Abstract
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are sent in print and are also available on-line. Monographs can be customized to meet the needs of a facility. A drug class review is now published monthly with The Formulary Monograph Service. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, contact Wolters Kluwer customer service at 866-397-3433. The June 2016 monograph topics are elbasvir/grazoprevir, ixekizumab, brivaracetam, reslizumab, and sofosbuvir/velpatasvir. The Safety MUE is on reslizumab.
INDICATIONS
Uridine triacetate (formally PN401) is a uridine replacement agent approved for the emergency treatment of fluorouracil or capecitabine overdose (regardless of the presence of symptoms) or early-onset severe or life-threatening cardiac or central nervous system (CNS) toxicity and/or early-onset unusually severe adverse reactions (eg, gastrointestinal [GI] toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration in adult and pediatric patients.1 Uridine triacetate is not recommended for nonemergent treatment of adverse reactions related to fluorouracil or capecitabine. Safety and efficacy of uridine triacetate initiated more than 96 hours after the end of fluorouracil or capecitabine administration have not been established.1 Uridine triacetate is also approved for the treatment of hereditary orotic aciduria under a different trade name (see Table 1).2
Table 1.
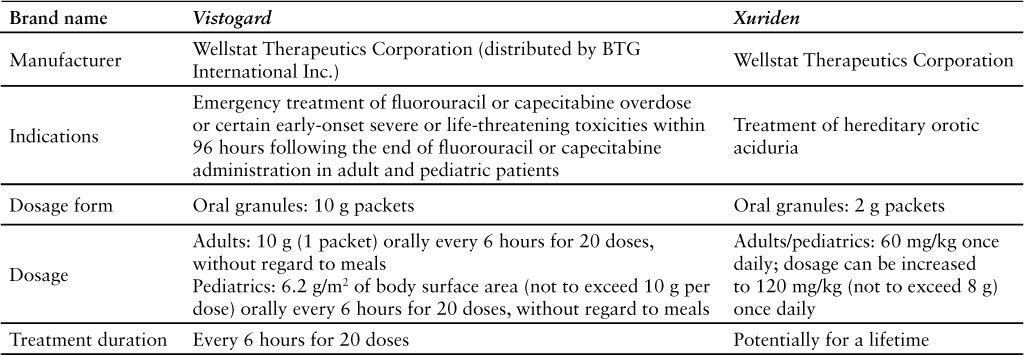
This evaluation will focus on the use of uridine triacetate for the emergency treatment of fluorouracil or capecitabine overdose or toxicity in adult and pediatric patients. Fluorouracil is approved for the treatment of carcinoma of the colon, rectum, breast, stomach, and pancreas. Fluorouracil blocks the methylation reaction of deoxyuridylic acid to thymidylic acid, which interferes with the synthesis of DNA and RNA.3 Capecitabine is a prodrug of 5′-deoxy-5-fluorouridine that undergoes metabolism within the body to fluorouracil; it is used to treat colorectal cancer and metastatic breast cancer.4
CLINICAL PHARMACOLOGY
Uridine triacetate is an acetylated prodrug of uridine. Once in the body, nonspecific esterases deacetylate uridine triacetate to form uridine. The resulting increased uridine levels competitively inhibit cell damage and cell death caused by fluorouracil.1,2
Fluorouracil is metabolized to fluorodeoxyuridine monophosphate (F-dUMP), fluorodeoxyuridine triphosphate (F-dUTP), and floxuridine triphosphate (FUTP). F-dUMP inhibits the synthesis of thymidine, which is needed for the repair and synthesis of DNA. FUTP takes the place of uridine triphosphate during RNA synthesis and causes a disruption of RNA activity. Uridine triacetate is converted to uridine triphosphate in the body. Uridine triphosphate competes with FUTP for RNA binding, thereby decreasing the risk of FUTP-induced toxicity.1,5
PHARMACOKINETICS
Uridine triacetate delivers 4- to 6-fold more uridine into systemic circulation than equimolar doses of uridine alone. Following oral administration, time to peak plasma concentration (Tmax) is 2 to 3 hours.1
In healthy adults given a 6 g dose of the Xuriden formulation, no distinguishable difference was seen in the overall rate and extent of uridine exposure for fed compared to fasted conditions.1,2
Uridine triacetate is distributed extensively within the body; it is taken up into mammalian cells by nucleotide transporters and also crosses the blood-brain barrier.1
Uridine is renally excreted but is also metabolized by pyrimidine catabolic pathways present in most tissues. The half-life of uridine triacetate is 2 to 2.5 hours.1
COMPARATIVE EFFICACY
Indication: Antagonist of Fluorouracil or Capecitabine
Studies
Drug: Uridine triacetate
Reference: Kelsen DP, et al, 19976
Study Design: Phase 1 study
Study Funding: Pro-Neuron Inc., National Cancer Institute
Patients: 38 patients with advanced cancer (pancreatic, bile duct, appendiceal, gastric, adenocarcinoma of unknown origin, sarcoma).
