Abstract
Percutaneous coronary intervention (PCI) of chronic total occlusion (CTO) poses a management dilemma for the interventional cardiologist. Effective wiring technique is the key to success of PCI in CTO, which requires more patience and skill of the operator. The author herein intends to explore in detail the different wiring strategies such as antegrade approach, dissection and reentry, retrograde and hybrid approach. Hopefully, this review would enhance the understanding of this complex procedure and, consequently, promote safe and effective PCI.
Keywords: Percutaneous coronary intervention, Chronic total occlusion, Antegrade approach, Dissection and reentry, Retrograde approach
1. Introduction
Percutaneous coronary intervention (PCI) of chronic total occlusion (CTO) is a well accepted revascularization procedure representing 10% of PCI procedure.1, 2, 3 Effective wiring technique is the key to success of PCI in CTO.4 However, success of wire crossing is mainly dependent on operator's experience and skill.5 In this review the author elaborates the histopathology of CTO, basic and special wire techniques including anatomical consideration, fundamentals of wire handling and manipulation, and review of current CTO-PCI strategies such as antegrade, retrograde, and hybrid approach.
2. CTO structure: pathological and intravascular ultrasound (IVUS) findings
There are some important pathological and IVUS findings, which provide helpful information in crossing a CTO and performing optimal dilation after successful recanalization.6, 7 In the pathological studies, neovascular channels (NCs) with diameter of 100–200 μ are frequently found (85%) especially in CTOs older than 1 year. NCs in pre-existing plaque often connect with vasa vasorum in the adventitia; this is especially true in an old CTO with no well-defined stump, in which a guidewire can easily reach the subintima. Conversely, NCs that develop in old thrombus communicate with distal lumen (recanalization channels); a tapered CTO on angiogram represents the CTO with these recanalization channels, which may serve as a route for the guidewire to reach the distal vessel.8, 9 Tissue composition in CTO consists of relatively soft tissues (composed by scattered fibrous tissue, lipid core, and NCs) and hard tissues composed by dense fibrous tissue and calcium. The dense fibrous tissue forms fibrous bundles not only cross-sectionally but also longitudinally, which partition soft tissues (Fig. 1). These partitions might restrict wire movement from one soft tissue plane to another and cause the wire to slip into a single soft tissue plane into the subintima to create a dissection plane.
Fig. 1.
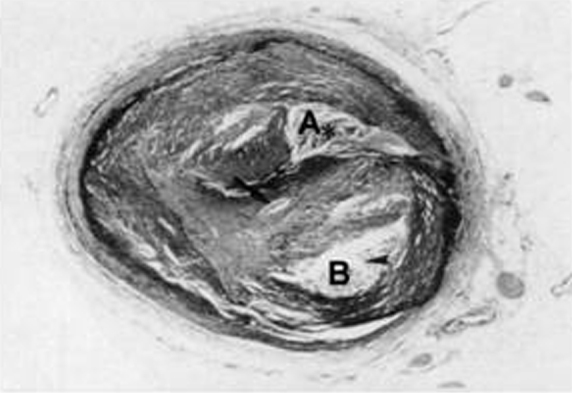
In this slice, two sections consisting of relatively scattered fibrous tissue (A, B) are seen. There are bundles between A and B. Though a guidewire tends to pass though relatively soft tissue like A or B, it is difficult for the guidewire to penetrate the fibrous bundle.
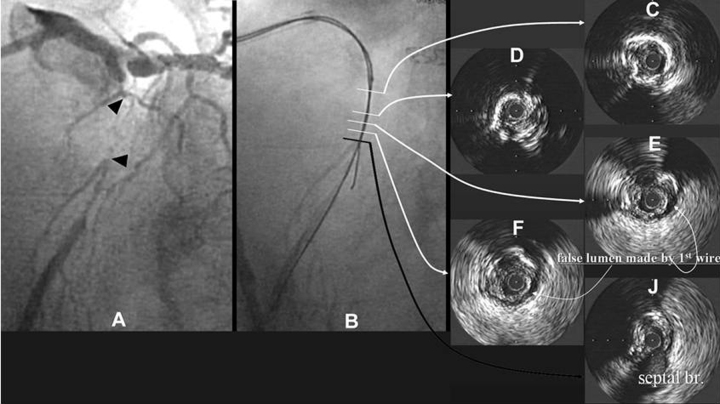
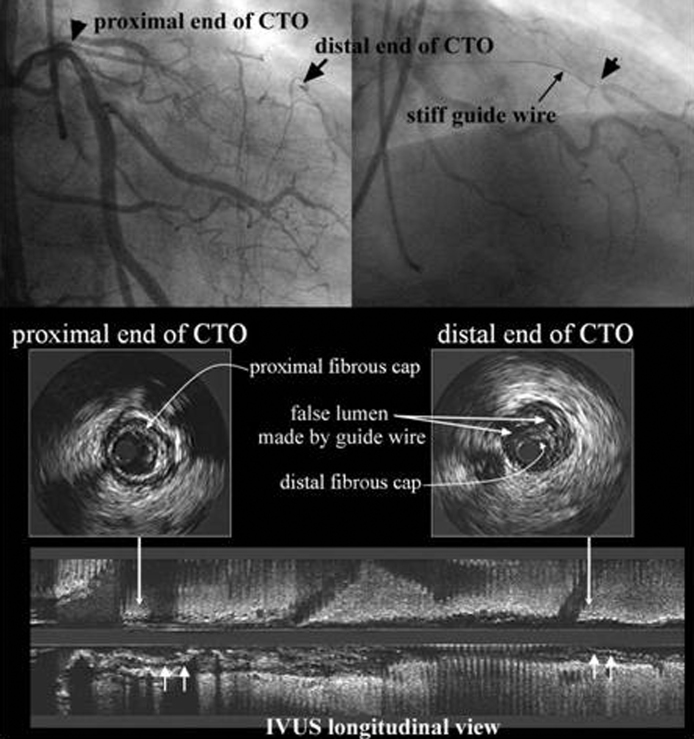
Recent IVUS findings indicate that subintimal space made by a guidewire is a strong factor of unsuccessful recanalization of CTOs. A typical case with subintimal space indicated by IVUS is shown in Fig. 2. Once the subintimal space is created by a guidewire, the wire tends to slip into the space repeatedly and extend it along the circumference of media like E and F in Fig. 2. If this happens, it is difficult to reach the distal true lumen. The most difficult part of CTO procedure is to penetrate tissue at the distal end of CTO to reach distal true lumen. Recent IVUS findings also indicate a thick fibrous membrane rarely exists at a distal end of CTO in contrast to a proximal end. The major reason, why it is difficult to penetrate into the distal true lumen in spite of absence of the thick fibrous cap is considered to be the false lumen made around the distal true lumen by a guidewire as shown in Fig. 3.
