Abstract
Daily living skills (DLS), such as personal hygiene, meal preparation, and money management, are important to independent living. Research suggests that many individuals with autism spectrum disorder exhibit impairments in daily living skills relative to their cognitive skills. This study examined predictors of daily living skills attainment and trajectories of daily living skills in a longitudinal sample referred for possible autism spectrum disorder and followed from 2 to 21 years of age. Consistent with previous studies, participants with autism spectrum disorder and nonspectrum diagnoses showed continual development of daily living skills throughout childhood and adolescence. Early childhood nonverbal mental age was the strongest predictor of daily living skills attainment for both diagnostic groups. Group-based modeling suggested two distinct trajectories of daily living skills development for participants with autism spectrum disorder. Skill levels for both groups of young adults with autism spectrum disorder remained considerably below age level expectations. Whereas the “High-DLS” group gained approximately 12 years in daily living skills from T2 to T21, the “Low-DLS” group’s daily living skills improved 3–4 years over the 16- to 19-year study period. Nonverbal mental age, receptive language, and social-communication impairment at 2 years predicted High- versus Low-DLS group membership. Receiving greater than 20 h of parent-implemented intervention before age 3 was also associated with daily living skills trajectory. Results suggest that daily living skills should be a focus of treatment plans for individuals with autism spectrum disorder, particularly adolescents transitioning to young adulthood.
Keywords: adaptive behavior, adults, autism spectrum disorders
Adaptive behavior encompasses daily activities important to functional independence, including communication, social, and daily living skills (DLS). Adaptive behavior is defined by typical performance, rather than ability (Sparrow et al., 2005). Thus, one can have low adaptive behavior despite average cognitive functioning, making it necessary to separately address adaptive skills in interventions for individuals with autism spectrum disorder (ASD; Klin et al., 2007). DLS, such as personal hygiene, meal preparation, and money and time management, are important to living independently and obtaining employment. Promoting personal self-care and employment may reduce the lifetime costs associated with having a child with ASD, as well as contribute to the individual’s well-being (Järbrink et al., 2007). Increased understanding of trajectories of DLS and predictors of outcomes in this area can provide insights into intervention targets that will help to promote functional independence in adults with ASD.
Individuals with ASD often demonstrate a relative strength in DLS compared to social and communicative adaptive skills (e.g. Farley et al., 2009; McGovern and Sigman, 2005; Sparrow et al., 2005; Venter et al., 1992; see Klin et al., 2007 for exception). Nonetheless, reports often indicate that many individuals with ASD exhibit significant DLS impairments relative to their cognitive skills. For example, in a sample of adolescents with ASD (aged 10–17 years), 56.4% were classified as having a “DLS Deficit” (i.e. significantly lower DLS than expected based on their full-scale IQ (FSIQ)). Despite FSIQ ≥85, approximately one-quarter of the sample had DLS scores below 70 (Duncan and Bishop, 2013). Similarly, in a sample of 41 adults with FSIQ or nonverbal IQ (NVIQ) above 70, Farley et al. (2009) reported a mean DLS of 76.2 (standard deviation (SD) = 34.9), despite IQ estimates in the average range (M = 86.66, SD = 15.44). In another study, 19 adults with autism had significantly lower DLS (M = 65.1, SD = 35.0) compared to adults with developmental language disorders (M = 99.9, SD = 15.3), despite no group differences in verbal or NVIQ (Howlin et al., 2000; Mawhood et al., 2000). Notably, the mean DLS score for the autism group was considerably lower than their mean NVIQ (82.78, SD = 13.14), whereas the language disorder group demonstrated higher DLS compared to NVIQ (M = 78.42, SD = 10.44). These results highlight a need for better understanding of the “DLS-deficit” in ASD.
Longitudinal studies show that individuals with ASD make gains in DLS across childhood and into young adulthood (Freeman et al., 1999; Gillespie-Lynch et al., 2012; Gray et al., 2014; McGovern and Sigman, 2005; Szatmari et al., 2009; Venter et al., 1992). In one sample, DLS improved in childhood and then flattened in late adolescence, suggesting a slowing of gains over time (Szatmari et al., 2009). In a somewhat older sample, Smith et al. (2012) reported that both individuals with ASD and individuals with Down syndrome made gains in DLS across adolescence and their early 20s. However, while individuals with Down syndrome continued to gain skills across adulthood, DLS attainment appeared to plateau in the ASD group around their late 20s. Authors encouraged caution in interpreting the latter result, given that most of their sample was under 30 years at the final time point. Nonetheless, this plateau was not due to ASD participants having obtained mastery in DLS; on average, adults were not independently completing over one-third of the DLS measured by their questionnaire.
Higher IQ in childhood and/or adolescence has been the most consistent and strongest predictor of better adult DLS outcome (Gillespie-Lynch et al., 2012; Gray et al., 2014; Venter et al., 1992). Studies including individuals with lower IQ suggest fewer or slower gains compared to higher IQ counterparts (Freeman et al., 1999; McGovern and Sigman, 2005; Smith et al., 2012). Preschool (i.e. before age 5; Gillespie-Lynch et al., 2012; Venter et al., 1992) and early school age (i.e. 6–8 years; Szatmari et al., 2009) language skills have also been associated with adolescent and adult DLS levels. Change in language and IQ between early childhood and late adolescence has been indicated to more strongly predict adult DLS than either measure alone (Gillespie-Lynch et al., 2012). Szatmari et al. (2009) reported that adolescents who had structural language impairment at 6–8 years of age also had considerably lower DLS scores than those without language impairment at 17.55 years (raw total difference of 22.53 points), even after controlling for NVIQ, although both groups showed similarly shaped trajectories. Another study also found that living in a more advantaged area predicted poorer DLS 16–17 years later (Gray et al., 2014); authors noted that this was counterintuitive and did not speculate further.
