Abstract
Background
Hantaan virus (HTNV), a negative sense tripartite RNA virus of the Family Bunyaviridae, is the most prevalent hantavirus in the Republic of Korea (ROK). It is the causative agent of Hemorrhagic Fever with Renal Syndrome (HFRS) in humans and maintained in the striped field mouse, Apodemus agrarius, the primary zoonotic host. Clinical HFRS cases have been reported commonly in HFRS-endemic areas of Gyeonggi province. Recently, the death of a member of the ROK military from Gangwon province due to HFRS prompted an investigation of the epidemiology and distribution of hantaviruses in Gangwon and Gyeonggi provinces that border the demilitarized zone separating North and South Korea.
Methodology and Principal Findings
To elucidate the geographic distribution and molecular diversity of HTNV, whole genome sequences of HTNV Large (L), Medium (M), and Small (S) segments were acquired from lung tissues of A. agrarius captured from 2003–2014. Consistent with the clinical incidence of HFRS established by the Korea Centers for Disease Control & Prevention (KCDC), the prevalence of HTNV in naturally infected mice in Gangwon province was lower than for Gyeonggi province. Whole genomic sequences of 34 HTNV strains were identified and a phylogenetic analysis showed geographic diversity of the virus in the limited areas. Reassortment analysis first suggested an occurrence of genetic exchange of HTNV genomes in nature, ROK.
Conclusion/Significance
This study is the first report to demonstrate the molecular prevalence of HTNV in Gangwon province. Whole genome sequencing of HTNV showed well-supported geographic lineages and the molecular diversity in the northern region of ROK due to a natural reassortment of HTNV genomes. These observations contribute to a better understanding of the genetic diversity and molecular evolution of hantaviruses. Also, the full-length of HTNV tripartite genomes will provide a database for phylogeographic analysis of spatial and temporal outbreaks of hantavirus infection.
Author Summary
Hemorrhagic Fever with Renal Syndrome (HFRS) and Hantavirus Pulmonary Syndrome (HPS) are endemic zoonotic infectious diseases caused by hantaviruses that belong to the Family Bunyaviridae containing negative-sense tripartite RNA genomes. Hantaviruses pose a critical emerging public health threat, with up to 200,000 clinical cases reported annually worldwide with 1–36% case fatality rates. In humans, hantavirus-borne diseases are contracted by the inhalation of viruses aerosolized from rodent excreta. However, there is no effective therapeutic or vaccine to prevent from the disease. Whole genome sequences of Hantaan virus (HTNV) were acquired from lung tissues of Apodemus agrarius captured in HFRS-endemic areas of the Republic of Korea (ROK). Phylogenetic analyses demonstrated that sequences of the HTNV tripartite genomes clustered geographically, showing broad diversity of HTNV throughout the areas surveyed. Reassortment analysis first suggested a natural occurrence of the HTNV genetic exchange in the ROK. These observations contribute to a better understanding of the genetic diversity and molecular evolution of hantaviruses in HFRS-endemic regions. The complete sequences of HTNV genomes will provide a database for the phylogeographic analysis and surveillance of endemic hantavirus-borne diseases.
Introduction
Viruses in the Hantavirus genus of the family Bunyaviridae are negative-sense single-stranded RNA virus containing Large (L), Medium (M), and Small (S) segments [1]. Hantaviruses pose an emerging public health threat, with about 200,000 cases of human disease annually worldwide and fatality rates of 1–36% [2, 3]. Old World hantaviruses, e.g., Hantaan virus (HTNV), Puumala virus (PUUV), Seoul virus (SEOV), and Dobrava-Belgrade virus (DOBV), are etiologic agents of Hemorrhagic Fever with Renal Syndrome (HFRS) in Eurasia [4]. In America, Hantavirus Pulmonary Syndrome (HPS) results from infections with New World hantaviruses, e.g., Sin Nombre virus (SNV) and Andes virus (ANDV) [5]. In humans, HFRS and HPS are contracted by inhaling aerosolized infectious particles from rodent salvia, urine, and feces [6]. Hantavirus infections are highly endemic and cause severe diseases in humans. However, there are no effective therapies or vaccines against these viruses.
HTNV is harbored by striped field mice (Apodemus agrarius), which constitute about 70% of the wild rodent population in the Republic of Korea (ROK) [7]. HFRS incidences increase during the spring and fall, due to the dynamics of rodent populations [8]. Observance of HFRS cases for military personnel and civilians near the demilitarized zone (DMZ) led to an investigation of the geographic distribution and molecular epidemiology of HTNV in Gangwon and Gyeonggi provinces [9–11]. Over decades, our studies have demonstrated the molecular similarities and diversity of hantaviruses using viral genomic sequences identified from HFRS patients and rodents where the patients were exposed in Gyeonggi province, the highest endemic area in the ROK [8, 9, 12–15]. However, the molecular prevalence of hantaviruses in Gangwon province remains unknown.
Reassortment is a genetic event that results in the exchange of genome segments, and it is a major molecular mechanism to confer genetic diversity [16]. Influenza virus, a negative-sense segmented RNA virus, frequently generates reassortants by switching genomes of viruses from different hosts. Reassortment can play an important role in viral fitness, transmission, and pathogenesis of segmented RNA viruses [17]. The genetic reassortment of hantaviruses has been reported naturally and experimentally [18, 19]. A natural reassortment of SNV within deer mice (Peromyscus maniculatus) occurred with the exchange of the M segment. Genetic exchange of the M segment of DOBV was observed between low pathogenic DOBV-Aa and highly pathogenic DOBV-Af in vitro. In addition, HTNV strains appeared to be highly divergent in the limited region of Guizhou in China, with the generation of S segment reassortants [20].
In this study, A. agrarius was collected in Gangwon and Gyeonggi provinces from 2003–2014. To investigate the molecular epidemiology and distribution of HTNV, serological and molecular screening of HTNV was performed from lung tissues of the rodents. Using 34 of complete sequences of HTNV tripartite genomes, phylogenetic analyses show well-supported geographic clusters of the L, M, and S segments in the ROK. Reassortment analysis first demonstrated that HTNV in Dagmar North (DN), Paju, consists of the heterogeneous L segment and homogeneous M and S segments, suggesting that the reassortment of HTNV may occur in nature. This study provides a better understanding of the genetic diversity and molecular evolution of HTNV tripartite genomes in the ROK. The whole genome sequences of HTNV will establish a database for the phylogeographic analysis and surveillance of endemic outbreaks of hantavirus infection.
