ABSTRACT
The subcellular compartments of eukaryotic cells are characterized by different redox environments. Whereas the cytosol, nucleus and mitochondria are more reducing, the endoplasmic reticulum represents a more oxidizing environment. As the redox level controls the formation of intra- and inter-molecular disulfide bonds, the folding of proteins is tightly linked to its environment. The proteostasis network of each compartment needs to be adapted to the compartmental redox properties. In addition to chaperones, also members of the thioredoxin superfamily can influence the folding of proteins by regulation of cysteine reduction/oxidation. This review will focus on thioredoxin superfamily members and chaperones of C. elegans, which play an important role at the interface between redox and protein homeostasis. Additionally, this review will highlight recent methodological developments on in vivo and in vitro assessment of the redox state and their application to provide insights into the high complexity of redox and proteostasis networks of C. elegans.
KEYWORDS: aging, chaperones, endoplasmic reticulum, ERO-1, PDI, proteostasis, redox homeostasis, thioredoxin, trx-domain, unfolded protein response
Introduction
Proteins need to fold into a unique 3-dimensional structure in order to become biologically active. In vivo folding of proteins is constantly challenged. First, the cell is a highly crowded environment with a macromolecule concentration of 300 to 400 mg/ml.1 These molecular crowding effects could result in unfavorable interactions, especially during protein synthesis where nascent polypeptides may get exposed. Second, several stress situations such as heat shock, aging or the expression of aggregation- prone proteins can result in protein un- and misfolding and consequently protein aggregation.2,3 To maintain cellular homeostasis, cells are equipped with a protein homeostasis network that supports de novo folding and refolding of denatured proteins, prevents misfolding and aggregation, reverses aggregation by chaperone mediated disaggregation and supports the clearance of damaged proteins via the ubiquitin proteasome system or by autophagy.3,4 The dominant players of the protein homeostasis network are molecular chaperones or heat shock proteins (Hsp). Historically, chaperones can be grouped into several classes based on their molecular weight such as Hsp40 (now referred to as J-protein), Hsp60, Hsp70, Hsp90 and Hsp100.3,5,6
Several stress-responsive pathways evolved during evolution to protect the organism from damage caused by e.g. heat shock, oxidative stress or the expression of aggregation-prone proteins.3,7,8 The most prominent examples for such pathways are the cytosolic heat shock response and the unfolded protein response (UPR) of the ER and mitochondria.3,7,8 These stress responses activate the expression of cytoprotective genes, such as chaperones and proteases, in a compartment-specific manner. Simultaneously, protein synthesis rates decline to reduce the influx of new proteins that may require the assistance of chaperones and thus compete with the existing chaperone substrate load.3,7,s8 Interestingly it could be shown, that with the progression of aging and with the onset of protein aggregation in models of neurodegenerative diseases the induction of stress responses is hampered.2,9,10 The failure to induce stress-responsive pathways upon exposure to proteotoxic conditions increases the concentration of misfolded and aggregated proteins over time and contributes to the aging process. In addition, changes in redox state and oxidative stress due to accumulation of reactive oxygen species (ROS) and subsequent damage of DNA, lipids and proteins were thought for a long time to contribute to the aging process as postulated in the free-radical theory of aging.11,12 However, the role of ROS in the aging process is still controversial. Many data suggest that ROS may react to age-dependent damages as second messenger and aging is in general accompanied by changes of the whole redox circuit. (For reviews see13,14). Although the induction and initial trigger of aging processes are still unclear, it is established that the subcellular redox levels change during the aging process.15,16
Compartments exhibit different redox states such as the oxidative environment of the ER and reducing conditions in the mitochondria, cytosol and nucleus.12,17 The redox state is defined by the ratio of oxidants and antioxidants such as glutathione (GSH) to glutathione disulfide (GSSG). As the sulfur containing amino acids cysteine and methionine are redox sensitive, the redox state is important for protein structures and influences their folding. For example, high GSH to GSSG ratios that are found in the cytosol or the nucleus define a more reductive environment and keep cysteines in their reduced state.17 Low ratios of GSH to GSSG, as observed in the ER, create an oxidative environment and favor the formation of disulfide bonds.17
Precise protein folding and maturation is central for the maintenance of a functional (sub-) proteome. Therefore, each organelle is equipped with a network of enzymes to cope with oxidative and proteotoxic challenges.12,18 Thioredoxins and glutaredoxins e.g., keep proteins in their reduced state, while specific reductases like thioredoxin reductases and glutathione disulfide reductases as well as glutathione guarantee a recycling of the oxidized enzymes back to their reduced state through e.g. NADPH oxidation.12 Protein disulfide isomerases play an important role in the folding of cysteine-containing proteins in the ER.19 Additionally, cells have evolved a network of enzymes, such as superoxide dismutases, catalases and peroxidases, which detoxify the cell from reactive oxygen species that are generated during aerobic respiration at the mitochondria. A more detailed description of the redox system of C. elegans is reviewed by Johnston and Ebert.12
As the redox environment determines the protein structure, alterations in the redox state affect protein conformation and can cause misfolding or rearrangement that could provide a new redox-activated function. Both scenarios imply that regulation of redox homeostasis is tightly linked to the maintenance of protein homeostasis. In this review we will focus on members of the thioredoxin superfamily of C. elegans, which play an important role in both redox and protein homeostasis. As also many chaperones contain cysteine residues, we will provide insights into the redox-regulation of chaperones. Finally, this review will summarize also recent methodologies, which have been used to study the redox state in vivo and in vitro to gain more insight into the redox challenges of e.g., aging organisms.
The thioredoxin superfamily – mediators between Redox and Protein Homeostasis
The thioredoxin (trx) superfamily comprises many different enzymes such as thioredoxins (e.g. TRX-1, TRX-2 in C. elegans), glutaredoxins (e.g., GLRX-3, GLRX-10 in C. elegans) and protein disulfide isomerases (e.g. PDI-1, PDI-2 in C. elegans), which are involved in disulfide reduction and isomerization, among others.12,20 As a common feature all members share at least one domain with the so called “trx-fold,” which is composed of central β-sheets, surrounded by α-helices.20 An additional characteristic of thioredoxin superfamily proteins is a CXXC motif in the active site. This motif differs between the members and can be used for their classification: thioredoxins contain a conserved CGPC motif, while PDIs have CGHC.21 However, those are not exclusive. For both families also slightly altered motifs were described.22 Furthermore, the CXXC motif can also be absent.22,23 Based on a Pfam database search (http://pfam.xfam.org) and NCBI protein blast (www.ncbi.nlm.nih.gov/BLAST/) against the human thioredoxin, TRX1, as well as C. elegans TRX-1, we could identify 37 related thioredoxin superfamily members in C. elegans. Out of those, 8 members harbor the canonical CGPC (Trx-like) motif, 8 the CGHC PDI-like motif and one member contains both sequences (Table 1). Only some of them have been functionally characterized until today and were reported to play roles in development, axon branching and protein folding, among others (Table 1). According to their diverse functions, these thioredoxin superfamily members are also differently expressed. Whereas PDIs are located in the ER, thioredoxin proteins can also act in other compartments (Fig. 1). However, preliminary data suggest that some members of the PDI family are not exclusively located in the ER in other species.24,25 In this section we highlight members of the thioredoxin superfamily with a canonical motif, which have important roles at the interface between redox and protein homeostasis.
Table 1.
Thioredoxin superfamily members with canonical active site sequences based on Pfam database and NCBI protein blast against human TRX1 and C. elegans TRX-1. */**Based on *www.wormbase.org or **NCBI protein blast against human genome. ***Dnj-27 has also CGHC motif.
| Thioredoxin family members with CGPC motif | ||
|---|---|---|
| Thioredoxin family member in C. elegans | Mammalian homolog*/** | Function in C. elegans |
| DNJ-27*** | DNAJC10 | UPRER/ERAD64 |
| PNG-1 | NGLY1 | Axon branching69 Deglycosylation76 |
| TRX-1 | TRX1 | Life span regulation66 Neuronal regulation121 Dauer formation70 |
| TRX-2 | TRX2 | Neuronal regulation121 Induced upon UPRMito65 |
| TRX-3 | NXNL2 | Pathogen protection63 |
| TRX-4 | TRX1 | Predicted protein disulfide oxidoreductase activity* |
| TRX-5 | NXNL2 | unknown |
| TXL-1 | TXNL1 | unknown |
| Y55F3AR.2 | TXNL1 | Predicted protein disulfide oxidoreductase activity* |
| Thioredoxin family members with CGHC motif of PDIs | ||
| C14B9.2 | PDIA4 | Induced upon UPRER122 predicted function in oxidative protein folding* |
| F35G2.1 | QSOX1/QSOX2 | Predicted thiol oxidase activity* |
| PDI-1 | PDIA1 | Disulfide isomerase50 |
| PDI-2 | PDIA1 | Development51 Extracell. matrix formation51 Disulfide isomerase50 |
| PDI-3 | PDIA3 | Disulfide isomerase50 Extracell. matrix assembly50 |
| PDI-6 | PDIA6 | Larval development 52 Predicted isomerase activity* |
| T10H10.2 | QSOX1/QSOX2 | Sma/Mab pathway123 Predicted thiol oxidase activity* |
| Y49E10.4 | PDIA6 | Predicted isomerase activity* Locomotion, molting cycle, vulva development, growth* |
Figure 1.