Some studies have also suggested that concurrent measures of language comprehension, verbal IQ, ASD symptoms (Duncan and Bishop, 2013; Venter et al., 1992), and behavior/emotion problems (Gray et al., 2014) are associated with adolescent and adult DLS. Interestingly, in a subset of participants over 18 (n = 22) Venter and colleagues did not find an association between adaptive scores and employment status, whereas Gray et al. (2014) found that individuals with more impaired DLS were significantly less likely to be employed or in post-secondary education. Living with a parent was associated with lower levels of DLS (Smith et al., 2012), while community, but not self-care, skills were associated with living independently (Gray et al., 2014).
To date, only three studies (Freeman et al., 1999; Smith et al., 2012; Szatmari et al., 2009) have examined trajectories of DLS (as opposed to descriptive studies or those comparing two time points or describing cross-sectional data). These studies varied in sample size (53–397), age range (10–53 years, with only one sample in which participants were seen at approximately the same ages), and measure (Vineland Adaptive Behavior Scales (VABS; Sparrow et al., 1984); Waisman Activities of Daily Living Scale (WADL; Maenner et al., 2013)). Each of these three studies was limited in their exploration of predictors of DLS (age, IQ, language, and residential status). Furthermore, none examined possible differences in trajectories of the separate components of DLS (i.e. personal care, domestic skills, and community skills), which could reveal important insights for treatment.
The purpose of this study was to examine predictors of DLS attainment and trajectories of separate DLS domains in a longitudinal sample of children referred for possible ASD at approximately 2 years of age. To this end, we first employ mixed modeling to investigate trajectories of DLS and the effects of early predictors (i.e. demographics, diagnosis, and cognitive and language skills at age 2) on DLS outcomes. In light of previous studies suggesting different patterns of DLS attainment in older individuals with and without ASD (Smith et al., 2012), this analysis included all children, regardless of whether their initial diagnosis was ASD or nonspectrum, in order to assess whether children with ASD showed different trajectories of DLS compared to children with nonspectrum diagnoses. We hypothesized that children with nonspectrum diagnoses would show greater gains in DLS compared to children with ASD, but that differences in DLS attainment would be accounted for by group differences in developmental level (i.e. the ASD group being more impaired with respect to nonverbal mental age (NVMA) and language). We then use group-based modeling to explore the variability of DLS development and predictors of outcome (e.g. demographics, cognitive and language skills, and ASD symptom severity) for children with ASD. This last analysis included only individuals whose most recent diagnosis was ASD, allowing us to examine whether there were different patterns of DLS development observed within individuals with ASD. Finally, comparisons of child characteristics and early intervention are used to examine patterns of group differences in early childhood and young adulthood.
Methods
Sample
Eligible participants were 192 children referred to agencies in North Carolina and Chicago for evaluation of possible autism prior to 37 months. Families completed in-person assessments at approximately ages 2, 3, 5, 9, 18, and 21 (Chicago families were not seen at age 5; a subset of individuals seen at age 18 were reassessed at 21). In addition, parent Vinelands were conducted at approximately 10 and 13 years of age. This sample has been described in detail elsewhere (Anderson et al., 2007, 2009, 2014).
Of the 192 eligible participants, 13 participants were excluded for having fewer than three Vineland assessments. Excluded participants were more likely to be African American (54% vs 30%, p = 0.09) and have maternal education less than a 4-year degree (91% vs 50%, p = 0.01). This resulted in 966 assessments from 179 participants available for analysis (see Table 1 for T2 participant characteristics). Information regarding sample size and available data at each time point is provided in Table S1 (available online).
Table 1.
Summary of T2 predictors used in GLMM.
| ASDa | NSa | |
|---|---|---|
| N | 152 | 27 |
| Age (M, SD) | 2.44 (0.42) | 2.54 (0.42) |
| NVMA (M, SD) | 1.62 (0.56) | 1.86 (0.81) |
| RL-AE (M, SD)b | 0.84 (0.51) | 1.42 (0.82) |
| Male (N, %) | 133 (88%) | 19 (70%) |
| Caucasian (N, %) | 102 (67%) | 20 (74%) |
| MatEd; BA+ (N, %)c | 77 (51%) | 9 (33%) |
GLMM = Generalized Linear Mixed Model; ASD = autism spectrum disorder; NS = nonspectrum; SD = standard deviation; NVMA = nonverbal mental age; RL-AE = receptive language age equivalent; MatEd = Maternal Education; BA+: Bachelor’s degree or higher.
Bold indicates p < 0.05.
Group reflects initial diagnosis at T2.
n = 151 ASD.
n = 146 ASD, n = 26 NS.
Procedures
In-person assessments generally included a parent interview (Autism Diagnostic Interview–Revised (ADI-R), Vineland) and youth assessment (Autism Diagnostic Observation Schedule (ADOS), cognitive test), as well as a battery of questionnaires. All assessments were completed free of charge. Both verbal and written feedback were provided to families. Parents provided written consent prior to each assessment as approved by relevant institutional review boards.
Measures
DLS
At each time point, DLS were assessed using the VABS (Vineland; Sparrow et al., 1984, 2005). The Vineland is a semi-structured parent interview of adaptive functioning. DLS are divided into three subdomains: Personal (e.g. toileting, dressing, hygiene), Domestic (e.g. household safety, food preparation), and Community (e.g. safety, telling time, money skills). For this study, subdomain age equivalents (AEs) were analyzed separately and averaged to obtain an overall DLS age equivalent (DLS-AE).
Autism severity
The sum of the Social and Nonverbal Communication domains (ADI-Soc + Comm) and the Restricted and Repetitive Behavior domain total (ADI-RRB) from the Current Behavior Algorithm of the ADI-R (Rutter et al., 2003) were used as parent-report estimates of ASD severity.