Methods
Ethics statement
Trapping of rodents was approved by US Forces Korea (USFK) in accordance with USFK Regulation 40–1 “Prevention, Surveillance, and Treatment of Hemorrhagic Fever with Renal Syndrome”. Wild rodents were euthanized by cardiac puncture and tissues were dissected under isoflurane anesthesia. All procedures and handling of rodents were conducted under an approved protocol by the Korea University Institutional Animal Care and Use Committee (KUIACUC, #2010–212).
Rodent trapping
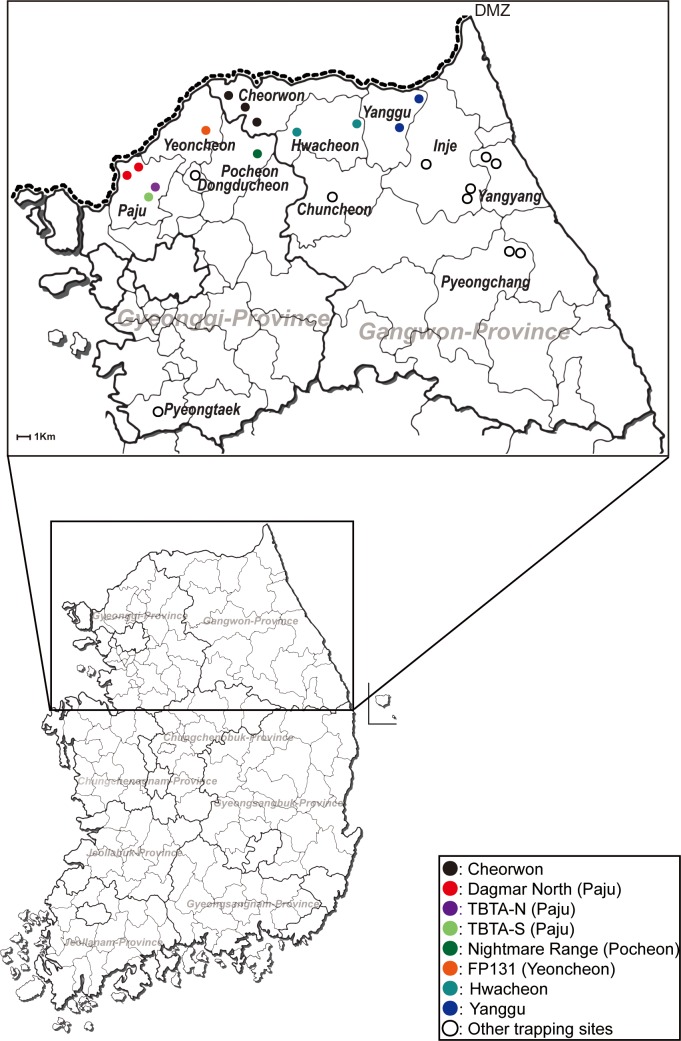
Rodents were captured in Gangwon and Gyeonggi provinces of the ROK, from 2003–2014 using live-capture Sherman traps (7.7 cm by 9 cm by 23 cm; H. B. Sherman, USA). A total of 100 traps were placed at intervals of about 4 m to 5 m at various US military training areas and ROK civilian sites in Cheorwon, Chuncheon, Hwacheon, Inje, Pyeongchang, Yanggu, and Yangyang in Gangwon province, and Dongducheon, Paju, Pocheon, Phyeongtaek, and Yeoncheon in Gyeonggi province each day over 2–3 consecutive days for each trapping period (Fig 1).
Fig 1. Geographic distribution of trapping sites for A. agrarius collected in Gangwon and Gyeonggi provinces, the Republic of Korea.
A map shows the trapping sites where A. agrarius was captured from 2003–2014. Color circles indicate the places where the positivity of HTNV was shown by RT-PCR; Dagmar North (DN), red; Twin Bridge Training Area North (TBTA-N), violet; TBTA South (TBTA-S), yellowish green; Nightmare Range (NR), green; Fire Point 131 (FP131), orange; Cheorwon, black; Hwacheon, cyan; opened circles indicate the places where no HTNV RNA was identified.
Immunofluorescence assay (IFA) test
Rodent sera, diluted 1:32, were placed into duplicate acetone-fixed wells of Vero E6 cells infected with HTNV, and the wells incubated at 37°C for 30 min. After incubation, the slides were washed with Phosphate-Buffered Saline (PBS) and then fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (ICN Pharmaceuticals,Inc., USA) added to each well and incubated at 37°C for 30 min. Following washes, the slides were examined for virus-specific fluorescence, using an Axioscope fluorescent microscope (Carl Zeiss AG, Germany).
RNA extraction and RT-PCR
Lung tissues were mechanically homogenized using a FastPrep-24 5G Instrument (MP Biomedicals, USA) with TRI Reagent Solution (Ambion, USA). Total RNA was extracted from lung tissues using a Hybrid R Kit (GeneAll, Korea) according to the manufacturer’s specifications. cDNA was synthesized using M-MLV (Promega, USA) with random hexamers or OSM55 (5'-TAGTAGTAGACTCC-3') [21]. First and nested PCR were performed in 25 μl reaction mixtures containing 200 μM dNTP (Elpis Biotech, USA), 0.25 U of Super-Therm Taq DNA polymerase (JMR Holdings, UK), 1.5 μl of DNA, and 5 pM of each primer. Oligonucleotide primer sequences for the first and nested PCR were specifically designed for HTNV. For the first and nested PCR, the initial denaturation was performed at 94°C for 4 min, followed by six cycles of denaturation at 94°C for 30 sec, annealing at 37°C for 30 sec, elongation at 72°C for 1 min, followed by 32 cycles of denaturation at 94°C for 30 sec, annealing at 42°C for 30 sec, elongation at 72°C for 1 min, and then elongation at 72°C for 5 min. PCR products were extracted using a PCR Purification Kit (Cosmo GENETECH, Korea), and DNA sequencing performed in an Automatic Sequencer, Model ABI 3730XL DNA Analyzer (Applied Biosystems, USA).