Fig. 2.
A typical case in which subintimal space was made by guidewire handling. After successful wiring (B), subintimal space was observed on IVUS (E and F). The major reason of the failed wire attempt was indicated to be calcification inside the CTO by IVUS.
Fig. 3.
In this case a very thin fibrous cap was seen at the distal end of CTO on IVUS compared to a thick fibrous cap at the proximal end. In spite of the thin distal cap, it was so difficult to get into the distal true lumen because of the intimal dissection made along the circumference of the distal true lumen by a guidewire.
2.1. Antegrade loose tissue tracking
Angiographically occluded lesions might contain loose tissue segments in both short and long duration CTO as suggested by histopathology and animal CTO model research.8, 9
In the loose tissue tracking technique, the tip of an intermediate-strength wire is bent at 45–60° at the distal 1–2 mm, so that wire tip can be controlled and directed and it does not penetrate hard atherosclerotic plaque. Usually, the loose tissue tracking is performed with 1.0 g tip strength hydrocoated wire. The aspects of wire handling and movement in loose tissue tracking tend to be similar to acute myocardial infarction cases; in that, the wire is advanced easily and smoothly, with minimal rotations of the wire tip.10, 11 In case of failure of the intermediate-strength wires to pierce the space between loose and dense fibrous tissues, an over-the-wire (OTW) balloon or microcatheter can be advanced and the wire is exchanged for stiffer tapered tip end because of its greater capability of penetrating the dense connective tissues into the distal true lumen than the conventional wires. When loose tissue tracking fails, the wire should be manipulated into the intimal plaque, subintimal space, or in a retrograde fashion depending on the convenience of the operators.
2.2. Antegrade intimal plaque tracking
Intentional intimal plaque tracking is preferable to subintimal as it yields higher success rate of entering true lumen at the distal vessel. The only way to know, whether the wire is in the intima is to pull it back 1–2 mm. An unusual and unmistakable sensation of being stuck is felt if the wire tip is in intima.11 The resistance of the intimal tissue surrounding the wire tip is relatively high but homogenous enabling navigation of the wire tip into the true lumen rather than subintimal space. Even with intimal plaque tracking, dense calcium or fibrocalcific plaque sometimes disturbs the wire crossing leading to procedural failure.10, 11
Computed tomography delineates very clearly calcification inside the CTO better enabling precise antegrade intentional intimal plaque tracking.12 IVUS better recognizes the optimal entry point of stumpless CTO with a side branch (SB) and evaluates, whether a guidewire would properly penetrate proximal cap,12, 13 verify guidewire location within an artery discriminating a true lumen from the false lumen before crossing the occlusion.14
2.3. Antegrade subintimal tracking
A free movement of wire tip during rotation and lesser resistance to advance is a mark of subintimal position (the wire turns around the vessel lumen, giving the appearance of lengthening the tip curve).11 The wire is considered completely in the false lumen, when the resistance of the wire tip to advancement decreases. Once the wire enters into the subintimal space, it is hard to redirect it into the true lumen without dedicated re-entry techniques and/or technologies, because the resistance of the subintimal tissue against the wire tip is much lower than toward the true lumen. Furthermore, enlargement of subintimal space pushes the plaque toward distal true lumen, resulting in its collapse. Both of these situations lead to failure of cross wire and increase the risk of myocardial injury.10
2.4. Retrograde intimal plaque tracking
Even if subintimal tracking is important in retrograde approach, 60% cases are actually intimal plaque tracking in IVUS study.15
2.5. Retrograde subintimal tracking
When antegrade wire is in subintimal space, it enables advancement of both antegrade and retrograde wires into the same place if retrograde wire is negotiated into the subintimal space. In retrograde subintimal tracking, contrast injection should be avoided as far as possible to a prevent enlargement of subintimal space and distal dissection. The author is of opinion of performing IVUS interrogation and balloon dilatation before contrast injection.
3. Strengthening guide catheter back up
When the guide catheter backs out during wire or device advancement, it is stabilized by placing another wire (wire anchoring) or smaller inflated balloon in SB proximal to CTO lesion (balloon anchoring), deep seating with a balloon, daughter-in-mother (5 in 6, 6 in 7 or 7 in 8) technique.11
4. Basic wire-handling technique
Needless to say the two fundamental elements of wire handling are rotating and “pushing” (feeding the wire forward). It is advisable to minimize wire rotation so that the point of penetration is not missed. Minimizing rotation of the wire also helps to minimize size of the channels made with the wire. It is logical to check for resistance at the wire tip. Once the wire is in the subintima, a crunching sensation is felt at its’ tip, when it is withdrawn 1–2 mm. Then one should bring the wire back and look for a new channel to continue down.
5. Guidewire selection
For a focal tapered CTO, one should start with soft tapered, polymer jacket wire for initial microchannel tracking. Tapered guidewires including Fielder XT (0.009 inch, Asahi Intec, Japan), Wizard 78 (0.078 inch, Lefeline, Japan), Gaia 1 (0.010 inch, Asahi Intec, Japan) are currently the initial choice. Intermediate type guidewires such as Miracle 3, 4.5, 6, Ultimate Bro 3, Gaia 2 (Asahi Intec, Japan), should be used in case of tortuous artery or microchannel tracking failure. Stiffer wires such as Conquest Pro 12 g, 20 g, Gaia 2 or 3, Miracle 12 (Asahi Intec, Japan) should be attempted in hard, dense, and blunt occlusions. No consensus exists over the best initial antegrade attempt. However, the author prefers a step-up approach with wires of moderately increased stiffness at the beginning, switching to more stiffer wires with penetration ability, those often being tapered.