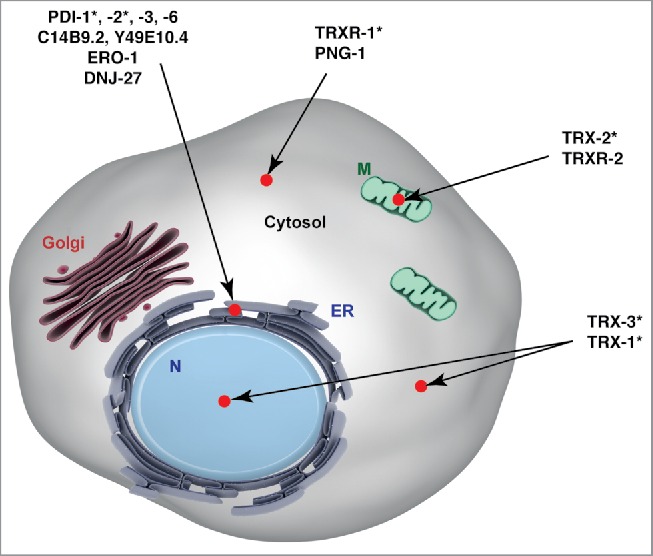
Subcellular and tissue localization of the Thioredoxin superfamily network. In C. elegans, members of the thioredoxin superfamily network have a sub-cellular and tissue specific localization. Characterized proteins are located in specific compartments, such as M, mitochondria (TRX-2, TRXR-2),65 ER, endoplasmic reticulum (PDI-1,2,3,51652; ERO-156; DNJ-27,64 C14B9.2, Y49E10.4), N, nucleus and cytosol (TRX-167; TRX-363), or only in the cytosol (TRXR-177, PNG-169). Most of these proteins have a broad expression pattern (PDI-350,6118; DNJ-2764, TRXR-277, PNG-169). However, some exhibit a tissue-specific expression*. TRX-1 is found in the intestine, ASI and ASJ neurons,62,67 TRXR-1 is expressed in the pharynx, intestine and vulva,77 TRX-2 is located AIYL/R and ASEL neurons,65 PDI-1/PDI-2 are expressed in hypodermis119,120 and TRX-3 is only present in the intestine.63 The tissue expression pattern of ERO-1, C14B9.2 and Y49E10.4 are still unknown.
PDIs and ERO-1 - the redox state guardians of the ER
Protein disulfide isomerases – PDI's
In the ER, membrane and secretory proteins are constantly synthesized and despite its size (one tenth of the entire cell), about one-third of the proteome is synthesized at the ER membrane.18 Consequently, the ER lumen is highly crowded with proteins that need to be folded in a coordinated way. The folding process needs to be assisted by several chaperones and glycosylation enzymes.26 Secreted and ER-resident proteins are enriched in disulfide bonds, which are critical for their conformational stability. The oxidizing environment of the ER is a prerequisite for the formation of disulfide bonds and is maintained by a network of redox enzymes such as oxidases and protein disulfide isomerases (PDIs).27-29 PDIs are multi-domain proteins. As common feature they share at least one trx-like domain, which harbors a conserved CXXC motif for thiol-disulfide exchange reaction.12 PDIs function as oxidoreductases, isomerases and additionally as molecular chaperones. They introduce disulfide bonds into nascent proteins, rearrange incorrect disulfide bonds and also support folding and prevention of aggregation of unfolded proteins.19,30 PDI is commonly defined as the main acceptor of oxidative equivalents from the Endoplasmic reticulum oxidase 1 (ERO-1) protein family that will be described in the next section.31,32
Endoplasmic reticulum oxidase 1 - ERO-1
ERO-1 the most conserved ER oxidase and largely controls oxidation in the ER.33,34 ERO-1 functions in the recycling of PDIs by catalyzing the re-oxidation of reduced PDIs for permanent transfer of the disulfides to substrate proteins. While oxidation of substrate protein occurs, ERO-1 consumes oxygen as the terminal electron acceptor producing H2O2.35 The -CxxC- outer active site positioned in an intrinsically flexible loop of ERO-1 transfers electrons from the active site of PDI to the -CxxC- inner active site, and the electrons are then used to reduce oxygen into hydrogen peroxide via flavin adenine dinucleotide cofactor (FAD).36,37 The formation of 2 regulatory disulfides Cys94-Cys131 and Cys99-Cys104 in the inactive latent state, prevents disulfide transferring from the inner active site to PDI via the outer active site (Fig. 2).38
Figure 2.
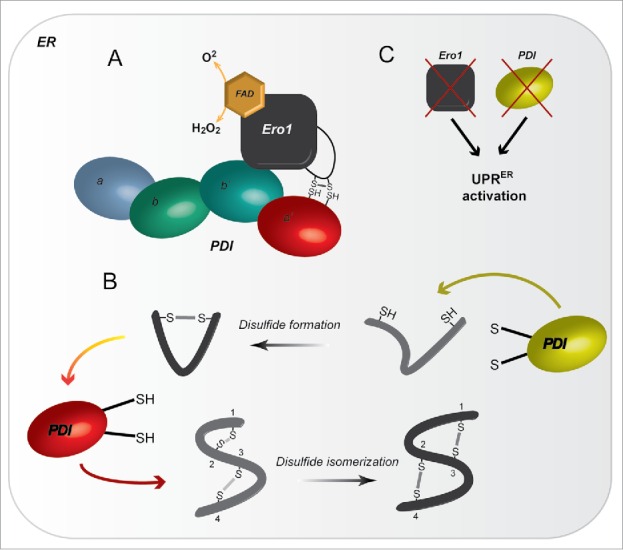
The interplay between Ero1 and PDIs. A) Ero1 in association with FAD is responsible for the re-oxidation of PDIs. Upon binding the b′ domain of PDI, a regulatory Cys-Cys flexible loop re-oxidizes the C- terminal active site a′ of PDI, resulting in H2O2 production. B) This reaction is crucial to maintain the levels of oxidized PDI (yellow), which is responsible to introduce disulfide bonds into newly synthetized proteins. PDIs have multiple functions, and in a reduced state (red), are able to catalyze efficiently the rearrangement of disulfide bonds within a protein. C) Ero1 and PDIs have a central role keeping the redox in the ER, their depletion leads to an UPRER activation.
Structural and functional studies have been conducted mainly on mammalian Ero1, where there are 2 isoforms known (α, β).39 Ero1-α is widely expressed, yet both isoforms have enzymatic activities that are controlled by regulatory disulfides formed among catalytic and non-catalytic cysteines to avoid futile oxidation cycles, which prevents excess hydrogen peroxide production.40-42
PDI and Ero1 represent for the ER a feedback regulatory system that is able to sense and respond to different redox conditions.43,44 The interplay between Ero1-α and PDI has been studied and some mechanical aspects are well characterized. The catalytic active Ero1-α preferentially oxidizes the C-terminal active site in the domain a' of PDI (Fig. 2).45,46 The initial substrate binding by the domain b' of PDI appears to be responsible for the interaction with Ero1-α for a functional disulfide cycle.47,48 However, the exact role of the interaction between PDI and Ero1 in the disulfide transfer chain, stimulating efficient oxidation of nascent proteins in the ER is still far from being completely understood.
The crucial role of PDI's and ERO-1 to maintain ER homeostasis in C. elegans
Sequence comparison and phylogenetic analysis demonstrated that the C. elegans genome encodes for 19 proteins with homologies to human PDIs.12 Eight of these members have the characteristic PDI-motif CGHC (Table 1). Among these proteins, PDI-1, PDI-2, PDI-3 and PDI-6 are the best characterized members. In vitro studies (RNase A refolding assay), confirmed that PDI-1, PDI-2 and PDI-3 are active disulfide isomerases, and whereas PDI-1 and PDI-3 are non-essential proteins, PDI-2 is required for post-embryonic development.50,51 Genetic manipulation of pdi-2 led to severe body morphology defects, uncoordinated movement, and adult sterility. Together they are synergistically essential for embryonic development in the metazoan.51 A fourth PDI member was recently assigned as pdi-6 that displays high sequence homology especially in its thioredoxin domains to the other 3 PDI proteins. Like pdi-2, pdi-6 is an essential gene required for development, and its knockout results in larval lethality (ok1373, wormbase: http://www.wormbase.org). RNAi-mediated knockdown of pdi-6 leads to larval arrest, molting defects and a striking reduction in fecundity.52
Depletion of the PDI family members induces the UPRER and in particular hsp-4 that encodes an ER Hsp70 chaperone (mammalian BiP), and is commonly used to monitor ER stress.53 Taking advantage of an hsp-4::gfp reporter strain, ER stress was also confirmed in response to pdi-6 RNAi and interestingly in a tissue-specific manner, such as in the intestine, hypodermis and pharyngeal cells.52 Notably, knockdown of pdi-3 did not induce the UPRER reporter while pdi-2 RNAi gave rise to robust reporter induction. This induction was observed in different tissues compared to pdi-6, indicating a tissue-specific response of ER stress.52 The effect of pdi-1 knockdown on UPRER is controversial and needs further investigation, as one group observed and another did not observe an induction.52,54 Given that the loss of some members of the PDI family leads to UPRER induction in C. elegans and as the UPRER is required for development,55 one could speculate that over-induction of UPRER might contribute to a deficient larval development in pdi knockdown or knockout animals.
C. elegans encodes for a single Ero1 homolog (ERO-1), and its depletion via RNAi had similar consequences as those reported for pdi members. By analyzing hsp-4 mRNA levels and using an hsp-4::gfp reporter it was confirmed that knockdown of ero-1 leads to UPRER activation.54,56,57 These findings indicate that the oxidases and PDIs play a central role in maintaining proteostasis of the ER.
