressed the increased resistance of flcn-1 nematodes to NaCl, while the pretreatment of wild-type and flcn-1 mutant worms with 200 mM NaCl increased their resistance upon PQ exposure.14 This could imply that the pretreatment of the animals with PQ depletes them from glycogen, generating ATP, and abrogating their ability to produce glycerol later on upon NaCl exposure. However, the pretreatment of the worms with NaCl depletes the glycogen stores and produces glycerol, a carbon source that could be used to produce ATP upon exposure to PQ.
The paradoxical role of AMPK in glycogen metabolism
The AMPK-dependent regulation of glycogen metabolism has long been a paradox. The acute activation of AMPK has been shown to inhibit glycogen synthase leading to glycogen degradation.24-27 However, the chronic activation of AMPK has been shown to lead to glycogen accumulation. Mechanistically, the chronic activation of AMPK has been shown to increase glucose uptake and result in the accumulation of glucose-6-phosphate, which allosterically activates glycogen synthase and leads to glycogen synthesis.28-30 Accordingly, the constitutive activation of AMPK via mutations in the γ2 and γ3 subunits has been associated with glycogen accumulation in the skeletal and cardiac muscles of pigs and mice.30-36 In agreement, and similarly to what we have reported,14 yeast snf1 mutants display decreased levels of glycogen as compared to the control.37 Importantly, whether the osmotic stress-dependent acute activation of AMPK leads to the activation of glycogen phosphorylase is still not clear and needs further investigation.
Using the nematode to understand the Birt-Hogg-Dubé disease
Birt-Hogg-Dubé is an autosmal dominant neoplastic syndrome characterized by skin lesions named fibrofolliculomas, pulmonary cysts, pneumothorax, and an increased presdisposition to renal cysts and tumors.38-51 BHD is caused by germline mutations in the BHD gene, which encodes FLCN, a 64KDa protein, expressed in most tissues.52 Since the discovery of the FLCN gene, diverse FLCN-related cellular functions have been reported. However, it remains unclear whether these biological processes are directly regulated by FLCN or they are simply a result of indirect effects related to FLCN.
Although mammalian model organisms such as mice and rats are highly advantageous to study disease-related biological processes in humans due the close anatomical and physiological similarities between systems, they have disadvantages including space, cost, and time-consuming transgenic technologies. The nematode C. elegans has emerged as an excellent model organism to study conserved signaling pathways. In fact, many biological processes are highly evolutionary conserved such that findings in C. elegans are applicable to humans. Importantly, deregulation in pathways that regulate proliferation, cell death, and metabolism is associated with tumor formation and dissemination. Although C. elegans nematodes do not develop tumors per se, the molecular pathways that lead to cancer in humans are conserved across evolution and lead to other phenotypic outcomes in the worm, which have been successfully used by researchers, to genetically determine molecular interactions and to screen for anticancer drugs.53-55
In analogy with the important role of glycogen in survival to stress, glycogen could also be an important molecule that fuels tumorigenesis. Glycogen accumulates in many cancer types including ovarian cancer,56 kidney cancer,57 colorectal cancer,58 bladder cancer,59 and others.60 In addition, higher glycogen levels were detected in breast, kidney, bladder, uterus, skin, ovary, and brain cancer cell lines60, and recent studies have demonstrated a critical role for glycogen in the survival of cancer cells to hypoxic environments and glucose restriction.57,61 Importantly, the inhibition of glycogen degradation induced apoptosis in pancreatic cancer cells and impaired the in vivo growth of tumor xenografts.57
The AMPK-dependent regulation of hyperosmotic stress that we observed in C. elegans is a very interesting aspect in regards to BHD disease, which is mostly manifested by enlarged renal cysts and tumors in all mammalian models including rats, mice, dogs, and men.40-42,44,48 Since the kidney is an organ chronically exposed to hyperosmotic stress, it is possible that the BHD renal tumors and cysts are formed because of an increased resistance to hyperosmotic conditions, which could lead to DNA damage. In support of this, loss of FLCN and VHL, which are both renal tumor suppressor genes, predispose to renal clear cell carcinomas which are glycogen-rich tumors. Based on our recent findings in C. elegans, we speculate that glycogen plays a dual role in BHD neoplasm: it supplies cancer cells with energy and helps them resist the renal hyperosmotic environment.
Concluding remarks
Future experiments aiming to determine which osmolytes are produced following glycogen degradation in animals are necessary to understand the role of glycogen in hyperosmotic stress resistance. Although we show that glycerol is a major osmoprotectant, other osmolytes resulting from the hyperosmotic-stress dependent degradation of glycogen could also contribute to the survival of cells/organisms.