IQ and language
T2-NVMA was obtained from the Mullen Scales of Early Learning (Mullen, 1995). Later cognitive assessments were chosen from a standard hierarchy including the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), Differential Abilities Scale (DAS; Elliott, 1990, 2007), and Mullen. AEs from the Vineland were used to estimate expressive (EL-AE) and receptive language (RL-AE). Because of the high correlation between T2 EL-AE and RL-AE (r = 0.80, p < 0.001), only RL-AE was included as a predictor; EL-AE was used in group comparison analyses.
Diagnosis
Using all available information, clinicians made best-estimate clinical diagnoses of ASD, other nonspectrum disability or psychiatric disorder, or typical development. Diagnoses were assigned at each in-person assessment except T3. Of the 179 individuals in this study, 152 received an initial diagnosis of ASD and 27 received a nonspectrum diagnosis at T2. Although most diagnoses were quite stable through late childhood and young adulthood, there was some variability over time (see Anderson et al., 2014; Lord et al., 2006). Within the T2 nonspectrum group, 30% received an ASD diagnosis at their most recent assessment. In contrast, 8% of the T2 ASD group ultimately received a nonspectrum diagnosis (54% of whom did not meet criteria for any clinical diagnosis at their most recent assessment).
Treatment
Information regarding types of treatment received prior to T3 was collected via a treatment log in which parents were asked to report the type, frequency, and length of any individual therapy their child received. Open-ended questions from the ADI-R were used to clarify treatment details. Two raters established 80% reliability on categorizing treatment types and computing hours for one-third of cases. The number of treatment hours was coded into four categories. A total of 23 children (17%) received mentored, parent-implemented structured teaching (MPST; a home program modeled after the TEACCH (Treatment and Education of Autistic and Related Communication Handicapped Children) extended diagnostic services; see Mesibov et al., 2005); total hours received prior to T3 ranged from 1 to 78 (M = 3.80, SD = 11.70 h). A total of 15% received applied behavior analysis (ABA), 85% speech, and 64% other (i.e. occupational, music, etc.). Based on the median number of MPST hours (not including children with 0 h), children were divided into “little to no MPST” (0–19 h) and “more MPST” (20 + h). For comparability across analyses, the same division was used for all treatment types.
Analyses
All analyses were restricted to individuals who had at least three Vineland assessments (the minimum number of time points recommended for estimation of quadratic trajectories; Burchinal et al., 1994). First, Generalized Linear Mixed Model (GLMM) in SPSS 19 was used to examine trajectories of DLS in the sample of children referred for ASD. Demographic (gender, race, and maternal education) and clinical features (diagnosis, NVMA, and RL-AE) from the first assessment (T2) were entered as predictors (see Table 1).
SAS 9.4 PROC TRAJ was used to examine the variability of development of overall DLS and DLS subdomains (Personal, Domestic, and Community) in 145 participants whose most recent diagnosis was ASD (including 82 diagnoses assigned at T18, 46 from T9, and 17 from an earlier assessment). Participants had 3–8 Vinelands available for analysis (M = 5.4, SD = 1.61). Bayesian Information Criterion (BIC) was compared between respective models to determine the optimal number of groups and order of trajectories (i.e. linear, quadratic; Nagin, 2005). Predictors were entered in a stepwise fashion: demographics (gender, race, maternal education) and T2 clinical features (NVMA, RL-AE, ADI-Soc + Comm, and ADI-RRB). Nonsignificant factors were dropped from the model before the next was entered. T-tests were used to determine the significance of individual parameter estimates for each predictor. Odds ratios (ORs) for each predictor were computed as eβ, where β equals the estimate for group j for a given risk factor (Nagin, 2005). The maximum posterior probability assignment rule was used to create profiles of group members; assignments were based on models without predictors to avoid circularity (Nagin, 2005). Group profiles were compared using independent samples t-tests. Multivariate analysis of variance (ANOVA) was used to control for group age differences (in AE and NVMA comparisons) and age and EL-AE (in comparisons of ADI-R totals due to significant associations with expressive language; Hus and Lord, 2013).
Results
Trajectories and predictors of DLS in children referred for ASD
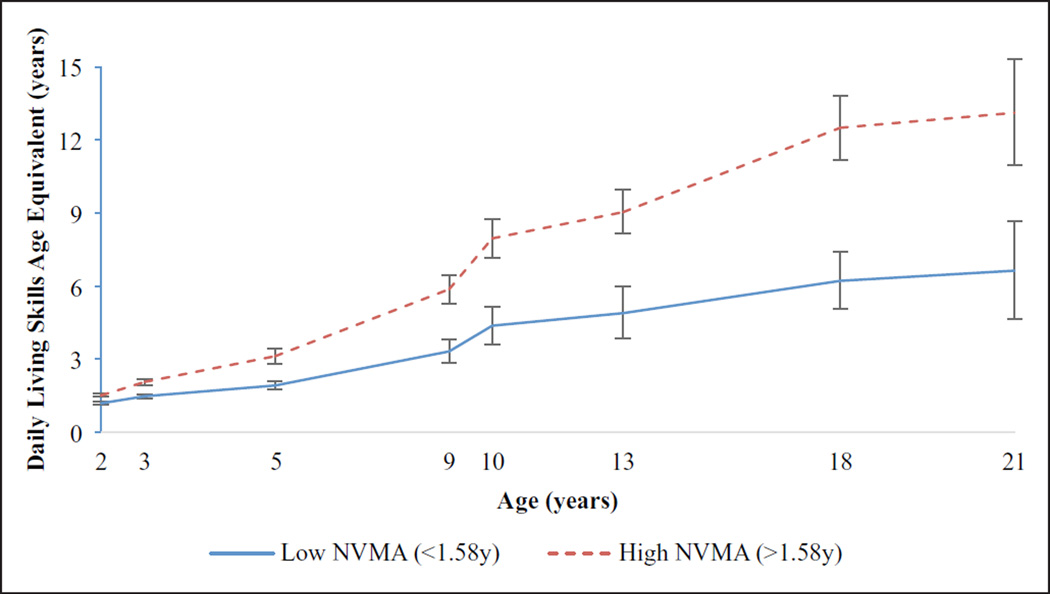
The full GLMM revealed a significant Time × NVMA interaction (p < 0.001), indicating that children with higher initial nonverbal problem solving skills showed more rapid progression of DLS. When T2-NVMA was excluded from the model, Time × RL-AE was significant (p < 0.001); children with higher receptive language skills showed more rapid gains of DLS. When RL-AE was excluded from the model, significant effects of Time and Time × Diagnosis emerged (p < 0.05), indicating that participants with ASD showed significantly slower progression of skills compared to the nonspectrum group. However, since the significant effects of diagnosis and initial receptive language were nonsignificant in the full model including NVMA, these effects are better explained by differences in NVMA between the ASD and nonspectrum group. Specifically, children with ASD showed lower initial NVMA compared to nonspectrum children (Table 1). When children were split into low and high NVMA groups based on the median (1.58 years), children in the low NVMA group demonstrated slower gains of DLS-AEs. Post hoc comparisons revealed that the low NVMA group had significantly lower DLS at all time points (all p < 0.001; Figure 1).