ER redox and protein homeostasis can also be perturbed by small molecules and compounds such as dithiothreitol, which is a reducing agent and tunicamycin, a glycosylation inhibitor.58 The activation of UPRER ameliorates the consequences of the ER stress induced by the inhibition of glycosylation. Consequently, UPRER induction in ero-1 depleted animals that were subjected to tunicamycin treatment led to an increased lifespan compared to RNAi control worms.59 Indeed, ERO-1 activates specifically the UPRER. In animals that carry a mutation in a crucial UPRER gene (ire-1), the depletion of ero-1 does not result in an hsp-4 (UPRER) induction.55,60,61
Thioredoxin superfamily members with classical CGPC motif
Thioredoxins (Trx) are conserved from bacteria to human and represent important key players in the maintenance of redox homeostasis by acting as general protein disulfide reductases.62 Thioredoxins and related proteins share a redox-active site sequence CXXC, where CGPC is described as canonical Trx-motif.12,63 The C. elegans genome encodes for 9 thioredoxin family members with the characteristic sequence motif (Table 1). However, only limited information is present about their function and characteristics.12,62–67 Thioredoxins are highly specialized in their tissue, cell and compartmental expression pattern (Fig. 1). While TRX-1 is expressed mainly in the intestine as well as in the amphid neurons ASI and ASJ, TRX-3 is intestine-specific.62,63,67 On the cellular level TRX-1, PNG-1 and TRX-3 are expressed in the cytosol and/or nucleus whereas TRX-2 and DNJ-27 are active in the mitochondrion and ER, respectively.63,64,67-69 TRX-1 was also detected in cilia, dendrites and axons of the ASJ neurons.67 Besides their function in redox homeostasis, thioredoxins have also been described to be involved in dauer formation, life span regulation and innate immunity.63,66,67,70
The J-protein DNJ-27 combines chaperone function with classical TRX and PDI active sites
A prominent member of the thioredoxin family that combines protein and redox homeostasis functions in one protein is the J-protein DNJ-27, which is the homolog of the mammalian ERdj5.64 Like its mammalian homolog, DNJ-27 harbors a N-terminal J-domain, 4 trx-like domains with the CXXC motif (CSHC, CPPC, trx motif CGPC, PDI motif CGHC) and 2 divergent trx-like domains without CXXC motif (Fig. 3).64 Both also display an ER retention signal (KDEL for ERdj5, HDEL for DNJ-27). Thus, DNJ-27 is presumed to be an ER-resident.64 DNJ-27 is induced upon treatment with the UPRER inducer tunicamycin and is controlled by the IRE-1/XBP-1 pathway, supporting its function in maintenance of ER homeostasis.64 A role in ER-associated protein degradation (ERAD) was demonstrated for mammalian ERdj5 and also suggested for DNJ-27.64,71 ERdj5 was described to reduce disulfide bonds of ERAD substrates in the ER prior to their retrotranslocation into the cytosol and subsequent proteasomal degradation.71
Figure 3.
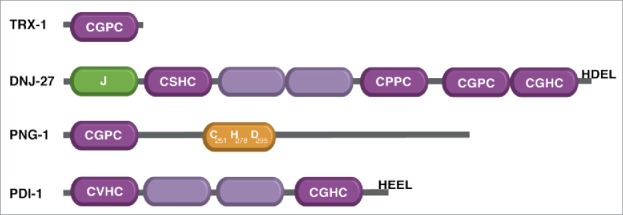
Domain comparison of TRX-1, DNJ-27, PNG-1 and PDI-1. Thioredoxin superfamily members have at least one trx-like domain. The trx-like domains with the respective CXXC motif are shown in dark purple. Trx-like domains, which lack a CXXC motif are illustrated in light purple. DNJ-27 and PNG-1 have a J-domain (green) and transglutaminase domain (yellow), respectively. The position of the catalytic triad within the transglutaminase domain of PNG-1 is indicated.76 Thioredoxins have a canonical CGPC motif whereas PDIs harbor the PDI-like motif CGHC in their active site. DNJ-27 combines both sequences in its structure. DNJ-27 and PDI-1 have an additional ER retention signal HDEL and HEEL, respectively.
In comparison to the other thioredoxins such as TRX-3 and TRX-1, DNJ-27 is ubiquitously expressed with higher expression levels in pharynx and vulva and lower expression in e.g., muscles, intestine and hypodermis.64 Knockdown of dnj-27 in C. elegans impaired the motility and increased protein aggregation of Alzheimer, Parkinson and Huntington's disease models, which express Aβ1-42, α-synuclein or a polyQ-stretch of 40 glutamines in the muscles, respectively.64 Consequently, overexpression of dnj-27 improved motility or reduced toxicity in these strains.64 These data support the idea that DNJ-27 is an important player to regulate cellular homeostasis.
Interestingly, although dnj-27 is expressed in the ER, its depletion by RNAi also results in cytosolic defects. Upon knockdown of dnj-27 by RNAi increased cytosolic degradation by autophagy and the proteasome could be detected.64 Presumably, the knockdown of dnj-27 leads to a higher influx of misfolded proteins from the ER into the cytosol, which in consequence is compensated by increased protein degradation rates. In line with that finding is the observation that depletion of dnj-27 in neurodegenerative disease models also results in an accumulation of cytosolic protein aggregates. It is suggested that upon depletion of dnj-27 the cytosolic clearance pathways become overwhelmed with the burden of misfolded and aggregated proteins resulting in increased aggregation propensity of the disease-causing proteins.64 These findings demonstrate a cross talk between cytosol and the ER.
Besides an increased protein aggregation in the cytosol, knockdown of dnj-27 also causes mitochondrial fragmentation.64 The ER and mitochondria are connected via mitochondrial-associated membranes.72 DNJ-27 might play an additional role in mitochondrial integrity maintenance.64 So far it is unknown whether DNJ-27 plays directly a role in mitochondrial dynamics or whether the induced proteotoxic stress upon dnj-27 knockdown caused the mitochondrial fragmentation.
For the mammalian ERdj5 diverse interaction partners such as BiP, EDEM1 and ERp72 were identified.71,73,74 Up to now, a few more substrates were detected such as the low-density lipoprotein receptor (LDLR), where ERdj5 aids in efficient folding and not LDLR degradation.74 Future studies will reveal more insight into the DNJ-27 interaction network and its substrates for its role as thioreductase and as J-protein in context of diverse neurodegenerative disease models and aging.
PNG-1 is a N-glycanase with disulfide reductase activity
Peptide:N-glycanases (PNGase) are conserved enzymes that hydrolyze N-linked glycoproteins in the cytosol and play a role in ERAD substrate processing.75,76 The nematode homolog PNG-1, however, lacks the PUB domain (Peptide:N-glycanase/UBA or UBX-containing proteins).76 In mammalian PNGase this PUB domain is responsible for ERAD substrate interaction.69 Thus, the role of PNG-1 in ERAD substrate processing remains to be demonstrated.76 Like the homologous proteins in humans, mouse and yeast, PNG-1 also contains the transglutaminase domain, which is required for PNGase function. In contrast, PNG-1 harbors an additional and unique N-terminal trx-like domain (Fig. 3).76 This trx-like domain has protein disulfide reductase activity, which expands the putative roles of PNG-1 in C. elegans in comparison to other PNGases in different eukaryotes.76 However, the in vivo function of this domain requires further characterization. PNG-1 has been described to act in axon branching, which could be dependent on its PNGase and thioredoxin domain.69 Using png-1 loss-of-function mutants, an increased axon branching was observed.69 PNG-1, together with its putative interaction partner RAD-23, may process retro-translocated glycopeptides in the cytosol and form bioactive peptides, which are involved in the inhibition of axon branching.69 Further studies are required to identify more substrates and experimental evidence that PNG-1 is involved in ERAD substrate processing despite the lack of the PUB domain.
Proteins of the thioredoxin network play important roles in C. Elegans AD and PD Models
The thioredoxin systems play an important role in protein redox state and protein function. Thioredoxins are dependent on their thioredoxin reductases (TRXR) to recover from substrate reduction. C. elegans encodes for 2 TRXR proteins, TRXR-1 and TRXR-2.77. A protective role of TRXR-2 was reported in an Alzheimer disease (AD) model of C. elegans, which expresses Aβ1-42 in muscle cells.65 TRXR-2 and its thioredoxin TRX-2 are expressed in mitochondria in several tissues and are up-regulated upon mitochondrial stress.65,77 Interestingly, only the knockdown of trxr-2 increased the paralysis onset of the Aβ-expressing model, while depletion of trx-2 had no effect.65 These data indicate an important role of the mitochondrial thioredoxin reductase trxr-2 in Aβ proteotoxicity. Using a cellular model of Parkinson disease (PD), a loss of TrxR2 (mammalian homolog of trxr-2) activity was monitored.78 Thus, TRXR-2/TrxR2 is essential for not only mitochondrial function, but also for maintenance of cellular proteostasis. An important role of the cytosolic TRXR-1 in PD was also demonstrated.79 TRX1 (mammalian homolog of TRX-1) and TRXR1 (mammalian homolog of TRXR-1) are less expressed in human brains of PD patients.79 Using C. elegans, it was shown that TRXR-1 has protective functions in dopaminergic neurons.79 Depletion mutants of trxr-1, which were treated with the dopaminergic neuron toxin 6-OHDA, lead to increased neuronal degradation in comparison to controls.79 These studies reveal a central protective role of cytosolic and mitochondrial thioredoxin reductases upon proteotoxic and neurotoxic stress.
Redox-regulation of Chaperones
Chaperones are the key players to maintain a balanced protein homeostasis. Many chaperones contain cysteines, which could be reduced or oxidized during redox changes and thus lead to conformational changes. These redox-induced conformational changes can be activating or inhibiting and permit redox-dependent chaperone regulation.80-83
DNJ-20 is a putative homolog of the mammalian ERdj3, which is another ER resident J-protein.80 Interestingly, the oxidation states of ERdj3s cysteines are important for its substrate binding. ERdj3 belongs to the class B of the J-protein family, which is characterized by a J-domain and a Glycin/Phenyl-rich domain, whereas class A J-proteins have an additional cysteine containing zinc-finger domain.84 ERdj3 harbors an atypical cysteine-rich domain with CXC and CXXC motifs, similar to domains in thioredoxins.80 These motifs are also present in the nematode homolog DNJ-20. In the native state, the cysteines of ERdj3 are oxidized in intramolecular disulfide-bridges.80 Alteration in the redox state, e.g. in response to a reducing environment, disrupts these bonds, changes the ERdj3 protein conformation and prevents substrate binding. For the C. elegans protein DNJ-20 such studies are still missing. It is not understood yet if cysteine reduction is simply an inactivation of ERdj3 or may lead to another functional conformation with new substrates or functions.