Whole genome sequencing
Viral cDNA was synthesized with random hexamers or OSM55. PCR was performed using specific primer sets covering the whole tripartite genome of HTNV. The PCR program was as follows: a cycle of 95°C for 5 min, 6 cycles of 95°C for 15 sec, 35°C for 30 sec, and 72°C for 20 sec, 30 cycles of 95°C for 15 sec, 42°C for 30 sec, and 72°C for 20 sec and then a cycle of 72°C for 3 min.
Phylogenetic analysis
The nucleotide sequences of HTNV L, M, and S segments were determined from virus-infected lungs of A. agrarius. Sequences were aligned and compared with HTNV sequences available in GenBank, using the ClustalW tool in the Lasergene program, version 5 (DNASTAR, USA). For the phylogenetic analysis, the Neighbor-Joining (NJ), Maximum Likelihood (ML), and Bayesian methods (MrBayes 3.2.2 Program) were used. Topologies were evaluated by a bootstrap analysis of 1000 iterations.
Reassortment analyses
Alignments of HTNV sequences were analyzed using RDP, GENECONV, MAXCHI, CHIMAERA, 3SEQ, BOOTSCAN, and SISCAN in the Recombination Detection Program 4 (RDP4) [22], with concatenated L, M, and S segments. P-values under 0.05 were considered statistically significant. All parameters were left at the default RDP settings. The whole genome sequences of HTNV from reassortants, parents, and in- and out-groups were aligned and used to construct maximum likelihood trees of the individual segment in MEGA 5.2 [23].
Results
Rodent collection
From 2003–2014, a total of 5,929 striped field mice, A. agrarius, were collected in Cheorwon, Chuncheon, Hwacheon, Inje, Pyeongchang, Yanggu, and Yangyang in Gangwon province, and Dongducheon, Paju, Pocheon, Phyeongtaek, and Yeoncheon in Gyeonggi province (Table 1). The geographic distribution of rodent trapping sites included military training sites [Dagmar North (DN), Twin Bridge Training Area North (TBTA-N), and Twin Bridge Training Area South (TBTA-S) in Paju; Nightmare Range (NR) in Pocheon; Fire Point 131 (FP131) in Yeoncheon] (Fig 1).
Table 1. Total number and screening results of A. agrarius collected from Gyeonggi and Gangwon provinces in the Republic of Korea, 2003–2014.
| Province | District | Year | No. A. agrarius sampled | No. seropositive/ No. sampled (%) | No. RT-PCR positive/ Number seropositive (%) |
|---|---|---|---|---|---|
| Gangwon | Cheorwon | 2014 | 67 | 6/67 (9.0) | 5/6 (83.3) |
| Hwacheon | 2014 | 63 | 6/63 (9.5) | 3/6 (50) | |
| Inje | 2013 | 52 | 2/52 (3.8) | 0/2 (0) | |
| 2014 | 16 | 1/16 (6.3) | 0/1 (0) | ||
| Pyeongchang | 2012 | 53 | 4/53 (7.5) | 0/4 (0) | |
| 2013 | 87 | 3/87 (3.4) | 0/3 (0) | ||
| 2014 | 34 | 0/34 (0) | Not determined | ||
| Yanggu | 2014 | 50 | 10/50 (20) | 10/10 (100) | |
| Yangyang | 2014 | 16 | 1/16 (6.3) | 0/1 (0) | |
| Chuncheon | 2012 | 26 | 1/26 (3.8) | 0/1 (0) | |
| 2013 | 64 | 2/64 (3.1) | 0/2 (0) | ||
| Gyeonggi | Dongducheon | 2012 | 21 | 0/21 (0) | Not determined |
| Paju | 2003 | 594 | 100/594 (16.8) | 81/100 (81) | |
| 2005 | 717 | 200/717 (27.9) | 135/200 (67.5) | ||
| 2010 | 628 | 109/628 (17.4) | 92/109 (84.4) | ||
| 2012 | 50 | 2/50 (4) | 2/2 (100) | ||
| 2013 | 22 | 6/22 (27) | 5/6 (83) | ||
| 2014 | 79 | 20/79 (25.3) | 20/20 (100) | ||
| Pocheon | 2003 | 140 | 16/140 (11.4) | 12/16 (75) | |
| 2005 | 87 | 12/87 (13.8) | 2/12 (16.7) | ||
| 2009 | 1,112 | 82/1,112 (7.4) | 62/82 (75.6) | ||
| 2014 | 29 | 5/9 (55.6) | 4/5 (80) | ||
| Pyeongtaek | 2003 | 24 | 0/24 (0) | Not determined | |
| 2009 | 837 | 17/837 (2) | 9/17 (52.9) | ||
| 2010 | 87 | 1/87 (1.1) | 0/1 (0) | ||
| 2012 | 33 | 0/33 (0) | Not determined | ||
| Yeoncheon | 2003 | 309 | 58/309 (18.8) | 53/58 (91.4) | |
| 2005 | 593 | 97/593 (16.4) | 38/97 (39.2) | ||
| 2013 | 24 | 10/24 (42) | 8/10 (80) | ||
| 2014 | 15 | 3/15 (20) | 3/3 (100) | ||
| Total | 5,929 | 774/5,929 (13.1) | 544/756 (70.3) | ||
Serological and molecular screening for HTNV in wild rodents
To examine the positivity of anti-HTNV IgG, immunofluorescence antibody (IFA) test was performed using sera collected from A. agrarius. A total of 774/5,929 (13.1%) A. agrarius were seropositive for anti-HTNV IgG. Among the 774 samples, 36 (4.7%) and 738 (95.3%) of the rodents were captured in Gangwon and Gyeonggi provinces, respectively. Fig 2 showed the number of A. agrarius which was positive for anti-HTNV IgG from trapping sites. Anti-HTNV IgG in A. agrarius from Chuncheon and Dongducheon was not detected. Total RNA was extracted from the lung tissues of seropositive samples and determined for the presence of HTNV RNA by RT-PCR. A 393-nucleotide partial sequence of HTNV M segment was recovered from 544/774 (70.3%) of the seropositive samples; including 18 (3.3%) and 526 (96.7%) A. agrarius in Gangwon and Gyeonggi provinces, respectively (Fig 2).
Fig 2. The serological and molecular prevalence of HTNV from A. agrarius in Gangwon and Gyeonggi provinces.
A total of 5,929 A. agrarius were captured in the Gangwon and Gyeonggi provinces from 2003–2014. A total of 774 A. agrarius were seropositive for anti-HTNV IgG (shown by black bars). Grey bars represent 544/774 A. agrarius that were positive for the partial HTNV M segment. Arabic number above the bars indicates the number of IFA and RT-PCR positive rodents, respectively.
