Abstract
Cardiac magnetic resonance (CMR) with its higher spatial resolution is considered the gold standard for evaluating ventricular mass, volumes, and ejection fraction. CMR can be used for accurate diagnosis of several conditions, especially cardiomyopathies. The purpose of this article is to review the utility of CMR in the diagnosis and management of nonischemic cardiomyopathies. We have reviewed both common and rare types of nonischemic cardiomyopathies in detail and elaborated on the specific CMR findings in each. We believe that CMR is an invaluable tool, not only in differentiating nonischemic from ischemic cardiomyopathy, but also in aiding the accurate diagnosis and management of the subtype of nonischemic cardiomyopathy. CMR should routinely be integrated in the diagnostic workup of various cardiomyopathies.
Keywords: Cardiac magnetic resonance, Nonischemic cardiomyopathy, Late gadolinium enhancement
1. Introduction
Cardiac magnetic resonance (CMR) is considered to be the gold standard for evaluating ventricular mass, volumes, and ejection fraction. CMR has an advantage in that it is not limited by poor acoustic windows, which can often limit echocardiographic studies, thereby enabling diagnosis of pathologies, which are otherwise not readily recognized by echocardiography. CMR can be used for assessing many pathologies, including aortic disease, coronary artery disease (CAD), cardiomyopathies, pericardial disease, and congenital heart disease.1 The purpose of this article is to review the utility of CMR in diagnosis and management of nonischemic cardiomyopathies.
2. Classification of cardiomyopathies
The current AHA classification of cardiomyopathies divides them into primary, which affect only the heart, and secondary, a much larger group in which myocardial involvement is part of a systemic (multiorgan) generalized disease process. Further subclassification is as follows2:
-
1.Primary
-
a.Genetic
-
i.Hypertrophic (obstructive) cardiomyopathy (HCM or HOCM)
-
ii.Arrhythmogenic right ventricular cardiomyopathy/Dysplasia (ARVC/D)
-
iii.Isolated ventricular noncompaction
-
iv.Glycogen storage disorders
-
v.Conduction defects
-
vi.Mitochondrial myopathies
-
vii.Ion channel disorders (e.g. Brugada's, Long QT)
-
i.
-
b.Mixed
-
i.Dilated cardiomyopathy
-
ii.Restrictive cardiomyopathy
-
i.
-
c.Acquired
-
i.Inflammatory (myocarditis)
-
ii.Stress provoked (Takotsubo)
-
iii.Peripartum
-
iv.Tachycardia induce
-
i.
-
a.
-
2.Secondary
-
a.Infiltrative, e.g. amyloidosis
-
b.Storage, e.g. hemochromatosis, Fabry's disease
-
c.Toxicity, e.g. alcohol, cocaine
-
d.Endomyocardial, e.g. endomyocardial fibroelastosis, Loeffler's syndrome
-
e.Inflammatory, e.g. sarcoidosis
-
f.Endocrine, e.g. DM, thyroid disorders, acromegaly, pheochromocytoma
-
g.Cardiofacial
-
h.Neuromuscular/neurological, e.g. Duchenne-Becker, Friedrich's ataxia
-
i.Nutritional deficiencies
-
j.Autoimmune/collagen e.g. RA, SLE
-
k.Electrolyte imbalance
-
l.Consequence of cancer therapy e.g. anthracyclines, such as doxorubicin, or alkylating agents, such as cisplatin and cyclophosphamide
-
a.
3. Differentiating nonischemic from ischemic cardiomyopathy
While attempting to diagnose the etiology of cardiomyopathy, it is important to exclude CAD as the etiology, given the differences in management. CMR technique of late gadolinium enhancement (LGE) becomes valuable in establishing the proper diagnosis. Gadolinium chelates are extracellular contrast agents that cannot cross myocyte cell membranes.3 Normal myocardium is densely packed with viable myocytes that do not permit entrance of gadolinium into the cell; thus there is little gadolinium enhancement of normal myocardium. However, in the setting of an acute myocardial infarction, myocardial cell membrane rupture will allow gadolinium to freely diffuse into the cell3 resulting in gadolinium hyperenhancement. The necrosis begins in the subendocardium and grows toward the epicardial area near the occluded artery.3 In chronic myocardial infarction, myocytes get replaced with collagenous scar tissue in the subendocardial region leading to increased gadolinium concentration and hyperenhancement in the subendocardium.3 Thus, ischemic cardiomyopathy tends to cause LGE in the subendocardium or transmurally and follows a vascular distribution, which lies in stark contrast to nonischemic cardiomyopathy, which generally does not correspond to any particular coronary artery distribution and is often located in the midwall or epicardial regions.4 Therefore, the pattern of LGE can be used to differentiate between cardiomyopathies of ischemic and nonischemic etiologies.