Notably, J-proteins of class A have a cysteine containing zinc finger domain, which could be redox-regulated. C. elegans encodes for 3 class A member proteins, DNJ-10, DNJ-12 and DNJ-19. So far, nothing is known about their redox sensitivity. Subcellular localization prediction by WoLF PSORT II (www.wolfpsort.org) revealed either a mitochondrial (DNJ-10, also reported85) or cytosolic (DNJ-12, DNJ-19) localization.86 In consequence, class A J-proteins are missing in the ER. It could be hypothesized whether the presence of the zinc-finger domain in class A proteins is responsible for its lack in the ER. Recently, it was demonstrated that a complex of class A and B J-protein together with HSP-1 and HSP-110 is required for efficient protein disaggregation of amorphous protein aggregates.87 Therefore, it would be interesting to analyze if either protein disaggregation of amorphous proteins is less efficient in the ER or if other complexes of chaperones are formed to compensate the lack of synergistic J-protein chaperone activity.
Yeast-two-hybrid studies showed that DNAJA1, which is the mammalian homolog of DNJ-12, could interact with TRX1.81 Additionally, oxidation of DNAJA1 by H2O2 in vitro caused a release of zinc, intermolecular disulfide formation and subsequent J-protein inactivation.81 Also other mammalian class A J-proteins, DNAJA2 (nematode DNJ-19) and DNJA3 (nematode DNJ-10), seem to be regulated by TRX1.88 As oxidative stress accompanies aging and neurodegenerative diseases, it has been speculated that the inactivation of this class of J-proteins may contribute to the increasing imbalance of proteostasis during aging and disease.81
Other chaperone family members were also reported to harbor oxidative-sensitive cysteines, such as HSP-1 (e.g., protein folding and dissaggregation87), CCT-4 (subunit of TCP/TriC complex, e.g. actin biogenesis89) and CDC-48 (e.g., targeting of retro-translocated ERAD substrates90).91 So far, it is unknown if these oxidative-sensitive cysteines have an activating or inhibiting function. For the mammalian homolog of HSP-1, Hsc70, it could be shown that oxidation of an internal thiol through glutathione accelerated the Hsc70 activity in protein aggregate prevention in vitro.82 Recently, it could be shown that also yeast BiP, an Hsp70 in the ER, has a cysteine which can be glutathionylated and cause enhanced chaperone holdase activity.92 Additionally, an activating thiol-based redox switch was observed for Hsp33 of E. coli, where oxidation of Hsp33 leads to chaperone activation.83
Taken together, the increasing number of observations that chaperones are subject to redox-regulation of their activities offers new possibilities for therapeutic intervention for neurodegenerative diseases
Methods to analyze redox capacity
The free radical concept of homeostasis and aging is based on the fact that ROS play an important role as intracellular signaling molecules. The levels of peroxide need to be maintained in controlled levels, as high concentrations of peroxide can be toxic.93 Proteins that are regulated by peroxide and other reactive oxygen and nitrogen species are generally enriched in oxidation-sensitive cysteine residues. Their thiol oxidation state might regulate the protein's activity, and by consequence, the pathway that the protein is involved.94 To evaluate the role that ROS play in a cellular context, a number of tools were developed that allow their quantification in vivo and in vitro (Table 2). Those techniques were utilized to analyze endogenous ROS levels during lifespan, in different sub-cellular compartments, e.g. mitochondria and in different organisms e.g., Drosophila.95 C. elegans has also been used as a model for redox proteomic analysis and fluorescent biosensors.15,91,96,97
Table 2.
Description of the most useful tools to investigate redox capacity in vivo and in vitro
| Method | Read-out | Detection | advantage/ limitation | References |
|---|---|---|---|---|
| OxICAT | Reduced peptides: 13C Oxidized peptides: 12C | Thiol groups | Identification of oxidative cysteines/ does not distinguish between compartments | 16,91,98,111,117 |
| roGFP | Excitation: 405/488 nm Emission: 509 nm | S-S bonds | Ratiometric measurements | 15,97,102,103 |
| HyPer | Excitation: 420/500 nm Emission: 516 nm | Hydrogen peroxide | Ratiometric measurements/pH sensitivity | 104-108,116 |
| Amplex UltraRed | Excitation: 544 nm Emission: 590 nm | Peroxide | Quantification of endogenous peroxide/ pH sensitivity | 16,109,110,115 |
| Killer-Red | Excitation: 585 nm Emission: 610 nm | Peroxide | Temporal and spatial ROS production / phototoxicity | 112 |
| SuperNova | Excitation: 579 nm Emission: 610 nm | Peroxide | Temporal and spatial ROS production / phototoxicity | 113 |
| miniSOG | Excitation: 448 nm Emission: 500/528 nm | Singlet oxygen | Temporal and spatial ROS production / phototoxicity | 114 |
Redox sensors
Thiol trapping technique - OxICAT
Reversible oxidation of cysteine residues enables redox-sensing proteins to regulate a wide range of physiological processes.98 Leichert and colleagues used an isotope-coded affinity tag (ICAT), a shotgun proteomic strategy approach, combined with a thiol trapping method to develop a novel quantitative technique, termed OxICAT.99 Apart from the cleavable biotin affinity tag, the ICAT reagent comprises an iodoacetamide element and a 9-carbon linker, with isotope light 12C- and heavy 13C-form.100 After acidic quenching, oxidized versus reduced cysteine residues are differentially labeled with the heavy and light ICAT reagents. To expose all accessible cysteines, extracts that succumbed to enzymatic digestion and the ICAT-labeled peptides were purified by streptavidin–biotin affinity chromatography. The purified peptides and, therefore, their oxidized cysteine residues, were identified by liquid chromatography tandem mass spectrometry (LC–MS/MS), and the ratio of oxidized to reduced cysteine residues (heavy to light) was determined. Peptides that initially contained reduced thiols would yield mass peaks corresponding to peptides labeled with light ICAT and initially oxidized peptides would yield a higher mass peak, corresponding to peptides labeled with heavy ICAT.99 The thiol groups on proteins are a major target of ROS and play an important role in redox signaling, so the absolute ratio of reduced to oxidized proteins in a sample is extremely relevant and can be obtained with the OxICAT method. This feature makes OxICAT properly tailored for determining any change in the redox state of proteins that were exposed to stress conditions, allowing exhaustive identification of all oxidized cysteine residues, and precise determination of the oxidized cysteine residues within polypeptides in one single analysis. By calculating the ratio Ox/Red peptides, the redox state of protein populations can be determined.99
Aging explored via OxICAT
OxICAT is a great tool to monitor the protein thiol redox state during the lifespan of C. elegans and determine cellular redox conditions.16,97 Taking advantage of this technique, oxidation sensitive cysteines in 40 different proteins were identified, that are involved in numerous biological pathways in worms.91 One of these proteins is the chaperone HSP-1 (Hsc70 homolog) that is highly oxidized in aged animals.16,91 The cytosolic redox proteome of C. elegans in general is more oxidized during development and during aging compared to the reproductive stage of young adults.
The ability to recover from an oxidative stress is linked to the lifespan extension. The ROS levels were compared between short and long-lived animals, daf-16 and daf-2 insulin-like signaling (ILS) mutants, respectively. While daf-2 mutants recovered from oxidative stress during early adulthood and maintained low ROS levels throughout their adult life, daf-16 mutants unsuccessfully recovered and showed significantly increased ROS levels in adult age.16 These data match ILS studies, where it was shown that this pathway could influence lifespan, especially when manipulated in young animals.101
Redox sensitive green fluorescent protein - roGFP
RoGFP is a genetically engineered version of GFP harboring 2 cysteine residues on the outer surface of the β-barrel, in an appropriate position to form disulfide bonds. These two cysteines are spatially close to the fluorophore, and the different structural conformations upon red/ox reactions, can be detected by its 2 excitation peaks at ˜405 and ˜488 nm and by the single emission peak at 509 nm.102 The formation or cleavage of a disulfide bond between those 2 cysteine residues induces changes in the roGFP excitation spectrum, the S-S formation in oxidizing conditions result in a higher excitation at ˜405 nm and lower excitation at ˜488 nm, and the opposite in reducing conditions. Thus, the dual excitation and single emission feature of roGFP can be employed for ratiometric measurements.103
Detecting hydrogen peroxide
HyPer
The H2O2 sensing property of HyPer is based on the E. coli transcription regulator OxyR to selectively detect small quantities of H2O2 by its regulatory domain, OxyR-RD.104 This domain comprises numerous cysteine residues and 2 of them sense H2O2. When oxidized, the Cys199 becomes adjacent to Cys208, which allows disulfide bond formation, leading to conformational changes in the OxyR-RD.105,106
In C. elegans, YFP was fused into the conformational changing region in OxyR-RD, between residues 205 and 206, creating a ratiometric sensor for H2O2 detection.107 HyPer has 2 excitation peaks at 420 nm and 500 nm, and one emission peak at 516 nm. Oxidation of HyPer by H2O2 results in a decrease in the 420 nm excitation peak and a proportional increase in the 500 nm excitation peak, making the sensor ratiometric. The advantages of HyPer are its reversibility, sensitivity (nanomolar range) and fast reaction rate, that makes it a precious tool to monitor real-time dynamics of H2O2 in a living organism.107,108
Amplex ultrared reagent
Peroxide levels can also be quantitatively accessed by incubating lysates of C. elegans with Amplex®UltraRed.109 The reagent reacts with hydrogen peroxide in a 1:1 stoichiometric ratio resulting in an increased fluorescence whose emission is measured at 590 nm (excitation at 544 nm).110
Together, these sensors offer a dynamic analysis due to their reversible oxidation and quantitative assessment of the redox state.