6. Overcoming the targets by the wire to cross CTO into distal true lumen
It involves piercing the proximal cap, traversing the body of the CTO, piercing the distal cap, re-entry into true distal lumen.
6.1. Piercing the proximal fibrous cap
The strategies involved for piercing the proximal cap include drilling, penetrating, and sliding. The drilling implies the gradual step-up of the wire stiffness according to the lesion complexity, relying on visual as well as tactile feedback information from the wire tip. The penetration strategy involves the direct pressure at a point with controlled and limited wire rotation of the stiffer wires like Miracle 12, conquest Pro 9-20 (Asahi Intec, Aichi, Japan) and Progress 200 (Abbott Vascular, USA). In sliding strategy, a hydrophilic wire (Fielder XT, XTR, XTA wire [Asahi Intec, Aichi, Japan], Wizard 78 [Lifeline, Japan], Gaia 1 [Asahi Intec, Aichi, Japan]) is slid into the distal segment. Overall, any method works pretty well for short, focal, non-calcified straight segment of CTO. However, for a longer, tortuous calcified occlusion, the stiffer, spring-tip wires having more torque are preferable. The author is of the opinion that combination of sliding and penetration over a microcatheter is more preferable. The workhorse microcatheter presently is the Finecross (130 cm, 150 cm, Terumo, Japan), whose small tip and M-coating enable it to negotiate smoothly through a narrow space in CTO. Moreover, newer catheters such as MicroCross, CenterCross and MultiCross (Roxwood Medical Inc., USA) are expected to further amplify the support for very demanding lesions. The Corsair (135 cm, 150 cm, Asahi Intec, Aichi, Japan) microcatheter designed for collateral channel (CC) tracking, can also be used antegradely. It has more support, even beyond a calcified segment. Sometimes balloon inflation of the over the OTW system as coaxial anchor balloon technique is used to penetrate the proximal cap. If the wire still cannot cross the proximal cap, IVUS guidance may be required.
6.1.1. IVUS-guided direct wire entry
The IVUS-guided direct wire entry technique involves the advancement of the IVUS catheter into proximal end of CTO with evaluation of the surrounding area. The tip of the IVUS catheter pinpoints the central area of main lumen at the start of the CTO lesion based on initial images.11
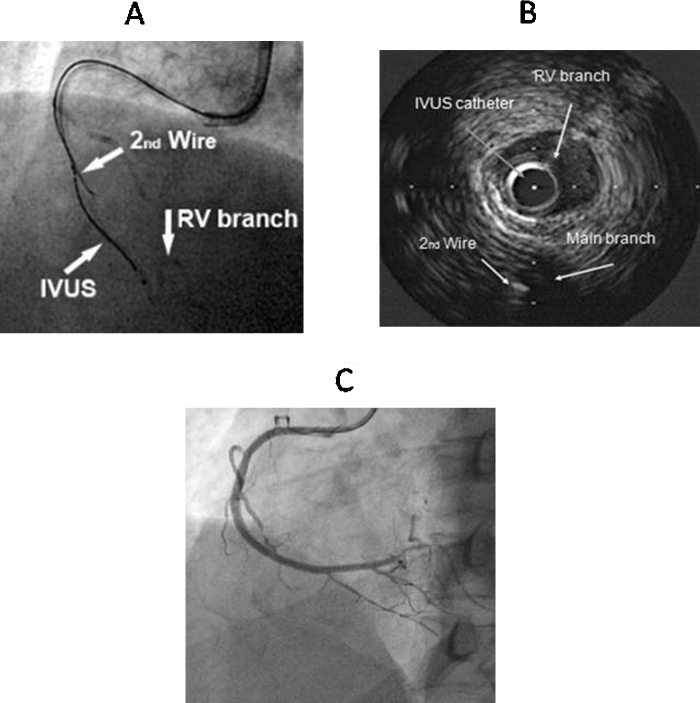
6.1.2. SB IVUS guidance
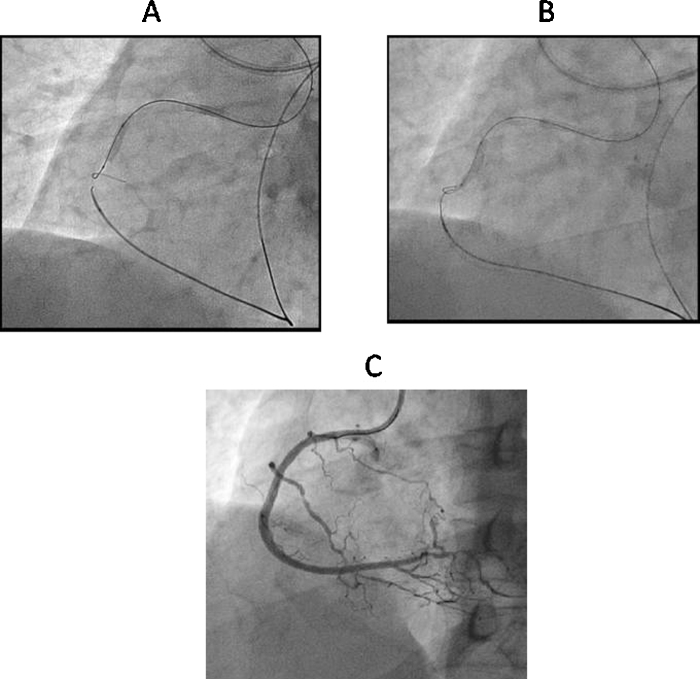
An IVUS catheter placed in proximal SB (Fig. 4) provides information about the location of occlusion cap and assists in negotiating the wire into the true lumen.16
Fig. 4.
SB IVUS guided wire crossing. (A) Manipulation of second guidewire using IVUS catheter in RV branch in CTO of RCA. (B) Second guidewire is in true lumen of RCA in IVUS imaging. (C) Final result after overlapping DES implantation.
