Abstract
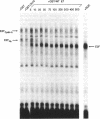
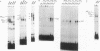
The adenovirus E1A gene product, the simian virus 40 large tumor antigen, and the human papillomavirus E7 protein share a short amino acid sequence that constitutes a domain required for the transforming activity of these proteins. These sequences are also required for these proteins to bind to the retinoblastoma gene product (pRb). Recent experiments have shown that E1A can dissociate complexes containing the transcription factor E2F bound to pRb, dependent on this conserved sequence element. We now show that the E7 protein and the simian virus 40 large tumor antigen can dissociate the E2F-pRb complex, dependent on this conserved sequence element. We also find that the E2F-pRb complex is absent in various human cervical carcinoma cell lines that either express the E7 protein or harbor an RB1 mutation, suggesting that the loss of the E2F-pRb interaction may be an important aspect in human cervical carcinogenesis. We suggest that the ability of E1A, the simian virus 40 large tumor antigen, and E7 to dissociate the E2F-pRb complex may be a common activity of these viral proteins that has evolved to stimulate quiescent cells into a proliferating state so that viral replication can proceed efficiently. In circumstances in which a lytic infection does not proceed, the consequence of this action may be to initiate the oncogenic process in a manner analogous to the mutation of the RB1 gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi S., Raychaudhuri P., Nevins J. R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990 Aug 24;62(4):659–669. doi: 10.1016/0092-8674(90)90112-r. [DOI] [PubMed] [Google Scholar]
- Bagchi S., Weinmann R., Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991 Jun 14;65(6):1063–1072. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- Bandara L. R., La Thangue N. B. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991 Jun 6;351(6326):494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- Blake M. C., Azizkhan J. C. Transcription factor E2F is required for efficient expression of the hamster dihydrofolate reductase gene in vitro and in vivo. Mol Cell Biol. 1989 Nov;9(11):4994–5002. doi: 10.1128/mcb.9.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein R., Shew J. Y., Chen P. L., Scully P., Lee W. H. Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science. 1990 Feb 9;247(4943):712–715. doi: 10.1126/science.2300823. [DOI] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Livingston D. M., Kaelin W. G., Jr The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell. 1991 Jun 14;65(6):1073–1082. doi: 10.1016/0092-8674(91)90559-h. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. H., Horowitz J. M., Gerber M. R., Wang X. F., Bogenmann E., Li F. P., Weinberg R. A. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Girardi A. J., Weinstein D., Moorhead P. S. SV40 transformation of human diploid cells. A parallel study of viral and karyologic parameters. Ann Med Exp Biol Fenn. 1966;44(2):242–254. [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S., Engel D. A., Shenk T. An adenovirus early region 4 gene product is required for induction of the infection-specific form of cellular E2F activity. Genes Dev. 1989 Jul;3(7):1062–1074. doi: 10.1101/gad.3.7.1062. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992 Feb;6(2):177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Horowitz J. M., Park S. H., Bogenmann E., Cheng J. C., Yandell D. W., Kaye F. J., Minna J. D., Dryja T. P., Weinberg R. A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Huang M. M., Hearing P. The adenovirus early region 4 open reading frame 6/7 protein regulates the DNA binding activity of the cellular transcription factor, E2F, through a direct complex. Genes Dev. 1989 Nov;3(11):1699–1710. doi: 10.1101/gad.3.11.1699. [DOI] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Mymryk J. S., Evelegh C. M., Cunniff N. F., Bayley S. T. Sequences in E1A proteins of human adenovirus 5 required for cell transformation, repression of a transcriptional enhancer, and induction of proliferating cell nuclear antigen. Virology. 1989 Jul;171(1):120–130. doi: 10.1016/0042-6822(89)90518-7. [DOI] [PubMed] [Google Scholar]
- Kim Y. K., Lee A. S. Identification of a 70-base-pair cell cycle regulatory unit within the promoter of the human thymidine kinase gene and its interaction with cellular factors. Mol Cell Biol. 1991 Apr;11(4):2296–2302. doi: 10.1128/mcb.11.4.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Miller J. S., Porter D., Roberts B. E. E1a regions of the human adenoviruses and of the highly oncogenic simian adenovirus 7 are closely related. J Virol. 1985 Feb;53(2):399–409. doi: 10.1128/jvi.53.2.399-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Loeken M. R., Brady J. The adenovirus EIIA enhancer. Analysis of regulatory sequences and changes in binding activity of ATF and EIIF following adenovirus infection. J Biol Chem. 1989 Apr 15;264(11):6572–6579. [PubMed] [Google Scholar]