Aging addressed via roGFP, HyPer and Amplex UltraRed
These different tools help to address numerous questions, such as how C. elegans responds to oxidative stress in distinct life stages. Monitoring of hydrogen peroxide levels (Amplex UltraRed) during the lifespan of wild type N2, daf-2 and daf-16 mutants revealed that all animals showed elevated levels of hydrogen peroxide during the developmental stage in the cytosol. The wild type animals (N2) exhibited reducing conditions in the young adult stage, and the long-living daf-2 mutant maintained these reducing conditions for longer in adult life compared to N2 and even more so compared to the short-lived daf-16 mutant.16 This aspect was also observed in a recent study, where daf-2 mutants revealed a reducing environment in a tissue-specific manner.97 Another study using the HyPer sensor assessed the peroxidation levels in C. elegans during aging under normal growth conditions or upon dietary restriction.111 This study demonstrated increasing peroxidation levels upon aging, which was delayed under dietary restriction conditions. Both studies showed that long-lived worms preserved a reducing environment for a longer time compared to N2 worms.16
These redox-sensing methods are also very attractive to study ER redox homeostasis upon proteostasis imbalances and aging in C. elegans, as it enables the precise monitoring of different events in distinct cellular compartments and tissues by fusing proteins of interest with specific promoters and signal sequences.15,97 The analysis of compartmental redox capacity of nematodes was achieved by targeting redox sensors to specific organelles such as the ER and cytosol in different tissues. It was demonstrated that the redox states in the cytosol and in the ER respond in an antagonistic manner, when challenged by proteotoxic stress.15 Whereas the ER becomes more reduced when proteostasis is perturbed e.g. upon expression of amyloidogenic proteins and during aging, the cytosol becomes more oxidizing in response to these challenges. The ER and cytosolic redox states are tightly linked to the UPRER. Induction of the UPRER is associated with a redox shift toward more reducing conditions in the ER lumen and toward more oxidizing conditions in the cytosol. These observations suggest that the cell can adjust the redox state in response to proteostasis imbalances across compartmental borders. It was also addressed how proteotoxic stress in specific tissues can affect the redox state of others, showing that the expression of an aggregation-prone protein, polyglutamine, in muscle cells induces a shift toward more oxidizing cytosolic conditions in neuronal cells and vice versa.15 The crosstalk between tissues, in response to disturbed proteostasis, suggests that the organism is able to sense redox changes across tissues and that complex signaling mechanism are involved in this regulation. Further studies are required to elucidate how this redox homeostasis signaling is controlled during aging and neurodegenerative diseases.
ROS-generating proteins - RGPs
More recently, genetically encoded ROS generating proteins have been applied not only for their phototoxic effects, but also to study ROS signaling and their role in specific cellular compartments. Using probes such as KillerRed (for the generation of peroxide), SuperNova (peroxide) or miniSOG (singlet oxygen), it is possible to control temporal and spatial production of ROS. These RPGs have been mainly employed as a means to ablate specific neurons. However, until today these tools were not developed yet for C. elegans to study the redox homeostasis. Covering this gap will greatly advance the knowledge and clarify the role that ROS play in a living organism.112-114
Despite all of the great advantages that those sensors offer, some limitations can be pointed out.115-117 HyPer and Amplex UltraRed are known to be pH sensitive, so precaution should be taken when measurements are in place.115,116 The expression of these genetic tools in specific subcellular compartments can also disrupt the normal cellular environment. Expression levels of these exogenous proteins should be carefully controlled to avoid an additional burden for the cellular folding machinery. The responsive cysteine residues in HyPer or roGFP can be mutated to demonstrate their redox sensing specificities in the applied experiments. The ratiometric properties of these sensors provide also an advantage as their read-out is independent of their concentration. Potential proteolysis of these sensors as it often occurs with the progression of aging does not affect their feasibility as redox reporters.
Concluding remarks
Aging and disease challenge cellular proteostasis, and organisms consequently lose the capacity to adequately respond to and recover from proteotoxic challenges. Using the model system C. elegans it was possible to gain insight into how proteotoxic stress is sensed by an animal in a tissue or cell specific manner and how stress-signaling pathways are integrated to mount an effective response. In this review we provided an overview of ongoing research on the interplay between the redox and protein homeostasis and how both are tightly regulated on an organismal, tissue, cell and especially cross-compartmental level. Despite the recent advances there are a number of open questions that can be addressed using the nematode as well as other complimentary model systems. Revealing the molecular mechanism underlying the interplay between oxidases and the numerous PDIs is central and crucial for understanding how efficient oxidative folding and redox homeostasis in the ER are maintained. What is the exact oxidation cascade in the formation of disulfide bonds? The high number of thioredoxin family members suggests tissue or cell specific roles in their substrate selection. C. elegans is a great model to explore the contribution of each enzyme in the redox cascade with spatial and temporal resolution. Little is known how redox imbalances are communicated between tissues within an organism and how aging or the organismal physiology such as the metabolic state of the animal affects these circuits. A large number of tools were developed to study redox homeostasis and the application of ROS-generating proteins holds great promise to further advance our understanding on the relationship of redox and protein homeostasis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
We would like to acknowledge funding by the DFG (Excellence Cluster 257 “NeuroCure,” SFB740 and SPP1623) and by the Forschungsverbund Berlin e.V.
References
- [1].Preissler S, Deuerling E. Ribosome-associated chaperones as key players in proteostasis. Trends Biochem Sci 2012; 37:274-83; PMID:22503700; http://dx.doi.org/ 10.1016/j.tibs.2012.03.002 [DOI] [PubMed] [Google Scholar]
- [2].Hipp MS, Park SH, Hartl FU. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol 2014; 24:506-14; PMID:24946960; http://dx.doi.org/ 10.1016/j.tcb.2014.05.003 [DOI] [PubMed] [Google Scholar]
- [3].Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Ulrich Hartl F. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 2013; 82:323-55; PMID:23746257; http://dx.doi.org/ 10.1146/annurev-biochem-060208-092442 [DOI] [PubMed] [Google Scholar]
- [4].Lilienbaum A. Relationship between the proteasomal system and autophagy. Int J Biochem Mol Biol 2013; 4:1-26; PMID:23638318 [PMC free article] [PubMed] [Google Scholar]
- [5].Imai J, Maruya M, Yashiroda H, Yahara I, Tanaka K. The molecular chaperone hsp90 plays a role in the assembly and maintenance of the 26s proteasome. Embo J 2003; 22:3557-67; PMID:12853471; http://dx.doi.org/ 10.1093/emboj/cdg349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rampelt H, Kirstein-Miles J, Nillegoda NB, Chi K, Scholz SR, Morimoto RI, Bukau B. Metazoan hsp70 machines use hsp110 to power protein disaggregation. EMBO J 2012; 31:4221-35; PMID:22990239; http://dx.doi.org/ 10.1038/emboj.2012.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brown MK, Naidoo N. The endoplasmic reticulum stress response in aging and age-related diseases. Front Physiol 2012; 3:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta 2013; 1833:410-6; PMID:22445420; http://dx.doi.org/ 10.1016/j.bbamcr.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morley JF. Regulation of longevity in caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell 2004; 15:657-64; PMID:14668486; http://dx.doi.org/ 10.1091/mbc.E03-07-0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in caenorhabditis elegans aging. Proc Natl Acad Sci U S A 2009; 106:14914-9; http://dx.doi.org/ 10.1073/pnas.0902882106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol 1956; 11:298-300; PMID:13332224; http://dx.doi.org/ 10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- [12].Johnston AD, Ebert PR. The redox system in c. Elegans, a phylogenetic approach. J Toxicol 2012; 2012:546915; PMID:22899914; http://dx.doi.org/ 10.1155/2012/546915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol 2011; 21:569-76; PMID:21824781; http://dx.doi.org/ 10.1016/j.tcb.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med 2012; 52:539-55; PMID:22080087; http://dx.doi.org/ 10.1016/j.freeradbiomed.2011.10.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kirstein J, Morito D, Kakihana T, Sugihara M, Minnen A, Hipp MS, Nussbaum-Krammer C, Kasturi P, Hartl FU, Nagata K, et al.. Proteotoxic stress and ageing triggers the loss of redox homeostasis across cellular compartments. Embo J 2015; 34:2334-49; PMID:26228940; http://dx.doi.org/ 10.15252/embj.201591711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Knoefler D, Thamsen M, Koniczek M, Niemuth NJ, Diederich AK, Jakob U. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol Cell 2012; 47:767-76; PMID:22819323; http://dx.doi.org/ 10.1016/j.molcel.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992; 257:1496-502; PMID:1523409; http://dx.doi.org/ 10.1126/science.1523409 [DOI] [PubMed] [Google Scholar]