Figure 1.
Changes in daily living skills from 2–21 years for median-split NVMA groups.
N = 179; group differences in DLS significant (p < 0.001) at all ages.
Trajectories and predictors of DLS in children with most recent ASD diagnoses
Trajectories of overall DLS
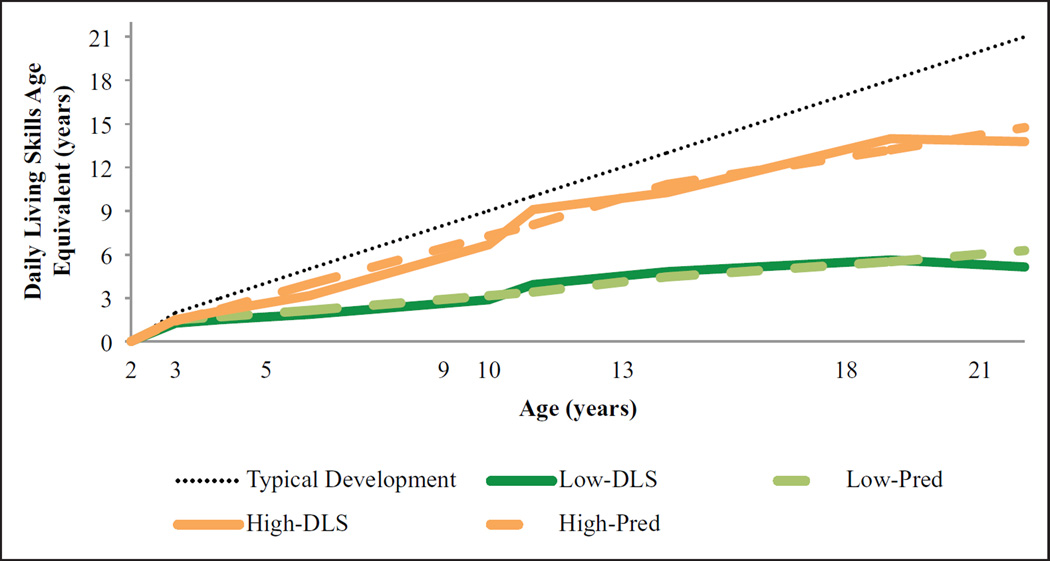
PROC TRAJ was used to examine the variability in DLS trajectories for individuals whose most recent diagnosis was ASD (N = 145). As shown in Figure 2, the best fitting model indicated two distinct trajectory groups. The first group, “Low-DLS,” comprised 66% of the sample (M p(assignment) = 0.94) and was characterized by a linear increase in DLS-AE (p < 0.0001). The remaining 34% of the sample was assigned to the “High-DLS” group (M p(assignment) = 0.99), which followed a quadratic trajectory (p < 0.0001). As shown in Table 2, the Low group had more impaired T2-DLS than the High group—t(140) = −4.55, p < 0.001—although the mean difference was only 2.64 months (standard error (SE) = 0.58). As children grew older, trajectories quickly diverged. By T21, the Low-DLS group had attained a DLS-AE of approximately 5.01 (SD = 2.54) years, whereas the High-DLS group achieved a DLS-AE of 13.69 (SD = 2.93) years (Table 2). Notably, the mean DLS-AE at T21 was slightly lower than the T18 mean for both groups. Paired sample t-tests for the subgroup of individuals with data at both assessments indicated a small, but significant, decline in DLS-AE for young adults in the Low-DLS group (Mdiff = −0.59, SE = 1.11; t(18) = −2.33, p < 0.05) and a nonsignificant increase for adults in the High-DLS group (Mdiff = 0.49, SE = 1.34; t(15) = 1.46, p > 0.05).
Figure 2.
Trajectories of daily living skills in ASD group.
Pred: predicted trajectory
N = 145 individuals whose most recent diagnosis was ASD.
Table 2.
Vineland subdomain scores in early childhood and adulthood by subdomain trajectory group.
| T2 | T3 | T18 | T21 | ||
|---|---|---|---|---|---|
| N = 142 | N = 139 | N = 85 | N = 36 | ||
| Overall DLS | Low (n = 94)a | 1.28 (0.28 | 1.55 (0.33) | 5.56 (2.31) | 5.01 (2.58) |
| High (n = 48) | 1.50 (0.26) | 2.14 (0.59) | 13.92 (3.22) | 13.69 (2.93) | |
| Personal | Low (n = 104) | 1.29 (0.35) | 1.68 (0.46) | 5.54 (2.79) | 6.02 (3.52) |
| High (n = 37) | 1.60 (0.38) | 2.36 (0.65) | 14.64 (2.73) | 16.10 (2.94) | |
| Domestic | Low (n = 99) | 1.40 (0.23) | 1.59 (0.48) | 6.78 (2.72) | 6.11 (3.49) |
| High (n = 41) | 1.42 (0.18) | 2.05 (0.70) | 15.72 (4.16) | 13.28 (2.83) | |
| Community | Low (n = 95) | 0.83 (0.46) | 1.17 (0.46) | 5.25 (2.69) | 4.96 (2.98) |
| High (n = 47) | 1.12 (0.43) | 1.89 (0.64) | 14.49 (3.28) | 15.46 (3.36) |
Bold indicates Low versus High comparison is significant at p < 0.05 or lower.