Whole genome sequencing
To obtain the whole tripartite genome sequences of HTNV, conventional PCR was performed using multiple primer sets. The whole genome sequencing of the HTNV L, M, and S segments was accomplished for 34 HTNV strains, including 3 strains from Cheorwon, 2 strains from Hwacheon, 6 strains from Yanggu, 6 strains from DN in Paju, 8 strains from TBTA-N and -S in Paju, 3 strains from NR in Pocheon, and 6 strains from FP131 in Yeoncheon (Table 2). The 5´ and 3´ end sequences of HTNV L, M, and S segments were determined by Rapid Amplification of cDNA Ends (RACE) experiments.
Table 2. Accession number of the whole genome sequences of HTNV collected from Gyeonggi and Gangwon provinces.
| No | Strain | Collection site | Accession number | ||
|---|---|---|---|---|---|
| L seg | M seg | S seg | |||
| 1 | Aa03-161 | FP131 (Yeoncheon) | KT934956 | KT934990 | KT935024 |
| 2 | Aa03-386 | FP131 (Yeoncheon) | KT934957 | KT934991 | KT935025 |
| 3 | Aa03-387 | FP131 (Yeoncheon) | KT934958 | KT934992 | KT935026 |
| 4 | Aa05-190 | DN (Paju) | KT934959 | KT934993 | KT935027 |
| 5 | Aa05-241 | DN (Paju) | KT934960 | KT934994 | KT935028 |
| 6 | Aa05-249 | DN (Paju) | KT934961 | KT934995 | KT935029 |
| 7 | Aa05-331 | FP131 (Yeoncheon) | KT934962 | KT934996 | KT935030 |
| 8 | Aa05-771 | FP131 (Yeoncheon) | KT934963 | KT934997 | KT935031 |
| 9 | Aa05-775 | FP131 (Yeoncheon) | KT934964 | KT934998 | KT935032 |
| 10 | Aa09-652 | NR (Pocheon) | KT934965 | KT934999 | KT935033 |
| 11 | Aa09-948 | NR (Pocheon) | KT934966 | KT935000 | KT935034 |
| 12 | Aa09-1000 | NR (Pocheon) | KT934967 | KT935001 | KT935035 |
| 13 | Aa10-123 | TBTA-S (Paju) | KT934968 | KT935002 | KT935036 |
| 14 | Aa10-288 | TBTA-S (Paju) | KT934969 | KT935003 | KT935037 |
| 15 | Aa10-434 | TBTA-N (Paju) | KT934970 | KT935004 | KT935038 |
| 16 | Aa10-518 | TBTA-N (Paju) | KT934971 | KT935005 | KT935039 |
| 17 | Aa10-561 | TBTA-N (Paju) | KT934972 | KT935006 | KT935040 |
| 18 | Aa10-679 | TBTA-S (Paju) | KT934973 | KT935007 | KT935041 |
| 19 | Aa14-172 | DN (Paju) | KT934974 | KT935008 | KT935042 |
| 20 | Aa14-188 | DN (Paju) | KT934975 | KT935009 | KT935043 |
| 21 | Aa14-198 | DN (Paju) | KT934976 | KT935010 | KT935044 |
| 22 | Aa14-204 | TBTA-S (Paju) | KT934977 | KT935011 | KT935045 |
| 23 | Aa14-207 | TBTA-S (Paju) | KT934978 | KT935012 | KT935046 |
| 24 | Aa14-266 | Hwacheon | KT934979 | KT935013 | KT935047 |
| 25 | Aa14-272 | Hwacheon | KT934980 | KT935014 | KT935048 |
| 26 | Aa14-362 | Cheorwon | KT934981 | KT935015 | KT935049 |
| 27 | Aa14-368 | Cheorwon | KT934982 | KT935016 | KT935050 |
