ABSTRACT
All eukaryotes store excess lipids in organelles known as lipid droplets (LDs), which play central roles in lipid metabolism. Understanding LD biogenesis and metabolism is critical for understanding the pathophysiology of lipid metabolic disorders like obesity and atherosclerosis. LDs are composed of a core of neutral lipids surrounded by a monolayer of phospholipids that often contains coat proteins. Nascent LDs bud from the endoplasmic reticulum (ER) but the mechanism is not known. In this commentary we discuss our recent finding that a conserved family of proteins called fat storage-inducing transmembrane (FIT) proteins is necessary for LDs budding from the ER. In cells lacking FIT proteins, LDs remain in the ER membrane. C. elegans has a single FIT protein (FITM-2), which we found is essential; almost all homozygous fitm-2 animals die as larvae and those that survive to adulthood give rise to embryos that die as L1 and L2 larvae. Homozygous fitm-2 animals have a number of abnormalities including a significant decrease in intestinal LDs and dramatic defects in muscle development. Understanding how FIT proteins mediate LD biogenesis and what roles they play in lipid metabolism and development are fascinating challenges for the future.
keywords: endoplasmic reticulum, fat storage-inducing transmembrane proteins, lipid droplets, lipid metabolism
Lipid droplets (LDs) are the major storage organelle for fat in most organisms.1,2 LDs play a key role in lipid homeostasis. However, recent findings suggest that LDs have many other functions; they play roles in protein degradation3,4 and the endoplasmic reticulum (ER) stress response,5 they act as sites for assembly of infectious virions,6 are involved in membrane trafficking and signal transduction, and act as a temporary storehouse of proteins.1,7,8 Understanding LD homeostasis, biogenesis and catabolism is critical for understanding the pathophysiology of lipid storage disorders like obesity, lipodystrophy (abnormal distribution of fat), type2-diabetes, insulin resistance, atherosclerosis and its associated diseases.9,10 These metabolic diseases have a wide range of crippling symptoms, the treatment options of which are few and not very effective.
We are still just beginning to understand the biogenesis of LDs. Before discussing what is known about this process, it is important to understand how LDs differ from other organelles. Most organelles are surrounded by a membrane bilayer that separates the lumen of the organelle from the cytoplasm. In contrast LDs have a phospholipid monolayer that surrounds a core of neutral lipids.11-14 LDs are surrounded by coat proteins; perilipins (PLIN) in mammals, and oleosins in plants, that regulate the access of lipid lipases to the neutral lipid core (for review see15-18). However, yeast and C. elegans lack LD coat proteins,19,20 suggesting that coat proteins are not necessary for LD formation or maintenance. Indeed, emulsions of neutral lipids and phospholipids without proteins form artificial LDs in vitro.
How do LDs form in cells? A number of models have been proposed but the simplest is one that has been termed the lens model. This model suggests that nascent LDs initially form blisters or lenses of neutral lipid that accumulate between the 2 leaflets of the bilayer membrane of the ER, where LD biogenesis occurs. As the lenses grow they eventually bud from the membrane and are then surrounded by a phospholipid monolayer11-14 (Fig. 1). The elegant aspect of this model is that LD formation could be driven largely by the properties of neutral lipids in membranes and may not require any proteins other than the enzymes that synthesize neutral lipids. Neutral lipids are highly hydrophobic and therefore probably remain mostly in the hydrophobic core of the membrane after they are synthesized. It is thought that when the concentration of neutral lipids in a bilayer gets high enough, they spontaneously “oil out” and begin to form a lens between the 2 leaflets of the ER membrane. Obtaining evidence for this model has been difficult, the lenses are thought to be short lived and small, perhaps less than 100 nm.
Figure 1.
