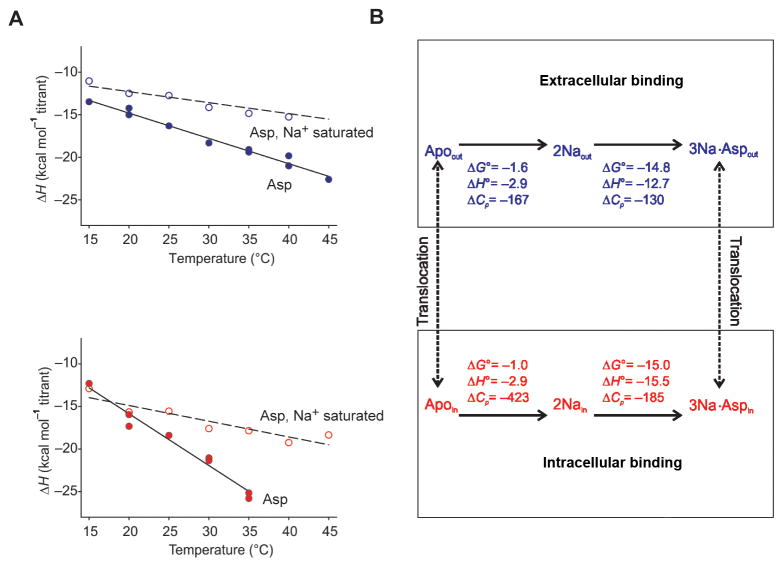
Fig. 6. Thermodynamics of ion and substrate binding to GltPh.
(A) Determination of binding ΔCp. Binding enthalpies for aspartate in the presence of 10 mM Na+ (solid circles) or 1 M Na+ (open circles) are plotted as functions of temperature for outwardly-(top, blue) and inwardly- (bottom, red) constrained GltPh. The data were fitted to straight lines with slopes corresponding to binding ΔCp of: −297 and −131 cal mol−1 K−1, respectively, for the outward-facing transporter and −608 and −184 cal mol−1 K−1, respectively, for the inward-facing transporter. (B) Thermodynamic scheme of the transport cycle of GltPh. Top and bottom show the thermodynamic schemes of Na+ and aspartate binding reactions with corresponding thermodynamic parameters• for the outward- and inward-facing states, respectively. The signs of the parameters correspond to the directions of the reactions indicated by the arrowheads. Broken lines represent conformational changes between the outward- and inward-facing states. The units are kcal mol−1 for ΔH and ΔG° and cal mol−1 K−1 for ΔCp. Figure was adapted from Reyes, et al. (15).