6.2. Traversing the body of CTO
Once the wire, with 1 mm of its tip curved to <45° crosses the proximal cap, it will be directed slowly by the left hand, while the right hand of the operator rotates it 180° back and forth. If the wire buckles, it should never be forced into the lesion, rather retracted, reoriented, and rotated. Constant forward pressure on the wire is more successful than aggressive tapping against the occlusion (“Jack hammering”), which does not transmit additional force.17 Advancement of the wire with rotation to full angle creates a larger area of dissection upon contrast injection through the guide catheter. Stiffer and stiffer with tapered tip wires are preferable for traversing the body of CTO. As there is no easy way to master this technique, it really is a question of trial and error and learning from the past mistakes. Recanalization channels inside the CTO, lesion calcification or occluded stents are guides to the vessel course. If the wire tip repeatedly creates a false lumen, one is unlikely to get a better result with the same or an identical wire. Using the parallel wire18 technique with a stiffer and tapered wire is a key component of successful strategy for this kind of case.
6.3. Penetrating the distal fibrous cap
The greater tactile feel of spring-type wires is especially important for penetration of distal fibrous cap of CTO and it does not create a false lumen. For the last few millimeters of the occlusion, microcatheter is advanced close to the wire tip. Low-force wires (Miracle 3, Gaia 1), with progressive use of stiffer, more powerful wires if resistance to penetration is encountered. Precise penetration into the distal true lumen would be feasible with careful analysis of two different orthogonal views and phase-adjusted contalateral injections.11 After the wire crosses, it is preferable to exchange it over advanced microcatheter to floppy-type wire for safety reasons. When the microcatheter does not enter deep enough into the occlusion, it should be exchanged for a Tornus (Asahi Intec, Aichi, Japan) catheter. The optimal point for the penetration of convex distal fibrous cap is its center, although the newly created proximal channel often leads laterally. If the wire has strayed in the subintimal space, parallel wire technique with or without double lumen microcatheter (Twin-Pass [Vascular Solutions Inc., USA], Crusade [Kaneka Medix Corp., Japan]) should be employed.
6.3.1. Wire assisted antegrade dissection and re-entry
The original subintimal tracking and re-entry (STAR) technique quite often used in CTO of peripheral vasculature19, 20, 21 was attempted in coronary occlusion by Colombo.22 It involves creating a cleavage subintimal plane by advancing a hydrophilic wire with a J-loop configuration to allow a blunt dissection between the anatomical planes of the vessel. A 1.5 OTW is used to support the wire. The wire is then manipulated so that it reenters into true lumen. The knuckled wire follows the subintimal path to a point, where the dissection can no longer be propagated and re-entry is achieved. However, location of the re-entry is unpredictable, and questions remains about true myocardial perfusion and long-term patency. Another iteration of the STAR technique is contrast-guided STAR technique,23 which consists of contrast injection via an OTW or microcatheter or by employing the “microchannel technique”,24 where contrast is injected to enlarge and connect the microchannels already existing within the occlusion. “Mini-STAR”25 variant creates significantly smaller subintimal spaces. The limited antegrade subintimal tracking (LAST) technique26 involves introduction of stiffer polymer-jacketed or non-jacketed penetration wire with the aim of redirecting to the distal true lumen after facilitating device advancement with knuckle wire technique.26
6.3.2. Dedicated device assisted antegrade dissection and re-entry
Recent technologies, “CrossBoss” catheter, Stingray balloon and Stingray re-entry guidewire (Boston Scientific, Natik, MA) have addressed the limitations of wire based re-entry methods and have provided a reproducible method for successfully gaining re-entry into the true lumen of artery. The CorssBoss, a metal-braided, OTW, support catheter with a 1 mm rounded tip, is advanced over a workhorse wire to the proximal cap. The wire is retracted into the catheter lumen, and then the catheter is rapidly rotated through the body of CTO. The CrossBoss can track into distal true lumen in 40% of cases,27 but when it is in more frequent subintimal position, the guidewire is introduced and the catheter is exchanged for the Stingray balloon, which is 1 mm thick, OTW, balloon catheter with three exit ports (one distal and two 180°, diametrically opposed, side ports). When the balloon is inflated to 3–4 atm, it effectively wraps the artery with an exit port that is directed toward the adventitia and an exit port that is always directed toward the lumen. A contralateral angiography is performed to pinpoint balloon-vessel relationship and Stingray guidewire is directed and exited through the luminal port, with a direct puncture technique (stick and drive technique). A contralateral angiogram is again performed to confirm the wire into the lumen; the wire is rotated 180° (away from the opposite wall) and is directed distally into the vessel. However in smaller and more tortuous vessels, the puncture (re-entry) is done with the stingray wire (the stick and swab technique). The stingray wire is removed and replaced by a Pilot 200 wire to re-enter and wire the distal vessel. In both cases (stick and drive and stick and swab), the stingray wire or the Pilot 200 are removed after advancing a microcatheter into the distal vessel and exchanged for a softer wire. Occasionally, subintimal hematoma, caused by subintimal wire entry, can compress the distal true lumen, requiring aspiration through an OTW or microcatheter for decompression to enable distal true lumen re-entry (subintimal transcatheter withdrawal [STRAW] technique).28
6.3.3. IVUS guided wire re-entry
IVUS is useful for identifying the site, where the wire has moved from the true to false lumen, assessing the length, depth, and circumferential extent of false lumen caused by the wire, identifying where and whether the wire has re-entered the true lumen. In general it helps in locating, whether the wire is in true or false lumen. True lumen is identified by presence of plaque or intima surrounding the IVUS catheter or from connection to SBs. In complex CTO, enlargement of subintimal space, created by first wire often collapses the distal true lumen, which cannot be observed with angiography. IVUS may be useful to guide the second wire into true lumen. This catheter should be advanced into the presumed subintimal space after 1.5 or 2.0 mm balloon dilatation at the CTO entrance.11, 16 Then a stiff wire (Conquest Pro 9-12 or Miracle 12, Asahi Intec, Japan) should be used as the second wire, which is oriented to true lumen under IVUS guidance. Multiple stenting is mandatory to cover fully the enlarged subintimal space. However, the need for subintimal dilatation creates an unwanted larger false lumen, and the monorail design of existing IVUS catheters precludes wire exchanges. Distal CTO lesion is not a good indication for this technique, because it cannot provide adequate space to insert IVUS catheter.