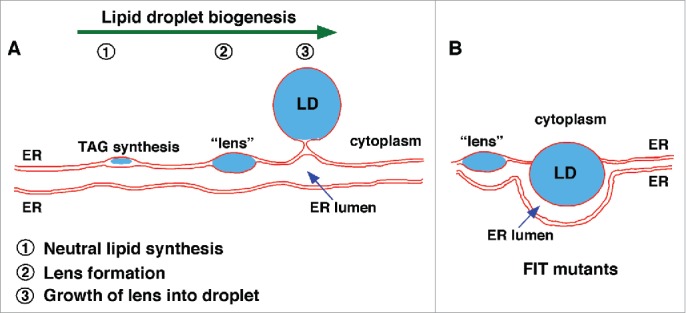
Model of LD biogenesis in wild-type cells and cells lacking FIT proteins. (A) Stages of LD biogenesis in wild-type cells. Neutral lipids are synthesized in the ER (1). When the amount of neutral lipid in the ER bilayer exceeds a threshold, lens-like structures form (2). Neutral lipid lenses grow and emerge out of the ER toward the cytoplasm, resulting in LDs consisting of a core of neutral lipids surrounded by a phospholipid monolayer. Mature LDs remain connected to the ER via membrane-bridge or separate from the ER. (B) In cells lacking FIT proteins LDs fail to bud from the ER and remain embedded in the ER membrane. They often also become wrapped by the ER membrane.
To address this problem, we used a yeast strain in which nascent LD biogenesis can be controlled.21 This strain lacks 3 of the 4 neutral lipid-synthesising enzymes in yeast and has the fourth under a regulatable promoter. When the promoter is off, the cells are devoid of neutral lipids and LDs. However, when the promoter is activated, neutral lipid biosynthesis and LD biogenesis begins within minutes.21 We used this strain in conjunction with EM tomography to confirm that nascent LDs in fact form lenses in the ER membrane.21 Whether lens formation requires any proteins other than the enzymes that synthesize neutral lipids remains unknown.
The finding that nascent LDs form lenses in the ER membrane raises an interesting question: why do LDs bud into the cytoplasm and not into the lumen of the ER (at least in most cell types)? There are probably many factors that determine the directionality of LD budding, including the shape of the ER membrane, the surface tension of the membrane, and the lipid composition of the ER. We have recently shown that a conserved family of proteins facilitates LD budding from the ER.21 These proteins, called fat storage-inducing transmembrane (FIT) proteins, are conserved and were first identified because, when over produced in mammalian cells, cause a dramatic increase in neutral lipid and LD production.22 Humans have 2 of these proteins, FIT1, which is expressed in muscle, and FIT2, found in all tissues.22 The yeast S. cerevisiae has 2 FIT2 homologs and lacks a homolog of FIT1. We found that in a yeast mutant lacking both FIT proteins, LDs fail to bud from the ER and often become wrapped by the ER membrane (Fig. 2). This function of FIT proteins is conserved since knock down of FIT2 in a human 3T3-L1 fibroblasts or the sole FIT protein in C. elegans (FITM-2) results in a similar cellular phenotype21 (Fig. 2).
Figure 2.
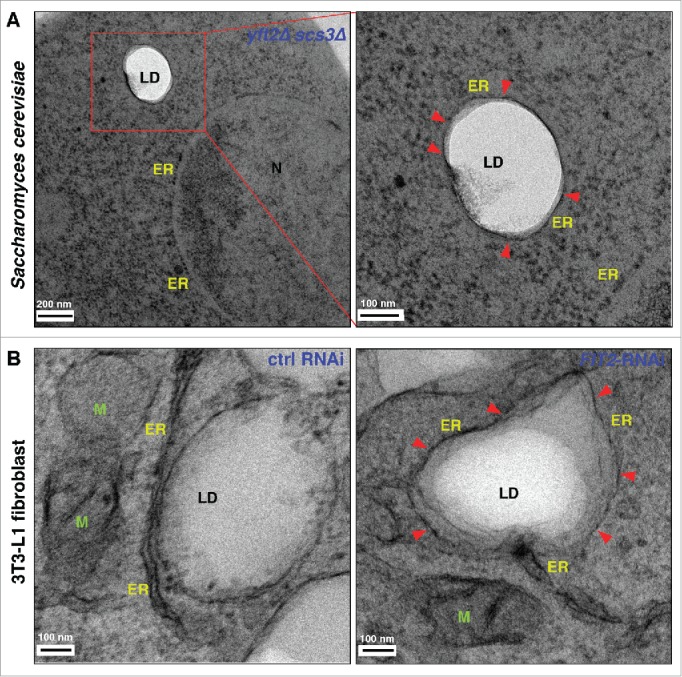
Lack of FIT proteins results in membrane wrapped LDs. (A) Yeast cells lacking FIT proteins (yft2Δ scs3Δ) were cryo-fixed using high pressure freezing and automatic freeze substitution and examined by electron microscopy. LDs are electron transparent. The ER membrane often wraps LDs in FIT mutants. Boxed region is shown in higher magnification. (B) 3T3-L1 fibroblast cells treated with control RNAi or FIT2-RNAi were subjected to chemical fixation and examined by electron microscopy. Arrowheads denote the ER membrane wrapping LDs. ER, endoplasmic reticulum; LD, lipid droplet; N, nucleus; M, mitochondria. Red arrowheads indicate ER membrane wrapping LDs.