Intervention: Patients received oral uridine triacetate as either a suspension or a tablet in escalating doses. In the first phase of the study, patients received a fixed dose of fluorouracil 600 mg/m2 as a rapid intravenous (IV) bolus followed by 10 doses of uridine triacetate given at 6-hour intervals 24 hours after fluorouracil administration. After determining the appropriate dose of uridine triacetate, a second group of patients received escalating doses of fluorouracil with a fixed dose of uridine triacetate.
Comments: This phase 1 study enrolled 38 patients with advanced cancer to determine the appropriate dose of uridine triacetate as a rescue agent for fluorouracil toxicity and to determine the maximum tolerated dose of fluorouracil when given with uridine triacetate. According to the study, 6 g orally every 6 hours for 10 doses beginning 24 hours after initiation of fluorouracil produced sustained plasma uridine concentrations greater than 50 mcmol/L. The maximum tolerated dose for fluorouracil was 1,000 mg/m2. Higher doses of fluorouracil were well tolerated when given with uridine triacetate.
Reference: Bamat M, et al, 20137
Study Design: Emergency investigational new drug (IND) and expanded access protocol
Study Funding: Wellstat Therapeutics Corporation
Patients: 98 patients with fluorouracil overdose or possible clearance defect.
Intervention: Following fluorouracil exposure, patients were treated at qualified clinical sites with uridine triacetate 10 g orally every 6 hours for 20 doses as soon as possible after overdose or recognition of clearance defect. Resumption of fluorouracil chemotherapy was possible.
Results: Full recovery occurred in 96 of 98 patients.
Endpoint(s)
Reductions or absence of fluorouracil-induced GI, hematologic, and other toxicities were observed following uridine triacetate treatment. Mild or no adverse events were associated with uridine triacetate.
Comments: The report presented at the 2010 American Society of Clinical Oncology Annual Meeting summarized findings from 28 of these patients.8 The product labeling for uridine triacetate includes results from 135 patients in 2 open-label trials (study 1 enrolled 60 patients and study 2 enrolled 75 patients); 117 were treated after an overdose of fluorouracil (n = 112) or capecitabine (n = 5) and 18 were treated for severe or life-threatening fluorouracil toxicities occurring within 96 hours following the end of fluorouracil administration. Of the 135 patients treated, 96% survived and 4% died.1
Limitations: Results of the Bamat et al study are only available as a meeting abstract.
Reference: Doroshow JH, et al, 20069
Study Design: Nonrandomized, open-label, phase 2 trial
Study Funding: Southwest Oncology Group (SWOG)
Patients: 65 patients with advanced gastric adenocarcinoma; data from 57 assessable patients were reported. Patients must not have received chemotherapy or biologic therapy for advanced disease; however, prior chemotherapy given adjuvantly or as a radiation sensitizer was allowed if it had been more than 6 months since the last treatment. Radiation therapy was allowed if it was completed at least 4 weeks prior to enrollment. Inclusion criteria included Zubrod performance status of 0 to 1 and adequate renal, hepatic, and hematologic function. Exclusion criteria included brain metastases, AIDS or HIV-associated disease, history of other malignancies except adequately treated basal or squamous skin cancers or stage I or II cancers in remission for 5 years, and pregnancy or breast-feeding.
Intervention: Each therapy cycle was 8 weeks, with 6 weeks of chemotherapy and 2 weeks of rest. Patients were given leucovorin (500 mg/m2) IV over 2 hours, followed 1 hour later by fluorouracil (1,200 mg/m2) IV over 30 minutes. Uridine triacetate 6 g every 8 hours orally was given starting 8 hours after completion of the fluorouracil infusion, for a total of 8 doses per week during weekly chemotherapy treatment.
Results
Primary Endpoint(s)
Use of uridine triacetate weekly allowed for a 2-fold increase in fluorouracil dose in the treatment of advanced gastric cancer.
Secondary Endpoint(s)
Median survival was 7.2 months, which was 2 months longer than in previous SWOG phase 2 trials of patients with advanced gastric cancer not given uridine.