| 28 | Aa14-386 | Cheorwon | KT934983 | KT935017 | KT935051 |
| 29 | Aa14-404 | Yanggu | KT934984 | KT935018 | KT935052 |
| 30 | Aa14-406 | Yanggu | KT934985 | KT935019 | KT935053 |
| 31 | Aa14-408 | Yanggu | KT934986 | KT935020 | KT935054 |
| 32 | Aa14-412 | Yanggu | KT934987 | KT935021 | KT935055 |
| 33 | Aa14-423 | Yanggu | KT934988 | KT935022 | KT935056 |
| 34 | Aa14-438 | Yanggu | KT934989 | KT935023 | KT935057 |
Phylogenetic analyses
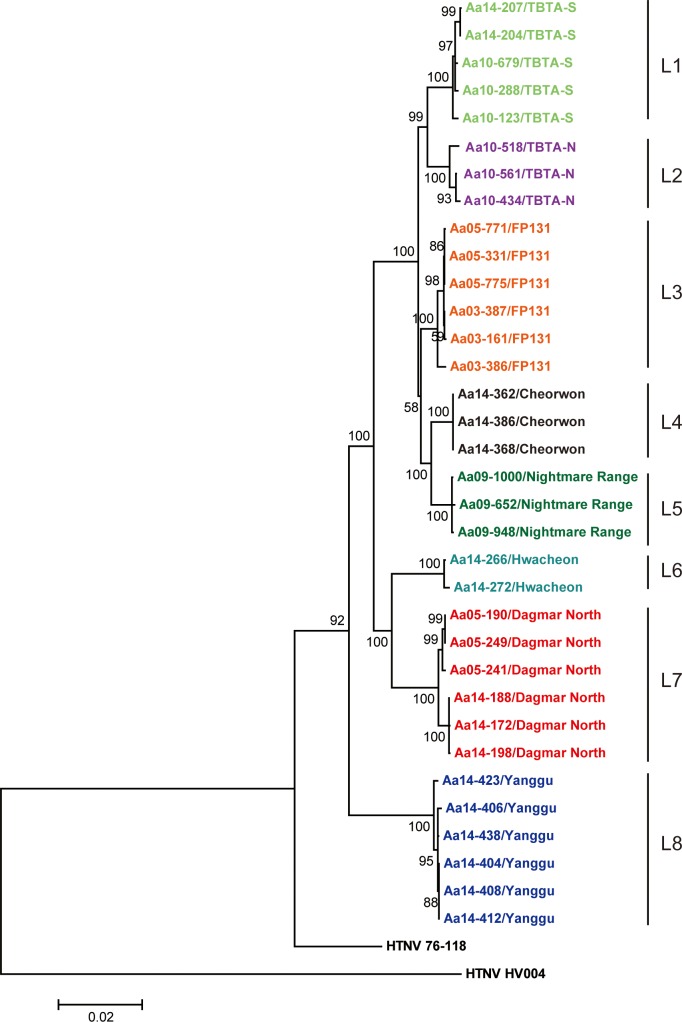
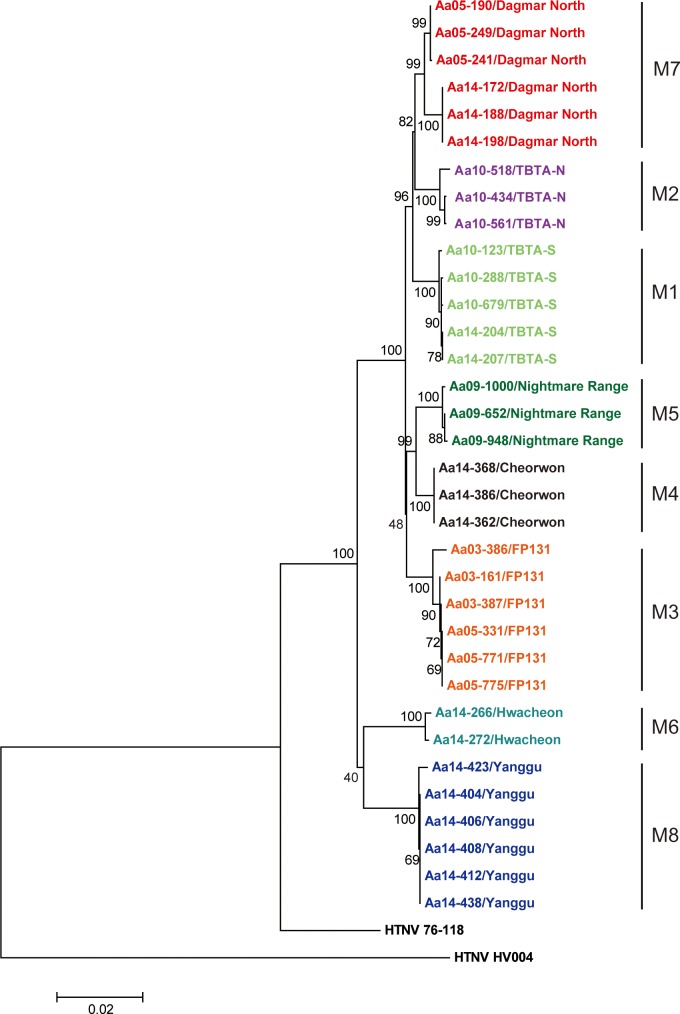
The full-length sequences of HTNV tripartite RNA genomes were phylogenetically analyzed by Neighbor-Joining (NJ), Maximum Likelihood (ML), and Bayesian methods. The genomic sequences of HTNV L, M, and S segments formed geographic clusters, respectively (Figs 3–5). For the HTNV L segment, the L4 group (Cheorwon) phylogenetically grouped closely with the L5 group (Pocheon). The L8 group (Yanggu) formed a group that was distinct from all other strains from Gyeonggi province. The L6 group (Hwacheon) formed a phylogenetic cluster with the L7 group (DN, Paju). Phylogenetic analyses of the HTNV M and S segments demonstrated that the M4 and S4 groups (Cheorwon) clustered with the M5 and S5 groups (Pocheon). The M and S segments of HTNV strains from Yanggu (M8 and S8) and Hwacheon (M7 and S7) in Gangwon province formed a geographic and distinct clusters from strains obtained in Gyeonggi province. The percent of nucleotide and amino acid sequence homologies of HTNV newly acquired was shown in S1–S3 Tables.
Fig 3. Phylogenetic analysis of whole HTNV L segment genomic sequences in Gangwon and Gyeonggi provinces.
The full-length sequences of the HTNV L segment were obtained from lung tissues of A. agrarius in Gangwon and Gyeonggi provinces. A phylogenetic tree was generated by the ML method. Topologies were evaluated by bootstrap analyses of 1000 iterations. The TN93 (Tamura-Nei)+G model of evolution was used, based on an alignment of the entire nucleotide sequence of the L segment, including strains HTN 76–118 (X55901) and HTN HV004 (JQ083393).
Fig 5. Phylogenetic analysis of whole HTNV S segment genomic sequences in Gangwon and Gyeonggi provinces.
The full-length sequences of the HTNV S segment were obtained from lung tissues of A. agrarius in Gangwon and Gyeonggi provinces. A phylogenetic tree was generated by the ML method. Topologies were evaluated by bootstrap analyses of 1000 iterations. The T92+G model of evolution was used, based on an alignment of the nucleotide sequence of the S segment, including strains HTN 76–118 (M14626) and HTN HV004 (JQ083395).