How FIT proteins affect LD budding from the ER is not yet understood. It has been found that FITs bind triacylglycerides (TAGs) and diacylglycerol, a precursor of TAGs23 and thus the FITs may play a role in lipid metabolism that somehow affects LD production. Indeed one of the 2 FIT proteins in yeast has been genetically implicated in lipid metabolism but its role is not known.24
Yeast cells lacking both FIT proteins have only modest growth defects and have normal levels of neutral lipids and LDs. Interestingly, this is not the case in metazoans, where elimination of FITs is lethal in mice and worms.21,25 Selective knockdown of FIT2 in adipose tissue renders mice lipodystrophic and insulin resistant.26 Most homozygous fitm-2 animals die as larvae; a minority of animals do survive to adulthood and give rise to viable embryos that die as L1 and L2 larvae.
Why FIT protein deletion is lethal in C. elegans is a fascinating mystery. It may be that a defect in LD budding from the ER disrupts ER function in C. elegans even though it does not in yeast. Lipoprotein production begins in the ER lumen in mammalian cells and probably in C. elegans as well. It could be that that this essential process is disrupted by the presence of LDs in the ER membrane. We have found a significant reduction in the number and size of LDs in intestinal cells,21 suggesting that fitm-2 animals have a profound lipodystrophy. It may be that lipid uptake by intestinal cells is disrupted in these cells, but this remains to be determined. Alternatively, it may be that the FIT protein in C. elegans plays an essential role in lipid metabolism that is not shared by the yeast proteins. It should be noted that a mutant lacking one of the 2 yeast FIT proteins, called Scs3p, has been implicated in lipid metabolism but its role is not known.
It is also possible that C. elegans lacking fitm-2 die because of a defect in muscle development. Remarkably, EM analysis of fitm-2 animal revealed improper development of body wall muscle. Homozygous fitm-2 worms showed very thin body wall muscle (average diameter 200–400 nm) compared to heterozygous fitm-2/+ worms (1–1.2 µm) (unpublished observations). Consistent with this fitm-2 worms showed severe locomotion defects compared to heterozygotes, suggesting impairment in muscle development in these animals. Moreover fitm-2 worms lacked LDs in the proximity of muscle fibers compared to fitm-2/+ worms (unpublished observations).
FIT protein may play an important role in muscle development in mammals as well. Mammals have a muscle-specific FIT protein (FIT1) that seems to be absent in worms;22 it may be that the sole FIT protein in worms performs the functions of FIT1 and FIT2 in mammals. It has recently been found that the expression of the FIT1 gene is altered in patients with facioscapulohumeral muscular dystrophy (FSHD, discussed in27). FSHD is characterized by muscle weakness and wasting in the facial, shoulder and upper arm regions. The symptoms of FSHD appear in adolescence with a prevalence rate of 1 in 20,000 people. FIT1 shows regulated expression during development in the skeletal muscles of pigs with highest expression at 4 months of age and at day 2 of differentiation in C2C12 myoblasts.27 MyoD1, a transcription factor that is crucial for myogenesis, transactivates the promoter of porcine FIT1 gene.27 MyoD1 binding sites are conserved among pig, rat, mice and humans suggesting a similar mechanism of activation of the FIT1 gene.27 The role of FIT proteins in muscle development and how lack of FIT proteins causes muscular dystrophy are important questions for future studies.