4. Hypertrophic cardiomyopathy
Transthoracic echocardiography (TTE) is considered the first line imaging modality for patients with hypertrophic cardiomyopathy (HCM). However, CMR can help in diagnosing variant types of HCM, including apical (Yamaguchi's) and lateral wall hypertrophies, otherwise not detected by TTE.5 CMR also has high accuracy in wall thickness measurements, which has important prognostic value. Cine-CMR with flow velocity encoding can be utilized to evaluate the flow dynamics and dynamic obstruction of the LV outflow tract in such patients. Several patterns of LGE have been described in HCM, which demonstrate areas of fibrosis.6 LGE has been associated with increased risk of re-entrant tachycardias, ventricular tachycardia, and sudden cardiac death.6
5. ARVC/D
ARVC/D is an inherited condition characterized pathologically by fibrofatty replacement of the ventricular wall, primarily RV, and clinically by life-threatening ventricular arrhythmias, heart failure, and sudden cardiac death.7 The 2010 revised task force criteria is used for diagnosis of ARVC,8 which includes parameters for regional RV dysfunction, RV volume, and RV global dysfunction.
6. Left ventricular noncompaction (LVNC)
LVNC, also known as left ventricular hypertrabeculation, is a congenital morphological disorder due to an arrest in the normal process of myocardial compaction during development, resulting in persistent prominent ventricular trabeculations and deep intertrabecular recesses. Diagnosis is based on clinical and morphological criteria. Diagnosis is usually established by TTE, but when imaging is suboptimal, CMR can be utilized. CMR criteria for diagnosis of LVNC include noncompacted to compacted myocardial thickness ratio of >2.3 (sensitivity, specificity, and positive and negative predictions of 86%, 99%, 75%, and 99%, respectively9), and trabeculated LV mass >20 percent of global LV mass (sensitivity of 94% and specificity of 94%).
7. Myocarditis
Myocarditis is an inflammation of the myocardium that can be caused by a variety of etiologies, commonly viral, but also toxins, drugs, and autoimmune processes.
Findings in CMR include myocardial edema, wall motion abnormalities, and patchy myocardial LGE. Tissue edema is best visualized in T2-weighted spin-echo CMR images.10 LGE in myocarditis is usually patchy, and involves the subepicardial regions.10 Since edema, and not fibrosis, is the cause for LGE in the acute phase of myocarditis; the area of LGE usually decreases with time. When both T2-weighted imaging and LGE were used in tandem for evaluation of patient's for myocarditis, the sensitivity, specificity, and diagnostic accuracy have been noted to be 76%, 95.5%, and 85%, respectively.10
8. Takotsubo cardiomyopathy
Takotsubo cardiomyopathy (stress cardiomyopathy, apical ballooning syndrome, broken heart syndrome) is a transient left ventricular ballooning syndrome that occurs in the absence of CAD, usually after emotional stress. The pathogenesis is thought to involve increased circulating catecholamines and exaggerated sympathetic stimulation. While CMR can help in diagnosis of Takotsubo cardiomyopathy, it is important to note that a coronary angiogram that demonstrates no CAD is often required for definitive diagnosis.11
CMR will demonstrate apical ballooning and wall motion abnormalities, including apical akinesis. An important feature in Takotsubo cardiomyopathy is that LGE is absent,12 which helps differentiate it from ACS (subendocardial enhancement) and myocarditis (patchy subepicardial enhancement).
9. Amyloidosis
Amyloidosis refers to an uncommon condition that results from systemic or organ specific extracellular deposition of insoluble fibrillar proteins. The percentage of amyloid patients with cardiac involvement depends on the type of amyloidosis, with cardiac involvement most common in primary/AL (up to 50%), familial/ATTR (10–50%), and secondary/AA (<5%) types. While evidence of amyloid deposits in the myocardium can be noted on ECG (low voltage in the limb leads), or echocardiography (increased LV wall thickness, small cavity size, diastolic dysfunction, biatrial enlargement, and pericardial effusion), CMR with LGE has been shown to have greater sensitivity and specificity than TTE. Cardiac amyloidosis appears as global subendocardial myocardial hyperenhancement on LGE. CMR with LGE has been reported to have sensitivity, specificity, positive predictive, and negative predictive values of between 86–88%, 86–90%, 88–95%, and 67–90%, respectively.13
10. Iron overload
Iron deposition in the myocardium secondary to iron overload can occur due to either primary (genetic) hemochromatosis or secondary (transfusion dependent) iron overload. CMR can detect myocardial iron deposits through T2-star (T2*) technique, in which the affected myocardium will appear dark (reduced T2*).14 An abnormally dark liver (due to iron deposition) can also be noted if included in the imaging window. It is also important to note that none of the other markers of iron overload, such as serum iron, ferritin, or liver iron are accurate predictors of myocardial iron deposition. This is especially important because cardiomyopathy secondary to iron overload is reversible if chelation therapy is initiated early, and the type of chelation therapy agent used depends on whether the prime target is extraction of iron from the heart or the liver.