- [18].Levine CG, Mitra D, Sharma A, Smith CL, Hegde RS. The efficiency of protein compartmentalization into the secretory pathway. Mol Biol Cell 2005; 16:279-91; PMID:15496459; http://dx.doi.org/ 10.1091/mbc.E04-06-0508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: Substrate interactions and functional properties. EMBO Rep 2005; 6:28-32; PMID:15643448; http://dx.doi.org/ 10.1038/sj.embor.7400311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pan JL, Bardwell JC. The origami of thioredoxin-like folds. Protein Sci 2006; 15:2217-27; PMID:17008712; http://dx.doi.org/ 10.1110/ps.062268106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carvalho AP, Fernandes PA, Ramos MJ. Similarities and differences in the thioredoxin superfamily. Prog Biophys Mol Biol 2006; 91:229-48; PMID:16098567; http://dx.doi.org/ 10.1016/j.pbiomolbio.2005.06.012 [DOI] [PubMed] [Google Scholar]
- [22].Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH. Thioredoxins, glutaredoxins, and peroxiredoxins–molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid Redox Signal 2013; 19:1539-605; PMID:23397885; http://dx.doi.org/ 10.1089/ars.2012.4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alanen HI, Williamson RA, Howard MJ, Hatahet FS, Salo KE, Kauppila A, Kellokumpu S, Ruddock LW. Erp27, a new non-catalytic endoplasmic reticulum-located human protein disulfide isomerase family member, interacts with erp57. J Biol Chem 2006; 281:33727-38; PMID:16940051; http://dx.doi.org/ 10.1074/jbc.M604314200 [DOI] [PubMed] [Google Scholar]
- [24].Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the pdi family: Unpredicted non-er locations and functions. J Cell Physiol 2002; 193:154-63; PMID:12384992; http://dx.doi.org/ 10.1002/jcp.10172 [DOI] [PubMed] [Google Scholar]
- [25].Coppari S, Altieri F, Ferraro A, Chichiarelli S, Eufemi M, Turano C. Nuclear localization and DNA interaction of protein disulfide isomerase erp57 in mammalian cells. J Cell Biochem 2002; 85:325-33; PMID:11948688; http://dx.doi.org/ 10.1002/jcb.10137 [DOI] [PubMed] [Google Scholar]
- [26].Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol 2004; 16:343-9; PMID:15261665; http://dx.doi.org/ 10.1016/j.ceb.2004.06.012 [DOI] [PubMed] [Google Scholar]
- [27].Oka OB, Bulleid NJ. Forming disulfides in the endoplasmic reticulum. Biochim Biophys Acta 2013; 1833:2425-9; PMID:23434683; http://dx.doi.org/ 10.1016/j.bbamcr.2013.02.007 [DOI] [PubMed] [Google Scholar]
- [28].Hebert DN, Molinari M. In and out of the er: Protein folding, quality control, degradation, and related human diseases. Physiol Rev 2007; 87:1377-408; PMID:17928587; http://dx.doi.org/ 10.1152/physrev.00050.2006 [DOI] [PubMed] [Google Scholar]
- [29].Araki K, Nagata K. Functional in vitro analysis of the ero1 protein and protein-disulfide isomerase pathway. J Biol Chem 2011; 286:32705-12; PMID:21757736; http://dx.doi.org/ 10.1074/jbc.M111.227181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gething MJ, Sambrook J. Protein folding in the cell. Nature 1992; 355:33-45; PMID:1731198; http://dx.doi.org/ 10.1038/355033a0 [DOI] [PubMed] [Google Scholar]
- [31].Riemer J, Bulleid N, Herrmann JM. Disulfide formation in the er and mitochondria: Two solutions to a common process. Science 2009; 324:1284-7; PMID:19498160; http://dx.doi.org/ 10.1126/science.1170653 [DOI] [PubMed] [Google Scholar]
- [32].Araki K, Inaba K. Structure, mechanism, and evolution of ero1 family enzymes. Antioxid Redox Signal 2012; 16:790-9; PMID:22145624; http://dx.doi.org/ 10.1089/ars.2011.4418 [DOI] [PubMed] [Google Scholar]
- [33].Appenzeller-Herzog C, Riemer J, Zito E, Chin KT, Ron D, Spiess M, Ellgaard L. Disulphide production by ero1 α-pdi relay is rapid and effectively regulated. Embo J 2010; 29:3318-29; PMID:20802462; http://dx.doi.org/ 10.1038/emboj.2010.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zito E, Melo EP, Yang Y, Wahlander A, Neubert TA, Ron D. Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol Cell 2010; 40:787-97; PMID:21145486; http://dx.doi.org/ 10.1016/j.molcel.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tavender TJ, Bulleid NJ. Molecular mechanisms regulating oxidative activity of the ero1 family in the endoplasmic reticulum. Antioxid Redox Signal 2010; 13:1177-87; PMID:20486761; http://dx.doi.org/ 10.1089/ars.2010.3230 [DOI] [PubMed] [Google Scholar]
- [36].Sevier CS, Kaiser CA. Ero1 and redox homeostasis in the endoplasmic reticulum. Bba-Mol Cell Res 2008; 1783:549-56. [DOI] [PubMed] [Google Scholar]
- [37].Tu BP, Weissman JS. The fad- and o-2-dependent reaction cycle of ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell 2002; 10:983-94; PMID:12453408; http://dx.doi.org/ 10.1016/S1097-2765(02)00696-2 [DOI] [PubMed] [Google Scholar]
- [38].Zhang LH, Niu YB, Zhu L, Fang JQ, Wang X, Wang L, Wang CC. Different interaction modes for protein-disulfide isomerase (pdi) as an efficient regulator and a specific substrate of endoplasmic reticulum oxidoreductin-1 α (ero1 α). J Biol Chem 2014; 289:31188-99; PMID:25258311; http://dx.doi.org/ 10.1074/jbc.M114.602961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang L, Zhu L, Wang CC. The endoplasmic reticulum sulfhydryl oxidase ero1 β drives efficient oxidative protein folding with loose regulation. Biochem J 2011; 434:113-21; PMID:21091435; http://dx.doi.org/ 10.1042/BJ20101357 [DOI] [PubMed] [Google Scholar]
- [40].Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R. Ero1-l, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J Biol Chem 2000; 275:4827-33; PMID:10671517; http://dx.doi.org/ 10.1074/jbc.275.7.4827 [DOI] [PubMed] [Google Scholar]
- [41].Appenzeller-Herzog C, Riemer J, Christensen B, Sorensen ES, Ellgaard L. A novel disulphide switch mechanism in ero1 α balances er oxidation in human cells. Embo J 2008; 27:2977-87; PMID:18833192; http://dx.doi.org/ 10.1038/emboj.2008.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baker KM, Chakravarthi S, Langton KP, Sheppard AM, Lu H, Bulleid NJ. Low reduction potential of ero1 α regulatory disulphides ensures tight control of substrate oxidation. Embo J 2008; 27:2988-97; PMID:18971943; http://dx.doi.org/ 10.1038/emboj.2008.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Benham AM, van Lith M, Sitia R, Braakman I. Ero1-pdi interactions, the response to redox flux and the implications for disulfide bond formation in the mammalian endoplasmic reticulum. Philos T R Soc B 2013; 368:20110403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kim S, Sideris DP, Sevier CS, Kaiser CA. Balanced ero1 activation and inactivation establishes er redox homeostasis. J Cell Biol 2012; 196:713-25; PMID:22412017; http://dx.doi.org/ 10.1083/jcb.201110090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chambers JE, Tavender TJ, Oka OBV, Warwood S, Knight D, Bulleid NJ. The reduction potential of the active site disulfides of human protein disulfide isomerase limits oxidation of the enzyme by ero1 α. J Biol Chem 2010; 285:29200-7; PMID:20657012; http://dx.doi.org/ 10.1074/jbc.M110.156596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Araki K, Lemura S, Kamiya Y, Ron D, Kato K, Natsume T, Nagata K. Ero1-α and pdis constitute a hierarchical electron transfer network of endoplasmic reticulum oxidoreductases. J Cell Biol 2013; 202:861-74; PMID:24043701; http://dx.doi.org/ 10.1083/jcb.201303027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang L, Li SJ, Sidhu A, Zhu L, Liang Y, Freedman RB, Wang CC. Reconstitution of human ero1-l α/protein-disulfide isomerase oxidative folding pathway in vitro. Position-dependent differences in role between the a and a' domains of protein-disulfide isomerase. J Biol Chem 2009; 284:199-206. [DOI] [PubMed] [Google Scholar]
- [48].Inaba K, Masui S, Iida H, Vavassori S, Sitia R, Suzuki M. Crystal structures of human ero1 α reveal the mechanisms of regulated and targeted oxidation of pdi. Embo J 2010; 29:3330-43; PMID:20834232; http://dx.doi.org/ 10.1038/emboj.2010.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Veijola J, Annunen P, Koivunen P, Page AP, Pihlajaniemi T, Kivirikko KI. Baculovirus expression of two protein disulphide isomerase isoforms from caenorhabditis elegans and characterization of prolyl 4-hydroxylases containing one of these polypeptides as their β subunit. Biochem J 1996; 317:721-9; PMID:8760355; http://dx.doi.org/ 10.1042/bj3170721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Eschenlauer SCP, Page AP. The caenorhabditis elegans erp60 homolog protein disulfide isomerase-3 has disulfide isomerase and transglutaminase-like cross-linking activity and is involved in the maintenance of body morphology. J Biol Chem 2003; 278:4227-37; PMID:12424233; http://dx.doi.org/ 10.1074/jbc.M210510200 [DOI] [PubMed] [Google Scholar]
- [51].Winter AD, McCormack G, Page AP. Protein disulfide isomerase activity is essential for viability and extracellular matrix formation in the nematode caenorhabditis elegans. Dev Biol 2007; 308:449-61; PMID:17586485; http://dx.doi.org/ 10.1016/j.ydbio.2007.05.041 [DOI] [PubMed] [Google Scholar]