The observation that fitm-2 mutants in C. elegans have defects in body wall muscle raises many questions about the primary defect in these animals. Is muscle development perturbed because of defects in lipid metabolism, lipid signaling, or LD formation? Future studies using genetic suppressor screens may help identify the other players in this process and may shed light on the molecular mechanism of specific muscular dystrophies. The larval lethality of these mutants suggests that other tissues are compromised as well. These phenotypes will be the focus of future investigations.
Disclosure of potential conflicts of interest
The authors declare no competing financial interests.
Funding
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. V.C. is supported by fellowship from Swiss National Science Foundation (Grant Nr; PA00P3_145358, P300P3_158454).
References
- [1].Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol (2006); 7:373-8; PMID:16550215; http://dx.doi.org/ 10.1038/nrm1912 [DOI] [PubMed] [Google Scholar]
- [2].Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res (2001); 40:325-438; PMID:11470496; http://dx.doi.org/ 10.1016/S0163-7827(01)00013-3 [DOI] [PubMed] [Google Scholar]
- [3].Hartman IZ, Liu P, Zehmer JK, Luby-Phelps K, Jo Y, Anderson RG, DeBose-Boyd RA. Sterol-induced dislocation of 3-hydroxy-3-methylglutaryl coenzyme A reductase from endoplasmic reticulum membranes into the cytosol through a subcellular compartment resembling lipid droplets. J Biol Chem (2010); 285:19288-98; PMID:20406816; http://dx.doi.org/ 10.1074/jbc.M110.134213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olzmann JA, Richter CM, Kopito RR. Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proc Natl Acad Sci U S A (2013); 110:1345-50; PMID:23297223; http://dx.doi.org/ 10.1073/pnas.1213738110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fei W, Wang H, Fu X, Bielby C, Yang H. Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Biochem J (2009); 424:61-7; PMID:19708857; http://dx.doi.org/ 10.1042/BJ20090785 [DOI] [PubMed] [Google Scholar]
- [6].Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol (2007); 9:1089-97; PMID:17721513; http://dx.doi.org/ 10.1038/ncb1631 [DOI] [PubMed] [Google Scholar]
- [7].Fujimoto T, Ohsaki Y. Cytoplasmic lipid droplets: rediscovery of an old structure as a unique platform. Ann N Y Acad Sci (2006); 1086:104-15; PMID:17185509; http://dx.doi.org/ 10.1196/annals.1377.010 [DOI] [PubMed] [Google Scholar]
- [8].Cermelli S, Guo Y, Gross SP, Welte MA. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol (2006); 16:1783-95; PMID:16979555; http://dx.doi.org/ 10.1016/j.cub.2006.07.062 [DOI] [PubMed] [Google Scholar]
- [9].Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology (2008); 149:942-9; PMID:18202123; http://dx.doi.org/ 10.1210/en.2007-1713 [DOI] [PubMed] [Google Scholar]
- [10].Guo Y, Cordes KR, Farese RV Jr, Walther TC. Lipid droplets at a glance. J Cell Sci (2009); 122:749-52; PMID:19261844; http://dx.doi.org/ 10.1242/jcs.037630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murphy DJ, Vance J. Mechanisms of lipid-body formation. Trends Biochem Sci (1999); 24:109-15; PMID:10203758; http://dx.doi.org/ 10.1016/S0968-0004(98)01349-8 [DOI] [PubMed] [Google Scholar]
- [12].Czabany T, Athenstaedt K, Daum G. Synthesis, storage and degradation of neutral lipids in yeast. Biochim Biophys Acta (2007); 1771:299-309; PMID:16916618; http://dx.doi.org/ 10.1016/j.bbalip.2006.07.001 [DOI] [PubMed] [Google Scholar]
- [13].Ohsaki Y, Suzuki M, Fujimoto T. Open questions in lipid droplet biology. Chem Biol (2014); 21:86-96; PMID:24239006; http://dx.doi.org/ 10.1016/j.chembiol.2013.08.009 [DOI] [PubMed] [Google Scholar]