- Loeken M. R., Khoury G., Brady J. Stimulation of the adenovirus E2 promoter by simian virus 40 T antigen or E1A occurs by different mechanisms. Mol Cell Biol. 1986 Jun;6(6):2020–2026. doi: 10.1128/mcb.6.6.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Cao X. R., Yen A., Chandler S., Driscoll B., Murphree A. L., T'Ang A., Fung Y. K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989 Dec 8;246(4935):1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudryj M., Devoto S. H., Hiebert S. W., Hunter T., Pines J., Nevins J. R. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991 Jun 28;65(7):1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- Mudryj M., Hiebert S. W., Nevins J. R. A role for the adenovirus inducible E2F transcription factor in a proliferation dependent signal transduction pathway. EMBO J. 1990 Jul;9(7):2179–2184. doi: 10.1002/j.1460-2075.1990.tb07387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree A. L., Benedict W. F. Retinoblastoma: clues to human oncogenesis. Science. 1984 Mar 9;223(4640):1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S. D., Hemstrom C., Virtanen A., Nevins J. R. An adenovirus E4 gene product trans-activates E2 transcription and stimulates stable E2F binding through a direct association with E2F. Proc Natl Acad Sci U S A. 1990 Mar;87(5):2008–2012. doi: 10.1073/pnas.87.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson B. E., Nasheuer H. P., Wang T. S. Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells. Mol Cell Biol. 1991 Apr;11(4):2081–2095. doi: 10.1128/mcb.11.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Bagchi S., Barnes J. A., Raychaudhuri P., Kraus V., Münger K., Howley P. M., Nevins J. R. Analysis of trans activation by human papillomavirus type 16 E7 and adenovirus 12S E1A suggests a common mechanism. J Virol. 1991 Dec;65(12):6922–6930. doi: 10.1128/jvi.65.12.6922-6930.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Bagchi S., Devoto S. H., Kraus V. B., Moran E., Nevins J. R. Domains of the adenovirus E1A protein required for oncogenic activity are also required for dissociation of E2F transcription factor complexes. Genes Dev. 1991 Jul;5(7):1200–1211. doi: 10.1101/gad.5.7.1200. [DOI] [PubMed] [Google Scholar]
- Reissmann P. T., Simon M. A., Lee W. H., Slamon D. J. Studies of the retinoblastoma gene in human sarcomas. Oncogene. 1989 Jul;4(7):839–843. [PubMed] [Google Scholar]
- Reuter S., Delius H., Kahn T., Hofmann B., zur Hausen H., Schwarz E. Characterization of a novel human papillomavirus DNA in the cervical carcinoma cell line ME180. J Virol. 1991 Oct;65(10):5564–5568. doi: 10.1128/jvi.65.10.5564-5568.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. W., Corrigan M., Yaciuk P., Whelan J., Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990 Sep;64(9):4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T'Ang A., Varley J. M., Chakraborty S., Murphree A. L., Fung Y. K. Structural rearrangement of the retinoblastoma gene in human breast carcinoma. Science. 1988 Oct 14;242(4876):263–266. doi: 10.1126/science.3175651. [DOI] [PubMed] [Google Scholar]
- Toguchida J., Ishizaki K., Sasaki M. S., Nakamura Y., Ikenaga M., Kato M., Sugimot M., Kotoura Y., Yamamuro T. Preferential mutation of paternally derived RB gene as the initial event in sporadic osteosarcoma. Nature. 1989 Mar 9;338(6211):156–158. doi: 10.1038/338156a0. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Armour J., Swallow J. E., Jeffreys A. J., Ponder B. A., T'Ang A., Fung Y. K., Brammar W. J., Walker R. A. The retinoblastoma gene is frequently altered leading to loss of expression in primary breast tumours. Oncogene. 1989 Jun;4(6):725–729. [PubMed] [Google Scholar]
- Weichselbaum R. R., Beckett M., Diamond A. Some retinoblastomas, osteosarcomas, and soft tissue sarcomas may share a common etiology. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2106–2109. doi: 10.1073/pnas.85.7.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Xu H. J., Hu S. X., Hashimoto T., Takahashi R., Benedict W. F. The retinoblastoma susceptibility gene product: a characteristic pattern in normal cells and abnormal expression in malignant cells. Oncogene. 1989 Jun;4(6):807–812. [PubMed] [Google Scholar]
- Yokota J., Akiyama T., Fung Y. K., Benedict W. F., Namba Y., Hanaoka M., Wada M., Terasaki T., Shimosato Y., Sugimura T. Altered expression of the retinoblastoma (RB) gene in small-cell carcinoma of the lung. Oncogene. 1988 Oct;3(4):471–475. [PubMed] [Google Scholar]
- Zerler B., Moran B., Maruyama K., Moomaw J., Grodzicker T., Ruley H. E. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. doi: 10.1128/mcb.6.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]