- [52].Eletto D, Eletto D, Dersh D, Gidalevitz T, Argon Y. Protein disulfide isomerase a6 controls the decay of ire1 α signaling via disulfide-dependent association. Mol Cell 2014; 53:562-76; PMID:24508390; http://dx.doi.org/ 10.1016/j.molcel.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Urano F, Calfon M, Yoneda T, Yun C, Kiraly M, Clark SG, Ron D. A survival pathway for caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol 2002; 158:639-46; PMID:12186849; http://dx.doi.org/ 10.1083/jcb.200203086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci 2004; 117:4055-66; PMID:15280428; http://dx.doi.org/ 10.1242/jcs.01275 [DOI] [PubMed] [Google Scholar]
- [55].Shen XH, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, et al.. Complementary signaling pathways regulate the unfolded protein response and are required for c-elegans development. Cell 2001; 107:893-903; PMID:11779465; http://dx.doi.org/ 10.1016/S0092-8674(01)00612-2 [DOI] [PubMed] [Google Scholar]
- [56].Glover-Cutter KM, Lin S, Blackwell TK. Integration of the unfolded protein and oxidative stress responses through skn-1/nrf. PLoS Genet 2013; 9:e1003701; PMID:24068940; http://dx.doi.org/ 10.1371/journal.pgen.1003701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ye YH, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the er lumen into the cytosol. Nature 2004; 429:841-7; PMID:15215856; http://dx.doi.org/ 10.1038/nature02656 [DOI] [PubMed] [Google Scholar]
- [58].Oslowski CM, Urano F. Measuring er stress and the unfolded protein response using mammalian tissue culture system. Method Enzymol 2011; 490:71-92; http://dx.doi.org/ 10.1016/B978-0-12-385114-7.00004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang YH, Jungreis R, Nagata K, Harding HP, Ron D. Chop induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 2004; 18:3066-77; PMID:15601821; http://dx.doi.org/ 10.1101/gad.1250704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Calfon M, Zeng HQ, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. Ire1 couples endoplasmic reticulum load to secretory capacity by processing the xbp-1 mrna. Nature 2002; 415:92-6; PMID:11780124; http://dx.doi.org/ 10.1038/415092a [DOI] [PubMed] [Google Scholar]
- [61].Harding HP, Zhang YH, Zeng HQ, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al.. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003; 11:619-33; PMID:12667446; http://dx.doi.org/ 10.1016/S1097-2765(03)00105-9 [DOI] [PubMed] [Google Scholar]
- [62].Jee C, Vanoaica L, Lee J, Park BJ, Ahnn J. Thioredoxin is related to life span regulation and oxidative stress response in caenorhabditis elegans. Genes Cells 2005; 10:1203-10; http://dx.doi.org/ 10.1111/j.1365-2443.2005.00913.x [DOI] [PubMed] [Google Scholar]
- [63].Jimenez-Hidalgo M, Kurz CL, Pedrajas JR, Naranjo-Galindo FJ, Gonzalez-Barrios M, Cabello J, Saez AG, Lozano E, Button EL, Veal EA, et al.. Functional characterization of thioredoxin 3 (trx-3), a caenorhabditis elegans intestine-specific thioredoxin. Free Radic Biol Med 2014; 68:205-19; PMID:24316195; http://dx.doi.org/ 10.1016/j.freeradbiomed.2013.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Munoz-Lobato F, Rodriguez-Palero MJ, Naranjo-Galindo FJ, Shephard F, Gaffney CJ, Szewczyk NJ, Hamamichi S, Caldwell KA, Caldwell GA, Link CD, et al.. Protective role of dnj-27/erdj5 in caenorhabditis elegans models of human neurodegenerative diseases. Antioxid Redox Signal 2014; 20:217-35; PMID:23641861; http://dx.doi.org/ 10.1089/ars.2012.5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cacho-Valadez B, Munoz-Lobato F, Pedrajas JR, Cabello J, Fierro-Gonzalez JC, Navas P, Swoboda P, Link CD, Miranda-Vizuete A. The characterization of the caenorhabditis elegans mitochondrial thioredoxin system uncovers an unexpected protective role of thioredoxin reductase 2 in β-amyloid peptide toxicity. Antioxid Redox Signal 2012; 16:1384-400; PMID:22220943; http://dx.doi.org/ 10.1089/ars.2011.4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fierro-Gonzalez JC, Gonzalez-Barrios M, Miranda-Vizuete A, Swoboda P. The thioredoxin trx-1 regulates adult lifespan extension induced by dietary restriction in caenorhabditis elegans. Biochem Biophys Res Commun 2011; 406:478-82; PMID:21334311; http://dx.doi.org/ 10.1016/j.bbrc.2011.02.079 [DOI] [PubMed] [Google Scholar]
- [67].Miranda-Vizuete A, González JCF, Gahmon G, Burghoorn J, Navas P, Swoboda P. Lifespan decrease in a caenorhabditis elegans mutant lacking trx-1, a thioredoxin expressed in asj sensory neurons. FEBS Lett 2006; 580:484-90; PMID:16387300; http://dx.doi.org/ 10.1016/j.febslet.2005.12.046 [DOI] [PubMed] [Google Scholar]
- [68].Schaar CE, Dues DJ, Spielbauer KK, Machiela E, Cooper JF, Senchuk M, Hekimi S, Van Raamsdonk JM. Mitochondrial and cytoplasmic ros have opposing effects on lifespan. PLoS Genet 2015; 11:e1004972; PMID:25671321; http://dx.doi.org/ 10.1371/journal.pgen.1004972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Habibi-Babadi N, Su A, de Carvalho CE, Colavita A. The n-glycanase png-1 acts to limit axon branching during organ formation in caenorhabditis elegans. J Neurosci 2010; 30:1766-76; PMID:20130186; http://dx.doi.org/ 10.1523/JNEUROSCI.4962-08.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fierro-Gonzalez JC, Cornils A, Alcedo J, Miranda-Vizuete A, Swoboda P. The thioredoxin trx-1 modulates the function of the insulin-like neuropeptide daf-28 during dauer formation in caenorhabditis elegans. PLoS One 2011; 6:e16561; PMID:21304598; http://dx.doi.org/ 10.1371/journal.pone.0016561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ushioda R, Hoseki J, Araki K, Jansen G, Thomas DY, Nagata K. Erdj5 is required as a disulfide reductase for degradation of misfolded proteins in the er. Science 2008; 321:569-72; PMID:18653895; http://dx.doi.org/ 10.1126/science.1159293 [DOI] [PubMed] [Google Scholar]
- [72].Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 1990; 265:7248-56; PMID:2332429 [PubMed] [Google Scholar]
- [73].Cunnea PM, Miranda-Vizuete A, Bertoli G, Simmen T, Damdimopoulos AE, Hermann S, Leinonen S, Huikko MP, Gustafsson JA, Sitia R, et al.. Erdj5, an endoplasmic reticulum (er)-resident protein containing dnaj and thioredoxin domains, is expressed in secretory cells or following er stress. J Biol Chem 2003; 278:1059-66; PMID:12411443; http://dx.doi.org/ 10.1074/jbc.M206995200 [DOI] [PubMed] [Google Scholar]
- [74].Oka OB, Pringle MA, Schopp IM, Braakman I, Bulleid NJ. Erdj5 is the er reductase that catalyzes the removal of non-native disulfides and correct folding of the ldl receptor. Mol Cell 2013; 50:793-804; PMID:23769672; http://dx.doi.org/ 10.1016/j.molcel.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kim I, Ahn J, Liu C, Tanabe K, Apodaca J, Suzuki T, Rao H. The png1-rad23 complex regulates glycoprotein turnover. J Cell Biol 2006; 172:211-9; PMID:16401726; http://dx.doi.org/ 10.1083/jcb.200507149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kato T, Kawahara A, Ashida H, Yamamoto K. Unique peptide:N-glycanase of caenorhabditis elegans has activity of protein disulphide reductase as well as of deglycosylation. J Biochem 2007; 142:175-81; PMID:17522090; http://dx.doi.org/ 10.1093/jb/mvm117 [DOI] [PubMed] [Google Scholar]
- [77].Li W, Bandyopadhyay J, Hwaang HS, Park BJ, Cho JH, Lee JI, Ahnn J, Lee SK. Two thioredoxin reductases, trxr-1 and trxr-2, have differential physiological roles in caenorhabditis elegans. Mol Cells 2012; 34:209-18; PMID:22836943; http://dx.doi.org/ 10.1007/s10059-012-0155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liu Y, Ma H, Zhang L, Cui Y, Liu X, Fang J. A small molecule probe reveals declined mitochondrial thioredoxin reductase activity in a parkinson's disease model. Chem Commun 2016; 52:2296-9; PMID:26725656; http://dx.doi.org/ 10.1039/C5CC09998F [DOI] [PubMed] [Google Scholar]
- [79].Arodin L, Miranda-Vizuete A, Swoboda P, Fernandes AP. Protective effects of the thioredoxin and glutaredoxin systems in dopamine-induced cell death. Free Radic Biol Med 2014; 73:328-36; PMID:24863694; http://dx.doi.org/ 10.1016/j.freeradbiomed.2014.05.011 [DOI] [PubMed] [Google Scholar]
- [80].Marcus NY, Marcus RA, Schmidt BZ, Haslam DB. Contribution of the hedj/erdj3 cysteine-rich domain to substrate interactions. Arch Biochem Biophys 2007; 468:147-58; PMID:17976514; http://dx.doi.org/ 10.1016/j.abb.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Choi HI, Lee SP, Kim KS, Hwang CY, Lee YR, Chae SK, Kim YS, Chae HZ, Kwon KS. Redox-regulated cochaperone activity of the human dnaj homolog hdj2. Free Radic Biol Med 2006; 40:651-9; PMID:16458196; http://dx.doi.org/ 10.1016/j.freeradbiomed.2005.09.018 [DOI] [PubMed] [Google Scholar]
- [82].Hoppe G, Chai YC, Crabb JW, Sears J. Protein s-glutathionylation in retinal pigment epithelium converts heat shock protein 70 to an active chaperone. Exp Eye Res 2004; 78:1085-92; PMID:15109915; http://dx.doi.org/ 10.1016/j.exer.2004.02.001 [DOI] [PubMed] [Google Scholar]
- [83].Graf PC, Martinez-Yamout M, VanHaerents S, Lilie H, Dyson HJ, Jakob U. Activation of the redox-regulated chaperone hsp33 by domain unfolding. J Biol Chem 2004; 279:20529-38; PMID:15023991; http://dx.doi.org/ 10.1074/jbc.M401764200 [DOI] [PubMed] [Google Scholar]
- [84].Kampinga HH, Craig EA. The hsp70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 2010; 11:579-92; PMID:20651708; http://dx.doi.org/ 10.1038/nrm2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Addo MG, Cossard R, Pichard D, Obiri-Danso K, Rotig A, Delahodde A. Caenorhabditis elegans, a pluricellular model organism to screen new genes involved in mitochondrial genome maintenance. Biochim Biophys Acta 2010; 1802:765-73; PMID:20580819; http://dx.doi.org/ 10.1016/j.bbadis.2010.05.007 [DOI] [PubMed] [Google Scholar]