Endpoint(s)
Despite the increased fluorouracil dose, results suggest a modest toxicity profile and a lessening of GI adverse effects (ie, diarrhea, stomatitis) with the addition of uridine triacetate.
Comments: This study focused on the use of uridine triacetate as a prophylactic agent to decrease the risks associated with fluorouracil therapy. The ability to safely increase the fluorouracil dose 2-fold suggests that the addition of uridine triacetate enhances the therapeutic index of the fluorouracil and leucovorin combination.
Limitations: The study did not test different uridine triacetate concentrations or dosing regimens. Other sources state that uridine triacetate 10 g every 6 hours for 20 doses was sufficient to treat fluorouracil toxicity in acute overdose situations.
CONTRAINDICATIONS, WAR NINGS, AND PRECAUTIONS
Contraindications
There are no contraindications to the use of uridine triacetate.1
If signs/symptoms of a hypersensitivity reaction to the medication and/or any of its excipients (ie, ethylcellulose, Opadry Clear [a proprietary dispersion of hydroxypropylmethylcellulose and Macrogol], natural orange juice flavor) occur, immediately discontinue the medication and begin supportive care.
Warnings and Precautions
There are no warnings or precautions associated with the use of uridine triacetate.1 Data regarding the safety of uridine triacetate during pregnancy in humans are insufficient. When administered to pregnant rats at half the maximum recommended human dose, no teratogenicity or effects on embryofetal development occurred.1
There are no data regarding the presence of uridine triacetate in human breast milk or its effect on the breast-fed infant or breast milk production.1
The safety and effectiveness of uridine triacetate in pediatric patients have been established, but are based on a small number of patients in open-label studies.1
The safety and effectiveness of uridine triacetate in patients 65 years and older have not been established because studies did not include sufficient numbers of patients older than 65 years to determine if they respond differently from younger patients.1
ADVERSE REACTIONS
The most common adverse reactions associated with uridine triacetate in clinical trials were vomiting (10%), nausea (5%), and diarrhea (3%). Overall, uridine triacetate was well tolerated.1
DRUG INTERACTIONS
Uridine triacetate is a weak inhibitor of P-glycoprotein (P-gp), but due to the potential for high drug concentrations in the GI tract, it may interfere with narrow therapeutic index P-gp substrates (eg, digoxin).1 In vivo data in humans are not available.1
RECOMMENDED MONITORING
Patients should be monitored for improvement in the acute signs/symptoms of fluorouracil toxicity (eg, encephalopathy, acute mental status change, mucositis, damage to GI mucosa, diarrhea, myelosuppression).
DOSING
The recommended adult dosage is 10 g (1 packet) orally every 6 hours for 20 doses, without regard to meals.1
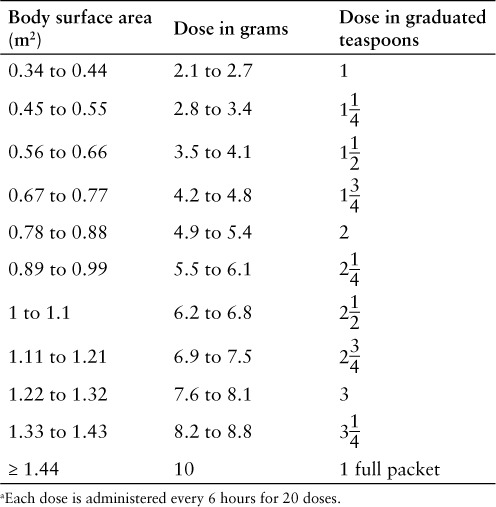
The recommended pediatric dosage is 6.2 g/m2 (not to exceed 10 g per dose; see Table 2) orally every 6 hours for 20 doses, without regard to meals. The dose should be measured with either a scale accurate to at least 0.1 g or a graduated teaspoon accurate to one-fourth teaspoon. Do not save any unused portion of granules for future doses.1
Table 2.