Group n at T2; varies for subsequent time points.
Baseline predictors of group membership were assessed in a stepwise fashion by entering factors individually to assess significance; only significant predictors were retained in the model. Initially, T2-RL-AE emerged as a significant predictor (p < 0.05; OR = 1.16); however, it was not significant (p > 0.05) in the final model including T2-NVMA (p < 0.001) and ADI-Soc + Comm (p < 0.01), likely reflecting the strong correlation between T2-RL-AE and T2-NVMA (see Table S2, available online). For every 1-month increase in T2-NVMA, the odds were 1.29 times greater that a child would be placed in the High-DLS group, whereas having a higher T2-ADI-Soc + Comm was associated with lower odds of being in the High-Personal group (OR = 0.88). As shown in Table 3, the High-DLS group had significantly higher T2-NVMA and language skills and less social-communication impairment (after controlling for age and expressive language). Children receiving MPST treatment by age 3 were also significantly more likely to be in the High-DLS group (OR = 5.40). Group differences persisted into adulthood (Table 4).
Table 3.
T2 child characteristics by subdomain trajectory group.
| Overall DLS | Personal | Domestic | Community | |||||
|---|---|---|---|---|---|---|---|---|
| Low (n = 95) | High (n = 50) | Low (n = 106) | High (n = 39) | Low (n = 100) | High (n = 45) | Low (n = 96) | High (n = 49) | |
| Age | 2.43 (0.39) | 2.52 (0.44) | 2.43 (0.38) | 2.53 (0.47) | 2.43 (0.39) | 2.52 (0.44) | 2.41 (0.38) | 2.56 (0.44) |
| NVMA | 1.41 (0.44) | 2.03 (0.56) | 1.45 (0.46) | 2.08 (0.58) | 1.44 (0.46) | 2.02 (0.58) | 1.40 (0.44) | 2.05 (0.55) |
| RL-AE | 0.67 (0.37) | 1.16 (0.55) | 0.71 (0.42) | 1.18 (0.54) | 0.73 (0.46) | 1.09 (0.50) | 0.64 (0.35) | 1.22 (0.52) |
| EL-AE | 0.47 (0.43) | 0.96 (0.50) | 0.51 (0.48) | 0.97 (0.45) | 0.54 (0.48) | 0.85 (0.52) | 0.44 (0.40) | 1.02 (0.50) |
| Soc + Comma | 30.94 (6.42) | 0.96 (0.50) | 30.6 (6.60) | 23.58 (7.99) | 29.94 (7.25) | 26.05 (7.84) | 31.17 (6.11) | 23.92 (8.11) |
| RRB | 3.56 (6.42) | 0.96 (0.50) | 3.56 (1.52) | 2.79 (1.75) | 3.45 (1.47) | 3.13 (1.90) | 3.51 (1.47) | 3.04 (1.85) |
| MPST (%)b | ||||||||
| 0–19 hrs | 96.7 | 84.4 | 96.1 | 82.4 | 95.8 | 85.0 | 96.7 | 84.1 |
| ≥ 20 hrs | 3.3 | 15.6 | 3.9 | 17.6 | 4.2 | 15.0 | 3.3 | 15.9 |
NVMA: nonverbal mental age; RL: receptive language; EL: expressive language; AE: age equivalent; Soc + Comm: Autism Diagnostic Interview Social + Nonverbal Communication domain total; RRB: Restricted and Repetitive Behavior domain total; MPST: mentored, parent-implemented structured therapy.
Bold indicates significant at p < 0.05 or lower.
Raw values are reported, but significance is based on comparison controlling for age and receptive language.
MPST was as of T3.
Table 4.
Adult participant characteristics by daily living skills trajectory group.
| T18 | T21 | |||
|---|---|---|---|---|
| Low (n = 49) | High (n = 36) | Low (n = 20) | High (n = 16) | |
| Age | 18.94 (1.04) | 19.28 (1.24) | 21.91 (1.09) | 22.49 (1.33) |
| DL-AE | 5.56 (2.31) | 13.92 (3.22) | 5.01 (2.58) | 13.69 (2.93) |
| NVIQa,b | 28.00 (19.18) | 90.82 (25.95) | ||
| RL-AE | 4.02 (3.58) | 10.67 (5.61) | 3.12 (2.37) | 9.91 (5.53) |
| EL-AE | 2.35 (1.97) | 12.15 (7.46) | 2.75 (2.72) | 8.87 (4.95) |
| Soc + Comma,c | 19.71 (5.92) | 9.00(5.15) | ||
| RRBa | 4.53 (2.3) | 3.56 (2.7) | ||
NVMA: nonverbal mental age; RL: receptive language; EL: expressive language; AE: age equivalent; Soc + Comm: Autism Diagnostic Interview Social + Nonverbal Communication domain total; RRB = Restricted and Repetitive Behavior domain total.
Bold indicates significant at p < 0.05.
Not administered at T21.
NVMA not reported for T18 high group due to use of tests providing ranges of AEs at higher skill levels.
Group differences also significant when controlling for EL-AE.
Post hoc exploration of early predictors of DLS trajectory groups
Examination of T2-NVMA by group membership indicated that, while the Low-DLS group had significantly lower NVMA than the High-DLS group (Mdiff = −7.48 months; t(142) = −7.39, p < 0.001), there was significant overlap (Figure S1, available online). To further understand early factors that may further contribute to DLS attainment, children were divided into groups based on DLS group (Low or High) and NVMA (at or above vs below T2 group median). Comparisons of children in the two groups with comparable NVMA are presented in the online supplement and Table S3 (available online).