11. Fabry's disease
Fabry's disease is an X-linked inherited disorder of lysosomal metabolism resulting in excess deposits of glycospingolipids within multiple organs. Cardiac manifestations of Fabry's disease include LV hypertrophy and fibrosis. CMR can demonstrate findings that are specific to Fabry's disease. In noncontrast T1 mapping, patients with Fabry's disease have lower septal T1, which helps differentiate from other causes of LVH, including hypertensive heart disease, aortic stenosis, amyloidosis, and HCM.15 T1 is inversely proportional to wall thickness (hypertrophy) and was abnormal even in patients with Fabry's disease who did not have LVH.15 The same study also showed pseudonormalization or elevation of T1 in the left ventricular inferolateral wall, correlating with the presence or absence of LGE.15 This was consistent with the pattern of LGE that is commonly seen in Fabry's disease: mesocardic and subepicardic areas of the basal and inferolateral segments of the left ventricle.
12. Cocaine/alcohol
Cocaine causes cardiac toxicity through ischemia (coronary vasospasm) and inflammation (hypersensitivity to cocaine/adulterants). Cocaine-induced cardiomyopathy is suspected when a strong history of cocaine abuse is present. CMR with tagging will show regional LV dysfunction, especially diastolic dysfunction. One study showed myocardial involvement detected on CMR in 83% of chronic cocaine users with manifestations, including edema, and LGE suggesting areas of fibrosis along both ischemic and nonischemic patterns,16 although the long-term prognostic implications of these findings are unclear.
Alcohol through multiple mechanisms also causes dilated cardiomyopathy. Features, such as LV dilation, reduced wall thickness, and systolic dysfunction, can be easily identified. Appropriate diagnosis is important as alcohol cessation can improve ejection fraction in 50% of affected patients while continued alcohol use will cause further decline in LV function.17 CMR is valuable when echocardiographic images are suboptimal; however, there are no features that distinguish alcoholic cardiomyopathy from other cardiomyopathies.17
13. Hypereosinophilic syndrome and endomyocardial fibrosis
Hypereosinophilic syndrome (HES) is characterized by increased eosinophils, which can be primary (myeloid or eosinophilic neoplasm), secondary (parasitic infections i.e. Loeffler's syndrome, or other nonstem cell tumors), or idiopathic. Endomyocardial fibrosis (EMF) is a disease characterized by fibrosis of the apical endomyocardium. The cardiac manifestations of EMF are similar to HES; however, the etiology is unclear because eosinophilia is not seen frequently.
Regardless of etiology, both HES and EMF present similarly in cardiac imaging. CMR findings include LGE in the subendocardial surfaces of areas affected by fibrosis, obliteration of the ventricular apices, occasional thrombus formation in the apices, and restrictive cardiomyopathy.18
14. Sarcoidosis
The exact incidence of cardiac involvement in sarcoidosis is unclear. Among patients with sarcoidosis, the prevalence of cardiac involvement, as noted among studies, has varied from 5% to 20%.19 Clinical manifestations include heart block, arrhythmias, restrictive cardiomyopathy, heart failure, and sudden cardiac death. Guidelines for diagnosis established by the Japan Society of Sarcoidosis and other Granulomatous Disorders rely on histological evidence, ECG abnormalities, echocardiographic abnormalities, and LGE on CMR.