Reassortment analysis
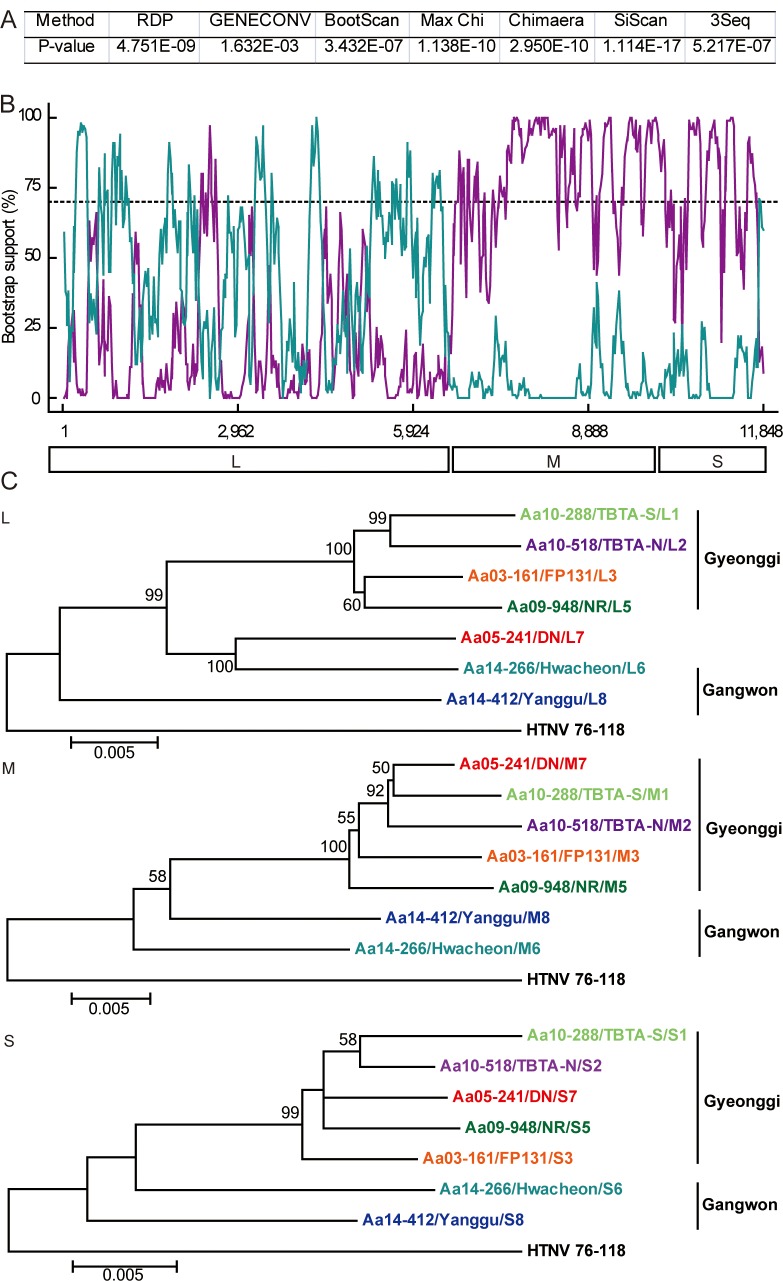
Phylogenetic analyses showed that the 7 group (DN, Paju) contained heterogeneous L segment which was clustered with the L6 group (Hwacheon) (Fig 3). However, the M7 and S7 segments of HTNV formed homogeneous clusters with the M2 and S2 groups (TBTA-N, Paju), respectively (Figs 4 and 5). To evaluate the possibility of a reassortment event in the HTNV genomes, the concatenated HTNV tripartite genomes from Gangwon and Gyeonggi provinces were aligned and analyzed using RDP4 software package. Fig 6A shows P-values ranging from 1.632E-02 to 1.114E-17 based on the various recombination/reassortment analysis programs. The RDP recombination consensus score (RDPRCS) of the 7 group (DN, Paju) is 0.522. Fig 6B shows high percent of bootstrap support for a grouping of the HTNV M and S segments from the 7 group (DN, Paju) and the 2 group (TBTA-N, Paju), whereas the L segment shows a high similarity between the 7 group (DN, Paju) and the 6 group (Hwacheon). Eight HTNV stains representing reassortants, parents, and in- and out- groups were used to generate maximum likelihood trees of the HTNV L, M, and S segments, respectively (Fig 6C). These results suggest that the 7 group (DN, Paju) were reassortants in nature based on the heterogeneity of the L segment, as the L segment formed a lineage with the 6 group (Hwacheon) in Gangwon province, while the M and S segments clustered with the 2 group (TBTA-N, Paju) in Gyeonggi province.
Fig 4. Phylogenetic analysis of whole HTNV M segment genomic sequences in Gangwon and Gyeonggi provinces.
The full-length sequences of the HTNV M segment were obtained from lung tissues of A. agrarius in Gangwon and Gyeonggi provinces. A phylogenetic tree was generated by the ML method. Topologies were evaluated by bootstrap analyses of 1000 iterations. The T92 (Tamura 3-parameter)+G model of evolution was used, based on an alignment of the entire nucleotide sequence of the M segment, including strains HTN 76–118 (M14627) and HTN HV004 (JQ083394).
Fig 6. Evidence of a possible genetic reassortment event between HTNV strains in the ROK.
(A) p-values from the RDP program. (B) The Bootscan plot was based on a pairwise distance model by the RDP4 algorithm. A Bootscan Support Percent of over 70% (cutoff value) was considered significant. Cyan color represents for the comparison of the 7 group (DN, Paju) to the 6 group (Hwacheon); Violet color represents for the comparison of the 7 group (DN, Paju) to the 2 group (TBTA-N, Paju). (C) A reassortant, in-, and out-groups of HTNV were phylogenetically analyzed by the construction of individual ML trees for the L, M, and S segments. The reassortant (the 7 group) is shown in red color. The 6 group (Hwacheon) and the 2 group (TBTA-N, Paju) are indicated by cyan and violet colors, respectively.
Discussion
HFRS is highly endemic in Gangwon and Gyeonggi provinces, ROK, affecting both military personnel and civilians. For decades, we conducted epidemiological and phylogeographic analyses of HTNV in Gyeonggi province. Recent clinical cases of HFRS in Gangwon province, including one deceased patient in 2013, prompted us to investigate the geographic distribution and diversity of HTNV in Gangwon province [24].
A total of 5,929 A. agrarius were collected in the endemic areas of Gangwon and Gyeonggi provinces from 2003–2014. IFA tests showed that 774 (13.1%) A. agrarius were seropositive for anti-HTNV IgG. HTNV-specific RT-PCR showed that 544/774 (70.3%) A. agrarius were positive for a partial HTNV M segment. Based on the serological and molecular tests, there was a lower prevalence of HTNV infections among A. agrarius in Gangwon province than in Gyeonggi province. Although the number of A. agrarius was much lower for Gangwon province, these data suggest that Gyeonggi province poses significantly higher HFRS health risks than Gangwon province [8, 9, 14, 15]. To support this, the incidence of HFRS patients in Gangwon province was lower than that in Gyeonggi province, demonstrating a correlation between rodent seroprevalence and human disease data [25].
Using the complete genomic sequences of 34 HTNV strains newly obtained from eight different trapping sites, the phylogenetic trees of HTNV L, M, and S segments demonstrated well-supported geographic clusters and showed the genetic diversity in the restricted areas. Diversification of HTNV, the Old World hantavirus, has been observed in China, a high HFRS-endemic area [20]. In Guizhou, high level of the molecular diversity of HTNV strains were observed. The ADNV, a New World hantavirus from southern South America, also exhibited high molecular diversity of the M segment, resulting in five different lineages based on their geographic origins in Argentina and Chile [26, 27]. Consistent with these observations, HTNV strains in the restricted areas of the ROK showed a high molecular diversity representing geographic distinct clusters.