- [86].Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. Wolf psort: Protein localization predictor. Nucleic Acids Res 2007; 35:W585-7; PMID:17517783; http://dx.doi.org/ 10.1093/nar/gkm259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Nillegoda NB, Kirstein J, Szlachcic A, Berynskyy M, Stank A, Stengel F, Arnsburg K, Gao X, Scior A, Aebersold R, et al.. Crucial hsp70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 2015; 524:247-51; PMID:26245380; http://dx.doi.org/ 10.1038/nature14884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Fu C, Wu C, Liu T, Ago T, Zhai P, Sadoshima J, Li H. Elucidation of thioredoxin target protein networks in mouse. Mol Cell Proteomics 2009; 8:1674-87; http://dx.doi.org/ 10.1074/mcp.M800580-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Saegusa K, Sato M, Sato K, Nakajima-Shimada J, Harada A, Sato K. Caenorhabditis elegans chaperonin cct/tric is required for actin and tubulin biogenesis and microvillus formation in intestinal epithelial cells. Mol Biol Cell 2014; 25:3095-104; PMID:25143409; http://dx.doi.org/ 10.1091/mbc.E13-09-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mouysset J, Kahler C, Hoppe T. A conserved role of caenorhabditis elegans cdc-48 in er-associated protein degradation. J Struct Biol 2006; 156:41-9; PMID:16647269; http://dx.doi.org/ 10.1016/j.jsb.2006.02.015 [DOI] [PubMed] [Google Scholar]
- [91].Kumsta C, Thamsen M, Jakob U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of caenorhabditis elegans. Antioxid Redox Signal 2011; 14:1023-37; PMID:20649472; http://dx.doi.org/ 10.1089/ars.2010.3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang J, Sevier CS. Formation and reversibility of bip cysteine oxidation facilitates cell survival during and post oxidative stress. J Biol Chem 2016; 291(14):7541-57; http://dx.doi.org/ 10.1074/jbc.M115.694810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].D'Autreaux B, Toledano MB. Ros as signalling molecules: Mechanisms that generate specificity in ros homeostasis. Nat Rev Mol Cell Biol 2007; 8:813-24; PMID:17848967; http://dx.doi.org/ 10.1038/nrm2256 [DOI] [PubMed] [Google Scholar]
- [94].Cross JV, Templeton DJ. Regulation of signal transduction through protein cysteine oxidation. Antioxid Redox Signal 2006; 8:1819-27; PMID:16987034; http://dx.doi.org/ 10.1089/ars.2006.8.1819 [DOI] [PubMed] [Google Scholar]
- [95].Albrecht SC, Barata AG, Grosshans J, Teleman AA, Dick TP. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab 2011; 14:819-29; PMID:22100409; http://dx.doi.org/ 10.1016/j.cmet.2011.10.010 [DOI] [PubMed] [Google Scholar]
- [96].Johnson TE. Caenorhabditis elegans 2007: The premier model for the study of aging. Exp Gerontol 2008; 43:1-4; PMID:17977684; http://dx.doi.org/ 10.1016/j.exger.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Romero-Aristizabal C, Marks DS, Fontana W, Apfeld J. Regulated spatial organization and sensitivity of cytosolic protein oxidation in caenorhabditis elegans. Nat Commun 2014; 5:5020; PMID:25262602; http://dx.doi.org/ 10.1038/ncomms6020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Putker M, Madl T, Vos HR, de Ruiter H, Visscher M, van den Berg MC, Kaplan M, Korswagen HC, Boelens R, Vermeulen M, et al.. Redox-dependent control of foxo/daf-16 by transportin-1. Mol Cell 2013; 49:730-42; PMID:23333309; http://dx.doi.org/ 10.1016/j.molcel.2012.12.014 [DOI] [PubMed] [Google Scholar]
- [99].Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A 2008; 105:8197-202; PMID:18287020; http://dx.doi.org/ 10.1073/pnas.0707723105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol 1999; 17:994-9; PMID:10504701; http://dx.doi.org/ 10.1038/13690 [DOI] [PubMed] [Google Scholar]
- [101].Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/igf-1 signaling in c-elegans. Science 2002; 298:830-4; PMID:12399591; http://dx.doi.org/ 10.1126/science.1074240 [DOI] [PubMed] [Google Scholar]
- [102].Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 2004; 279:13044-53; PMID:14722062; http://dx.doi.org/ 10.1074/jbc.M312846200 [DOI] [PubMed] [Google Scholar]
- [103].Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 2004; 279:22284-93; PMID:14985369; http://dx.doi.org/ 10.1074/jbc.M312847200 [DOI] [PubMed] [Google Scholar]
- [104].Zheng M, Aslund F, Storz G. Activation of the oxyr transcription factor by reversible disulfide bond formation. Science 1998; 279:1718-21; PMID:9497290; http://dx.doi.org/ 10.1126/science.279.5357.1718 [DOI] [PubMed] [Google Scholar]
- [105].Aslund F, Zheng M, Beckwith J, Storz G. Regulation of the oxyr transcription factor by hydrogen peroxide and the cellular thiol - disulfide status. Proc Natl Acad Sci U S A 1999; 96:6161-5; PMID:10339558; http://dx.doi.org/ 10.1073/pnas.96.11.6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Choi HJ, Kim SJ, Mukhopadhyay P, Cho S, Woo JR, Storz G, Ryu SE. Structural basis of the redox switch in the oxyr transcription factor. Cell 2001; 105:103-13; PMID:11301006; http://dx.doi.org/ 10.1016/S0092-8674(01)00300-2 [DOI] [PubMed] [Google Scholar]
- [107].Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 2006; 3:281-6; PMID:16554833; http://dx.doi.org/ 10.1038/nmeth866 [DOI] [PubMed] [Google Scholar]
- [108].Lee CJ, Lee SM, Mukhopadhyay P, Kim SJ, Lee SC, Ahn WS, Yu MH, Storz G, Ryu SE. Redox regulation of oxyr requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol 2004; 11:1179-85; PMID:15543158; http://dx.doi.org/ 10.1038/nsmb856 [DOI] [PubMed] [Google Scholar]
- [109].Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M. Impaired insulin/igf1 signaling extends life span by promoting mitochondrial l-proline catabolism to induce a transient ros signal. Cell Metab 2012; 15:451-65; PMID:22482728; http://dx.doi.org/ 10.1016/j.cmet.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Cohn CA, Simon SR, Schoonen MAA. Comparison of fluorescence-based techniques for the quantification of particle-induced hydroxyl radicals. Part Fibre Toxicol 2008; 5:2; PMID:18307787; http://dx.doi.org/ 10.1186/1743-8977-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Back P, De Vos WH, Depuydt GG, Matthijssens F, Vanfleteren JR, Braeckman BP. Exploring real-time in vivo redox biology of developing and aging caenorhabditis elegans. Free Radical Bio Med 2012; 52:850-9; http://dx.doi.org/ 10.1016/j.freeradbiomed.2011.11.037 [DOI] [PubMed] [Google Scholar]
- [112].Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol 2006; 24:95-9; PMID:16369538; http://dx.doi.org/ 10.1038/nbt1175 [DOI] [PubMed] [Google Scholar]
- [113].Takemoto K, Matsuda T, Sakai N, Fu D, Noda M, Uchiyama S, Kotera I, Arai Y, Horiuchi M, Fukui K, et al.. Supernova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci Rep 2013; 3:2629; PMID:24043132; http://dx.doi.org/ 10.1038/srep02629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol 2011; 9:e1001041; PMID:21483721; http://dx.doi.org/ 10.1371/journal.pbio.1001041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhu A, Romero R, Petty HR. Amplex ultrared enhances the sensitivity of fluorimetric pyruvate detection. Anal Biochem 2010; 403:123-5; PMID:20382105; http://dx.doi.org/ 10.1016/j.ab.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lukyanov KA, Belousov VV. Genetically encoded fluorescent redox sensors. Biochim Biophys Acta 2014; 1840:745-56; PMID:23726987; http://dx.doi.org/ 10.1016/j.bbagen.2013.05.030 [DOI] [PubMed] [Google Scholar]
- [117].Brandes N, Reichmann D, Tienson H, Leichert LI, Jakob U. Using quantitative redox proteomics to dissect the yeast redoxome. J Biol Chem 2011; 286:41893-903; PMID:21976664; http://dx.doi.org/ 10.1074/jbc.M111.296236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Caruso ME, Jenna S, Bouchecareilh M, Baillie DL, Boismenu D, Halawani D, Latterich M, Chevet E. Gtpase-mediated regulation of the unfolded protein response in caenorhabditis elegans is dependent on the aaa+ atpase cdc-48. Mol Cell Biol 2008; 28:4261-74; PMID:18458060; http://dx.doi.org/ 10.1128/MCB.02252-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Page AP. Cyclophilin and protein disulfide isomerase genes are co-transcribed in a functionally related manner in caenorhabditis elegans. DNA Cell Biol 1997; 16:1335-43; PMID:9407005; http://dx.doi.org/ 10.1089/dna.1997.16.1335 [DOI] [PubMed] [Google Scholar]
- [120].Myllyharju J, Kukkola L, Winter AD, Page AP. The exoskeleton collagens in caenorhabditis elegans are modified by prolyl 4-hydroxylases with unique combinations of subunits. J Biol Chem 2002; 277:29187-96; PMID:12036960; http://dx.doi.org/ 10.1074/jbc.M203824200 [DOI] [PubMed] [Google Scholar]
- [121].Schlotterer A, Hamann A, Kukudov G, Ibrahim Y, Heckmann B, Bozorgmehr F, Pfeiffer M, Hutter H, Stern D, Du X, et al.. Apurinic/apyrimidinic endonuclease 1, p53, and thioredoxin are linked in control of aging in c. Elegans. Aging Cell 2010; 9:420-32; PMID:20346071; http://dx.doi.org/ 10.1111/j.1474-9726.2010.00572.x [DOI] [PubMed] [Google Scholar]
- [122].Chen D, Thomas EL, Kapahi P, Mango SE. Hif-1 modulates dietary restriction-mediated lifespan extension via ire-1 in caenorhabditis elegans. PLoS Genet 2009; 5:e1000486; PMID:19461873; http://dx.doi.org/ 10.1371/journal.pgen.1000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Roberts AF, Gumienny TL, Gleason RJ, Wang H, Padgett RW. Regulation of genes affecting body size and innate immunity by the dbl-1/bmp-like pathway in caenorhabditis elegans. BMC Dev Biol 2010; 10:61; PMID:20529267; http://dx.doi.org/ 10.1186/1471-213X-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]