As a continuation of this work, it will be important to assess this pathway in cell culture systems and in BHD mouse models and to target it in cancer models to determine potential therapeutic benefits in the treatment of BHD disease in specific and kidney cancers in general.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Brocker C, Thompson DC, Vasiliou V. The role of hyperosmotic stress in inflammation and disease. Biomol Concepts 2012; 3:345-64; PMID:22977648; http://dx.doi.org/ 10.1515/bmc-2012-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 2007; 87:1441-74; PMID:17928589; http://dx.doi.org/ 10.1152/physrev.00056.2006 [DOI] [PubMed] [Google Scholar]
- [3].Christoph K, Beck FX, Neuhofer W. Osmoadaptation of Mammalian cells - an orchestrated network of protective genes. Current genomics 2007; 8:209-18; PMID:18645598; http://dx.doi.org/ 10.2174/138920207781386979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 2005; 208:2819-30; PMID:16043587; http://dx.doi.org/ 10.1242/jeb.01730 [DOI] [PubMed] [Google Scholar]
- [5].O'Rourke SM, Herskowitz I, O'Shea EK. Yeast go the whole HOG for the hyperosmotic response. Trends Genet 2002; 18:405-12; PMID:12142009; http://dx.doi.org/ 10.1016/S0168-9525(02)02723-3 [DOI] [PubMed] [Google Scholar]
- [6].Sheikh-Hamad D, Gustin MC. MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am J Physiol Renal Physiol 2004; 287:F1102-10; PMID:15522988; http://dx.doi.org/ 10.1152/ajprenal.00225.2004 [DOI] [PubMed] [Google Scholar]
- [7].Lamitina ST, Morrison R, Moeckel GW, Strange K. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am J Physiol Cell Physiol 2004; 286:C785-91; PMID:14644776; http://dx.doi.org/ 10.1152/ajpcell.00381.2003 [DOI] [PubMed] [Google Scholar]
- [8].Rohlfing AK, Miteva Y, Hannenhalli S, Lamitina T. Genetic and physiological activation of osmosensitive gene expression mimics transcriptional signatures of pathogen infection in C. elegans. PLoS One 2010; 5:e9010; PMID:20126308; http://dx.doi.org/ 10.1371/journal.pone.0009010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lamitina T, Huang CG, Strange K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc Natl Acad Sci U S A 2006; 103:12173-8; PMID:16880390; http://dx.doi.org/ 10.1073/pnas.0602987103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Solomon A, Bandhakavi S, Jabbar S, Shah R, Beitel GJ, Morimoto RI. Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics 2004; 167:161-70; PMID:15166144; http://dx.doi.org/ 10.1534/genetics.167.1.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wheeler JM, Thomas JH. Identification of a novel gene family involved in osmotic stress response in Caenorhabditis elegans. Genetics 2006; 174:1327-36; PMID:16980399; http://dx.doi.org/ 10.1534/genetics.106.059089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rohlfing AK, Miteva Y, Moronetti L, He L, Lamitina T. The Caenorhabditis elegans mucin-like protein OSM-8 negatively regulates osmosensitive physiology via the transmembrane protein PTR-23. PLoS Genet 2011; 7:e1001267; PMID:21253570; http://dx.doi.org/ 10.1371/journal.pgen.1001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Braeckman BP. Intermediary metabolism. In: Koen Houthoofd JRV, ed. wormbook, 2009; http://www.wormbook.org/chapters/www_intermetabolism/intermetabolism.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Possik E, Ajisebutu A, Manteghi S, Gingras MC, Vijayaraghavan T, Flamand M, Coull B, Schmeisser K, Duchaine T, van Steensel M, et al.. FLCN and AMPK confer resistance to hyperosmotic stress via remodeling of glycogen stores. PLoS Genet 2015; 11:e1005520; PMID:26439621; http://dx.doi.org/ 10.1371/journal.pgen.1005520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Possik E, Jalali Z, Nouet Y, Yan M, Gingras MC, Schmeisser K, Panaite L, Dupuy F, Kharitidi D, Chotard L, et al.. Folliculin regulates ampk-dependent autophagy and metabolic stress survival. PLoS Genet 2014; 10:e1004273; PMID:24763318; http://dx.doi.org/ 10.1371/journal.pgen.1004273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Possik