Uridine triacetate pediatric dose based on body surface area a 1
Uridine triacetate must be started within 96 hours after administration of the fluorouracil or capecitabine dose. Safety and efficacy of uridine triacetate administration after 96 hours have not been established.1
Administer treatment as soon as possible after an overdose or early-onset toxicity within 96 hours following the end of fluorouracil or capecitabine administration. Administer all 20 doses.1
The oral granules should be mixed with 3 to 4 ounces of soft food (eg, applesauce, yogurt, pudding) and ingested within 30 minutes of mixing. Instruct patients not to chew the granules and to drink at least 4 oz of water. If a patient vomits within 2 hours of taking a dose, they should take another complete dose as soon as possible after vomiting and then receive the next dose at the regularly scheduled time.1
If administration via nasogastric tube or gastrostomy tube is required, use approximately 4 fl oz of a food starch–based thickening product and mix with water until the thickener has fully dissolved. Crush the full contents of a 10 g packet of granules into a fine powder. Add the crushed granules to 4 oz of reconstituted food starch–based thickening product and mix thoroughly. For pediatric patients receiving less than 10 g, prepare the mixture using a ratio of no greater than 1 g per 10 mL of reconstituted food starch–based thickening product. Administer the mixture using the tube, and then flush the tube with water.1
PRODUCT AVAILABILITY
Uridine triacetate was approved by the FDA on December 11, 2015 for the treatment of fluorouracil or capecitabine overdose or toxicity.10 It is available as orange-flavored granules in single-dose packets containing 10 g of uridine triacetate. One carton contains 20 single-dose packets (course of therapy package) or 4 single-dose packets (24-hour pack).1
Store uridine triacetate at 25°C (77°F); excursions are permitted from 15°C to 30°C (59°F to 86°F).1
DRUG SAFETY/RISK EVALUATION AND MITIGATION STRATEGY (REMS)
No REMS is required for uridine triacetate.10
CONCLUSION
Uridine triacetate is an emergency treatment for patients who receive an overdose of fluorouracil or capecitabine or develop serious toxicity following treatment with these agents. In clinical trials, the majority of patients recovered fully after completing the course of uridine triacetate therapy, with mild or no adverse effects associated with its use.
Footnotes
Founder and Contributing Editor, The Formulary
Pharmacy Intern, Drug Information Center, College of Pharmacy, Washington State University Spokane
Drug Information Resident, College of Pharmacy, Washington State University Spokane
Director, Drug Information Center, and Professor of Pharmacy Practice, College of Pharmacy, Washington State University Spokane.
The authors indicate no relationships that could be perceived as a conflict of interest.
REFERENCES
- 1.Vistogard (uridine triacetate) [prescribing information] West Conshohocken, PA: BTG International Inc.; December 2015. [Google Scholar]
- 2.Xuriden (uridine triacetate) [prescribing information] Gaithersburg, MD: Wellstat Therapeutics Corporation; September 2015. [Google Scholar]
- 3.Adrucil (fluorouracil injection, solution) [prescribing information] Irvine, CA: Teva Parenteral Medicines Inc.; July 2007. [Google Scholar]
- 4.Xeloda (capecitabine) [prescribing information] South San Francisco, CA: Genentech USA Inc.; March 2015. [Google Scholar]
- 5.McEvilly M, Popelas C, Tremmel B. Use of uridine triacetate for the management of fluorouracil overdose. Am J Health Syst Pharm. 2011;68(19):1806–1809. doi: 10.2146/ajhp100434. [DOI] [PubMed] [Google Scholar]
- 6.Kelsen DP, Martin D, O'Neil J et al. Phase I trial of PN401, an oral prodrug of uridine, to prevent toxicity from fluorouracil in patients with advanced cancer. J Clin Oncol. 1997;15(4):1511–1517. doi: 10.1200/JCO.1997.15.4.1511. [DOI] [PubMed] [Google Scholar]
- 7.Bamat M, Tremmel R, Helton J, von Borstel R. Clinical experience with uridine triacetate for 5-fluorouracil overexposure: An update [abstract] Ann Oncol. 2013;24(suppl 4):iv71. [Google Scholar]
- 8.Bamat MK, Tremmel R, O'Neil JD, von Borstel R. Uridine triacetate: An orally administered, life-saving antidote for 5-FU overdose [abstract] J Clin Oncol. 2010;15(suppl) abstract 9084. [Google Scholar]
- 9.Doroshow J, McCoy S, Macdonald JS et al. Phase II trial of PN401, 5-FU, and leucovorin in unresectable or metastatic adenocarcinoma of the stomach: A Southwest Oncology Group study. Invest New Drugs. 2006;24(6):537–542. doi: 10.1007/s10637-006-9244-8. [DOI] [PubMed] [Google Scholar]
- 10.Kim G. NDA approval letter: Vistogard (uridine triacetate NDA 208159) US Food and Drug Administration website. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/208159Orig1s000ltr.pdf. Published December 11, 2015. Accessed January 25, 2016.