7. The retrograde approach
The retrograde approach was developed and pioneered by Katoh et al.29 It involves targeted collateral crossing, retrograde lesion crossing and management of the subintimal space with use of balloon dilatation for connecting antegrade and retrograde channels.30 Possible indications of this new approach could be subsets of previous antegrade failures. It could be the primary procedure in many situations such as ostial occlusions, long occlusions, heavy calcification, occlusions with ambiguous proximal cap, and occlusions with a diffusely diseased distal vessel.
Successful collateral crossing depends on CC selection, wire tip curve, and wire handling. The best CC would be clearly visible, less tortuous collaterals by super-selective injection, exemplified by Dr. Werner's CC grade 1 or 2 (CCs are graded as follows: CC0, no continuous connection, CC1, continuous thread-like connection; and CC2, continuous, small SB-like connection).31 After wire access to the desired collateral, the microcatheter [Corsair (Asahi Intec, Japan), Turnpike (Vascular Solutions, USA), Finecross (Terumo, Japan)] is placed at the start of the CC. Once the position has been secured, the workhorse wire is exchanged with an appropriate guidewire. Suitable for CC crossing, the best spring coil wires are Sion and Sion blue (Asahi Intec, Japan); polymer jacket wires are Fielder FC, XT, XTR (Asahi Intec, Japan), Pilot 50, and Whisper (Abbott Vascular, USA). A guidewire with an extremely small tip (<1 mm) curve (30–45°) is recommended for CC crossing. Once the distal end of CTO has been reached, the retrograde wire is often exchanged for a stiffer wire to be directed into the CTO lesions. Once the distal cap has been penetrated, the last move is to connect the antegrade and retrograde channels, for which one or combination of following strategies may be used.
7.1. Retrograde wire crossing
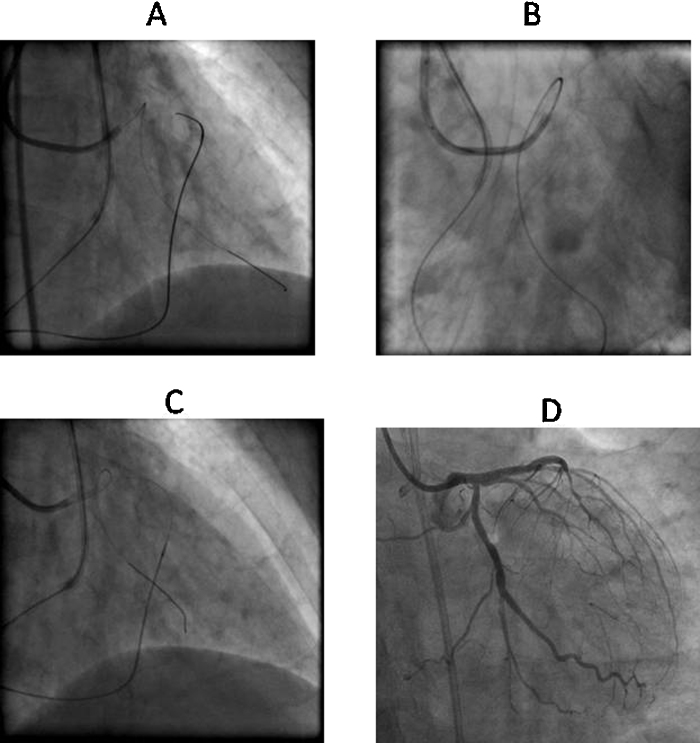
It involves a wire crossing the entire occlusion in a retrograde manner. The retrograde wire is deeply advanced into aorta or antegrade guide, then it is anchored by inflating a small balloon (2–2.5 mm) within antegrade guide catheter to facilitate crossing of the occlusion with the microcatheter. Finally, this retrograde wire is exchanged for a long wire to be externalized from the antegrade guide so that subsequent procedure can be accomplished in a antegrade manner (Fig. 5).32
Fig. 5.
Angiographic depiction of retrograde wire crossing technique. (A) Retrograde guidewire navigation in RCA with Corsair (Asahi Intec, Japan). (B) Retrograde true lumen puncture with Corsair. (C) Wire externalization with RG 3 (Asahi Intec, Japan). (D) Final result after DES implantation in LAD antegradely.
7.2. Kissing wire technique
This technique combines the antegrade and retrograde approach, although CTO is pierced through antegrade route.33 The retrograde wire serves as a landmark facilitating manipulation of antegrade wire allowing both the wires to kiss. Because of many diseased layers inside the occlusion, it is difficult to align both the wires.
7.3. Controlled antegrade and retrograde subintimal tracking (CART)
The CART technique consists of creating a subintimal dissection with limited extension by a retrograde balloon for advancement of antegrade wire into distal true lumen.29 Once the antegrade wire is deemed to be entering subintimal space, it is left in this position. The second wire, advancing retrogradely under the support of balloon or microcatheter, is positioned at the distal end of CTO, then penetrates from distal true lumen into the CTO, and finally into subintimal space at the CTO site. A small balloon (1.5–2 mm) is dilated in subintimal space retrogradely. The deflated balloon is kept in place to keep this subintimal space open. As both the dissections created by antegrade wire and retrograde balloon lie in the same subintimal space in CTO site, the antegrade wire is directed further into the distal true lumen along the retrograde deflated balloon. This follows ballooning and stent implantation in an antegrade fashion. The advantage of this technique is minimization of just subintimal tracking through the length of CTO lesion. The limitations include the need to introduce the wire into an often small and diffusely diseased distal lumen, failure of negotiation of retrograde balloon inside the occlusion, the inability to use IVUS to optimize the strategy, and creation of subintimal space extending to proximal true lumen of CTO.