E, Pause A. Measuring oxidative stress resistance of Caenorhabditis elegans in 96-well microtiter plates. J Vis Exp 2015:e52746; PMID:25993260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yan M, Gingras MC, Dunlop EA, Nouet Y, Dupuy F, Jalali Z, Possik E, Coull BJ, Kharitidi D, Dydensborg AB, et al.. The tumor suppressor folliculin regulates AMPK-dependent metabolic transformation. J Clin Invest 2014; 124:2640-50; PMID:24762438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Frazier HN 3rd, Roth MB. Adaptive sugar provisioning controls survival of C. elegans embryos in adverse environments. Curr Biol 2009; 19:859-63; PMID:19398339; http://dx.doi.org/ 10.1016/j.cub.2009.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].LaMacchia JC, Frazier HN 3rd, Roth MB. Glycogen fuels survival during hyposmotic-anoxic stress in caenorhabditis elegans. Genetics 2015; 201:65-74; PMID:26116152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].LaMacchia JC, Roth MB. Aquaporins 2 and 4 Regulate glycogen metabolism and survival during hyposmotic-anoxic stress in caenorhabditis elegans. Am J Physiol Cell Physiol 2015; 309:C92-6; ajpcell 00131 2015; PMID:26017147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baba M, Keller JR, Sun HW, Resch W, Kuchen S, Suh HC, Hasumi H, Hasumi Y, Kieffer-Kwon KR, Gonzalez CG, et al.. The folliculin-FNIP1 pathway deleted in human Birt-Hogg-Dube syndrome is required for murine B-cell development. Blood 2012; 120:1254-61; PMID:22709692; http://dx.doi.org/ 10.1182/blood-2012-02-410407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hasumi H, Baba M, Hasumi Y, Huang Y, Oh H, Hughes RM, Klein ME, Takikita S, Nagashima K, Schmidt LS, et al.. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN. J Natl Cancer Inst 2012; 104:1750-64; PMID:23150719; http://dx.doi.org/ 10.1093/jnci/djs418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Seibold GM, Eikmanns BJ. The glgX gene product of Corynebacterium glutamicum is required for glycogen degradation and for fast adaptation to hyperosmotic stress. Microbiology 2007; 153:2212-20; PMID:17600065; http://dx.doi.org/ 10.1099/mic.0.2006/005181-0 [DOI] [PubMed] [Google Scholar]
- [24].Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta 1989; 1012:81-6; PMID:2567185; http://dx.doi.org/ 10.1016/0167-4889(89)90014-1 [DOI] [PubMed] [Google Scholar]
- [25].Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, et al.. The alpha2-5'AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 2004; 53:3074-81; PMID:15561936; http://dx.doi.org/ 10.2337/diabetes.53.12.3074 [DOI] [PubMed] [Google Scholar]
- [26].Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes 2002; 51:284-92; PMID:11812734; http://dx.doi.org/ 10.2337/diabetes.51.2.284 [DOI] [PubMed] [Google Scholar]
- [27].Miyamoto L, Toyoda T, Hayashi T, Yonemitsu S, Nakano M, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Ogawa Y, et al.. Effect of acute activation of 5'-AMP-activated protein kinase on glycogen regulation in isolated rat skeletal muscle. J Appl Physiol (1985) 2007; 102:1007-13; PMID:17122373; http://dx.doi.org/ 10.1152/japplphysiol.01034.2006 [DOI] [PubMed] [Google Scholar]
- [28].Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 2011; 60:766-74; PMID:21282366; http://dx.doi.org/ 10.2337/db10-1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aschenbach WG, Hirshman MF, Fujii N, Sakamoto K, Howlett KF, Goodyear LJ. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes 2002; 51:567-73; PMID:11872652; http://dx.doi.org/ 10.2337/diabetes.51.3.567 [DOI] [PubMed] [Google Scholar]
- [30].Luptak I, Shen M, He H, Hirshman MF, Musi N, Goodyear LJ, Yan J, Wakimoto H, Morita H, Arad M, et al.. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest 2007; 117:1432-9; PMID:17431505; http://dx.doi.org/ 10.1172/JCI30658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest 2002; 109:357-62; PMID:11827995; http://dx.doi.org/ 10.1172/JCI0214571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ahmad F, Arad M, Musi N, He H, Wolf C, Branco D, Perez-Atayde AR, Stapleton D, Bali D, Xing Y, et al.. Increased alpha2 subunit-associated AMPK activity and PRKAG2 cardiomyopathy. Circulation 2005; 112:3140-8; PMID:16275868; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.105.550806 [DOI] [PubMed] [Google Scholar]