Reassortment, recombination, and genetic drift are molecular genetic mechanisms that confer the genetic diversity in RNA viruses in nature [28]. Segmented RNA viruses preferentially give rise to genetic reassortment rather than recombination. A reassortment event predicted by RDP4 was considered significant if it satisfied at least 2 criteria with a P-value (p) <0.05 and the RDPRCS was >0.6 [29]. When P <0.05 and the RDPRCS was between 0.4 and 0.6, the genetic event was considered possible. An RDPRCS under 0.4 with P <0.05 was a cause to reject the genetic event. The HTNV strains (the 7 group) from DN were likely reassortants since these strains showed P <0.05 and an RDPRCS of 0.522 (HTNV from Hwacheon, 0.322; HTNV from TBTA-N, 0.156).
The genetic reassortment of bunyaviruses in nature has been previously reported [30, 31]. Reassortments of SNV have more commonly been described in M segments than S or L segments in nature and in vitro [18, 31]. The L, M, and S segments of hantaviruses encode the viral RNA-dependent RNA-polymerase (the L protein), two surface glycoproteins (Gn and Gc), and the nucleocapsid (N), respectively [32]. The less common reassortants of L or S segments than M segment might be associated with the function of these viral proteins. The maintenance of L and S segments might be beneficial for replication, transcription, and assembly, resulting in the production of the progeny possessed appropriate viral fitness. Still, given our limited understanding of this genetic mechanism, the existence of reassortants that contain different combinations of segments should not be ignored. Infection with Guaroa virus generated the L segment reassortant, whereas the S segment reassortant was observed between Bunyamwera and California encephalitis viruses [33]. In this study, the reassortment analysis demonstrated that HTNV (the 7 group) from DN in Paju showed heterogeneous L segment, but homogeneous M and S segments. Whether the exchange of L segment is a determinant of the fitness and pathogenesis of HTNV remains to be studied.
In conclusion, we report the phylogeographic analysis of full-length HTNV sequences from A. agrarius collected in HFRS-endemic areas of Gangwon and Gyeonggi provinces, ROK. These results demonstrate the geographic diversity and a possible reassortment of HTNV in nature. This study greatly increases our understanding of the genetic diversity and molecular evolution of HTNV in the hantavirus-endemic areas. The whole sequences of HTNV tripartite genomes will provide a database for the phylogeographic analysis and surveillance of hantavirus infection from HFRS patients and natural reservoir hosts.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank to Dr. Luck Ju Baek for scientific discussion and rodent capture, Mr. Su-Am Kim for his expert with trapping rodents, and the Commanders and their soldiers of the 5th Medical Detachment, 168th Multifunctional Medical Battalion, 65th Medical Brigade for their support and collection of small mammals at US/ROK operated training sites and US military installations. We thank to Mr. Charles Hong of the Defense Threat Reduction Agency (DTRA) for scientific discussion.
Data Availability
All relevant data are within the paper. All sequences are available from the NCBI database (accession numbers KT934956-KT935057).
Funding Statement
This work was supported by a grant from Agency for Defense Development (UE134020ID) to JWS. Partial funding was provided by the Armed Forces Health Surveillance Center-Global Emerging Infections Surveillance and Response System (AFHSC-GEIS), Silver Spring, MD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Plyusnin A, Vapalahti O, Vaheri A. Hantaviruses: genome structure, expression and evolution. J Gen Virol. 1996;77 (Pt 11):2677–87. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 2.MacNeil A, Ksiazek TG, Rollin PE. Hantavirus pulmonary syndrome, United States, 1993–2009. Emerg Infect Dis. 2011;17(7):1195–201. Epub 2011/07/19. 10.3201/eid1707.101306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruger DH, Figueiredo LT, Song JW, Klempa B. Hantaviruses—globally emerging pathogens. J Clin Virol. 2015;64:128–36. Epub 2014/12/03. 10.1016/j.jcv.2014.08.033 . [DOI] [PubMed] [Google Scholar]
- 4.Krautkramer E, Zeier M, Plyusnin A. Hantavirus infection: an emerging infectious disease causing acute renal failure. Kidney Int. 2013;83(1):23–7. Epub 2012/11/16. 10.1038/ki.2012.360 . [DOI] [PubMed] [Google Scholar]
- 5.Macneil A, Nichol ST, Spiropoulou CF. Hantavirus pulmonary syndrome. Virus Res. 2011;162(1–2):138–47. Epub 2011/09/29. 10.1016/j.virusres.2011.09.017 . [DOI] [PubMed] [Google Scholar]
- 6.Lahdevirta J. Nephropathia epidemica in Finland. A clinical histological and epidemiological study. Annals of clinical research. 1971;3:1–54. Epub 1971/01/01. . [PubMed] [Google Scholar]
- 7.Song JW, Song KJ, Baek LJ, Lee YJ, Jung KM, Go EY, et al. Serological Study on Hantavirus Infection of Wild Rodents Captured in the Mountains of Kangwon Province in Korea. Journal of Bacteriology and Virology. 1998;28(3):287–93. [Google Scholar]
- 8.Klein TA, Kim HC, Chong ST, O'Guinn ML, Lee JS, Turell MJ, et al. Hantaan virus surveillance in small mammals at firing points 10 and 60, Yeoncheon, Gyeonggi Province, Republic of Korea. Vector Borne Zoonotic Dis. 2012;12(8):674–82. Epub 2012/05/23. 10.1089/vbz.2011.0618 . [DOI] [PubMed] [Google Scholar]