Trajectories and predictors of DLS subdomains in children with most recent ASD diagnoses
To examine whether there were differences in trajectories of DLS subdomains (i.e. Personal, Domestic, and Community), separate models were fit for each variable. Results were highly similar to the overall DLS-AE, with two distinct trajectory groups for each subdomain. There were slight differences in trajectory shape for the second (high) group, although these are difficult to interpret given the differences in the scales over time. There was also some variation in predictors of group membership as they were entered individually, although the final models looked quite similar. Results are discussed in the online supplement (see Figures S2, S3, and S4, available online, and Tables 2 and 3).
Intraindividual variability in subdomain trajectories
Although the majority of individuals fell in the same trajectory group across all subdomains (i.e. “All-Low” = 88, “All-High” = 32), 25 individuals showed different trajectories across subdomains (“Mixed”). Of these participants, 72% fell in the Low-Personal group, 48% in the Low-Domestic group, and 32% in the Low-Community group. As shown in Table S4 (available online), individuals in the All-Low group were more impaired on cognitive, language, and social-communication measures than both the Mixed and All-High groups. The Mixed group had lower NVMA than the All-High group. More participants in the All-High group had ≥20 h of MPST by T3 than the All-Low group; the Mixed group did not differ from either group on amount of MPST.
Discussion
Consistent with previous longitudinal studies (Freeman et al., 1999; Smith et al., 2012; Szatmari et al., 2009), participants with ASD and nonspectrum diagnoses in our sample showed continual development of DLS throughout childhood and adolescence. For both diagnostic groups, early childhood NVMA was a significant predictor of DLS attainment. Receptive language skills also predicted DLS attainment for both diagnostic groups, but this effect did not emerge until NVMA was removed from the model. When both NVMA and receptive language skills were removed, a significant Time × Diagnosis interaction emerged, indicating that children with ASD showed a slower progression of DLS compared to children with non-spectrum diagnoses. Post hoc analysis revealed that, irrespective of diagnosis, children with lower NVMA showed slower progression of DLS attainment over time; children with lower NVMA showed significantly more impaired DLS-AEs than children with higher NVMA across all assessments (Figure 1). These results suggest that, while children with ASD may be at risk for slower DLS development than children with other developmental disabilities, this is largely attributable to children with ASD having more delayed nonverbal problem solving and/or receptive language skills. As such, interventions targeting DLS would benefit young children with early cognitive delays, regardless of diagnosis. It is also noteworthy that, in this analysis, the diagnostic groups were defined by T2 diagnosis; because 30% of the nonspectrum group was diagnosed with ASD and 8% of the ASD group received non-ASD diagnoses at their most recent assessment, results might have differed if these diagnoses had been considered.
Group-based modeling suggested two distinct trajectories of DLS development for participants with ASD: a “High” group, gaining an average of approximately 12 years in DLS and a “Low” group, showing modest improvements in DLS (3–4 years) across the 16- to 19-year study period. Although group DLS-AEs differed by only 3 months at age 2, there was a gap of more than 8 years at adult assessments (aged 18 and 21 years). Similar patterns were observed for each DLS subdomain.
Predictors of group membership for overall DLS and subdomain trajectory groups included T2-NVMA, receptive language skills, and social-communication impairment (measured by the ADI-R). Children in the “Low” trajectory groups demonstrated greater impairments in nonverbal problem solving, language skills, and ASD symptoms at age 2 compared to children in the “High” trajectory groups. Moreover, children who received early MPST intervention before age 3 were significantly more likely to fall in the trajectory groups that made greater gains in DLS (Table 3).
Although ABA, speech, and other types of intervention were not significantly associated with DLS attainment (data not shown), the lack of knowledge regarding specific treatment goals for each child cautions against interpretation of a null finding. It may be that goals of the parent-implemented intervention were more focused on teaching DLS. However, it is important to acknowledge that this study design does not allow us to conclude that MPST directly affected attainment of DLS. Future research is needed to directly investigate the relationship between intervention and DLS attainment, paying careful attention to goals targeting specific skill domains, such as personal care, food preparation, and safety. Some studies have reported increased rates of adaptive skill acquisition for toddlers and school-age children whose treatment programs comprised goals and objectives to improve DLS (Ben-Itzchak et al., 2014; Drahota et al., 2011). Interestingly, the cognitive behavioral program implemented by Drahota and colleagues also included a parent component; considering results of this study suggested that MPST, but not other therapies, had significant influence on DLS attainment; future studies should further investigate the significance of parent involvement in treatments targeting DLS. In addition, research is needed to develop programs for supporting development of DLS in adolescent and adults (Smith et al., 2012).
Childhood cognitive ability has been an important determinant of adult outcomes across multiple measures of DLS (Gray et al., 2014; Smith et al., 2012). Given that NVMA was a robust predictor of DLS attainment across analyses, post hoc comparisons were conducted to further explore early childhood predictors of DLS trajectory group membership for children showing similar NVMA. As shown in Table S3 (available online), children with the highest NVMA in the Low-DLS group were compared to children with the lowest NVMA in the High-DLS group. At age 2, the Low-DLS/High-NVMA group had greater social-communication impairments (on the ADI-R) than the High-DLS/Low-NVMA group, but did not differ on other clinical features (controlling for age and, in the case of ADI-R, expressive language). By age 3, children in the High-DLS/Low-NVMA group had more developed daily living and expressive language skills than the Low-DLS/High-NVMA group; differences in social-communication were no longer significant after controlling for age and language. At 5 years of age, differences in DLS-AE continued to widen, accompanied by significant discrepancies in expressive and receptive language. These results suggest that, consistent with prior research, development of early language skills is an important predictor of DLS attainment (Gillespie-Lynch et al., 2012; Venter et al., 1992). Notably, group differences in language skills were not a function of general cognitive ability (subgroups were created so that T2-NVMA would be comparable and NVMA differences did not emerge at T3 or T5). Moreover, while ADI-R social-communication impairments appeared to be a distinguishing factor at age 2, differences in language seemed to account for differences in ADI-R scores at ages 3 and 5.