- [14].Wilfling F, Haas JT, Walther TC, Farese RV Jr. Lipid droplet biogenesis. Curr Opin Cell Biol (2014); 29:39-45; PMID:24736091; http://dx.doi.org/ 10.1016/j.ceb.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res (2007); 48:2547-59; PMID:17878492; http://dx.doi.org/ 10.1194/jlr.R700014-JLR200 [DOI] [PubMed] [Google Scholar]
- [16].Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett (2006); 580:5484-91; PMID:16962104; http://dx.doi.org/ 10.1016/j.febslet.2006.08.040 [DOI] [PubMed] [Google Scholar]
- [17].Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life (2004); 56:379-85; PMID:15545214; http://dx.doi.org/ 10.1080/15216540400009968 [DOI] [PubMed] [Google Scholar]
- [18].Sztalryd C, Kimmel AR. Perilipins: lipid droplet coat proteins adapted for tissue-specific energy storage and utilization, and lipid cytoprotection. Biochimie (2014); 96:96-101; PMID:24036367; http://dx.doi.org/ 10.1016/j.biochi.2013.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee JH, Kong J, Jang JY, Han JS, Ji Y, Lee J, Kim JB. Lipid droplet protein LID-1 mediates ATGL-1-dependent lipolysis during fasting in Caenorhabditis elegans. Mol Cell Biol (2014); 34:4165-76; PMID:25202121; http://dx.doi.org/ 10.1128/MCB.00722-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zweytick D, Athenstaedt K, Daum G. Intracellular lipid particles of eukaryotic cells. Biochim Biophys Acta (2000); 1469:101-20; PMID:10998572; http://dx.doi.org/ 10.1016/S0005-2736(00)00294-7 [DOI] [PubMed] [Google Scholar]
- [21].Choudhary V, Ojha N, Golden A, Prinz WA. A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J Cell Biol (2015); 211:261-71; PMID:26504167; http://dx.doi.org/ 10.1083/jcb.201505067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kadereit B, Kumar P, Wang WJ, Miranda D, Snapp EL, Severina N, Torregroza I, Evans T, Silver DL. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci U S A (2008); 105:94-99; PMID:18160536; http://dx.doi.org/ 10.1073/pnas.0708579105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gross DA, Zhan C, Silver DL. Direct binding of triglyceride to fat storage-inducing transmembrane proteins 1 and 2 is important for lipid droplet formation. Proc Natl Acad Sci U S A (2011); 108:19581-6; PMID:22106267; http://dx.doi.org/ 10.1073/pnas.1110817108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moir RD, Gross DA, Silver DL, Willis IM. SCS3 and YFT2 link transcription of phospholipid biosynthetic genes to ER stress and the UPR. PLoS Genet (2012); 8:e1002890; PMID:22927826; http://dx.doi.org/ 10.1371/journal.pgen.1002890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goh VJ, Tan JS, Tan BC, Seow C, Ong WY, Lim YC, Sun L, Ghosh S, Silver DL. Postnatal deletion of Fat storage-inducing Transmembrane Protein 2 (FIT2/FITM2) causes lethal enteropathy. J Biol Chem (2015); 290(42):25686-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ]26[.Miranda DA, Kim JH, Nguyen LN, Cheng W, Tan BC, Goh VJ, Tan JS, Yaligar J, Kn BP, Velan SS, et al.. Fat storage-inducing transmembrane protein 2 is required for normal fat storage in adipose tissue. J Biol Chem (2014); 289:9560-72; PMID:24519944; http://dx.doi.org/ 10.1074/jbc.M114.547687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ]27[.Yan C, Xia X, He J, Ren Z, Xu D, Xiong Y, Zuo B. MyoD Is a Novel Activator of Porcine FIT1 Gene by Interacting with the Canonical E-Box Element during Myogenesis. Int J Mol Sci (2015); 16:25014-30; PMID:26492245; http://dx.doi.org/ 10.3390/ijms161025014 [DOI] [PMC free article] [PubMed] [Google Scholar]