- [33].Zou L, Shen M, Arad M, He H, Lofgren B, Ingwall JS, Seidman CE, Seidman JG, Tian R. N488I mutation of the gamma2-subunit results in bidirectional changes in AMP-activated protein kinase activity. Circ Res 2005; 97:323-8; PMID:16051890; http://dx.doi.org/ 10.1161/01.RES.0000179035.20319.c2 [DOI] [PubMed] [Google Scholar]
- [34].Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, et al.. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 2000; 288:1248-51; PMID:10818001; http://dx.doi.org/ 10.1126/science.288.5469.1248 [DOI] [PubMed] [Google Scholar]
- [35].Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 2002; 115:2433-42; PMID:12006627 [DOI] [PubMed] [Google Scholar]
- [36].Yu H, Hirshman MF, Fujii N, Pomerleau JM, Peter LE, Goodyear LJ. Muscle-specific overexpression of wild type and R225Q mutant AMP-activated protein kinase gamma3-subunit differentially regulates glycogen accumulation. Am J Physiol Endocrinol Metab 2006; 291:E557-65; PMID:16638825; http://dx.doi.org/ 10.1152/ajpendo.00073.2006 [DOI] [PubMed] [Google Scholar]
- [37].Wang Z, Wilson WA, Fujino MA, Roach PJ. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol 2001; 21:5742-52; PMID:11486014; http://dx.doi.org/ 10.1128/MCB.21.17.5742-5752.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hornstein OP, Knickenberg M. Perifollicular fibromatosis cutis with polyps of the colon–a cutaneo-intestinal syndrome sui generis. Arch Dermatol Res 1975; 253:161-75; PMID:1200700; http://dx.doi.org/ 10.1007/BF00582068 [DOI] [PubMed] [Google Scholar]
- [39].Birt AR, Hogg GR, Dube WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977; 113:1674-7; PMID:596896; http://dx.doi.org/ 10.1001/archderm.1977.01640120042005 [DOI] [PubMed] [Google Scholar]
- [40].Toro JR, Glenn G, Duray P, Darling T, Weirich G, Zbar B, Linehan M, Turner ML. Birt-Hogg-Dube syndrome: a novel marker of kidney neoplasia. Arch Dermatol 1999; 135:1195-202; PMID:10522666 [DOI] [PubMed] [Google Scholar]
- [41].Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, Merino MJ. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol 2002; 26:1542-52; PMID:12459621; http://dx.doi.org/ 10.1097/00000478-200212000-00002 [DOI] [PubMed] [Google Scholar]
- [42].Zbar B, Alvord WG, Glenn G, Turner M, Pavlovich CP, Schmidt L, Walther M, Choyke P, Weirich G, Hewitt SM, et al.. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dube syndrome. Cancer Epidemiol Biomarkers Prev 2002; 11:393-400; PMID:11927500 [PubMed] [Google Scholar]
- [43].Tobino K, Gunji Y, Kurihara M, Kunogi M, Koike K, Tomiyama N, Johkoh T, Kodama Y, Iwakami S, Kikkawa M, et al.. Characteristics of pulmonary cysts in Birt-Hogg-Dube syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol 2011; 77:403-9; PMID:19782489; http://dx.doi.org/ 10.1016/j.ejrad.2009.09.004 [DOI] [PubMed] [Google Scholar]
- [44].Gupta P, Eshaghi N, Kamba TT, Ghole V, Garcia-Morales F. Radiological findings in Birt-Hogg-Dube syndrome: a rare differential for pulmonary cysts and renal tumors. Clin Imaging 2007; 31:40-3; PMID:17189846; http://dx.doi.org/ 10.1016/j.clinimag.2006.09.023 [DOI] [PubMed] [Google Scholar]
- [45].Kupres KA, Krivda SJ, Turiansky GW. Numerous asymptomatic facial papules and multiple pulmonary cysts: a case of Birt-Hogg-Dube syndrome. Cutis 2003; 72:127-31; PMID:12953936 [PubMed] [Google Scholar]
- [46].Furuya M, Tanaka R, Koga S, Yatabe Y, Gotoda H, Takagi S, Hsu YH, Fujii T, Okada A, Kuroda N, et al.. Pulmonary cysts of Birt-Hogg-Dube syndrome: a clinicopathologic and immunohistochemical study of 9 families. Am J Surg Pathol 2012; 36:589-600; PMID:22441547; http://dx.doi.org/ 10.1097/PAS.0b013e3182475240 [DOI] [PubMed] [Google Scholar]