It is noteworthy that group-based trajectories in the ASD sample suggested a slowing of DLS attainment in later adolescence, particularly for domestic skills (e.g. household safety, chores, food preparation). Notably, the Low-DLS group showed a small, but significant, decline in DLS between 18 and 21 years of age. The High-Domestic group also showed a (nonsignificant) decline in domestic skills during the same period, resulting in a considerably lower Domestic-AE compared to other subdomains at T21 (i.e. 13.28 years vs 16.10 and 15.46 years for Personal and Community subdomains, respectively). Given the small sample size with data available at both time points, it is difficult to interpret the small declines observed in DLS-AE between 18 and 21 years of age. However, we draw attention to this observation because previous research has showed a slowing of improvements in internalizing behaviors and ASD symptoms following exit from high school (Taylor and Seltzer, 2010). Taken together, these results emphasize the need for additional support for adolescents transitioning to adulthood. It is important to highlight that adaptive behavior (as defined on the Vineland; Sparrow et al., 2005) is defined by typical performance and the expectations or standards of caregivers or those reporting on their performance. Thus, the change in DLS-AE is not assumed to reflect a loss of skills. Rather, a decline in DLS may reflect changes in performance or changes in caregivers’ expectations of performance as the individual transitions to adulthood.
Regardless of change between T18 and T21, it is important to note that, even for the group with the highest DLS attainment, mean DLS-AE remained approximately 7 years below age level at T21. The Low-DLS group showed an even greater deficit of approximately 16 years compared to their chronological age at T21. This clearly highlights the need for high school and transition programs to specifically target these skills in order to promote functional independence. In this sample, the attainment of Domestic skills was lower than other skills in the High group, whereas the Low group showed more impairment in Community skills compared to other subdomains (Table 2). Future studies are needed to more carefully analyze the specific areas of daily living in which young adults with ASD struggle the most in order to inform development of targeted intervention programs. It is likely that priorities will vary depending on individuals’ cognitive and language abilities.
An important finding separate from the attainment of DLS is that group differences in ADI-R totals were sometimes nonsignificant when controlling for age and language skills. When ADI-R totals were entered as predictors, the significance of language skills was often reduced. These findings highlight the strong associations between ADI-R totals (particularly Soc + Comm) and language skills and have implications for interpretation of studies using the ADI-R as a measure of ASD severity; Hus and Lord, 2013). Adult studies using ADI-R scores without appropriate covariates to compare ASD severity in “high” and “low” functioning individuals may be confounded by group differences in language ability (see Hus and Lord, 2013 for further discussion).
Strengths and limitations
This study included a relatively large sample of participants whose DLS were assessed at approximately similar ages across multiple time points. In addition to exploration of factors previously demonstrated to influence DLS attainment (e.g. cognitive and language ability), this study included diagnosis (ASD vs nonspectrum) and ASD symptoms as predictors of DLS attainment. Finally, this is the first study to separately examine trajectories of DLS subdomains and to assess the role of early intervention on adult DLS outcomes.
There are limitations to this study that warrant acknowledgment. Foremost, significance levels reported do not take into account multiple comparisons. Because this is one of the first studies of trajectories of DLS development, we felt it was important to provide a comprehensive assessment of factors influencing DLS attainment to inform future studies.
Second, the present sample included participants with at least three assessments. Excluded participants were more likely to be African American and less educated, consistent with higher overall attrition rates of African Americans with lower maternal education in this sample (Anderson et al., 2014; Carr and Lord, 2013). Interpretations are somewhat limited by small sample sizes in some groups. For example, while inclusion of all children referred for ASD evaluation afforded exploration of the relative contributions of initial diagnosis versus demographic/developmental factors, the small number of children with initial nonspectrum diagnoses limits comparisons.
The relatively small size of the sample assessed at 21 years of age also limits interpretation of observed declines in DLS. Similarly, post hoc analyses were based on a small subsample of children in different DLS trajectory groups with comparable NVMA. Few children in our sample received early intervention; more recently collected samples may have higher levels of early intervention. However, it is noteworthy that intervention effects were specific to the mentored, parent-implemented treatment; receiving speech, ABA, and other types of intervention did not influence DLS group membership (data available upon request). Larger population-based studies are needed to better explore the influence of sociodemographic and developmental factors on DLS trajectories in representative samples.
Finally, this study relied on caregiver report of DLS and did not include a direct assessment of DLS. While the Vineland is commonly used for this purpose, it is not known how results (e.g. the shape of subdomain trajectories) may be influenced by the properties of this measure. Nonetheless, this study is among the first longitudinal investigations of DLS across childhood and into young adulthood and the first to separately examine subdomains of DLS. Thus, despite limitations, these findings provide insights for future research and clinical care.
Conclusion
Both individuals with ASD and nonspectrum disorders showed progression of DLS from 2 to 21 years of age. Individuals with ASD showed slower development of DLS than individuals with other nonspectrum diagnoses, likely a reflection of more impaired nonverbal cognition. Within the ASD group, although gains in DLS are made across childhood and into young adulthood, attainment is significantly affected by early cognitive and language skills, as well as severity of ASD symptoms. Early intervention may play a significant role in encouraging development of DLS. Nevertheless, results suggest that DLS should be a focus of treatment plans for individuals with ASD of all ages; even among young adults who have made the greatest gains, DLS were often considerably below age expectations. Future research is needed to investigate the relationship between interventions targeting specific aspects of DLS (i.e. personal, domestic, and community skills) and DLS outcomes, particularly for adolescents and adults with ASD.