7.4. Reverse CART technique
This approach involves antegrade access to proximal cap, retrograde access to distal cap, creation of subintimal space by antegrade balloon dilation (after overlapping with the retrograde catheter), thereby facilitating the crossing of occlusion with the retrograde wire. Next, this retrograde wire is externalized through the guide catheter and is used for antegrade angioplasty.34 The reverse CART is the most common technique used currently.35 The optimal wire position is when both antegrade and retrograde wires lie within the subintimal space. This technique is more predictable and reproducible as compared to CART. Undersizing the antegrade balloon makes the creation of common subintimal space much more difficult that can be prevented by IVUS-guided balloon sizing and positioning.36 Recoiling of common subintimal space sometimes occurs even after successful creation of the connecting channel. Moreover, medial disruption caused by antegrade balloon potentially causes bidirectional expansion of subintimal dissection making retrograde wire crossing difficult.11 Stent reverse CART37 technique involves deployment of a stent within the antegrade dissected plane to create open target for retrograde crossing. An alternative method is to use a guide-extension device such as Guideliner (Vascular Solutions, Minneapolis, MN, USA), Guidezilla (Boston Scientific, USA) or Guidion (IMDS, Netherlands) into antegradely created space to help connect the retrograde wire to the antegrade guide (Child-in-Mother reverse CART).37, 38 Unlike a stent, a catheter may be removed or repositioned if the connection between the antegrade and the retrograde true lumen fails. To minimize the length of subintimal stenting, currently contemporary reverse CART39 is the preferred technique, wherein following inflation of smaller antegrade balloon close to distal end of CTO, the Gaia series of guidewires enable the precise intentional retrograde wire control (Fig. 6).
Fig. 6.
Angiographic depiction of contemporary reverse CART. (A) Inflation of balloon over antegrade wire followed by navigation of retrograde wire (Gaia 2 [Asahi Intec, Japan]) with Corsair in RCA CTO. (B) Retrograde wiring with Gaia 2. (C) Final result after 2 overlapping DES deployment antegradely in RCA.
The crossing retrograde wire is exchanged for externalization wire after a microcatheter or channel dilator is delivered into antegrade guide catheter. The wire used for externalization needs to be as long as possible. Extending short guidewires is unadvisable for this purpose because of the possibility of kinking or separation. The wire should be lubricious and kink resistant but not so supportive as to damage tortuous and fragile CC. In general, a 330 cm RG 3 (Asahi Intec, Japan) guidewire is preferable. Sometimes retrograde advancement of wire into antegrade guide catheter fails in event of aorto-ostial lesions, extremely tortuous artery, or whenever there is poor retrograde wire control. This difficulty in antegrade wiring is overcome by snaring. It is best to snare the tip or soft part of the wire. Snaring on the stiffer part of externalization wires leads to kinking that can shear the antegrade guide tip. The 3-snare system, referred to as tulip snare (EN Snare; Merit Medical Systems, South Jordan, USA), is the most useful snaring system for externalization of wire during retrograde procedure.38
7.5. Knuckle wire technique
The knuckle wire technique is used to dissect the artery from distal cap to proximal cap of CTO. In this technique, subintimal dissected plane is created by forming a loop in retrograde wire, then antegrade wire in subintimal space is led inside this space (Fig. 7). A knuckle wire is advanced without torquing. For this purpose, polymer jacketed tapered (Fielder XT) [Asahi, Japan]) or non-tapered wire [Pilot 100, 200 (Abbott Vascular, USA)] can be used. As the wire is advanced, the support catheter should be kept within 10–20 mm of the tip of the knuckle to provide the support.38 The major limitation is that the longitudinal dissected plane cannot be controlled.
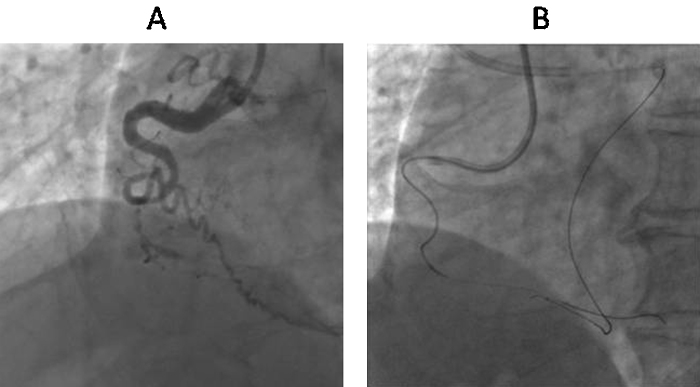
Fig. 7.
Angiographic depiction of knuckle wire technique. (A) Very tortuous RCA CTO. (B) Knuckle formation on retrograde Fielder XT (Asahi Intec, Japan) wire by fashioning a broad curve followed by navigating forward.
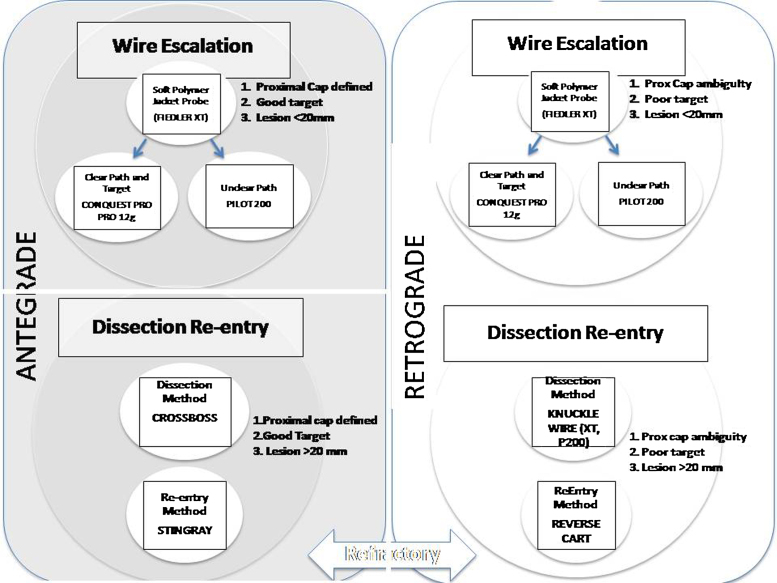
8. Hybrid strategy
Hybrid CTO PCI involves a standard, simplified process to approach CTO PCI dictated by anatomy, harmonizing antegrade and retrograde techniques, harmonizing lesion wire or device-based crossing with dissection and re-entry methods.40 The initial directional strategy of antegrade or retrograde is dependent on the anatomical variables such as ability to identify and safely and effectively engage proximal cap, lesion length, significant branches, size and quality of target at the distal cap, suitability and anticipated ease of retrograde interventional collaterals.40 The following initial strategy is determined based on these results.40
-
1.