- [47].Van Denhove A, Guillot-Pouget I, Giraud S, Isaac S, Freymond N, Calender A, Pacheco Y, Devouassoux G. [Multiple spontaneous pneumothoraces revealing Birt-Hogg-Dube syndrome]. Rev Mal Respir 2011; 28:355-9; PMID:21482341; http://dx.doi.org/ 10.1016/j.rmr.2010.08.015 [DOI] [PubMed] [Google Scholar]
- [48].Petersson F, Gatalica Z, Grossmann P, Perez Montiel MD, Alvarado Cabrero I, Bulimbasic S, Swatek A, Straka L, Tichy T, Hora M, et al.. Sporadic hybrid oncocytic/chromophobe tumor of the kidney: a clinicopathologic, histomorphologic, immunohistochemical, ultrastructural, and molecular cytogenetic study of 14 cases. Virchows Arch 2010; 456:355-65; PMID:20300772; http://dx.doi.org/ 10.1007/s00428-010-0898-4 [DOI] [PubMed] [Google Scholar]
- [49].Koga S, Furuya M, Takahashi Y, Tanaka R, Yamaguchi A, Yasufuku K, Hiroshima K, Kurihara M, Yoshino I, Aoki I, et al.. Lung cysts in Birt-Hogg-Dube syndrome: histopathological characteristics and aberrant sequence repeats. Pathol Int 2009; 59:720-8; PMID:19788617; http://dx.doi.org/ 10.1111/j.1440-1827.2009.02434.x [DOI] [PubMed] [Google Scholar]
- [50].Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, Turner M, Choyke P, Merino MJ, Pinto PA, et al.. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet 2008; 45:321-31; PMID:18234728; http://dx.doi.org/ 10.1136/jmg.2007.054304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G, Turner ML, Duray P, Merino M, Choyke P, Pavlovich CP, et al.. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell 2002; 2:157-64; PMID:12204536; http://dx.doi.org/ 10.1016/S1535-6108(02)00104-6 [DOI] [PubMed] [Google Scholar]
- [52].Tee AR, Pause A. Birt-Hogg-Dube: tumour suppressor function and signalling dynamics central to folliculin. Fam Cancer 2013; 12:367-72; PMID:23096221; http://dx.doi.org/ 10.1007/s10689-012-9576-9 [DOI] [PubMed] [Google Scholar]
- [53].Rodriguez M, Snoek LB, De Bono M, Kammenga JE. Worms under stress: C. elegans stress response and its relevance to complex human disease and aging. Trends Genet 2013; 29:367-74; PMID:23428113; http://dx.doi.org/ 10.1016/j.tig.2013.01.010 [DOI] [PubMed] [Google Scholar]
- [54].Lapierre LR, Hansen M. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol Metab 2012; 23:637-44; PMID:22939742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol 2006; 190:191-202; PMID:16899554; http://dx.doi.org/ 10.1677/joe.1.06856 [DOI] [PubMed] [Google Scholar]
- [56].Iida Y, Aoki K, Asakura T, Ueda K, Yanaihara N, Takakura S, Yamada K, Okamoto A, Tanaka T, Ohkawa K. Hypoxia promotes glycogen synthesis and accumulation in human ovarian clear cell carcinoma. Int J Oncol 2012; 40:2122-30; PMID:22447231 [DOI] [PubMed] [Google Scholar]
- [57].Favaro E, Bensaad K, Chong MG, Tennant DA, Ferguson DJ, Snell C, Steers G, Turley H, Li JL, Gunther UL, et al.. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab 2012; 16:751-64; PMID:23177934; http://dx.doi.org/ 10.1016/j.cmet.2012.10.017 [DOI] [PubMed] [Google Scholar]
- [58].Takahashi S, Satomi A, Yano K, Kawase H, Tanimizu T, Tuji Y, Murakami S, Hirayama R. Estimation of glycogen levels in human colorectal cancer tissue: relationship with cell cycle and tumor outgrowth. J Gastroenterol 1999; 34:474-80; PMID:10452680; http://dx.doi.org/ 10.1007/s005350050299 [DOI] [PubMed] [Google Scholar]
- [59].Mourad WA, Mackay B, Ordonez NG, Ro JY, Swanson DA. Clear cell melanoma of the bladder. Ultrastruct Pathol 1993; 17:463-8; PMID:8266605; http://dx.doi.org/ 10.3109/01913129309027791 [DOI] [PubMed] [Google Scholar]
- [60].Rousset M, Zweibaum A, Fogh J. Presence of glycogen and growth-related variations in 58 cultured human tumor cell lines of various tissue origins. Cancer Res 1981; 41:1165-70; PMID:7459858 [PubMed] [Google Scholar]
- [61].Pelletier J, Bellot G, Gounon P, Lacas-Gervais S, Pouyssegur J, Mazure NM. Glycogen synthesis is induced in hypoxia by the hypoxia-inducible factor and promotes cancer cell survival. Front Oncol 2012; 2:18; PMID:22649778; http://dx.doi.org/ 10.3389/fonc.2012.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]