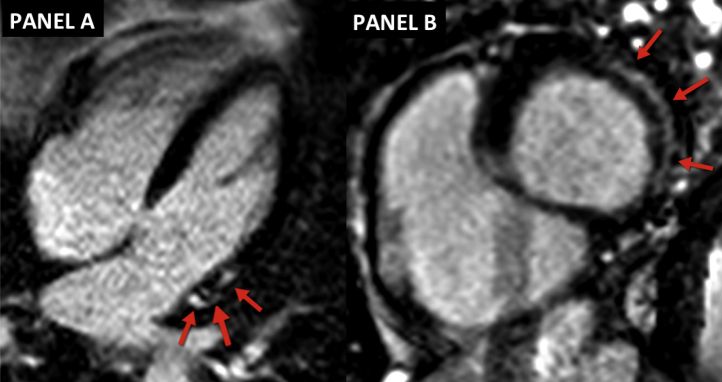
CMR has been shown to have high sensitivity and specificity in detecting cardiac involvement in sarcoidosis. LGE CMR will demonstrate mesocardial or subepicardial enhancement with predilection for the basal and lateral segments of the left ventricle (Fig. 1). In addition, the presence of LGE in cardiac sarcoidosis has been shown to have prognostic value, with an increased risk of MACE and cardiac death.20 Additional findings noted on CMR include regional wall motion abnormalities on cine-CMR, and inflammation and edema in the acute phase on T1-weighted images.
Fig. 1.
Cardiac sarcoidosis: Red arrows demonstrate areas of sub-epicardial LGE in the basal and lateral segments of the left ventricle in the 4-chamber view (A) and the basal short-axis view (B).
15. Other diseases
Many other disease processes can cause secondary cardiomyopathy. However, as their findings are nonspecific they will be discussed briefly here. Endocrine disorders, such as diabetes mellitus, thyroid diseases, pheochromocytoma, and growth hormone excess, are all associated with dilated cardiomyopathy. Cushing's syndrome commonly presents as HCM, but can on occasion present with dilated cardiomyopathy. Thiamine deficiency can also cause high output cardiac failure (wet beri-beri) and eventual dilated cardiomyopathy. Duchenne's muscular dystrophy and chemotherapeutic agents, such as doxorubicin, also cause dilated cardiomyopathy. In all the above noted diseases, CMR can show hypertrophic or dilated cardiomyopathy, and LGE in areas of fibrosis, but the patterns are not specific to any disease.
16. Conclusion
CMR is an invaluable tool, not only in differentiating nonischemic from ischemic cardiomyopathy, but also in aiding the accurate diagnosis of the subtype of nonischemic cardiomyopathy. CMR should routinely be integrated in the diagnostic workup of various cardiomyopathies.
Conflicts of interest
The authors have none to declare.
References
- 1.Pennell D.J. Clinical indications for cardiovascular magnetic resonance (CMR): consensus Panel report. Eur Heart J. 2004;25:1940–1965. doi: 10.1016/j.ehj.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 2.Maron B.J. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 3.Mahrholdt H. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 4.Lim R.P., Srichai M.B., Lee V.S. Non-ischemic causes of delayed myocardial hyperenhancement on MRI. Am J Roentgenol. 2007;188:1675–1681. doi: 10.2214/AJR.06.1224. [DOI] [PubMed] [Google Scholar]
- 5.Sardanelli F. MRI in hypertrophic cardiomyopathy: a morphofunctional study. J Comput Assist Tomogr. 1993;17:862–872. doi: 10.1097/00004728-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Moon J.C. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561–1567. doi: 10.1016/s0735-1097(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 7.Sen-Chowdhry S. Arrhythmogenic right ventricular cardiomyopathy: clinical presentation, diagnosis, and management. Am J Med. 2004;117:685–695. doi: 10.1016/j.amjmed.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Marcus F.I. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen S.E. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–105. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Aty H. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 11.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Eitel I. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J. 2008;29:2651–2659. doi: 10.1093/eurheartj/ehn433. [DOI] [PubMed] [Google Scholar]
- 13.Ruberg F.L. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am J Cardiol. 2009;103:544–549. doi: 10.1016/j.amjcard.2008.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson L. Cardiovascular T2-star (T 2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 15.Sado D.M. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ: Cardiovasc Imaging. 2013;6:392–398. doi: 10.1161/CIRCIMAGING.112.000070. [DOI] [PubMed] [Google Scholar]
- 16.Aquaro G.D. Silent myocardial damage in cocaine addicts. Heart. 2011 doi: 10.1136/hrt.2011.226977. [DOI] [PubMed] [Google Scholar]
- 17.Francone M. Role of cardiac magnetic resonance in the evaluation of dilated cardiomyopathy: diagnostic contribution and prognostic significance. ISRN Radiol. 2014;2014 doi: 10.1155/2014/365404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings K.W. A Pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging 1. Radiographics. 2009;29:89–103. doi: 10.1148/rg.291085052. [DOI] [PubMed] [Google Scholar]
- 19.Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. Am J Roentgenol. 2005;184:249–254. doi: 10.2214/ajr.184.1.01840249. [DOI] [PubMed] [Google Scholar]
- 20.Greulich S. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC: Cardiovasc Imaging. 2013;6:501–511. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]