Antegrade wire escalation: clear proximal cap, short lesion less than 20 mm, good target, interventional collateral irrelevant.
-
2.
Antegrade dissection re-entry: clear proximal cap, long lesion, good target, interventional collateral irrelevant.
-
3.
Retrograde wire escalation: ambiguous proximal cap, short lesion, good or poor target, suitable interventional collaterals.
-
4.
Retrograde dissection and re-entry: ambiguous proximal cap, long lesion, good or poor target, suitable interventional collaterals (Fig. 8).41
Fig. 8.
9. Conclusion
All CTO procedures require endless delicacy and patience that differentiate them from other lesions. Herein we have not dealt with how to reduce and prevent complications associated with CTO lesions, but concentrated entirely on wire handling. Guidewire crossing is the most important component of successful PCI for CTO. Acquiring wire handling skills for tackling these lesions is a gradual process. A solid grasp of how to read the lesions and a good understanding of all the pitfalls and important details is crucially important before one begins to tackle CTO. Advances in guidewire technology and development of innovative techniques in the last few years have resulted success rates of more than 80–90% allowing operators to attempt PCI for very complex CTOs. Still the journey in crossing CTO is incomplete and there is a need for further technical innovation.
Conflicts of interest
The author has none to declare.
References
- 1.Bell M.R., Berger P.B., Menke K.K., Holmes D.R. Balloon angioplasty of chronic total occlusion: what does it cost in radiation exposure, time, and materials. Catheter Cardiovasc Diagn. 1992;25:10–15. doi: 10.1002/ccd.1810250104. [DOI] [PubMed] [Google Scholar]
- 2.Puma J.A., Sketch M.H., Tcheng J.E. Percutaneous revascularization of chronic coronary occlusion: an overview. J Am Coll Cardiol. 1995;26:1–11. doi: 10.1016/0735-1097(95)00156-t. [DOI] [PubMed] [Google Scholar]
- 3.Laarman G., Plante S., de Feyter P.J. PTCA of chronically occluded coronaries. Am Heart J. 1990;119:1153–1160. doi: 10.1016/s0002-8703(05)80247-9. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchikane E. Complex PCI: PCI for the chronic total occlusion. Coron Interv. 2003;2:8–13. [Google Scholar]
- 5.Mitsudo K. Igakushoin; Tokyo: 2004. PTCA Technique-Chronic Total Occlusion. [Google Scholar]
- 6.Kimura B.J., Bhargava T.S.V., DeMaria A.N. Subintimal wire position during angioplasty of a chronic total coronary occlusion: detection and subsequent procedural guidance by intravascular ultrasound. Catheter Cardiovasc Diagn. 1995;35:262–265. doi: 10.1002/ccd.1810350323. [DOI] [PubMed] [Google Scholar]
- 7.Gatzoulis L., Watson R.J., Jordan L.B. Three-dimensional forward-viewing intravascular ultrasound imaging of human arteries in vitro. Ultrasound Med Biol. 2001;27:969. doi: 10.1016/s0301-5629(01)00371-4. [DOI] [PubMed] [Google Scholar]
- 8.Katsuragawa M., Fujiwara H., Miyamae M., Sasayama S. Histologic studies in percutaneous transluminal coronary angioplasty for chronic total occlusion: comparison of tapering and abrupt types of occlusion and short and long occluded segments. J Am Coll Cardiol. 1993;21:604–611. doi: 10.1016/0735-1097(93)90091-e. [DOI] [PubMed] [Google Scholar]
- 9.Srivatsa S.S., Edwards W.D., Boos C.M. Histological correlates of angiographic chronic total coronary occlusion: influence of occlusion duration on neovascular channel patterns and intimal composition. J Am Coll Cardiol. 1997;5:955–963. doi: 10.1016/s0735-1097(97)00035-1. [DOI] [PubMed] [Google Scholar]
- 10.Sumitsuji S., Inoue K., Ochiai M. Fundamental wire technique and current standard strategy of percutaneous intervention for chronic total occlusion with histopathological insights. J Am Coll Cardiol Interv. 2011;4:941–951. doi: 10.1016/j.jcin.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen T.N., Sumitji S., Han Y., Saito S. 4th ed. John Wiley and Sons; 2012. Chronic Total Occlusion. Practical Handbook of Advanced Interventional Cardiology: Tips and Tricks. [Google Scholar]
- 12.Ehara M., Terashima M., Kawai M. Impact of multislice computed tomography to estimate difficulty in wire crossing in percutaneous coronary intervention for chronic total occlusion. J Invasive Cardiol. 2009;21:575–582. [PubMed] [Google Scholar]
- 13.Furuichi S., Airoldi F., Colombo A. Intravascular ultrasound-guided wiring for chronic total occlusion. Catheter Cardiovasc Interv. 2007;708:56–59. doi: 10.1002/ccd.21219. [DOI] [PubMed] [Google Scholar]
- 14.Ito S., Suzuki T., Ito T. Novel technique using intravascular ultrasound-guided guidewire cross in coronary intervention for uncrossable chronic total occlusion. Circ J. 2004;68:1088–1092. doi: 10.1253/circj.68.1088. [DOI] [PubMed] [Google Scholar]
- 15.Tsujita K., Maehara A., Mintz G.S. Intravascular ultrasound comparison of the retrograde versus antegrade approach percutaneous intervention for chronic total coronary occlusion. J Am Coll Cardiol. 2009;2:846–856. doi: 10.1016/j.jcin.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Dash D., Li L. Intravascular ultrasound guided percutaneous coronary intervention for chronic total occlusion. Curr Cardiol Rev. 2015;11:323–327. doi: 10.2174/1573403X11666150909105827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito S., Tanaka S., Hiroe Y. Angioplasty for chronic total occlusion by using tapered-tip guidewires. Catheter Cardiovasc Interv. 2003;59:305–311. doi: 10.1002/ccd.10505. [DOI] [PubMed] [Google Scholar]