Supplementary Material
Acknowledgments
We gratefully acknowledge the participants and their families for their ongoing participation in this study. We would also like to thank Shanping Qiu for her assistance with data management.
Funding
This study was funded by National Institute of Mental Health (NIMH) 1 R01 MH81873-01A1 to C.L.
Footnotes
Declaration of conflicting interests
Catherine Lord acknowledges receipt of royalties for the Autism Diagnostic Interview–Revised (ADI-R); profits from this study were donated to charity.
References
- Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2014;55(5):485–494. doi: 10.1111/jcpp.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, et al. Patterns of growth in verbal abilities among children with autism spectrum disorder. Journal of Consulting and Clinical Psychology. 2007;75(4):594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Oti RS, Lord C, et al. Patterns of growth in adaptive social abilities among children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2009;37(7):1019–1034. doi: 10.1007/s10802-009-9326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Itzchak E, Watson LR, Zachor DA. Cognitive ability is associated with different outcome trajectories in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2014;44(9):2221–2229. doi: 10.1007/s10803-014-2091-0. [DOI] [PubMed] [Google Scholar]
- Burchinal MR, Bailey DB, Snyder P. Using growth curve analysis to evaluate child change in longitudinal investigations. Journal of Early Intervention. 1994;18(4):403–423. [Google Scholar]
- Carr T, Lord C. Longitudinal study of perceived negative impact in African American and Caucasian mothers of children with autism spectrum disorder. Autism. 2013;17(4):405–417. doi: 10.1177/1362361311435155. [DOI] [PubMed] [Google Scholar]
- Drahota A, Wood JJ, Sze KM, et al. Effects of cognitive behavioral therapy on daily living skills in children with high-functioning autism and concurrent anxiety disorders. Journal of Autism and Developmental Disorders. 2011;41(3):257–265. doi: 10.1007/s10803-010-1037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Bishop SL. Understanding the gap between cognitive abilities and daily living skills in adolescents with autism spectrum disorders with average intelligence. Autism. 2013 doi: 10.1177/1362361313510068. Epub ahead of print 25 November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- Elliott CD. Differential Ability Scales—Second Edition (DAS-II) San Antonio, TX: The Psychological Corporation; 2007. [Google Scholar]
- Farley MA, McMahon WM, Fombonne E, et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Research. 2009;2(2):109–118. doi: 10.1002/aur.69. [DOI] [PubMed] [Google Scholar]
- Freeman BJ, Del’Homme M, Guthrie D, et al. Vineland adaptive behavior scale scores as a function of age and initial IQ in 210 autistic children. Journal of Autism and Developmental Disorders. 1999;29(5):379–384. doi: 10.1023/a:1023078827457. [DOI] [PubMed] [Google Scholar]
- Gillespie-Lynch K, Sepeta L, Wang Y, et al. Early childhood predictors of the social competence of adults with autism. Journal of Autism and Developmental Disorders. 2012;42(2):161–174. doi: 10.1007/s10803-011-1222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Keating CM, Taffe JR, et al. Adult outcomes in autism: community inclusion and living skills. Journal of Autism and Developmental Disorders. 2014;44:3006–3015. doi: 10.1007/s10803-014-2159-x. [DOI] [PubMed] [Google Scholar]
- Howlin P, Mawhood L, Rutter M. Autism and developmental receptive language disorder – A follow-up comparison in early adult life. II: Social, behavioural, and psychiatric Outcomes. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41(5):561–578. doi: 10.1111/1469-7610.00643. [DOI] [PubMed] [Google Scholar]
- Hus V, Lord C. Effects of child characteristics on the Autism Diagnostic Interview-Revised: implications for use of scores as a measure of ASD severity. Journal of Autism and Developmental Disorders. 2013;43(2):371–381. doi: 10.1007/s10803-012-1576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbrink K, McCrone P, Fombonne E, et al. Cost-impact of young adults with high-functioning autistic spectrum disorder. Research in Developmental Disabilities. 2007;28(1):94–104. doi: 10.1016/j.ridd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Klin A, Saulnier CA, Sparrow SS, et al. Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: the Vineland and the ADOS. Journal of Autism and Developmental Disorders. 2007;37(4):748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, et al. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Smith LE, Hong J, et al. Evaluation of an activities of daily living scale for adolescents and adults with developmental disabilities. Disability and Health Journal. 2013;6(1):8–17. doi: 10.1016/j.dhjo.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern CW, Sigman M. Continuity and change from early childhood to adolescence in autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46(4):401–408. doi: 10.1111/j.1469-7610.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Mawhood L, Howlin P, Rutter M. Autism and developmental receptive language disorder—a comparative follow-up in early adult life. I: cognitive and language outcomes. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2000;41(5):547–559. doi: 10.1111/1469-7610.00642. [DOI] [PubMed] [Google Scholar]
- Mesibov G, Shea V, Schopler E. The TEACCH Approach to Autism Spectrum Disorders. New York: Kluwer Academic/Plenum; 2005. [Google Scholar]
- Mullen E. The Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service, Inc.; 1995. [Google Scholar]
- Nagin DS. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Smith LE, Maenner MJ, Seltzer MM. Developmental trajectories in adolescents and adults with autism: the case of daily living skills. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(6):622–631. doi: 10.1016/j.jaac.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, Inc.; 1984. [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2nd. Circle Pines, MN: American Guidance Service, Inc.; 2005. [Google Scholar]
- Szatmari P, Bryson S, Duku E, et al. Similar developmental trajectories in autism and Asperger syndrome: from early childhood to adolescence. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50(12):1459–1467. doi: 10.1111/j.1469-7610.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Seltzer MM. Changes in the autism behavioral phenotype during the transition to adulthood. Journal of Autism and Developmental Disorders. 2010;40(12):1431–1446. doi: 10.1007/s10803-010-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter A, Lord C, Schopler E. A follow-up study of high-functioning autistic children. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1992;33(3):489–507. doi: 10.1111/j.1469-7610.1992.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.