- 9.Song JW, Moon SS, Gu SH, Song KJ, Baek LJ, Kim HC, et al. Hemorrhagic fever with renal syndrome in 4 US soldiers, South Korea, 2005. Emerg Infect Dis. 2009;15(11):1833–6. Epub 2009/11/07. 10.3201/eid1511.090076 ; PubMed Central PMCID: PMCPmc2857219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S-H, Chung B-H, Lee W-C, Choi I-S. Epidemiology of hemorrhagic fever with renal syndrome in Korea, 2001–2010. Journal of Korean medical science. 2013;28(10):1552–4. 10.3346/jkms.2013.28.10.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song JY, Chun BC, Kim SD, Baek LJ, Kim S-H, Sohn JW, et al. Epidemiology of hemorrhagic fever with renal syndrome in endemic area of the Republic of Korea, 1995–1998. Journal of Korean medical science. 2006;21(4):614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein TA, Kang HJ, Gu SH, Moon S, Shim SH, Park YM, et al. Hantaan virus surveillance targeting small mammals at Dagmar North Training Area, Gyeonggi Province, Republic of Korea, 2001–2005. J Vector Ecol. 2011;36(2):373–81. Epub 2011/12/02. 10.1111/j.1948-7134.2011.00178.x . [DOI] [PubMed] [Google Scholar]
- 13.O'Guinn ML, Klein TA, Lee JS, Kim HC, Baek LJ, Chong ST, et al. Ecological surveillance of small mammals at Firing Points 10 and 60, Gyeonggi Province, Republic of Korea, 2001–2005. J Vector Ecol. 2008;33(2):370–84. Epub 2009/03/07. . [DOI] [PubMed] [Google Scholar]
- 14.Sames WJ, Klein TA, Kim HC, Chong ST, Lee IY, Gu SH, et al. Ecology of Hantaan virus at Twin Bridges Training Area, Gyeonggi Province, Republic of Korea, 2005–2007. J Vector Ecol. 2009;34(2):225–31. Epub 2010/09/15. 10.1111/j.1948-7134.2009.00030.x . [DOI] [PubMed] [Google Scholar]
- 15.Klein TA, Kim HC, Chong ST, Kim JA, Lee SY, Kim WK, et al. Hantaan virus surveillance targeting small mammals at nightmare range, a high elevation military training area, gyeonggi province, republic of Korea. PLoS One. 2015;10(4):e0118483 Epub 2015/04/16. 10.1371/journal.pone.0118483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010;7(6):440–51. Epub 2010/06/15. 10.1016/j.chom.2010.05.009 ; PubMed Central PMCID: PMCPmc2892379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijaykrishna D, Mukerji R, Smith GJ. RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion. PLoS pathogens. 2015;11(7):e1004902 10.1371/journal.ppat.1004902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson WW, Monroe MC, St Jeor SC, Thayer WP, Rowe JE, Peters CJ, et al. Naturally occurring Sin Nombre virus genetic reassortants. Virology. 1995;214(2):602–10. Epub 1995/12/20. 10.1006/viro.1995.0071 . [DOI] [PubMed] [Google Scholar]
- 19.Kirsanovs S, Klempa B, Franke R, Lee MH, Schonrich G, Rang A, et al. Genetic reassortment between high-virulent and low-virulent Dobrava-Belgrade virus strains. Virus Genes. 2010;41(3):319–28. Epub 2010/08/25. 10.1007/s11262-010-0523-2 . [DOI] [PubMed] [Google Scholar]
- 20.Zou Y, Hu J, Wang ZX, Wang DM, Li MH, Ren GD, et al. Molecular diversity and phylogeny of Hantaan virus in Guizhou, China: evidence for Guizhou as a radiation center of the present Hantaan virus. J Gen Virol. 2008;89(Pt 8):1987–97. Epub 2008/07/18. 10.1099/vir.0.2008/000497-0 . [DOI] [PubMed] [Google Scholar]
- 21.Song JW, Gu SH, Bennett SN, Arai S, Puorger M, Hilbe M, et al. Seewis virus, a genetically distinct hantavirus in the Eurasian common shrew (Sorex araneus). Virol J. 2007;4:114 Epub 2007/10/31. 10.1186/1743-422x-4-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evolution. 2015;1(1):vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9. Epub 2011/05/07. 10.1093/molbev/msr121 ; PubMed Central PMCID: PMCPmc3203626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epidemiological investigation for HFRS dead soldier. 2013. Available from: http://news.kbs.co.kr/news/view.do?ref=A&ncd=2770142.
- 25.Lee SH, Chung BH, Lee WC, Choi IS. Epidemiology of hemorrhagic fever with renal syndrome in Korea, 2001–2010. J Korean Med Sci. 2013;28(10):1552–4. 10.3346/jkms.2013.28.10.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levis S, Morzunov SP, Rowe JE, Enria D, Pini N, Calderon G, et al. Genetic diversity and epidemiology of hantaviruses in Argentina. J Infect Dis. 1998;177(3):529–38. Epub 1998/03/14. . [DOI] [PubMed] [Google Scholar]
- 27.Padula PJ, Colavecchia SB, Martinez VP, Gonzalez Della Valle MO, Edelstein A, Miguel SD, et al. Genetic diversity, distribution, and serological features of hantavirus infection in five countries in South America. J Clin Microbiol. 2000;38(8):3029–35. Epub 2000/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon-Loriere E, Holmes EC. Why do RNA viruses recombine? Nat Rev Microbiol. 2011;9(8):617–26. Epub 2011/07/05. 10.1038/nrmicro2614 ; PubMed Central PMCID: PMCPmc3324781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Z, Deng F, Han N, Wang H, Sun S, Zhang Y, et al. Reassortment and migration analysis of Crimean-Congo haemorrhagic fever virus. J Gen Virol. 2013;94(Pt 11):2536–48. Epub 2013/08/14. . [DOI] [PubMed] [Google Scholar]
- 30.Li D, Schmaljohn AL, Anderson K, Schmaljohn CS. Complete nucleotide sequences of the M and S segments of two hantavirus isolates from California: evidence for reassortment in nature among viruses related to hantavirus pulmonary syndrome. Virology. 1995;206(2):973–83. Epub 1995/02/01. 10.1006/viro.1995.1020 . [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez LL, Owens JH, Peters CJ, Nichol ST. Genetic reassortment among viruses causing hantavirus pulmonary syndrome. Virology. 1998;242(1):99–106. Epub 1998/03/17. 10.1006/viro.1997.8990 . [DOI] [PubMed] [Google Scholar]
- 32.Vaheri A, Strandin T, Hepojoki J, Sironen T, Henttonen H, Makela S, et al. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol. 2013;11(8):539–50. Epub 2013/09/11. . [DOI] [PubMed] [Google Scholar]
- 33.Briese T, Calisher CH, Higgs S. Viruses of the family Bunyaviridae: are all available isolates reassortants? Virology. 2013;446(1–2):207–16. Epub 2013/10/01. 10.1016/j.virol.2013.07.030 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper. All sequences are available from the NCBI database (accession numbers KT934956-KT935057).