- 18.Katoh O., Reifart N. New double wire technique to stent ostial lesions. Cathet Cardiovasc Diagn. 1997;40:400–402. doi: 10.1002/(sici)1097-0304(199704)40:4<400::aid-ccd18>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Bolia A., Brennan J., Bell P.R. Recanalisation of femoro-popliteal occlusions: improving success rate by subintimal recanalisation. Clin Radiol. 1989;40:325. doi: 10.1016/s0009-9260(89)80231-4. [DOI] [PubMed] [Google Scholar]
- 20.Reekers J.A., Bolia A. Percutaneous intentional extraluminal (subintimal) recanalisation: how to do it yourself. Eur J Radiol. 1998;28:192–198. doi: 10.1016/s0720-048x(98)00114-4. [DOI] [PubMed] [Google Scholar]
- 21.Ingle H., Nasim A., Bolia A. Subintimal angioplasty of isolated infragenicular vessels in lower limb ischemia: long term results. J Endovasc Ther. 2002;9:411–416. doi: 10.1177/152660280200900404. [DOI] [PubMed] [Google Scholar]
- 22.Colombo A., Mikhail G.W., Michev I. Treating chronic total occlusion using subintimal tracking and reentry: the STAR technique. Catheter Cardiovasc Interv. 2005;64:407–411. doi: 10.1002/ccd.20307. [DOI] [PubMed] [Google Scholar]
- 23.Carlino A., Godino C., Latib A. Subintimal tracking and re-entry technique with contrast guidance: a safer approach. Catheter Cardiovasc Interv. 2008;72:790–796. doi: 10.1002/ccd.21699. [DOI] [PubMed] [Google Scholar]
- 24.Carlino A., Latib A., Godino C. Recanalization of by intraocclusion injection of contrast: the microchannel technique. Catheter Cardiovasc Interv. 2008;71:20–26. doi: 10.1002/ccd.21396. [DOI] [PubMed] [Google Scholar]
- 25.Galassi A.R., Tomasello S.D., Costanzo L. Mini-STAR as bail out strategy for percutaneous coronary intervention of chronic total occlusion. Catheter Cardiovasc Interv. 2012;79:30–40. doi: 10.1002/ccd.22998. [DOI] [PubMed] [Google Scholar]
- 26.Lombardi W.L. Retrograde PCI: what will they think of next? J Invasive Cardiol. 2009;21:543. [PubMed] [Google Scholar]
- 27.Whitlow P.L., Burke M.N., Lombardi W.L. Use of a novel crossing and re-entry system in coronary chronic total occlusions that have failed standard crossing techniques: results of the FAST-CTO (Facilitated Antegrade Steering technique in Chronic Total Occlusions) trial. JACC Cardiovasc Interv. 2012;5:393–401. doi: 10.1016/j.jcin.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Galassi A., Grantham A., Kandzari D. Percutaneous treatment of coronary chronic total occlusion: technical approach. Interv Cardiol Rev. 2014;9:201–207. doi: 10.15420/icr.2014.9.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surmely J.F., Tsuchikane E., Katoh O. New concept of CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART technique. J Invasive Cardiol. 2006;18:334–338. [PubMed] [Google Scholar]
- 30.Brilakis E.S., Grantham J.A., Thompson C.A. The retrograde approach to coronary artery chronic total occlusions: a practical approach. Catheter Cardiovasc Interv. 2012;79:3–19. doi: 10.1002/ccd.23004. [DOI] [PubMed] [Google Scholar]
- 31.Werner G.S., Ferrari M., Heinke S. Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation. 2003;107:1972–1977. doi: 10.1161/01.CIR.0000061953.72662.3A. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchikane E., Katoh O., Kimura M. The first clinical experience with a novel catheter for collateral tracking in retrograde approach for chronic coronary total occlusions. JACC Cardiovasc Interv. 2009;74:555–563. doi: 10.1016/j.jcin.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Hsu J.T., Tamai H., Kyo E. Traditional antegrade approach versus combined antegrade and retrograde approach in the percutaneous treatment of coronary chronic total occlusions. Catheter Cardiovasc Interv. 2009;79:555–563. doi: 10.1002/ccd.22035. [DOI] [PubMed] [Google Scholar]
- 34.Cohen R., Hattab M., Elhadad S. Retrograde approach reverse CART technique with a single guide for chronic total occlusion of the right coronary via an anomalous left circumflex artery. J Invasive Cardiol. 2011;23:E92–E94. [PubMed] [Google Scholar]
- 35.Karmpaliotis D., Michael T.T., Brilakis E.S. Retrograde coronary chronic total occlusion revascularization: procedural and in-hospital outcome from a multicenter registry in the United States. JACC Cardiovasc Interv. 2012;5:1273–1279. doi: 10.1016/j.jcin.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Rathore S., Katoh O., Tsuchikane E. Mini-focus issue: chronic total occlusion a novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking. JACC Cardiovasc Interv. 2010;3:155–164. doi: 10.1016/j.jcin.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Joyal D., Thompson C.A., Grantham J.A. The retrograde technique for recanalization of chronic total occlusion: a step-by-step approach. JACC Cardiovasc Interv. 2012;5:1–11. doi: 10.1016/j.jcin.2011.10.011. 38. [DOI] [PubMed] [Google Scholar]
- 38.Dash D. Retrograde coronary total occlusion intervention. Curr Cardiol Rev. 2015;11:291–298. doi: 10.2174/1573403X11666150909110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dash D. Retrograde coronary chronic total occlusion intervention using a novel reverse controlled antegrade and retrograde subintimal tracking. J Interv Cardiol. 2016 doi: 10.1111/joic.12268. [DOI] [PubMed] [Google Scholar]
- 40.Thompson C.A. The hybrid approach for percutaneous revascularization of coronary chronic total occlusion. Interv Cardiol Clin. 2012;1:349–353. doi: 10.1016/j.iccl.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Brilakis E.S., Grantham A., Rinfret S. A percutaneous treatment algorithm for crossing coronary chronic total occlusion. JACC Cardiovasc Interv. 2012;5:367–379. doi: 10.1016/j.jcin.2012.02.006. [DOI] [PubMed] [Google Scholar]